Abstract
Background
The thyroid hormone receptor alpha gene (THRA) is alternately spliced to generate thyroid receptor alpha1 (TRα1) or a non hormone-binding variant protein (α2), whose function is unknown. Here, we describe the first patients with a mutation in both TRα1 and α2 and compare them to cases of Resistance to Thyroid Hormone (RTH) with defective TRα1 alone, to delineate their relative roles.
Findings
The index case and her two sons presented in childhood with growth failure, developmental delay and constipation which improved with thyroxine treatment despite normal circulating thyroid hormone (TH) levels. They exhibit similar clinical (macrocephaly, broad facies, skin tags, motor dyspraxia, slow speech), biochemical (subnormal FT4/FT3 ratio, low reverse T3, raised creatine kinase, mild anaemia) and radiological (thickened calvarium) features to TRα1-mediated RTH cases, although they harbour a heterozygous, missense mutation (A263V) in both TRα1 and α2 proteins. Impaired transcriptional activity of A263V mutant TRα1 and dominant negative inhibition of its wild type counterpart, as well as reduced TH-responsive target gene expression in patient-derived blood cells, are reversible at higher T3 levels. In contrast, A263V mutant α2 function mirrors its normal counterpart.
Interpretation
Resemblance of RTH due to mutation in both TRα1 and α2 proteins with TRα1-RTH suggests that TRα1 is the principal functional product of the THRA locus. As observed in vitro, thyroxine treatment alleviates hormone resistance in vivo, possibly ameliorating the phenotype. Recognition and therapeutic intervention in future TRα-RTH cases may be of clinical importance.
Key terms: Thyroid hormone receptor alpha, dominant negative, Resistance to Thyroid Hormone
Introduction
The physiological actions of thyroid hormones (TH) are mediated by nuclear receptors (TRα, TRβ) encoded by separate (THRA, THRB) genes, which regulate target tissue gene expression. Alternate splicing of the THRA locus generates TRα1 and α2 subtypes with identical aminoterminal and DNA binding domains but divergent carboxyterminal regions; TRα1 binds TH and is most highly expressed in myocardium, skeletal muscle, gastrointestinal tract and the central nervous system; aminoterminally truncated forms of TRα1 (p48, p28) expressed in mitochondria influence its function (1,2). The non hormone-binding variant α2 protein is widely expressed, but its function is not understood (2). With upregulated target genes, unliganded TR binds to regulatory DNA sequences (thyroid response elements, TREs) in their promoters (usually as a heterodimer with retinoid X receptor), recruiting a multiprotein corepressor complex which mediates inhibition of basal gene transcription; thyroid hormone occupancy of receptor results in corepressor dissociation and binding of a coactivator complex, leading to transcriptional activation (3).
The incidence of Resistance to Thyroid Hormone mediated by defective TRβ (RTHβ) is ~1 in 40,000, and several hundred heterozygous, β receptor mutations which localise to three hotspots within its ligand binding domain (LBDs), have been identified in this disorder (4). Consistent with dominant inheritance of RTHβ, mutant β receptors inhibit the function of their wild type receptor counterparts in a dominant negative manner; constitutive target gene repression due to failure of corepressor dissociation from mutant TRβ represents a likely mechanism for such dominant negative inhibition (5). Based on the marked homology (95%) of their LBDs, analogous mutations in human TRα were anticipated, but only three different frameshift/stop or premature stop mutations which localise to an TRα1-specific exon and selectively disrupt its carboxyterminal transcription activation function, have been described hitherto (6,7,8,9). However, murine models with different TRα1 mutations (10,11,12,13) have been generated and exhibit varying phenotypes, suggesting molecular and clinical heterogeneity of the human disorder.
Here, we describe the first family with a THRA defect, resulting in mutation of both TRα1 and the α2 splice variant protein. Their clinical and biochemical features are homologous to previous cases with defective TRα1 alone and define characteristics of this disorder. Notably, consistent with lack of discernible difference between wild type and mutant α2 protein function, we are unable to identify any added clinical phenotypes. We document reversal of mutant receptor dysfunction at higher T3 levels in vitro and amelioration of hormone resistance with thyroxine treatment in vivo.
Patients and Methods
Case Descriptions
The index case (P1, female 60 yrs) exhibited features (increased body weight, poor linear growth, constipation, large, prominent tongue) suggesting hypothyroidism age 2 years, but thyroid hormone levels were within the normal range. Nevertheless, she was treated with thyroxine, with improvement in growth and constipation, and has remained on this since.
Her eldest son (P2, age 30 yrs) was delivered by caesarean section due to macrocephaly. At six weeks, he required conversion from breast to bottle feeding to correct poor nutritional intake. His subsequent growth and developmental milestones (using a ball, speech) were delayed and, in view of the resemblance of clinical features to those previously noted in his mother, thyroxine treatment was commenced age 3 years, despite thyroid hormone levels being within the normal range. Although growth and development improved, motor coordination remained poor, causing imbalance, “clumsiness” and poor handwriting and he attended a specialist school for children with motor dyspraxia. Thyroxine therapy was continued throughout childhood and adult life except for an interval (26 to 29 yrs), when he noted constipation, weight gain, lethargy and low mood off treatment.
