Abstract
Answering the question as to why we age is tantamount to answering the question of what is life itself. There are countless theories as to why and how we age, but, until recently, the very definition of aging – senescence – was still uncertain. Here, we summarize the main views of the different models of senescence, with a special emphasis on the biochemical processes that accompany aging.
Though inherently complex, aging is characterized by numerous changes that take place at different levels of the biological hierarchy. We therefore explore some of the most relevant changes that take place during aging and, finally, we overview the current status of emergent aging therapies and what the future holds for this field of research.
From this multi-dimensional approach, it becomes clear that an integrative approach that couples aging research with systems biology, capable of providing novel insights into how and why we age, is necessary.
Keywords: Aging, Senescence, Anti-aging therapies, Biochemistry, Biology
1. Introduction
Aging is a topic that has captivated both scientists and philosophers throughout history. For Plato (428–347 BC), those who lived longer reached a philosophical understanding of mortal life, which lead to the desire in understanding everlasting ideas and truths, beyond the mortal world (Baars, 2012): “for wisdom and assured true conviction, a man is fortunate if he acquires them even on the verge of old age” (Cary et al., 1852). But perhaps the most accurate depiction of the human perception of aging comes from Giacomo Leopardi (1798–1837): “Old age is the supreme evil, because it deprives us of all pleasures, leaving us only the appetite for them, and it brings with it all sufferings. Nevertheless, we fear death, and we desire old age” (Leopardi et al., 1905).
In its broadest sense, aging merely refers to the changes that occur during an organisms' life-span, though the rate at which these take place varies widely (Kirkwood, 2005). Consequently, such definition comprises changes that are not necessarily deleterious, such as wrinkles and graying hair in humans, which do not affect the individual's viability. As Anton and co-workers put it (Anton et al., 2005), the phenotype is the end result of the interaction between genotype and external factors:
To differentiate these innocuous changes from those leading to increased risk of disease, disability or death, biogerontologists tend to use a more precise term – senescence – when describing aging (Dollemore, 2002). Senescence is, therefore, the progressive deterioration of bodily functions over time and normal human aging has been associated with a loss of complexity in a wide range of physiological processes and anatomic structures (Goldberger et al., 2002), including blood pressure (Kaplan et al., 1991), stride intervals (Hausdorff et al., 1997; Terrier and Dériaz, 2011), respiratory cycles (Peng et al., 2002; Schumann et al., 2010) and vision (Azemin et al., 2012), among others, such as postural dynamics (Manor et al., 2010), ultimately leading to decreased fertility and increased risk or mortality (Chesser, 2015; Lopez-Otin et al., 2013). Herein, however, we will refer to the more inclusive term “aging”, due to its extensive use in the literature. Though aging may be defined as the breakdown of self-organizing systems and reduced ability to adapt to the environment (Vasto et al., 2010), this is still a rather complex biological process with poorly understood mechanism(s) of regulation. Explanations of the aging mechanisms have become unexpectedly complicated. Where gerontologists once looked for a single, all-encompassing theory that could explain aging, such as a single gene or the decline of the immune system, they are now finding that multiple processes, combining and interacting on many levels, are on the basis of the aging process (Dollemore, 2002; Guarente, 2014) These processes take place not only at a cellular and molecular level, but also on tissues and organ systems. The relatively young science of aging is now becoming increasingly aware of the biochemical mechanisms that cause or react to aging (Yin and Chen, 2005). Hence, gerontology research currently stands on chemistry and biochemistry, as these are at the core of the aging processes. Advanced analytical studies are underway to observe and identify age-related changes in living organisms. Simultaneously, new synthetic and medicinal chemistry methodologies are yielding small molecule tools for the complete elucidation of complex biological pathways, as well as potential lifespan extending therapeutics (Ostler, 2012). However, to better understand how these could contribute to extend the knowledge of the mechanisms of aging, it is necessary to explore what are the prevailing theories as to why and how we age. Thus, we will extensively review and evaluate the prevalent theories of aging focusing on the major chemical, biological, psychological and pathological aspects of the process. The discussion of the different models of senescence will highlight the urgent need for system-wide approaches that provide a new, integrative view on aging research.
2. Theories of aging and how they shape the definitions of senescence
Many widespread theories as to why aging takes place abound. Generally, these consider it a programmed development (Tower, 2015a), though many disagree and the debate is still ongoing (Blagosklonny, 2013; Goldsmith, 2014, 2012, 2013). By 1990, Medvedev attempted to rationally classify the numerous theories of aging, which exceeded 300 (Medvedev, 1990). Aging has been attributed to molecular cross-linking (Bjorksten, 1968), free radical-induced damages (Harman, 1993), changes in immunological functions (Effros, 2005), telomere shortening (Kruk et al., 1995) and the presence of senescence genes in the DNA (Warner et al., 1987). More recently, however, a unified theory encompassing genes, the performance of genetic maintenance and repair systems, milieu and chance is becoming increasingly accepted (Rattan, 2006), highlighting the need for a systematic and integrative analysis of the aging process. The vast amount of research carried out concerning aging and aging-related processes makes it almost impossible to give a complete overview of the aging theories that have been put forth. Most of these, if not all, can, however, be classified into two categories: error theories and program hypotheses, which will be explored in the following sections. A third category – combined theories –, which contains certain elements of both groups, can be considered (Fig. 1). Such categorization is subjective and others have been suggested (Baltes et al., 2012; de Magalhães, 2005; Jin, 2010; Vina et al., 2007; Weinert and Timiras, 2003). As such, only a brief description of these prevailing theories will be discussed. However, despite whatever the theory, all aim at answering one question: what is the cause of aging? No matter the working hypothesis, one must consider that the underlying assumption that there is one single cause for aging may not be correct. Moreover, gerontologists may have to face the possibility that there may not be a universal cause of aging valid for all living organisms.
Fig. 1.
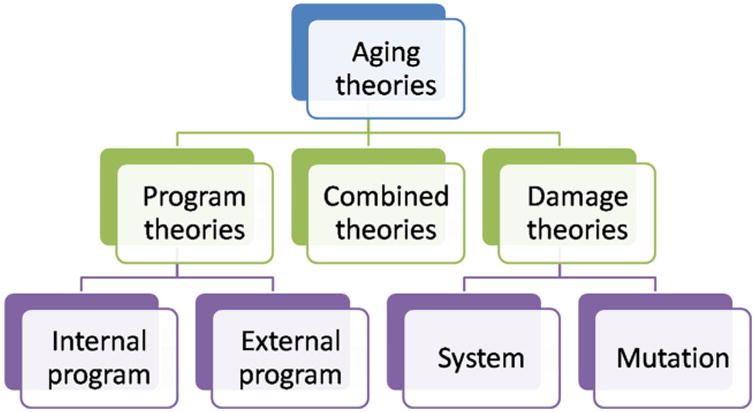
Categorization of the main theories of aging. Classification based on the worked developed by (Semsei, 2000) and (de Magalhães, 2013).
