Abstract
Hypertension, obesity, and old age are major risk factors for left ventricular (LV) diastolic dysfunction (LVDD), but easily applicable screening tools for people at risk are lacking. We investigated whether HF1, a urinary biomarker consisting of 85 peptides, can predict over a 5-year time span mildly impaired diastolic LV function as assessed by echocardiography. In 645 white Flemish (50.5% women; 50.9 years [mean]), we measured HF1 by capillary electrophoresis coupled with mass spectrometry in 2005–2010. We measured early (E) and late (A) peak velocities of the transmitral blood flow and early (e') and late (a') mitral annular peak velocities and their ratios in 2009–2013. In multivariable-adjusted analyses, per 1-standard deviation increment in HF1, e' was −0.193 cm/s lower (95% confidence interval: −0.352 to −0.033; P = .018) and E/e' 0.174 units higher (0.005–0.342; P = .043). Of 645 participants, 179 (27.8%) had LVDD at follow-up, based on impaired relaxation in 69 patients (38.5%) or an elevated filling pressure in the presence of a normal (74 [43.8%]) or low (36 [20.1%]) age-specific E/A ratio. For a 1-standard deviation increment in HF1, the adjusted odds ratio was 1.37 (confidence interval, 1.07–1.76; P = .013). The integrated discrimination (+1.14%) and net reclassification (+31.7%) improvement of the optimized HF1 threshold (−0.350) in discriminating normal from abnormal diastolic LV function at follow-up over and beyond other risk factors was significant (P ≤ .024). In conclusion, HF1 may allow screening for LVDD over a 5-year horizon in asymptomatic people.
Keywords: Diastolic left ventricular function, population science, screening, urinary proteomics
Highlights
-
•
Asymptomatic diastolic left ventricular dysfunction (LVDD) affects 25% of people.
-
•
LVDD is a forerunner of cardiovascular complications.
-
•
HF1 is a urinary peptidomic classifier mainly consisting of dysregulated collagen fragments.
-
•
HF1 predicts LVDD over a 5-year time span and may allow screening for this high-risk condition.
Introduction
Diastolic heart failure (DHF) represents half of all heart failure cases1 and has a 30% death rate within 1 year of the first hospital admission.2 Subclinical left ventricular (LV) diastolic dysfunction (LVDD) has 25% prevalence in the general population.3, 4 Hypertension, obesity, old age, and insulin resistance are among the major risk factors.3, 4 LVDD is an insidious condition evolving to DHF.5 Screening for LVDD at the point of entry in health care is extremely challenging because it requires awareness of predisposing risk factors, clinical interpretation of vague symptoms and signs, and LV imaging demonstrating functional or structural LV changes. The observation that natriuretic peptide levels in LVDD patients are often within normal limits3, 4, 6 complicates the matters further and justifies the quest for novel biomarkers specific for LVDD at an early stage long before it progresses to DHF.
Capillary electrophoresis coupled with high-resolution mass spectrometry (CE-MS) enables detection of over 5000 distinct peptides in urine samples.7, 8 We previously identified a multidimensional urinary classifier, HF1, mainly consisting of dysregulated collagen fragments,10, 11, 9 which in case-control studies9 and in the general population10, 11 was reproducibly associated with subclinical LVDD. In patients progressing from LVDD to DHF, the LV wall undergoes fibrosis characterized by increased interstitial deposition12 and cross-linking of collagen I at the detriment of collagen III.13, 14 We hypothesized that urinary markers of collagen turnover, and circulating serum markers of collagen degradation might predict LVDD3, 4 over and beyond known risk factors and might therefore represent easily applicable screening tools in primary care. We tested our hypothesis in participants enrolled in the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO10, 11, 9), in whom we related the echocardiographically assessed diastolic LV function (2009–2013) to HF1, and the serum markers of collagen degradation measured approximately 5 years earlier (2005–2010).
Methods
Study Participants
FLEMENGHO is a family-based population study,15, 16 which complies with the Helsinki declaration17 and received ethical approval from the Ethics Committee of the University Hospitals Leuven (approval number B32220083510). For the current analysis, we selected 655 people, whose urinary proteome had been measured in 2005–2010 (baseline) and who had undergone echocardiography in 2009–2013 (follow-up). The participation rate at echocardiography was 80.0%. We excluded 10 participants, whose diastolic LV function at follow-up could not be reliably assessed, because of atrial fibrillation (N = 6) or paced heart rhythm (N = 4). Thus, the number of participants statistically analyzed totaled 645.
Clinical Measurements
Body mass index (BMI) was weight in kilogram divided by height in meters squared. Waist circumference was determined using a measuring tape. Abdominal obesity was a waist circumference of ≥88 cm in women and ≥102 cm in men.18 Blood pressure was the average of five consecutive auscultatory readings. Hypertension was a blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic or use of antihypertensive drugs. Estimated glomerular filtration rate (eGFR) was derived from serum creatinine (Crt) by the chronic kidney disease epidemiology collaboration equation.19 Diabetes mellitus was a self-reported diagnosis, a fasting plasma glucose (Glyc) ≥7 mmol/L, or use of antidiabetic agents.20 Using validate questionnaires21 and published tables,22 we computed the energy spent in physical activity from body weight, time devoted to work, walking, sports and leisure time activities, and type of physical activity.
Echocardiography
Detailed information on the acquisition and offline analysis of the echocardiographic images is available in previous publications.10, 9 In short, echocardiographic measurements, obtained with a Vivid7 Pro device (GE Vingmed, Horten, Norway) interfaced with a 2.5–3.5-MHz phased-array probe, were averaged over three heart cycles. Diastolic LV function was assessed by the EchoPac software, version 4.0.4 (GE Vingmed, Horten, Norway). In keeping with guidelines,23 we determined peak early (E) and late (A) diastolic velocities of the transmitral blood flow from the pulsed Doppler signal and peak early (e') and late (a') mitral annular movement by tissue Doppler imaging with velocities averaged over four acquisition sites (septal, lateral, inferior, and posterior). Reproducibility across the four tissue Doppler imaging sampling sides ranged from 4.5% to 5.3% for e' and from 4.0% to 4.5% for a'. Patients with LVDD had an abnormally low age-specific transmitral E/A ratio indicative of impaired relaxation or a mildly-to-moderately elevated LV filling pressure (E/e' > 8.5) with normal or decreased age-specific E/A ratio. These age-specific criteria in a healthy reference sample drawn from FLEMENGHO3 were replicated in an independent European population study.4
Biomarkers
Participants collected 24-hour urine samples within 1 week of the echocardiographic examination at the field center. For measurement of HF1 and microalbuminuria, aliquots (0.7 mL) were stored at −80°C for 8 years (median) and thawed immediately before analysis. Detailed information about urine sample preparation, proteome analysis by CE-MS, data processing, and sequencing has been published before.7, 8 Peptides were combined into a single summary variable, using the MosaCluster software, version 1.6.5. HF1 was originally derived in a case-control study including participants with mild and moderate LVDD. It consists of 85 peptides (Table S1), mainly collagen fragments. HF1 is a robust10, 9 urinary biomarker validated before in case-control studies9 and in the general population.10 HF1 is normally distributed, higher values being associated with worse outcomes.10, 24, 25
At baseline (2005–2010), venous blood samples were drawn after at least 8 hours of fasting. Carboxyterminal telopeptide of collagen I (CITP) and tissue inhibitor of the matrix metalloproteinase type I (TIMP-I) were measured as circulating markers of collagen 1 degradation in 607 participants (94.1%). CITP was quantified by a quantitative enzyme immunoassay (Orion Diagnostica, Espoo, Finland) and TIMP-I (GE Healthcare Life Sciences, Buckinghamshire, UK) by sandwich enzyme-linked immunosorbent assay.14 The detection limits were 0.3 μg/L for CITP (interassay and intra-assay coefficients of variability, 13.1% and 10.0%) and 1.25 ng/mL for TIMP-I (12.8% and 2.6%).14 In 591 participants (91.6%), N-terminal pro b-type natriuretic peptide (NT-proBNP) was also measured in plasma at baseline by a competitive enzyme immunoassay for research use (Biomedica Gruppe, Vienna, Austria).