A second son (P3, age 26 yrs), delivered by elective caesarean section, with a large tongue and similar facial appearance to P2, exhibited somnolence, delayed linear growth, speech and motor development which improved following thyroxine treatment from age 3 yrs. Significant motor incoordination, also requiring specialist schooling, has persisted.
A third sibling is unaffected, with normal growth and development.
Methods
All investigations were part of an ethically approved protocol (Cambridgeshire LREC 98/154) and/or clinically indicated, being undertaken with prior informed patient consent. Serial biochemical and physiological measurements were made in patients off and on thyroxine therapy as described previously (6,9). Molecular genetic analysis of THRA and functional characterisation of mutant TRα were undertaken as described previously (6,9) and in supplementary data. Statistical analysis of data was undertaken using a two-tailed t-test, in Excel, version 14.3.9). Structural modelling of the mutant TRα and TRβ was undertaken using MacPyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, L.L.C (www.PyMOL.org).
Results
Clinical Investigation
All patients were on thyroxine treatment when referred, and were assessed on therapy and six weeks after its discontinuation.
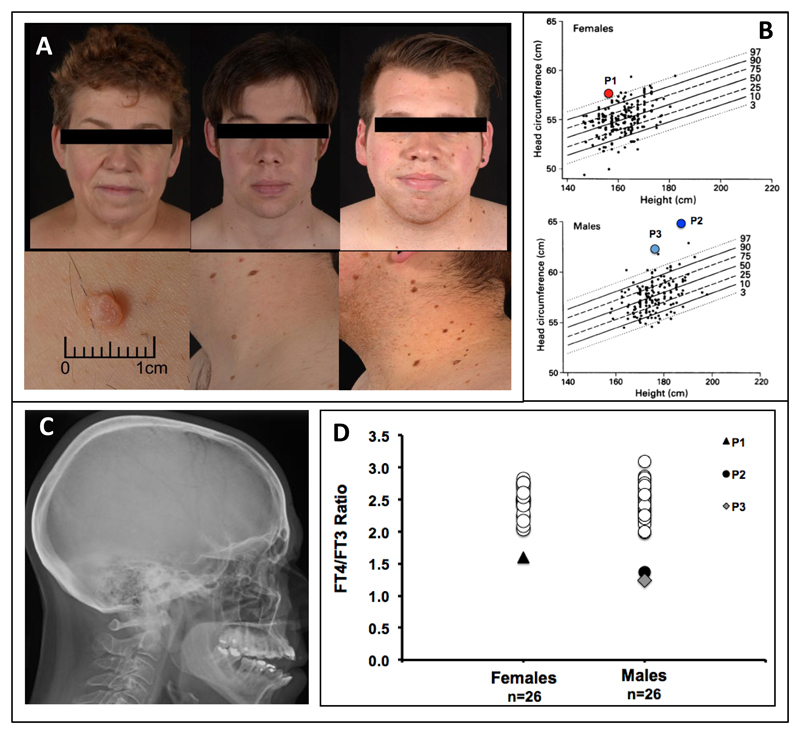
P1’s stature is appropriate (height 1·57m, mid-parental height (MPH) 1·69m) and proportionate (sitting height 83·8cm (-1·3 SDS), subischial leg length 73·2cm (-0·3 SDS), but head circumference is increased (57·5cm, >97th centile, Figure 1B). P2 is proportionately tall (height 1·86m (MPH 1·78m); sitting height 97·3cm (+1·1 SDS), subischial leg length 88·7 cm (+1·6 SDS)), with markedly increased head circumference (64·8cm, >97th centile, Figure 1B). P3 is also proportionate (height 1·77m (MPH 1·78m); sitting height 94·5cm (+0·3 SDS), subischial leg length 82·.5cm (+0·15 SDS), but macrocephalic (head circumference 62cm, >97th centile, Figure 1B). These individuals have a broad face, flattened nasal bridges and prominent tongue, with numerous skin tags and moles (P2, P3) (Fig 1A). Their speech is dysarthric (P2, P3); IQ has not been formally tested, but was low average (88) at school in P3; all have achieved a third level qualification.
Figure 1. Phenotypic Features of the Patients.
Photographs of the patients (Panel A) illustrate broad facies, flattened nasal bridge (P1 left, P3 right) full lips (P2 middle, P3 right) and long philtrum. Multiple skin tags are evident (P2 and P3). Head circumferences (Panel B) for height and gender (adapted with permission from reference 27) are markedly increased. Skull radiograph of P1 (Panel C) shows thickened calvarium. FT4:FT3 ratios, compared with gender-matched healthy subjects of similar age (females 35-64yrs, males 20-40yrs), are subnormal (Panel D).
All patients had a thickened skull vault (cranial hyperostosis) (Figure 1C). Bone mineral density (BMD) assessed by DXA scan and quantitative CT (qCT) was slightly reduced at the hip (T scores: DXA -1·0; qCT total hip -1·7, femoral neck -0·5) or normal at lumbar spine (T score +1·9) in P1. In contrast, BMD at these sites was increased in both P2 and P3 (DXA T scores: P2, Hip +0·8, Lumbar spine + 1·9, P3 Hip +1·4, Lumbar spine +1·9), being more evident on qCT (T scores: P2, total hip +1·52 or 94th centile for age, femoral neck T score + 1·7 or 96th centile for age; P3 total hip +1·9 or 97th centile for age, femoral neck +2·8 or 99th centile for age).
