Abstract
Objective
Many cancer survivors experience fatigue as a nagging symptom lasting years after treatment. To learn of the relevant biological pathways involved in fatigue among cancer survivors, we tested for an association between fatigue levels and leukocyte gene expression profiles and determined the specific mediating immune cell types.
Methods
A sample of N=89 Hispanic/Latino adults diagnosed with colorectal cancer aged 60.5 years, 2.9 years since diagnosis, and 62% male provided blood for transcriptome profiling and completed a validated measure of fatigue (Multidimensional Fatigue Inventory-Short Form). We applied genome-wide transcriptional profiling of leukocyte RNA to identify gene expression activity associated with fatigue, tested for activity of specific transcription factors involved in previously established markers of inflammation and immunologic activation, and identified the specific cell types mediating these transcriptional alterations.
Results
In analyses adjusting for demographic and behavioral health risk factors, results linked fatigue to increased activation of B lymphocytes and CD8+ T cells, as well as several transcription factors involved in immune activation (NF-κB, STAT, and CREB). Results also replicated several specific genomic effects previously observed in fatigued cancer survivors, including up-regulated expression of alpha-synuclein (SNCA) and hemoglobin subunits (HBA and HBB).
Conclusions
Cancer survivors’ heightened fatigue levels may be partially explained by activation of specific immune cell subsets, providing a potential molecular biomarker for clinical interventions targeting the remediation of fatigue.
Keywords: Cancer, Colorectal, Fatigue, Genomics, Hispanic, Inflammation, Leukocyte, Oncology, Survivorship, Transcriptome
Many cancer survivors experience fatigue as a nagging symptom lasting years after treatment. Fatigue is experienced as physical and/or mental tiredness to exhaustion that is disproportional to level of exertion.1 Cancer survivors report fatigue as the symptom most disruptive to daily life.2 National prevalence of fatigue is around 37% for survivors,3 and over 30% can experience fatigue up to 10 years after diagnosis.4 Proposed culprits include immune and neuroendocrine perturbations from radiation, chemotherapy, biological-agent treatments, and the tumor environment itself. Although our understanding of fatigue is limited, some promising biological mechanisms have been identified, including alteration of immune system activity.5,6
Immune cell signaling across inflammatory pathways offers initial insight about biological mechanisms of fatigue. Fatigued survivors, either proximal or distal to their treatment period, show elevated concentrations of pro-inflammatory markers in peripheral blood, compared to those not fatigued. Markers have included interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP).7,8 However, some studies do not replicate all signals,9 and mixed findings arise for other inflammatory markers such as interleukin-1β (IL-1B) and interleukin-6 (IL-6).5,10
Studies investigating gene expression in immune cells (leukocytes) have also provided insight into the deeper molecular drivers of fatigue. For example, fatigued breast cancer survivors show increased expression of genes bearing response elements for the pro-inflammatory transcription factor, Nuclear Factor-κB (NF-κB) compared to those not fatigued.11 Studies have also identified specific individual target genes that are associated with fatigue in cancer patients, mainly in those exposed to radiation therapy. These include the upregulation of genes coding for alpha-synuclein (SNCA) and hemoglobin subunits (HB family genes).12,13 B and T lymphocytes have been implicated as cellular mediators of these dynamics.13–15 However, most of these findings remain provisional as they have not been replicated in independent samples or in different types of cancer. Moreover, it remains unclear how these molecular correlates relate to one another (i.e., are they different manifestations of a single common underlying syndrome, or do they reflect multiple distinct risk factors that are each sufficient to induce fatigue?).
To test the generality of previously observed molecular correlates of fatigue, we examined the gene expression profile of fatigue in colorectal cancer survivors. Cancer survivorship is defined here as the period from cancer treatment onward. We applied genome-wide transcriptional profiling of leukocyte RNA to identify gene transcriptional correlates of fatigue (controlling for relevant demographic and behavioral covariates). We used those transcriptome data as inputs into bioinformatic analyses of transcription factors involved in inflammation and immunologic activation and tested for specific cell types mediating the transcriptional alterations. Guided by findings from previous studies among cancer survivors, we hypothesized that fatigue would map on to a gene expression profile indicative of greater inflammation (e.g., NF-κB activity), greater activation of stress-related neuroendocrine pathways (e.g., CREB/ATF factors mediating beta-adrenergic influences from the sympathetic nervous system, and glucocorticoid receptors mediating influences from the hypothalamic-pituitary-adrenal axis), and reduced activity of Type I interferon antiviral activity (e.g., IRF factors).
