Abstract
Accelerated epigenetic aging, the difference between the DNA methylation-predicted age (DNAm age) and the chronological age, is associated with a myriad of diseases. This study investigates the relationship between epigenetic aging and risk and protective factors of PTSD. Genome-wide DNA methylation analysis was performed in 211 individuals including combat-exposed Australian veterans (discovery cohort, n = 96 males) and trauma-exposed civilian males from the Grady Trauma Project (replication cohort, n = 115 males). Primary measures included the Clinician Administered PTSD Scale for DSM-5 and the Connor-Davidson Resilience Scale (CD-RISC). DNAm age prediction was performed using the validated epigenetic clock calculator. Veterans with PTSD had increased PTSD symptom severity (P-value = 3.75 × 10−34) and lower CD-RISC scores (P-value = 7.5 × 10−8) than veterans without PTSD. DNAm age was significantly correlated with the chronological age (P-value = 3.3 × 10−6), but DNAm age acceleration was not different between the PTSD and non-PTSD groups (P-value = 0.24). Evaluating potential protective factors, we found that DNAm age acceleration was significantly associated with CD-RISC resilience scores in veterans with PTSD, these results remained significant after multiple testing correction (P-value = 0.023; r = 0.32). This finding was also replicated in an independent trauma-exposed civilian cohort (P-value = 0.02; r = 0.23). Post-hoc factor analyses revealed that this association was likely driven by “self-efficacy” items within the CD-RISC (P-value = 0.015; r = 0.35). These results suggest that among individuals already suffering from PTSD, some aspects of increased resilience might come at a biological cost.
Keywords: Epigenetic aging, Biomarkers, Veterans, Stress, PTSD, Resilience
1. Introduction
Posttraumatic stress disorder (PTSD) is a severely debilitating disorder that can develop after experiencing a traumatic event such as sexual assault, natural disaster, life-threatening accidents or combat exposure. Most people experience at least one traumatic event during their lifetime, however only around 14% of those exposed to traumatic events develop PTSD (Yehuda, 1999). A key unresolved question in PTSD research is why certain individuals are at risk of developing PTSD after traumatic exposure, while others appear to be more resilient to the effects of trauma (Voges and Romney, 2003; Yehuda, 2004). Several risk factors for PTSD such as family history of psychiatric disease, childhood trauma, sociodemographic and socioeconomic factors have been established (Breslau, 1999; Breslau and Davis, 1992; Brewin et al., 2000). In addition, psychological features including hostility, neuroticism and better social functioning have also been identified as predictors of PTSD symptoms (McNally, 2003; McNally et al., 2003; Yuan et al., 2011).
How traumatic events are cognitively processed and interpreted influences adjustment and the subsequent development of PTSD (Ehlers and Clark, 2000; Lee et al., 2016). Allostasis is the active process by which the body responds to daily events and maintain homeostasis while allostatic load or overload is the wear and tear resulting from either too much stress or from inefficient management of allostasis, such as not turning off the stress response when it is no longer needed (McEwen, 2004, 2017; McEwen and Gianaros, 2011). In the context of allostasis, resilience is broadly defined as the ability to adapt successfully in the face of adversity, trauma, tragedy or significant threat (Charney, 2004; Feder et al., 2009). Individuals who are more resilient are less stressed, less lonely, have better social adaptation skills, and experience greater psychological comfort, therefore resilience has overall positive effects on mental health (Lee et al., 2016). While the majority of research has aimed to identify risk factors for PTSD, fewer studies have specifically looked at resilience in PTSD and how it might moderate the risk of disease. In summary, the neurobiology of risk and resilience in PTSD is dynamic, complex and multilayered with a wide-range of interacting factors.
A major research avenue in the field of PTSD is understanding the biology underlying the disorder. For PTSD, it is clear that both genetic and environmental factors interact with each other to influence disease risk (Klengel et al., 2014). The influence of environmental factors on the genome can occur via different means, including epigenetic processes. Epigenetics involves functional alterations in the chromatin structure that can trigger long-lasting modifications in gene expression without creating changes in the DNA sequence (Dudley et al., 2011). Among different epigenetic processes such as DNA methylation and histone methylation and acetylation, DNA methylation is one of the common epigenetic mechanism that is involved in physical and mental health and wellbeing (Alegria-Torres et al., 2011; Mitchell et al., 2016; Szyf et al., 2016). Recently, we performed a genome-wide study in veterans and identified novel genes that showed DNA methylation differences in PTSD (Mehta et al., 2017). Another aspect of genome-wide epigenetic modifications seen in several disorders, including stress-related disorders, has been demonstrated by the seminal work of Horvath (2013) who described a robust ‘epigenetic clock’, i.e. a DNA-methylation based predictor of aging. Horvath found a composite multi-tissue predictor comprised of 353 cytosine-phosphate-guanosine sites (CpGs) across the genome (‘epigenetic clock’) that was shown to strongly correlate with chronological age across multiple tissues in humans (Horvath, 2013), suggesting its usefulness as a biomarker in aging-related research. Using this predictor, accelerated epigenetic aging (Δ - age), defined as the difference between DNA methylation-predicted age (DNAm age) and chronological age, has been associated with aging-related and other phenotypes, including obesity, Down syndrome, Parkinson's disease, Alzheimer's and mortality (Chen et al., 2016; Horvath et al., 2015; Horvath and Ritz, 2015; Levine et al., 2015; Marioni et al., 2015; Nevalainen et al., 2017; Quach et al., 2017). Furthermore, another epigenetic age predictor was developed by Hannum and colleagues, using 71 CpG sites that was optimized for whole blood samples (Hannum et al., 2013). The correlations between the Horvath and Hannum age predictors have been shown to be fairly strong, with correlations of up to 0.76 (Chen et al., 2016). We and others previously used the Horvath epigenetic age predictor and demonstrated that cumulative lifetime stress accelerated epigenetic aging, an effect that was driven by glucocorticoid-induced epigenetic changes (Zannas et al., 2015). Therefore, epigenetic aging is likely to be a key mechanism linking chronic stress with accelerated aging and heightened disease risk for stress-related disorders. In the same study, we found no significant association between PTSD and Horvath DNA methylation age (Zannas et al., 2015). To the best of our knowledge, only a handful of other studies have tested the association between PTSD and epigenetic aging (Boks et al., 2015; Wolf et al., 2016, 2018), while the role of epigenetic aging in resilience in humans has not yet been studied. In the longitudinal study of Dutch military personnel deployed to Afghanistan, the authors used the Horvath epigenetic age predictor and demonstrated that trauma was not associated with decreased telomere length but was associated with accelerated DNAm aging. However, contrary to author expectations, PTSD symptoms were associated with increased telomere length and decreased epigenetic aging (Boks et al., 2015). In the second study, the authors assessed both the Horvath and the Hannum epigenetic age predictors and found that the Hannum DNAm age was associated with lifetime PTSD severity and accelerated DNAm age was associated with reduced integrity of the corpus callosum genu and poor working memory performance (Wolf et al., 2016). In a recent study, Wolf and colleagues (Wolf et al., 2018) found that only PTSD hyperarousal symptoms but not total PTSD symptom severity nor trauma exposure was associated with accelerated epigenetic aging. Morevoer, the authors reported that accelerated epigenetic aging was also associated with a 13% increased risk for all-cause mortality over a 6.5 year medical record review period (Wolf et al., 2018).