Off thyroxine treatment, all patients had normal TSH, marginally low (P1, P3) or low-normal (P2) FT4 and normal (P1, P2) or marginally high (P3) FT3 levels (Table 1); however, their FT4:FT3 ratio was uniformly low (Figure 1D), with subnormal reverse T3 levels (Table 1). Their resting energy expenditure (REE) was markedly reduced with raised skeletal muscle creatine kinase (CK) isoenzyme levels and mild, normocytic, anaemia (Table 1).
Table 1. Biochemical and Metabolic Measurements in the Patients.
Abbreviations: CK-MM: Skeletal muscle isoenzyme of creatine kinase; P1NP: procollagen type 1 N propeptide; CTx: C-terminal cross-linking telopeptide of type 1 collagen; NTx/Cr: N-terminal cross-linking telopeptide of type 1 collagen to creatinine ratio. BCE: Bone collagen equivalents.
| Variable | P1 Female, aged 61 years |
P2 Male, aged 30 years |
P3 Male, aged 26 years |
Reference Values | |||
|---|---|---|---|---|---|---|---|
| Thyroxine Dose (mcg/day) | Nil | 75 | Nil | 150 | Nil | 150 | |
| Weight (kg) | 70.3 | 69.6 | 84 | 84.4 | 104.3 | 106 | |
| BMI (kg/m2) | 28.52 | 28.24 | 24.28 | 24.40 | 33.29 | 33.83 | |
| Sleeping heart rate (bpm) | 58 | 62 | 53 | 54 | 55 | 51 | 40-68* |
| Resting Energy Expenditure | -1.93 | -1.82 | -3.06 | -1.75 | -3.35 | -0.3 | Z score |
| TSH (mU/L) | 4.6 | 0.12 | 4.8 | <0.03 | 3.2 | 0.54 | 0.35-5.5 |
| fT4 (pmol/L) | 9.4 | 13.9 | 10.5 | 17 | 9.7 | 11 | 10-19.8 |
| Total T4 (nmol/L) | 60 | 96.1 | 76.6 | 130 | 66.3 | 83.6 | 69-141 |
| fT3 (pmol/L) | 4.4 | 6.3 | 6.4 | 8.7 | 6.8 | 8 | 3.5-6.5 |
| Total T3 (nmol/L) | 1.3 | 1.6 | 1.7 | 2.3 | 2.1 | 2.4 | 0.9-2.8 |
| rT3 (ng/dl) | <5 | 7 | 5 | 12 | <5 | <5 | 8-25 |
| Thyroglobulin (ug/L) | 14 | 1.1 | 69.7 | 2.6 | 23.6 | 3.2 | |
| Total CK (U/L)** | 364 | 178 | 385 | 242 | 184 | 295 | 26-192 U/L |
| SHBG (nmol/L) | 45.5 | 45.7 | 28.2 | 32.8 | 14 | 13.6 | Male 10-57nmol/L ; Female 18-144nmol/L |
| Total Cholesterol (mmol/L) | 7.9 | 7.2 | 5.2 | 4.3 | 4.2 | 4 | < 5 mmol/L for a healthy adult - UK |
| LDL cholesterol (mmol/L) | 5.06 | 4.72 | 3.2 | 2.53 | 2.34 | 2.09 | < 3 mmol/L or less for healthy adult - UK |
| IGF-1 (nmol/L) | 9.9 | 11.4 | 24.3 | 26.9 | 32.9 | 31.7 | Female 11.8-28.6; Male 16.3-39.3 |
| Bone Turnover Markers*** | |||||||
| Formation | |||||||
| Bone-specific Alkaline Phosphatase (ng/ml) | 12.6 | 14.8 | 17.4 | 15.4 | 10.7 | 11.8 | Female (post menopausal) 3.8-22.6; Male 5.7-32.9 |
| Osteocalcin (ng/ml) | 9.7 | 10.7 | 13 | 16.8 | 12.7 | 12.9 | Female (post menopausal) 15-46; Male (aged 18-30) 24-70 |
| P1NP (ng/ml) | 26.3 | 27.3 | 55.2 | 56.3 | 52.8 | 46.6 | Female (post menopausal) 20.25-76.31 |
| Resorption | |||||||
| CTx (ng/ml) | 0.389 | 0.339 | 0.7 | 0.839 | 0.585 | 0.525 | Female (post menopausal) 0.104-1.008; Male (aged 30-50) 0.096-0.584 |
| NTx/Cr (nmol BCE/mmol Cr) | 24.5 | 30.8 | 25.3 | 24.7 | 19.5 | 28.2 | Male 21-83 |
| RBC mass | 3.74 | 3.7 | 4.23 | 4.27 | 4.17 | 4.16 | Female 3.8-5.3 x 1012/L ; Male 4.20-5.80 1012/L |
| MCV (fL) | 94.8 | 95.5 | 92.5 | 92.6 | 88 | 88.2 | 80-100 |
| Haemoglobin (g/dl) | 12 | 12 | 12.9 | 12.9 | 12.5 | 12.4 | Female 11.5-16; Male 13-17 |
Sleeping heart rate from 148 healthy volunteers
Only CK-MM isoenzyme detected
Bone turnover marker measurements on chronic thyroxine therapy
Molecular Genetic Studies
THRA sequencing indicated heterozygosity for a nucleotide substitution (GCG to GTG), corresponding to an Alanine to Valine change at codon 263 (A263V) in the sequence common to both TRα1 and α2 variant proteins; the mutation segregates with abnormal phenotype and thyroid biochemistry, being present in patients (P1, P2, P3) but absent in unaffected family members (sibling, father) (Supplemental data Fig 1) and from normal genome databases (dBSNP, 1000 Genomes, NHLBI exome server).
