Abstract
Living cells detect and process external signals using signaling pathways that are affected by random fluctuations. These variations cause the behavior of individual cells to fluctuate over time (behavioral variability) and generate phenotypic differences between genetically identical individuals (phenotypic diversity). These two noise sources reduce our ability to predict biological behavior because they diversify cellular responses to identical signals. Here, we review recent experimental and theoretical advances in understanding the mechanistic origin and functional consequences of such variation in Escherichia coli chemotaxis—a well-understood model of signal transduction and behavior. After briefly summarizing the architecture and logic of the chemo-taxis system, we discuss determinants of behavior and chemotactic performance of individual cells. Then, we review how cell-to-cell differences in protein abundance map onto differences in individual chemotactic abilities and how phenotypic variability affects the performance of the population. We conclude with open questions to be addressed by future research.
Keywords: fluctuations, navigation, signal transduction, single-cell behavior, chemical sensing, adaptation
1. INTRODUCTION
Chemotaxis signaling pathways allow bacteria to sense and respond to external chemical signals. In the case of Escherichia coli, decades of research have made this system into one of the best-characterized signal transduction pathways in biology. Many design principles of the pathway are shared with other biological systems, and, as a result, this system has become a paradigm for the study of complex cellular functions, including signal detection, amplification and processing, adaptation and memory, decision making, and motility and navigation.
Many of these results were originally obtained using population-averaged methods. Although these approaches were effective in accurately dissecting the pathway, they also necessarily discarded information about behavioral variability and phenotypic heterogeneity. Still, from Antony van Leeuwenhoek’s initial observation of so-called animalcules (42) in the 17th century to Howard Berg’s (14) three-dimensional (3D) tracking of individual E. coli cells in 1972, the individual nature of single bacteria has always captured the interest of adventurous researchers. Over the past decade, improved single-cell experimental techniques have provided quantitative insights that have rapidly increased interest in this branch of research.
In this article, we focus on chemotaxis from the single-cell perspective. Comprehensive reviews of bacterial chemotaxis in E. coli are already available, so for simplicity we summarize the essential details of the pathway and of its modeling in the introduction. For more in-depth information, we refer the reader to the many recent reviews that describe this system from the molecular and cellular (10, 23, 67, 106, 121), biophysical (39, 100, 125, 136), evolutionary (149), and historical (58) perspectives. After the introduction, we focus on the behavior and chemotactic performance of the individual cell and how intracellular fluctuations are thought to affect them. Then, we discuss what makes two isogenic cells that express the same chemotaxis genes exhibit different chemotactic phenotypes. Finally, we examine the functional consequences of nongenetic diversity for the individual cell and for the population.
1.1. The Bacterial Chemotaxis Strategy
At 1–2 microns long, an E. coli cell is too small to detect gradients of small molecules of interest over its body length (15). To surmount this problem, it performs a random walk by alternating straight motions (runs) with abrupt changes of direction (tumbles). If a cell detects an increase in attractant, tumbles are suppressed, which extends runs in the desired direction (14). Detecting an increase in repellent increases the probability to tumble. Over many runs and tumbles, this strategy results in a net motion toward attractants and away from repellents (Figure 1a). Temporal comparison of signal concentrations, which determines whether an increase is detected, require memory (14, 94). In E. coli, this is implemented as a negative integral feedback (11, 115, 150). The run-and-tumble strategy is used by many organisms such as worms, fly larvae, and even robots to navigate gradients whenever local directional information is unreliable (see Reference 90 and references therein).
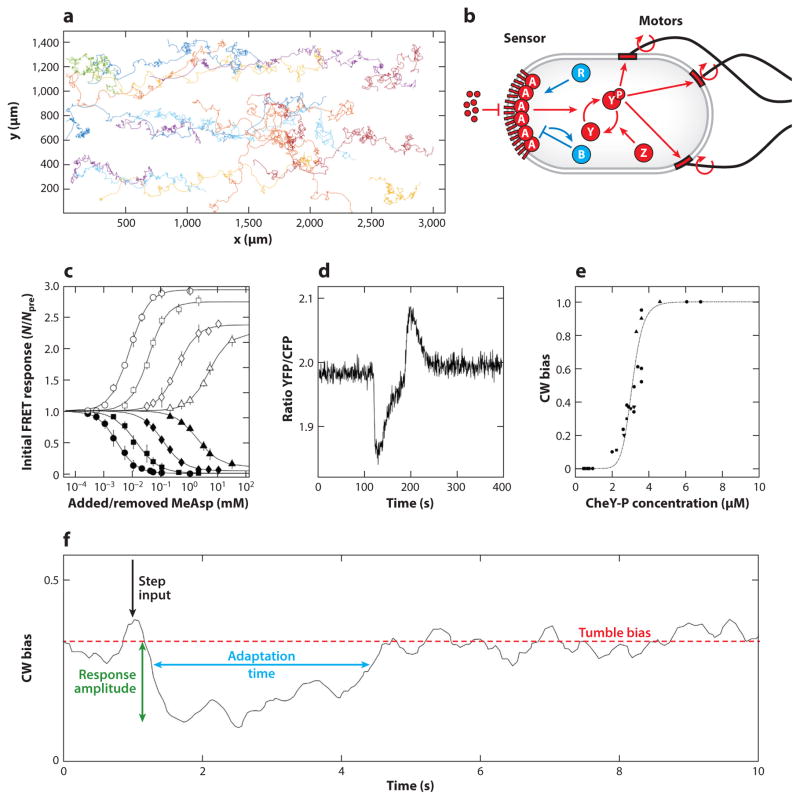
Figure 1.
Bacterial chemotaxis. (a) Trajectories of individual Escherichia coli cells (RP437) climbing a linear gradient of attractant [0.1 mM/mm α-methyl-DL-aspartate (MeAsp) increasing with x; 4 min trajectories at 20 frames/s]. (b) Signals are detected through transmembrane receptors, which, together with the adaptor protein CheW and the kinase CheA, form a complex that amplifies the signals via allosteric interactions. Changes in kinase activity are rapidly communicated to the flagellar motors through phosphorylation of the response regulator CheY. The phosphatase CheZ opposes the kinase activity of CheA. At a slower timescale, adaptation of the receptor complex to its steady-state activity level is mediated by CheR and CheB, which methylate and demethylate receptors at rates that depend on receptor activity level. Methylation desensitizes the receptors to ligand while respectively increasing receptor activity. Abbreviations: A, CheA; B, CheB; P, P, phosphorylated form of the protein; R, CheR; Y, CheY; Z, CheZ. (c) Initial response of the kinase activity in a population of ~400 cells to step increase (closed symbols) and decrease (open symbols) of MeAsp after complete adaptation to background concentrations (from left to right) of 0, 0.1, 0.5, and 5 mM, measured using Förster resonance energy transfer (FRET) between CheY-YFP and CheZ-CFP. Abbreviations: N, number of FRET pairs after the stimulus; Npre, number of FRET pairs before the stimulus. (d ) Kinase (FRET) activity as a function of time in response to addition and removal of 10 μM of MeAsp. Abbreviations: CFP, cyan fluorescent protein; YFP, yellow fluorescent protein. (e) Probability of individual motors to spin clockwise (CW) as a function of CheY-P concentration, Yp, measured simultaneously in individual cells. Line: with K = 3.1 μM and n = 10.3. (f ) Step response of the E. coli chemotaxis system to L-aspartate or MeAsp measured using the tethered cell assay and averaging over 227 records comprising 5,040 reversals of 10 cells responding to either signal. Colors indicate key functional parameters of the chemotaxis system. Panels b, c, d, e, and f are adapted with permission from References 55, 122, 124, 36, and 115, respectively.
1.2. The Chemotaxis Signaling Pathway
Homologs of the main molecular components of the chemotaxis signaling pathway are conserved across many species (4). Transmembrane chemoreceptors bind ligands in the periplasmic region and control the activity of the histidine auto-kinase CheA via the coupling protein CheW. The chemoreceptors form homodimers, which assemble into trimers of dimers (73). Two trimers of dimers and two CheW proteins control a dimer of CheA and are arranged hexagonally in large signaling clusters localized at the poles and at future division sites (23, 24, 67, 88, 106). CheA phosphorylates the response regulator CheY into the active form CheY-P, which diffuses throughout the cell and interacts with the flagellar motors to raise the probability of tumbling. The phosphatase CheZ localizes near the receptor clusters by binding to the short form of the kinase CheAs and rapidly dephosphorylates CheY-P, increasing the time-resolution of information transfer between the receptors and the motors (0.1–0.5 s). Receptors can have either active or inactive conformations. Binding of an attractant to the receptors causes conformational changes in the receptors that shut down the activity of the associated kinase CheA. Cooperative interactions within the clusters of receptors strongly amplify this input signal (22, 45, 72, 98, 106, 122, 123) (Figure 1c). The resulting sudden decrease in CheA activity (Figure 1d) causes a drop in CheY-P concentration and a corresponding decrease in the probability to tumble (Figure 1e,f ).
