Abstract
Background
Mandibular advancement splints (MASs) can effectively treat obstructive sleep apnea (OSA); however, no validated and reliable prediction method for treatment outcome currently exists. The efficacy of MAS may relate to anatomic factors, including craniofacial size and upper-airway soft-tissue volume and anatomic balance between them. We aimed to assess whether craniofacial and oral measurements are associated with MAS treatment outcome.
Methods
Dental impressions and lateral cephalometric radiographs were obtained from patients with OSA prior to commencing MAS treatment. Intertooth distances and palatal depths were measured on dental casts, and standard cephalometric analysis was performed with the addition of cross-sectional area (CSA) of the tongue and bony oral enclosure. Treatment outcome was determined by polysomnography.
Results
Of 53 patients, 25 were complete responders (posttreatment apnea-hypopnea index [AHI] < 5/h), 17 were partial responders (≥ 50% AHI reduction), and 11 were nonresponders (< 50% AHI reduction). Cephalometric analyses did not reveal any significant differences between responders and nonresponders. Oral cavity measurements or CSA did not differ with treatment outcome; however, there was a trend toward a larger tongue CSA in complete vs partial and nonresponders (39.5 ± 1.3 cm2 vs 35.5 ± 0.5 cm2, P = .09). Tongue/oral enclosure CSA ratio, indicating a larger tongue for a given oral cavity size, was greater in complete responders (P = .012, n = 30).
Conclusions
Oral dimensions do not appear to differ between patients who respond and those who do not respond to MAS treatment. However, the larger tongue for a given oral cavity size in responders suggests that MAS may help to correct anatomic imbalance. Further research to assess whether the ratio between tongue and bony oral enclosure size may be useful in selecting patients for MAS treatment is warranted.
Abbreviations
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- CSA
cross-sectional area
- ICC
intraclass correlation coefficient
- MAS
mandibular advancement splint
- OSA
obstructive sleep apnea
- %ΔAHI
apnea-hypopnea index changes with treatment
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder with major public health significance, but treatment remains challenging. 1 Continuous positive airway pressure (CPAP) is the current treatment gold standard but often is suboptimal because of highly variable patient adherence.2, 3, 4 Patients often consider oral appliance therapy a more acceptable alternative,5, 6 and the potential association with greater compliance and, therefore, clinical effectiveness have led to growing interest in this treatment modality.
Mandibular advancement splints (MASs) are the most commonly used oral appliances for OSA treatment.7, 8, 9, 10 The American Academy of Sleep Medicine clinical practice parameters recommend MAS as an alternative to CPAP to treat mild to moderate OSA and severe OSA when CPAP is refused or not tolerated. 11 A clinically important treatment outcome can be achieved in approximately two-thirds of patients.8, 9, 12, 13 However, because treatment requires titration over a period of months, some patients find it inconvenient because outcome cannot be determined beforehand. The ability to predict efficacy in individual patients is an important clinical issue to resolve because considerable health resource wastage could be avoided if outcome could be established before therapy is initiated.
The pathogenesis of OSA is complex, but craniofacial structure is an important interacting factor. Bony and soft-tissue craniofacial abnormalities14, 15, 16, 17 as well as restricted oral dimensions 18 have been reported in OSA. Restriction of hard-tissue dimensions and enlargement of upper-airway soft tissues are both likely to compromise upper-airway space, resulting in a smaller and more collapsible airway. Ultimately, the relative size of the bony compartment compared with the amount of soft tissue contained within it will determine the amount of pressure exerted by surrounding tissues on the airway and influence its collapsibility, a concept that has been termed “anatomic balance.” 19 Although the mechanisms by which MAS improve OSA are still not well understood, an increase in upper-airway dimensions appears to be an important effect, 20 and craniofacial size is likely to be a mediator of this effect. Cephalometric studies have reported that craniofacial measurements, such as a longer maxilla, smaller overjet, shorter soft palate, mandibular plane-to-hyoid distance, facial height, and reduced retropalatal airway space, are associated with MAS treatment success.9, 21, 22, 23 The balance between craniofacial oral restriction and enlarged soft-tissue structures (anatomic balance) may limit the effectiveness of the upper-airway response to MAS. MAS treatment outcome may be related to differences in these anatomic factors either individually or in combination, which may dictate the influence of MAS on the upper-airway space and subsequently on upper-airway collapsibility.
We assessed whether craniofacial bony and soft-tissue structures and oral cavity dimensions are associated with MAS treatment outcome. We sought to test the hypothesis that maxillary and mandibular dimensions, as measured from dental casts, and craniofacial structure, as measured by lateral cephalometry, differ between those who respond to MAS treatment and those who do not.
Materials and Methods
Subjects
Patients who received a new diagnosis of OSA were prospectively recruited from two tertiary sleep disorders clinics. Ethics approval was obtained from institutional ethics committees (Northern Sydney Central Coast and South West Sydney Area Health Services, protocol numbers 0610-192M and X07-0168), and written informed consent was obtained from all participants. Inclusion criteria were a diagnosis of OSA (apnea-hypopnea index [AHI] > 10/h), aged > 18 years, suitability for MAS treatment (good dental health, > 10 teeth/dental arch, and absence of periodontal disease or temporomandibular joint dysfunction). Exclusion criteria were other sleep disorders, comorbid medical or psychiatric disorders, and hypnotic sedative use. On average, recruitment into the study occurred 2 months after the diagnostic sleep study.
MAS Treatment
The oral appliance was a customized two-piece splint (SomnoDent MAS; SomnoMed Ltd; Crows Nest, New South Wales, Australia), with design features previously reported.8, 9, 10, 13, 24, 25 Prior to treatment, dental impressions and a lateral cephalometric radiograph were obtained. The appliance was incrementally titrated over a 6- to 8-week acclimatization period until the maximum comfortable limit of mandibular protrusion was reached. This level of mandibular protrusion was recorded in absolute millimeters as well as a percentage of the maximum voluntary protrusion that could be achieved at baseline.
