Abstract
Background/Aims
Laser treatment of burns scars is considered by some providers to be standard of care. However there is little evidence-based research as to the true benefit. A number of factors hinder evaluation of the benefit of laser treatment. These include significant heterogeneity in patient response and possible delayed effects from the laser treatment. Moreover, laser treatments are often provided sequentially using different types of equipment and settings, so there are effectively a large number of overall treatment options that need to be compared. We propose a trial capable of coping with these issues, and that also attempts to take advantage of the heterogeneous response in order to estimate optimal treatment plans personalized to each individual patient. It will be the first large-scale randomized trial to compare the effectiveness of laser treatments for burns scars, and, to our knowledge, the very first example of the utility of a Sequential Multiple Assignment Randomized Trial (SMART) in plastic surgery.
Methods
We propose using a Sequential Multiple Assignment Randomized Trial (SMART) design to investigate the effect of various permutations of laser treatment on hypertrophic burn scars. We will compare and test hypotheses regarding laser treatment effects at a general population level. Simultaneously we hope to use the data generated to discover possible beneficial personalized treatment plans, tailored to individual patient characteristics.
Results
We show that the proposed trial has good power to detect laser treatment effect at the overall population level, despite comparing a large number of treatment combinations. The trial will simultaneously provide high quality data appropriate for estimating precision-medicine treatment rules. We detail population level comparisons of interest, and corresponding sample size calculations. We provide simulations to suggest the power of the trial to detect laser effect and also the possible benefits of personalization of laser treatment to individual characteristics.
Conclusions
We propose, to our knowledge, the first use of a Sequential Multiple Assignment Randomized Trial (SMART) in surgery. The trial is rigorously designed so that it is reasonably straightforward to implement, and powered to answer general overall questions of interest. The trial is also designed to provide data that is suitable for the estimation of beneficial precision-medicine treatment rules that depend both on individual patient characteristics and on-going real-time patient response to treatment.
Keywords: Hypertrophic scarring, burn, surgery, plastic surgery, laser treatment, pulsed-dye laser, CO2 laser, clinical trial, Q-learning, outcome weighted learning, sequential multiple assignment randomized trial, SMART, factorial experiment, individualized treatment rule, ITR, dynamic treatment regime, dynamic treatment rule, DTR, precision-medicine
Background/Aims
Overview
The Laser Induced BioEngineered Remodeling of Thermally Injured skin (LIBERTI) trial is a proposed clinical trial in burn scar surgery, which combines both conventional and precision-medicine aspects. The trial will investigate and evaluate the efficacy of certain sequences of laser treatment on hypertrophic burn scars. There are two types of main goals. The first is to answer important unknown clinical questions regarding the efficacy of laser treatment at an overall population level. The second is to provide high-quality data suitable for estimating precision-medicine, patient-specific, treatment rules.
We show a Sequential Multiple Assignment Randomized Trial (SMART) design may be used to achieve both goals. Analyzing the results using classic regression methods will allow us to test null hypotheses that laser treatment has no effect on average. Recording appropriate patient variables before and during the trial, including ones reflecting ongoing response to treatment, will allow us to data-mine precision-medicine rules, whereby treatment may be beneficially tailored to each individual patient depending on their individual characteristics.
LIBERTI will be the first large-scale randomized trial of laser treatments for hypertrophic burn scars. Moreover, LIBERTI will to our knowledge be the very first SMART in surgery, and so certainly the first SMART study to attempt to uncover patient-specific treatment rules in this field.
Burn injury and laser treatment
The American Burns Association estimates that at least 40,000 patients are hospitalized each year in the United States through burn injury, mostly at 128 specialized burns centers throughout the country.1 Hypertrophic burn scars are the cause of significant on-going morbidity in such burn victims.2,3 Physical sequelae include itching, pain, stiffness and contracture,2,3 while psychological sequelae include depression, post traumatic stress disorder and great social anxiety.4–6 Up to 70% of burns victims develop such scars, but optimal therapy combinations, treatment timings, and indications remain unknown.2 Clinical trials are much needed to establish the efficacy of currently used treatments.2,7,8
Two laser treatments hypothesized to ameliorate hypertrophic scars are vascular-specific pulsed-dye laser, and ablative fractional CO2 laser.3,9 These two therapies operate through different mechanisms. Pulsed-dye lasers essentially vaporize capillaries by targeting hemoglobin which reduces vascularity.3,9 CO2 lasers target water and excise multiple small vertical columns into the scar which promotes collagen formation and scar remodeling.3,9 Some plastic surgeons familiar with these therapies consider them effective for remodeling burn scars, but clear evidence is currently lacking, a major gap in the research.7 Specifically there is a considerable lack of large-scale randomized trials involving laser treatments.7
Problems evaluating treatment effects