If the concentration of attractant stays constant, the kinase activity in a population of cells slowly (1–30 s) adapts back to prestimulus levels (Figure 1d), therefore restoring the original behavior of the population of bacteria (Figure 1f ) (115). Adaptation is mediated by two antagonistic enzymes (Figure 1b): CheR adds methyl groups at multiple glutamate residues on inactive receptors, which tends to reactivate them, whereas CheB removes methyl groups and tends to deactivate active receptors. Phosphorylation of CheB by CheA enhances its activity. The dependency of CheR and CheB activity on the activity (conformation) of the receptors (7, 21, 134) has two important consequences. First, it ensures that adaptation is ligand-specific (i.e., adaptation to one ligand does not lead to the adaptation to other signals) (7, 80). Second, it ensures that, at steady state, when methylation and demethylation balance each other, the system returns to the same mean activity level (5, 11) (Figure 1f ). Mathematically, this architecture implements a negative integral feedback, with only one stable fixed point (150), a feature that is reflected in the bilobed average impulse response of the chemotaxis system to methyl-aspartate, which integrates to zero (115). Adaptation is further enhanced by the presence of a tethering site on the Tsr and Tar receptors that is distinct from the sites of methylation and helps recruit CheR and CheB to the receptors (7, 30, 98, 107, 148). This enables one enzyme to act on a so-called assistance neighborhood of receptors (49, 57, 80, 87, 108) and may also facilitate the mobility of CheR and CheB within the cluster (85, 108). In addition to activating the receptors, methylation desensitizes the receptors (116, 122). Finally, the packing of receptors slowly decreases upon prolonged stimulation with an attractant, contributing to the desensitization of the cell by reducing cooperativity (54). When combined with adaptation, these features enable the system to maintain the same response to relative signals over a wide range of background signals (81, 99) until gradual saturation of the methylation sites leads to imperfect adaptation in a ligand-dependent manner (80, 102). For recent reviews, see References 106, 125, and 136.
Escherichia coli swims by rotating flagella. When motors spin counterclockwise (CCW), the flagella form a corkscrew bundle that propels the cell forward in a run. Clockwise (CW) spinning of one or more of the motors favors the disruption of the bundle and causes tumbling (97, 139). Spontaneous switching of the motors between CCW and CW rotation results in characteristic run-and-tumble trajectories (Figure 1a). The average probability to spin CW (and therefore the average probability to tumble) is ultrasensitive with respect to the concentration of CheY-P, allowing cells to translate continuous concentration signal into discrete output (36) (Figure 1e). On much longer timescales, the motor itself adapts to persistent stimuli through changes in the number of FliM subunits that are incorporated in the motor and that bind CheY-P at the base of the motor (151). In addition to responding to CheY-P, the motor also responds to the mechanical load (13, 82, 145) and to the metabolic state of the cell (20, 53).
1.3. Assays Used to Quantify Bacterial Chemotaxis Behavior
A wealth of assays have been developed over the last few decades to analyze chemotaxis behavior at multiple scales. Key techniques are summarized here. Chemotaxis was initially assessed by quantifying the expansion of rings of cells chasing a self-generated gradient of nutrients on soft agar or by counting the relative number of cells able to swim from a reservoir into a capillary filled with attractant (2, 3). Light microscopy can be used to track the motion of individual cells in 2D and 3D (14, 94, 131) in environments controlled by microfluidics (110, 144) (Figure 1a). Tracking free-swimming behavior can be combined with quantification of flagellar state (139) and protein abundances in the same individual cells (44). Holding a swimming cell with optical trap(s) (101) can provide additional information about hydrodynamics (32) and flagellar states (97). Measurement of internal kinase activity (Figure 1b) is possible using Förster resonance energy transfer (FRET) between fluorescently labeled CheY and CheZ, providing direct measurement of receptor activity upstream of the motor (122, 123) (Figure 1c,d). Performing this measurement in single cells is now possible (38, 70, 140). The output of kinase activity—switching of individual motors—can be monitored by tethering a cell to glass by its flagella (115, 118) or by attaching a bead to a flagellum (36, 75, 105, 111) or to the motor hook (13) (Figure 1f ). Finally, fluorescence polarization, sensitive to homo-FRET between YFPs fused to receptors, provides in vivo measurements of biophysical changes in the receptor cluster (54, 141). In many cases, measurements have been performed on many single cells, then averaged to obtain parameter values of a typical cell. Thus, many of the techniques required to explore the differences between individual cells had been established before a general interest in nongenetic individuality arose.
1.4. Mathematical Modeling of the Bacterial Chemotaxis Pathway in Escherichia coli
The classical picture of the bacterial chemotaxis system that emerges from these studies is that of two highly nonlinear modules (Figure 1b–e). The sensory module, consisting of transmembrane receptors, receptor-associated kinases, and adaptation enzymes, amplifies input signals (Figure 1c). At the output, the motility module converts changes in the amount of CheY-P into the probability to run or tumble through the high sensitivity of the flagellar motors (Figure 1e). Cellular memory is implemented through the interaction of (de)methylases acting to alter receptor sensitivities back to a baseline. The resulting negative integral feedback architecture of this adaptation is precise (Figure 1d), which helps retain sensitivity over a wide dynamic range of signal concentrations. Over the years, these features have been encapsulated into a minimal model of the chemotaxis signaling pathway.
The receptor–kinase complex cooperatively changes conformation, switching between active and inactive states to determine the fractional kinase activity (22, 45, 72, 98, 122, 123, 136) , where is the free-energy difference between the active and inactive states in units of kBT , which depends linearly on the average methylation state 0 ≤ m ≤ 8 of the dimers with constants ε and ε0. L is the ligand concentration, i indexes receptor types, and Ni and are their effective cooperativity and dissociation constants. The activity a(m, L) determines the concentration of the response regulator CheY-P (74, 122) as Yp (a) = αa, where α is a constant. CheY-P determines the Poisson switching rates between CW and CCW rotation of the motors, with basal switching rate ω0, motor cooperativity g, and dissociation constant Kd (137). The methylation level follows adaptation kinetics (11, 47, 116) , where VR is constant and VB (a) depends on a because of the CheB phosphorylation feedback. In population measurements, VB (a) was found to be constant for small values of a and to increase linearly with activity for large values (a > 0.7) (116). The Michaelis-Menten form of the (de)methylation rates assumes that CheR (CheB) binds methylation sites only on receptors in the inactive (active) conformations (11, 47). Assuming instead that the enzymes work in the linear regime or that only the catalytic step depends on receptor activity, these rates become linear functions of activity (27, 35, 57).
This minimal model makes several simplifying assumptions, most notably that ligand binding, receptor conformation changes, and phosphorylation cascade (0.1–0.5 s) reach quasi-equilibrium on the slow timescale of (de)methylation reactions (1–30 s). These simplifying assumptions have been relaxed in many studies to examine various aspects of the signaling pathway in more detail, including allosteric interactions (see review in Reference 136) and their fluctuations (38, 119); adaptation dynamics and how they are affected by fluctuations (29, 38, 47, 70, 105, 108), energy dissipation (79, 113), saturation of the methylation sites that cause deviations from perfect adaptation (57, 80, 102, 108), nonlinearity of the CheB-P feedback (35), and localization to and motion within the clusters of receptors of CheR and CheB (85, 108); kinetics of dephosphorylation (27, 138); motor adaptation (43, 151); and coordination between motors (60, 97, 120).
2. CHEMOTAXIS OF AN INDIVIDUAL ESCHERICHIA COLI BACTERIUM
Although some of the experiments used to derive the minimal model of chemotaxis involved single-cell measurements, the data were in general averaged over a large number of cells to reduce noise. The extent to which the various assumptions of this model hold at the single-cell level is not a settled issue, given that averaging can mask fluctuations and nonlinear dependencies. In this section, we examine recent steps toward the characterization of single-cell chemotaxis behavior.
2.1. Spontaneous Fluctuations in the Sensory Module of an Adapted Cell
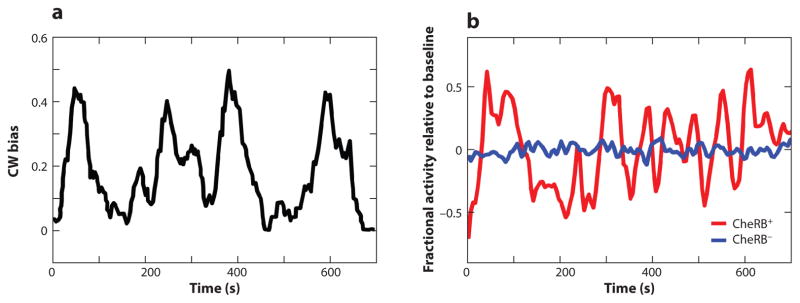
Cells experience significant fluctuations in unstimulated tumble bias over long timescales (76) (Figure 2a). This phenomenon, which is known as behavioral variability, is masked by measurements averaged over an entire population. Artificially increasing CheR copy number above wild-type levels reduces these fluctuations and blocking adaption or decoupling the motor from the signaling pathway eliminates them (76), suggesting that stochastic fluctuations in the futile cycle of methylation/demethylation mediated by CheR and CheB can generate behavioral variability (76, 105). When the enzymes of a futile cycle work at saturation, the output of the cycle can become very sensitive to small variations in the number or activity of the enzymes (56). Since there are only ~200–400 CheR and CheB molecules and ~60,000 potential methylation sites in a cell (86), CheR and CheB are likely to operate near saturation. This led to the theoretical proposal that saturated enzyme kinetics in the adaptation system of the chemotaxis pathway could contribute to the large variations in the behavior of adapted cells (47).
Figure 2.
(a) Clockwise (CW) bias of an individual motor on a wild-type cell averaged with a 30-s sliding window. Panel adapted with permission from Reference 76. (b) Single-cell Förster resonance energy transfer measurements of the fractional kinase activity minus its mean in a wild-type and in a CheRB− cell. Data are averaged with a 15-s sliding window. Panel adapted with permission from Reference 70.