Statistical Analysis
For database management and statistical analysis, we used the SAS system, version 9.4 (SAS Institute Inc., Cary, NC). Means were compared using the large-sample z-test and proportions by Fisher's exact test. We normalized the distributions of the energy spent in physical activity and 24-hour microalbuminuria by a logarithmic transformation. Our statistical methods also included multivariable-adjusted linear and logistic regression with as dependent variables the echocardiographic indexes reflecting diastolic LV function on a continuous or binary scale. We adjusted for baseline covariables of physiological relevance, identified in previous analyses, including sex, age, BMI, systolic and diastolic blood pressure, heart rate, serum Crt, fasting blood Glyc, and treatment with inhibitors of the renin system and β-blockers. In sensitivity analyses, we replaced BMI by waist circumference and additionally accounted for 24-microalbuminuria, energy spent in physical activity.21, 22 We determined optimal discrimination limits for HF1 by maximizing Youden's index (the maximum of sensitivity plus specificity minus 1). Finally, we assessed the incremental value of the urinary biomarkers in discriminating between normal and abnormal LV function, using the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI).26
Results
Baseline Characteristics of Participants
Of 645 participants, 326 (50.5%) were women. Mean values (standard deviation) were 50.9 ± 14.7 years for age, 26.5 ± 4.4 kg/m2 for BMI, 103.5 ± 8.9 cm for waist circumference, and 128.8 ± 16.8/79.8 ± 9.3 mm Hg for systolic/diastolic blood pressure. Of all participants, 268 (41.6%) had hypertension, of whom 160 (59.7%) were on antihypertensive drug treatment; 18 (2.8%) had a history of diabetes mellitus; and 30 (4.7%) reported previous coronary heart disease. The antihypertensive drug classes used at baseline were diuretics in 59 (9.2%), β-blockers in 100 (15.5%), inhibitors of the renin-angiotensin system in 51 (7.9%), and calcium-channel blockers in 26 (4.0%) participants. Table 1 lists the characteristics of participants by quartiles of the HF1 distribution. Age, BMI, waist circumference, systolic pressure, the prevalence of hypertension, the use of antihypertensive drugs, or being overweight, plasma Glyc, serum Crt, and TIMP-I all increased (P ≤ .004) with higher HF1 category, whereas the opposite was the case for eGFR (P < .0001). Across the HF1 quartiles, there were no differences (P ≥ .12) in the prevalence of abdominal obesity, the energy spent in physical activity, or 24-hour microalbuminuria.
Table 1.
Baseline characteristics of 645 participants by quartiles of the HF1 distribution
| Characteristic |
Categories of the Urinary HF1 Biomarker |
P | |||
|---|---|---|---|---|---|
| Limits, Score | <−1.623 | −1.623 to −1.047 | −1.046 to −0.445 | >−0.445 | |
| Number of subjects (%) | |||||
| All patients in category | 162 | 161 | 161 | 161 | |
| Women | 83 (51.2) | 84 (52.2) | 85 (52.8) | 74 (46.0) | .60 |
| Smokers | 40 (24.7) | 33 (20.5) | 22 (13.7) | 26 (16.2) | .057 |
| Drinking alcohol | 60 (37.0) | 57 (35.4) | 61 (37.9) | 60 (37.3) | .60 |
| Hypertension | 44 (27.2) | 60 (37.3) | 62 (38.5) | 102 (63.4)§ | <.0001 |
| Antihypertensive treatment | 18 (11.1) | 27 (16.8) | 38 (23.6) | 77 (47.8)§ | <.0001 |
| Body mass index ≥25 kg/m2 | 81 (50.0) | 95 (59.0) | 99 (61.5) | 125 (77.6) | <.0001 |
| Abdominal obesity | 122 (75.3) | 131 (81.4) | 126 (78.3) | 133 (82.6) | .37 |
| History of coronary heart disease | 3 (1.9) | 10 (6.2)∗ | 7 (4.4) | 14 (8.7) | .043 |
| Diabetes mellitus | 2 (1.2) | 0 | 4 (2.5)∗ | 12 (7.5)∗ | .0003 |
| Mean of characteristic | |||||
| Age, y | 44.0 ± 14.0 | 49.9 ± 14.2‡ | 52.0 ± 13.5 | 57.5 ± 13.9‡ | <.0001 |
| Body mass index, kg/m2 | 25.3 ± 3.7 | 26.2 ± 3.7∗ | 26.4 ± 4.5 | 28.3 ± 5.0‡ | <.0001 |
| Waist circumference, cm | 101.7 ± 7.8 | 102.9 ± 7.4 | 103.1 ± 9.9 | 106.2 ± 10.3† | <.0001 |
| Energy spent in physical activity, Kcal | 1725 (1300–2108) | 1788 (1400–2221) | 1781 (1367–2207) | 1809 (1411–2200) | .60 |
| Blood pressure | |||||
| Systolic pressure, mm Hg | 125.2 ± 14.0 | 128.4 ± 18.9 | 128.1 ± 16.1 | 133.3 ± 17.2† | .0003 |
| Diastolic pressure, mm Hg | 78.6 ± 8.8 | 79.6 ± 9.9 | 80.1 ± 9.2 | 81.0 ± 9.4 | .13 |
| Heart rate, beats per minute | 60.2 ± 9.1 | 60.3 ± 9.6 | 60.0 ± 9.8 | 59.8 ± 10.6 | .97 |
| Biochemical data | |||||
| Total cholesterol, mmol/L | 5.22 ± 1.51 | 5.45 ± 1.10 | 5.29 ± 0.95 | 5.29 ± 0.97 | .36 |
| Plasma glucose, mmol/L | 4.83 ± 0.52 | 4.83 ± 0.43 | 4.92 ± 0.66 | 5.10 ± 1.23 | .004 |
| Serum creatinine, μmol/L | 81.8 ± 13.4 | 83.4 ± 12.9 | 83.5 ± 13.0 | 88.1 ± 21.1∗ | .002 |
| eGFR, ml/min/1.73 m2 | 87.5 ± 14.9 | 82.1 ± 16.5† | 80.6 ± 13.8 | 75.8 ± 16.3† | <.0001 |
| 24-h microalbuminuria, mg | 5.29 (3.89–6.59) | 5.73 (3.79–7.10) | 5.83 (4.39–7.35) | 6.38 (4.30–7.91) | .12 |
| CITP, μg/L | 5.44 ± 1.62 | 5.30 ± 1.80 | 5.63 ± 2.00 | 5.82 ± 2.56 | .13 |
| TIMP-I, ng/mL | 603 ± 154 | 670 ± 192‡ | 679 ± 154 | 730 ± 224∗ | <.0001 |
| NT-proBNP, pmol/L | 212 (158–284) | 199 (143–288) | 217 (148–318) | 202 (130–284) | .51 |
eGFR, estimated glomerular filtration rate calculated according to the CKD-EPI formula; CITP, carboxyterminal telopeptide of collagen I; TIMP-I, tissue inhibitor of the matrix metalloproteinase type I; NT-proBNP, N-terminal pro b-type natriuretic peptide; SD, standard deviation.
Abdominal obesity was a waist circumference of ≥88 cm in women and ≥102 cm in men. Blood pressure was the average of five consecutive auscultatory readings. Heart rate was determined after ≥15 minutes recumbent rest. Hypertension was a blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic, or use of antihypertensive drugs. CITP and TIMP-I were available in 149, 153, 155, and 150 participants of the 1st, 2nd, 3rd, and 4th quartile, respectively (607 in total). NT-proBNP was measured in 144, 149, 150, and 148 participants of the 1st, 2nd, 3rd, and 4th quartile, respectively (591 in total). Means are arithmetic means (SD) or geometric means (interquartile range). P values denote the significance of the differences in prevalence rates or means across quartiles of the HF1 distribution.
Significance of the difference with the adjacent lower quartile: P ≤ .05.
P ≤ .01.
P ≤ .001.
P < .0001.