Determining the effectiveness of laser treatment is challenging for various reasons.7 Firstly, laser treatment is not usually provided alone. Standard non-surgical medical treatments such as silicone gels, compression garments and medical ointments are often provided over the same treatment period. Secondly, laser treatments may be prescribed repeatedly in sequence. Moreover, in a sequence of laser treatments the laser type used may differ.10,11 Therefore it is difficult to isolate the effects of each particular instance of laser treatment, and to isolate the effect of laser treatment from the standard non-surgical treatments.7
A further difficulty comes from the considerable heterogeneity across patients and their treatment responses.7 Many patient characteristics and other factors are expected to produce significant differences in laser treatment response. For example, the outcome is likely to be affected by how the burn was sustained with, say, electrical burns being fundamentally different from flame burns.12,13 The amount of melatonin in the skin is known to affect response to laser treatment, with, for example, hypopigmentation being an issue for darker skin types.14 Even genetic factors, particularly regarding the inflammatory response,15,16 will play a part in scar formation, and hence treatment. Nevertheless, the presence of such variation in treatment response suggests even somewhat basic precision-medicine treatment plans might offer certain patients much benefit, and hence a SMART design with one aim of finding such plans will hopefully give good results.17
SMART studies
In recent years much consideration has been given to the possibilities of personalized- or precision-medicine.18,19 Therefore the secondary aims of a trial may now include estimating treatment rules individualized to each patient’s particular characteristics, including their on-going treatment response.20,21 In the biostatistical literature, such rules are often referred to as dynamic treatment regimes.22–24
To examine new questions about the development of dynamic treatment regimes, a subtype of randomized clinical trials, the Sequential Multiple Assignment Randomized Trial (SMART), has been introduced.25–28 In a SMART patients may be repeatedly re-randomized to different treatments at multiple scheduled intervention points. These randomizations may be between all treatment options with equal probability, or to only certain treatment paths that reflect clinical or ethical practice, such as switches to second line chemotherapy options in cancer therapy when progression occurs.29,30 SMARTs may be regarded as a form of factorial experiment, with the treatments being the factors.17,31,32 We expand on the precise meaning of 'dynamic treatment regime', and the relation to SMARTs, in the online supplement. Additionally there are very many excellent references in the biostatistical literature.22–24,29,33–35
This paper describes our design of LIBERTI, a SMART in the field of plastic surgery. It is the first SMART, to our knowledge, in surgery. The trial design, analysis plan and proposed implementation address both issues relevant to many SMARTs, such as data collection and randomization procedures, and also issues more particular to the study of laser treatments, such as the inability to isolate treatments.
Method
Broad overview
We would like to design a trial that both gives good indication of the benefits, or disadvantages, of laser treatment for hypertrophic burns scars in the general population, and also provides high-quality measurements and data that is suitable for data-mining and estimating personalized treatment regimes. We would like this trial to mimic existing clinical practice as close as possible, for both the obvious reasons of allowing an intent-to-treat type analysis, as well as for ease of implementing the trial. Indeed having a trial similar to current practice will maximize the likelihood of successful implementation.
Current practice
Current treatment practice at the University of North Carolina Burn Reconstruction & Aesthetic Center for hypertrophic burns scars may be simplified/summarized as follows. After initial intensive care and surgery patients have a consultation with a plastic surgeon at the burn center. Typically one or two laser treatment periods of either CO2 or else pulsed-dye laser are then offered over a following period of up to a year. Each laser treatment period is three to four months long. During each treatment period each patient receives multiple sessions of laser treatment from a single assigned laser. The total number of periods, and which laser is used in each treatment period is chosen according to the surgeons' intuition. Each patient is also provided ongoing non-surgical medical treatment, irrespective of whether they receive laser therapy.
Study interventions/measurements
In order for the LIBERTI study to match current practice as closely as possible we propose to enroll each patient for a two-year period. During the first year laser treatments, as well as the standard non-surgical medical treatments, will be provided. The second year will be a year of follow-up with no surgical treatments. A second year of follow-up is important as we are concerned with both short-term scar improvement, or otherwise, from laser treatment, but even more with long-term improvement after scheduled treatment has ended.
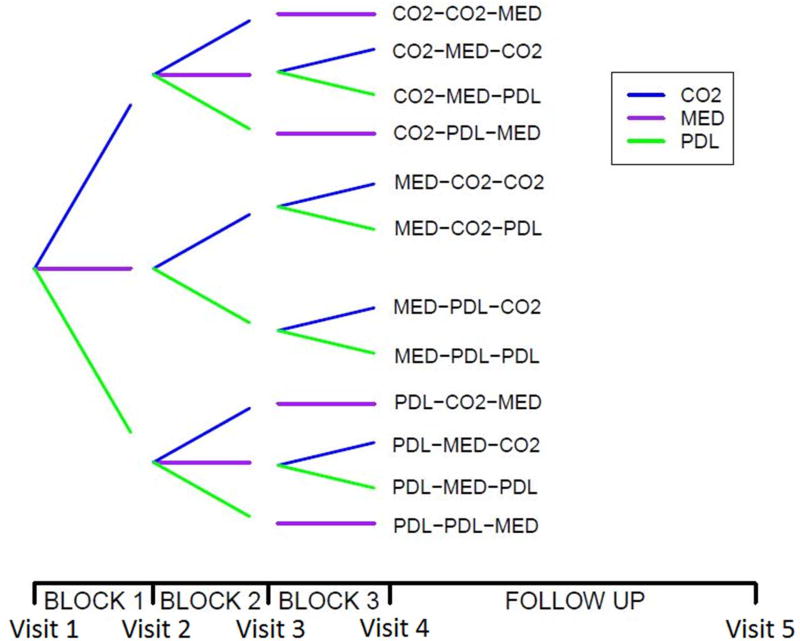
Year 1 of the study is divided into three consecutive four-month blocks. Patients will be randomized so that laser treatment, augmented with non-surgical medical therapy, is given in two of these blocks, and solely non-surgical medical therapy is given in the other block. The treatment path options are shown in Figure 1, along with a timeline for patients’ progression through the trial. The burn center’s attending plastic surgeon will provide all surgical treatments.