The extent to which the futile cycle of methylation-demethylation amplifies fluctuations in receptor activity ultimately depends on how CheR and CheB interact with the receptors in the cluster. For example, noise is reduced if one assumes that the enzymes work in the linear regime or that only the catalytic step of the (de)methylation reactions depends on receptor activity (27, 57). While tethering of CheR/CheB to receptors enhances precise adaptation (49, 57), it also enhances fluctuations around the steady state by helping distribute enzymatic activity across the cluster (85) and amplifying fluctuations in the number of localized enzymes (108). The CheB-P feedback loop (Figure 1b) reduces the sensitivity of the steady-state activity to cell-to-cell variability in protein abundances while only slightly damping fluctuations (47, 74, 108).
Improvements in light microscopy have recently made it possible to perform FRET measurements of the kinase activity in individual cells (38, 70). In unstimulated cells with wild-type receptor complexes, the kinase activity exhibited substantial fluctuations with a correlation time of ~10–20 s, which were reduced in cells lacking cheR and cheB, confirming the contribution of (de)methylation to the noise in kinase activity. While the amplitude of these fluctuations was larger (~40%) than previously estimated from measurements of individual motors and population FRET (10–20%) (47, 104, 105, 116, 120, 137), the correlation time was similar to previous estimates. Mutations that blocked the CheB phosphorylation feedback without disrupting demethylation yielded populations with increased cell-to-cell variability in the steady-state kinase activity. This is consistent with saturated adaptation kinetics and the predicted role of the CheB-P feedback in reducing cell-to-cell variability (70).
Interestingly, the same data revealed a second source of signaling noise besides adaptation kinetics. The extra noise in kinase activity has a very long correlation time (~100 s compared to ~10 s for methylation noise) and was traced back to allosteric interactions within receptor clusters (38, 70). The strength of these methylation-independent fluctuations depends strongly on the activity and composition of the receptor clusters. Fluctuations are larger when cells lacking cheR and cheB are positioned in the middle of the kinase activity dose response curve (using ligands or expressing receptors with blocked methyl levels), or when cells express tsr as the sole receptor. In the latter case, receptor clusters are homogeneous and have higher gain (122, 123), probably because of the higher number of receptors that can participate in the response of one cluster.
Analysis of the response function and spontaneous fluctuations using the fluctuation-dissipation theorem to extract an effective temperature (38) confirmed a previously observed relation between fluctuations and adaptation time (47, 105) and indicated that fluctuations in the kinase activity arise from multiple factors: thermal fluctuations in the receptors that are amplified by allosteric interactions and slowed down by the delayed response function of the receptor clusters, and the dynamics of the methylation system that generates out-of-equilibrium fluctuations. Thus, while cooperativity between receptors enhances signal amplification and (de)methylation enables cells to maintain their sensitivity as they adapt to background signals, these two mechanisms also increase the spontaneous fluctuations in kinase activity, which are then transported to the motors by the response regulator.
2.2. Behavioral Consequences of the Noise in Kinase Activity
A significant consequence of the fluctuations discussed above is that they generate variability in the behavior of an individual cell over time (76). In the absence of fluctuations in CheY activity (e.g., in cells where wild-type CheY is replaced with a constitutively active protein), the durations of the clockwise (CW) and counterclockwise (CCW) events depend only on the mechanical load on the motor. At low load, the events are exponentially distributed (9, 18, 146), whereas at higher loads, the distributions become gamma distributed (75, 145), revealing the energetic contribution of the motor torque to the motor operation (135, 145). In contrast, in wild-type cells, the fluctuations in CheY-P produce long tails in the distribution of CCW event durations (47, 76, 104, 137).
To what extent CW and CCW events of the individual motors translate into runs and tumbles depends on how well E. coli synchronizes its motors. Indeed, a single motor switching to CW can be sufficient to cause a freely swimming cell to tumble (97, 139). Theoretical work has shown that fluctuations in CheY-P could help synchronize the stochastic motors (120), consistent with measurements of two motors on the same cell immobilized on glass (63, 120, 133). Measurements of flagellar dynamics in swimming cells held between two optical traps further support this hypothesis (97). Besides CheY-P fluctuations, other mechanisms that may contribute to motor synchronization are hydrodynamic forces (60), motor loads (145), and the exchange of motor subunits with the cytoplasm (82).
Synchronization between the flagellar motors enhances the probability that long CCW events translate into long runs (120). Thus, fluctuations in kinase activity are likely to generate long tails in the distribution of run durations, which affects how a cell explores its environment. We consider the consequences of these long tails for the chemotactic performance of individual cells in Section 2.3.
2.3. Dynamical Parameters of Chemotaxis Signaling and Their Role in Shaping Performance
Several key parameters define a cell’s chemotactic behavior (Figure 1f ). These include the cooperativity of the receptor (N), which controls the amplitude of the response to ligand, its adaptation time τ, and its steady-state output, the tumble bias TB (time averaged probability to change direction). Other important parameters are the swimming speed ν, the effective rotational diffusion coefficient DR, which dictates how fast the cell loses its original swimming direction during runs, and the persistence α, which controls how much a tumble randomizes the new run direction. Because of its role in modulating run-and-tumble statistics, the noise in the activity of the receptor clusters σ is also an important determinant of the chemotactic behavior of individual cells.
Basic relationships between macroscopic measures of behavior such as diffusion (unbiased expansion) and drift velocity (directional motion up a gradient), and cellular physical quantities such as run velocity, mean run duration, and persistence were established early on (71, 93, 114). These results were expanded by taking into account the role of adaptation (28, 31, 34, 40, 51, 89, 147), revealing how the linear response of the chemotaxis system (the shape of its so-called response function) influences performance. While a response function that does not integrate to zero can increase drift speed along a static gradient (40, 147), precise adaptation (115) helps cells stay close to point sources (34) and maximizes the minimum performance in random environments (28). Besides the precision of the adaptation mechanism, the timescale of adaptation is also important. The effect of adaptation time on chemotaxis was investigated using stochastic integration of a minimal nonlinear model of bacterial chemotaxis (see Section 1.4) to simulate individual cells in gradients. While a short adaptation time helps a bacterium stay close to signal peaks, a long adaptation time enhances its exploration and helps it climb gradients (47, 48, 55, 66, 120, 143). These simulations also showed that a key determinant of drift velocity is the tumble bias.
How tumble bias affects drift velocity has recently been examined closely. Because receptor clusters and flagellar motors exhibit very steep input-output relationships (Figure 1c,e), it has often been assumed that the adapted level of CheY-P (which determines the tumble bias of a cell) should be maintained by precise adaptation within the middle of the motor response curve where the probability to spin CW is most sensitive to variations in CheY-P. In this interpretation, the motor was viewed as an amplifier of CheY-P signal. However, examination of how the drift velocity up a gradient of attractant depends on the adapted level of CheY-P suggests that the relevant variable is not the tumble bias ( gray line in Figure 3a) but rather the run-direction decorrelation time—how long a cell is able to sustain a run in a given direction before a tumble or rotational diffusion kicks in. When a cell is swimming in liquid, this duration is limited by rotational diffusion (89) and decreases to zero with increasing CheY-P (black line in Figure 3a). Theory and simulations (lines and dots in Figure 3b) predict that drift velocity will be maximized in the middle of this transition, where the CW bias is very low and contrast between run duration up and down the gradient is maximized (red and green lines in Figure 3a) (43). This hypothesis was recently supported by experiments that measured the drift velocity of individual E. coli cells swimming in liquid gradients of α-methyl-DL-aspartate (MeAsp) and serine. In a gradient of MeAsp, drift velocity increased with decreasing tumble bias all the way down to a tumble bias as small as 0.005 (144 and Figure 4b). However, cells that never tumble because they lack CheY or express very low levels of CheR performed worse than wild-type cells with very low tumble bias (144). Similarly, when E. coli cells were monitored swimming up a gradient of serine, imperfect adaptation to serine caused the mean CheY-P level to operate at a lower level than it would if the cell adapted precisely, resulting in increased drift speed (147). Taken together, these results suggest the intriguing possibility that the flagellar motors operate more as a threshold that triggers runs and tumbles when CheY-P crosses the threshold rather than as an amplifier of CheY-P fluctuations (43). Note that a corollary of this interpretation is that strong amplification at the input is needed for CheY-P to be able to reach the response threshold when needed.
Figure 3.
(a) Clockwise bias ( gray) and run-direction decorrelation time (black)—how long a cell is able to sustain a run in a given direction before a tumble or rotational diffusion kicks in—as a function of adapted mean CheY-P concentration for a cell in a shallow gradient of α-methyl-DL-aspartate Green indicates time-dependent change of the intracellular concentration of CheY-P in a cell that swims up a gradient of attractant. Red indicates the same quantity but for a cell that swims down the same gradient of attractant. Two phenotypes with low (0.01) and high (0.5) tumble bias are shown. It is assumed that at the start of the run the concentration of CheY-P is at its mean adapted value. The intersection with the black curve indicates when the cell is expected to tumble. The difference between the duration of runs up and down the gradient, and therefore the drift velocity, is larger for cells with a mean adapted CheY-P level located where the black curve is steepest. (b) Drift velocity as a function of adapted mean CheY-P concentration for cells with different adaptation times. Dots are from agent-based simulations of a model similar to that in Section 1.4, and curves are analytic predictions. Adapted with permission from Reference 43.
Figure 4.
