Abstract
Background and aims
Calcific aortic valve disease (CAVD) is the most common valve disease. Although micronutrients are known to contribute to cardiovascular disease, the relationship with CAVD remains poorly evaluated. We examined the association of serum levels of magnesium, phosphorus, and calcium with prevalence, incidence, and progression of aortic valve calcification (AVC).
Methods
We conducted a prospective study in a population-based sample of Japanese men aged 40–79 years without known cardiovascular disease and chronic kidney disease at baseline, and quantified AVC from serial computed tomographic images with the Agatston method.
Results
Of 938 participants at baseline (mean age, 63.7±9.9 years), AVC prevalence was observed in 173 (18.4%). Of 596 participants without baseline AVC at follow-up (median duration, 5.1 years), AVC incidence was observed in 138 (23.2%). After adjustment for demographics, behaviors and cardiovascular risk factors, relative risks (95% confidence intervals) in the highest versus lowest categories of serum magnesium, phosphorus, and calcium were 0.62 (0.44–0.86), 1.45 (1.02–2.04), and 1.43 (0.95–2.15), respectively, for AVC prevalence and 0.62 (0.42–0.92), 1.93 (1.28–2.91), and 1.09 (0.77–1.55), respectively, for AVC incidence. Their linear trends of serum magnesium and phosphorus were also all statistically significant. Of 131 participants with baseline AVC, there was no association of any serum micronutrients with AVC progression.
Conclusions
Serum magnesium was inversely associated, while serum phosphorus was positively associated, with AVC prevalence and incidence, suggesting that these serum micronutrients may be potential candidates for risk prediction or prevention of CAVD, and warranting further studies.
Keywords: aortic valve disease, calcification, magnesium, phosphorus, prospective study, epidemiology
1. INTRODUCTION
Calcific aortic valve disease (CAVD) is the most common heart valve disease worldwide.1 CAVD was historically thought to result from passive degeneration because of aging in association with calcium accumulation.2 However, CAVD is now recognized as an independent predictor for cardiovascular diseases (CVD)3,4 and as a predecessor to aortic stenosis,5,6 the third most common CVD in developed countries,5 and the most common cause of heart valve replacement.7 In addition, there is no known effective therapy or prevention for CAVD, with large randomized trials failing to reduce the progression of aortic stenosis.8,9 Thus, the cellular mechanisms, risk factors, and therapeutic interventions for CAVD are widely studied.1,5
Magnesium, phosphorus, and calcium are micronutrients traditionally characterized in relation to bone health or chronic kidney disease (CKD), and meanwhile they are also considered to be associated with the risk of CVD10–14 and subclinical coronary atherosclerosis.15 Serum magnesium has broad physiological roles in the cardiovascular system; for example, low serum magnesium is associated with impaired glucose homeostasis and insulin action, elevated blood pressure, metabolic disorder, endothelial dysfunction, and excessive inflammation.16,17 Elevated serum phosphorus and calcium was also hypothesized to promote atherogenesis through vascular calcification.18,19
Despite their relevance to CVD, there are limited data on the association of these micronutrients with CAVD. Subclinical early stage of CAVD is characterized by aortic valve calcification (AVC), with only two studies in the general population examining the relationship of serum phosphate or calcium with AVC prevalence,20,21 incidence, and progression.21 In addition, few studies have examined the association of serum magnesium with AVC. Therefore, in the present study, we aimed to examine the associations of serum levels of magnesium, phosphorus, and calcium with AVC prevalence, incidence, and progression in a Japanese general population using quantitative AVC scores evaluated by serial computed tomography (CT) scans during a median follow-up of 5.1 years. Given that the subclinical early stage of CAVD (AVC) is an alternative key target for prevention and treatment,5,22 this investigation in apparently healthy participants is of great interest.
2. PATIENTS AND METHODS
2.1. Study participants
The Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA) is an ongoing prospective population-based study of a random sample from a general Japanese population.23 In brief, 1094 Japanese men aged 40–79 years, residents of Kusatsu City, Shiga in Japan, participated in the baseline survey from 2006 to 2008. After excluding those with a history of aortic valve replacement (n = 4), myocardial infarction or stroke (n = 66), CKD (n = 41), with triglycerides ≥ 400 mg/dL (n = 15; as we used Friedwald’s formula to estimate low density lipoprotein cholesterol levels24), and with missing information on CT data, serum micronutrients, and other covariates (n = 30), a total of 938 men were analyzed for AVC prevalence. Participants to the baseline survey were invited for a follow-up examination between 2010–2014. After excluding those who died or were lost to follow-up (n = 202) and those with missing CT data (n = 9), a total of 727 participants were included for analysis of AVC incidence or progression. The present study was approved by the Institutional Review Board of Shiga University of Medical Science (No. 17–19, 17–83; Otsu, Japan), and all participants provided written informed consent.
2.2. Exposure and covariate measurement
Venipuncture was performed early in the clinic visit after fasting for at least at 12 hour. We separated serum by centrifugation (3000 revolutions/min, for 15 min) at 4°C within 90 min. Samples for routine tests were sent to the laboratory, and other samples were frozen at −70°C until analysis. Serum magnesium, phosphorus, and calcium were quantified using the xylidyl blue-I method, molybdate blue colorimetric method (Sekisui Medical, Tokyo, Japan), and arsenazo III method (NIPRO, Osaka, Japan), respectively. The intra and interassay coefficients of variation for serum micronutrients levels were <2.4%. Serum albumin levels were measured using the bromocresol green assay (Wako Pure Chemical Industries, Osaka, Japan). The intra and interassay coefficients of variation for serum albumin levels were <2.8%. As typically performed in the clinical setting, serum calcium was adjusted for serum albumin using the following equation: corrected calcium = measured total calcium (mg/dL) + 0.8 × [4.0 − serum albumin (g/dL)] if the serum albumin levels are <4 g/dL.14 Herein, all results reported for calcium are based on the serum albumin–corrected variable.