To date, no study has interrogated the association between epigenetic aging and protective factors in stress-related disorders. Therefore, the aim of the present study was to investigate the relationship between epigenetic aging and PTSD and identify underlying risk and potential protective factors.
2. Methods
2.1. Samples
The individuals included in the study were part of a larger cohort of Vietnam veterans (N = 299) recruited by the Gallipoli Medical Research Foundation (GMRF) at Greenslopes Private Hospital (GPH). Clinical and life experience data, including psychiatric and physical health diagnoses and combat-trauma exposure has been collected for these veterans (McLeay et al., 2017). Interview-based data has been supplemented by pre-military and military data from Army records, including information about combat exposure. The study was approved by the Greenslopes Research and Ethics Committee and Queensland University of Technology (QUT) Human Research Ethics Committee and all participants provided written informed consent.
2.2. Clinical assessments
Structured clinical history included demographics and information regarding smoking, diet and exercise, lifetime history of alcohol consumption, past and current illnesses, medications and family medical history. Structured military and combat history questions such as term of service, number of times served, role and duration in the defence force were also administered for the veterans.
Severity of PTSD symptoms was assessed by clinical psychologists using the Clinician Administered PTSD Scale for DSM-5 (CAPS-5) (Weathers et al., 2014) which is a gold-standard for PTSD assessment. Half the veterans had current PTSD symptoms and 25% meet DSM-5 diagnostic criteria for current PTSD. Of this larger sample of 299 veterans, for the current study we selected a sub-sample of veterans with high levels of PTSD symptom severity (n = 48) and veterans with low levels of PTSD symptom severity and no previous PTSD diagnosis (n = 48) as cases and controls respectively, to increase the power to detect biological differences between the groups. Care was taken to match the case-control groups for environmental factors and demographics (Table 1).
Table 1.
Demographics and characteristics of the 96 veterans included in the study.
| Phenotype | Mean [SE]/N [%] |
P-value |
||
|---|---|---|---|---|
| Overall sample (n = 96) | Non PTSD (n = 48) | PTSD (n = 48) | PTSD group difference | |
| Service type: Army | 79 [82.3%] | 37 | 42 | 0.408 |
| : Airforce | 14 [14.6%] | 9 | 5 | |
| : Navy | 3 [3.1%] | 2 | 1 | |
| Age (in years) | 68.67 [0.45] | 69.40 [0.64] | 67.94 [0.61] | 0.102 |
| DNAm Age (in years) | 75.82 [0.53] | 75.66 [0.78] | 76.0 [0.72] | 0.754 |
| Accelerated epigenetic aging (delta age) | 7.16 [0.51] | 6.27 [0.66] | 8.06 [0.77] | 0.47 |
| BMI | 29.53 [0.46] | 28.99 [0.35] | 30.5 [0.37] | 0.38 |
| Marital status: Married | 82 [85%] | 41 [85.4%] | 42 [87.5%] | 0.433 |
| : Divorced | 8 [8.3%] | 4 [8.3%] | 4 [8.3%] | |
| : Other (Single/Widowed) | 5 [5.2%] | 3 [6.3%] | 2 [4.2%] | |
| Children | 76 [79%] | 40 [83%] | 36 [75%] | 0.177 |
| Employment status: Retired | 63 [66%] | 33 [68.8%] | 30 [62.5%] | 0.433 |
| : Full-time working | 8 [8%] | 6 [12.5%] | 2 [4.2%] | |
| : Part-time working | 10 [10%] | 5 [10.4%] | 5 [10.4%] | |
| : Other | 15 [16%] | 4 [8.3%] | 11 [22.9%] | |
| PTSD Symptom Severity score (CAPS-5) | 13.55 [1.42] | 2.52 [0.32] | 15.64 [0.78] | 3.75 × 10−34 |
| CD-RISC total score (Resilience) | 72.87 [1.59] | 79.12 [0.94] | 68.28 [1.22] | 7.5 × 10−8 |
| MoCA total score (Montral cognitive assessment) | 26.14 [0.27] | 26.53 [0.19] | 25.98 [0.21] | 0.3 |
| DASS21 Depression score (Depression anxiety stress scale 21) | 4.83 [0.53] | 2.25 [0.23] | 6.41 [0.39] | 9.47 × 10−13 |
| DASS21 Anxiety score (Depression anxiety stress scale 21) | 4.49 [0.51] | 1.24 [0.15] | 6.14 [0.35] | 1.98 × 10−17 |
| DASS21 Stress score (Depression anxiety stress scale 21) | 8.09 [0.61] | 4.34 [0.29] | 10.42 [0.38] | 1.05 × 10−16 |
| PHQ9 (Patient health questionnaire 9) total score | 6.61 [0.65] | 2.83 [0.28] | 8.37 [0.44] | 7.25 × 10−17 |
| Suicide ideation current | 14 [15%] | 0 [0%] | 14 [29.2%] | 4 × 10−5 |
| Alcohol abuse current | 2 [2%] | 0 [0%] | 2 [4.2%] | 0.439 |
| Current smoker | 6 [6%] | 1 [2.1%] | 5 [10.4%] | 0.102 |
| Current medications | 32 [33%] | 2 [4.2%] | 30 [62.5%] | 1.2 × 10−10 |
Common comorbidities were assessed using the Mini International Neuropsychiatric Interview DSM IV (MINI), an instrument designed to assess major Axis 1 disorders with high validity and reliability (Lecrubier et al., 1997; Sheehan et al., 1997). The Depression Anxiety Stress Scale 21 (DASS-21) is a self-report scale that measures through subscales three different constructs: stress, depression and anxiety (Lovibond and Lovibond, 1995). Higher scores for DASS-21 reflect increased symptoms of depression, anxiety and stress, respectively. The Cronbach's Alpha DASS-21 was high with an α = 0.95. The Connor-Davidson Resilience Scale (CD RISC) was used to measure resilience via a range of coping strategies that have been shown to be successful mediators in dealing with adversity (Connor and Davidson, 2003). The scale has good psychometric properties (Bezdjian et al., 2016), with a Cronbach's α = 0.93.