Performance consequences of phenotypic diversity. (a) Conceptual overview of the connection between protein expression, phenotype, performance, and fitness. The genetic network architecture converts protein expression to phenotypic parameters, while the environment converts these phenotypic parameters to performance profiles. Finally, performance is converted to fitness through a selection function. Panel a is adapted with permission from Reference 55. (b) Genetically identical cells categorized by tumble bias (shown in inset) display differential performance in a race up a gradient of α-methyl-DL-aspartate (colored dots). A quantitative model (solid lines; see Section 1.4) that only assumed cell-to-cell differences in gene expression was sufficient to recapitulate the results. (c) Population performance (right) is the convolution of a population’s phenotypic distribution (left) and the performance of each phenotype (center). Because of this, nonlinearities in the phenotype-to-performance function (center, dark beige) can result in two populations with the same mean performance (left, red and blue) that display very different population performance (right). Thus, knowing the performance of the average phenotype is in general not sufficient to predict how the population will perform. Panels b and c are adapted with permission from Reference 144.
Early theoretical analysis of signal detection and amplification demonstrated that uncertainty in the detection of the signal scales inversely with the number of independent sample measurements the cell can make (15). Thus, rebinding the same molecule or waiting too long before unbinding reduces precision (16, 50). These considerations have led to several theoretical studies examining the physical limits of chemical sensing both at equilibrium and out of equilibrium. (See Reference 8 for a recent review.) Amplification depends on the size and composition of the receptor clusters (122), which in turn depend on gene expression and the nutrient environment (17, 86). The gain of an individual cell, however, is not independent of its adaptation time and noise. These interdependencies arise because, in physical systems fluctuations, response and energy dissipation (e.g., energy consumed to perform one methylation-demethylation cycle) are fundamentally linked (16, 29, 47, 79, 108, 113). Important consequences of such constraints, demonstrated by single-cell experiments, are that noisier cells tend to adapt slower (105), and larger receptor clusters that provide more gain (as obtained by expressing Tsr receptors only) are also noisier (38, 70). Finally, the precision and speed of adaption in the bacterial chemotaxis system depend on the amount of energy dissipated by methylation and demethylation (79, 113). Thus, precise adaptation does not come for free.
Given the energetic cost of reducing fluctuations and the magnitude of the spontaneous fluctuations observed in the kinase activity of a single cell (38, 70, 76), it is not clear that the bacterial chemotaxis system of E. coli was selected to reduce fluctuations around the mean adapted activity of the receptor–kinase complex. An interesting alternative could be that the system was selected for strong signal amplification (17). While increasing gain increases spontaneous fluctuations, these fluctuations and those from the adaptation mechanism take place at longer timescales [tens of seconds (38, 70)] than those of signal detection and kinase response (<1 s). When there is no signal, these slow fluctuations could enhance exploration by enabling individual cells to search their environment over multiple scales (47, 48, 76, 96, 137). When climbing gradients, noise can still be beneficial because long tails in the run-duration distribution enhance gradient detection without interfering much with localization at concentration peaks (52, 120). Thus, while excessive noise can abolish a cell’s ability to perform chemotaxis, some noise appears to be beneficial.
So far, we have discussed how sensing affects motion and how the negative integral feedback mediated by adaptation tends to bring the cell back to its adapted state. This, however, is only half the picture. Also inherent in run-and-tumble motion is an important positive feedback of the cell’s own motion onto the ligand change the cell senses. A direct consequence of this positive feedback is that motion up the gradient lowers CheY-P and the probability to tumble, which in turn boosts drift up the gradient. This positive feedback can profoundly affect chemotactic performance under certain conditions because it can drive large asymmetric transients in the organism’s internal state that selectively amplify runs in the correct direction. Theory predicts that this nonequilibrium process, not described by mean-field theory, could result in fast ratchet-like gradient climbing behavior that mitigates the classical drawback of run-and-tumble navigation: wasteful runs in the wrong direction (90). This effect is likely to be further enhanced by the modulation of directional persistence upon tumbling: Cells swimming up the gradient exhibit higher directional persistence upon tumbling than cells going down the gradient, which further enhances drift velocity (112, 139, 142).
3. PHENOTYPIC DIVERSITY
In addition to the behavioral variability that a single cell exhibits over time, clonal cells also exhibit substantial cell-to-cell variability in their chemotactic behavior. Nongenetic individuality was identified early on by measuring the amount of time it took individual cells to return to their steady-state behavior after a step increase in attractant (126). Single cells differed significantly in their recovery time, demonstrating that a key dynamical parameter of the system (adaptation time) varied substantially between clonal cells much more than could be explained from possible genetic variations accumulated in an overnight culture. This finding has since been validated using various single-cell experimental methods (70, 76, 95, 105). Besides adaptation time, other parameters of the chemotaxis pathway have been found to vary substantially from cell to cell in isogenic populations, including tumble bias (44, 105, 144), input gain (70), number of flagella (37, 97, 139), and cell velocity (44, 131, 144).
Cell-to-cell variations in the values of these functional parameters could have multiple origins. An important one is variations in the abundances of chemotaxis proteins (84, 126), which can arise from noise in gene expression (130) as well as from the partitioning of cellular components during cell division (62). Complementation of null strains with proteins expressed from inducible promoters quantified how the concentrations of different chemotaxis proteins affect functional parameters and behavior of cell populations (5, 36, 74, 122, 123). For example, by increasing expression of CheR or CheY, the average tumble bias can be tuned from 0 to higher values. Together, these results supported the hypothesis that differences in swimming behavior of unaltered, wild-type individuals could be explained in part by differences in protein levels.
Given this hypothesis, what precisely is the relationship between protein levels and the larger-scale behavior of the cell? How are expression levels and their correlations shaped through genetically encoded, evolvable factors such as ribosome binding site strength or operon ordering? How could these factors yield the experimentally observed diversity in swimming behavior and chemotaxis? In the chemotaxis operon in E. coli, it was found that the organization of the genes cheRBYZ on a single multicistronic operon ensures that gene expression noise in these proteins is correlated, preserving the ratio of proteins in each cell while allowing the total amount to vary (74, 91, 92). Constraining CheR/CheB and CheY/CheZ ratios has the effect of constraining behaviors within a functional range of tumble bias, suggesting that the order of these genes may have evolved to further constrain chemotactic diversity along certain axes of phenotype space. Another regulatory mechanism that helps maintain the population of cells within a functioning range is a temperature-sensitive secondary RNA structure upstream of the cheR gene. This element modulates the protein expression ratio between the receptor proteins and the adaptation enzymes CheR and CheB, which helps the population cope with temperature variations (103).
Despite the existence of regulatory mechanisms that constrain phenotypic heterogeneity, populations still exhibit marked diversity, the function of which is an open question. Linking molecular diversity to behavioral diversity (Figure 4a) requires quantifying the swimming behavior and protein content of an individual cell within a small time window. Recently, this was achieved by tracking cells in a dilute solution of the unpolymerized form of the hydrogel poly-ethylene glycol diacrylate to quantify individual behaviors. Then, the field of view was briefly exposed to UV light, polymerizing the media into a soft hydrogel. The immobilized cells were then fluorescently imaged at higher magnification to observe fluorescently labeled CheR and CheB. By using independent inducible promoters to generate a wide range of CheR and CheB expression profiles in the population, this approach generated a map between protein levels and tumble bias built from single-cell data (44). Surprisingly, the mean and variance in tumble bias could be adjusted independently, depending on the expression levels of CheR and CheB. Since mutations in non-coding sequences can alter these expression levels (55, 68), this finding suggests that variance in a phenotypic parameter may itself be genetically encoded and therefore evolvable.
4. FUNCTIONAL CONSEQUENCES OF NONGENETIC DIVERSITY
It is often assumed that substantial nongenetic heterogeneity carries some adaptive significance (1). A common hypothesis is that different phenotypes in a population are adapted to different environments, and the distribution of these phenotypes reflects the historical likelihood of a particular strain encountering these environments (26, 77). However, observing cell-to-cell differences in behavior does not necessarily imply that these differences contribute to fitness. If the fitness differences between cells with different behaviors are too small to be acted upon by natural selection, the observed behavioral noise could persist without having any functional significance. This makes it challenging to assess whether the observed differences in function are sufficiently large to confer a selective advantage.
Partial progress can be made by separating fitness into two components: performance and selection. Performance measures how well a cell does a well-defined, non-reproduction-related task. Selection, in contrast, describes how this measure of performance is converted to survival and eventual reproduction (Figure 4a). Using this approach, researchers developed an experimentally constrained model of noise in gene expression to generate virtual cells with different numbers of chemotaxis proteins (55). The protein content of each cell was then converted to phenotypic parameters such as tumble bias and adaptation time using a single-cell model of the signaling pathway (see Section 1.4), which was then used to simulate cell behavior (55). These virtual cells were challenged with different tasks, such as navigating to a source of nutrient as quickly as possible (colonizing) or accumulating nutrients while exploring an environment (foraging). In both the colonizing and foraging tasks, simulated cells that started near the goal benefited from a higher tumble bias and shorter adaptation time, while cells placed far away from the goal performed better when they had low tumble bias and longer adaptation times. The task of navigating is further complicated by the fact that each environment was not static: In time-varying environments, cells can become “out of phase” with the environment if the environment changes faster than the cells can adapt (28, 116, 117, 152). The experimentally observed inverse correlation between tumble bias and adaptation time (5, 43, 105) means that individual cells cannot be optimal at both tasks, resulting in a functional trade-off. Indeed, increasing environmental diversity favored populations containing so-called specialists that each performed very well in one environment but poorly in the other over generalists that had intermediate performance in both environments. Thus, behavioral differences in navigation can have significant effects on the performance of navigation-based tasks such as colonization and foraging, and cell-to-cell variations in protein abundances may be a mechanism for survival of cell colonies across diverse environments (46, 55).