(1) Program theories
Programmed aging theories, sometimes referred to as active or adaptive aging theories, suggest that there is a deliberate deterioration with age because a limited life span results in evolutionary benefits (Goldsmith, 2012).
For many years, programmed aging has been debated and some studies have substantiated this hypothesis. For example, Ünal et al. (2011) have suggested that there are mechanisms that preserve the integrity of spores of aging diploid yeast cells. Through these mechanisms, aging diploid cells that are induced to sporulate appear to lose all age-associated damage to a point that is no longer detectable, though the assumption that these findings can be extrapolated to higher organisms has been put into question (Biliński et al., 2012).
Yet, though development and morphogenesis can be easily understood as programmed, as they are the end-result of a determined sequence of molecular and cellular events designed to produce a given phenotype (Austad, 2004), aging is mostly thought of as decay. If aging is indeed programmed, the purposes of such program remain unclear. Some have suggested that aging may constitute an altruistic plan (Longo et al., 2005), by eliminating post-reproductive age individuals, who would compete for resources, by avoiding overpopulation and by promoting adaptation through a succession of generations (Kirkwood Thomas and Melov, 2011). The supporters of this view underscore that the similarities between the biochemical pathways that regulate aging in organisms such as yeasts, flies and mice, together with evidence consistent with programmed death in salmon and other organisms, hint at the possibility that programmed aging can occur in higher eukaryotes (Longo et al., 2005). Moreover, this plan could be the result of “aging genes” (de Magalhães, 2013). Nonetheless, if this was the case, than certainly such mechanisms would be susceptible to inactivation, and, despite many gene mutations have been described as life-extending mutations (Barbieri et al., 2003; Fontana et al., 2010; Friedman and Johnson, 1988; Meléndez et al., 2003) none has been reported that abolishes the process of aging (Kirkwood, 2011). It should be noted that, in some model organisms, genes have been demonstrated to play a pivotal role in aging. In fact, the first described mutation to yield a significant extension in the lifespan of Caenorhabditis elegans was in the age-I gene, which was shown to result in a 65% increase in mean lifespan and a 110% increase in maximum lifespan of this organism (Johnson, 1990). Since then, many mutations that result in lifespan extension in C. elegans have been identified, most of which involving genes that are homologs of the of components of the insulin/IGF (insulin-like growth factor) pathway (Mattson, 2003), namely, daf-2/daf-16 (Kenyon, 2010) and sir2.1 (Guarente and Kenyon, 2000), which, interestingly, have been shown to interact to extend lifespan in C. elegans (Berdichevsky et al., 2006).
Composed mostly of post-mitotic cells, C. elegans is one of the most widely studied model organisms. With a lifespan ranging from days to a few weeks, it has been noted that, under caloric restriction (CR) and/or crowded conditions, C. elegans can enter an alternative stasis-like developmental pathway, called dauer (Riddle et al., 1981). This pathway consists of a developmental arrest, leading to an increased adult phase (de Magalhães, 2013; Kenyon et al., 1993; Meléndez et al., 2003). This arrest suggests that, at least partially, aging and development are coupled in C. elegans, as well as in other invertebrates (Brakefield et al., 2005). However, in addition to the severity of the restriction (30–70% fewer calories than the control group), the degree of lifespan lengthening in C. elegans depends on numerous factors, namely, age at onset of restriction (Weinert and Timiras, 2003). Though providing some key insights into longevity, invertebrates are, nevertheless, distant animal models and are likely unrepresentative of human biology and physiology.
The endocrine system has also been viewed as involved in “telling the time”. Because the levels of hormones such as growth hormone (GH) and its corresponding downstream target insulinlike growth factor I (IGF-1) decline with age, the idea that such changes cause aging has been suggested a few decades ago (Hammerman, 1987; Ho et al., 1987; Rudman, 1985), and, in rats, deficiency in growth hormone production (loss of function mutations at the Pit-I locus) has been linked to lifespan extension and delayed immune aging (Flurkey et al., 2001). Due to the fact that the brain regulates the endocrine system, the neuroendocrine theory of aging has emerged as the main hormone-based theory of aging (Finch, 2014; Meites, 2012), and, not surprisingly, many anti-aging products aim at restoring the levels of specific hormones in older people (Elewa and Zouboulis, 2014; Sah et al., 2013). Some studies have supported the idea that the insulin pathway is associated with human longevity, as individuals with mutated Prop-I gene – a pituitary transcription factor whose mutation causes dwarfism (Kržišnik et al., 2010) – may live longer and patients with GH and IGF-1 deficiencies have shown signs of early aging, despite actually living longer (Anisimov and Bartke, 2013; Brownborg et al., 1996). Some have proposed that such mechanisms could be activated by decreasing cellular replication (Kushner, 2013) or that it may operate on the basis of antioxidant regulation (Vitale et al., 2013). Whatever the mechanism, it is now clear that the early assumption that the aging process is driven by hormone changes that occur with age is unsubstantiated. If anything, the decrease in GH/IGF-1 signaling increases lifespan, not the contrary (de Magalhães, 2013) and, more broadly, hormonal changes may regulate aging as an indirect consequence of the developmental program. The imbalance on chemical processes caused by differential gene expression and hormonal changes may contribute to aging, but, so far, such assertions remain in the realm of speculation. Furthermore, the significant lifespan differences observed in numerous species, under identical conditions, seems to indicate that there is no pre-determined timeline for aging. Thus, under certain conditions, it may be possible to prolong or to curtail lifespan, leading to the hypothesis that aging is not predetermined, but rather the end-result of a “wear-and-tear” mechanism.
(2) Damage theories
Evolutionary biologists may argue that aging occurs due to the absence of natural selection at the post-reproductive stage of life (Johnson et al., 1999). Hence, aging is not programmed; instead, it is the absence of selection for maintenance (Medawar, 1952). Although such aging theories are subjectively appealing, as they convey a cure for aging, the accumulation of damage is a spontaneous entropy-driven process, and, as such, its kinetics can be genetically and environmentally modulated, resulting in the wide range of life-spans we observe (Aledo and Blanco, 2015).
Among the damage theories, a prevailing idea is that of oxidative damage (Harman, 1981). Reactive oxygen species (ROS) – partially reduced intermediates of oxygen that can be radical or non-radical molecules (Zelickson et al., 2013) – are generated during metabolism through a number of inter-related reactions Eqs. (1)–(4) (Novo and Parola, 2008) and are considered to lead to the cumulative DNA, protein and lipid damage (Piedrafita et al., 2015; Rinnerthaler et al., 2015; Thanan et al., 2014) (Fig. 2) observed over a lifetime (Freitas et al., 2013) (Fig. 3). Approximately 2–3% of oxygen taken up is chemically reduced by the addition of single electrons. Incomplete reduction of oxygen can generate a variety of biologically relevant ROS such as, hydrogen peroxide, the anion radical superoxide and the hydroxyl radical (Johnson et al., 1999). The electron transport chain in the mitochondria, the nicotinamide adenine dinucleotide phosphate oxidases (NADPH oxidase) and the 5-lipoxygenase as the three major sources of ROS in living cells (Novo and Parola, 2008). Multiple studies have highlighted the relatively haphazard molecular damage that ROS cause to lipids (Shah et al., 2001), proteins (Mishra et al., 2011) and nucleic acids (Dizdaroglu, 1992) and exposure to ROS have been demonstrated to trigger specific mechanisms aimed at neutralizing their effects (Silva et al., 2015).