Figure 1.
Schematic diagram illustrating the allowed patient treatment sequences. CO2 indicates an assigned treatment block of CO2 laser, in conjunction with non-surgical medical therapy, PDL of pulsed-dye laser, in conjunction with non-surgical medical therapy, and MED of solely non-surgical medical therapy with no laser treatment.
We offer all patients the maximum number of two four-month blocks of laser treatment periods during the year. Initial patient consultation suggested that patients would be unwilling to enroll if there was some chance of not receiving the maximum laser treatment they might otherwise receive outside of the trial.
We plan to have three four-month treatment blocks, even though laser treatment is only provided in two out of the three, for two main reasons. Observing some patients with no laser treatment in one of the three four-month blocks will, along with appropriate modeling assumptions, firstly give insights into the effects of varying when laser treatment is given after burn injury, and secondly allow some estimation of the effect of no laser treatments in a study where all patients must receive some.
The patients will be examined at baseline, at the end of each four-month treatment block, and also at the end of Year 2. Therefore measurements will occur at 0, 4, 8, 12 and 24 months. Examinations after each treatment block will give some insight into short-term laser effects, as well as provide measurements of response to treatment that might be crucial prescriptive variables in a precision-medicine regime. Examinations at the end of Year 2 will give some insight into the long-term laser effect a significant period after active treatment has ended.
In total upwards of thirty variables will be collected from each patient, over ten of which are time varying and must be recorded at every visit. Measurements will be taken by a nurse at the burn center, who is blinded to treatments.
The main outcome of interest will be the well-validated Vancouver Scar Score, which gives a single numerical score from an evaluation of many separate scar attributes.36 Standard demographics, injury/treatment factors and skin/burn measurements37,38 will be used as controlling variables in the analysis, and as possible prescriptive variables to determine a dynamic treatment regime. For example, Asian skin pigments might be an indication away from pulsed-dye laser, with some authors suggesting less effect of pulsed-dye laser on darker skin,39 whereas a higher score on the vascularity attribute of the Vancouver Scar Score might be an indication towards pulsed-dye laser, which has been hypothesized to reduce redness.40 We will collect quality of life measures41 and alternative scar scores36 as secondary outcomes of interest. The quality of life measures may also be important prescriptive variables. For example, treatment at each stage should be chosen, at least in part, to address a patient's personal concerns that might be reflected in quality of life measures. Better quality of life might suggest that less intensive treatment is necessary. Another important prescriptive variable may be the Patient Observer Scar Assessment Scale,42 which factors patients’ own perceptions into the score, and therefore suggests issues of particular concern for each individual that should be targeted. More details are provided in the online supplement.
Randomization/blinding
For each treatment block, each patient is randomized to CO2 laser in conjunction with non-surgical medical therapy, pulsed-dye laser in conjunction with non-surgical medical therapy, or else just non-surgical medical therapy, with the restriction that all patients must receive exactly two blocks of laser treatment during the first year of treatment. Patients will be permuted-block randomized with equal probability between all allowed treatment sequences.43
For this SMART, randomizations of patients to treatment are independent of on-going patient response. Therefore at enrollment we may already randomize each patient to a sequence of therapies for each of the three treatment blocks. More specifically for every twelve patients, we will randomly order the twelve total treatment options over the course of the first year, and then assign the twelve patients to these in sequence of enrollment. This block randomization will hopefully remove temporal biases in the trial and ensure that any drift in patient characteristics over the trial's duration will not bias the results. We believe that stratification within this randomization would be unnecessarily difficult. We have little quantitative evidence regarding best stratification variables. Furthermore both the trial logistics and the intended estimation of precision-medicine dynamic treatment regimes, would be substantially complicated by more elaborate randomization schemes.
The nature of laser treatment is such that neither the provider nor the patient can be blinded to it during the treatment session itself. However the investigator taking the measurements throughout the study will be different from the providing clinician and will be blinded to all patients’ particular treatments. Furthermore the patient and provider themselves will only be made aware of each treatment block's assignment as late as possible, that is only at the beginning of the treatment block itself. Therefore even when the first treatment is revealed for a particular patient, the patient and provider will not know further treatments until the beginning of the relevant sessions. This partial blinding and the large number of possible treatment sequences will ensure that the provider will essentially be unable to predict the randomization for any given patient.
Patients
The study population will be all patients referred to the burn center at the University of North Carolina that satisfy the inclusion criteria. These criteria are essentially just suitability for laser treatment and willingness to participate in a study. Exclusion criteria are the intention to have further treatment outside of the study while enrolled, previous adverse reaction to anesthesia and being pregnant. Therefore the intended target population is essentially all referrals to the burns center. All suitable patients will be asked by the surgeon to consider consenting to the trial. Consent will be obtained once and upfront. No patient incentives are planned, as treatment in the trial is very similar to what patients would receive anyway, and in an informal survey many patients indicated to the clinician that they would be happy to consent without incentives. Excluding measurements at the two-year follow-up visit, all study measurements will be made immediately prior to treatment, thus necessitating no extra visits, and ensuring these measurements are obtained for all patients who receive treatment in the trial.