Materials and Methods
Participants and procedure
A subset of participants from the Hispanic Colorectal Cancer Study (HCCS) provided a blood sample and completed additional self-report measures for this study from 2015–2016. HCCS is a population-based cohort study of individuals self-identified as Hispanic or Latino with a confirmed diagnosis of colorectal cancer (CRC) at any stage.16 All men and women over 21 years of age with a first-time diagnosis of CRC (International Classification of Diseases for Oncology, Third Edition codes: C18–C20) who were able to communicate in English or Spanish were eligible for participation. Cases were identified from the California Cancer Registry and/or directly from two hospitals in Los Angeles (LAC+USC County Hospital and USC Norris Comprehensive Cancer Center). HCCS participants completed risk factor questionnaires, but only participants who agreed to provide an additional blood sample, which was optional, were eligible to participate in the present study. The University of Southern California Institutional Review Board approved this study, and all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration.
Assessments
Fatigue
Fatigue was assessed using the 6-item Multidimensional Fatigue Inventory-Short Form General Fatigue scale (MFSI-SF).17 MFSI-SF is a self-report measure of multiple dimensions of fatigue developed for use with cancer survivors.18 Response options range from 1 (not al all) to 5 (extremely) with higher scores coded to indicate a greater level of fatigue. Example items used in our study include “I am worn out,” “I feel fatigued,” and “I feel run down.” In the present study, the scale’s Cronbach’s alpha was .94. The abbreviated scale was used to reduce respondent burden as participants were completing lengthy questionnaires for the HCCS study. Gene expression analyses utilized z-score standardized scores on the fatigue scale to provide a metric for the identification of differentially expressed genes comparable to that used in previous research (i.e., ≥ 1.5-fold difference in average RNA expression over the 4-SD range of normal variation in scale scores).19
Demographic, biometric, and behavioral covariates
We extracted birthdate and sex from the questionnaires administered to patients and calculated age coincident with the date of blood collection. Socioeconomic assessments included highest education and annual household income. Body mass index (BMI) was calculated from self-reported height and weight (kg/m2). A dummy variable for smoking history was coded as 1 if participants reported ever smoking regularly. A dummy variable for alcohol history was coded as 1 if participants reported alcohol use during the last decade of the assessment period.
Blood Processing and Transcriptome Profiling
A phlebotomist administered venipuncture to draw a fasting blood sample from participants into vacutainer tubes. Samples were centrifuged at 4C at approximately 1300g for 20 minutes. Buffy coat was extracted then stored at −80C. Samples were subsequently shipped in batch to the UCLA Social Genomics Core Laboratory. RNA was extracted (Qiagen RNEasy), tested for suitable mass (Nanodrop ND1000) and integrity (Agilent TapeStation), converted to fluorescent cRNA (Ambion TotalPrep), and hybridized to Illumina Human HT-12 v4 BeadArrays following the manufacturer’s standard protocol in the UCLA Neuroscience Genomics Core Laboratory. All samples were assayed in a single batch, and 88 of the 89 assayed samples yielded valid results according to quality assurance metrics (e.g., median probe fluorescence intensity >80 units).
Reference Cell Transcriptome Profiling
To facilitate identification of the specific leukocyte subtype(s) giving rise to fatigue-related transcriptome alterations, we isolated sets of major leukocyte subpopulations from anonymous donor PBMC samples and conducted genome-wide transcriptional profiling to identify subtype-specific gene transcripts that could provide reference profiles for Transcript Origin Analyses.20 Given previous data linking fatigue to pro-inflammatory signaling processes in myeloid-lineage immune cells,11 the cell isolation protocol specifically differentiated among subpopulations of monocytes and 3 distinct dendritic cell (DC) phenotypes: CD1c+(BDCA1+) Myeloid DCs (DC1), CD303+(BDCA2+) Plasmacytoid DCs (DC2), and CD141+(BDCA3+) Myeloid DCs (DC3).21 Isolations were conducted using a FACS Aria III (B–D Immunocytometry) flow cytometer to separate 650 × 106 peripheral blood mononuclear cells (PBMC) into discrete populations of B lymphocytes (CD19+), CD4+ T lymphocytes (CD3+/CD4+), CD8+ T lymphocytes (CD3+/CD8+), Natural Killer (NK) cells (CD3−/CD14−/CD56+), monocytes (CD14+), DC1 (BDCA1+/CD19−), DC2 (BDCA2+), and DC3 (BDCA3+/CD14−).21 B, T, and NK cells were isolated from total PBMC. Monocytes and DCs were isolated from PBMC that had been pre-depleted of CD3+ and CD19+ cells by immunomagnetic positive selection (MACS microbeads; Miltenyi Biotech) in order to enhance flow cytometry positive event rates (i.e., reduce the length of time required to accrue a viable number of DCs, which generally have a prevalence < 1% of total PBMC).