Fig. 2.
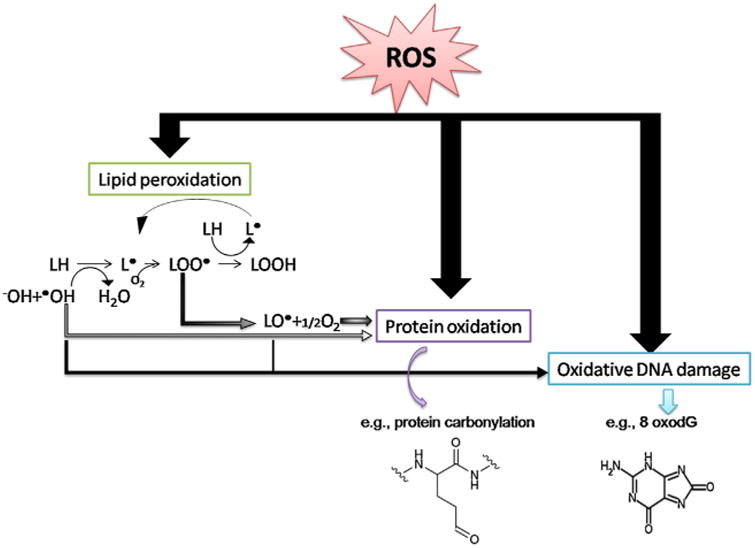
General mechanism of oxidative damage to biomolecules. Oxidative damage to lipids yields lipid peroxidation products, mainly localized at the cellular membrane, which results in a loss of membrane properties/function. Their reactive end products can induce damage to other molecules, such as proteins and DNA. In nuclear and mitochondrial DNA, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) is one of the predominant forms of free radical-induced oxidative lesions (Valavanidis et al., 2009). Potential outcomes include dysfunction of the affected biomolecules and interference with signaling pathways. Adapted from (Thanan et al., 2014).
Fig. 3.
The cumulative effect of ROS over time. ROS accumulation, oxidative stress and the imbalance of the normal redox state increases exponentially with age, accompanied by a marked decline of the cell repair machinery. Note that, despite only depicting the general stress response pathways, a typical Golgi pathway has yet to be described. Nonetheless, multiple stress factors may influence gene expression in the nucleus and cell homeostasis via alterations in the function of the Golgi apparatus (Kourtis and Tavernarakis, 2011). The figure was partly created using Servier medical art image bank (Servier, France).
- The four electron reduction reactions forming H2O from O2.
(1.1) (1.2) (1.3) (1.4) - Reactions involving H2O2
(2.1) (2.2) (2.3) - ROS reactions in the presence of transition metal ions (Fenton's reaction)
(3.1) (3.2) (3.3) (3.4) (3.5) - Carbon-centered free radicals generated through ROS
(4.1) (4.2) (4.3) (4.4)
Additionally, oxidative stress is known to affect both translation and protein turnover (Vogel et al., 2011) and has been demonstrated to contribute to cell signaling in a controlled fashion (Cassina et al., 2000; Inoue et al., 2003; Sata et al., 1997). The supposition that aging may be caused by ROS has been further substantiated by studies involving transgenic animals for genes encoding antioxidants. The life-span of Drosophila melanogaster has been extended by overexpression of both superoxide dismutase (SOD) and catalase, both antioxidant enzymes (Orr and Sohal, 1994; Tower, 2015b), and such gene modulation can be achieved through dietary intake (Wang et al., 2015). Oppositely, mice knocked out for GPX1 (encoding glutathione peroxidase), SOD1, SOD2 or SOD3 did not display a rapid aging phenotype, either resulting in normal mice (Ho et al., 1997) or animals which expired within a short time period due to cardiac failure (Melov et al., 1998). This may be because, as demonstrated in C. elegans, SOD overexpression increases life span not through an enhanced removal of O2•−, but by activating longevity-promoting transcription factors (Cabreiro et al., 2011).
Due to the fact that mitochondria are the major producer of ROS in mammalian cells, mitochondrial DNA (mtDNA) is therefore particularly susceptible to oxidative damage (Cui et al., 2011) and the main purine product of oxidative DNA base damage is 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-oxodG). This, upon replication, can cause characteristic G:T transversions at a relatively low frequency (Hanes et al., 2006), which results in mutations that lead to defective Electron Transport Chain (ETC) components. The subsequent incorporation of these into the ETC causes further increase in ROS species, ultimately leading to a “vicious cycle” of ROS production and mtDNA mutations, eventually leading to cellular damage levels incompatible with life (Alexeyev, 2009). Mitochondrial maintenance is, therefore, essential to preserve cellular homeostasis and impaired mitochondrial maintenance has been described as a shared hallmark of numerous human pathologies and aging (Artal-Sanz and Tavernarakis, 2009). Though this mechanism is not fully understood, recent studies have demonstrated that mitophagy, a specific and selective type of autophagy that targets mitochondria for degradation, interact with mitochondrial biogenesis in order to regulate mitochondrial content as well as longevity in C. elegans (Palikaras et al., 2015).
Nonetheless, one of the most considered aspect of ROS-induced damage in DNA and aging is DNA methylation levels. These vary with age, and it is commonly considered that DNA hypomethylation is a typical aspect of the aging process (Afanas'ev, 2014). ROS are active intermediates of DNA methylation, as well as of histone modification. These reactive oxygen species may play a role in epigenetic processes (physiological phenotypic variations caused by external or environmental factors that switch genes on/off) through reactions of nucleophilic substitution at the DNA level. Consequently, it has been suggested that a better preservation of DNA methylation levels, slower cell metabolism and improved control in signal transmission through epigenetic mechanisms could be key processes involved in human longevity. In other words, ROS signaling in senescent cells probably causes DNA hypomethylation, although there are still insufficient data to sustain such hypothesis (Gentilini et al., 2013). There are, nonetheless, some evidences supporting the role of ROS in the aging process, namely, studies on the effect of ionizing radiation on living cells, dietary manipulations and works highlighting the partaking of free radicals in the pathogenesis of specific diseases (Harman, 1993).