Plasma glucose levels were determined from NaF-treated plasma using a hexokinase glucose–6 phosphate–dehydrogenase enzymatic assay, hemoglobin A1c (HbA1c) was measured by latex agglutination immunoassay (Kyowa Medix, Tokyo, Japan). Lipid measurements were standardized according to the protocol of the Centers for Disease Control and Prevention/Cholesterol Reference Method Laboratory Network. Total cholesterol and triglycerides were measured using enzymatic assays, and high-density lipoprotein cholesterol was determined using a direct method. Low-density lipoprotein cholesterol levels were estimated using Friedewald’s formula.24 C-reactive protein was measured by nephelometry using a BN II analyzer with an interassay coefficient of variation ranging from 4.5% to 4.6%. Cystatin C was measured using a colloidal gold enhanced immunoturbidimetry method (Alfresa Pharma, Osaka, Japan) with intra and interassay coefficients of variation <1.7%. Based on the glomerular filtration rate estimating equation using cystatin C for Japanese men,25 glomerular filtration rate was calculated as follows: glomerular filtration rate (mL/min/ 1.73 m2) = (104 × Cystatin C−1.019 × 0.996age) − 8.
A self-administered questionnaire was used to obtain information on demography, smoking habits, alcohol drinking, socioeconomic status, and medication use and history. After the participants completed the questionnaires, trained nurses confirmed them with the participants. The body mass index was calculated as weight (kg) divided by height squared (m2). Cumulative pack-year smoking was estimated by multiplying the average number of packs smoked daily by the number of smoking years. Using an automated sphygmomanometer (BP-8800; Omron Health Care, Tokyo, Japan), the mean of two consecutive measurements on the right arm with participants in a seated position after a 5-minute rest were used to determine blood pressure. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or as the use of anti-hypertensive medications. Diabetes mellitus was defined as a hemoglobin A1c ≥6.1% (per the Japan Diabetes Society protocol; equivalent to ≥6.5% in the National Glycohemoglobin Standardization Program),26 a fasting blood glucose ≥126 mg/dl, or the use of antidiabetic medications. Step counts were recorded over seven consequent days by a pedometer (DIGI-WALKER DW-200; Yamasa Tokei Keiki, Tokyo, Japan), and then the daily average steps were calculated.
2.3. AVC assessment
The detailed method for cardiac CT in SESSA was previously reported.23,27 In brief, subclinical CAVD, characterized by AVC, at baseline was measured by either electron-beam CT (EBCT) using a C-150 scanner (Imatron, San Francisco, CA, USA) or a 16-channel multidetector row CT (MDCT) with an Aquilion scanner (Toshiba, Tokyo, Japan), while that at follow-up was measured by MDCT. Images were obtained from the level of the root of the aorta through the heart at a slice thickness of 3 mm with a scan time of 100 ms (EBCT) or 320 ms (MDCT). We acquired images at 70% of the cardiac cycle using electrocardiogram triggering during a single breath-hold. A DICOM workstation and AccuImage software (AccuImage Diagnosis, San Francisco, CA, USA) were used to quantify calcium scores. The calcium score was calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield units within this area, as described by the Agatston method.28 AVC was identified according to the methods from the Multi-Ethnic Study of Atherosclerosis (MESA);21,29–31 any calcified lesion residing within the aortic valve leaflets. Calcification of the aortic annulus, aortic sinuses, ascending aorta, or coronary arteries was excluded. All CT images were assessed by one trained medical technologist blinded to the clinical information of the participants. The intrareader reproducibility, evaluated among a random sample of 10% of CT images, was 0.97 for EBCT and 0.98 for MDCT. Since a stratified analysis by CT-type showed similar results, the EBCT and MDCT images were considered equivalent. Other studies have reported comparable findings for AVC assessment by EBCT and MDCT.32,33
2.4. Statistical analysis
Data for participants’ characteristics are shown as mean and standard deviation (SD) for continuous variables with approximately normal distributions, as medians and interquartile ranges for continuous variables with skewed distributions, and as percentages for categorical variables. Skewed distributed variables such as pack-year smoking, C-reactive protein, and AVC score were log transformed for analysis. Characteristics were compared according to AVC prevalence or incidence using an unpaired Student’s t-test, Mann–Whitney U-test, or χ2 test.
AVC prevalence was defined as AVC score >0.21,29 AVC incidence was also defined as detectable AVC (AVC score >0) at follow-up among participants with no AVC (AVC score = 0) at baseline;21,30,31 these data were treated as dichotomous outcomes. Further, as the best method for modeling AVC progression is unknown, it was modeled multiplicatively as a log transformation of the difference in AVC score between baseline and follow-up surveys among those with detectable AVC at baseline, because of a skewed distribution;21 this data was treated as a continuous outcome.