Continuous Analyses
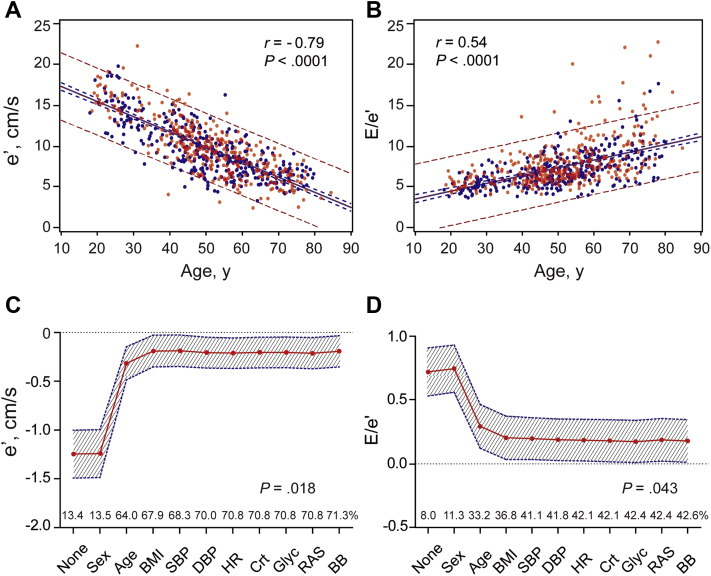
Median follow-up time was 4.8 years (interquartile range, 4.4–5.1 years; 5th–95th percentile interval, 3.7–5.4 years). Table S2 shows that the left atrial volume index, A, and a' peak velocities, and the E/e' ratio were greater (P ≤ .010) with higher baseline HF1 category, whereas the opposite was the case (P < .0001) for the E and e' peak velocities and the E/A and e'/a' ratios. e' (r = −0.79; Figure 1, panel A), E/e' (r = 0.54; Figure 1, panel B), and HF1 (r = 0.36) were strongly dependent on age (P < .0001). Stepwise cumulative adjustment for the covariables (Figure 1, panels C and D) weakened the associations of e' and E/e' at follow-up with baseline HF1, baseline age having the biggest impact (Figure 1, panels C and D). With all adjustments applied, per 1-standard deviation increment in HF1, e' was −0.193 cm/s lower (95% confidence interval [CI], −0.352 to −0.033; P = .018) and E/e' 0.174 units higher (CI, 0.005–0.342; P = .043).
Figure 1.
Simple correlations of e′ (A) and E/e′ (B) with age. In panels A and B, red and blue dots indicate women and men, respectively. Full lines represent the regression slopes, and dotted lines represent the 95% confidence boundaries for the prediction of the mean values (blue) and individual values (red) of e′ and E/e′ at any given age. Associations of e′ (C) and E/e′ (D) with HF1 weaken as covariables were stepwise introduced in the regression model but remained significant after full adjustment. The association sizes are expressed for a 1-standard deviation increment in HF1. The shaded area denotes the 95% confidence boundary of the parameter estimates. The percentage of explained variance is plotted along the horizontal axis. Stepwise cumulative adjustment was implemented for sex, age, BMI, SBP, DBP, heart rate (HR), serum creatinine (Crt), fasting blood glucose (Glyc), and treatment with inhibitors of the renin system (RAS) and β-blockers (BB).
The corresponding association sizes were −0.005 (CI, −0.159 to 0.148; P = .94) for CITP and −0.042 (CI, −0.205 to 0.120; P = .61) for TIMP-I. When in the sensitivity analysis, we replaced BMI by waist circumference and additionally adjusted for energy spent in physical activity and 24-microalbuminuria, e' was −0.215 cm/s lower (CI, −0.377 to −0.053; P = .009) and E/e' 0.179 units higher (CI, 0.009–0.349; P = .039). None of the other echocardiographic variables (E, A, E/A, e'/a') was associated with HF1, CITP, or TIMP-I.
Categorical Analyses
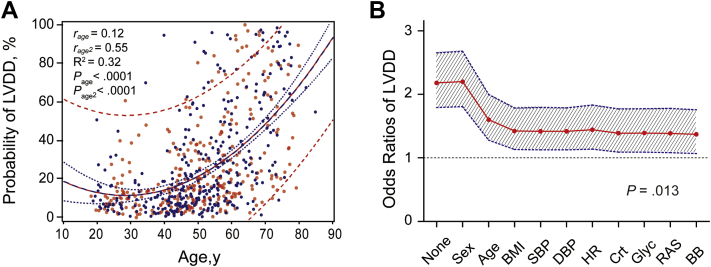
Next, we analyzed the relative risk of LVDD in relation to HF1. Of 645 participants, 179 (27.8%) had LVDD at follow-up, based on impaired relaxation in 69 patients (38.5%) or an elevated filling pressure in the presence of a normal (74 [43.8%]) or low (36 [20.1%]) age-specific E/A ratio. The probability of having LVDD curvilinearly increased with age (model R2 = 0.32; Figure 2, panel A). Age strongly influenced the parameter estimates for the association of the risk of LVDD with baseline HF1 (Figure 2, panel B). In multivariable-adjusted analyses, per 1-standard deviation increment, the odds ratios were 1.37 (CI, 1.07–1.76; P = .013) for HF1, 1.16 (CI, 0.91–1.49; P = .13) for CITP, and 1.26 (CI, 0.96–1.66; P = .09) for TIMP-I. For a doubling of NT-proBNP, the odds ratio was 1.16 (CI, 0.87–1.55; P = .30). HF1 in the presence of NT-proBNP yielded an odds ratio of 1.39 (CI, 1.07–1.81; P = .013). When in the sensitivity analysis, we replaced BMI by waist circumference and additionally adjusted for energy spent in physical activity and 24-microalbuminuria, the odds ratios were 1.41 (CI, 1.10–1.82; P = .0068) for HF1 and 1.43 (CI, 1.10–1.86; P = .0071) for HF1 in the presence of NT-proBNP.
Figure 2.
The probability of having LVDD curvilinearly increased with age (A). rage and rage2 are the partial correlation coefficients for the linear and squared terms of age and Page and Page2 the corresponding significance levels. R2 is the coefficient of multiple determination. In panel A, red and blue dots indicate women and men, respectively. The full line represents the regression slope, and the dotted lines represent the 95% confidence boundaries for the prediction of the mean probabilities (blue) and individual probabilities (red) of LVDD at any given age. The odds of having LVDD in relation to HF1 weakened as covariables were stepwise introduced in the logistic model but remained significant after full adjustment (B). Odds ratios are expressed for a 1-standard deviation increment in HF1. The shaded area denotes the 95% confidence boundary of the parameter estimates. Stepwise cumulative were implemented for sex, age, BMI, SBP, DBP, heart rate (HR), serum creatinine (Crt), fasting blood glucose (Glyc), and treatment with inhibitors of the renin system (RAS) and β-blockers (BB).
Added Diagnostic Accuracy
From baseline to follow-up, HF1 increased from −1.00 ± 0.90 to −0.86 ± 0.90 (P = .0004). At baseline, the optimized HF1 threshold was −0.350. Among all participants, sensitivity was 43.0%, and specificity was 86.1%. Of 469 participants with baseline HF1 <−0.350, 90 (19.2%) progressed to ≥ −0.350 at follow-up. Conversely, of 129 participants with baseline HF1 at baseline ≥ −0.350, 62 (48.1%) reverted to a value of < −0.350 at follow-up. When stratified by age (≥50 versus <50 years), BMI (≥25 versus <25 kg/m2) or hypertension versus normotension, sensitivity, and specificity were consistently higher in the low compared with the high-risk group, whereas the misclassification rate showed the opposite trend (Table 2). In all and aged participants, both IDI and NRI reached significance (P ≤ .024), whereas this was also the case for NRI in overweight, normal weight, and normotensive people (P ≤ .007).
Table 2.