These genetic links between aging and oxidative damage have been described for animals in which most matured cells are postmitotic. Such cells may be more susceptible to the cumulative damage of ROS due to the inability of replacing themselves. Interestingly, it has been demonstrated that such susceptibility to damage may vary greatly in mammals, even in those with identical sizes (Montgomery et al., 2011). The most vulnerable organs in these organisms are the heart, brain and the skeletal muscle. This is because these are energy-rich tissues and, in the case of the brain, susceptibility to ROS-induced damaged may stem from the abundance of redox-active compounds (de Magalhães, 2013). Although ROS are usually considered as damaging compounds, studies have confirmed that these play an important role in multiple cellular functions (Miki and Funato, 2012; Ray et al., 2012; Sena and Chandel, 2012), such as regulation of the mitogen-activated protein kinase (MAPK) signaling pathways (Cuadrado and Nebreda, 2010) and of iron regulatory proteins −1 and −2 (IRP1 and IRP2, involved in iron homeostasis) expression levels (Recalcati et al., 2010). Furthermore, a corollary of this strict view of ROS as damaging compounds is that antioxidants should curtail their effect in aging and general health (Viña et al., 2016). However, multiple studies have demonstrated that this is not always the case (Fortmann et al., 2013; Grodstein et al., 2013; Higashida et al., 2011). ROS, including in mitochondria, are not necessarily detrimental and, in fact, some health-benefits, including a positive role in life-span under stress conditions, have been reported (Lee et al., 2010). Hence, low levels of these may induce an adaptive response that ultimately leads to the general improvement of systemic defense mechanisms, a concept termed mitochondrial hormesis or mitohormesis (Kawagishi and Finkel, 2014; Ristow, 2014).
Consequently, aging may be the result of a deregulation of the ROS signaling pathways and not of the reactive species themselves (de Magalhães, 2013). However, whether considering the available data for mitotic or postmitotic cells, evidences for a direct link between ROS and aging is still frail, at best.
While affecting DNA and lipids (Fig. 2), oxidative damage to proteins is irreversible and irreparable (Thanan et al., 2014) and must be degraded by the proteasome. The proteasome is the most important proteolytic machinery in eukaryotic cells, largely responsible for the removal of oxidized proteins and the prevention of its aggregation (Nyström, 2005). However it has been shown that the activity of proteasome is impaired during aging leading to the accumulation of oxidizing proteins, aggresome and lipofuscin, so called the age pigment.
In fact, protein aggregation is the common defining feature in age-associated neurodegenerative diseases, such as Parkinson's and Alzheimer's (David, 2012). According to this view, aging is, then, the rising collapse of protein homeostasis and is dependent on the interplay between proteostasis network components, which have a marked consequence on the long-term health of the cell (Douglas and Dillin, 2010). This proteostasis networks are capable of buffering the constant flux of protein misfolding, which is caused by the inherently error-prone characteristics of the protein synthesis and degradation mechanisms. However, such networks also undergo deterioration over time, thus making cells more vulnerable to protein-induced toxic stress (Morimoto, 2004). Cells are capable of counteracting disease protein aggregation in their early stages of life by reducing disease protein flux via increased folding and degradation control, reduced protein synthesis and favorable protein processing (Douglas and Dillin, 2010). Nonetheless, these are highly complex mechanisms (Hetz and Glimcher, 2011) and their exact nature remains largely unknown (Jarosz et al., 2010). Attempts have been made to increase proteasome expression and activity, which lead to 15–20% increase in longevity in cellular models. However, despite some compelling evidence that there is the formation of protein aggregates in some age-related illnesses, it is unclear whether protein aggregation induces aging or vice-versa (Moronetti Mazzeo et al., 2012).
The main function of the proteolytic degradation system of damaged proteins is the prevention of the accumulation of the most damaged proteins. If the damaged protein is not recognized and degraded via proteosomal activity, further oxidation can take place, as well as covalent crosslinking to other protein by-products of lipid peroxidation, such as 4-hydroxy-2-trans-nonenal (HNE) (Friguet and Szweda, 1997) and malondialdehyde (MDA) (Voitkun and Zhitkovich, 1999), two abundant bifunctional aldehydic oxidation products. When not sufficiently rapid degraded and/or when the cell is exposed to extreme oxidative stress, there is an increased probability for the cells to reach a different stage. At this stage, the proteins are not longer degradable by the proteasome. This results in the formation of protein aggregates, hydrophobic and insoluble in nature, referred to as “aggresomes” (Amidi et al., 2007). The formation of aggresomes can be thermodynamically driven by their exposed hydrophobic residues and the by-products of lipid peroxidation (like MDA or HNE) can cause covalent cross linking (Jung et al., 2009) The proteasomal activity decreases in aging cells and it has been shown that proteasomal inhibition in young cells leads to the enhanced formation of (polyubiquinated) protein aggregates (Powell et al., 2005). Interestingly, there have been some indications that healthy centenarians exhibit levels of both proteasomal activity and of oxidatively modified proteins identical to those found in younger control groups (Chondrogianni et al., 2000). These observations have led to the idea of artificially activating the proteasomal system as an anti-aging strategy (Chondrogianni and Gonos, 2008). Despite showing markedly increased proteolysis, with higher turnover of damaged/modified proteins and enhanced recovery after externally applied oxidative stress, such strategies are still far from feasible. Only a few of the post-translational modifications of ribosomal subunits that affect proteasome activities during aging have been investigated and many areas of proteasome regulation are not entirely elucidated, including specific regulators and the transcriptional regulation of the proteasome activation pathways.
Similarly to oxidative damage, nitrosative damage – that caused by reactive nitrogen species (RNS), such as nitric oxide – has been suggested to also contribute to age-related diseases, namely, hepatic steatosis and apoptosis (Abdelmegeed et al., 2016), as well as functional and structural changes in the cardiovascular system (Novella et al., 2013; Surikow et al., 2015). Additionally, it has also been associated to impairments in sleep homeostasis (Rytkönen et al., 2010), psychological disorders (Maurya et al., 2016) and dementia (Mangialasche et al., 2009). However, the mechanisms by which RNS may interact with cellular components, such as the mitochondrion, are still unclear, particularly in vivo (Zelickson et al., 2013). Consequently, there is the need to better understand how these species are formed and the processes through which they affect mitochondrial and cellular function.
Advanced glycation end-products (AGEs) are a complex and highly heterogeneous group of compounds capable of inducing cellular oxidative damage. They are formed when reducing sugar reacts in a non-enzymatic way with proteins, lipids or DNA (Fig. 3), dubbed the Maillard reaction (Luevano-Contreras and Chapman-Novakofski, 2010). This reaction plays a critical role in the food industry, as its products add desirable taste and coloring to foods (Rufián-Henares and Pastoriza, 2016). In vivo, AGEs have received increasing attention due to the fact that these have been associated with specific chronic diseases, namely, diabetes (Forbes et al., 2004), cardiovascular pathologies (Bucala et al., 1994) and, more recently, to cognitive impairment (West et al., 2014; Yaffe et al., 2011). Their biological deleterious effects can be attributed to their pro-oxidative, inflammatory and chemical actions (Ahmed, 2005), which are exerted by two distinct mechanisms. One is independent of the receptor, while the other involves the receptor for AGEs (RAGE) (Luevano-Contreras and Chapman-Novakofski, 2010) (Fig. 4). The interaction of RAGE and AGEs ultimately leads to a positive feedback cycle (Ishibashi et al., 2014; Lohwasser et al., 2006; Nakamura et al., 2009; Tanaka et al., 2000), increasing RAGE expression. Additionally, the AGEs-RAGE interaction activates NADPH oxidase, which is upregulated, thus increasing intracellular oxidative stress (Luevano-Contreras and Chapman-Novakofski, 2010). Despite some interesting works describing the accumulation of AGEs in age-related illnesses (Srikanth et al., 2011; West et al., 2014), and in the elderly (Peppa et al., 2008; Uribarri et al., 2007; Vlassara et al., 2009), there is still no established direct link between these and senescence.