Recently, the connection between changes in gene expression and phenotypic diversity suggested by these simulations was experimentally demonstrated. The link between protein abundance, swimming behavior, and performance was achieved using a microfluidic device that allowed simultaneous measurements of phenotype (tumble bias) and performance (distance traveled in a fixed amount of time) in freely swimming individual cells (144). In experiments with and without a gradient, cells with lower tumble bias phenotypes dramatically outperformed genetically identical cells with higher tumble bias in the same population. Reducing CheR or CheY shifted the distribution of tumble bias to lower values, connecting gene expression to behavior, as previously demonstrated in simulations (55) and experiments (44). Additionally, this decrease in tumble bias increased population performance, directly linking phenotypic diversity to functional performance. A model of the bacterial chemotaxis system with a single set of parameters could reproduce the data for all phenotypes simultaneously (Figure 4b), further establishing the causal relationship between cell-to-cell differences in protein expression and phenotypic diversity in behavior (144).
These data were used to determine the full empirical relationship between phenotype, TB, and performance, ϕ(TB), which turned out to be convex. A consequence of this shape is that it allows a population of cells to perform better than what would naively be expected on the basis of their mean phenotype. This is due to Jensen’s inequality (65), which stipulates that, for a nonlinear function ϕ of some variable x, the expectation of the mean of x may be greater or less than the mean of the expectation of x, depending on the relative convexity or concavity of the function. Thus, the performance of a population, ϕ =Σx P(x)ϕ(x), is a convolution of two components: the phenotypic distribution of the population, P(x), and the function that relates phenotype to performance, ϕ(x). Here, x is the value of a particular phenotype, which in this case was the tumble bias. The consequences of Jensen’s inequality are profound, as it implies that rare phenotypic subpopulations can have a disproportionate effect on overall population performance, provided that the performance function is concave with respect to phenotype (Figure 4c). In addition, these nonlinear relationships appear to be a general feature of biological systems, as they have been observed in neurons, plants, birds, and insects (see references in 144).
5. FUTURE PROSPECTS
The recent experiments on the single-cell aspects of cellular navigation open up many exciting avenues for future research. Here, we cover some of the more intriguing possibilities.
5.1. Sources of Diversity Beyond Protein Number
Necessarily, almost all work on chemotaxis to date has used mutant strains that are always motile. However, expression of the proteins involved in motility and chemotaxis is normally a highly regulated process with distinct stages (33, 69). Motility is known to be affected by the growth stage of a cell (6, 127), as well as its metabolic state through the second messenger c-di-GMP (64). Changes in swimming speed (by manipulation of intracellular levels of cyclic di-GMP) (20) and proton motive force (by adjusting environmental pH) (61) were recently discovered to affect chemotaxis, implicating them as potential modulators of chemotactic variability alongside noise in gene expression. While the nutrient environment experienced during exponential growth is known to affect many cellular properties, including size and shape (129), as well as the number of chemotaxis proteins within a cell (86), how different nutrient environments affect cell-to-cell diversity of chemotaxis is currently not known. Epigenetic regulation, such as small noncoding RNAs (41), and insertion sequence elements (83) both affect motility but have unknown effects on phenotypic variability in chemotaxis. Finally, while this review focuses on nongenetic cell-to-cell differences and behavioral fluctuations over time, there could be short-term inheritance effects, since the number of proteins inherited by each daughter cell will be some function of the number present in the mother. This is also true for the length and number of flagella, which will affect properties such as rotational diffusion and swimming speed. All these differences will certainly play a role in shaping the nongenetic diversity of a clonal or nearly clonal population, but it is not yet clear how they will affect the relationship between phenotype and chemotactic performance.
5.2. Spatial Structuring of Motile Clonal Populations
One consequence of the link between nongenetic diversity and gradient-climbing performance is that spatial structure will spontaneously develop as a clonal population navigates its environment. For example, in gradient in a liquid environment, cells will become ordered, with low tumble bias cells at the front and higher tumble bias cells at the back (144). This means that what looks to be a well-mixed population may not be and will depend on the chemotaxis ability of individuals compared to the time-scale of mixing forces encountered, for example, in nutrient plumes encountered in the ocean (128, 132). To better understand macroscopic motion of bacterial populations, “phenotypic unmixing” will have to be considered in greater depth.
5.3. Collective Behavior—Is Diversity Suppressed or Exploited When Cells Perform Tasks as a Group?
Most of the experiments on single cells have been done at low density to facilitate individual cellular measurements. However, bacteria are often found living at high density in biofilms or in the gut and produce signaling molecules that affect the behavior of neighboring cells (109). Cells perform chemotaxis toward one such molecule, AI-2, raising the question of how this molecule affects nongenetic individuality in chemotaxis (59, 78).
In a possibly related phenomenon, when E. coli are placed in a small space at high density with access to nutrients, consumption of the nutrients creates a self-sustaining gradient that the bacteria follow (2, 112). The result is a traveling wave of cells that moves at constant speed. How can a uniform wave speed be maintained when the population is composed of individuals with different chemotactic abilities? One possibility is that the wave selects for a single phenotype, suppressing diversity. Another possibility is that the wave is not selective, but somehow within the wave, diversity is suppressed, perhaps through signaling. A third possibility is that diversity is present in the wave, and the performance characteristics of each phenotype are somehow utilized to improve the overall performance of the wave.
5.4. Selection and Evolution of Nongenetic Diversity in Chemotaxis
Ultimately, the evolutionary importance of nongenetic diversity concerns its potential to be altered by natural selection. On its face, this appears to be a contradiction: How can a nongenetic trait be a heritable component of an individual? As discussed in Section 3, simulations have shown that mutations affecting ribosome binding sites, promoters, and operon ordering can all influence nongenetic diversity by controlling the mean and variance of protein expression (55). The particular combination of these noncoding sequences combine to create a single genotype. Each individual with this genotype is a random realization from the underlying phenotypic distribution but has the capacity, through its (clonal) offspring, to generate the entire phenotypic distribution. This idea recently received experimental support with the demonstration that mutations in regulatory elements can directly alter noise in gene expression (68). The next step, demonstrating that differences in gene expression lead to differences in the performance of biologically relevant tasks (144), was discussed in Section 4. There is experimental evidence that phenotypic heterogeneity improves fitness and facilitates adaptive evolution in the context of selection for increased phenotypic diversity (12) and antibiotic resistance (19). Furthermore, these populations evolved bimodal expression of the advantageous phenotypes, showing that selection can increase nongenetic diversity (12, 19). What remains to be shown is that selection can shape functional diversity in continuous traits through mutations in noncoding regions, as previously predicted (55). On the basis of our recent results (144), repeated selection of the highest-performing cells in a liquid environment should favor mutations that reduce both the mean and variation in tumble bias phenotype. If this turns out to be the case, there will be unequivocal support for the hypothesis that evolvability contributes to the evolution of species (25).
Acknowledgments
We thank Junjiajia Long for help with the figures, Thomas Shimizu for comments on an early version of this review, and NIGMS (grant 1R01GM106189) and the Allen Distinguished Investigator Program (grant 11562) for support.