Restriction fragment length polymorphism (RFLP) analysis and direct sequencing of TRα1 and α2 cDNAs derived from primary blood mononuclear cells (PBMCs) from P1, confirmed that A263V mutant TRα1 and α2 mRNAs are coexpressed together with wild type transcripts in vivo (Supplementary Fig 2). Lack of antibodies that reliably distinguish TRα1 and α2 subtypes and wild type versus missense mutant receptors, precluded testing for their expression at protein level.
Functional properties of Ala263Val mutant TRα1 and α2
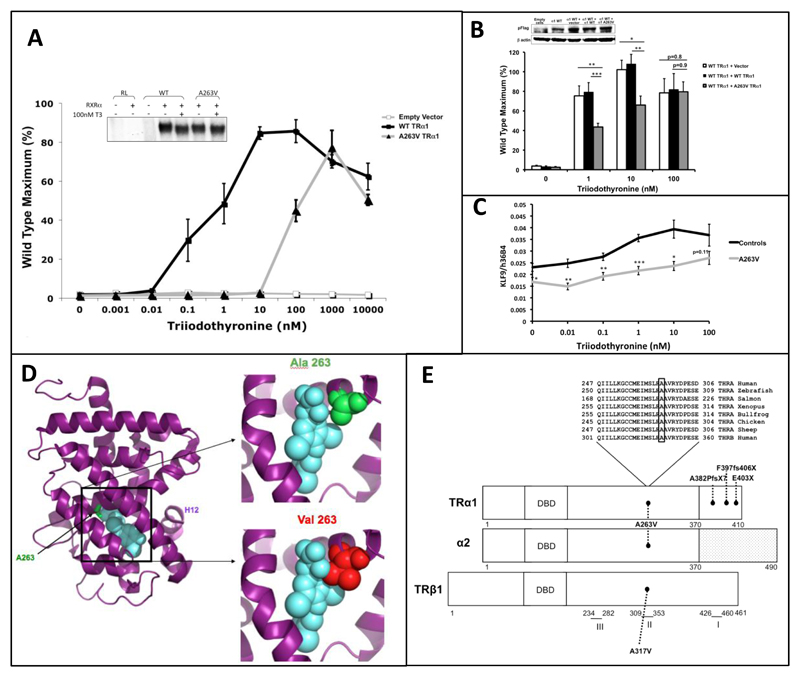
Radiolabelled T3 binding by the mutant receptor is markedly reduced (Supplementary Fig 3). In transfection assays, A263V mutant TRα1 mediates transcriptional activation of a thyroid hormone-responsive target gene minimally at low (0.001-10 nM) T3 levels (Fig 2A), but at higher T3 concentrations (100nM-10μM) mutant receptor function is comparable to WT receptor. A263V mutant TRα1 can bind to DNA (Fig 2A inset) and, when coexpressed with either wild type TRα1 (Fig 2B) or wild type TRβ1 (supplementary Fig 9), the mutant receptor inhibits the transcriptional activity of its wild type counterpart function in a dominant negative manner. Higher (100 nM) T3 concentrations reverse such dominant negative inhibition by A263V mutant TRα1 in vitro (Fig 2B) and also reverse reduced expression of a thyroid hormone-responsive target gene (KLF9) in patient-derived peripheral blood mononuclear cells studied ex vivo (Fig 2C). Consistent with reversal of dominant negative inhibition by mutant TRα1 at higher T3 levels, higher (1000 nM) T3 concentrations fully dissociate A263V mutant TRα1 from corepressor (NCoR) and recruit coactivator (TRAP220) in assays which measure receptor interactions with cofactors (Supplementary Fig 4).
Figure 2. Functional Properties and Molecular Modelling of A263V TRα1.
JEG-3 cells were transfected with empty, WT or A263V TRα1 expression vectors together with a thyroid hormone responsive reporter gene, assaying T3-dependent activation (Panel A); the inset shows an electrophoretic mobility shift assay with comparable interaction of unliganded or hormone bound – WT/RXR and A263V mutant TRα/RXR heterodimers with a direct repeat thyroid response element from the malic enzyme gene. Dominant negative inhibition was tested (Panel B) in cells cotransfected with reporter gene and equal combinations of expression vectors. Panel C. Quantitative RT-PCR (internal control: 36B4, acidic ribosomal phosphoprotein) showing expression of KLF9 in peripheral blood mononuclear cells from the patients (A263V) or control subjects with increasing T3 concentrations. For Panels B and C * p<0·05; **p<0·01; ***p<0·001.