All flow sorting was conducted in the UCLA Janis Giorgi Immunocytometry Core Laboratory and yielded cell populations that were > 99% pure. Sorts were conducted on 4 independent PBMC samples (each isolated from Red Cross Leukopak buffy coat specimens by standard ficol density gradient centrifugation), with 2 samples pooled to create 2 biological replicate samples of each cell type for subsequent transcriptome profiling. Transcriptome profiling was conducted in a single batch as described above using Illumina Human HT-12 v4 BeadArrays. All samples yielded valid data, and data are publicly available as Gene Expression Omnibus GSE101489.
Statistical Analyses
Gene expression values were quantile-normalized,22 and then log2-transformed for analysis using standard linear statistical models to estimate the association of transcript abundance with (z-score transformed) MFSI-SF scores while controlling for age, sex, BMI, history of smoking (1/0), and history of alcohol consumption (1/0). Differentially expressed genes were identified as those showing ≥ 1.5-fold difference in expression over the 4-SD range of normal variation in MFSI-SF scores.
A 2-sample variant of the Transcription Element Listening System (TELiS23) was applied to the sets of up- and down-regulated genes to test whether the observed differences in gene expression could be driven by specific a priori-hypothesized transcription factors involved in inflammation and immunologic activation (NF-κB, AP-1, CREB/ATF, GR, IRF, and STAT). These analyses tested the (log) ratio of transcription factor-binding motif prevalence for each factor in up- vs. down-regulated promoters, pooled across 9 alternative technical specifications involving parametric variations of promoter length (−300 bp, −600 bp, −1000 to +200 bp relative to the transcription start site) and TFBM detection stringency (MatSim .80, .90, .95).23
To identify specific cell types that contributed to the empirically observed differences in gene expression, we applied Transcript Origin Analysis (TOA)20 to separate lists of up- and down-regulated genes. TOA tested for significant over-representation of genes preponderantly expressed by a specific subset of PBMCs (i.e., monocytes, dendritic cells, CD4+ T cells, CD8+ T cells, B cells, or Natural Killer cells) using cell-specific reference transcriptomes derived by flow cytometric isolation as described above. We also tested whether MFSI-SF scores were associated with average expression of 19 gene transcripts previously utilized as a generalized index of pro-inflammatory gene expression (IL1A, IL1B, IL6, IL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, RELB) or 34 gene transcripts previously utilized as a generalized index of Type I interferon activity (GBP1, IFI16, IFI27, IFI27L1-2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1-3, IFIT5, IFIT1L, IFITM1-3, IFITM4P, IFITM5, IFNB1, IRF2, IRF7-8, MX1-2, OAS1-3, OASL) and antibody synthesis (IGJ, IGLL1, IGLL3), as previously detailed.19 In all analyses, standard errors for observed bioinformatic statistics were derived from 200 cycles of bootstrap resampling of linear model residual vectors, while accounting for potential correlation among residuals across transcripts.
Results
Sample Characteristics
The sample comprised 89 colorectal cancer survivors with an average age of 60.5 years, majority male, moderately overweight BMI, and 2.9 years since diagnosis (Table 1). Over half the sample had a history of smoking regularly. Average MFSI-SF scores (M=7.4, SD=6.3) were similar to previous levels found among survivor samples (range=5–12)24 and for population-based norms (M=8.4, SD=3.6)25 but lower than advanced-stage cancer patient samples (range=13–19).26 18% of our sample showed MFSI-SF global scores >12 which is the national norm for chronically unhealthy individuals.25
Table 1.