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13:497–508. doi: 10.1038/nrmicro3491. [DOI] [PubMed] [Google Scholar]
- 2.Adler J. Chemotaxis in bacteria. Science. 1966;153:708–16. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- 3.Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 4.Alexander RP, Zhulin IB. Evolutionary genomics reveals conserved structural determinants of signaling and adaptation in microbial chemoreceptors. PNAS. 2007;104:2885–90. doi: 10.1073/pnas.0609359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alon U, Surette MG, Barkai N, Leibler S. Robustness in bacterial chemotaxis. Nature. 1999;397:168–71. doi: 10.1038/16483. [DOI] [PubMed] [Google Scholar]
- 6.Amsler CD, Cho M, Matsumura P. Multiple factors underlying the maximum motility of Escherichia coli as cultures enter post-exponential growth. J Bacteriol. 1993;175:6238–44. doi: 10.1128/jb.175.19.6238-6244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antommattei FM, Munzner JB, Weis RM. Ligand-specific activation of Escherichia coli chemoreceptor transmethylation. J Bacteriol. 2004;186:7556–63. doi: 10.1128/JB.186.22.7556-7563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aquino G, Wingreen NS, Endres RG. Know the single-receptor sensing limit? Think again. J Stat Phys. 2016;162:1353–64. doi: 10.1007/s10955-015-1412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai F, Branch RW, Nicolau DV, Jr, Pilizota T, Steel BC, et al. Conformational spread as a mechanism for cooperativity in the bacterial flagellar switch. Science. 2010;327:685–89. doi: 10.1126/science.1182105. [DOI] [PubMed] [Google Scholar]
- 10.Bardy SL, Briegel A, Rainville S, Krell T. Recent advances and future prospects in bacterial and ar-chaeal locomotion and signal transduction. J Bacteriol. 2018 doi: 10.1128/JB.00203-17. In press. https://doi.org/10.1128/JB.00203-17. [DOI] [PMC free article] [PubMed]
- 11.Barkai N, Leibler S. Robustness in simple biochemical networks. Nature. 1997;387:913–17. doi: 10.1038/43199. [DOI] [PubMed] [Google Scholar]
- 12.Beaumont HJE, Gallie J, Kost C, Ferguson GC, Rainey PB. Experimental evolution of bet hedging. Nature. 2009;462:90–93. doi: 10.1038/nature08504. [DOI] [PubMed] [Google Scholar]
- 13.Berg HC. The rotary motor of bacterial flagella. Annu Rev Biochem. 2003;72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- 14.Berg HC, Brown DA. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239:500–4. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 15.Berg HC, Purcell EM. Physics of chemoreception. Biophys J. 1977;20:193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bialek W, Setayeshgar S. Physical limits to biochemical signaling. PNAS. 2005;102:10040–45. doi: 10.1073/pnas.0504321102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitbol AF, Wingreen NS. Fundamental constraints on the abundances of chemotaxis proteins. Biophys J. 2015;108:1293–305. doi: 10.1016/j.bpj.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block SM, Segall JE, Berg HC. Adaptation kinetics in bacterial chemotaxis. J Bacteriol. 1983;154:312–23. doi: 10.1128/jb.154.1.312-323.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bódi Z, Farkas Z, Nevozhay D, Kalapis D, Lázár V, et al. Phenotypic heterogeneity promotes adaptive evolution. PLOS Biol. 2017;15:e2000644. doi: 10.1371/journal.pbio.2000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, et al. Second messenger-mediated adjustment of bacterial swimming velocity. Cell. 2010;141:107–16. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Borczuk A, Staub A, Stock J. Demethylation of bacterial chemoreceptors is inhibited by attractant stimuli in the complete absence of the regulatory domain of the demethylating enzyme. Biochem Biophys Res Commun. 1986;141:918–23. doi: 10.1016/s0006-291x(86)80130-9. [DOI] [PubMed] [Google Scholar]
- 22.Bray D, Levin MD, Morton-Firth CJ. Receptor clustering as a cellular mechanism to control sensitivity. Nature. 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- 23.Briegel A, Jensen G. Progress and potential of electron cryotomography as illustrated by its application to bacterial chemoreceptor arrays. Annu Rev Biophys. 2017;46:1–21. doi: 10.1146/annurev-biophys-070816-033555. [DOI] [PubMed] [Google Scholar]
- 24.Briegel A, Li X, Bilwes AM, Hughes KT, Jensen GJ, Crane BR. Bacterial chemoreceptor arrays are hexagonally packed trimers of receptor dimers networked by rings of kinase and coupling proteins. PNAS. 2012;109:3766–71. doi: 10.1073/pnas.1115719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookfield JFY. Evolution and evolvability: celebrating Darwin 200. Biol Lett. 2009;5:44–46. doi: 10.1098/rsbl.2008.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull JJ. Evolution of phenotypic variance. Evolution. 1987;41:303–15. doi: 10.1111/j.1558-5646.1987.tb05799.x. [DOI] [PubMed] [Google Scholar]
- 27.Celani A, Shimizu TS, Vergassola M. Molecular and functional aspects of bacterial chemotaxis. J Stat Phys. 2011;144:219–40. [Google Scholar]
- 28.Celani A, Vergassola M. Bacterial strategies for chemotaxis response. PNAS. 2010;107:1391–96. doi: 10.1073/pnas.0909673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celani A, Vergassola M. Nonlinearity, fluctuations, and response in sensory systems. Phys Rev Lett. 2012;108:258102. doi: 10.1103/PhysRevLett.108.258102. [DOI] [PubMed] [Google Scholar]
- 30.Chalah A, Weis RM. Site-specific and synergistic stimulation of methylation on the bacterial chemotaxis receptor Tsr by serine and CheW. BMC Microbiol. 2005;5:12. doi: 10.1186/1471-2180-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatterjee S, da Silveira RA, Kafri Y. Chemotaxis when bacteria remember: drift versus diffusion. PLOS Comput Biol. 2011;7:e1002283. doi: 10.1371/journal.pcbi.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chattopadhyay S, Wu XL. The effect of long-range hydrodynamic interaction on the swimming of a single bacterium. Biophys J. 2009;96:2023–28. doi: 10.1016/j.bpj.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chilcott GS, Hughes KT. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol Mol Biol Rev. 2000;64:694–708. doi: 10.1128/mmbr.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark DA, Grant LC. The bacterial chemotactic response reflects a compromise between transient and steady-state behavior. PNAS. 2005;102:9150–55. doi: 10.1073/pnas.0407659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausznitzer D, Oleksiuk O, Lovdok L, Sourjik V, Endres RG. Chemotactic response and adaptation dynamics in Escherichia coli. PLOS Comput Biol. 2010;6:e1000784. doi: 10.1371/journal.pcbi.1000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cluzel P, Surette M, Leibler S. An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Science. 2000;287:1652–55. doi: 10.1126/science.287.5458.1652. [DOI] [PubMed] [Google Scholar]
- 37.Cohen-Ben-Lulu GN, Francis NR, Shimoni E, Noy D, Davidov Y, et al. The bacterial flagellar switch complex is getting more complex. EMBO J. 2008;27:1134–44. doi: 10.1038/emboj.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colin R, Rosazza C, Vaknin A, Sourjik V. Multiple sources of slow activity fluctuations in a bacterial chemosensory network. eLife. 2017;6:e26796. doi: 10.7554/eLife.26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colin R, Sourjik V. Emergent properties of bacterial chemotaxis pathway. Curr Opin Microbiol. 2017;39:24–33. doi: 10.1016/j.mib.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 40.de Gennes PG. Chemotaxis: the role of internal delays. Eur Biophys J. 2004;33:691–93. doi: 10.1007/s00249-004-0426-z. [DOI] [PubMed] [Google Scholar]
- 41.De Lay N, Gottesman S. A complex network of small non-coding RNAs regulate motility in Escherichia coli. Mol Microbiol. 2012;86:524–38. doi: 10.1111/j.1365-2958.2012.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobell C. Antony van Leeuwenhoek and His “Little Animals”. New York: Dover; 1960 (1932). [Google Scholar]
- 43.Dufour YS, Fu X, Hernandez-Nunez L, Emonet T. Limits of feedback control in bacterial chemo-taxis. PLOS Comput Biol. 2014;10:e1003694. doi: 10.1371/journal.pcbi.1003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dufour YS, Gillet S, Frankel NW, Weibel DB, Emonet T. Direct correlation between motile behavior and protein abundance in single cells. PLOS Comput Biol. 2016;12:e1005041. doi: 10.1371/journal.pcbi.1005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duke TA, Bray D. Heightened sensitivity of a lattice of membrane receptors. PNAS. 1999;96:10104–8. doi: 10.1073/pnas.96.18.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgington MP, Tindall MJ. Understanding the link between single cell and population scale responses of Escherichia coli in differing ligand gradients. Comput Struct Biotechnol J. 2015;13:528–38. doi: 10.1016/j.csbj.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emonet T, Cluzel P. Relationship between cellular response and behavioral variability in bacterial chemotaxis. PNAS. 2008;105:3304–9. doi: 10.1073/pnas.0705463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emonet T, Macal CM, North MJ, Wickersham CE, Cluzel P. AgentCell: a digital single-cell assay for bacterial chemotaxis. Bioinformatics. 2005;21:2714–21. doi: 10.1093/bioinformatics/bti391. [DOI] [PubMed] [Google Scholar]
- 49.Endres RG, Wingreen NS. Precise adaptation in bacterial chemotaxis through “assistance neighborhoods. PNAS. 2006;103:13040–44. doi: 10.1073/pnas.0603101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endres RG, Wingreen NS. Accuracy of direct gradient sensing by single cells. PNAS. 2008;105:15749–54. doi: 10.1073/pnas.0804688105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erban R, Othmer HG. From individual to collective behavior in bacterial chemotaxis. SIAM J Appl Math. 2005;65:361–91. [Google Scholar]
- 52.Flores M, Shimizu TS, ten Wolde PR, Tostevin F. Signaling noise enhances chemotactic drift of E. coli. Phys Rev Lett. 2012;109:148101. doi: 10.1103/PhysRevLett.109.148101. [DOI] [PubMed] [Google Scholar]
- 53.Fraiberg M, Afanzar O, Cassidy CK, Gabashvili A, Schulten K, et al. CheY’s acetylation sites responsible for generating clockwise flagellar rotation in Escherichia coli. Mol Microbiol. 2015;95:231–44. doi: 10.1111/mmi.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank V, Vaknin A. Prolonged stimuli alter the bacterial chemosensory clusters. Mol Microbiol. 2013;88:634–44. doi: 10.1111/mmi.12215. [DOI] [PubMed] [Google Scholar]