Classification parameters by HF1 at baseline
| Groups | Classification Parameters Using −0.350 as Optimized HF1 Threshold |
||||||
|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Misclassification Rate | Integrated Discrimination Improvement (%) | Net Reclassification Improvement (%) | |
| All participants (N = 645) | 43.0 | 86.1 | 54.2 | 79.7 | 25.9 | 1.14 (0.15 to 2.13)∗ | 31.7 (14.9 to 18.5)‡ |
| Age ≥50 y (N = 345) | 45.9 | 81.4 | 67.3 | 64.3 | 34.8 | 1.66 (0.30 to 3.02)∗ | 39.2 (19.3 to 59.1)§ |
| Age <50 y (N = 300) | 77.3 | 89.2 | 85.7 | 93.6 | 15.7 | 0.07 (−0.40 to 0.54) | 19.1 (−21.5 to 59.7) |
| BMI ≥25 kg/m2 (N = 400) | 43.1 | 82.3 | 59.6 | 70.3 | 32.6 | 0.81 (−0.09 to 1.71) | 26.7 (7.31 to 46.1)† |
| BMI <25 kg/m2 (N = 245) | 57.1 | 90.4 | 36.4 | 92.5 | 15.0 | 3.75 (−0.39 to 7.88) | 48.6 (9.81 to 87.4)∗ |
| Hypertension (N = 268) | 47.3 | 76.3 | 64.9 | 60.9 | 37.7 | 0.78 (−0.29 to 1.85) | 21.0 (−2.44 to 44.4) |
| Normotension (N = 377) | 68.0 | 90.2 | 66.7 | 89.7 | 17.5 | 2.77 (−0.18 to 5.73) | 44.4 (17.8 to 71.1)† |
BMI, body mass index.
The basic reference models included as covariables sex, age, BMI, systolic and diastolic blood pressure, heart rate, serum creatinine, fasting blood glucose, and treatment with inhibitors of the renin system and β-blockers. The integrated discrimination improvement (IDI) is the difference between the discrimination slopes of basic models and basic models extended with HF1. The discrimination slope is the difference in predicted probabilities (%) between cases and controls. Cases and controls are participants with and without LVDD, respectively. The net reclassification improvement (NRI) is the sum of the percentages of participants reclassified correctly as cases and controls.
Significance: P ≤ .05.
P ≤ .01.
P ≤ .001.
P ≤ .0001.
Discussion
The objective of our present study was to evaluate whether the multidimensional urinary biomarker HF1 could discriminate over a 5-year horizon between normal LV function and mildly impaired LVDD. The key findings can be summarized as follows: (1) in continuous analyses, lower e' and greater E/e' at follow-up were associated with higher baseline HF1; (2) in categorical analyses, HF1 predicted subclinical LVDD and; (3) over a 5-years horizon, HF1 improved discrimination between people with normal and mildly impaired diastolic LV function. Two previous FLEMENGHO reports25, 27 illustrate the clinical relevance of the early diagnosis of LVDD. Higher HF125 and lower e'27 over a median follow-up of 5–6 years predicted the incidence of cardiovascular complications. An unexpected finding was that over this short follow-up period, HF1 was more predictive than systolic blood pressure.25 This observation probably reflects the time course of events, systolic blood pressure being a major driver of LVDD. In the Framingham Heart Study, remote blood pressure (average of all reading 11–20 years before current) and recent blood pressure (average of all readings 1–10 years before current) predicted cardiovascular disease incrementally over current blood pressure.28 Explanations offered by the Framingham investigators were that antecedent blood pressure is a forerunner of cardiovascular target organ damage, which is on the path to hard cardiovascular complications and that the relation between cardiovascular risk and blood pressure weakens over time, for instance by the initiation of antihypertensive drug treatment.28
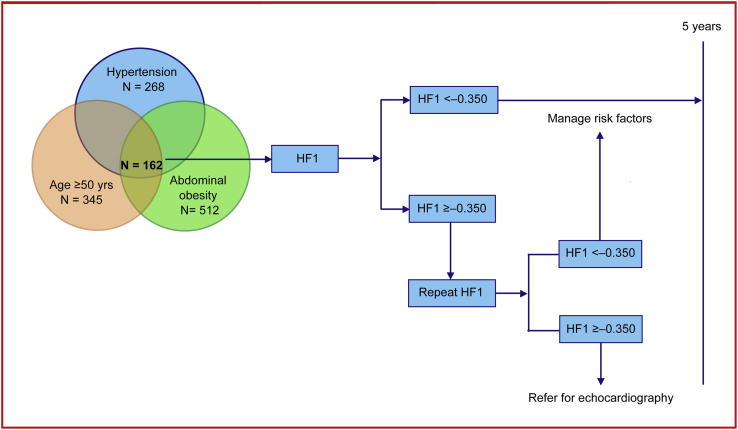
While the diagnosis of DHF remains challenging in a hospital environment, this is even more the case for asymptomatic LVDD at the point of entry in health care. Echocardiography is the diagnostic approach recommended by guidelines, which requires highly skilled observers and is costly and impossible to implement on a large scale. Hence, screening by means of biomarkers in primary care is an option to be favored. Figure 3 proposes how HF1 might be applied in clinical practice in asymptomatic high-risk individuals. In the presence of one or more clinical risk factors for LVDD, in particular the combination of seniority, overweight or abdominal obesity, and hypertension (N = 162; 25.1% of our study population), HF1 might be used as a screening tool. If the value is < −0.350, managing risk factors over a 5-year time span is the intervention to be recommended. In contrast, if HF1 is ≥ −0.350, a second test might inform the health-care provider whether continuing managing risk factors for 5 years is sufficient or whether the patient should be referred for echocardiography. An added benefit is that HF1 predicts worsening of renal function24 and the 5-year incidence of cardiovascular and cardiac events.25 NT-proBNP is the biomarker most frequently used in clinical practice, but its distribution shows large overlap between individuals with normal diastolic LV function, LVDD, or even DHF.10, 27 In our present study, in line with previous publications,3, 4, 6 NT-proBNP did not add to the prediction of LVDD over and beyond classical risk factors. Moreover, HF1 in the presence of NT-proBNP fully retained its prognostic value. Another issue to be considered is the risk assessment of individuals not at high risk. In such participants, HF1 values ≥ −0.350 tended to have higher sensitivity than in high-risk individuals (Table 2). The large amount of prognostic information carried by old age and higher BMI (Figure 1, Figure 2) explain this observation. Thus, although a positive test in low-risk individuals is predictive, it cannot be recommended, because its application on a large scale is impracticable.
Figure 3.
Proposal for the clinical application of HF1 over a 5-year horizon, pending confirmation in an independent cohort, and ultimately testing in a proof-of-concept randomized clinical trial.
A major advantage of running proteomics on urine samples is the comfort for the patient because all what is needed is a fresh mid-morning urine sample of 5 mL. Urinary proteins remain stable for a time long enough to perform the proteome analysis in a reliable manner.29 Two independent sets of experiments demonstrated that the urinary proteome does not undergo significant changes when urine is stored for 3 days at 4°C30 or for 6 hours at room temperature.31 Moreover, for studies running over several years, urine can be stored at −20°C without significant alteration of the proteome.29 The urinary proteome is well characterized, and reference standards are available.8 CE-MS that provides sufficient sensitivity and high reproducibility is capable to resolve up to 6000 different peptides per sample within approximately 45 minutes.32 On the other hand, urinary proteomic analysis remains substantially more costly than other diagnostic tests employed in the management of patients at risk of or already having LVDD. However, a cost-effectiveness analysis within the setting of the German health insurance system suggested that CKD273, a multidimensional classifier used for the early diagnosis of decline in the eGFR performs better than microalbuminuria.33 Markov models were constructed for diabetic patients free of chronic kidney disease or other diabetic complication and assumed follow-up from 45 until 85 years or death. By using CKD273 instead of microalbuminuria, the overall cost per patient was €17,567 ($20,731) lower, and the number of patients progressing to dialysis decreased by 30%.33 Whether or not the Markov models would materialize in clinical care is currently being tested in a randomized clinical trial.34
Our current and previous findings are in line with the pathophysiologic concepts underlying deterioration of diastolic LV function. In patients with LVDD, the LV wall undergoes fibrosis characterized by increased interstitial deposition12 and cross-linking of collagen I at the detriment of collagen III.13, 14 Small increases in the collagen I/III ratio augment myocardial stiffness, thereby reducing early diastolic LV filling and increasing LV filling pressure.35, 36 Of the peptides with known amino acid sequence, which make up HF1, 60.0%9 consist of dysregulated collagen fragments. Recent cross-sectional analyses of sequenced urinary peptides in FLEMENGHO participants demonstrated that LVDD was associated with higher levels of urinary collagen I fragments, lower levels of urinary collagen III degradation products, and higher levels of circulating tissue inhibitor of matrix metalloproteinase type I, an inhibitor of collagen-degrading enzymes.11 Combined, these data suggest that HF1 reflects collagen degradation, and in this way reveals relevant molecular processes that subsequently lead to remodeling of the extracellular matrix and fibrosis of the myocardium.