Fig. 4.
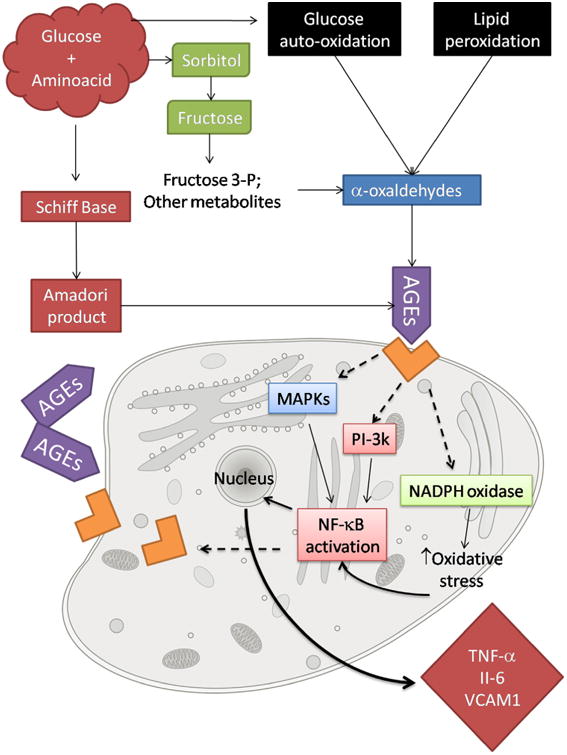
The formation of AGEs and their mechanism of action at a cellular level. The positive feedback loop of NF-κB activation with subsequent RAGE expression is highlighted.
Another prevailing damage theory as the cause for aging is that of genome instability, thoroughly reviewed elsewhere (Lopez-Otin et al., 2013). Both stability and integrity of DNA are challenged on a continuous basis by numerous endogenous and exogenous factors, including DNA replication errors and physical, chemical and biological agents (Lopez-Otin et al., 2013). Organisms have evolved a complex system of DNA repair mechanisms which, in most cases, deal effectively with these damages inflicted to DNA. However, if defective, these mechanisms can result in genome instability and yield premature aging syndromes. DNA helicases play an essential role in the maintenance of genomic stability and, in fact, a number of mutations in human helicase genes have been linked to chromosomal instability diseases characterized by age-related ailments (Suhasini and Brosh, 2013), including Xeroderma Pigmentosum (XP), Cockayne Syndrome (CS), and Werner Syndrome (WS) (Brosh, 2013; Fang Evandro et al., 2014). Nuclear events, including transcription coupled repair (TCR), nucleotide excision repair (NER) and, perhaps more familiar to the wider audience, telomere maintenance, are thought to be individually affected by CS-A/CS-B, XP-B/XP-D and WRN helicases, respectively (Uchiumi et al., 2015).
Most supporters of the genomic instability theory of aging refer to telomere shortening (Kruk et al., 1995) (Fig. 4). Telomeres are the repeated DNA sequences at the ends of linear chromosomes, which are unable to be fully replicated by DNA polymerases (Johnson et al., 1999). Consequently, telomeres shorten with each cell division, unless maintained by telomerase, a ribonucleoprotein enzyme (Fig. 5). Nonetheless, most mammals lack this enzyme and telomere exhaustion is, in fact, the root of the so-called Hayflick limit, the maximum proliferative capacity of some types of in vitro-cultured cells (Hayflick and Moorhead, 1961). This happens due to the presence of shelterin, a multiprotein complex that bounds telomeres, functioning as a barrier against DNA repair proteins. Furthermore, the introduction of telomerase in normal human cells as yielded immortal cell lines (Bodnar et al., 1998; Stampfer and Garbe, 2015).
Fig. 5.
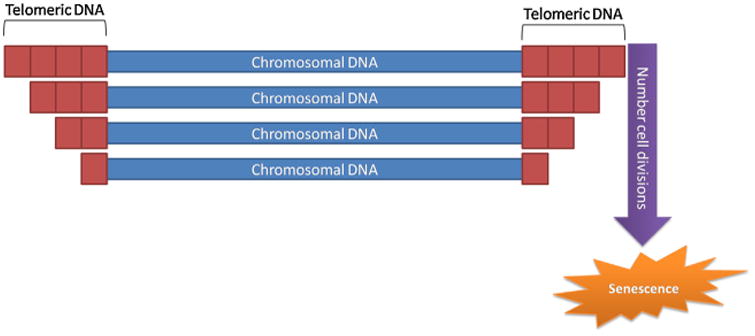
Model of telomere shortening on aging.
Although the presence of shelterin may, at first glance, seem detrimental to the cell, such mechanisms prevent chromosome fusions (Meena et al., 2015). Despite all this apparently overwhelming evidence for telomere shortening as a major driver of aging, many works have casted a shadow of doubt over this assertion. Studies of both cross-sectional and longitudinal samples have revealed that, if donor health status and biopsy conditions are controlled, no significant correlation between the age of the donor and replicative life-span of the culture cells can be determined (Holliday, 2014). Additionally, the premature aging process of progeroid fibroblasts (enhanced aging cells) have been demonstrated to share only part of the in vitro aging process of normal fibroblasts (Toda et al., 1998), though recent experimental work showed that telomerase confers protection of accelerated aging in Werner syndrome lineage-specific stem cells (Cheung et al., 2014). Finally, contrary to what expected, CD28– T cells, which exhibit shortened telomeres and markedly decreased proliferative capacity in culture, accumulate with age (Effros, 1998). Hence, telomere shortening may be involved in aging, but surely is not the sole cause of senescence and its action mechanism, though seemingly simple in principle, remains to be fully understood.
Mutations and deletions in mitochondrial DNA (mtDNA) may also contribute to aging. This type of DNA is extremely gene dense and encodes numerous factors that are critical for oxidative phosphorylation. Hence, mutations in mtDNA– which are believed to be ten times higher than that of nuclear DNA (Jeppesen et al., 2011) – cause a wide range of human mitochondrial diseases and have been implicated in age-related diseases and aging (Park and Larsson, 2011), which is further enhanced by the oxidative microenvironment of the mitochondria and by the lack of protective histones, involved in the repair mechanisms of nuclear DNA (Pinto and Moraes, 2015). Causative evidences of the role of mtDNA damage in aging come from studies on mice that are deficient in mitochondrial DNA polymerase γ. These mutants exhibit aspects of premature aging and reduced life-span (Vermulst et al., 2008). In humans, deletions taking place through clonal expansion of single mutation events in aged brains have been described (Williams et al., 2013), interestingly, in regions of the brain highly susceptible to oxidative damage (Pickrell et al., 2011). However, the implication of mtDNA mutations in aging is controversial, due to the multiplicity of mitochondrial genomes (Lopez-Otin et al., 2013). This means that mutant and wild-type genomes can coexist within the same cell, a phenomenon called “heteroplasmy”, and, recently, the degree of heteroplasmy has been suggested as a simple and noninvasive predictor of age-related neurologic and movement impairments (Tranah et al., 2015). Despite this possible coexistence of mtDNA's and of a globally low level of mtDNA mutations, single-cell analyses have revealed that the load of individual aging cells becomes significant (Khrapko et al., 1999) and may ultimately reach a state of homoplasmy, in which one mutant genome dominates (Lopez-Otin et al., 2013). Though the mechanisms by which mitochondrial dysfunction lead to diseases have been described (Ylikallio and Suomalainen, 2012), how mtDNA mutations may induce aging is not completely clarified (Pinto and Moraes, 2015). One key limitation of this theory is that how a focal impairment of mitochondrial function can spread throughout the tissue remains unexplained. Thus, it is clear that further studies are required to better elucidate how mtDNA mutations eventually lead to aging.