2.3. Experimental procedures
All experimental procedures have previously been described (Mehta et al., 2017). Briefly, the blood samples were sent to the Australian Genome Research Facility (AGRF) and stored at −20 °C. Genomic DNA was extracted from a 2 ml blood sample, using MACHEREY-NAGEL NucleoSpin L (MACHEREY-NAGEL GmbH & Co. KG, Dueren, NRW, Germany). Quality assessment of the samples was performed by resolution on a 0.8% agarose gel at 130 V for 60 min. Samples were bisulphite converted with Zymo EZ DNA Methylation kit as previously described (Mehta et al., 2013; Wockner et al., 2014). All the Illumina quality controls met performance metrics which included sample-independent, sample dependent, staining, extension, target removal, hybridization, bisulphite conversion I and II, specificity, non-polymorphic and negative controls.
2.4. Replication sample
Replication was performed using the same approach in the Grady Trauma Project (GTP). Details of the cohort are described in Zannas A et al., 2015 (Zannas et al., 2015); briefly, the Grady Trauma Project (GTP) is a large study conducted in Atlanta, Georgia, that includes participants from a predominantly African American, urban population of low socioeconomic status (Binder et al., 2008; Gillespie et al., 2009). This population is characterized by high prevalence and severity of trauma over the lifetime and is thus particularly relevant for examining the effects of stressors on epigenetic markers. For replication we used a subset of 115 males that were matched for adult trauma levels (all had two or more types of adult trauma) assessed by the Trauma Events Inventory (Hovens et al., 2000), this subgroup has been previously described (Mehta et al., 2017). For resilience, as in the discovery sample, the Connor-Davidson Resilience Scale (CD RISC) was used. The GTP samples were run on the Illumina 450 k arrays as previously detailed in Zannas et al. (2015) (Zannas et al., 2015). Methylation beta values after corrections for batch effects were used for the analysis.
2.5. Statistical analysis
Differences in group demographics and clinical variables was performed using Chi square/ANOVA tests in R. Correlations were calculated using the non-parametric Spearman's coefficient in R. Generalized regression models were used to test the associations between DNAm age with PTSD and resilience. The interaction of CD-RISC scores x PTSD group was modelled using the CD-RISC, PTSD group main effects and the 17 SVA's as covariates.
Raw beta values from EPIC Illumina arrays were exported into R for statistical analysis. The level of methylation was determined by calculating a “β value” (the ratio of the fluorescent signals for the methylated vs. unmethylated sites). The methylation status for each probe was recorded as a β-value that ranged between 0 and 1, where values close to 1 represent high levels of methylation and where values close to 0 represent low levels of methylation. For methylation analysis, the raw data was background and control-normalized using the Bioconductor MINFI package (1.4.0) (Aryee et al., 2014). Cell counts were analyzed using the Houseman method (Houseman et al., 2012).
DNA methylation-based age prediction was performed using the online DNA methylation age calculator (https://dnamage.genetics.ucla.edu/) developed by Horvath (2013). For analysis purposes, the DNAm-age was regressed against the chronological age (age acceleration residuals) and the resulting residuals together with other covariates were used in the generalized regression models.
Data were analyzed using an established analysis pipeline comprising of custom statistical programs and scripts (Barfield et al., 2014; Mehta et al., 2011, 2013) written in R and Linux. Surrogate variable analyses revealed 17 significant SVA vectors which were used as covariates in the model to correct for technical artefacts and hidden confounds (Leek and Storey, 2007). We have previously used these SVA vectors previously to correct for ethnicity, cell counts, current medication, BMI and smoking (Mehta et al., 2017). Results were corrected for multiple testing using 10% false discovery rate.
Factor analysis of the CD-RISC resilience scores was performed in R using the psych package (Revelle, 2017). Initially, exploratory analysis using the spree plot and parallel analysis were used to determine the number of significant factors. The minimum residual solution was transformed into an oblique solution using an oblimin transformation and loadings >0.4 were considered relevant for the factor.
3. Results
3.1. Demographics of samples
A total of 96 male Australian veterans from the Vietnam War were included in the study. Demographics and characteristics of the individuals are shown in Table 1. The veterans had an average age of 69 years [SE = 0.45]. A total of 85% veterans were currently married and 79% of the veterans had children. Among the veterans, 8% were working full-time, 10% were working part-time and 66% veterans were currently retired. Veterans with a current diagnosis of PTSD had increased PTSD symptom severity, higher depressive, anxiety and stress scores, increased rates of suicide ideation and significantly higher CD-RISC resilience scores compared to veterans without PTSD (Table 1). In the overall sample, PTSD symptom severity was negatively correlated with CD-RISC scores (r = −0.58, P-value = 5.41 × 10−10).
3.2. Relationship between DNA methylation age and PTSD
In the full sample of veterans (n = 96), DNAm age was significantly correlated with the chronological age (r = 0.50, P-value = 3.3e-6). No significant differences were present in the DNAm age acceleration between the PTSD and non-PTSD groups (P-value = 0.24, PTSD: mean [se] = 8.06 [0.77], non-PTSD: mean [se] = 6.27 [0.66]). In addition, PTSD symptom severity was not associated with DNAm age acceleration (P-value = 0.47). PTSD symptom severity was also not associated with predicted cell counts (P-values - CD8T:0.88, CD4T:0.38, NK: 0.17, Bcells:0.39, Monocytes:0.87, Granulocytes:0.30).