As the prevalence or incidence of AVC was >10% in our cohort, the odds ratio could not be used to assess relative risk (RR). Thus, we used a Poisson regression with a robust error variance34 to estimate RR and 95% confidence interval (CI) according to quartiles of serum micronutrients levels for prevalence, and their tertiles for incidence. Quartiles or tertiles of serum micronutrients levels were used because there are no established cutoff points for these measures in relation to CAVD, and a sufficient sample size was obtained for each group. Similar to previous reports,20,21 multivariate models included covariates selected prior to analyses based on the biologic plausibility that they may confound the associations of serum micronutrients with AVC.2 In Model 1, we adjusted for age. In Model 2, we also adjusted for behavioral characteristics (pack-year smoking, drinking habit [yes/no], daily steps), body mass index, and glomerular filtration rate. In Model 3, we further adjusted for hypertension (yes/no), low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, lipid lowering therapy (yes/no), diabetes mellitus (yes/no), and C-reactive protein. We included CT-type (EBCT or MDCT) as a covariate in all models to account for CT scanner changes.21,30,31 Additionally, we included follow-up duration as a covariate in all models in the longitudinal analysis. As further adjustment for education year did not significantly affect the findings, this variable was not included in the model. Tests for trend across categories were also based on assigning the median value for each category and modeling this variable as a continuous variable. In addition, we evaluated a mutually-adjusted model containing all variables in multivariate analysis for AVC prevalence and incidence. Linear regression was used to assess the associations of serum micronutrients levels with AVC progression. The same modeling as above was used for progression with additional adjustment for baseline AVC score. Analyses were performed using statistical software (STATA v14.0; Stata Corp LP, College Station, TX, USA). Two-tailed p values of <0.05 were considered statistically significant.
3. RESULTS
3.1. AVC prevalence
The baseline characteristics of all participants according to AVC prevalence are shown in Table 1. We found AVC prevalence of 173 (18.4%) in all participants at baseline. The participants with AVC were older and had less favorable risk factor distributions than those without AVC, including hypertension, diabetes mellitus, medication status, lower glomerular filtration rate and daily steps, and higher C-reactive protein levels. Among serum micronutrients, those with AVC had lower serum levels of magnesium, and higher serum levels of phosphorus and calcium, than those without AVC. The characteristics according to quartiles of serum micronutrients levels are shown in Supplemental Tables 1–3. The participants with higher levels of serum magnesium had lower diastolic blood pressure, less prevalence of diabetes mellitus, and higher low-density lipoprotein cholesterol levels. Higher serum phosphorus levels were associated with younger age, more frequent current smokers, and higher levels of serum magnesium and calcium. Participants with higher levels of serum calcium tend to be older, had unfavorable CVD risk factor profiles, including higher pack-year smoking, hypertension, diabetes mellitus, and lower glomerular filtration rate, and had higher levels of serum phosphorus.
Table 1.
Baseline characteristics of study participants with or without aortic valve calcification (SESSA, Shiga, Japan, 2006–08 at baseline).
| Variables | Overall n = 938 |
Without AVC n = 765 |
With AVC n = 173 |
p value |
|---|---|---|---|---|
| Age, years | 63.7 (9.9) | 62.2 (10.0) | 70.6 (6.3) | <0.001 |
| Body mass index, kg/m2 | 23.5 (3.0) | 23.4 (3.0) | 23.7 (3.1) | 0.334 |
| Smoking status, % | 0.787 | |||
| Current | 32.5 | 32.8 | 31.2 | |
| Former | 49.6 | 49.7 | 49.1 | |
| Pack-year smoking | 24.0 (5.0, 43.8) | 24.0 (5.0, 43.0) | 26.4 (7.5, 47.5) | 0.312 |
| Alcohol drinker, % | 77.6 | 78.6 | 73.4 | 0.087 |
| Education year, years | 12.5 (3.1) | 12.7 (3.0) | 11.7 (3.6) | <0.001 |
| Hypertension, % | 53.0 | 49.3 | 69.4 | <0.001 |
| Systolic blood pressure, mmHg | 135.8 (18.7) | 134.3 (18.6) | 142.2 (17.7) | <0.001 |
| Diastolic blood pressure, mmHg | 79.5 (10.9) | 79.6 (11.0) | 79.2 (10.6) | 0.695 |
| Anti-hypertensive medications, % | 28.4 | 25.5 | 41.0 | <0.001 |
| Diabetes mellitus, % | 20.4 | 18.8 | 27.2 | 0.014 |
| Fasting glucose, mg/dL | 102.2 (21.1) | 101.8 (20.3) | 104.4 (24.2) | 0.135 |
| Anti-diabetic medications, % | 9.3 | 7.7 | 16.2 | 0.001 |
| LDL cholesterol, mg/dL | 125.3 (31.2) | 124.4 (30.3) | 129.2 (34.7) | 0.069 |
| HDL cholesterol, mg/dL | 59.0 (16.9) | 59.4 (17.1) | 57.1 (15.9) | 0.103 |
| Lipid-lowering therapy, % | 12.4 | 10.7 | 19.7 | 0.001 |
| Daily steps | 7999.2 (3151.2) | 8204.5 (3084.6) | 7091.5 (3289.0) | <0.001 |
| Glomerular filtration rate, mL/min/1.73 m2 | 75.6 (15.6) | 77.5 (15.0) | 67.1 (15.3) | <0.001 |
| C-reactive protein, mg/L | 0.44 (0.21, 0.89) | 0.42 (0.20, 0.83) | 0.58 (0.26, 1.14) | 0.004 |
| Serum magnesium, mg/dL | 1.98 (0.18) | 1.99 (0.17) | 1.95 (0.20) | 0.012 |
| Serum phosphorus, mg/dL | 3.12 (0.36) | 3.10 (0.36) | 3.17 (0.38) | 0.037 |
| Serum calcium, mg/dL | 8.74 (0.28) | 8.72 (0.28) | 8.84 (0.29) | <0.001 |
| Baseline AVC score | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 37.1 (12.4, 118.1) | <0.001 |
The prevalence of AVC was defined as AVC score >0. Data are expressed as mean (standard deviation), median (25th, 75th), or percentage. Calcium levels were corrected for serum albumin concentration. Differences in characteristics were evaluated using the unpaired Student’s t-test, Mann–Whitney U-test, or χ2 test.
AVC, aortic valve calcification; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SESSA, Shiga Epidemiological Study of Subclinical Atherosclerosis.