(3) Combined theories
One of the first efforts at developing a unified theory for aging was carried out by Strehler (1976) in 1976. He formulated four postulates: (1) aging is universal, and, as such, a phenomenon associated with aging must occur in all individuals of a species, albeit in different degrees; (2) aging must be intrinsic: the causes must be endogenous and they do not depend on extrinsic factors; (3) aging is progressive and must occur incrementally throughout the life-span and; (4) aging must be deleterious, i.e., a phenomenon associated with aging will only be considered a part of the aging process if it is holds no advantages for the individual.
Soon thereafter, stemming from these postulates, a membrane hypothesis of aging was developed (Zs.-Nagy, 1978), based on the fact that cell membranes become more rigid during aging and that a decrease of intracellular potassium content could lead to a sort of “rejuvenation”. In other words, aging was related to changes in the cells' ability to transfer chemicals, heat and electrical processes.
In the early 1980s, Cutler put forth the dysdifferentiative hypothesis of mammalian aging and longevity (Cutler, 1982), based on the notion that the underlying cause for most of the vast complexities of the aging process was the drifting away of cells from their proper state of differentiation, as dysdifferentiated cells are responsible for the initiation of a cascade of changes in the entire organism and that the sum of these is aging (Taylor and Johnson, 2008). Though some studies were carried with the dysdifferentiative hypothesis of aging as their underlying premise (Kator et al., 1985; Ono et al., 1985), this idea has been largely abandoned in favor of some of the previously described views in the process of senescence.
More recently, a new integrative theory has been proposed, based on the notion that aging is not fundamentally a chemical process, but rather a biophysical, electrical in nature, mechanism. The fading electricity theory of aging (De Loof et al., 2013) postulates that, as cells gradually lose their ability to produce their own electricity, the biochemical processes that have been suggested as the drivers of aging come into play, eventually leading to death by senescence. Though certainly plausible, this theory lacks data supporting this hypothesis. It does raise, nonetheless, an interesting aspect in aging research: scientists should not limit themselves to the biochemical and genetic causes of aging. All biophysical activities of the living cell should be taken into consideration, namely, the bioelectrical one, as a possible cause for senescence.
While multiple theories of aging have been proposed, there is no consensus on the matter to date. In fact, many of the suggested mechanisms seem to, in one way or another, interact with each other (Jin, 2010). Hence, an integrative analysis of the quantitative available evidence at the different levels of biological hierarchy is necessary to fundamentally understand how the aging process takes place. Multiple attempts at finding synergies and combining diverse views and theories of aging have been made (Barja, 2013; Bengtson et al., 1999; Gems, 2000; Miquel, 1991; Weinert and Timiras, 2003), though none has established itself as a prevailing detailed and comprehensive view of what is, and, most importantly, how aging occurs. Nonetheless, combined theories view aging as a highly networked process on a systems level, regulated through feedback loops between levels of biological organization (Kriete et al., 2006) (Fig. 6).
Fig. 6.
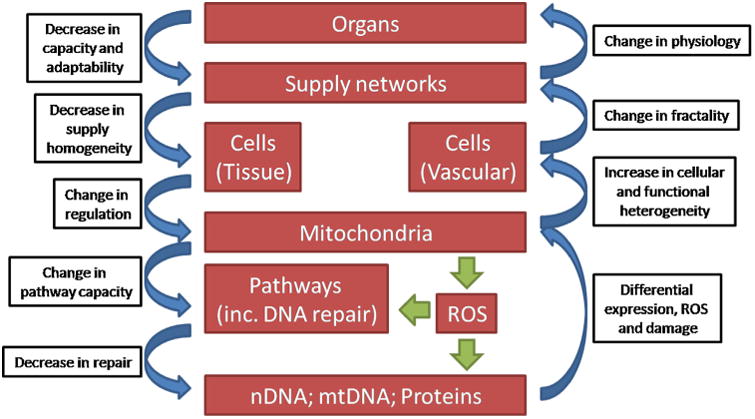
An integrated physiological view of the functional and structural changes observed during aging. Multiple feedback loops exist at both the genomic and the organ levels, suggesting that aging is an accelerated process, countered only by the robustness of each level. Adapted from (Kriete et al., 2006).
3. The chemical interplay
Aging has been dubbed as a war raged between chemical and biochemical processes (Clarke, 2003), though a more accurate description might be that of a complex and rather interconnected gear mechanism. However, on the basis of this perspective, aging is fundamentally the end-result of unwanted chemical processes, which yields spontaneous side products of normal metabolism, including mutated, less active, and potentially toxic species of lipids, proteins, RNA, DNA and small molecules (Clarke, 2003). Hence, organisms endure to the extent that they can minimize the accumulation of these modified biomolecules (Yin and Chen, 2005). Such minimization processes rely on enzyme-mediated reactions, which are the backbone of the metabolic pathways involved in energy generation, biosynthesis, and signal transduction (Vogel et al., 2004). Therefore, an optimization of these processes could, in theory, make life indefinite. What seems to work against biochemistry is chemistry itself. While enzymes may act as catalysts to speed up these reactions, it becomes difficult to slow them down, and, consequently, side reactions continue, leading to the build-up of undesirable side products (Clarke, 2003). These products are not limited to small molecules and include complex biomolecules, such as proteins and nucleic acids. Because nearly all biomolecules are thermodynamically unstable (Ross and Subramanian, 1981), they are susceptible of undergoing non-enzymatic conversion. These conversions can impact orderly biochemical process, which is at the heart of the damage-based theories of aging. These modified molecules can sometimes be repaired, though such mechanisms are rarely 100% effective (Yin and Chen, 2005). Fig. 7 illustrates how the pathways of spontaneous degradation, repair, and replacement described for aged proteins (Clarke, 2003; Grimaud et al., 2001; Ruan et al., 2002; Schiene and Fischer, 2000). In Fig. 8, the pathways of spontaneous chemical degradation of the aspartyl and asparaginyl residues in proteins, as well as of the methyltransferase-mediated repair mechanism, are described as an example.
Fig. 7.