3.3. Relationship between DNA methylation age and resilience
Given the significant difference in resilience scores between the PTSD and non-PTSD groups (Table 1), we next sought to investigate the contribution of potential protective factors associated with DNAm age using CD-RISC scores. The CD-RISC resilience scores were not associated with predicted cell counts (P-values - CD8T:0.37, CD4T:0.47, NK: 0.42, Bcells:0.12, Monocytes:0.41, Granulocytes:0.71).
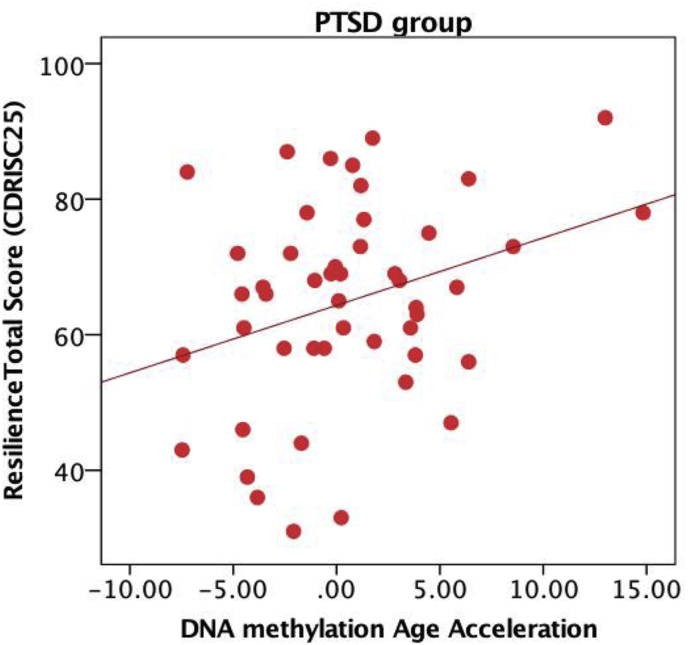
In the overall sample, DNAm age acceleration was not associated with resilience scores (P-value = 0.18). Given a significant interaction of CD-RISC scores x PTSD group (p = 0.015), we subsequently stratified the samples. Upon stratification by PTSD diagnosis, we observed that DNAm age acceleration was significantly associated with resilience scores in the PTSD group and remained significant after multiple testing correction (r = 0.32 and P-value = 0.023). No significant correlation was observed in the non-PTSD group (r = −0.19 and P-value = 0.4). Contrary to expectations, we observed that DNAm age acceleration was positively correlated with resilience scores in the PTSD group, such that increased epigenetic age acceleration was associated with increased resilience among veterans with PTSD (Fig. 1).
Fig. 1.
Epigenetic aging and resilience scores: DNAm age acceleration was positively correlated with resilience scores in the PTSD group (r = 0.32 and P-value = 0.023).
Next, we performed post-hoc analyses to investigate if specific latent factors within the Connor-Davidson Resilience Scale might drive the observed positive relationship between DNAm age acceleration and resilience scores in PTSD. Exploratory factor analysis in R using the psych package identified two significant factors, summarizing ‘Hardiness/Adaptability’ and ‘Self-efficacy’ items (Supplementary Figure 1). The 2-factor model fit yielded a Kaiser-Meyer Olkin measure of sampling adequacy of 0.96 and significant Bartlett's Test measure of Sphericity (P-value < 8.2e-05). Of the two factors, only the Self-efficacy factor was significantly associated with DNAm age acceleration in the PTSD group (r = 0.35, P-value = 0.015).
3.4. Replication of the epigenetic clock and resilience findings in a civilian cohort
We sought to replicate our results in an independent population from the Grady Trauma Project (GTP), which comprises of a population from the suburbs of Atlanta that had been exposed to significant traumatic events (Gillespie et al., 2009) and test if observed associations between DNA methylation age and resilience scores were specific to the veteran cohort or could be generalized to a non-combat population. We have previously assessed epigenetic aging in this cohort (Zannas et al., 2015) and for this replication we used a male subset of the larger sample (n = 115). We hypothesized that the relationship between age acceleration and resilience scores would be significantly different between the PTSD and non-PTSD groups.
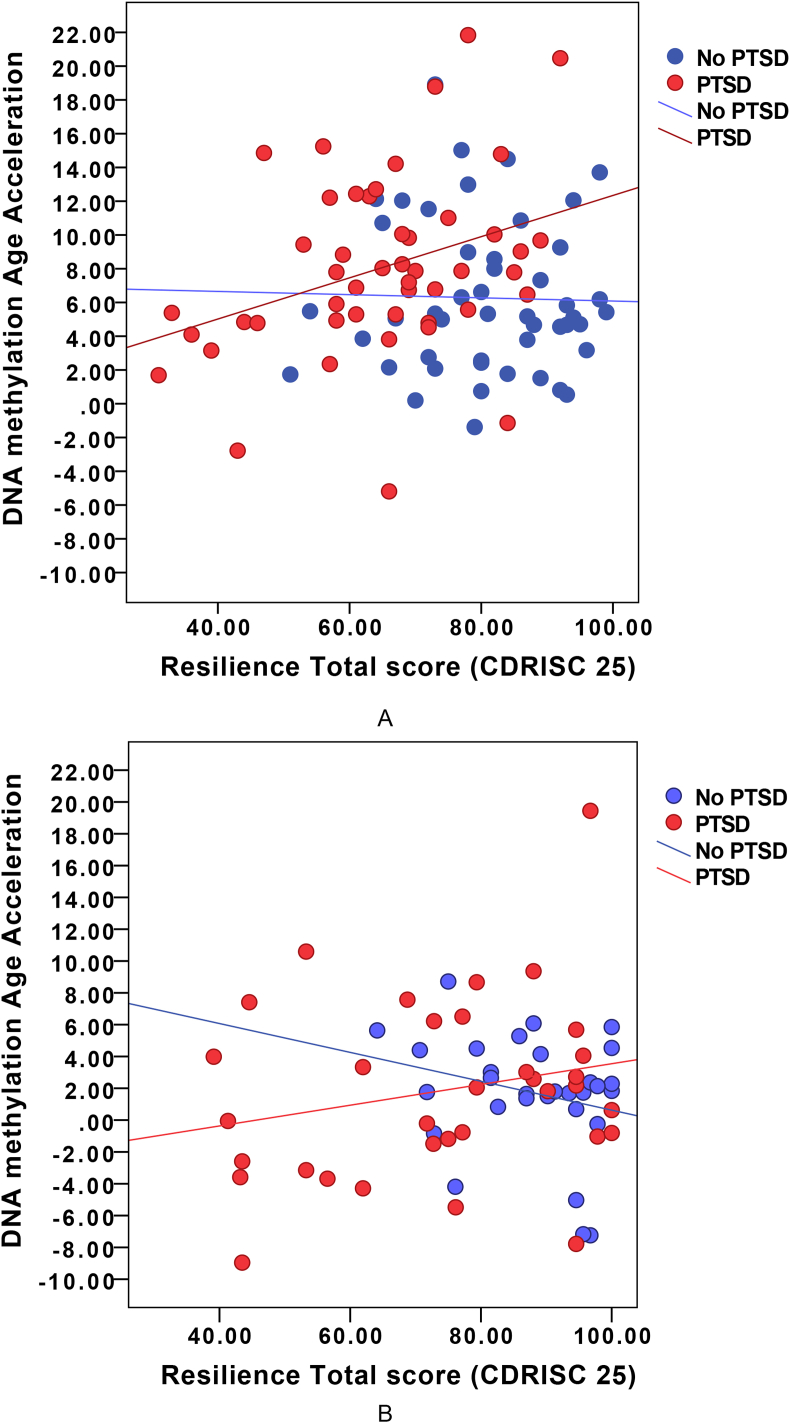
The replication sample comprised of 115 males (n = 70 PTSD and n = 45 non-PTSD) aged between 19 and 65, with a mean age of 44 [1.14]. We tested whether the association between DNAm age acceleration and resilience scores were driven by the PTSD diagnosis status. We observed significant differences in association between age acceleration and resilience scores between PTSD and non-PTSD groups in the GTP cohort (P-value = 0.02). The positive association between age acceleration and resilience scores were also observed in the GTP PTSD group (r = 0.23), similar to that in the discovery sample of veterans (Fig. 2). For the non-PTSD group however, in the GTP sample, we observed a stronger negative correlation between age acceleration and resilience (r = −0.25). The results in controls (non-PTSD) from the GTP indicate that as per hypothesis, DNAm age acceleration and resilience are inversely correlated, i.e. increased DNAm age acceleration is associated with decreased resilience. For veterans without PTSD, the same negative correlation between DNAm age and resilience was also observed as described above, but the correlation was slightly blunted (r = −0.19).
Fig. 2.