Extrapolating from previous patient demographics we expect to recruit a heterogeneous population at an estimated rate of ten patients a month. We propose a sample size of 180 patients, which both power calculations and simulation studies suggest is sufficient. More sample size and power calculation details are given both below and in the online supplement.
There are twelve possible treatment paths during the study, as shown in Figure 1. As the providers are in equipoise regarding actual laser effect and difference between lasers, we will divide the patients equally between all the paths. Therefore over the course of the trial fifteen patients in total will be assigned to each of the twelve different possible treatment paths. This might seem a small number per treatment path but the aggregate information obtained should be appreciable.
Missing/compliance
The debilitating nature of serious burns injuries means that few people fail to complete the treatment course recommended by the providers at the burns center. It is the clinicians’ expectation therefore that there will be very little dropout or missing data at the end of the study. Nevertheless to be cautious we inflate by 10% the sample size initially given by our power calculations. In the event of missing data we will perform complete case analyses, and also subsequently estimate how the missingness may have influenced the results using standard methods such as imputation.44 As the treatment is very similar to non-trial treatment offered at the burns center currently, and from sample survey responses, we believe it will not be necessary to offer incentives to persuade patients to enroll and continue in the trial to avoid dropout or missing data.
Results
Model/analyses
We outline our main analyses for investigating the effect of laser treatment on the primary outcome of change in Vancouver Scar Score below. More details, especially involving statistics methodology and regression formulas, are provided in the online supplement. This trial has aims that require three separate types of analyses. These are evaluation of short-term laser effect, evaluation of long-term laser effect and estimation of a precision-medicine dynamic treatment regime.
We estimate 180 participants will give good power to ensure all three of our aims are satisfied. We allow for an estimated worst scenario of 10% dropout of the original 180 patients. Different power calculations are required for each of our analyses, appropriate to each particular analysis method proposed.45–47
Short-term laser effect evaluation
We may use the four-month outcomes that are measured after completion of the first treatment block to investigate the short-term laser effect. After the first treatment block we will have already completed a mini classical randomized clinical trial, where patients are randomized between three simple treatment arms of treatment with CO2 laser for four-months, treatment with pulsed-dye laser for four-months, or non-surgical medical therapy for four months.
We will test the following hypotheses regarding the short-term change in Vancouver Scar Score between entry to the study and the end of the first four-month treatment block:
Short-Term Null Hypothesis 1: No significant difference on average in short-term scar score change between pulsed-dye laser and non-surgical medical therapy.
Short-Term Null Hypothesis 2: No significant difference on average in short-term scar score change between CO2 laser and non-surgical medical therapy.
Short-Term Null Hypothesis 3: No significant difference on average in short-term scar score change between pulsed-dye laser and CO2 laser.
We may use three standard t-tests to compare pairwise the average outcomes for the treatment groups and examine if we have good evidence to reject each of the null hypotheses. Using t-tests with overall type I error rate of 5%, we estimate we will have 80% power to detect a minimal clinically significant difference of 0.9 Vancouver Scar Score units in treatment effect after four months between pulsed-dye laser and non-surgical medical treatment alone, between CO2 laser treatment and non-surgical treatment alone, or between pulsed-dye laser treatment and CO2 laser treatment.
Such pooling of treatment groups between different treatment paths in a SMART and testing simple hypotheses is a standard and very simple way of approximating power and appropriate sample size for a SMART.17,28,48
More statistical details are provided in the supplement.
Long-term laser effect evaluation
We will use the 24-month outcome that is measured following at least one year of no laser treatment to indicate the long-term laser effect. We believe this will be a good surrogate outcome for the long-term effect. Our analyses and conclusions should bear in mind however that, in general, hypertrophic scarring tends to improve with time. We will therefore compare the results we obtain regarding the short-term and the long-term laser effects to see if they are consistent.
We may evaluate evidence for long-term laser effect through testing hypotheses in a classical statistical manner. However unlike the short-term effect evaluation, we no longer have a sizable population split between a small number of distinct treatment arms. Indeed for considering short-term laser effect evaluation we estimate having 180 patients equally divided between CO2 laser treatment, pulsed-dye laser treatment and just non-surgical medical treatment, giving 60 patients per treatment arm. For long-term laser effect evaluation we will be considering the outcomes from essentially 12 different treatment paths, each with only 15 patients. Statistically speaking, comparing such a large number of treatments, each received by such few patients, will be rather unlikely to give definitive conclusions or evidence of treatment effect.
Alternatively the study may also be viewed as a factorial experiment, with the treatment blocks giving three factors, and the three treatment choices in each block giving three levels.17,31,32 In this view a sizable number of patients is assigned overall to any particular level of a factor, that is a sizable number is assigned to any given treatment in any given block. However, the constraints on treatment combinations, means that this is not a fully-crossed design by any means, since some combinations of factors are not observed, and this will cause difficulties in the analysis.