Hispanic/Latino cancer survivor sample characteristics, N=89
| Variable | M (SD) or % |
|---|---|
| Age in years | 60.5 (10.3) |
| Sex (male) | 62% |
| Education | |
| <High school graduate | 41.6% |
| High school graduate | 25.8% |
| Some college | 27.0% |
| Bachelor degree or above | 5.6% |
| Household income | |
| $29,000 USD or less | 58.7% |
| >$29,000 USD | 44.3% |
| Years since dx | 2.9 (1.7) |
| Alcohol use history (yes) | 36.0% |
| Smoking history (yes) | 52.0% |
| Body Mass Index (BMI) | 30.5 (7.1) |
| Fatigue (MFSI-SF) | 7.4 (6.3) |
Notes. MFSI-SF: Multidimensional Fatigue Symptom Inventory-Short Form presented as a sum score of 6 items for a possible range from 0 to 24.
Gene Expression Correlates of Fatigue
Analyses estimating the magnitude of association between leukocyte gene expression and MFSI-SF scores identified 280 gene transcripts showing ≥ 1.5-fold difference in expression over the 4-SD range of normal variation in MFSI-SF scores after controlling for age, sex, BMI, smoking, and alcohol use (155 up-regulated and 120 down-regulated). Up-regulated genes (Supplemental Table 1) included several transcripts associated with fatigue, such as the amyloid subunit synuclein-α (SNCA) and multiple hemoglobin subunits (HBA1, HBA2, HBB, HBG2).12–15 Other up-regulated transcripts included indicators of immunologic activation (HLA-B, HLA-H, LTB) and related transcriptional regulators (EGR1, ETS1, ATF4, HMGB1, HMGB2, JUND, KLF2, EIF1), as well as several small nucleolar RNAs (SNORA45, SNORA63, SNORD3A, SNORD3C, SNORD3D, SNORD13, SNORD89). Down-regulated genes were difficult to characterize due to the preponderance of unannotated/un-named transcripts (LOC/HS./ORF) but included the lymphocyte differentiation marker LY6E, several keratin-associated proteins and cadherins (KRTAP6-3, KRTAP19-6, KRTAP21-1, KRTAP21-2, CHD5), and indicators of Type I interferon activity (ISG15, IFI6).
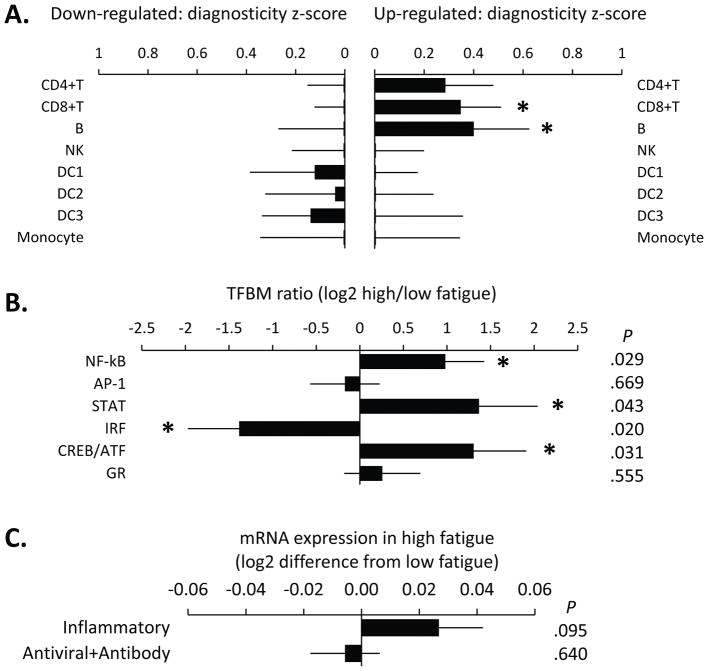
To identity the specific cell types mediating fatigue-related transcriptional alterations within the overall leukocyte pool, we conducted Transcript Origin Analysis (TOA) using reference cell transcriptome profiles derived from immunomagnetically isolated leukocyte subsets (CD4+ T cells, CD8+ T cells, B cells, NK cells, monocytes, and DC1, DC2, and DC3 dendritic cells). Results identified B lymphocytes, CD8+ T lymphocytes, and to a lesser extent, CD4+ T lymphocytes as the primary cellular origins of gene transcripts up-regulated in association with MFSI-SF scores (Figure 1a). No individual cell type emerged as a preponderant contributor of gene transcripts that were down-regulated in fatigue.