DNAm age acceleration and resilience score – a) DNAm age acceleration was significantly associated with resilience scores (shown in red) in the PTSD group in the Australian Vietnam combat veterans (r = 0.32) and b) DNAm age acceleration was significantly associated with resilience scores (shown in red) in the PTSD group in the replication cohort from the Grady Trauma project (r = 0.23). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These results corroborate our initial findings that increased resilience scores are associated with increased DNAm age acceleration in PTSD and the opposite effect is observed in controls (non-PTSD), with increased resilience scores associated with decreased DNAm age in both the veterans and the civilian population.
4. Discussion
In recent years, extensive research effort has been made to identify biological markers that can predict healthy aging as well as disease risk and negative health outcomes associated with aging. In this study, for the first time we assessed the link between the epigenetic clock, a biological marker of aging, and resilience in veterans. The epigenetic clock has been widely described to be associated with detrimental health and a myriad of disorders including obesity, Down syndrome, Parkinson's disease, Huntington's disease, and cognitive and physical fitness in the elderly (Chen et al., 2016; Horvath et al., 2015; Horvath and Ritz, 2015; Levine et al., 2015; Marioni et al., 2015; Nevalainen et al., 2017; Quach et al., 2017).
PTSD occurs due to a complex interplay of risk and protective factors that interact with each other to modulate risk of disease. Identification of risk and protective factors for PTSD will help to identify individuals with the greatest necessity for early intervention and facilitate research aimed at improving the efficacy of treatment. In a recent study, higher resilience was associated with improved social functioning after PTSD and depression severity, childhood maltreatment, physical health, gender, education, marital status, and employment were simultaneously adjusted for (Wingo et al., 2017). Other studies have illustrated the importance of resilience as a protective factor against PTSD symptoms in high-risk groups (Lee et al., 2016); however, models of resilience include risk as well as protective factors that may interact to reduce negative consequences and facilitate positive consequences (Fergus and Zimmerman, 2005).
Here, we examined resilience measures and demonstrated that while in controls (non-PTSD), increased resilience was associated with decreased DNAm age as expected, among veterans diagnosed with PTSD, increased epigenetic aging was associated with increased resilience, contrary to our expectations. Importantly, in this study, the link between epigenetic aging and resilience among veterans with PTSD was also replicated in an independent sample from a civilian population with high levels of trauma-exposure. In this civilian population, we also observed that increased resilience was associated with increased DNAm age acceleration among individuals with PTSD. These results are in line with previous work showing that elevated allostatic load was observed in women with high stress histories and particularly those with PTSD (Glover et al., 2006). Stress response associated with allostatic load can follow different trajectories including an inability to turn off allostatic responses after stress termination and inadequate responses by some allostatic systems that trigger compensatory increases in other systems (Korte et al., 2005; McEwen, 1998, 2003). For example, one study demonstrated that among children in low socioeconomic strata, self-control acts as a “double-edged sword,” facilitating psychosocial adjustment, whilst simultaneously undermining physical health as reflected by increased epigenetic aging (Miller et al., 2015). Our findings also suggest that the allostatic load among individuals with PTSD is distinct from people without PTSD. Among individuals with PTSD, it is likely that there is already an increased allostatic load such that for these individuals, increased resilience further elevates the allostatic load and thereby resulting in an allostatic overload, resulting in a cascading negative impact on overall health.