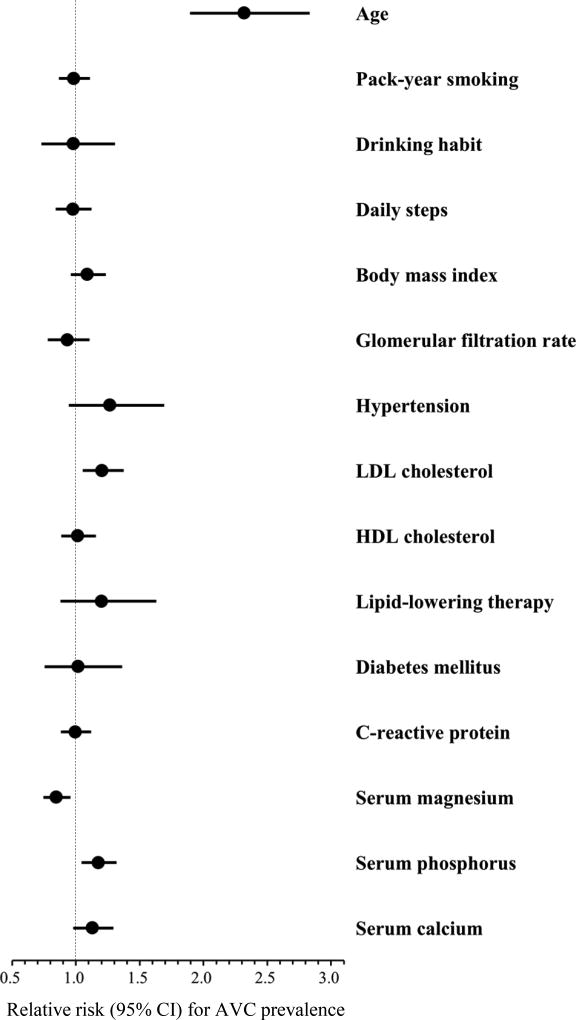
The association of serum micronutrients levels with AVC prevalence is shown in Table 2. Serum magnesium had an inverse association with AVC prevalence (all p for trend <0.05), and the relative risks (RR) for AVC prevalence in the highest versus lowest category of serum magnesium were significantly lower after further adjustment for confounders (Model 2) as well as for CVD risk factors (RR, 0.62; 95% confidence interval [CI], 0.44–0.86 in Model 3). By contrast, serum phosphorus showed a positive association with AVC prevalence (all p for trend <0.05), while the RRs for AVC prevalence in the highest versus lowest category of serum phosphorus were significantly higher in all models (RR, 1.45; 95% CI, 1.02–2.04 in Model 3). Age-adjusted RR for AVC prevalence in the highest versus lowest category of serum calcium was significantly higher (Model 1). However, an additional adjustment for confounders and CVD risk factors attenuated this association (RR, 1.43; 95% CI, 0.95–2.15 in Model 3). Similar results were observed for the association of 1-standard deviation (SD) higher in serum micronutrients levels with AVC prevalence. In addition to serum micronutrients, age and low-density lipoprotein cholesterol levels were positively associated with AVC prevalence (Fig.1).
Table 2.
Serum magnesium, phosphorus, and calcium levels and prevalence of aortic valve calcification (SESSA, Shiga, Japan, 2006–08 at baseline)
| Quartiles of serum micronutrients (n = 938) |
p for trend |
1-SD higher | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Q1 | Q2 | Q3 | Q4 | |||
| Magnesium (mg/dL) | ||||||
| Median (range) | 1.8 (1.3–1.8) | 1.9 (1.9–1.9) | 2.0 (2.0–2.0) | 2.1 (2.1–2.5) | ||
| no. of event / total | 48 / 200 | 38 / 191 | 37 / 230 | 50 / 317 | ||
| Model 1, RR (95% CI) | 1 (ref) | 0.83 (0.58–1.19) | 0.77 (0.54–1.10) | 0.68 (0.49–0.94)a | 0.022 | 0.86 (0.77–0.97)a |
| Model 2, RR (95% CI) | 1 (ref) | 0.82 (0.57–1.17) | 0.77 (0.53–1.11) | 0.64 (0.45–0.89)b | 0.010 | 0.84 (0.74–0.95)b |
| Model 3, RR (95% CI) | 1 (ref) | 0.80 (0.56–1.15) | 0.75 (0.52–1.09) | 0.62 (0.44–0.86)b | 0.006 | 0.83 (0.73–0.94)b |
| Phosphorus (mg/dL) | ||||||
| Median (range) | 2.7 (1.7–2.8) | 3.0 (2.9–3.0) | 3.2 (3.1–3.3) | 3.6 (3.4–4.4) | ||
| no. of event / total | 39 / 230 | 30 / 192 | 53 / 272 | 51 / 244 | ||
| Model 1, RR (95% CI) | 1 (ref) | 0.99 (0.66–1.50) | 1.16 (0.81–1.65) | 1.43 (1.01–2.02)a | 0.036 | 1.19 (1.06–1.34)b |
| Model 2, RR (95% CI) | 1 (ref) | 1.01 (0.67–1.53) | 1.14 (0.80–1.63) | 1.45 (1.02–2.05)a | 0.035 | 1.19 (1.06–1.33)b |
| Model 3, RR (95% CI) | 1 (ref) | 1.01 (0.67–1.53) | 1.15 (0.81–1.63) | 1.45 (1.02–2.04)a | 0.035 | 1.18 (1.05–1.33)b |
| Calcium (mg/dL) | ||||||
| Median (range) | 8.4 (7.8–8.5) | 8.6 (8.6–8.7) | 8.8 (8.8–8.9) | 9.0 (9.0–9.6) | ||
| no. of event / total | 26 / 230 | 26 / 218 | 54 / 253 | 67 / 237 | ||
| Model 1, RR (95% CI) | 1 (ref) | 0.83 (0.51–1.35) | 1.29 (0.85–1.95) | 1.61 (1.09–2.40)a | 0.001 | 1.21 (1.05–1.38)b |
| Model 2, RR (95% CI) | 1 (ref) | 0.81 (0.50–1.3) | 1.24 (0.82–1.88) | 1.42 (0.95–2.13) | 0.020 | 1.14 (0.99–1.31) |
| Model 3, RR (95% CI) | 1 (ref) | 0.83 (0.51–1.34) | 1.22 (0.80–1.85) | 1.43 (0.95–2.15) | 0.021 | 1.13 (0.98–1.31) |
The prevalence of AVC was defined as AVC score >0. Calcium levels were corrected for serum albumin concentration. Model 1 was adjusted for age. Model 2 was adjusted for age, pack-year smoking, drinking habit, daily steps, body mass index, and glomerular filtration rate. Model 3 was adjusted for Model 2 plus hypertension, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, lipid lowering therapy, diabetes mellitus, and C-reactive protein. In addition, CT-type was included as a covariate in all models.