Strong points of our study are the assessment of Doppler indexes as early signs of subclinical LVDD and the adjustment of our analyses for a large number of covariables measured simultaneously with the urinary biomarker. What our study additionally highlighted is the strong age dependency of both diastolic LV function and the HF1 marker and providing a justification for repeating the measurement of HF1 if its value is ≥ −0.350, as is currently recommended for albuminuria in the field of chronic kidney disease.37 Of note, HF1 retained its prognostic significance over and beyond established LVDD risk factors, in particular age and abdominal obesity. In all and older participants, HF1 improved both IDI and NRI. However, our study must also be interpreted within the context of its limitations. Although as outlined cardiovascular outcome data25, 27 add a perspective to our current observations, an observational study cannot fully prove the utility of a biomarker. Further validation of HF1 as screening tool in a randomized clinical trial is therefore necessary. Such approach is presently being implemented to validate CKD27338 in the multicenter double-blind placebo-controlled PRIORITY trial (proteomic prediction and renin-angiotensin aldosterone system inhibition prevention of early diabetic nephropathy in type II diabetic patients with normal albumin excretion).34 Second, notwithstanding the consistency of the association between LVDD and HF1 in a discovery and test case-control study9 and subsequently in the general population10 and the pathophysiological plausibility in mechanistic studies,11 replication in an independent cohort would enhance the clinical relevance of our findings. Third, a sensitivity ranging from 43.0% to 77.3% might be considered as low. However, ECG is a commonly used instrument with a sensitivity of only 35% to diagnose echocardiographically confirmed LV hypertrophy.39 Finally, our present study cannot prove the cardiac origin of the urinary collagen fragments that contribute to HF1. However, in a tissue proteomic study,40 we applied liquid chromatography-tandem mass spectrometry to analyze biopsies from explanted human hearts, 15 with ischemic cardiomyopathy, 14 with dilated cardiomyopathy, and 12 healthy donor hearts discarded from implantation (control). In both ischemic and dilated cardiomyopathy, the tissue proteomic signature consistently showed higher abundance of proteins involved in the organization of the extracellular matrix, which is in agreement with the contribution of dysregulated collagen fragments to HF1.41
In conclusion, in a general population, HF1 allows screening for LVDD. Our current observations support the concept of porting easily obtainable multidimensional urinary biomarkers8 to clinical practice to enable a personalized approach to the diagnosis, prevention, and treatment of LVDD, a high-risk condition27 that affects 25% of the general population.3, 4 Such biomarkers might be particularly useful in primary health care, particularly in older high-risk patients with hypertension or abdominal obesity and serve as a decision tool informing doctors when referral for echocardiography is indicated.
Footnotes
Conflict of interest: E.N.K. is an employee of Mosaiques-Diagnostics GmbH. H.M. is the co-founder and co-owner of Mosaiques-Diagnostics GmbH. The other authors declare no conflict of interest.
Funding: The European Union (HEALTH-F7-305507 HOMAGE and the European Research Council Advanced Researcher Grant-2011-294713-EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13 and G.088013) currently support the Studies Coordinating Centre in Leuven. The authors gratefully acknowledge the clerical assistance of V De Leebeeck and R Wolfs and the technical support of L Custers, MJ Jehoul, and H Truyens in data collection.
Supplemental Material can be found at www.ashjournal.com.
Appendix
Expanded Methods
Participants collected 24-hour urine samples within 1 week of the clinical examination at the field center. Aliquots (0.7 mL) were stored at −80°C and thawed immediately before analysis. To remove higher molecular mass proteins, such as albumin and immunoglobulin G, the samples were ultrafiltered using Centrisart ultracentrifugation devices (20 kDa MWCO; Sartorius, Göttingen, Germany) at 2000 g relative centrifugal force until 1.1 mL of filtrate was obtained. This filtrate was then applied onto a PD-10 desalting column (GE Healthcare, Uppsala, Sweden) equilibrated in 0.01% NH4OH in HPLC-grade H2O (Roth, Germany) to decrease matrix effects by removing urea, electrolytes, and salts and to enrich peptides. Finally, all samples were lyophilized, stored at 4°C, and suspended in HPLC-grade H2O shortly before capillary electrophoresis coupled with mass spectrometry (CE-MS).
As described in detail elsewhere,1, 2, 3 CE-MS analyses were performed using a P/ACE MDQ capillary electrophoresis system (Beckman Coulter, Brea, California, USA) online coupled to a micrOTOF MS (Bruker Daltonic, Bremen, Germany). The ESI sprayer (Agilent Technologies, Palo Alto, CA, USA) was grounded, and the ion spray interface potential was set between 4 and 4.5 kV. Data acquisition and mass spectrometry acquisition methods were automatically controlled by the capillary electrophoresis via contact-close relays. Spectra were accumulated every 3 seconds, over a range of charge states (m/z) 350–3000. Previous publications described the accuracy, precision, selectivity, sensitivity, reproducibility, and stability of the CE-MS measurements in detail.3, 4 Mass spectra were processed using MosaiquesVisu software, including peak picking, deconvolution, and de-isotoping.5 Migration time and peak intensity were normalized, using internal polypeptide standards.6 These fragments result from normal biological processes and appear to be unaffected by any disease state studied to date based on over 30,000 samples in the Mosaiques database.7 The resulting peak list characterizes each polypeptide by its molecular mass, normalized capillary electrophoresis migration time, and normalized signal intensity. All detected polypeptides were deposited, matched, and annotated in a Microsoft SQL database, allowing further analysis and comparison of multiple patient groups.
For targeted sequencing, urine samples were analyzed on a Dionex Ultimate 3000 RSLS nano flow system (Dionex, Camberly, UK) or on a Beckman CE, interfaced with an Orbitrap Velos MS instrument (Thermo Scientific, Waltham, MA, USA).3 The data files were analyzed using Proteome Discoverer 1.2 (precursor mass tolerance, 5 ppm; fragment mass tolerance, 0.05 Da) and were searched against the UniProt human nonredundant database without enzyme specificity. No fixed modifications were selected. Oxidation of methionine and proline were considered as variable modifications. The criteria for accepting sequences were high confidence (Xcorr ≥1.9) and absence of unmodified cysteine. A strict correlation between peptide charge at the working pH of two and capillary electrophoresis migration time was used to avoid falsely characterized peptides.8
Peptide fragments identified in previous study were combined into a single summary variable, using the support-vector machine based MosaCluster software, version 1.6.5. As published previously,9 HF1 combined information from 85 peptide fragments identified in 19 patients with diastolic LV dysfunction and 19 controls.
Table S1.