Factor analysis of the CD-RISC scores revealed two significant factors reflecting ‘Adaptability’ and ‘Self-efficacy’, these were similar to other factor analyses of the same resilience inventory (Arias Gonzalez et al., 2015; Campbell-Sills and Stein, 2007; Green et al., 2014). Of the two factors, we observed that the ‘self-efficacy’ factor was significantly associated with DNAm age acceleration in the PTSD group (r = 035). Self-efficacy is defined as an individual's perceived ability to organize and employ one's skills and execute the necessary actions to achieve desired outcomes (Bandura, 1997; Bandura et al., 2001). Previously, Boks and colleagues (Boks et al., 2015) demonstrated that PTSD symptoms were significantly associated with increased telomere length and decreased DNAm aging. The authors discussed differences between short-term and long-term trauma response in aging parameters. The authors noted that their results were consistent with a model whereby aging parameters increase in response to trauma only for those who have already manifested symptoms of PTSD; and for these individuals with PTSD it is likely that this maladaptive state of age decrease subsides afterwards. Wolf and colleagues (Wolf et al., 2016) demonstrated that accelerated epigenetic aging may be manifested in degradation in the neural integrity of microstructural cells in the genu in the corpus callosum and working memory deficits and the authors discussed that PTSD may levy a heavy toll on the body reflected, in part, as accelerated cellular aging in the epigenome. Our findings are also in line with previous studies that highlight the notion that self-efficacy is not uniformly beneficial, and that higher levels of self-efficacy can sometimes lead to increases in neuroendocrine and psychological stress responses and decreases in performance, a widely overlooked phenomenon (Schonfeld et al., 2017).
This study has several strengths and limitations. This study assessed only males and replication of these findings in females is warranted. Furthermore, DNA methylation can be affected by several different factors and while we have attempted to account for these, it is likely that there are other unaccounted covariates than remain to be addressed. In this study, we assessed epigenetic aging using the Horvath age predictor only, other similar predictors of epigenetic aging are available. Despite a small sample size and an extreme study-design of a subset of PTSD cases and controls from a larger cohort, we were able to replicate our findings in an independent male civilian cohort, demonstrating the robustness of these results. Larger longitudinal studies investigating the trajectories of epigenetic aging and resilience in PTSD will shed further light on these findings.
In conclusion, our study sheds light on the role of epigenetic aging in PTSD. This is the first study to evaluate the relationship between epigenetic aging in PTSD by examining the influence of potential protective factors such as resilience. Contrary to our expectations, we found that increased resilience in PTSD is associated with accelerated epigenetic aging and we found that this relationship may be related to underlying self-efficacy. Our findings fit well with the theory of allostatic overload as a result of cumulative physiological wear and tear via repeated efforts to adapt to stressors over time. Additional larger, longitudinal studies assessing the effects of stress and epigenetic aging over time would be beneficial to better understand the disease trajectory and biological processes that shape risk and resilience in PTSD.
5. Declarations of interest
All authors report no potential conflicts of interest.
Acknowledgements
The authors thank the Gallipoli Medical Research Foundation for their support, especially Miriam Dwyer and Dr Sarah McLeay for their project management support. The authors would also like to acknowledge Dr Madeline Romaniuk for psychological expertise and Dr John Gibson and the team at the Keith Payne Unit, and the staff and investigators at Greenslopes Private Hospital for their valuable contribution to the study. All authors extend their gratitude to the study participants for their generous provision of data and time. The PTSD Initiative was funded by the Queensland Branch of the Returned & Services League of Australia (RSL QLD). The Gallipoli Medical Research Foundation wishes to thank the RSL QLD for their generous donation, and Sullivan Nicolaides Pathology and Queensland X-Ray for their in-kind support. The authors thank QUT and IHBI for financial and administrative support. Divya Mehta was funded via the QUT Vice Chancellors Research Fellowship.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ynstr.2018.04.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1 – Exploratory factor analysis of the Connor Davidson resilience scores in R using the psych package identified two significant factors. The Cronbach alpha was 0.93, indicating internal consistency and reliability. The 2-factor model fit yielded a Kaiser-Meyer Olkin measure of sampling adequacy of 0.96 and significant Bartlett's Test measure of Sphericity (chi-square of 344, P-value < 8.2e-05), indicating a good fit. a) Scree plot and parallel analysis indicating a 2-factor solution is optimal. b) Factor analysis diagram depicting the loadings of the sub-items within the factors. TC1 comprised ‘Hardiness/Adaptability’ and itemsTC2 comprised ‘Self-efficacy’ items.
References
- Alegria-Torres J.A., Baccarelli A., Bollati V. Epigenetics and lifestyle. Epigenomics. 2011;3:267–277. doi: 10.2217/epi.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias Gonzalez V.B., Crespo Sierra M.T., Arias Martinez B., Martinez-Molina A., Ponce F.P. An in-depth psychometric analysis of the Connor-Davidson Resilience Scale: calibration with Rasch-Andrich model. Health Qual. Life Outcome. 2015;13:154. doi: 10.1186/s12955-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., Irizarry R.A. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. The anatomy of stages of change. Am. J. Health Promot. 1997;12:8–10. doi: 10.4278/0890-1171-12.1.8. [DOI] [PubMed] [Google Scholar]
- Bandura A., Barbaranelli C., Caprara G.V., Pastorelli C. Self-efficacy beliefs as shapers of children's aspirations and career trajectories. Child Dev. 2001;72:187–206. doi: 10.1111/1467-8624.00273. [DOI] [PubMed] [Google Scholar]