Crystallographic modelling of the TRα1 ligand binding domain (LBD) bound to T3 (blue) (Panel D), highlighting the normal amino acid (alanine 263, green), with substitution of the larger valine residue (red) predicting steric hindrance to T3 binding. Panel E. Schematic representation showing the similar domain structure of TRα1, variant α2 and TRβ1 proteins. Conservation of alanine at position 263 in THRA from different species or THRB, suggests its functional importance. The A263V mutation is common to both TRα1 and α2, whereas THRA mutations described previously (E403X, F397fs406X, A382PfsX7) are unique to TRα1. Three clusters of TRβ mutations (I, amino acids 426-460; II, 309-353; III, 234-282) are associated with RTHβ and a homologous TRβ mutation (A317V) localises to one of these.
The Alanine to Valine mutation involves a phylogenetically highly conserved amino acid in THRA (Fig 2E) and structural modelling predicts that this substitution impairs T3 binding via steric hindrance (Fig 2D).
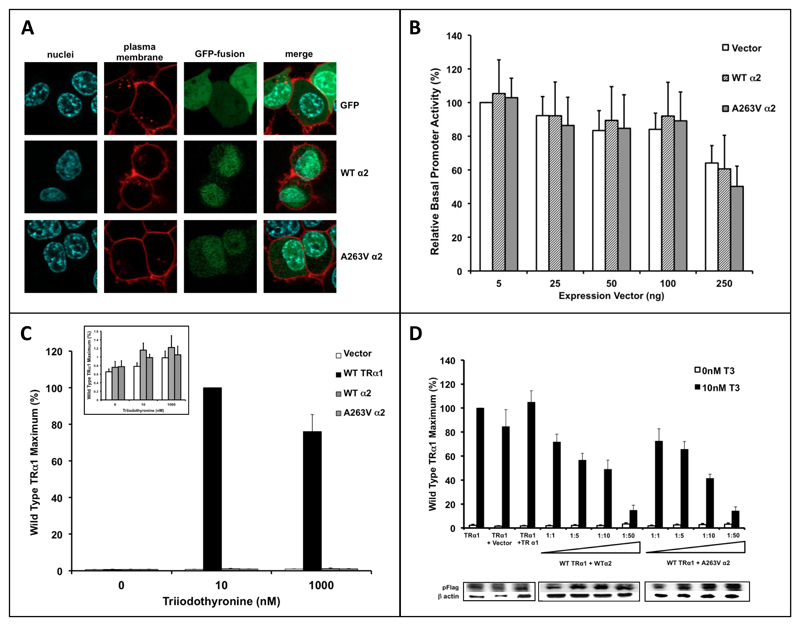
When studied in the variant α2 protein context, the A263V mutation exhibits no discernible effect, with similar cellular localisation (Fig 3A), negligible repression (Fig 3B) or activation of reporter gene activity (Fig 3C) in the absence or presence of T3 and weak dominant negative activity when overexpressed (Fig 3D), no different to its wild type α2 counterpart.
Figure 3. Functional Properties of A263V TRα2.
Panel A shows 293 cells transfected with either GFP or GFP-tagged WTα2 or A263V mutant α2 expression vectors with visualisation of nuclei (blue), plasma membrane (red), GFP-fusion (green) by immunofluorescence and a composite merged image.
Transcriptional function of WTα2 and A263V mutant α2 proteins was tested in JEG-3 cells cotransfected with reporter gene and increasing amounts (5 to 250ng) of empty, WT or A263V α2 expression vectors in the absence of ligand (Panel B), or a fixed amount of empty, TRα1, WT α2 and A263V mutant α2 expression vectors with increasing T3 concentrations (Panel C), or a fixed amount of WT TRα1 and increasing ratio (1:1 to 1:50) of α2 expression vectors (Panel D).
Response to thyroxine therapy
Following thyroxine therapy in replacement dosage (1·1-1·8mcg/kg) a rise in FT4 and FT3 in all patients was associated with subnormal/suppressed TSH levels, reduction in circulating thyroglobulin and variable rise in reverse T3 levels (Table 1). REE rose in all cases but remains subnormal, with a smaller increment in P1 who takes thyroxine in lower (75mcg) dosage. Concurrently, LDL cholesterol levels fell in all patients (P1 5·0 to 4·7mmol/L; P2 3·2 to 2·5 mmol/L; P3 2·3 to 2·0 mmol/L); CK levels fell in P1 (364 to 178U/L) and P2 (385 to 242 U/L); there was an increase in many, but not all, markers of bone turnover, but no change in the sleeping heart rate (Table 1). All patients noted symptomatic improvement after restarting thyroxine: specifically, paraesthesiae suggestive of carpal tunnel syndrome resolved in P1; both P2 and P3 reported reduced motor incoordination and constipation.
Homologous mutation (A317V) in THRB is associated with RTHβ
The A263V mutation in TRα1 corresponds to an A317V substitution in TRβ and we have identified its first occurrence in a family with RTHβ. A thyroglossal cyst prompted investigation of the index case at age 4yrs, identifying elevated thyroid hormones with non-suppressed TSH. Affected family members (mother, siblings S1, S2) exhibit the same biochemical profile together with raised rT3 and Tg levels and this segregates with heterozygosity for the A317V mutation in TRβ (Supplementary data Table). Recognised features of RTHβ are present in affected cases (S1, frequent upper respiratory tract infections; Proband, S2 failure to thrive; S1, hyperactivity and mild learning difficulties; Proband, S1 increased appetite) and their REE is uniformly elevated (Proband 142%, S1 152%, S2 122%, Mother 127% (Normal 100 ± 5%). A317V mutant TRβ dysfunction resembles A263V mutant TRα1, with severely reduced ligand binding (Supplementary Fig 3), impaired hormone-dependent transactivation (Supplementary Fig 5) and dominant negative activity which is reversible at higher T3 levels (Supplementary Fig 6). Structural modelling (Supplementary Fig 7) provides a basis for its impaired T3 binding. This amino acid change involves a residue which is known to be mutated to Threonine in RTHβ (14) and localises to one of the recognised RTHβ mutation clusters in the LBD (Fig 2E).