p value: a < 0.05; b < 0.01; c < 0.001.
CI, confidence interval; CT, computed tomography; RR, relative risk; SD, standard deviation; other abbreviations are shown in Table 1.
Figure 1. Association of demographics, behavioral and cardiovascular risk factors, and serum micronutrients with AVC prevalence.
Circle markers and horizontal lines indicate relative risks and 95% CI for AVC prevalence, respectively. Relative risks for continuous variables are expressed as per 1 standard deviation higher in values of each factor, with the following exception: pack-year smoking and C-reactive protein (per 1-log higher). All variables listed were mutually adjusted and further adjusted for computed tomography type. AVC, aortic valve calcification; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
3.2. AVC incidence and progression
The participants’ characteristics according to AVC incidence are shown in Supplemental Table 4. During a median follow-up of 5.1 years (interquartile range, 3.6, 6.0), we found AVC incidence of 138 (23.2%) in participants with no AVC at baseline (n = 596). The participants with AVC incidence were older and had more comorbidities than those free of AVC incidence, including body mass index, hypertension, diabetes mellitus, higher low-density lipoprotein cholesterol levels, lower high-density lipoprotein cholesterol levels and glomerular filtration rate, and medication status.
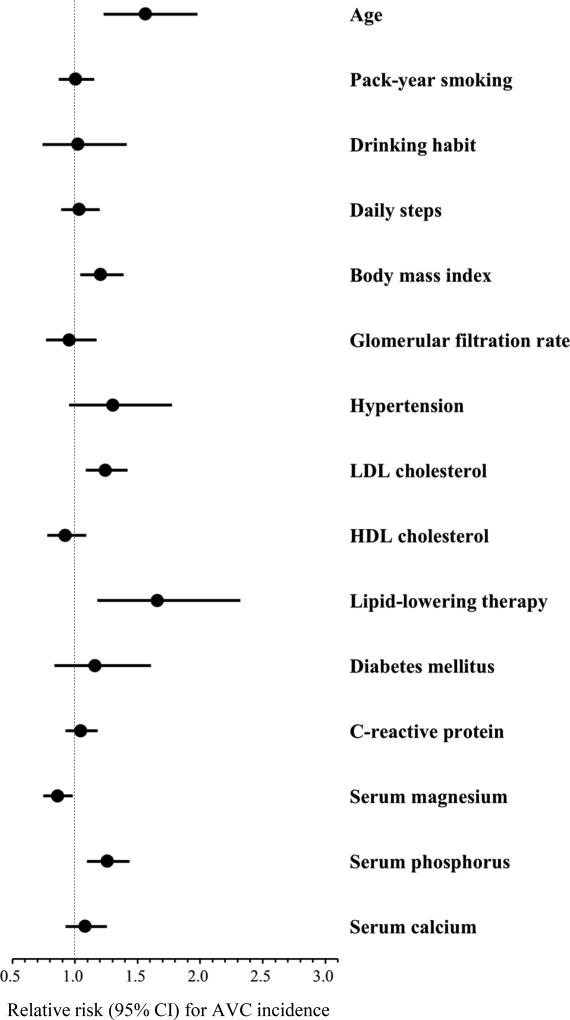
The association of serum micronutrients levels with AVC incidence is shown in Table 3. After further adjustment for confounders (Model 2) and CVD risk factors (Model 3), serum magnesium was inversely associated with AVC incidence. RRs for AVC incidence were significantly lower in the highest compared with lowest category of serum magnesium, while their inverse linear trends were also statistically significant in Models 2 and 3 (RR, 0.62; 95% CI, 0.42–0.92; p for trend = 0.012 in Model 3). Serum phosphorus showed a positive association with AVC incidence (all p for trend <0.01), and RRs for AVC incidence in the highest compared with lowest category of serum phosphorus were significantly higher in all models (RR, 1.93; 95% CI, 1.28–2.91 in Model 3). There was no association of serum calcium levels with AVC incidence. Similar results were observed for the association of 1-SD higher in serum micronutrients levels with AVC incidence. In addition to serum micronutrients, age, body mass index, low-density lipoprotein cholesterol levels, and lipid-lowering therapy were positively associated with AVC incidence (Fig. 2). Among participants with AVC at baseline (n = 131), the median (interquartile range) value of the annualized change in AVC score was 14.8 (3.1, 41.6) Agatston units per year. In this group, there was no relationship of any serum micronutrients levels with AVC progression (Supplemental Table 5).