- Barfield R.T., Almli L.M., Kilaru V., Smith A.K., Mercer K.B., Duncan R., Klengel T., Mehta D., Binder E.B., Epstein M.P., Ressler K.J., Conneely K.N. Accounting for population stratification in DNA methylation studies. Genet. Epidemiol. 2014;38:231–241. doi: 10.1002/gepi.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian S., Schneider K.G., Burchett D., Baker M.T., Garb H.N. 2016. Resilience in the United States Air Force: Psychometric Properties of the Connor-Davidson Resilience Scale (CD-risc) Psychological Assessment. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B., Schwartz A.C., Cubells J.F., Ressler K.J. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J. Am. Med. Assoc. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks M.P., van Mierlo H.C., Rutten B.P., Radstake T.R., De Witte L., Geuze E., Horvath S., Schalkwyk L.C., Vinkers C.H., Broen J.C., Vermetten E. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512. doi: 10.1016/j.psyneuen.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Breslau N. Are baseline depressive symptoms associated with smoking initiation in adolescents? West. J. Med. 1999;170:265. [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Davis G.C. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am. J. Psychiatr. 1992;149:671–675. doi: 10.1176/ajp.149.5.671. [DOI] [PubMed] [Google Scholar]
- Brewin C.R., Andrews B., Valentine J.D. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J. Consult. Clin. Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L., Stein M.B. Psychometric analysis and refinement of the connor-davidson resilience scale (CD-RISC): validation of a 10-item measure of resilience. J. Trauma Stress. 2007;20:1019–1028. doi: 10.1002/jts.20271. [DOI] [PubMed] [Google Scholar]
- Charney D.S. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatr. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chen B.H., Marioni R.E., Colicino E., Peters M.J., Ward-Caviness C.K., Tsai P.C., Roetker N.S., Just A.C., Demerath E.W., Guan W., Bressler J., Fornage M., Studenski S., Vandiver A.R., Moore A.Z., Tanaka T., Kiel D.P., Liang L., Vokonas P., Schwartz J., Lunetta K.L., Murabito J.M., Bandinelli S., Hernandez D.G., Melzer D., Nalls M., Pilling L.C., Price T.R., Singleton A.B., Gieger C., Holle R., Kretschmer A., Kronenberg F., Kunze S., Linseisen J., Meisinger C., Rathmann W., Waldenberger M., Visscher P.M., Shah S., Wray N.R., McRae A.F., Franco O.H., Hofman A., Uitterlinden A.G., Absher D., Assimes T., Levine M.E., Lu A.T., Tsao P.S., Hou L., Manson J.E., Carty C.L., LaCroix A.Z., Reiner A.P., Spector T.D., Feinberg A.P., Levy D., Baccarelli A., van Meurs J., Bell J.T., Peters A., Deary I.J., Pankow J.S., Ferrucci L., Horvath S. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 2016;8:1844–1865. doi: 10.18632/aging.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K.M., Davidson J.R.T. vol. 18. 2003. pp. 76–82. (Development of a New Resilience Scale: the Connor-Davidson Resilience Scale (CD-risc) Depression and Anxiety). [DOI] [PubMed] [Google Scholar]
- Dudley K.J., Li X., Kobor M.S., Kippin T.E., Bredy T.W. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci. Biobehav. Rev. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Clark D.M. A cognitive model of posttraumatic stress disorder. Behav. Res. Ther. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Feder A., Nestler E.J., Charney D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergus S., Zimmerman M.A. Adolescent resilience: a framework for understanding healthy development in the face of risk. Annu. Rev. Publ. Health. 2005;26:399–419. doi: 10.1146/annurev.publhealth.26.021304.144357. [DOI] [PubMed] [Google Scholar]
- Gillespie C.F., Bradley B., Mercer K., Smith A.K., Conneely K., Gapen M., Weiss T., Schwartz A.C., Cubells J.F., Ressler K.J. Trauma exposure and stress-related disorders in inner city primary care patients. Gen. Hosp. Psychiatr. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D.A., Stuber M., Poland R.E. Allostatic load in women with and without PTSD symptoms. Psychiatry. 2006;69:191–203. doi: 10.1521/psyc.2006.69.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.T., Hayward L.C., Williams A.M., Dennis P.A., Bryan B.C., Taber K.H., Mid-Atlantic Mental Illness Research E., Clinical Center W., Davidson J.R., Beckham J.C., Calhoun P.S. Examining the factor structure of the connor-davidson resilience scale (CD-RISC) in a post-9/11. U.S. Military Veteran Sample Assess. 2014;21:443–451. doi: 10.1177/1073191114524014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G., Guinney J., Zhao L., Zhang L., Hughes G., Sadda S., Klotzle B., Bibikova M., Fan J.B., Gao Y., Deconde R., Chen M., Rajapakse I., Friend S., Ideker T., Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Ritz B.R. Increased epigenetic age and granulocyte counts in the blood of Parkinson's disease patients. Aging (Albany NY) 2015;7:1130–1142. doi: 10.18632/aging.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Garagnani P., Bacalini M.G., Pirazzini C., Salvioli S., Gentilini D., Di Blasio A.M., Giuliani C., Tung S., Vinters H.V., Franceschi C. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14:491–495. doi: 10.1111/acel.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman E.A., Accomando W.P., Koestler D.C., Christensen B.C., Marsit C.J., Nelson H.H., Wiencke J.K., Kelsey K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinf. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens J.E., Bramsen I., van der Ploeg H.M., Reuling I.E. Test-retest reliability of the trauma and life events self-report inventory. Psychol. Rep. 2000;87:750–752. doi: 10.2466/pr0.2000.87.3.750. [DOI] [PubMed] [Google Scholar]
- Klengel T., Pape J., Binder E.B., Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014;80:115–132. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Korte S.M., Koolhaas J.M., Wingfield J.C., McEwen B.S. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D.V., Weiller E., Amorim P., Bonora I., Harnett Sheehan K., Janavs J., Dunbar G.C. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI European. Psychiatry. 1997;12:224–231. [Google Scholar]
- Lee J.K., Choi H.G., Kim J.Y., Nam J., Kang H.T., Koh S.B., Oh S.S. Self-resilience as a protective factor against development of post-traumatic stress disorder symptoms in police officers. Ann. Occup. Environ. Med. 2016;28:58. doi: 10.1186/s40557-016-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek J.T., Storey J.D. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet. 2007;3:1724–1735. doi: 10.1371/journal.pgen.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M.E., Lu A.T., Bennett D.A., Horvath S. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer's disease related cognitive functioning. Aging (Albany NY) 2015;7:1198–1211. doi: 10.18632/aging.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond S.H., Lovibond P.F. vol. 33. 1995. pp. 335–343. (The Structure of Negative Emotional States: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories Behaviour Research and Therapy). [DOI] [PubMed] [Google Scholar]