Polysomnography and Treatment Outcome
Treatment outcome was assessed by polysomnography performed as previously described 9 and scored according to standard criteria.26, 27 Treatment outcome was determined by degree of improvement in AHI according to our previously reported rigorous definition.8, 9, 10, 13 “Complete responders” were patients with > 50% improvement in AHI as well as a posttreatment AHI < 5/h. “Partial responders” showed > 50% AHI reduction but still had residual OSA (AHI > 5/h), and “nonresponders” had < 50% AHI reduction.
Dental Measurements
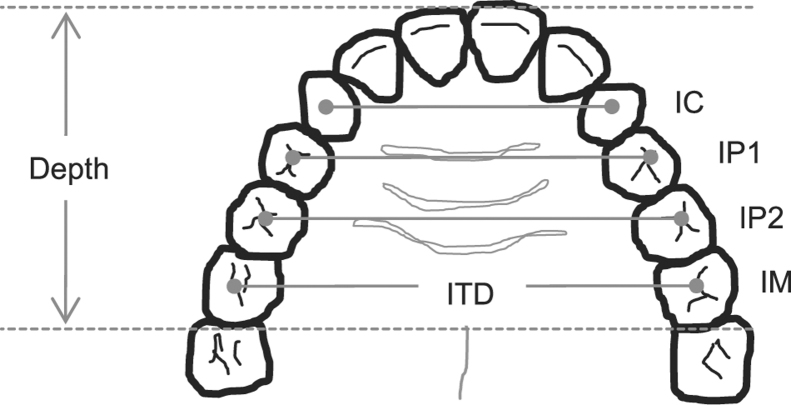
Plaster models made from impressions of the upper and lower dental arches were used to obtain linear measurements of oral cavity size. 18 The following intertooth distances were measured with vernier calipers (Beerendonk; Aesculap; Tuttlingen, Germany): intercanine (between centroids of the canines), interpremolar (between centroids of first and second premolars), and intermolar (between centroids of the first molars). Maxillary and mandibular depth, defined as distance between the most labial point of the central incisors and the point bisecting the line joining the distal midpoints of either the maxillary or the mandibular first molars, were measured with a Korkhaus three-dimensional bow divider (Dentaurm; Ispingen, Germany) (Fig 1). The cross-sectional shape of the palate at the levels of the canine, first and second premolars, and first molars was recorded by a profile gauge placed through the centroids of each set of teeth. Corresponding palatal heights were measured off the gauge using a ruler.
Figure 1.
Schematic diagram of dental arch illustrating the linear dental measurements taken from the upper- and lower-dental arch study models. IC = intercanine; IP1 = first interpremolar; IP2 = second interpremolar; IM = intermolar; ITD = intertooth distance. Maximal palatal height also was measured on the upper dental arch along each line intersecting the centroids of each set of teeth.
Cephalometry
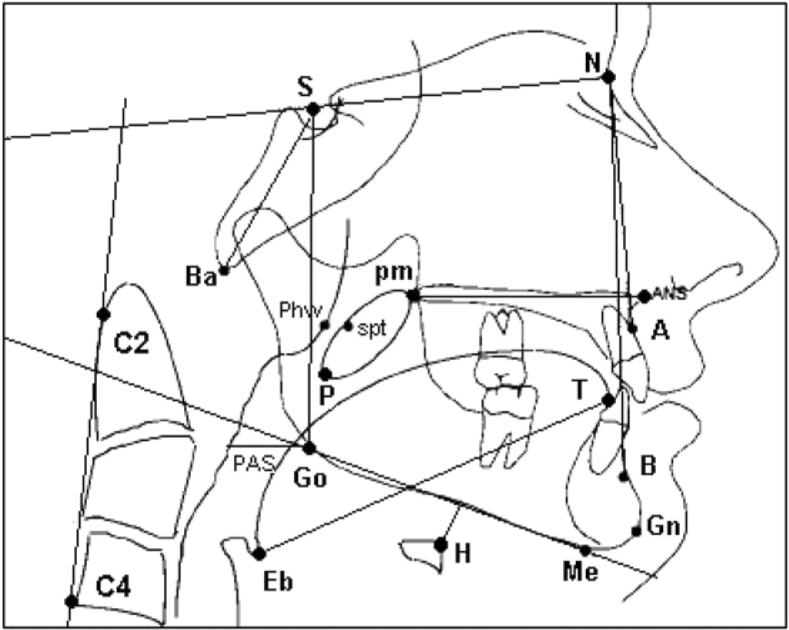
Lateral cephalometric radiographs were performed in a standardized fashion (Orthrolix L.D. unit; Philips Oral Healthcare Inc; Snoqualmie, Washington) at 76 kV and 14 mA at 0.6 s exposure time. Radiographs were analyzed using Dolphin Imaging, premium version 10.0 (Dolphin; Los Angeles, California) and Image J version 1.36 (National Institutes of Health; Bethesda, Maryland) analysis software. Standard cephalometric analysis of bony, soft-tissue, and upper-airway structures were performed14, 18, 28, 29 (Fig 2). Linear and angular measurements are defined in Table 1.
Figure 2.
Schematic of lateral cephalogram illustrating identified cephalometric landmarks. A = subspinal; ANS = anterior nasal spine; B = supramentale; Ba = basion; C2 = tangent point on the dorsal surface of C2 vertebrato a line from C4; C4 = C4 vertebra inferoposterior; Eb = epiglottis base; Gn = gnathion; Go = gonion; H = hyoidale; Me = menton; N = nasion; P = soft-palate tip; PAS = posterior airway space; phw = posterior pharyngeal wall; pm = posterior nasal spine; S = sella; spt = soft palate tangent; T = tongue tip.