List of polypeptides included in the HF1 classifier
| Polypeptide |
Cases |
Controls |
R | P-value (Unadjusted) | ||
|---|---|---|---|---|---|---|
| ID | Mass (Da) | CE Time (min) | MA (%) | MA (%) | ||
| 81,272 | 2211.98 | 33.23 | 0 (0) | 2.67 (0.42) | 0 | 1.99E-03 |
| 12,9821 | 3333.36 | 19.42 | 0 (0) | 2.39 (0.47) | 0 | 8.72E-04 |
| 8725 | 949.4 | 25.79 | 1.94 (0.05) | 2.28 (0.63) | 0.067 | 2.22E-04 |
| 123,106 | 3130.43 | 30.82 | 1.98 (0.05) | 2.63 (0.47) | 0.080 | 2.57E-03 |
| 1577 | 840.41 | 23.17 | 1.65 (0.05) | 1.85 (0.47) | 0.095 | 3.29E-03 |
| 103,493 | 2658.22 | 19.5 | 3.36 (0.05) | 3.29 (0.47) | 0.109 | 4.71E-03 |
| 44,146 | 1518.6 | 19.37 | 1.91 (0.11) | 2.49 (0.58) | 0.145 | 1.33E-03 |
| 4845 | 900.27 | 43.66 | 1.55 (0.16) | 2.44 (0.63) | 0.161 | 1.33E-03 |
| 37,610 | 1421.59 | 38.71 | 1.73 (0.11) | 1.87 (0.53) | 0.192 | 6.07E-03 |
| 83,441 | 2248.97 | 33.69 | 3.45 (0.11) | 3.56 (0.53) | 0.201 | 4.88E-03 |
| 74,703 | 2087.84 | 19.42 | 2.64 (0.11) | 2.7 (0.53) | 0.203 | 6.76E-03 |
| 101,157 | 2616.16 | 28.39 | 197 (0.11) | 1.98 (0.53) | 0.206 | 6.76E-03 |
| 103,022 | 2649.2 | 34.85 | 2.52 (0.16) | 2.56 (0.68) | 0.232 | 2.50E-03 |
| 57,360 | 1734.66 | 19.9 | 2.2 (0.16) | 2.24 (0.58) | 0.271 | 1.03E-02 |
| 46,091 | 1554.66 | 28.59 | 2.08 (0.16) | 2.24 (0.53) | 0.280 | 1.18E-02 |
| 32,022 | 1319.58 | 20.89 | 1.99 (0.21) | 2.21 (0.58) | 0.326 | 1.57E-02 |
| 102,269 | 2638.18 | 28.42 | 2.3 (0.26) | 2.49 (0.68) | 0.353 | 1.26E-02 |
| 82,708 | 2235.04 | 34.17 | 2.57 (0.32) | 2.68 (0.84) | 0.365 | 2.53E-03 |
| 188,895 | 11,967.55 | 20.47 | 2.68 (0.26) | 2.94 (0.63) | 0.376 | 9.50E-03 |
| 98,089 | 2559.18 | 19.41 | 2.97 (0.32) | 3 (0.84) | 0.377 | 3.76E-03 |
| 138143 | 3593.47 | 20.2 | 2.67 (0.26) | 2.68 (0.68) | 0.381 | 1.50E-02 |
| 167,786 | 4771.07 | 20.2 | 2.74 (0.37) | 3.13 (0.79) | 0.410 | 4.34E-03 |
| 61,984 | 1835.71 | 19.91 | 2.64 (0.53) | 3.12 (1) | 0.448 | 1.33E-04 |
| 46,369 | 1560.7 | 29.79 | 2.78 (0.32) | 2.84 (0.68) | 0.461 | 2.27E-02 |
| 143,947 | 3801.77 | 33.46 | 2.26 (0.37) | 2.24 (0.79) | 0.473 | 2.67E-02 |
| 39,275 | 1445.62 | 37.36 | 2.59 (0.47) | 2.96 (0.79) | 0.521 | 4.87E-03 |
| 56,493 | 1716.66 | 20.18 | 2.56 (0.47) | 2.74 (0.79) | 0.556 | 2.11E-02 |
| 41,972 | 1478.61 | 39.3 | 2.75 (0.53) | 2.95 (0.84) | 0.588 | 3.16E-03 |
| 24,168 | 1195.48 | 37.51 | 2.8 (0.58) | 3.26 (0.84) | 0.593 | 3.12E-03 |
| 107,858 | 2751.34 | 29.23 | 2.36 (0.63) | 2.69 (0.89) | 0.621 | 3.00E-03 |
| 23,356 | 1179.52 | 37.49 | 2.63 (0.58) | 2.9 (0.84) | 0.626 | 2.67E-02 |
| 97,599 | 2547.99 | 21.44 | 2.59 (0.58) | 2.66 (0.89) | 0.635 | 3.15E-02 |
| 8695 | 949.22 | 34.33 | 2.46 (0.53) | 3.01 (0.68) | 0.637 | 2.78E-02 |
| 23,697 | 1186.53 | 22.39 | 2.8 (0.68) | 2.88 (1) | 0.661 | 2.08E-02 |
| 36,566 | 1401.38 | 36.56 | 2.77 (0.58) | 3.27 (0.74) | 0.664 | 8.74E-03 |
| 153,832 | 4196.75 | 20.84 | 2.41 (0.68) | 2.59 (0.95) | 0.666 | 4.93E-03 |
| 26,670 | 1235.56 | 26.65 | 3.02 (0.63) | 3.3 (0.84) | 0.686 | 1.08E-02 |
| 58,050 | 1749.81 | 30.61 | 2.57 (0.63) | 2.79 (0.84) | 0.691 | 3.04E-02 |
| 28,005 | 1255.48 | 35.77 | 3.08 (0.68) | 3.4 (0.84) | 0.733 | 3.19E-02 |
| 159,396 | 4409.89 | 20 | 2.72 (0.74) | 3.23 (0.84) | 0.742 | 2.68E-02 |
| 69,979 | 1996.79 | 20.98 | 2.86 (0.79) | 3.17 (0.95) | 0.750 | 8.53E-03 |
| 40,737 | 1462.62 | 39.42 | 3.33 (0.84) | 3.68 (1) | 0.760 | 2.62E-04 |
| 65,368 | 1901.82 | 43.83 | 3.17 (0.79) | 3.61 (0.89) | 0.779 | 1.52E-02 |
| 128,086 | 3286.55 | 30.92 | 3.13 (0.79) | 3.51 (0.89) | 0.792 | 6.91E-04 |
| 73,434 | 2067.82 | 20.62 | 3.1 (0.84) | 3.28 (1) | 0.794 | 1.42E-02 |
| 148,086 | 3986.65 | 20.6 | 3.53 (0.84) | 3.82 (0.95) | 0.817 | 2.75E-03 |
| 108,574 | 2764.21 | 42.63 | 3.56 (0.79) | 3.85 (0.89) | 0.821 | 2.43E-02 |
| 90,344 | 2377.1 | 20.8 | 3.12 (0.89) | 3.46 (0.95) | 0.845 | 1.95E-02 |
| 36,759 | 1405.61 | 39.04 | 2.94 (0.89) | 3.18 (0.95) | 0.866 | 1.02E-02 |
| 147,541 | 3968.6 | 21.09 | 3.14 (0.89) | 3.57 (0.89) | 0.880 | 1.77E-03 |
| 28,561 | 1265.59 | 27.09 | 3.36 (0.89) | 3.79 (0.89) | 0.887 | 1.10E-02 |
| 107,460 | 2742.25 | 28.98 | 2.91 (0.95) | 3.11 (1) | 0.889 | 1.19E-02 |
| 32,171 | 1321.59 | 28.37 | 4.07 (0.95) | 4.27 (1) | 0.906 | 1.82E-02 |
| 39,322 | 1446.64 | 39.43 | 3.2 (1) | 3.49 (1) | 0.917 | 3.19E-02 |
| 35,339 | 1378.61 | 28.82 | 3.36 (1) | 3.53 (1) | 0.952 | 1.54E-02 |
| 81,196 | 2210.95 | 33.61 | 3.72 (1) | 13.59 () | 1.036 | 2.15E-02 |
| 41,601 | 1469.67 | 23.69 | 3.72 (1) | 3.56 (1) | 1.045 | 2.33E-02 |
| 62,866 | 1854.81 | 40.92 | 3.89 (1) | 3.71 (1) | 1.048 | 1.98E-02 |
| 99,021 | 2570.19 | 42.56 | 3.88 (1) | 3.7 (1) | 1.049 | 1.19E-02 |
| 79,136 | 2175 | 33.28 | 3.74 (1) | 3.49 (1) | 1.072 | 1.09E-02 |
| 50,840 | 1623.73 | 24.12 | 4.17 (0.95) | 3.86 (0.95) | 1.080 | 9.77E-03 |
| 72,533 | 2046.92 | 32.58 | 3.49 (0.95) | 3.21 (0.95) | 1.087 | 1.06E-02 |
| 57,537 | 1737.78 | 23.73 | 4.02 (1) | 3.82 (0.95) | 1.108 | 2.15E-02 |
| 50,212 | 1613.82 | 23.99 | 2.7 (0.89) | 2.43 (0.89) | 1.111 | 3.30E-02 |
| 60,149 | 1794.8 | 23.92 | 3.72 (1) | 3.47 (0.95) | 1.128 | 6.20E-03 |
| 103,198 | 2654.19 | 23.92 | 2.94 (0.89) | 2.47 (0.89) | 1.190 | 5.52E-03 |
| 104,786 | 2679.2 | 23.53 | 3.58 (1) | 3.34 (0.89) | 1.204 | 7.89E-03 |
| 33,135 | 1338.6 | 23.99 | 2.86 (1) | 2.65 (0.89) | 1.213 | 1.20E-02 |
| 73,291 | 2064.92 | 24.46 | 2.75 (0.84) | 2.37 (0.79) | 1.234 | 3.25E-02 |
| 45,021 | 1532.62 | 26.35 | 2.82 (1) | 2.55 (0.89) | 1.243 | 1.67E-02 |
| 99,475 | 2577.25 | 24.67 | 2.78 (0.95) | 0.892.38 () | 1.247 | 6.05E-03 |
| 40,294 | 1452.66 | 23.61 | 2.85 (1) | 0.842.62 () | 1.295 | 2.17E-03 |
| 35,424 | 1380.64 | 23.83 | 2.79 (0.95) | 0.792.56 () | 1.311 | 7.17E-03 |
| 131,294 | 3375.57 | 31.92 | 2.87 (1) | 2.71 (0.79) | 1.341 | 1.80E-02 |
| 111,564 | 2841.26 | 24.54 | 3.21 (0.89) | 2.67 (0.79) | 1.354 | 4.98E-03 |
| 104,195 | 2663.2 | 23.51 | 2.61 (0.89) | 2.29 (0.74) | 1.371 | 2.07E-02 |
| 28,747 | 1268.57 | 27.25 | 3.44 (1) | 3.32 (0.74) | 1.400 | 1.01E-02 |
| 44,802 | 1526.69 | 23.92 | 2.51 (0.79) | 2.1 (0.63) | 1.499 | 1.10E-02 |
| 113,452 | 2889.35 | 24.08 | 2.47 (0.89) | 2.29 (0.58) | 1.655 | 7.34E-03 |
| 69,681 | 1989.88 | 32.44 | 2.43 (0.84) | 2.51 (0.42) | 1.936 | 2.03E-02 |
| 55,516 | 1696.72 | 23.95 | 2.54 (0.79) | 2.39 (0.42) | 1.999 | 1.59E-02 |
| 80,360 | 2196.02 | 33.16 | 2.74 (0.68) | 2.73 (0.26) | 2.625 | 1.15E-02 |
| 82,784 | 2236.98 | 27.14 | 2.28 (0.63) | 2.31 (0.21) | 2.961 | 1.29E-02 |
| 56,806 | 1723.52 | 37.74 | 2.31 (0.53) | 2.52 (0.11) | 4.417 | 1.03E-02 |