Table 3.
Serum magnesium, phosphorus, and calcium levels and incidence of aortic valve calcification (SESSA, Shiga, Japan, 2006–08 at baseline and 2010–2014 at follow-up)
| Tertiles of serum micronutrients (n = 596) | p for trend | 1-SD higher | |||
|---|---|---|---|---|---|
|
| |||||
| T1 | T2 | T3 | |||
| Magnesium (mg/dL) | |||||
| Median (range) | 1.8 (1.3–1.8) | 2.0 (1.9–2.0) | 2.1 (2.1–2.5) | ||
| no. of event / total | 29 / 111 | 65 / 271 | 44 / 214 | ||
| Model 1, RR (95% CI) | 1 (ref) | 0.95 (0.66–1.38) | 0.79 (0.53–1.17) | 0.189 | 0.91 (0.79–1.05) |
| Model 2, RR (95% CI) | 1 (ref) | 0.90 (0.64–1.29) | 0.66 (0.45–0.97)a | 0.022 | 0.86 (0.75–0.98)a |
| Model 3, RR (95% CI) | 1 (ref) | 0.84 (0.59–1.20) | 0.62 (0.42–0.92)a | 0.012 | 0.85 (0.74–0.97)a |
| Phosphorus (mg/dL) | |||||
| Median (range) | 2.7 (1.7–2.8) | 3.0 (2.9–3.1) | 3.4 (3.2–4.4) | ||
| no. of event / total | 25 / 148 | 46 / 197 | 67 / 251 | ||
| Model 1, RR (95% CI) | 1 (ref) | 1.38 (0.89–2.13) | 1.72 (1.15–2.59)b | 0.006 | 1.21 (1.05–1.39)b |
| Model 2, RR (95% CI) | 1 (ref) | 1.41 (0.91–2.17) | 1.76 (1.16–2.65)b | 0.005 | 1.22 (1.06–1.40)b |
| Model 3, RR (95% CI) | 1 (ref) | 1.41 (0.92–2.18) | 1.93 (1.28–2.91)b | 0.001 | 1.25 (1.10–1.42)b |
| Calcium (mg/dL) | |||||
| Median (range) | 8.4 (7.8–8.5) | 8.7 (8.6–8.8) | 8.9 (8.9–9.6) | ||
| no. of event / total | 39 / 198 | 38 / 193 | 61 / 205 | ||
| Model 1, RR (95% CI) | 1 (ref) | 0.88 (0.59–1.30) | 1.19 (0.85–1.67) | 0.261 | 1.15 (0.99–1.33) |
| Model 2, RR (95% CI) | 1 (ref) | 0.81 (0.55–1.18) | 1.07 (0.76–1.51) | 0.560 | 1.10 (0.94–1.28) |
| Model 3, RR (95% CI) | 1 (ref) | 0.79 (0.54–1.14) | 1.09 (0.77–1.55) | 0.496 | 1.08 (0.93–1.26) |
The incidence of AVC was defined as detectable AVC (AVC score >0) at follow–up among those with no AVC (AVC score = 0) at baseline. Calcium levels were corrected for serum albumin concentration. Model 1 was adjusted for age. Model 2 was adjusted for age, pack-year smoking, drinking habit, daily steps, body mass index, and glomerular filtration rate. Model 3 was adjusted for Model 2 plus hypertension, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, lipid lowering therapy, diabetes mellitus, and C-reactive protein. CT–type and follow–up duration were also included as covariates in all models.
p value: a < 0.05; b < 0.01; c < 0.001.
CI, confidence interval; CT, computed tomography; RR, relative risk; SD, standard deviation; other abbreviations are shown in Table 1.
Figure 2. Association of demographics, behavioral and cardiovascular risk factors, and serum micronutrients with AVC incidence.
Circle markers and horizontal lines indicate relative risks and 95% CI for AVC incidence, respectively. Relative risks for continuous variables are expressed as per 1 standard deviation higher in values of each factor, with the following exception: pack-year smoking and C-reactive protein (per 1-log higher). All variables listed were mutually adjusted and further adjusted for computed tomography type and follow-up duration. Abbreviations are shown in Fig. 1.
4. DISCUSSION
In this prospective community-based study in Japanese men without apparent CVD and CKD with a median follow-up of 5.1 years, we found that serum magnesium levels were inversely associated, whereas serum phosphate levels were positively associated, with AVC prevalence and incidence independent of health behaviors and CVD risk factors. As CAVD shares histological and epidemiological features with vascular atherosclerosis,35,36 our findings are in line with previous reports linking lower serum magnesium levels to greater risk of CVD outcomes.10–12 Low serum magnesium levels were also reported to be associated with adverse CVD risk factor profiles.16,17 Indeed, we found that lower serum magnesium levels were related to unfavorable CVD risk factor distributions including blood pressure and fasting glucose (Supplemental Table 1). However, in our analysis, the inverse associations of serum magnesium with AVC prevalence or incidence remained significant after adjustment for health behaviors and CVD risk factors (some of which may be on the causal pathway between serum magnesium and CAVD). Additionally, experimental studies in cultured human endothelium and animals reported that magnesium deficiency promoted endothelial dysfunction and systemic inflammation,37,38 which are factors involved in the early pathogenic process of CAVD.1,2 Thus, magnesium may have beneficial effects on the onset of CAVD via additional mechanisms beyond known traditional pathways.