Table 1.
Definitions of Cephalometric Measurements
| Linear Measurement | Definition | Angular Measurement | Definition |
|---|---|---|---|
| Maxillary length | Cd-A | SNA | S-N-A |
| Mandibular length | Cd-Gn | SNB | S-N-B |
| Ramus height | Cd-Go | ANB | A-N-B |
| Corpus length | Go-Gn | Ramal plane angle | Po Or-ramus tangent |
| Upper-facial height | N-ANS | Mandibular plane angle | SN-Go-Me |
| Lower-facial height | ANS-Me | Gonial angle | Ar-Go-Me |
| Anterior facial height | N-Me | Hyoid angle | Go-H-Me |
| Posterior facial height | S-Go | Upper-incisor inclination | U1i-ANS-PNS |
| Hyoid position | Lower-incisor inclination | L1i-MP | |
| Vertical | MP-H | Occlusion plane angle | OccPl-SN |
| Horizontal anterior | RGN-H | Craniocervical angulation | C2si-S-N |
| Horizontal posterior | C3ia-H | … | … |
| Airway widths | Measured parallel to B-Go plane | Area | |
| Superior posterior | Narrowest width behind soft palate | Oral enclosure | PNS-ANS-Go-Me |
| Posterior inferior | Width base of tongue to posterior pharyngeal wall | … | … |
| Middle airway | Width through P | … | … |
| Soft tissue | |||
| Tongue length | TT-Eb | … | … |
| Tongue height | Maximum height, perpendicular to TT-Eb | … | … |
| Soft-palate length | PNS-P | … | … |
A = subspinal; ANB = angle from A point to nasion to B point; ANS = anterior nasal spine; Ar = articulare; B = supramentale; C2si = C2 vertebrae superoinferior; C3ia = C3 vertebrae inferoanterior; Cd = condylion; Eb = epiglottis base; Gn = gnathion; Go = gonion; H = hyoidale; L1i = lower-incisor tip; Me = menton; MP = mandibular plane; N = nasion; OccP1 = occlusal plane; Occlusion plane angle = angle formed between occlusal plane line and sella to nasion line; Or = orbitale; P = soft-palate tip; PNS = posterior nasal spine; Po = ponion; RGN = retrognathion; S = sella; SN = sella-nasion distance; SNA = angle from sella to nasion to point A; SNB = angle from sella to nasion to point B; TT = tongue tip; U1i = upper-incisor tip.
Tongue cross-sectional area (CSA) was quantified as the region within the outline of the dorsum of the tongue surface and lines that connect tongue tip, retrognathion, hyoid bone, and the epiglottis base. 28 Tongue CSA was only assessed when there was adequate visibility of the entire outline of the structure. The bony oral enclosure CSA was the area of the box formed by the posterior nasal spine, anterior nasal spine, gonion, and menton (Fig 3). These boundary points were selected to reflect the dimensions of the oral cavity. All cephalometric measurements were made by a single assessor, with intrarater reliability assessed in a patient subset by repeat measurements after 6 weeks.
Figure 3.
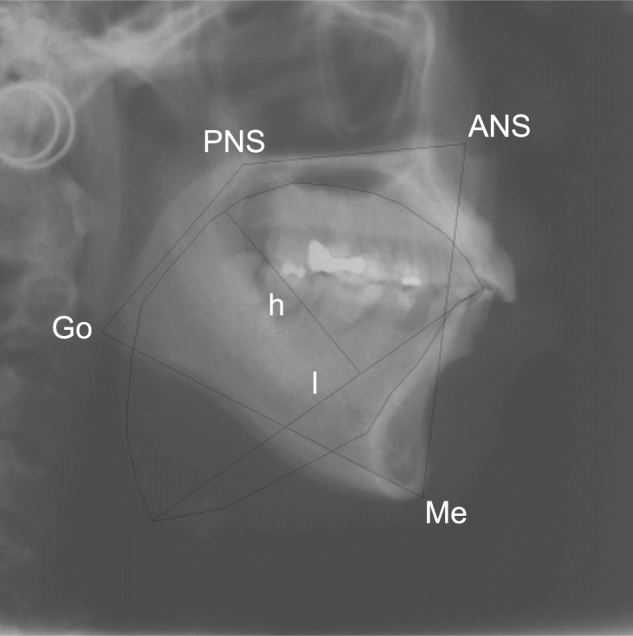
Lateral cephalogram showing the trace of the outline of the tongue of which cross-sectional area (CSA) was determined. Tongue height and length also are marked. The oral enclosure box, the sides of which are lines drawn between the points PNS, ANS, Me, and Go, is shown. A ratio of the CSA of the tongue and oral enclosure area was calculated. h = tongue height; l = tongue length; PNS = posterior nasal spine. See Figure 2 legend for expansion of other abbreviations.
Statistical Analysis
Statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc; Chicago, Illinois) software. Continuous variables were compared between treatment outcome groups using one-way analysis of variance and Tukey post hoc test. Kruskal-Wallis test was used for nonnormally distributed data. Correlation (Pearson r or Spearman ρ, as appropriate) was used to explore associations between cephalometric and dental cast-measured variables and AHI changes with treatment (%ΔAHI). Multivariate forward linear regression analysis was used to investigate relationships between %ΔAHI and cephalometric and dental variables. Intrarater reliability was assessed using intraclass correlation coefficients (ICCs). Statistical significance was accepted at P < .05.