Discussion
Many clinical features in our patients (growth retardation, developmental delay, constipation, macrocephaly, large tongue) suggested hypothyroidism, despite normal circulating thyroid hormones. However, they exhibit a subnormal FT4/FT3 ratio, low reverse T3, raised muscle CK and mild anaemia. This phenotype segregates with a THRA defect, which results in mutation (A263V) of both TRα1 and variant α2 proteins. However, whereas A263V mutant TRα1 function is impaired and it inhibits wild type receptor action in a dominant negative manner, A263V mutant α2 exhibits similar properties to WTα2 with no added gain or loss-of-function. Consistent with this, the clinical and biochemical features in our patients are strikingly similar to the phenotype of previous cases with defective TRα1 alone (6,7,8,9), with no added characteristics attributable to any alteration in α2 function. Our observations accord with the lack of phenotype linked specifically to α2 deficiency in a murine knockout model (15). Although a sporadic case, with a different THRA mutation (N359Y) involving both α1 and α2 subtypes has been described (16), this patient exhibits many dissimilar features (e.g. clavicular agenesis, humeroradial synostosis, syndactyly, chronic diarrohea, primary hyperparathyroidism) which are not present in the murine α2 knockout model (15) and it is not clear whether these added abnormalities are due to the THRA mutation alone (17).
Crystallographic modelling, suggesting that the Alanine to Valine substitution inhibits T3 occupancy of receptor via steric hindrance, provides a basis for observed deleteriousness (impaired hormone binding and transcriptional function) of the A263V mutation in the TRα1 context. In contrast, an effect of this amino acid change on α2 function is difficult to envisage. Wild type α2 protein does not bind T3 (18), is devoid of intrinsic transcriptional activity and is a weak dominant negative inhibitor of TRα1 function (19,20), perhaps because it interacts poorly with RXR and corepressor (20,21), making additional loss-of-function from the A263V mutation unlikely. Conversely, although blocking phosphorylation of residues in the α2 carboxyterminus is known to mediate gain of dominant negative inhibitory function (22), the A263V mutation is located well outside this domain.
Numerous skin tags and moles were present in our patients and we have also observed this feature in other reported cases (supplementary Fig 8; 8); whilst present in the general population, the universal occurrence of this feature in defective TRα cases, even in childhood, suggests it may be an added characteristic of the disorder, although its absence would not exclude the diagnosis. The type 3 deiodinase enzyme (DIO3), is present in human and mouse skin (23) and its expression is known to be TRα1-regulated (24), such that tissue DIO3 activity might be diminished in humans with defective TRα. Topical inhibition of DIO3 activity enhances keratinocyte proliferation (23) and, by analogy, we speculate that cutaneous DIO3 deficiency in our patients with defective TRα1 might mediate this phenotype. Altered TH metabolism, due to DIO3 deficiency or documented upregulation of hepatic DIO1 in a murine model of this disorder (25), may also mediate the altered thyroid biochemical pattern, with disproportionately low FT4 and high FT3 concentrations, low FT4/FT3 ratios and subnormal rT3 levels, seen in our cases.
Unlike previous cases with highly deleterious TRα1 defects, resulting in non-functional mutant receptors with irreversible dominant negative activity (6,7,9), higher T3 concentrations reverse A263V mutant TRα1 dysfunction and dominant negative activity in vitro. T3 exposure restores subnormal expression of KLF9, a TH-responsive target gene, in mutation-containing primary blood mononuclear cells from patients, suggesting that dominant negative inhibition by mutant TRα1 can also be overcome in vivo. We correlate these observations with improvement in some peripheral markers of TH action (REE, CK) following thyroxine therapy in our patients; moreover, hormone treatment in physiological dosage (P1 0·9 mcg/kg; P2 1·8mcg/kg; P3 1·4 mcg/kg), readily raised T3 and suppressed TSH levels (P1, P2), suggesting that the pituitary-thyroid axis remains TH sensitive in these cases. Commencement of thyroxine therapy in childhood due to remarkable clinical prescience, improved their growth and development in childhood and has also alleviated symptoms (median entrapment neuropathy, constipation, motor incoordination) in adult life, without abnormal elevation in bone turnover markers as recorded in a previous case (9). Raising TH levels in transgenic mice harbouring mutant TRα1 (R384C) with a similar, 10-fold, reduction in T3 binding affinity, can also reverse neurological abnormalities (26).
The A263V mutation in TRα1 involves a residue that is also highly conserved in TRβ, predicting occurrence of the homologous defect in RTHβ; we have indeed identified the equivalent amino acid mutation (A317V) in TRβ, with this mutant receptor exhibiting very similar dysfunction in vitro, but associated with different biochemical and clinical features (raised TH and rT3 with elevated REE) in vivo. These markedly divergent phenotypes underscore the importance of TRβ in mediating negative feedback within the hypothalamo-pituitary thyroid axis and TRα in mediating hormone action in the periphery (muscle, myocardium, gastrointestinal tract).