A further complication to the analysis of long-term laser effect is that we are very interested in comparing the outcomes for patients given laser treatment with outcomes for patients who receive no laser treatment. However, as discussed above, we do not have any patients who will be assigned to no laser treatment at all during the study. The obvious way round this issue is to introduce modeling assumptions, such as additive effects, and extrapolate. However we are uncertain what modeling assumptions are appropriate to model scar score response to laser treatments. Therefore we will take a slightly different approach. We will assume a regression equation that connects the average outcomes for patients who are given any particular combination of treatments in each of the three blocks, including combinations unobserved in the trial such as CO2 laser in all three blocks, or such as just non-surgical medical therapy in all blocks. Rather than make specific assumptions on how the treatments in each block interact, we may impose constraints on a fully flexible model that force the model to identify overall better and worse average treatments in each block. The regression equation from this constrained model may, or may not, be suitable for extrapolating average outcomes of unobserved combinations, but at the minimum it will give a model that allows powerful and straightforward exact tests for the following null hypotheses:
Long-Term Null Hypothesis 1: No significant difference in average long-term scar score change between any laser treatment and non-surgical medical treatment.
Long-Term Null Hypothesis 2: No significant difference in average long-term scar score change between CO2 and pulsed-dye laser treatments.
We will be able to test these null hypotheses by applying standard F-tests to certain combinations of coefficients in the regression equation. These coefficients correspond to the main effects when the clinical trial is regarded as a factorial experiment. The main effects may then be interpreted as marginal effects, such as the effect that would be expected for a patient when treatment in one treatment block is fixed, but the patient is randomized between all treatments in the other two blocks as in the trial. The Hierarchical Ordering Principle suggests a test structured around the main effects is sensible. This principle states lower order effects are likely to be more important than higher order ones if no specific knowledge indicates otherwise.31,49 The model we propose seems clinically reasonable for modeling laser effects.
The model appears statistically reasonable in that it appears to have very good power for rejecting our hypotheses, despite having comparatively many degrees of freedom, as is needed to realistically model the effects of twelve treatment sequences. Testing such a model demonstrates one way of statistically examining overall treatment effects in SMART studies in a classical manner.
More precise statistical details are provided in the supplement.
Precision medicine dynamic treatment regime estimation
The precise estimation of individualized precision-medicine dynamic treatment regimes would then provide answers automatically to our above hypotheses regarding both short-term and long-term laser effect through averaging the dynamic treatment regimes over the characteristics of the general population. However, it is also the most challenging part of this study due to the complicated estimations that will need to be performed, and the large number of variables that may interact together.
We intend to analyze the data from LIBERTI using recently developed precision-medicine analysis techniques. We intend to make two separate explorations using the machine-learning techniques of Q-learning and outcome-weighted-learning, both of which provide powerful techniques to data-mine dynamic treatment regimes from data provided by a SMART. We will not further specify the precise analyses to be used, for we expect significant theoretical developments while the study is running, and we will avail ourselves of these at LIBERTI’s conclusion.
The results of these analyses, and comparisons with the results of the more classical regression analysis described above, will hopefully provide insights into possible benefits of personalizing treatment in this field.
The sample size of 180 patients is predicted to be large enough to provide good insights into an individualized dynamic treatment regime, based on published simulation results for similar studies.50 We have further made numerous simulation studies for this particular trial that suggest we will have good power.
We explain more regarding methods of data-mining dynamic treatment regimes, detail some of our simulation studies, and show some of the potential benefits of a dynamic treatment regime analysis in the online supplement.
Conclusions
Expectations/current status
The LIBERTI trial design received both approval from the University of North Carolina’s institutional review board, and support from grant bodies, allowing the trial to start recruiting.
Although we had received positive feedback from potential participants, regarding their willingness to be in such a study, we have experienced some unexpected issues with insurance providers. We had believed insurance providers would be supportive of the study, as the treatment provided closely matches existing treatment plans subjects would receive outside the study. However, this assumption now appears to be erroneous. We are currently in discussions as whether incentives, and therefore further funding, would be needed to bypass this issue.
Meanwhile we continue to collect data using a study framework very similar to LIBERTI without the randomization of treatments. Patients are currently asked to consent to give their data as they would in the study but the treatments are determined by the usual clinician intuition, as has already been standard practice. An advantage of having designed the trial to closely follow current clinical practice is that we may temporarily turn LIBERTI into an observational study, in particular using the same data-gathering set-up. We intend to use this data to provide further evidence of the possible benefits of precision-medicine in this field, and to ensure that all stakeholders may be convinced of the benefits of the LIBERTI study. Irrespectively, we believe this design provides a template for a study answering questions of interest, and expect that these roadblocks recently encountered may soon be removed.
In summary, we have provided a trial design and analysis plan that allows comparisons of a large number of treatment options without drastic loss of power, and additionally allows the data-mining of precision-medicine individualized dynamic treatment regimes. We have designed a SMART, which will both answer concrete questions in a classical way, as well as provide data highly suitable for calculating individualized dynamic treatment regimes.