Results
Patient Characteristics and Treatment Efficacy
Baseline patient characteristics are shown in Table 2. MAS treatment overall reduced the AHI from 33.0 ± 14.4/h to 10.8 ± 12.5/h (Table 2), with ∼ 79% of patients achieving a clinically important response (≥ 50% AHI reduction). There was no difference among treatment response groups (complete responders, partial responders, and nonresponders) in the level of mandibular advancement provided by MAS. Treatment nonresponders were significantly older with more severe OSA than complete responders (Table 2).
Table 2.
Patient Characteristics
| Treatment Response Groups |
||||
|---|---|---|---|---|
| Variable | All Patients | Complete Responders | Partial Responders | Nonresponders |
| Patients, No. | 53 | 25 | 17 | 11 |
| Sex, M (F) | 42 (11) | 17 (8) | 14 (3) | 11 (0) |
| Age, y | 49.5 ± 11.8 | 46.2 ± 10.8a | 49.1 ± 11.4 | 57.3 ± 12.3 |
| Height, cm | 173.8 ± 9.3 | 172.7 ± 10.0 | 174.6 ± 9.7 | 174.7 ± 6.9 |
| Weight, kg | 86.9 ± 16.3 | 85.7 ± 16.2 | 88.9 ± 15.8 | 86.6 ± 18.4 |
| BMI, kg/m2 | 28.7 ± 4.2 | 28.7 ± 4.5 | 29.1 ± 4.1 | 28.2 ± 4.3 |
| Skeletal class | ||||
| I | 26 | 12 | 9 | 5 |
| II | 22 | 10 | 7 | 5 |
| III | 5 | 3 | 1 | 1 |
| Baseline AHI, events/h | 33.0 ± 14.4 | 28.4 ± 14.4a | 34.8 ± 15.1 | 40.6 ± 12.7 |
| AHI with MAS, events/h | 10.8 ± 12.5 | 2.5 ± 1.4a , b | 10.8 ± 4.8c | 29.4 ± 15.1 |
| Baseline MinSao 2, % | 83.4 ± 8.1 | 84.2 ± 7.8 | 83.2 ± 8.4 | 81.9 ± 9.1 |
| MinSao 2 with MAS | 86.7 ± 6.6 | 89.9 ± 3.4a | 86.2 ± 5.9 | 80.1 ± 8.2 |
| Mandibular advancement, mm | 9.5 ± 2.0 | 9.4 ± 2.1 | 10.1 ± 1.8 | 8.9 ± 2.1 |
| Mandibular advancement, % of baseline maximum protrusion | 116.2 ± 25.1 | 114.7 ± 22.9 | 118.4 ± 31.5 | 116.4 ± 20.2 |
Data are presented as mean ± SD, unless otherwise indicated. AHI = apnea-hypopnea index; F = female; M = male; MAS = mandibular advancement splint; MinSao 2 = minimum arterial oxygen saturation.
P < .05 complete responder vs nonresponder groups.
P < .05 complete responder vs partial responder groups.
P < .05 partial responder vs nonresponder groups.
Cephalometric Measurements
Cephalometric variables are presented in Table 3. There were no differences in cephalometric linear, angular, or area measurements between complete responders, partial responders, and nonresponders. Maxillary length and upper-facial height was significantly shorter in complete responders than in partial responders. Upper-facial height modestly correlated with %ΔAHI with MAS treatment (r = 0.311, P = .024) such that a greater AHI reduction was associated with a shorter upper-facial height. Multiple linear regression did not identify any variables that predicted posttreatment AHI improvement.
Table 3.
Lateral Cephalometric Measurements by MAS Treatment Outcome
| Variable | Complete Responders | Partial Responders | Nonresponders |
|---|---|---|---|
| Linear measurement, mm | |||
| Maxillary length | 83.8 ± 1.1a | 87.6 ± 1.2 | 86.1 ± 1.1 |
| Mandibular length | 118.8 ± 1.8 | 119.8 ± 2.0 | 121.8 ± 2.0 |
| Ramus height | 56.1 ± 1.3 | 56.6 ± 1.3 | 57.6 ± 1.8 |
| Corpus length | 78.1 ± 1.3 | 79.0 ± 1.8 | 80.0 ± 1.6 |
| Upper-facial height | 51.2 ± 3.5a | 54.5 ± 1.0 | 54.3 ± 0.9 |
| Lower-facial height | 70.2 ± 1.3 | 66.3 ± 1.8 | 70.6 ± 2.3 |
| Anterior facial height | 119.7 ± 1.5 | 118.7 ± 2.1 | 122.9 ± 2.3 |
| Posterior facial height | 85.0 ± 1.5 | 84.9 ± 1.5 | 87.1 ± 1.6 |
| Hyoid position | |||
| Vertical | 24.1 ± 1.3 | 27.2 ± 1.7 | 25.7 ± 1.6 |
| Horizontal anterior | 42.0 ± 1.6 | 43.3 ± 1.9 | 41.4 ± 1.8 |
| Horizontal posterior | 36.7 ± 1.6 | 37.6 ± 0.9 | 38.4 ± 1.6 |
| Airway widths | |||
| Superior posterior | 73.3 ± 4.6 | 65.1 ± 8.7 | 69.1 ± 10.4 |