With the identification of patients with equivalent TRα1 and TRβ defects, it is tempting to speculate that other cases with TRα1 mutations, homologous to the ~125 different known receptor mutations in RTHβ, exist. Furthermore, maternal inheritance of the TRα mutation in this family and paternal inheritance of the α receptor defect in a previous kindred (7), suggests that transmission of TRα mutations may not be as compromised in the human context as in some murine models (11). With thyroid function tests being near-normal in RTHα, the clinical and biochemical characteristics of this family, together with features of previous cases, define a phenotypic signature for this syndrome (see Research in Context), enabling early identification of future cases, which will be of importance if thyroxine therapy proves to be widely beneficial in this disorder.
Research in Context.
Systematic Review
Cases of Resistance to Thyroid Hormone with thyroid hormone receptor alpha gene defects (RTHα) identified hitherto, involve mutations which selectively disrupt thyroid hormone receptor alpha1 (TRα1) function, manifesting with typical features of hypothyroidism but paradoxically near-normal circulating thyroid hormone levels (6,7,8,9).
Interpretation
In this study we have described the first patients with a mutation common to TRα1 and the variant α2 protein derived from the same genetic locus. Their clinical (growth and developmental retardation, constipation, macrocephaly) and biochemical features (subnormal FT4/FT3 ratio, low reverse T3) are strikingly similar to previous RTHα1 cases, with no added phenotype attributable to mutant α2. We have shown that thyroid hormone reverses mutant receptor dysfunction in vitro and alleviates hormone resistance in vivo; remarkably prescient commencement of thyroxine therapy in childhood in these cases, may have ameliorated their clinical phenotype. Future identification of other RTHα patients, based on common characteristics of these and previous cases which now define the syndrome, will be of clinical importance if early thyroxine therapy proves to be widely beneficial.
Supplementary Material
Acknowledgements
Our research is supported by funding from the Wellcome Trust (100585/Z/12/Z to NS, 095564/Z/11/Z to KC) and NIHR Cambridge Biomedical Research Centre (CM, MG, KC). W.E.V is financially supported by a Marie Curie Intra-European Fellowship (330183) and a research grant from the Foundation for Development of Internal Medicine in Europe.
Funding: Wellcome Trust; NIHR Cambridge Biomedical Research Centre; Marie Curie Intra-European Fellowship; Foundation for Development of Internal Medicine in Europe.
Role of the Funding Sources: Our funding sources had no role in study design, collection, analysis or interpretation of the data, in writing the report or in the decision to submit the manuscript for publication.
Footnotes
Disclosure statement: The authors have nothing to disclose
Conflicts of interest: There are no conflicts of interest.
Contributors
CM, MA, EV, ES, NS, MG and KC (study design, data collection, interpretation, analysis and writing) and AO, KP, OR, GL, DH, DC, AE, CB, SA (data collection, interpretation and analysis) contributed to this manuscript.
Contributor Information
Amaka C. Offiah, Academic Unit of Child Health, University of Sheffield
Ken Poole, Department of Rheumatology, Addenbrooke’s Hospital, Cambridge.
David Halsall, Department of Clinical Biochemistry, Addenbrooke’s Hospital, Cambridge.
Mark Gurnell, University of Cambridge Metabolic Research Laboratories, Wellcome Trust-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge.
Krishna Chatterjee, University of Cambridge Metabolic Research Laboratories, Wellcome Trust-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge.
References
- 1.Pessemesse L, Schlernitzauer A, Sar C, et al. Depletion of the p43 mitochondrial T3 receptor in mice affects skeletal muscle development and activity. The FASEB Journal. 2012;26:748–756. doi: 10.1096/fj.11-195933. [DOI] [PubMed] [Google Scholar]
- 2.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 3.Horlein AJ, Heinzel T, Rosenfeld MG. Gene regulation by thyroid hormone receptors. Curr Opin Endocrinol Diabetes. 1996;3:412–416. [Google Scholar]
- 4.Refetoff S, Dunitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Practice and Research Clinical Endocrinology and Metabolism. 2007;21:277–305. doi: 10.1016/j.beem.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Gurnell M, Visser TJ, Beck-Peccoz P, Chatterjee VK. Resistance to Thyroid Hormone. In: Jameson JL, de Groot LJ, editors. Endocrinology. 6th edition. Saunders; Philadelphia: 2010. [Google Scholar]
- 6.Bochukova E, *, Schoenmakers N, *, Agostini M, et al. A dominant negative mutation in the thyroid hormone receptor α gene. N Engl J Med. 2012;366:243–249. doi: 10.1056/NEJMoa1110296. [DOI] [PubMed] [Google Scholar]
- 7.van Mullem AA, Van Heerebeek R, et al. Clinical phenotype and mutant TRα1. N Engl J Med. 2012;366:1451–1453. doi: 10.1056/NEJMc1113940. [DOI] [PubMed] [Google Scholar]