Supplementary Material
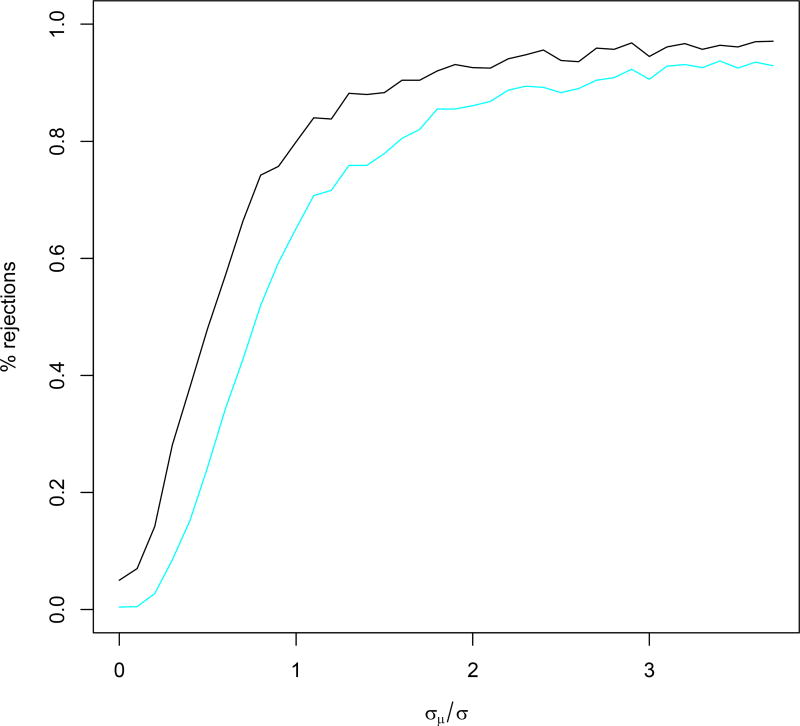
Figure 2.
Power curves showing, in black, percentage of times H01 was rejected in simulations, and, in blue, percentage of times both firstly H01 and then H02 was rejected in simulations, as functions of σμ/σ.
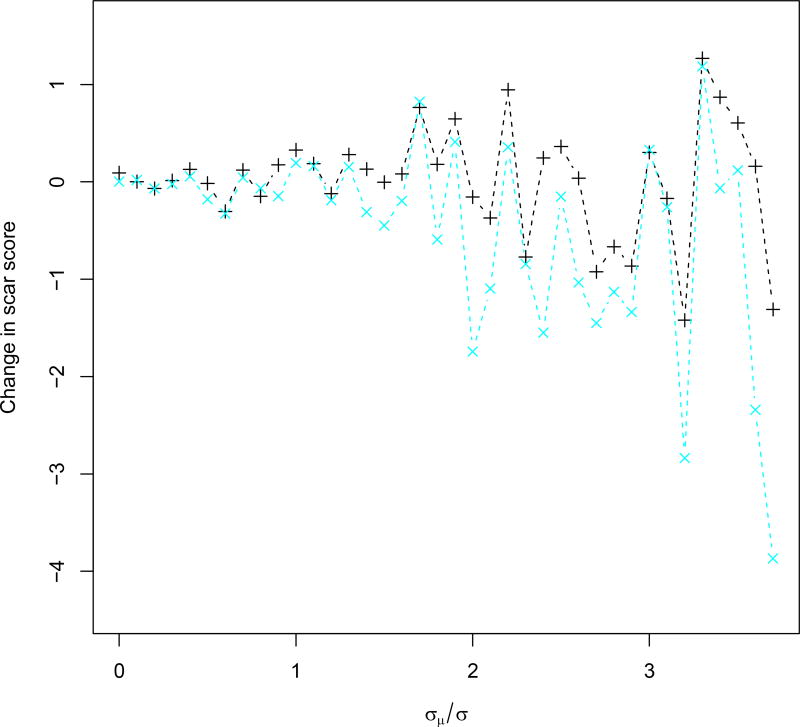
Figure 3.
Simulated change of patient scar scores for patients in trial, shown as black ‘+’, and for patients subsequently following the discovered DTR, shown as blue ‘x’, plotted against σμ/σ.
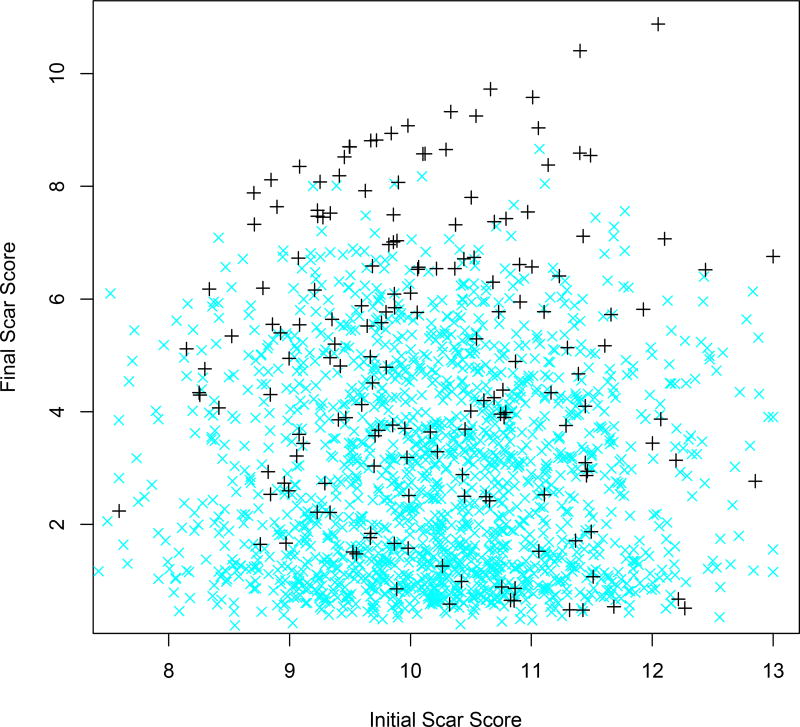
Figure 4.