| 129,182 | 3320.51 | 24.25 | 2.07 (0.47) | 2.1 (0.05) | 9.266 | 4.71E-03 |
ID, polypeptide identifier (SQL number); %, percentage of samples, in which the polypeptide could be detected; MA, mean signal amplitude of the polypeptides.
R was calculated as ∑ (ln signal amplitude × frequency/number of participants) in controls divided by ∑ (ln signal amplitude × frequency/number of participants) in cases. The polypeptides were ordered by ascending R. Published under CC BY-NC-ND license.
Table S2.
Echocardiographic measurements by quartiles of the HF1 distribution
| Characteristic |
Categories of the Urinary HF1 Biomarker |
|||
|---|---|---|---|---|
| Limits, Score | <−1.623 | −1.623 to −1.047 | −1.046 to −0.445 | >−0.445 |
| Conventional echocardiography | ||||
| Left atrial volume index, mL/m2 | 24.6 ± 5.46 | 26.2 ± 6.79∗ | 26.2 ± 7.32 | 27.5 ± 7.22 |
| Left ventricular mass index, g/m2 | 89.6 ± 16.7 | 97.0 ± 21.4‡ | 96.6 ± 21.2 | 104.3 ± 24.1† |
| Doppler data | ||||
| E peak, cm/s | 70.8 ± 14.8 | 68.6 ± 15.8 | 65.9 ± 15.4 | 62.1 ± 15.0∗ |
| A peak, cm/s | 56.4 ± 13.8 | 60.6 ± 15.9∗ | 62.2 ± 14.5 | 65.5 ± 15.7 |
| E/A ratio | 1.35 ± 0.50 | 1.21 ± 0.44∗ | 1.12 ± 0.40 | 1.01 ± 0.40∗ |
| e′ peak, cm/s | 11.4 ± 3.26 | 10.0 ± 3.44‡ | 9.40 ± 2.98 | 8.14 ± 3.11‡ |
| a′ peak, cm/s | 9.08 ± 2.02 | 9.66 ± 2.06∗ | 9.75 ± 2.32 | 9.77 ± 2.03 |
| e′/a′ ratio | 1.40 ± 0.71 | 1.14 ± 0.59‡ | 1.07 ± 0.55 | 0.90 ± 0.45† |
| E/e′ ratio | 6.56 ± 1.81 | 7.44 ± 2.79‡ | 7.44 ± 2.06 | 8.36 ± 3.00† |
Significance of the difference with the adjacent lower quartile: P ≤ .05.
P ≤ .01.
P ≤ .001.
References
- 1.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 2.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Kuznetsova T., Herbots L., López B., Jin Y., Richart T., Thijs L. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 4.Kloch-Badelek M., Kuznetsova T., Sakiewicz W., Tikhonoff V., Ryabikov A., González A., on behalf of the European Project on Genes in Hypertension (EPOGH) Investigators Prevalence of diastolic left ventricular dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovasc Ultrasound. 2012;10:10. doi: 10.1186/1476-7120-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuznetsova T., Thijs L., Knez J., Cauwenberghs N., Petit T., Gu Y.M. Longitudinal changes in left ventricular diastolic dysfunction in a general population. Circ Cardiovasc Imaging. 2015;8:e002882. doi: 10.1161/CIRCIMAGING.114.002882. [DOI] [PubMed] [Google Scholar]
- 6.Redfield M.M. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–1877. doi: 10.1056/NEJMcp1511175. [DOI] [PubMed] [Google Scholar]
- 7.Jantos-Siwy J., Schiffer E., Brand K., Schumann G., Rossing K., Delles C. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res. 2009;8:268–281. doi: 10.1021/pr800401m. [DOI] [PubMed] [Google Scholar]
- 8.Mischak H., Kolch W., Aivalotis M., Bouyssie D., Court M., Dihazi H. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin Appl. 2010;4:464–478. doi: 10.1002/prca.200900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuznetsova T., Mischak H., Mullen W., Staessen J.A. Urinary proteome analysis in hypertensive patients with left ventricular diastolic dysfunction. Eur Heart J. 2012;33:2342–2350. doi: 10.1093/eurheartj/ehs185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z.Y., Staessen J.A., Thijs L., Gu Y., Liu Y., Jacobs L. Left ventricular diastolic function in relation to the urinary proteome: a proof-of-concept study in a general population. Int J Cardiol. 2014;176:158–165. doi: 10.1016/j.ijcard.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z.Y., Ravassa S., Yang W.Y., Petit T., Pejchinovski M., Zürbig P. Diastolic left ventricular function in relation to urinary and serum collagen biomarkers in a general population. PLoS One. 2016;11:e0167582. doi: 10.1371/journal.pone.0167582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess O.M., Schneider J., Koch R., Bamert C., Grimm J., Krayenbuehl H.P. Diastolic function and myocardial structure in patients with myocardial hypertrophy. Circulation. 1981;63:360–371. doi: 10.1161/01.cir.63.2.360. [DOI] [PubMed] [Google Scholar]
- 13.Weber K.T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 14.López B., Ravassa S., González A., Zubillaga E., Bonavila C., Bergés M. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol. 2016;67:251–260. doi: 10.1016/j.jacc.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 15.Wei F.F., Drummen N.E.A., Schutte A.E., Thijs L., Jacobs L., Petit T. Vitamin K dependent protection of renal function in multi-ethnic population studies. EBioMedicine. 2016;4:162–169. doi: 10.1016/j.ebiom.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W.Y., Efremov L., Mujaj B., Zhang Z.Y., Wei F.F., Huang Q.F. Association of office and ambulatory blood pressure with blood lead in workers prior to occupational exposure. J Am Soc Hypertens. 2018;12:14–24. doi: 10.1016/j.jash.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–374. [PMC free article] [PubMed] [Google Scholar]
- 18.Mancia G., Fagard R., Narkiewicz K., Redón J., Zanchetti A., Böhm M. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro A.F., III, Feldman H.I., for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 21.Staessen J.A., Fagard R., Amery A. Life style as a determinant of blood pressure in the general population. Am J Hypertens. 1994;7:685–694. doi: 10.1093/ajh/7.8.685. [DOI] [PubMed] [Google Scholar]
- 22.Åstrand P.O., Rodahl K. McGraw-Hill Book Company; New York, NY: 1986. Textbook of work physiology. Physiological bases of exercise; pp. 486–522. [Google Scholar]
- 23.Gottdiener J.S., Bednarz J., Devereux R., Gardin J., Klein A., Manning W.J. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. A report from the American Society of Echocardiography's guidelines and standard committee and the task force on echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y.M., Thijs L., Liu Y.P., Zhang Z.Y., Jacobs J., Koeck T. The urinary proteome as correlate and predictor of renal function in a population study. Nephrol Dial Transplant. 2014;29:2260–2268. doi: 10.1093/ndt/gfu234. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z.Y., Thijs L., Petit T., Gu Y.M., Jacobs L., Yang W.Y. The urinary proteome and systolic blood pressure as predictors of 5-year cardiovascular and cardiac outcomes in a general population. Hypertension. 2015;66:52–60. doi: 10.1161/HYPERTENSIONAHA.115.05296. [DOI] [PubMed] [Google Scholar]