Figure 1. Gene expression, cellular origin, and transcriptome representation of fatigue scores (MFSI-SF).
(A) Transcript Origin Analyses (TOA) identifying major cell types mediating fatigue-related transcriptional alterations within the overall leukocyte pool that show ≥1.5-fold difference in expression over the 4-SD range of normal variation in fatigue scores after controlling for age, sex, BMI, smoking, and alcohol use (280 up-regulated gene transcripts with 155 up-regulated and 120 down-regulated listed in Table S1). (B) Transcription Element Listening System (TELiS) analyses comparing the prevalence of transcription factor-binding motifs in the promoters of genes that were up- vs. down-regulated in association with fatigue scores. (C) Comparing the expression of previously defined gene sets comprising 19 cardinal inflammatory and antiviral+antibody gene transcripts. TFBM=Transcriptional factor-binding motif.
To identify specific transcription control pathways that might mediate the observed differences in gene expression, we conducted Transcription Element Listening System (TELiS) analyses comparing the prevalence of transcription factor-binding motifs in the promoters of genes that were up- vs. down-regulated in association with MFSI-SF scores. Results indicated up-regulation of NF-κB, CREB/ATF, and STAT family factors, down-regulated activity of IRF factors, and no indication of differential activity for AP-1 or GR (Figure 1b).
Previous studies have linked cancer-related fatigue to increased pro-inflammatory signaling,11 so we also tested whether MFSI-SF scores might be associated with increased expression of a previously defined set of 19 cardinal pro-inflammatory gene transcripts. Results showed a positive association at the trend level (p = .095; Figure 1c). Although not hypothesized a priori, we also tested a previously defined gene set tracking Type I interferon antiviral activity and antibody-related transcription (which is often found to track inversely with pro-inflammatory gene expression27) and did not find a significant association with MFSI-SF scores in this sample.
Discussion
Based on the study results, we find that heightened levels of fatigue among colorectal cancer survivors are associated with up-regulated activity in the adaptive immune system, including increased activation of B lymphocytes and CD8+ T lymphocytes, elevated activity of transcription factors involved in lymphocyte activation and inflammation (NF-κB, STAT, and CREB/ATF), and reduced activity of interferon response factors (IRF). At the level of individual gene transcripts, these alterations included up-regulation of α-synuclein (SNCA) and hemoglobin subunits (HBA and HBB), which corroborates results previously observed in cancer-related fatigue.12,13 Our study is the first to replicate previous exploratory findings as a priori hypotheses, the first to document correlates of fatigue in colorectal cancer, and the first to extend such results in a Hispanic/Latino sample, lending to enhanced generalizability of the findings across race/ethnicity. Moreover, the simultaneous documentation of transcriptional alterations at the level of specific individual genes (e.g., SNCA, HBA/HBB), up-stream transcription factors (e.g., NF-κB, STAT, CREB/ATF, IRF), and their broader cellular context (e.g., B lymphocytes, CD8+ T cells) suggests that each of these processes may reflect different manifestations of a single common underlying syndrome involving dysregulation of the adaptive immune response.