| Posterior inferior | 96.8 ± 8.0 | 83.7 ± 7.4 | 100.0 ± 9.6 |
| Middle airway | 87.9 ± 6.0 | 78.1 ± 8.4 | 97.1 ± 7.7 |
| Soft tissue | |||
| Tongue length | 80.1 ± 1.2 | 79.2 ± 1.7 | 78 ± 2.0 |
| Tongue height (n = 30) | 4.3 ± 0.1 | 4.2 ± 0.1 | 4.0 ± 0.2 |
| Soft-palate length | 38.6 ± 0.8 | 37.8 ± 1.1 | 36.3 ± 1.0 |
| Angular measurements, ° | |||
| SNA | 82.5 ± 0.8 | 81.7 ± 1.2 | 83.8 ± 1.7 |
| SNB | 79.2 ± 0.8 | 77.6 ± 1.3 | 79.9 ± 1.0 |
| ANB | 3.3 ± 0.6 | 4.1 ± 0.9 | 3.9 ± 0.7 |
| Ramal plane angle | 82.2 ± 1.3 | 80.1 ± 1.4 | 82.0 ± 1.6 |
| Mandibular plane angle | 35.1 ± 1.5 | 34.0 ± 1.9 | 35.1 ± 1.8 |
| Gonial angle | 127.8 ± 1.2 | 127.0 ± 1.7 | 128.0 ± 2.4 |
| Hyoid angle | 111.2 ± 2.6 | 104.7 ± 3.8 | 108.3 ± 3.5 |
| Upper-incisor inclination | 110.6 ± 1.5 | 109.1 ± 2.5 | 111.8 ± 2.7 |
| Lower-incisor inclination | 87.0 ± 1.1 | 88.9 ± 2.0 | 87.4 ± 2.4 |
| Occlusion plane angle | 14.6 ± 1.1 | 16.0 ± 1.6 | 14.8 ± 1.3 |
| Craniocervical angulation | 111.2 ± 1.9 | 111.0 ± 2.1 | 109.9 ± 2.3 |
| Area measurement, mm2 | |||
| Oral enclosure (n = 30) | 40.3 ± 1.6 | 38.6 ± 1.3 | 42.1 ± 2.0 |
Oral Dimensions
Dental measurements of oral cavity size are presented in Table 4. There were no significant differences in intertooth distances, palatal height, or depths among treatment response groups. Maxillary intermolar distance correlated with %ΔAHI (r = 0.290, P = .035) such that a smaller intertooth distance was associated with a greater AHI reduction. No other dental measurements were related to treatment outcome. Multiple linear regression analysis did not reveal any oral measurements that were predictive of treatment outcome.
Table 4.
Dental Measurements by MAS Treatment Outcome
| Variable, mm | Complete Responders | Partial Responders | Nonresponders |
|---|---|---|---|
| Maxillary intertooth distances | |||
| Canine | 34.7 ± 0.5 | 34.1 ± 0.6 | 35.7 ± 0.7 |
| First premolar | 35.9 ± 0.7 | 36.6 ± 0.6 | 37.3 ± 0.5 |
| Second premolar | 41.8 ± 0.9 | 42.3 ± 0.6 | 43.2 ± 0.6 |
| Molar | 47.4 ± 0.9 | 47.9 ± 0.7 | 49.4 ± 0.8 |
| Mandibular intertooth distances | |||
| Canine | 26.1 ± 0.5 | 25.3 ± 0.6 | 26.2 ± 0.7 |
| First premolar | 31.0 ± 0.5 | 32.1 ± 0.8 | 32.5 ± 0.7 |
| Second premolar | 36.5 ± 0.7 | 38.0 ± 0.9 | 37.9 ± 0.9 |
| Molar | 43.7 ± 0.9 | 44.0 ± 0.8 | 44.6 ± 1.0 |
| Palatal heights | |||
| Canine | 5.2 ± 0.2 | 5.6 ± 0.4 | 4.9 ± 0.4 |
| First premolar | 11.4 ± 0.5 | 11.4 ± 0.5 | 10.4 ± 0.5 |
| Second premolar | 16.0 ± 0.5 | 15.6 ± 0.6 | 14.7 ± 0.5 |
| Molar | 18.1 ± 0.3 | 18.0 ± 0.5 | 17.7 ± 0.4 |
| Palatal depths | |||
| Maxillary | 35.9 ± 0.5 | 35.9 ± 0.9 | 35.6 ± 0.6 |
| Mandibular | 30.6 ± 0.8 | 30.0 ± 1.3 | 29.4 ± 0.9 |
N = 53. Data are presented as mean ± SEM. No significant differences were found in measurements. See Table 2 legend for expansion of abbreviation.
Tongue Area and Anatomic Balance
Tongue CSA was obtained in a subset of 30 patients. This subset did not differ in any baseline characteristics to patients in whom tongue area was not measureable. Tongue area did not significantly differ among complete (39.5 ± 1.3 cm2), partial (35.7 ± 1.3 cm2), and nonresponders (35.5 ± 0.5 cm2), although there was a trend toward significance (P = .09). However, tongue area correlated with %ΔAHI (r = −0.478, P = .008), with larger tongue CSA associated with greater AHI reduction.
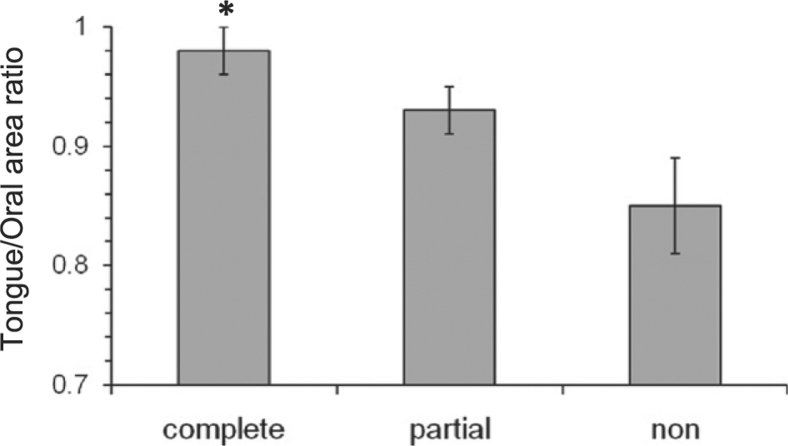
The tongue/oral CSA ratio significantly differed between complete and nonresponders (P = .012) such that complete responders had a larger tongue area for a given oral cavity size (Fig 4). Oral cavity area itself did not differ among treatment outcome groups (Table 2).