- 26.Pencina M.J., D'Agostino R.B., Sr., D'Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 27.Kuznetsova T., Thijs L., Knez J., Herbots L., Zhang Z., Staessen J.A. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc. 2014;3:e000789. doi: 10.1161/JAHA.114.000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasan R.S., Massaro J.M., Wilson P.W.F., Seshadri S., Wolf P.A., Levy D. Antecedent blood pressure and risk of cardiovascular disease: the Framingham heart study. Circulation. 2002;105:48–53. doi: 10.1161/hc0102.101774. [DOI] [PubMed] [Google Scholar]
- 29.Fliser D., Novak J., Thongboonkerd V., Argilés A., Jankowski V., Girolami M.A. Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol. 2007;18:1057–1071. doi: 10.1681/ASN.2006090956. [DOI] [PubMed] [Google Scholar]
- 30.Schaub S., Wilkins J., Weiler T., Sangster K., Rush D., Nickerson P. Urine protein profiling with surface-enhanced laser-desorption/ionization time-of-flight mass spectrometry. Kidney Int. 2004;65:323–332. doi: 10.1111/j.1523-1755.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 31.Theodorescu D., Wittke S., Ross M.M., Walden M., Conaway M., Just I. Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol. 2006;7:230–240. doi: 10.1016/S1470-2045(06)70584-8. [DOI] [PubMed] [Google Scholar]
- 32.Mischak H., Coon J.J., Novak J., Weissinger E.M., Schanstra J.P., Dominiczak A.F. Capillary electrophoresis-mass spectrometry as a powerful tool in biomarker discovery and clinical diagnosis: an update of recent developments. Mass Spectrom Rev. 2009;28:703–724. doi: 10.1002/mas.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahlmann F.H., Critselis E., Pontillo C., Fluehe L. Comparison of the cost-effectiveness of the urinary based CKD273 biomarker panel and current clinical practices in the management of chronic kidney disease progression. Nephrol Dial Transplant. 2015;30(Suppl 3):iii303. [Google Scholar]
- 34.Lindhardt M., Persson F., Currie G., Pontillo C., Beige J., Delles C. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): essential study design and rationale of a randomised clinical multicentre trial. Br Med J Open. 2016;6:e010310. doi: 10.1136/bmjopen-2015-010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauschinger M., Knopf D., Petschauer S., Doerner A., Poller W., Schwimmbeck P.L. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- 36.Westermann D., Lindner D., Kasner M., Zietsch C., Sawatis K., Escher F. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 37.KDIGO Board members KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 38.Nkuipou-Kenfack E., Zürbig P., Mischak H. The long path towards implementation of clinical proteomics: exemplified based on CKD273. Proteomics Clin Appl. 2016;11:5–6. doi: 10.1002/prca.201600104. [DOI] [PubMed] [Google Scholar]
- 39.Chaturvedi N., Athanassopoulos G., McKeigue P.M., Marmot M.G., Nihoyannopoulos P. Echocardiographic measures of left ventricular structure and their relation with rest and ambulatory blood pressure in blacks and whites in the United Kingdom. J Am Coll Cardiol. 1994;24:1499–1505. doi: 10.1016/0735-1097(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 40.Huang Q.F., Trenson S., Zhang Z.Y., Yang W.Y., Van Aelst L., Nkuipou-Kenfack E. Urinary proteomics in predicting heart transplantation outcomes (uPROPHET) - rationale and database description. PLoS One. 2017;12:e0184443. doi: 10.1371/journal.pone.0184443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z.Y., Trenson S., Yang W.Y., Zoidakis J., Nkuipou-Kenfack E., Huang Q.F. OMICS as Tool to Address the Burden of Non-Communicable Age-Related Disease in Populations in Epidemiological Transition. Leuven University Press; Leuven, Belgium: 2017. Myocardial proteomic signatures in end-stage dilated and ischemic cardiomyopathy compared with normal human hearts; pp. 315–375. [Google Scholar]
References
- 1.Zhang Z.Y., Staessen J.A., Thijs L., Gu Y., Liu Y., Jacobs L. Left ventricular diastolic function in relation to the urinary proteome: a proof-of-concept study in a general population. Int J Cardiol. 2014;176:158–165. doi: 10.1016/j.ijcard.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z.Y., Thijs L., Petit T., Gu Y.M., Jacobs L., Yang W.Y. The urinary proteome and systolic blood pressure as predictors of 5-year cardiovascular and cardiac outcomes in a general population. Hypertension. 2015;66:52–60. doi: 10.1161/HYPERTENSIONAHA.115.05296. [DOI] [PubMed] [Google Scholar]
- 3.Klein J., Papadopoulos T., Mischak H., Mullen W. Comparison of CE-MS/MS and LC-MS/MS sequencing demonstrates significant complementarity in natural peptide identification in human urine. Electrophoresis. 2014;35:1060–1064. doi: 10.1002/elps.201300327. [DOI] [PubMed] [Google Scholar]
- 4.Mischak H., Vlahou A., Ioannidis J.P. Technical aspects and inter-laboratory variability in native peptide profiling: the CE-MS experience. Clin Biochem. 2013;46:432–443. doi: 10.1016/j.clinbiochem.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Neuhoff N.V., Kaiser T., Wittke S., Krebs R., Pitt A., Burchard A. Mass spectrometry for the detection of differentially expressed proteins: a comparison of surface-enhanced laser desorption/ionization and capillary electrophoresis/mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:149–156. doi: 10.1002/rcm.1294. [DOI] [PubMed] [Google Scholar]
- 6.Jantos-Siwy J., Schiffer E., Brand K., Schumann G., Rossing K., Delles C. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res. 2009;8:268–281. doi: 10.1021/pr800401m. [DOI] [PubMed] [Google Scholar]
- 7.Coon J.J., Zürbig P., Dakna M., Dominiczak A.F., Decramer S., Fliser D. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteomics Clin Appl. 2008;2:964–973. doi: 10.1002/prca.200800024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zürbig P., Renfrow M.B., Schiffer E., Novak J., Walden M., Wittke S. Biomarker discovery by CE-MS enables sequence analysis via MS/MS with platform-independent separation. Electrophoresis. 2006;27:2111–2125. doi: 10.1002/elps.200500827. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsova T., Mischak H., Mullen W., Staessen J.A. Urinary proteome analysis in hypertensive patients with left ventricular diastolic dysfunction. Eur Heart J. 2012;33:2342–2350. doi: 10.1093/eurheartj/ehs185. [DOI] [PMC free article] [PubMed] [Google Scholar]