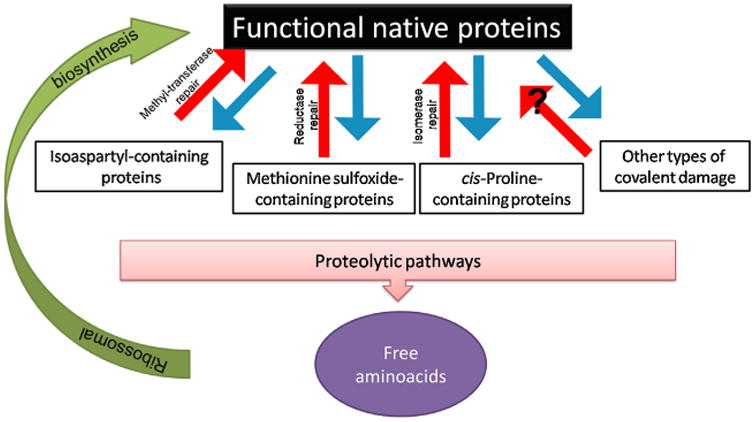
Pathways of non-enzymatic degradation, repair, and replacement of aged proteins. Functional proteins can be covalently altered by a number of pathways (blue arrows). Enzymatic mechanisms exist that are capable of directly repairing, at least partially, this damage (red arrows), though, so far, no repair mechanisms have been described for many other types of damage. Altered proteins can be proteolytically digested to free amino acids and these can be used for synthesizing new functional proteins (green arrow). Adapted from (Clarke, 2003).
Fig. 8.
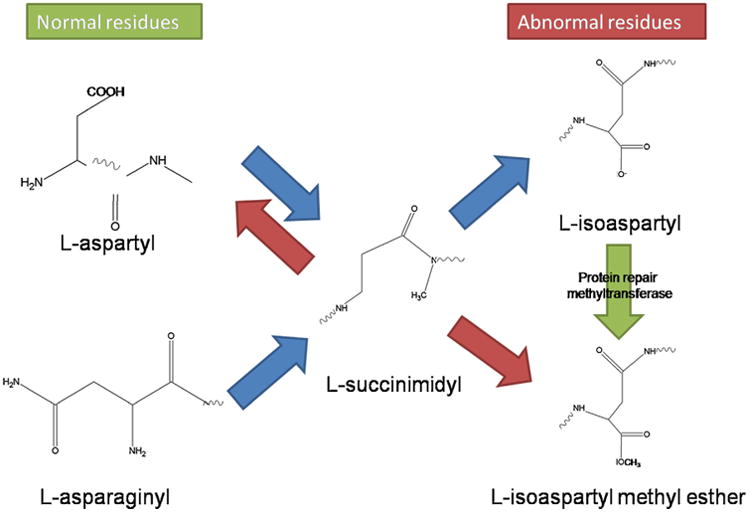
Pathways of non-enzymatic chemical degradation of aspartyl and asparaginyl residues in proteins and of the methyltransferase-mediated repair mechanism. Spontaneous degradation of normal l-aspartyl and l-asparaginyl residues lead to the formation of a ring succinimidyl intermediate. This can spontaneously hydrolyze to either the l-aspartyl residue or the abnormal l-isoaspartyl residue. The l-isoaspartyl residue is specifically recognized by the protein l-isoaspartate (d-aspartate) O-methyltransferase. The result is the formation of an unstable methyl ester that is converted back to l-succinimidyl. Net repair occurs when the l-succinimidyl residue is hydrolyzed to the l-aspartyl form. With the exception of the repair methyltransferase step, all the reactions are non-enzymatic. Adapted from (Clarke, 2003).
While the understanding of aging mechanisms has become much more complicated than ever before, it seems clear that phenomena such as oxidative stress and associated damages are neither parallel with alterations observed during aging nor correlated with maximum life span, due to the existence of these repair and defense mechanisms (Sohal et al., 2002). It is the remaining alterations after repairing which may define the extension of the age-related changes, namely, the interactions of these with other biomolecules. This results in rather complex, interconnected processes, as highlighted in Fig. 9, which describes the predicted and known interactions of some of the molecules whose expression changes more dramatically during aging (see Table 1) and the more common reactive oxygen species.
Fig. 9.
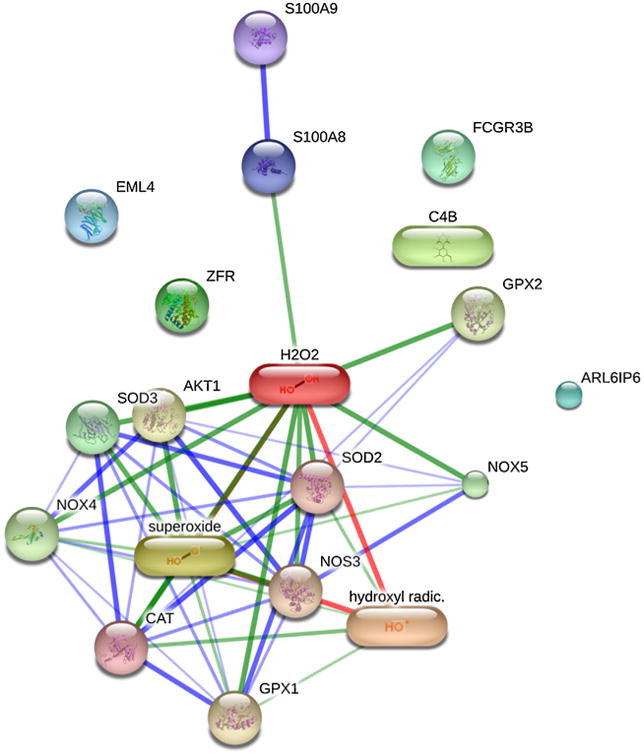
Confidence view of the predicted and know protein and chemical interactions. Stronger associations are represented by thicker lines. Protein-protein interactions are shown in blue, chemical-protein interactions in green and interactions between chemicals in red. ZFR – zinc finger RNA binding protein. FCGR3B – Fc fragment of IgG, low affinity IIIb, receptor (CD16b). ARL6IP6 – ADP-ribosylation-like factor 6 interacting protein 6. EML4 – echinoderm microtubule associated protein like 4. S100A8 – S100 calcium binding protein A8. C4B – complement component 4B. Predicted functional partners include catalase, SOD2 – superoxide dismutase 2; SOD3 – superoxide dismutase 3; NOS3 – nitric oxide synthase 3; GPX1 – glutathione peroxidase 1; AKT1 – v-akt murine thymoma viral oncogene homolog 1; GPX2 – glutathione peroxidase 2; NOX4 – NADPH oxidase 4; NOX5 – NADPH oxidase, EF-hand calcium binding domain 5; TYRP1 – tyrosinase-related protein 1. A more comprehensive and in-depth study of all the modified proteins during aging may yield strong known and/or predicted protein and chemical interactions. Data obtained using STITCH (Kuhn et al., 2014).
Table 1.