- Marioni R.E., Shah S., McRae A.F., Ritchie S.J., Muniz-Terrera G., Harris S.E., Gibson J., Redmond P., Cox S.R., Pattie A., Corley J., Taylor A., Murphy L., Starr J.M., Horvath S., Visscher P.M., Wray N.R., Deary I.J. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol. 2015;44:1388–1396. doi: 10.1093/ije/dyu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52:10–16. doi: 10.1016/s0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatr. 2017;74:551–552. doi: 10.1001/jamapsychiatry.2017.0270. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeay S.C., Harvey W.M., Romaniuk M.N., Crawford D.H., Colquhoun D.M., Young R.M., Dwyer M., Gibson J.M., O'Sullivan R.A., Cooksley G., Strakosch C.R., Thomson R.M., Voisey J., Lawford B.R. Physical comorbidities of post-traumatic stress disorder in Australian Vietnam War veterans. Med. J. Aust. 2017;206:251–257. doi: 10.5694/mja16.00935. [DOI] [PubMed] [Google Scholar]
- McNally R.J. Psychological mechanisms in acute response to trauma. Biol. Psychiatr. 2003;53:779–788. doi: 10.1016/s0006-3223(02)01663-3. [DOI] [PubMed] [Google Scholar]
- McNally R.J., Bryant R.A., Ehlers A. Does early psychological intervention promote recovery from posttraumatic stress? Psychol. Sci. Publ. Interest. 2003;4:45–79. doi: 10.1111/1529-1006.01421. [DOI] [PubMed] [Google Scholar]
- Mehta D., Gonik M., Klengel T., Rex-Haffner M., Menke A., Rubel J., Mercer K.B., Putz B., Bradley B., Holsboer F., Ressler K.J., Muller-Myhsok B., Binder E.B. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch. Gen. Psychiatr. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D., Klengel T., Conneely K.N., Smith A.K., Altmann A., Pace T.W., Rex-Haffner M., Loeschner A., Gonik M., Mercer K.B., Bradley B., Muller-Myhsok B., Ressler K.J., Binder E.B. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D., Bruenig D., Carrillo-Roa T., Lawford B., Harvey W., Morris C.P., Smith A.K., Binder E.B., Young R.M., Voisey J. Genomewide DNA methylation analysis in combat veterans reveals a novel locus for PTSD. Acta Psychiatr. Scand. 2017;136:493–505. doi: 10.1111/acps.12778. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Yu T., Chen E., Brody G.H. Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proc. Natl. Acad. Sci. U. S. A. 2015;112:10325–10330. doi: 10.1073/pnas.1505063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Schneper L.M., Notterman D.A. DNA methylation, early life environment, and health outcomes. Pediatr. Res. 2016;79:212–219. doi: 10.1038/pr.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T., Kananen L., Marttila S., Jylhava J., Mononen N., Kahonen M., Raitakari O.T., Hervonen A., Jylha M., Lehtimaki T., Hurme M. Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clin. Epigenet. 2017;9:20. doi: 10.1186/s13148-016-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach A., Levine M.E., Tanaka T., Lu A.T., Chen B.H., Ferrucci L., Ritz B., Bandinelli S., Neuhouser M.L., Beasley J.M., Snetselaar L., Wallace R.B., Tsao P.S., Absher D., Assimes T.L., Stewart J.D., Li Y., Hou L., Baccarelli A.A., Whitsel E.A., Horvath S. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY) 2017;9:419–446. doi: 10.18632/aging.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W. Northwestern University; Evanston, Illinois, USA: 2017. Psych: Procedures for Personality and Psychological Research.https://cran.r-project.org/package=psych Version = 1.7.5. [Google Scholar]
- Schonfeld P., Preusser F., Margraf J. Costs and benefits of self-efficacy: differences of the stress response and clinical implications. Neurosci. Biobehav. Rev. 2017;75:40–52. doi: 10.1016/j.neubiorev.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Harnett Sheehan K., Janavs J., Weiller E., Keskiner A., Schinka J., Knapp E., Sheehan M.F., Dunbar G.C. The validity of the Mini international neuropsychiatric interview (MINI) according to the SCID-P and its reliability European. Psychiatry. 1997;12:232–241. [Google Scholar]
- Szyf M., Tang Y.Y., Hill K.G., Musci R. The dynamic epigenome and its implications for behavioral interventions: a role for epigenetics to inform disorder prevention and health promotion. Transl. Behav. Med. 2016;6:55–62. doi: 10.1007/s13142-016-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges M.A., Romney D.M. Risk and resiliency factors in posttraumatic stress disorder. Ann. Gen. Hosp. Psychiatr. 2003;2:4. doi: 10.1186/1475-2832-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Marx B.P., Friedman M.J., Schnurr P.P. Posttraumatic stress disorder in DSM-5: new criteria, new measures, and implications for assessment. Psychol. Injury Law. 2014;7:93–107. [Google Scholar]
- Wingo A.P., Briscione M., Norrholm S.D., Jovanovic T., McCullough S.A., Skelton K., Bradley B. Psychological resilience is associated with more intact social functioning in veterans with post-traumatic stress disorder and depression. Psychiatr. Res. 2017;249:206–211. doi: 10.1016/j.psychres.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Wockner L.F., Noble E.P., Lawford B.R., Young R.M., Morris C.P., Whitehall V.L., Voisey J. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl. Psychiatry. 2014;4:e339. doi: 10.1038/tp.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E.J., Logue M.W., Hayes J.P., Sadeh N., Schichman S.A., Stone A., Salat D.H., Milberg W., McGlinchey R., Miller M.W. Accelerated DNA methylation age: associations with PTSD and neural integrity. Psychoneuroendocrinology. 2016;63:155–162. doi: 10.1016/j.psyneuen.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E.J., Logue M.W., Stoop T.B., Schichman S.A., Stone A., Sadeh N., Hayes J.P., Miller M.W. Accelerated DNA methylation age: associations with posttraumatic stress DIsorder and mortality. Psychosom. Med. 2018;80:42–48. doi: 10.1097/PSY.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Biological factors associated with susceptibility to posttraumatic stress disorder. Can. J. Psychiatr. 1999;44:34–39. doi: 10.1177/070674379904400104. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Risk and resilience in posttraumatic stress disorder. J. Clin. Psychiatr. 2004;65(Suppl. 1):29–36. [PubMed] [Google Scholar]
- Yuan C., Wang Z., Inslicht S.S., McCaslin S.E., Metzler T.J., Henn-Haase C., Apfel B.A., Tong H., Neylan T.C., Fang Y., Marmar C.R. Protective factors for posttraumatic stress disorder symptoms in a prospective study of police officers. Psychiatr. Res. 2011;188:45–50. doi: 10.1016/j.psychres.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas A.S., Arloth J., Carrillo-Roa T., Iurato S., Roh S., Ressler K.J., Nemeroff C.B., Smith A.K., Bradley B., Heim C., Menke A., Lange J.F., Bruckl T., Ising M., Wray N.R., Erhardt A., Binder E.B., Mehta D. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015;16:pp.266. doi: 10.1186/s13059-015-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.