- 55.Frankel NW, Pontius W, Dufour YS, Long J, Hernandez-Nunez L, Emonet T. Adaptability of non-genetic diversity in bacterial chemotaxis. eLife. 2014;3:e03526. doi: 10.7554/eLife.03526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldbeter A, Koshland DE., Jr An amplified sensitivity arising from covalent modification in biological systems. PNAS. 1981;78:6840–44. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen CH, Endres RG, Wingreen NS. Chemotaxis in Escherichia coli: a molecular model for robust precise adaptation. PLOS Comput Biol. 2008;4:e1. doi: 10.1371/journal.pcbi.0040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hazelbauer GL. Bacterial chemotaxis: the early years of molecular studies. Annu Rev Microbiol. 2012;66:285–303. doi: 10.1146/annurev-micro-092611-150120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hegde M, Englert DL, Schrock S, Cohn WB, Vogt C, et al. Chemotaxis to the quorum-sensing signal AI-2 requires the Tsr chemoreceptor and the periplasmic LsrB AI-2-binding protein. J Bacteriol. 2011;193:768–73. doi: 10.1128/JB.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu B, Tu Y. Coordinated switching of bacterial flagellar motors: evidence for direct motor-motor coupling? Phys Rev Lett. 2013;110:158703. doi: 10.1103/PhysRevLett.110.158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu B, Tu Y. Behaviors and strategies of bacterial navigation in chemical and nonchemical gradients. PLOS Comput Biol. 2014;10:e1003672. doi: 10.1371/journal.pcbi.1003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huh D, Paulsson J. Non-genetic heterogeneity from stochastic partitioning at cell division. Nat Genet. 2011;43:95–100. doi: 10.1038/ng.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishihara A, Segall JE, Block SM, Berg HC. Coordination of flagella on filamentous cells of Escherichia coli. J Bacteriol. 1983;155:228–37. doi: 10.1128/jb.155.1.228-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenal U, Reinders A, Lori C. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol. 2017;15:271–84. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- 65.Jensen JLWV. Sur les fonctions convexes et les inégalités entre les valeurs moyennes. Acta Math. 1906;30:175–93. [Google Scholar]
- 66.Jiang L, Ouyang Q, Tu Y. Quantitative modeling of Escherichia coli chemotactic motion in environments varying in space and time. PLOS Comput Biol. 2010;6:e1000735. doi: 10.1371/journal.pcbi.1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones CW, Armitage JP. Positioning of bacterial chemoreceptors. Trends Microbiol. 2015;23:247–56. doi: 10.1016/j.tim.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Jones DL, Brewster RC, Phillips R. Promoter architecture dictates cell-to-cell variability in gene expression. Science. 2014;346:1533–36. doi: 10.1126/science.1255301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalir S, McClure J, Pabbaraju K, Southward C, Ronen M, et al. Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science. 2001;292:2080–83. doi: 10.1126/science.1058758. [DOI] [PubMed] [Google Scholar]
- 70.Keegstra JM, Kamino K, Anquez F, Lazova MD, Emonet T, Shimizu TS. Phenotypic diversity and temporal variability in a bacterial signaling network revealed by single-cell FRET. eLife. 2017;6:e27455. doi: 10.7554/eLife.27455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keller EF, Segel LA. Traveling bands of chemotactic bacteria: a theoretical analysis. J Theor Biol. 1971;30:235–48. doi: 10.1016/0022-5193(71)90051-8. [DOI] [PubMed] [Google Scholar]
- 72.Keymer JE, Endres RG, Skoge M, Meir Y, Wingreen NS. Chemosensing in Escherichia coli: two regimes of two-state receptors. PNAS. 2006;103:1786–91. doi: 10.1073/pnas.0507438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim KK, Yokota H, Kim SH. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature. 1999;400:787–92. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 74.Kollmann M, Lovdok L, Bartholome K, Timmer J, Sourjik V. Design principles of a bacterial signalling network. Nature. 2005;438:504–7. doi: 10.1038/nature04228. [DOI] [PubMed] [Google Scholar]
- 75.Korobkova E, Emonet T, Park H, Cluzel P. Hidden stochastic nature of a single bacterial motor. Phys Rev Lett. 2006;96:58105. doi: 10.1103/PhysRevLett.96.058105. [DOI] [PubMed] [Google Scholar]
- 76.Korobkova E, Emonet T, Vilar JM, Shimizu TS, Cluzel P. From molecular noise to behavioural variability in a single bacterium. Nature. 2004;428:574–78. doi: 10.1038/nature02404. [DOI] [PubMed] [Google Scholar]
- 77.Kussell E, Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309:2075–78. doi: 10.1126/science.1114383. [DOI] [PubMed] [Google Scholar]
- 78.Laganenka L, Colin R, Sourjik V. Chemotaxis towards autoinducer 2 mediates autoaggregation in Escherichia coli. Nat Commun. 2016;7:12984. doi: 10.1038/ncomms12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lan G, Sartori P, Neumann S, Sourjik V, Tu Y. The energy-speed-accuracy tradeoff in sensory adaptation. Nat Phys. 2012;8:422–28. doi: 10.1038/nphys2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lan G, Schulmeister S, Sourjik V, Tu Y. Adapt locally and act globally: strategy to maintain high chemoreceptor sensitivity in complex environments. Mol Syst Biol. 2011;7:475. doi: 10.1038/msb.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazova MD, Ahmed T, Bellomo D, Stocker R, Shimizu TS. Response rescaling in bacterial chemotaxis. PNAS. 2011;108:13870–75. doi: 10.1073/pnas.1108608108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leake MC, Chandler JH, Wadhams GH, Bai F, Berry RM, Armitage JP. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature. 2006;443:355–58. doi: 10.1038/nature05135. [DOI] [PubMed] [Google Scholar]
- 83.Lee C, Park C. Mutations upregulating the flhDC operon of Escherichia coli K-12. J Microbiol. 2013;51:140–44. doi: 10.1007/s12275-013-2212-z. [DOI] [PubMed] [Google Scholar]
- 84.Levin MD, Morton-Firth CJ, Abouhamad WN, Bourret RB, Bray D. Origins of individual swimming behavior in bacteria. Biophys J. 1998;74:175–81. doi: 10.1016/S0006-3495(98)77777-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levin MD, Shimizu TS, Bray D. Binding and diffusion of CheR molecules within a cluster of membrane receptors. Biophys J. 2002;82:1809–17. doi: 10.1016/S0006-3495(02)75531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li M, Hazelbauer GL. Cellular stoichiometry of the components of the chemotaxis signaling complex. J Bacteriol. 2004;186:3687–94. doi: 10.1128/JB.186.12.3687-3694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li M, Hazelbauer GL. Adaptational assistance in clusters of bacterial chemoreceptors. Mol Microbiol. 2005;56:1617–26. doi: 10.1111/j.1365-2958.2005.04641.x. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, Hu B, Morado DR, Jani S, Manson MD, Margolin W. Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells. PNAS. 2012;109:E1481–88. doi: 10.1073/pnas.1200781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Locsei JT. Persistence of direction increases the drift velocity of run and tumble chemotaxis. J Math Biol. 2007;55:41–60. doi: 10.1007/s00285-007-0080-z. [DOI] [PubMed] [Google Scholar]
- 90.Long J, Zucker SW, Emonet T. Feedback between motion and sensation provides nonlinear boost in run-and-tumble navigation. PLOS Comput Biol. 2017;13:e1005429. doi: 10.1371/journal.pcbi.1005429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Løvdok L, Bentele K, Vladimirov N, Müller A, Pop FS, et al. Role of translational coupling in robustness of bacterial chemotaxis pathway. PLOS Biol. 2009;7:e1000171. doi: 10.1371/journal.pbio.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Løvdok L, Kollmann M, Sourjik V. Co-expression of signaling proteins improves robustness of the bacterial chemotaxis pathway. J Biotechnol. 2007;129:173–80. doi: 10.1016/j.jbiotec.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 93.Lovely PS, Dahlquist FW. Statistical measures of bacterial motility and chemotaxis. J Theor Biol. 1975;50:477–96. doi: 10.1016/0022-5193(75)90094-6. [DOI] [PubMed] [Google Scholar]
- 94.Macnab RM, Koshland DE., Jr The gradient-sensing mechanism in bacterial chemotaxis. PNAS. 1972;69:2509–12. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Masson JB, Voisinne G, Wong-Ng J, Celani A, Vergassola M. Noninvasive inference of the molecular chemotactic response using bacterial trajectories. PNAS. 2012;109:1802–7. doi: 10.1073/pnas.1116772109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matthaus F, Jagodic M, Dobnikar J. E. coli superdiffusion and chemotaxis-search strategy, precision, and motility. Biophys J. 2009;97:946–57. doi: 10.1016/j.bpj.2009.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mears PJ, Koirala S, Rao CV, Golding I, Chemla YR. Escherichia coli swimming is robust against variations in flagellar number. eLife. 2014;3:e01916. doi: 10.7554/eLife.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mello BA, Tu Y. An allosteric model for heterogeneous receptor complexes: understanding bacterial chemotaxis responses to multiple stimuli. PNAS. 2005;102:17354–59. doi: 10.1073/pnas.0506961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mesibov R, Ordal GW, Adler J. The range of attractant concentrations for bacterial chemotaxis and the threshold and size of response over this range: Weber law and related phenomena. J Gen Physiol. 1973;62:203–23. doi: 10.1085/jgp.62.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Micali G, Endres RG. Bacterial chemotaxis: information processing, thermodynamics, and behavior. Curr Opin Microbiol. 2016;30:8–15. doi: 10.1016/j.mib.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Min TL, Mears PJ, Chubiz LM, Rao CV, Golding I, Chemla YR. High-resolution, long-term characterization of bacterial motility using optical tweezers. Nat Methods. 2009;6:831–35. doi: 10.1038/nmeth.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neumann S, Vladimirov N, Krembel AK, Wingreen NS, Sourjik V. Imprecision of adaptation in Escherichia coli chemotaxis. PLOS ONE. 2014;9:e84904. doi: 10.1371/journal.pone.0084904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oleksiuk O, Jakovljevic V, Vladimirov N, Carvalho R, Paster E, et al. Thermal robustness of signaling in bacterial chemotaxis. Cell. 2011;145:312–21. doi: 10.1016/j.cell.