Figure 4.
Tongue/oral area ratio by mandibular advancement splint (MAS) treatment outcome. Tongue/oral area ratio in shown for complete (n = 13), partial (n = 13), and nonresponders (n = 4) to MAS treatment. Tongue/oral area ratio was significantly greater in complete responders than in nonresponders, *P = .012. Data are presented as mean ± SEM.
Age, BMI, tongue area, oral area, and tongue/oral CSA ratio were considered as independent variables for predicting %ΔAHI using multiple linear regression. A model containing age and tongue area explained ∼ 38% of the variance in treatment outcome (Table 5).
Table 5.
Multiple Regression Analyses for %ΔAHI (n = 30)
| Variable | B | SE B | P Value |
|---|---|---|---|
| Model 1: R 2 = 0.244, P = .005 | |||
| Age | 0.69 | 0.23 | .005 |
| Model 2: R 2 = 0.379, P = .023 | |||
| Age | 0.59 | 0.21 | .011 |
| Tongue area | −1.53 | 0.63 | .023 |
%ΔAHI = apnea-hypopnea index changes with treatment; B = unstandardized coefficient. Variables not in the equation are BMI, oral area, and tongue/oral CSA ratio.
Intrarater Reliability
Duplicate cephalometric measurements showed high reproducibility (median ICC, 0.885; 95% CI, 0.807-0.964; n = 10). Similarly, intertooth distances and depth and palatal height measurements showed high repeatability (median ICC, 0.905; 95% CI, 0.846-0.965; n = 10). Repeated soft-tissue measurements of tongue area and height also showed excellent reproducibility, with an average ICC of 0.902 and 0.939, respectively (n = 5).
Discussion
To our knowledge, this study is the first to combine craniofacial bony and soft-tissue variables and dental oral measurements and to associate them with MAS treatment outcome. This novel approach encompasses the assessment of multiple anatomic factors that likely relate to treatment success. A key finding of this study is that although oral dimensions do not appear to differ among treatment outcome groups, treatment responders appear to have a larger tongue for a given oral cavity size. This finding gives rise to the concept that MAS treatment increases oral cavity size, counteracting an imbalance between soft-tissue dimensions and the surrounding bony enclosure.
Predicting MAS effectiveness for OSA treatment remains an ongoing clinical challenge. Our understanding of the mechanisms of action of OSA improvement by MAS is still in its infancy. Using MRI, we have recently investigated the effects of mandibular advancement on upper-airway structure by sophisticated three-dimensional analysis of upper airways and surrounding soft-tissue structures. 20 Although responders displayed larger upper-airway volume increases with application of MAS, no baseline airway or soft-tissue variables discriminated between those who responded to MAS treatment and those who did not.
A variety of mechanisms underlie OSA, including ventilatory control instability, anatomic compromise, and upper-airway dilator muscle response. Recent evidence suggests that the mechanisms underlying apnea are highly variable, with some patients having primarily an anatomic problem. It has been shown recently that in addition to individual anatomic factors, relationships between soft-tissue and bony enclosure size are altered, a concept termed anatomic balance.19, 28 A larger tongue for a given oral cavity size has been demonstrated in patients with OSA,28, 30 and it is believed that this situation contributes to increased upper-airway collapsibility by airway narrowing and increased extraluminal tissue pressure. Therefore, our finding that complete responders have a larger tongue for oral enclosure size is interesting and suggests that a mechanism of action of MAS is to increase oral size and improve anatomic balance. Patients with smaller tongue volumes relative to oral cavity size may have other (eg, nonanatomic) factors underlying apnea. This possibility warrants further investigation.
Tongue size was larger in complete responders to MAS treatment in relative terms, and there was a trend in absolute terms. Intuitively, an oral enclosure containing a smaller tongue would be believed to respond better to mandibular advancement. Schaaf et al 31 also found that relative and not absolute tongue size in a tongue/bony confines ratio predicted clinical success in genioglossus advancement surgery to treat OSA secondary to glossoptosis. Our earlier functional studies of MAS treatment outcome prediction identified patients whose airway collapses in the oropharyngeal region as responders to treatment. 32 A larger tongue may be indicative of propensity to collapse in this region and may indicate an oropharyngeal-collapser and, therefore, MAS treatment-responder. Similarly, patients with primary compromise at the level of the velopharynx could be predicted to respond well to uvulopalatopharyngoplasty. Our previous MRI study did not identify a larger tongue in treatment responders; however, anatomic balance was not assessed. 20
Oral dimensions were assessed through a novel approach of assessing dental cast measurements in addition to cephalometric imaging analysis. Although oral dimensions appear to differ between patients with OSA and control subjects, 18 there was no relationship between these measures and MAS treatment outcome. Despite these negative results, these findings represent an important step in continuing the development of our understanding of the mechanisms of MAS efficacy for OSA treatment.
Our study sample represented a typical cohort of patients with OSA who were middle-aged, were slightly overweight, and presented with a range of OSA severity from mild to severe. MAS treatment has been shown to be effective even in some patients with severe OSA, although it is indicated primarily for use in milder cases.33, 34 In our sample, nonresponders were older than partial and complete responders. Age was a predictive variable for MAS treatment outcome in our patient subset in which tongue CSA could be assessed. Younger age has been associated with treatment success, 35 but this is not always the case, and age is not always a predictive factor for treatment outcome in oral appliance studies. However, older age and higher AHI in the nonresponders may represent more severe progression of the disease, which will not respond as well to oral appliance treatment. Some studies have reported decreased pharyngeal CSA and upper-airway dilator muscle activity with age, but these findings are not consistent.36, 37 Changes in mandibular shape with aging 38 and increases in pharyngeal collapsibility also have been observed, 39 and both factors may reduce the efficacy of mandibular advancement. Nonresponders have a higher BMI than responders. 40 Mandibular advancement enlarges the upper-airway lumen and decreases collapsibility in lean but not in obese subjects. 41 This differential response may be due to excess tissue surrounding the upper airway, which is unable to be displaced effectively to increase lumen size.