Plot of simulated patient final scar scores against corresponding initial scar scores, for patients in trial, shown as black ‘+’, and for patients subsequently following the discovered DTR, shown as blue ‘x’.
Acknowledgments
The Authors would like to acknowledge the exceptionally helpful contribution of the editor and three anonymous referees, whose comments vastly improved and reworked the manuscript.
Funding
NIH grant P01 CA142538
NIH grant UL1 TR001111
Footnotes
Declaration of conflicting interests
The Authors declare that there are no conflicts of interest.
References
- 1.American Burn Association. Burn incidence fact sheet [Internet] Chicago, IL: American Burn Association; 2017. [Accessed 1 November 2017]. http://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/ [Google Scholar]
- 2.Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388:1427–1436. doi: 10.1016/S0140-6736(16)31406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hultman CS, Edkins RE, Wu C, et al. Prospective, before-after cohort study to assess the efficacy of laser therapy on hypertrophic burn scars. Ann Plast Surg. 2013;70:521–526. doi: 10.1097/SAP.0b013e31827eac5e. [DOI] [PubMed] [Google Scholar]
- 4.Arno AI, Guaglitz GG, Barret JP, et al. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40:1255–1266. doi: 10.1016/j.burns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown BC, McKenna SP, Siddhi K, et al. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61:1049–1058. doi: 10.1016/j.bjps.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg. 2014;72:S198–S201. doi: 10.1097/SAP.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 8.Porter C, Tompkins RG, Finnerty CC, et al. The metabolic stress response to burn trauma: current understanding and therapies. Lancet. 2016;388:1417–1426. doi: 10.1016/S0140-6736(16)31469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hultman CS, Edkins R, Lee CN, et al. Shine on: review of laser- and light-based therapies for the treatment of burn scars. Dermatol Res Pract. 2012;2012:243651. doi: 10.1155/2012/243651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloemen MC, van der Veer WM, Ulrich MM, et al. Prevention and curative management of hypertrophic scar formation. Burns. 2009;35:463–475. doi: 10.1016/j.burns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Kerwin LY, El Tal AK, Stiff MA, et al. Scar prevention and remodeling: a review of the medical, surgical, topical and light treatment approaches. Int J Dermatol. 2014;53:922–936. doi: 10.1111/ijd.12436. [DOI] [PubMed] [Google Scholar]
- 12.Fontana CR, Bonini D, Bagnato VS. A 12-month follow-up of hypopigmentation after laser hair removal. J Cosmet Laser Ther. 2013;15:80–84. doi: 10.3109/14764172.2012.758378. [DOI] [PubMed] [Google Scholar]
- 13.Kidd M, Hultman CS, Van Aalst J, et al. The contemporary management of electrical injuries: resuscitation, reconstruction, rehabilitation. Ann Plast Surg. 2007;58:273–278. doi: 10.1097/01.sap.0000250837.18740.d9. [DOI] [PubMed] [Google Scholar]
- 14.Duke J, Wood F, Semmens J, et al. An assessment of burn injury hospitalisations of adolescents and young adults in Western Australia, 1983–2008. Burns. 2012;38:128–135. doi: 10.1016/j.burns.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Barber RC, Chang LY, Purdue GF, et al. Detecting genetic predisposition for complicated clinical outcomes after burn injury. Burns. 2006;32:821–827. doi: 10.1016/j.burns.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Schwacha MG, Holland LT, Chaudry IH, et al. Genetic variability in the immune-inflammatory response after major burn injury. Shock. 2005;23:123–128. doi: 10.1097/01.shk.0000148073.19717.a9. [DOI] [PubMed] [Google Scholar]
- 17.Collins LM, Nahum-Shani I, Almirall D. Optimization of behavioral dynamic treatment regimens based on the sequential, multiple assignment, randomized trial (SMART) Clin Trials. 2014;11:426–434. doi: 10.1177/1740774514536795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 19.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. N Engl J Med. 2012;366:489–491. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 20.Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309:1351–1352. doi: 10.1001/jama.2013.393. [DOI] [PubMed] [Google Scholar]
- 21.Alyass A, Turcotte M, Meyre D. From big data analysis to personalized medicine for all: challenges and opportunities. BMC Med Genomics. 2015;8:33. doi: 10.1186/s12920-015-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moodie EE, Richardson TS, Stephens DA. Demystifying optimal dynamic treatment regimes. Biometrics. 2007;63:447–455. doi: 10.1111/j.1541-0420.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 23.Laber EB, Lizotte DJ, Qian M, et al. Dynamic treatment regimes: Technical challenges and applications. Electron J Stat. 2014;8:1225–1272. doi: 10.1214/14-ejs920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy SA. Optimal dynamic treatment regimes. J R Stat Soc Ser B. 2003;65:331–355. [Google Scholar]