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park H, Oikonomou P, Guet CC, Cluzel P. Noise underlies switching behavior of the bacterial flagellum. Biophys J. 2011;101:2336–40. doi: 10.1016/j.bpj.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park H, Pontius W, Guet CC, Marko JF, Emonet T, Cluzel P. Interdependence of behavioural variability and response to small stimuli in bacteria. Nature. 2010;468:819–23. doi: 10.1038/nature09551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parkinson JS, Hazelbauer GL, Falke JJ. Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol. 2015;23:257–66. doi: 10.1016/j.tim.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perez E, West AH, Stock AM, Djordjevic S. Discrimination between different methylation states of chemotaxis receptor Tar by receptor methyltransferase CheR. Biochemistry. 2004;43:953–61. doi: 10.1021/bi035455q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pontius W, Sneddon MW, Emonet T. Adaptation dynamics in densely clustered chemoreceptors. PLOS Comput Biol. 2013;9:e1003230. doi: 10.1371/journal.pcbi.1003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Popat R, Cornforth DM, McNally L, Brown SP. Collective sensing and collective responses in quorum-sensing bacteria. J R Soc Interface. 2015;12:20140882. doi: 10.1098/rsif.2014.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rusconi R, Garren M, Stocker R. Microfluidics expanding the frontiers of microbial ecology. Annu Rev Biophys. 2014;43:65–91. doi: 10.1146/annurev-biophys-051013-022916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryu WS, Berry RM, Berg HC. Torque-generating units of the flagellar motor of Escherichia coli have a high duty ratio. Nature. 2000;403:444–47. doi: 10.1038/35000233. [DOI] [PubMed] [Google Scholar]
- 112.Saragosti J, Calvez V, Bournaveas N, Perthame B, Buguin A, Silberzan P. Directional persistence of chemotactic bacteria in a traveling concentration wave. PNAS. 2011;108:16235–40. doi: 10.1073/pnas.1101996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sartori P, Tu Y. Free energy cost of reducing noise while maintaining a high sensitivity. Phys Rev Lett. 2015;115:118102. doi: 10.1103/PhysRevLett.115.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schnitzer MJ. Theory of continuum random walks and application to chemotaxis. Phys Rev E. 1993;48:2553–68. doi: 10.1103/physreve.48.2553. [DOI] [PubMed] [Google Scholar]
- 115.Segall JE, Block SM, Berg HC. Temporal comparisons in bacterial chemotaxis. PNAS. 1986;83:8987–91. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shimizu TS, Tu Y, Berg HC. A modular gradient-sensing network for chemotaxis in Escherichia coli revealed by responses to time-varying stimuli. Mol Syst Biol. 2010;6:382. doi: 10.1038/msb.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Si G, Wu T, Ouyang Q, Tu Y. Pathway-based mean-field model for Escherichia coli chemotaxis. Phys Rev Lett. 2012;109:048101. doi: 10.1103/PhysRevLett.109.048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Silverman MR, Simon MI. Flagellar assembly mutants in Escherichia coli. J Bacteriol. 1972;112:986–93. doi: 10.1128/jb.112.2.986-993.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skoge M, Meir Y, Wingreen NS. Dynamics of cooperativity in chemical sensing among cell-surface receptors. Phys Rev Lett. 2011;107:178101. doi: 10.1103/PhysRevLett.107.178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sneddon MW, Pontius W, Emonet T. Stochastic coordination of multiple actuators reduces latency and improves chemotactic response in bacteria. PNAS. 2012;109:805–10. doi: 10.1073/pnas.1113706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sourjik V, Armitage JP. Spatial organization in bacterial chemotaxis. EMBO J. 2010;29:2724–33. doi: 10.1038/emboj.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sourjik V, Berg HC. Receptor sensitivity in bacterial chemotaxis. PNAS. 2002;99:123–27. doi: 10.1073/pnas.011589998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sourjik V, Berg HC. Functional interactions between receptors in bacterial chemotaxis. Nature. 2004;428:437–41. doi: 10.1038/nature02406. [DOI] [PubMed] [Google Scholar]
- 124.Sourjik V, Vaknin A, Shimizu TS, Berg HC. In vivo measurement by FRET of pathway activity in bacterial chemotaxis. Methods Enzymol. 2007;423:365–91. doi: 10.1016/S0076-6879(07)23017-4. [DOI] [PubMed] [Google Scholar]
- 125.Sourjik V, Wingreen NS. Responding to chemical gradients: bacterial chemotaxis. Curr Opin Cell Biol. 2012;24:262–68. doi: 10.1016/j.ceb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spudich JL, Koshland DE., Jr Non-genetic individuality: chance in the single cell. Nature. 1976;262:467–71. doi: 10.1038/262467a0. [DOI] [PubMed] [Google Scholar]
- 127.Staropoli JF, Alon U. Computerized analysis of chemotaxis at different stages of bacterial growth. Biophys J. 2000;78:513–19. doi: 10.1016/S0006-3495(00)76613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stocker R. Marine microbes see a sea of gradients. Science. 2012;338:628–33. doi: 10.1126/science.1208929. [DOI] [PubMed] [Google Scholar]
- 129.Taheri-Araghi S, Bradde S, Sauls John T, Hill Norbert S, Levin Petra A, et al. Cell-size control and homeostasis in bacteria. Curr Biol. 2015;25:385–91. doi: 10.1016/j.cub.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–38. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taute KM, Gude S, Tans SJ, Shimizu TS. High-throughput 3D tracking of bacteria on a standard phase contrast microscope. Nat Commun. 2015;6:8776. doi: 10.1038/ncomms9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taylor JR, Stocker R. Trade-offs of chemotactic foraging in turbulent water. Science. 2012;338:675–79. doi: 10.1126/science.1219417. [DOI] [PubMed] [Google Scholar]
- 133.Terasawa S, Fukuoka H, Inoue Y, Sagawa T, Takahashi H, Ishijima A. Coordinated reversal of flagellar motors on a single Escherichia coli cell. Biophys J. 2011;100:2193–200. doi: 10.1016/j.bpj.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Terwilliger TC, Wang JY, Koshland DE. Kinetics of receptor modification: the multiply methylated aspartate receptors involved in bacterial chemotaxis. J Biol Chem. 1986;261:814–20. [PubMed] [Google Scholar]
- 135.Tu Y. The nonequilibrium mechanism for ultrasensitivity in a biological switch: sensing by Maxwell’s demons. PNAS. 2008;105:11737–41. doi: 10.1073/pnas.0804641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tu Y. Quantitative modeling of bacterial chemotaxis: signal amplification and accurate adaptation. Annu Rev Biophys. 2013;42:337–59. doi: 10.1146/annurev-biophys-083012-130358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tu Y, Grinstein G. How white noise generates power-law switching in bacterial flagellar motors. Phys Rev Lett. 2005;94:208101. doi: 10.1103/PhysRevLett.94.208101. [DOI] [PubMed] [Google Scholar]
- 138.Tu Y, Shimizu TS, Berg HC. Modeling the chemotactic response of Escherichia coli to time-varying stimuli. PNAS. 2008;105:14855–60. doi: 10.1073/pnas.0807569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Turner L, Ping L, Neubauer M, Berg HC. Visualizing flagella while tracking bacteria. Biophys J. 2016;111:630–39. doi: 10.1016/j.bpj.2016.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vaknin A, Berg HC. Single-cell FRET imaging of phosphatase activity in the Escherichia coli chemotaxis system. PNAS. 2004;101:17072–77. doi: 10.1073/pnas.0407812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vaknin A, Berg HC. Osmotic stress mechanically perturbs chemoreceptors in Escherichia coli. PNAS. 2006;103:592–96. doi: 10.1073/pnas.0510047103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vladimirov N, Lebiedz D, Sourjik V. Predicted auxiliary navigation mechanism of peritrichously flagellated chemotactic bacteria. PLOS Comput Biol. 2010;6:e1000717. doi: 10.1371/journal.pcbi.1000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vladimirov N, Lovdok L, Lebiedz D, Sourjik V. Dependence of bacterial chemotaxis on gradient shape and adaptation rate. PLOS Comput Biol. 2008;4:e1000242. doi: 10.1371/journal.pcbi.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Waite AJ, Frankel NW, Dufour YS, Johnston JF, Long J, Emonet T. Non-genetic diversity modulates population performance. Mol Syst Biol. 2016;12:895. doi: 10.15252/msb.20167044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang F, Shi H, He R, Wang RJ, Zhang RJ, Yuan JH. Non-equilibrium effect in the allosteric regulation of the bacterial flagellar switch. Nat Phys. 2017;13:710–14. [Google Scholar]
- 146.Wang F, Yuan J, Berg HC. Switching dynamics of the bacterial flagellar motor near zero load. PNAS. 2014;111:15752–55. doi: 10.1073/pnas.1418548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wong-Ng J, Melbinger A, Celani A, Vergassola M. The role of adaptation in bacterial speed races. PLOS Comput Biol. 2016;12:e1004974. doi: 10.1371/journal.pcbi.1004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu J, Li J, Li G, Long DG, Weis RM. The receptor binding site for the methyltransferase of bacterial chemotaxis is distinct from the sites of methylation. Biochemistry. 1996;35:4984–93. doi: 10.1021/bi9530189. [DOI] [PubMed] [Google Scholar]
- 149.Wuichet K, Cantwell BJ, Zhulin IB. Evolution and phyletic distribution of two-component signal transduction systems. Curr Opin Microbiol. 2010;13:219–25. doi: 10.1016/j.mib.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yi T-M, Huang Y, Simon MI, Doyle J. Robust perfect adaptation in bacterial chemotaxis through integral feedback control. PNAS. 2000;97:4649–53. doi: 10.1073/pnas.97.9.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yuan J, Branch RW, Hosu BG, Berg HC. Adaptation at the output of the chemotaxis signalling pathway. Nature. 2012;484:233–36. doi: 10.1038/nature10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu X, Si G, Deng N, Ouyang Q, Wu T, et al. Frequency-dependent Escherichia coli chemotaxis behavior. Phys Rev Lett. 2012;108:128101. doi: 10.1103/PhysRevLett.108.128101. [DOI] [PMC free article] [PubMed] [Google Scholar]