The present results are particularly striking in replicating associations of several specific transcripts with cancer-related fatigue, including the amyloid subcomponent synuclein-α (SNCA) and several hemoglobin subunits (HBA1, HBA2, HBB, HBG2).12,13,15 The present findings also converge to some degree with previous observations relating cancer-associated fatigue to increased activity of pro-inflammatory signaling pathways (e.g., activation of NF-κB, CREB/ATF, and STAT family TFs, as well as a trend toward increased activity of the a priori-specified composite of 19 canonical pro-inflammatory gene transcripts). However, the present data showed no evidence of altered activity of the anti-inflammatory glucocorticoid signaling pathway (GR) or of the monocyte lineage cell types that mediate classical inflammatory reactions. Instead, the present results implicated lymphoid lineage cells, particularly B lymphocytes and CD8+ T lymphocytes. Previous studies have also implicated B cells in the leukocyte transcriptomic correlates of fatigue.14,15 Consistent with that observation, SNCA is known to influence B cell homeostasis and differentiation in addition to its better known role in neuronal integrity as the non-Aβ component of Alzheimer’s Disease amyloid.28
Past research assessing inflammatory markers using genome-wide transcription profiling have focused on breast cancer patients, and these studies have demonstrated variability in methodology, particularly fatigue measures and sampling techniques. 5,6,29 The discontinuity of the present results with the more classical myeloid/inflammatory profile observed in previous studies of post-treatment fatigue in breast cancer11 may represent distinct pathophysiological pathways in distinct cancer types (i.e., colorectal cancer in this study vs. breast cancer in the previous study) and/or differences in the time elapsed after radiation and other cancer treatments. In particular, colorectal cancer may involve more extensive surgery and more extended radiation and chemotherapy exposures, and those differences may contribute to differences in the immunoregulatory gene expression profiles observed in this study relative to previous studies of early stage breast cancer patients. Additionally, racial and ethnic differences in the experience of cancer survivorship, including higher reported obesity rates (defined by the CDC as an adult BMI>30.0), may affect the relevant biological pathways and associated gene expression profiles involved in fatigue among Hispanic/Latino CRC survivors.30,31 More studies on racial and ethnically diverse cancer survivors with a variety of cancer diagnoses are needed to elucidate the possible influence of these factors on symptoms post-treatment. 31
The present results also differ from previous observations in linking cancer-related fatigue to reductions in Type I interferon-related signaling, including down-regulation of specific interferon response genes (e.g., ISG15, IFI6) as well as reduced activity of IRF transcription factors. However, we observed no significant down-regulation of a broader set of 34 pre-specified interferon and antibody-related genes, suggesting that the observed down-regulation is specific to a narrow range of interferon-related transcripts. The biological significance of this observation remains to be clarified in future research, but it could represent a compensatory side-effect of the observed increases in NF-κB signaling activity (as NF-κB is known to reciprocally cross-regulate IRF activity).32,33 Alternatively, this observation may reflect a selective deficit in Type I interferon containment of a chronic viral infection that subsequently contributes to fatigue (e.g., as hypothesized in chronic fatigue syndromes). Future research assessing the role of subclinical viral infections such as Epstein-Barr Virus or Cytomegalovirus may help clarify the basis for these observations.
Study Limitations
One limitation involves the cross-sectional study design, which precludes drawing any conclusions regarding the direction of causal relationships among gene expression activity and fatigue. The observed associations may reflect a causal effect of immunologic activation on CNS processes involved in arousal and motivation (e.g., as in the “sickness behavior” literature).4,34,35 However, the profile of transcription factor alterations observed here (elevated pro-inflammatory and reduced antiviral) is reminiscent of the stress-induced “Conserved Transcriptional Response to Adversity” profile,27 suggesting that at least some of the observed transcriptomic correlates of fatigue could potentially reflect the effects of fatigue-related distress on immune cell gene regulation. Associations may also reflect the effects of stressful life circumstances that causally influence both experienced fatigue and immune cell gene expression. It is also important to note that no assessments of clinical health outcomes or immune function are available in this sample, so the implications of the observed transcriptome differences for health remain to be determined in future studies.
Clinical Implications
The present findings identify a systematic alteration in lymphocyte gene regulation that may serve as a target for future interventions to reduce cancer-associated fatigue. Based on these findings, therapeutic strategies the reduce CD8+ T cell and/or B cell activation should be considered for potential use in cases of severe or persistent cancer-related fatigue (e.g., monoclonal antibodies targeting cell type-specific activation receptors). Moreover, the RNA-based measures of CD8+ T cell and B cell activation, activation-related transcription factor activity (e.g., NF-κB, STAT, CREB), and specific replicated transcript alterations (SNCA, HBA, HBB) identified in this report may potentially be used as biomarkers to gauge the impact of pharmacologic or behavioral interventions to reduce fatigue.36 The establishment of replicated molecular biomarkers thus opens new horizons for the clinical management of cancer-related fatigue.
Supplementary Material
Supplemental Table 1. Genes differentially expressed as a function of high versus low fatigue
Acknowledgments
Funding
Funding and resource support was received from the National Institute on Aging via a grant to the University of Southern California/University of California Los Angeles Center on Biodemography and Population Health (P30AG017265 to D.B.), from the National Cancer Institute (R01CA155101 to J.F.) from the National Center for Complementary and Integrative Health (L30AT008380 to D.B.), from the American Mindfulness Research Association, and from the Keck School of Medicine via institutional funds to J.F. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards P30CA016042 and 5P30AI028697. The ideas and opinions expressed herein are those of the authors, and endorsement of those opinions by the funders is not intended nor should be inferred.