To be clinically useful, prediction methods for MAS treatment outcome need to be quick, inexpensive, accessible, and accurate. Lateral cephalometry often is used in clinical practice to image the oral cavity and pharynx because it is inexpensive, is widely available with minimal radiation exposure, and can identify craniofacial characteristics associated with OSA. 42 Dental casts commonly are used in the clinical setting to measure oral dimensions for treatment planning. These casts are used directly for fabrication of MAS. The shape of the dental arches through assessing intertooth distances 18 also is a measure of dental arch constriction. However, despite the suitability of these techniques for potential clinical prediction methods, we did not identify any variables that predicted MAS treatment success. This lack of predictive value possibly is due to the multifactorial nature of MAS treatment response, as anatomic measurements are not the sole determinant of treatment efficacy. Other factors, such as upper-airway dilator muscle activity, also may be important, 43 and it is likely that the relative contributions of anatomic and functional effects will differ among patients. Therefore, it is likely that a combination of both functional and structural assessments is needed to predict MAS treatment outcome accurately.
Study Limitations
The two-dimensional nature of cephalometric imaging is always a limitation when assessing three-dimensional structures. However, dental cast measurements also were used to provide some transverse measurements of oral dimensions. Our goal was to identify simple and accessible screening procedures; thus, more-sophisticated three-dimensional imaging techniques, such as MRI, were not considered because these would be unfeasible in routine clinical practice. Imaging also was performed during wakefulness; however, this condition should not influence skeletal or oral measurements. Our sample size was only modest, particularly the subset in which tongue measurements were able to be assessed. In particular, the subset only contained four nonresponders for comparison. Although it did not reach statistical significance, there was a trend for similar findings between complete and partial responders. Despite these limitations, we believe that our findings are novel and will usefully inform future research in the field.
Conclusions
The present study suggests that oral cavity dimensions do not significantly differ between those who respond to MAS treatment and those who do not; however, assessing soft-tissue volumes relative to their bony confinements may have predictive value. Responders appear to have a larger tongue volume for a given oral cavity size, suggesting that MAS help to correct anatomic imbalance. Further studies are needed to determine whether lateral cephalometric assessment of the ratio between tongue and bony enclosure size is useful in predicting response to oral appliance therapy.
Acknowledgments
Author contributions: Ms Mostafiz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ms Mostafiz: contributed to the study design, performance of all measurements, and manuscript preparation.
Dr Dalci: contributed to the data collection and manuscript preparation.
Dr Sutherland: contributed to the data analysis and manuscript preparation.
Dr Malhotra: contributed to the study conception and design and manuscript preparation.
Dr Srinivasan: contributed to the data collection and manuscript preparation.
Dr Darendeliler: contributed to the study design and manuscript preparation.
Dr Cistulli: contributed to the study conception and design and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Malhotra has consulting and research income from Philips Oral Healthcare Inc, SGS Pharma, SHC, Ethicon, Medtronic, Pfizer, Merck, Itamar Medical, Sepracor, Apnicure, Cephalon, and Apnex Medical. Dr Cistulli contributed to the development of the MAS used in this study (SomnoDentMAS; SomnoMed Ltd). He has consulted for and has been on the advisory board for SomnoMed and is a consultant to ExploraMed. He has received research support from ResMed and is a board member of the ResMed Foundation, a nonprofit, charitable organization. Ms Mostafiz and Drs Dalci, Sutherland, Srinivasan, and Darendeliler have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding sources played no role in the conduct of the study.
Other contributions: We thank the clinical research operations group at the Woolcock Institute of Medical Research for its assistance in collection of patient data.