- 8.van Mullem AA, Chrysis D, Eythimiadou A, et al. Clinical phenotype of a new type of thyroid hormone resistance caused by a mutation of the TRα1 receptor: consequences of LT4 treatment. J Clin Endocrinol Metab. 2013;98(7):3029–3038. doi: 10.1210/jc.2013-1050. [DOI] [PubMed] [Google Scholar]
- 9.Moran C, Schoenmakers N, Agostini M, et al. An adult female with resistance to thyroid hormone mediated by defective thyroid hormone receptor α. J Clin Endocrinol Metab. 2013;98(11):4254–61. doi: 10.1210/jc.2013-2215. [DOI] [PubMed] [Google Scholar]
- 10.Tinnikov A, Nordstrom K, Thoren P, et al. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor α1. EMBO J. 2002;21:5079–5087. doi: 10.1093/emboj/cdf523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneshige M, Suzuki H, Kaneshige K, et al. A targeted dominant negative mutation of the thyroid hormone α1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Aacd Sci USA. 2001;98:15095–15100. doi: 10.1073/pnas.261565798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YY, Schultz JJ, Brent GA. A thyroid hormone receptor alpha gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem. 2003;278:38913–38920. doi: 10.1074/jbc.M306120200. [DOI] [PubMed] [Google Scholar]
- 13.Quignodon L, Vincent S, Winter H, Samarut J, Flamant F. A point mutation in the activation function 2 domain of thyroid hormone receptor α1 expressed after CRE-mediated recombination partially recapitulates hypothyroidism. Mol Endocrinol. 2007;21:2350–2360. doi: 10.1210/me.2007-0176. [DOI] [PubMed] [Google Scholar]
- 14.Sunthornthepvarakul T, Angsusingha K, Likitmaskul S, Ngowngarmratana S, Refetoff S. Mutation in the thyroid hormone receptor β gene (A317T) in a Thai subject with resistance to thyroid hormone. Thyroid. 1997;7(6):905–907. doi: 10.1089/thy.1997.7.905. [DOI] [PubMed] [Google Scholar]
- 15.Saltó C, Kindblom JM, Johansson C, et al. Ablation of TRα2 and a concomitant overexpression of α1 yields a mixed hypo- and hyperthyroid phenotype in mice. Mol Endocrinol. 2001;15:2115–2128. doi: 10.1210/mend.15.12.0750. [DOI] [PubMed] [Google Scholar]
- 16.Espiard S, Savagner F, D’herbomez M, Rose C, Rodien P, Wemeau J-L. Polymalformation, dyserythropoietic anemia, primary hyperparathyroidism and diarrhoea in a patient with mutation of the thyroid hormone receptor alpha gene (THRα). 95th Annual meeting of the Endocrine Society USA; 2013. SUN-431 (Abstract) [Google Scholar]
- 17.Refetoff S, Bassett JD, Beck-Peccoz P, et al. Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. J Clin Endoc Metab. 2014;3:1–3. doi: 10.1159/000358180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazar M, Hodin R, Chin W. Human carboxyl-terminal variant of α-type c-erbA inhibits trans-activation by thyroid hormone receptors without binding thyroid hormone. Proc Natl Acad Sci USA. 1989;86(20):7771–7774. doi: 10.1073/pnas.86.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rentoumis A, Chatterjee K, Madison L, et al. Negative and positive transcriptional regulation by thyroid hormone receptor isoforms. Mol Endocrinol. 1990;4(10):1522–1531. doi: 10.1210/mend-4-10-1522. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Burgos-Trinidad M, Wu Y, Koenig R. Thyroid hormone receptor variant α2. J Biol Chem. 1996;271:28235–28242. doi: 10.1074/jbc.271.45.28235. [DOI] [PubMed] [Google Scholar]
- 21.Tagami T, Kopp P, Johnson W, Arseven OK, Jameson JL. The thyroid hormone receptor variant α2 is a weak antagonist because it is deficient in interactions with nuclear receptor corepressors. Endocrinology. 1998;139(5):2535–2544. doi: 10.1210/endo.139.5.6011. [DOI] [PubMed] [Google Scholar]
- 22.Katz D, Reginato R, Lazar M. Functional regulation of the thyroid hormone receptor variant, TRα2 by phosphorylation. Mol Cell Biol. 1995;15(5):2341–2348. doi: 10.1128/mcb.15.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang M, Rodgers K, O’Mara R, et al. The thyroid hormone degrading type 3 deiodinase is the primary deiodinase active in murine epidermis. Thyroid. 2011;21:1263–1268. doi: 10.1089/thy.2011.0105. [DOI] [PubMed] [Google Scholar]
- 24.Barca-Mayo O, Liao X, Alonso M, et al. Thyroid hormone receptor α and regulation of type 3 deiodinase. Mol Endocrinol. 2011;25(4):575–583. doi: 10.1210/me.2010-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingjam MC, Cheng S. A targeted dominant negative mutation of the thyroid hormone α1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci USA. 2001;98(26):15095–15100. doi: 10.1073/pnas.261565798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venero C, Guadano-Ferraz A, Herrero AI, et al. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor α1 can be ameliorated by T3 treatment. Genes & Dev. 2005;19:2152–2163. doi: 10.1101/gad.346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bushby KMD, Cole T, Matthews JNS, Goodship JA. Centiles for adult head circumference. Arch Dis Child. 1992;67:1286–1287. doi: 10.1136/adc.67.10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.