The authors are indebted to the individuals who participated in this study. The authors thank the following individuals for their assistance with logistic support and management, interviewing patients, and data entry: Nathalie Nguyen, Julissa Ramirez, Yaquelin Perez, Daniel Collin, Alicia Rivera, and Lauren Gerstmann; and Jesusa Arevalo and Jeffrey Ma for assistance in transcriptome profiling assays.
Footnotes
Conflict of interest statement: All authors declare that there are no conflicts of interest.
Author Contributions
David Black: Project conceptualization (Principal Investigator), methodology, resources, funding acquisition, lead of manuscript writing. Georgia Christodoulou: Data curation, manuscript writing. Steve Cole: Statistical analysis, data curation and interpretation, manuscript writing. Jane Figueiredo: Data collection supervision, resources, funding acquisition, and manuscript writing.
References
- 1.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–9. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 2.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 3.Cella D, Davis K, Breitbart W, et al. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–91. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–8. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 5.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(Suppl):S48–57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–27. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Orre IJ, Murison R, Dahl AA, et al. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav Immun. 2009;23:868–74. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Aziz N, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–11. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–22. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao C, Beitler JJ, Higgins KA, et al. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav Immun. 2016;52:145–52. doi: 10.1016/j.bbi.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower JE, Ganz PA, Irwin MR, et al. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25:147–50. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saligan LN, Hsiao CP, Wang D, et al. Upregulation of alpha-synuclein during localized radiation therapy signals the association of cancer-related fatigue with the activation of inflammatory and neuroprotective pathways. Brain Behav Immun. 2013;27:63–70. doi: 10.1016/j.bbi.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karim S, Mirza Z, Chaudhary AG, et al. Assessment of Radiation Induced Therapeutic Effect and Cytotoxicity in Cancer Patients Based on Transcriptomic Profiling. Int J Mol Sci. 2016;17:250. doi: 10.3390/ijms17020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao CP, Reddy SY, Chen MK, et al. Genomic Profile of Fatigued Men Receiving Localized Radiation Therapy. Biol Res Nurs. 2016;18:281–9. doi: 10.1177/1099800415618786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J. 2009;9:333–40. doi: 10.1038/tpj.2009.27. [DOI] [PubMed] [Google Scholar]
- 16.Schmit SL, Schumacher FR, Edlund CK, et al. Genome-wide association study of colorectal cancer in Hispanics. Carcinogenesis. 2016;37:547–56. doi: 10.1093/carcin/bgw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein KD, Martin SC, Hann DM, et al. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–52. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 19.Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci U S A. 2013;110:13684–9. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole SW, Hawkley LC, Arevalo JM, et al. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108:3080–5. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 22.Bolstad BM, Irizarry RA, Astrand M, et al. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 23.Cole SW, Yan W, Galic Z, et al. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21:803–10. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26:140–4. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 25.Lin JM, Brimmer DJ, Maloney EM, et al. Further validation of the Multidimensional Fatigue Inventory in a US adult population sample. Popul Health Metr. 2009;7:18. doi: 10.1186/1478-7954-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Raaf PJ, Sleijfer S, Lamers CH, et al. Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: an explorative study. Cancer. 2012;118:6005–11. doi: 10.1002/cncr.27613. [DOI] [PubMed] [Google Scholar]
- 27.Slavich GM, Cole SW. The Emerging Field of Human Social Genomics. Clin Psychol Sci. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao W, Shameli A, Harding CV, et al. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by alpha-synuclein, a key player in Parkinson’s disease. Immunobiology. 2014;219:836–44. doi: 10.1016/j.imbio.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saligan LN, Olson K, Filler K, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23:2461–78. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz KH, Neuhouser ML, Agurs-Collins T, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105:1344–54. doi: 10.1093/jnci/djt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aziz NM. Cancer survivorship research: challenge and opportunity. J Nutr. 2002;132:3494S–3503S. doi: 10.1093/jn/132.11.3494S. [DOI] [PubMed] [Google Scholar]
- 32.Amit I, Garber M, Chevrier N, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–63. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–87. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 34.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bower JE. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014 doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller AH, Ancoli-Israel S, Bower JE, et al. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–82. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Genes differentially expressed as a function of high versus low fatigue