Footnotes
Funding/Support: This study was supported by the National Health and Medical Research Council of Australia[Project Grant 457557]. SomnoMed Ltd provided the oral appliances for this study. Ms Mostafiz was supported by a Harvard Medical School Office of Enrichment Program grant and an American Association of Dental Research Student Research Fellowship.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 pt 1):1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 3.Weaver TE, Kribbs NB, Pack AI. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 4.Kribbs NB, Pack AI, Kline LR. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 5.Chan AS, Lee RW, Cistulli PA. Non-positive airway pressure modalities: mandibular advancement devices/positional therapy. Proc Am Thorac Soc. 2008;5(2):179–184. doi: 10.1513/pats.200707-104MG. [DOI] [PubMed] [Google Scholar]
- 6.Hoekema A, Hovinga B, Stegenga B, De Bont LG. Craniofacial morphology and obstructive sleep apnoea: a cephalometric analysis. J Oral Rehabil. 2003;30(7):690–696. doi: 10.1046/j.1365-2842.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–262. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 8.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166(5):743–748. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 9.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(6):1457–1461. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 10.Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166(6):860–864. doi: 10.1164/rccm.200204-342OC. [DOI] [PubMed] [Google Scholar]
- 11.Kushida CA, Littner MR, Morgenthaler T. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 12.Menn SJ, Loube DI, Morgan TD, Mitler MM, Berger JS, Erman MK. The mandibular repositioning device: role in the treatment of obstructive sleep apnea. Sleep. 1996;19(10):794–800. doi: 10.1093/sleep/19.10.794. [DOI] [PubMed] [Google Scholar]
- 13.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27(5):934–941. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 14.Battagel JM, Johal A, Kotecha B. A cephalometric comparison of subjects with snoring and obstructive sleep apnoea. Eur J Orthod. 2000;22(4):353–365. doi: 10.1093/ejo/22.4.353. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson KA, Ono T, Lowe AA, Ryan CF, Fleetham JA. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108(2):375–381. doi: 10.1378/chest.108.2.375. [DOI] [PubMed] [Google Scholar]
- 16.Lowe AA, Ozbek MM, Miyamoto K, Pae EK, Fleetham JA. Cephalometric and demographic characteristics of obstructive sleep apnea: an evaluation with partial least squares analysis. Angle Orthod. 1997;67(2):143–153. doi: 10.1043/0003-3219(1997)067<0143:CADCOO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Tangugsorn V, Krogstad O, Espeland L, Lyberg T. Obstructive sleep apnea (OSA): a cephalometric analysis of severe and non-severe OSA patients. Part II: A predictive discriminant function analysis. Int J Adult Orthodon Orthognath Surg. 2000;15(3):179–191. [PubMed] [Google Scholar]
- 18.Seto BH, Gotsopoulos H, Sims MR, Cistulli PA. Maxillary morphology in obstructive sleep apnoea syndrome. Eur J Orthod. 2001;23(6):703–714. doi: 10.1093/ejo/23.6.703. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165(2):260–265. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 20.Chan AS, Sutherland K, Schwab RJ. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65(8):726–732. doi: 10.1136/thx.2009.131094. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Lowe AA. Factors related to the efficacy of an adjustable oral appliance for the treatment of obstructive sleep apnea. Chin J Dent Res. 2000;3(3):15–23. [PubMed] [Google Scholar]
- 22.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114(6):1630–1635. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 23.Mayer G, Meier-Ewert K. Cephalometric predictors for orthopaedic mandibular advancement in obstructive sleep apnoea. Eur J Orthod. 1995;17(1):35–43. doi: 10.1093/ejo/17.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Zeng B, Ng AT, Darendeliler MA, Petocz P, Cistulli PA. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175(7):726–730. doi: 10.1164/rccm.200608-1205OC. [DOI] [PubMed] [Google Scholar]
- 25.Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31(4):543–547. doi: 10.1093/sleep/31.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Association ASD EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Government Printing Office; Washington, DC: 1968. NIH publication 204. [Google Scholar]
- 28.Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108(6):1009–1015. doi: 10.1097/ALN.0b013e318173f103. [DOI] [PubMed] [Google Scholar]
- 29.Battagel JM, Johal A, Smith AM, Kotecha B. Postural variation in oropharyngeal dimensions in subjects with sleep disordered breathing: a cephalometric study. Eur J Orthod. 2002;24(3):263–276. doi: 10.1093/ejo/24.3.263. [DOI] [PubMed] [Google Scholar]
- 30.Iida-Kondo C, Yoshino N, Kurabayashi T, Mataki S, Hasegawa M, Kurosaki N. Comparison of tongue volume/oral cavity volume ratio between obstructive sleep apnea syndrome patients and normal adults using magnetic resonance imaging. J Med Dent Sci. 2006;53(2):119–126. [PubMed] [Google Scholar]
- 31.Schaaf WE, Jr, Wootten CT, Donnelly LF, Ying J, Shott SR. Findings on MR sleep studies as biomarkers to predict outcome of genioglossus advancement in the treatment of obstructive sleep apnea in children and young adults. AJR Am J Roentgenol. 2010;194(5):1204–1209. doi: 10.2214/AJR.09.3254. [DOI] [PubMed] [Google Scholar]
- 32.Ng AT, Qian J, Cistulli PA. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 2006;29(5):666–671. [PubMed] [Google Scholar]
- 33.Lee CH, Mo JH, Choi IJ. The mandibular advancement device and patient selection in the treatment of obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2009;135(5):439–444. doi: 10.1001/archoto.2009.31. [DOI] [PubMed] [Google Scholar]
- 34.Monasterio C, Navarro A, Farreras S. Effectiveness of a mandibular advancement prosthesis in the treatment of obstructive sleep apnea syndrome [in Spanish] Arch Bronconeumol. 2000;36(7):371–376. doi: 10.1016/s0300-2896(15)30136-8. [DOI] [PubMed] [Google Scholar]
- 35.Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132(2):693–699. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- 36.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10(9):2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 37.Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88(5):1831–1839. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra A, Huang Y, Fogel R. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119(1) doi: 10.1016/j.amjmed.2005.01.077. 72.e9-72.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eikermann M, Jordan AS, Chamberlin NL. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131(6):1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker-Engström ML, Ringqvist I, Vestling O, Wilhelmsson B, Tegelberg A. A prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnea. Sleep Breath. 2003;7(3):119–130. doi: 10.1007/s11325-003-0119-3. [DOI] [PubMed] [Google Scholar]
- 41.Isono S, Tanaka A, Tagaito Y, Sho Y, Nishino T. Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology. 1997;87(5):1055–1062. doi: 10.1097/00000542-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Togeiro SM, Chaves CM, Jr, Palombini L, Tufik S, Hora F, Nery LE. Evaluation of the upper airway in obstructive sleep apnoea. Indian J Med Res. 2010;131:230–235. [PubMed] [Google Scholar]
- 43.Johal A, Gill G, Ferman A, McLaughlin K. The effect of mandibular advancement appliances on awake upper airway and masticatory muscle activity in patients with obstructive sleep apnoea. Clin Physiol Funct Imaging. 2007;27(1):47–53. doi: 10.1111/j.1475-097X.2007.00714.x. [DOI] [PubMed] [Google Scholar]