- 25.Lavori PW, Dawson R. A design for testing clinical strategies: biased adaptive within-subject randomization. J R Stat Soc Ser A. 2000;163:29–38. [Google Scholar]
- 26.Lavori PW, Dawson R. Dynamic treatment regimes: practical design considerations. Clin Trials. 2004;1:9–20. doi: 10.1191/1740774s04cn002oa. [DOI] [PubMed] [Google Scholar]
- 27.Dawson R, Lavori PW. Placebo free designs for evaluating new mental health treatments: the use of adaptive treatment strategies. Stat Med. 2004;23:3249–3262. doi: 10.1002/sim.1920. [DOI] [PubMed] [Google Scholar]
- 28.Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24:1455–1481. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 29.Zhao YQ, Laber EB. Estimation of optimal dynamic treatment regimes. Clin Trials. 2014;11:400–407. doi: 10.1177/1740774514532570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahum-Shani I, Qian M, Almirall D, et al. Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods. 2012;17:457–477. doi: 10.1037/a0029372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy SA, Bingham D. Screening experiments for developing dynamic treatment regimes. J Am Stat Assoc. 2009;104:391–408. doi: 10.1198/jasa.2009.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Box GE, Hunter WG, Hunter JS. Statistics for experimenters: an introduction to design, data analysis, and model building. Hoboken, NJ: Wiley; 1978. [Google Scholar]
- 33.Kosorok MR, Moodie EE, editors. Adaptive treatment strategies in practice: planning trials and analyzing data for personalized medicine. Philadelphia: SIAM; 2016. [Google Scholar]
- 34.Schulte PJ, Tsiatis AA, Laber EB, et al. Q-and A-learning methods for estimating optimal dynamic treatment regimes. Stat Sci. 2014;29:640–661. doi: 10.1214/13-STS450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty B, Moodie EE. Statistical methods for dynamic treatment regimes. New York: Springer; 2013. [Google Scholar]
- 36.Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43. [PMC free article] [PubMed] [Google Scholar]
- 37.Hultman CS, Saou MA, Roach ST, et al. To heal and restore broken bodies: a retrospective, descriptive study of the role and impact of pastoral care in the treatment of patients with burn injury. Ann Plast Surg. 2014;72:289–294. doi: 10.1097/SAP.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 38.Liu A, Moy RL, Ozog DM. Current methods employed in the prevention and minimization of surgical scars. Dermatol Surg. 2011;37:1740–1746. doi: 10.1111/j.1524-4725.2011.02166.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan HH, Wong DS, Ho WS, et al. The use of pulsed dye laser for the prevention and treatment of hypertrophic scars in Chinese persons. Dermatol Surg. 2004;30:987–994. doi: 10.1111/j.1524-4725.2004.30303.x. [DOI] [PubMed] [Google Scholar]
- 40.Batta K, Goodyear HM, Moss C, et al. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: results of a 1-year analysis. Lancet. 2002;360:521–527. doi: 10.1016/S0140-6736(02)09741-6. [DOI] [PubMed] [Google Scholar]
- 41.Edwards RR, Smith MT, Klick B, et al. Symptoms of depression and anxiety as unique predictors of pain-related outcomes following burn injury. Ann Behav Med. 2007;34:313–322. doi: 10.1007/BF02874556. [DOI] [PubMed] [Google Scholar]
- 42.Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:1960–1965. doi: 10.1097/01.prs.0000122207.28773.56. [DOI] [PubMed] [Google Scholar]
- 43.Anisimov VV, Yeung WY, Coad DS. Imbalance properties of centre-stratified permuted-block and complete randomisation for several treatments in a clinical trial. Stat Med. 2017;36:1302–1318. doi: 10.1002/sim.7206. [DOI] [PubMed] [Google Scholar]
- 44.Little RJ, Rubin DB. Statistical analysis with missing data. 3. Hoboken, NJ: Wiley; 2012. [Google Scholar]
- 45.Hsu H, Lachenbruch PA. Wiley Encyclopedia of Clinical Trials. Hoboken, NJ: Wiley; 2008. Paired t test. [Google Scholar]
- 46.Morrison DF. Multivariate analysis, overview. Hoboken, NJ: Wiley; 1998. [Google Scholar]
- 47.Selvin S. Encyclopedia of Biostatistics. Hoboken, NJ: Wiley; 2005. F Distributions. [Google Scholar]
- 48.Li Z, Murphy SA. Sample size formulae for two-stage randomized trials with survival outcomes. Biometrika. 2011;98:503–518. doi: 10.1093/biomet/asr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CJ, Hamada MS. Experiments: planning, analysis, and optimization. Hoboken, NJ: Wiley; 2011. [Google Scholar]
- 50.Zhao Y, Zeng D, Socinski MA, et al. Reinforcement learning strategies for clinical trials in nonsmall cell lung cancer. Biometrics. 2011;67:1422–1433. doi: 10.1111/j.1541-0420.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.