We also found that serum phosphorus levels were positively and independently associated with AVC prevalence and incidence. The majority of all phosphorus detected in the extracellular fluid space is in the form of inorganic phosphate.39 Linefsky et al. reported a significant cross-sectional association of high serum phosphate levels with echocardiographic AVC prevalence among participants aged ≥65 years in the Cardiovascular Health Study.20 Further, in the Multi-Ethnic Study of Atherosclerosis (MESA), they also found a significant association of serum phosphate levels with prevalence, but not with incidence, for CT-based AVC, among participants aged 45–84 years.21 The lack of association of serum phosphate with AVC incidence may relate to the relatively short follow-up periods (mean, 2.4 years) and low incidence of AVC (4.1%) in these participants. Another reason for these contrasting findings may relate to differences in the sample demographics (e.g., AVC distribution and CVD risk factor profiles) and methodology (e.g., MESA investigation included participants with CKD) between the studies. In support of our findings, no independent relationship of serum calcium levels with AVC was previously reported in the Cardiovascular Health Study.20 As only 3 population-based studies20,21 have investigated the association of serum micronutrients with AVC, further studies are required to confirm these associations in other populations and to identify mechanisms underlying the contrasting findings.
Serum magnesium and phosphorus showed significant associations with AVC incidence, but not with its progression. Similarly, previous epidemiological studies have reported that CVD risk factors had a great impact on its incidence, but a less impact on its progression.30,31 These findings may support the hypothesis that these factors contribute to lesion formation or early stage pathology of CAVD, whereas late stage progression may be more affected by direct paracrine actions, systemic regulators of calcification, or myofibroblast transdifferentiation toward an osteoblastic phenotype.30,40 Alternatively, the small number of participants with baseline AVC may have limited statistical power to show a relation of serum micronutrients to AVC progression. Overall, our results are consistent with previous reports, although these epidemiological studies are essentially limited in its ability to draw mechanistic conclusions.
Levels of serum magnesium, phosphorus, and calcium are influenced by numerous metabolic pathways, as well as by dietary intake. Serum phosphorus and calcium levels are weakly related to dietary intake in individuals without CKD.41,42 Further, serum magnesium is responsive to supplementation and long-term changes in dietary intake,43,44 although the correlation of dietary intake with serum levels is low,10,11 suggesting other regulatory and homeostatic mechanisms, primarily through renal reabsorption and excretion.45 Importantly, however, no more than approximately 60% of Japanese men meet the Recommended Daily Allowance for dietary magnesium intake.46
This is the first study to demonstrate that serum magnesium levels were inversely associated, while serum phosphorus levels were positively associated, with AVC prevalence or incidence independent of possible confounders and CVD risk factors in the community-based prospective design. Given the subclinical early stage of CAVD characterized by AVC, the clinical implication of the present study is that serum magnesium and phosphate may be involved in the onset or early stage pathophysiology of CAVD and that these serum micronutrients may be useful candidates for risk prediction or prevention targets for CAVD. Thus, the roles of magnesium and phosphorus in CVAD warrant further studies.
There are several limitations in our study. First, measurements of serum micronutrients took place at baseline, and may not accurately reflect their long-term distributions during the follow-up period. Further, although we carefully controlled for the major known confounders, our findings, at least in part, may be explained by differences in unknown confounders. Second, participants without follow-up had less alcohol drinking and fewer daily steps compared with those with follow-up, although there were no significant differences in serum micronutrient measures between the groups (Supplemental Table 6). Speculatively, participants without follow-up may have been at higher risk for AVC progression (because of a higher baseline AVC score). Additionally, we may have underestimated the association of serum micronutrients with AVC progression, as the loss of participants to follow-up reduced the available sample size in this higher-risk strata. Finally, as only Japanese men were included in this study, our results are restricted to men of a single ethnic group.
Supplementary Material
HIGHLIGHTS.
Calcific aortic valve disease (CAVD) is the most common heart valve disease worldwide.
Subclinical early stage of CAVD is characterized by aortic valve calcification (AVC).
Our population-based study examined relations of serum micronutrients levels to AVC prevalence, incidence, and progression.
Serum magnesium were inversely related, while serum phosphorus were positively related, to AVC prevalence or incidence.
Serum micronutrients may be potential candidates for risk prediction or prevention of CAVD and warrant further studies.
Acknowledgments
The authors thank staff members of the SESSA, and Ms. Muramatsu for support with AVC analysis. SESSA Research Group member are listed in Supplemental Data.
Financial support
This research was supported by the Uehara Memorial Foundation; by the Young Investigator Grant of the Shimane University; by the Grants-in-aid for Scientific Research (A) 13307016, (A) 17209023, (A) 21249043, (A) 23249036, and (A) 25253046 from the Ministry of Education, Culture, Sports, Science and Technology, Japan; by Glaxo-Smith Klein; and by National Institutes of Health (NIH), USA [R01HL068200].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
T.H., K.M., A.F., A.K., N.M., T.Y., M.H., and H.U. contributed to the conception and design. T.H., A.F., A.K., N.M., A.S., M.Z., and SESSA Research Group member collected the data. T.H., K.M., and H.U. analyzed and interpreted the data. T.H. drafted the article. All authors critically revised the article for intellectual content. All authors approved the final version.
References
- 1.Rajamannan NM, Moura L. The lipid hypothesis in calcific aortic valve disease: The role of the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:774–776. doi: 10.1161/ATVBAHA.116.307435. [DOI] [PubMed] [Google Scholar]
- 2.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, Heckbert SR, Otto CM, Probstfield JL, Kronmal RA, O'Brien KD. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012;5:619–625. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 5.Saikrishnan N, Kumar G, Sawaya FJ, Lerakis S, Yoganathan AP. Accurate assessment of aortic stenosis: A review of diagnostic modalities and hemodynamics. Circulation. 2014;129:244–253. doi: 10.1161/CIRCULATIONAHA.113.002310. [DOI] [PubMed] [Google Scholar]
- 6.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 7.Iung B, Vahanian A. Degenerative calcific aortic stenosis: A natural history. Heart. 2012;98(Suppl 4):iv7–13. doi: 10.1136/heartjnl-2012-302395. [DOI] [PubMed] [Google Scholar]
- 8.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 9.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 10.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 1998;136:480–490. doi: 10.1016/s0002-8703(98)70224-8. [DOI] [PubMed] [Google Scholar]
- 11.Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc. 2013;2:e000114. doi: 10.1161/JAHA.113.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2013;98:160–173. doi: 10.3945/ajcn.112.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 2008;156:556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 15.Shin S, Kim KJ, Chang HJ, Cho I, Kim YJ, Choi BW, Rhee Y, Lim SK, Yang WI, Shim CY, Ha JW, Jang Y, Chung N. Impact of serum calcium and phosphate on coronary atherosclerosis detected by cardiac computed tomography. Eur Heart J. 2012;33:2873–2881. doi: 10.1093/eurheartj/ehs152. [DOI] [PubMed] [Google Scholar]
- 16.Shechter M. Magnesium and cardiovascular system. Magnes Res. 2010;23:60–72. doi: 10.1684/mrh.2010.0202. [DOI] [PubMed] [Google Scholar]
- 17.Rude RK. Magnesium. New York, NY: Informa Healthcare; 2010. [Google Scholar]
- 18.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid IR, Bolland MJ, Avenell A, Grey A. Cardiovascular effects of calcium supplementation. Osteoporos Int. 2011;22:1649–1658. doi: 10.1007/s00198-011-1599-9. [DOI] [PubMed] [Google Scholar]
- 20.Linefsky JP, O'Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS, Siscovick DS, Kestenbaum B. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: The cardiovascular health study. J Am Coll Cardiol. 2011;58:291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linefsky JP, O'Brien KD, Sachs M, Katz R, Eng J, Michos ED, Budoff MJ, de Boer I, Kestenbaum B. Serum phosphate is associated with aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2014;233:331–337. doi: 10.1016/j.atherosclerosis.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens DS, Katz R, Johnson E, Shavelle DM, Probstfield JL, Takasu J, Crouse JR, Carr JJ, Kronmal R, Budoff MJ, O'Brien KD. Interaction of age with lipoproteins as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:1200–1207. doi: 10.1001/archinte.168.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamazoe M, Hisamatsu T, Miura K, Kadowaki S, Zaid M, Kadota A, Torii S, Miyazawa I, Fujiyoshi A, Arima H, Sekikawa A, Maegawa H, Horie M, Ueshima H. Relationship of insulin resistance to prevalence and progression of coronary artery calcification beyond metabolic syndrome components: Shiga Epidemiological Study of Subclinical Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1703–1708. doi: 10.1161/ATVBAHA.116.307612. [DOI] [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S Collaborators Developing the Japanese Equation for Estimated GFR. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61:197–203. doi: 10.1053/j.ajkd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in japan from japan diabetes society to national glycohemoglobin standardization program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hisamatsu T, Miura K, Arima H, et al. Smoking, smoking cessation, and measures of subclinical atherosclerosis in multiple vascular beds in japanese men. J Am Heart Assoc. 2016;5:e003738. doi: 10.1161/JAHA.116.003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 29.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 30.Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O'Brien KD. Incidence and progression of aortic valve calcium in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2010;105:701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz R, Budoff MJ, Takasu J, Shavelle DM, Bertoni A, Blumenthal RS, Ouyang P, Wong ND, O'Brien KD. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: The Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes. 2009;58:813–819. doi: 10.2337/db08-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O'Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of ct measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the Multi-Ethnic Study of Atherosclerosis. Acad Radiol. 2006;13:166–172. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 33.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, O'Brien KD, Carr JJ. Effect of scanner type on the reproducibility of extracoronary measures of calcification: The Multi-Ethnic Study of Atherosclerosis. Acad Radiol. 2007;14:1043–1049. doi: 10.1016/j.acra.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 35.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 36.Agmon Y, Khandheria BK, Meissner I, Sicks JR, O'Fallon WM, Wiebers DO, Whisnant JP, Seward JB, Tajik AJ. Aortic valve sclerosis and aortic atherosclerosis: Different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol. 2001;38:827–834. doi: 10.1016/s0735-1097(01)01422-x. [DOI] [PubMed] [Google Scholar]
- 37.Ferre S, Baldoli E, Leidi M, Maier JA. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim Biophys Acta. 2010;1802:952–958. doi: 10.1016/j.bbadis.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Kharitonova M, Iezhitsa I, Zheltova A, Ozerov A, Spasov A, Skalny A. Comparative angioprotective effects of magnesium compounds. J Trace Elem Med Biol. 2015;29:227–234. doi: 10.1016/j.jtemb.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Vinod KB. Serum inorganic phosphorus. Boston, MA: Butterworths; 1990. [PubMed] [Google Scholar]
- 40.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012;81:1116–1122. doi: 10.1038/ki.2011.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klevay LM, Milne DB. Low dietary magnesium increases supraventricular ectopy. Am J Clin Nutr. 2002;75:550–554. doi: 10.1093/ajcn/75.3.550. [DOI] [PubMed] [Google Scholar]
- 44.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050–1056. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 45.Arnaud MJ. Update on the assessment of magnesium status. Br J Nutr. 2008;99(Suppl 3):S24–36. doi: 10.1017/S000711450800682X. [DOI] [PubMed] [Google Scholar]
- 46.Akizawa Y, Koizumi S, Itokawa Y, Ojima T, Nakamura Y, Tamura T, Kusaka Y. Daily magnesium intake and serum magnesium concentration among Japanese people. J Epidemiol. 2008;18:151–159. doi: 10.2188/jea.JE2007381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.