Molecular age-related changes. The level of variation is also indicated, as well as the statistical significance. Data compiled based on the information available at the Aging Digital Atlas (Craig et al., 2015).
| Measured variable | Variation (%) P value | Observations | Ref. |
|---|---|---|---|
| Calcium binding protein A8 | ↑1228.2% p < 0.01 |
Expression in the parietal lobe from elderly (aged 69–99 years old) compared to cells from younger persons (aged 20–52 years old) | Cribbs et al. (2012) |
| Major histocompatibility complex, class II, DQalpha 1 | ↑496.7% p < 0.01 |
||
| CD163 molecule | ↑515.7% p < 0.01 |
||
| miR-320b | ↑1049.9% p = 1.33 × 10−8 |
Expression from blood samples of German elderly (mean age: 98.9 years) compared to younger controls (mean: 43.8 years) | ElSharawy et al. (2012) |
| miR-320d | ↑529.9% p = 6.93 × 10−7 |
||
| miR-106a | ↓98.7% p = 1.20 × 10−10 |
||
| miR-668 | ↑251.4% p = 5.67 × 10−12 |
Significantly increased expression in foreskin cells from elderly when compared with younger controls. | Hackl et al. (2010) |
| miR-144 | ↑390.2% p = 1.00 × 10−25 |
||
| miR-100 | ↓49.1% p = 1 28 × 10−16 |
||
| transmembrane protein 33 | ↓63.9% p = 8.62 × 10−3 |
Decrease in the expression of TMEM33 in oocytes donated from women aged 37–39 years) compared to those from women aged 25–35 years | Grondahl et al. (2010) |
| zinc finger RNA binding protein | ↑150.0% p = 5.68 × 10−3 |
4. Models of senescence—what changes?
Aging is intrinsically complex and is characterized by numerous changes that take place at different levels of the biological hierarchy. There is no clear evidence which molecular, cellular or physiological changes are the most important drivers of the aging process and/or how they influence one another. Each mechanism tends to be – at least in part – supported by data indicating that it may play a role in the overall process. Nonetheless, the magnitude of an isolated mechanism is usually modest (Kirkwood, 2011). Consequently, such confined approaches may hinder a full appreciation of how different molecular, cellular and physiological components interact with each other. An important effort to circumvent this limitation has been the development of the Digital Aging Atlas (http://ageing-map.org), which aims at integrating the multiplicity of reported age-related changes into a unified, freely accessible resource (Craig et al., 2015). Ultimately, the goal of an integrative approach will be the compilation of the acquired knowledge into a single depiction of how the aging process takes place, ideally capable of characterizing the phenotype at a systemic/organism level (Cevenini et al., 2010). Such approaches will inevitably rely on the identification of pivotal genes, biochemical pathways and interactions involved in the aging process, as well as on the study of hereditable genetic diseases that result in premature aging and physiological experiments targeted at correlating caloric intake and “speed” of aging. Cell and molecular biology will play a key role in unveiling the basis of the changes that organisms undergo during senescence and the multitude of available data, especially from high-throughput studies (de Magalhães et al., 2009), will be managed through systems biology approaches, where computational and mathematical modeling could decisively contribute to the understanding of the old problem of aging (Hou et al., 2012).
In the following sections, we will explore some of the most notorious molecular, physiological and pathological changes associated with aging, though many more exist and such description could not but be invidious.
(1) Molecular changes
Approximately 25–32% of the overall variation in adult life-span may be attributed to genetic variation, making it a particularly important feature for survival at advanced age (Hjelmborg et al., 2006). As such, there has been a remarkable effort into the elucidation of the molecular mechanisms of senescence, searching for “signatures” that can be definitively associated to the aging process and many gene-centric studies have identified genes whose expression is altered in senescent cells (Zhang, 2007). Table 1 highlights some of the most expressive findings in age-related changes at the molecular level. However, what these studies fail to unequivocally demonstrate is whether such alterations in gene expression are unique and causal to senescence or if they are a mere nonspecific consequence of cessation of cell proliferation. Adding to this complexity, studies carried out in animal models may contribute in a very limited way in our understanding of aging in humans, as senescence pathways vary significantly among cells from different species. For example, mouse fibroblasts express telomerase and exhibit very long telomeres, unlike human fibroblasts (Greenberg et al., 1998; Kipling and Cooke, 1990). In culture, mouse fibroblasts undergo senescence, which is independent of telomere shortening (Banito and Lowe Scott, 2013; Sherr and DePinho, 2000). Within the same species, cells can vary significantly in their senescence pathways (Zhang, 2007). For example, human fibroblasts undergo senescence after a finite number of divisions and telomerase expression has been proven to avoid this arrest (Yamashita et al., 2012). However, human mammary epithelial cells reach a growth arrest state unrelated to telomere shortening, but mediated by the tumor suppressing protein p16 (Stampfer et al., 2013b). The importance of p16 in growth arrested was demonstrated by the immortalization of these cells by short hairpin RNA (shRNA) targeting p16 (Stampfer et al., 2013a). Thus, these data strongly suggest that there are multiple pathways to senescence (Zhang, 2007). In this post-genome era, where –omics approaches allow for the detailed and comprehensive characterization of molecular changes during aging (da Costa et al., 2016), it will be possible to link such changes to cellular and physiological processes (Craig et al., 2015). However, it should be noted that different platforms and/or methodologies by which these molecular changes are evaluated often yield disparate results, and even distinct nomenclatures can have an impact on the final conclusions (da Costa et al., 2016). Consequently, it is of the utmost importance to create standardized methods for data acquisition and analysis, and, despite many attempts (Kohl et al., 2014; Sun et al., 2014; Weis, 2005; Zheng et al., 2015), these have so far failed to be universally implemented. The recent technological advances observed in –omics research allow for the simultaneous measurement of millions of biochemical entities (Zierer et al., 2015). Reductionist association studies have shown a high degree of correlation between –omics data with aging and age-related diseases, it is becoming increasingly evident that integrated network and -omics analyses targeting the aging process at a systems level could provide information previously unattainable, namely, pathways involved and interactions with key internal and external factors and variables (Valdes et al., 2013; Van Assche et al., 2015; Zierer et al., 2015).
Another key aspect of –omics research is that software packages and databases are continuously updated, and, therefore, studies should not end in a stable list of proteins and genes, but, rather, should periodically be revisited (Zhou et al., 2016). Not only could the initial studies benefit from new annotated information, but the original raw data could enclose previously unreported and valuable results, as evident from studies disclosing new findings from earlier published data (Mann and Edsinger, 2014; Matic et al., 2012). Nonetheless, no standard guidelines for such comprehensive re-analysis exist, although some efforts have been developed towards that goal (Zhou et al., 2016) and a successful implementation of such strategies could hold potential key discoveries towards the understanding of the aging mechanism(s).
(2) Physiological changes
Physiological changes occur with aging in all organ systems. Cardiac output decreases and blood pressure increases, often leading to arteriosclerosis. Degenerative changes occur in multiple joints and, combined with loss of muscle mass, movement becomes impaired in elderly (Boss and Seegmiller, 1981). Consequently, numerous studies have focused on the physiological changes that occur with age and, while listing all would be a herculean endeavor, some of the most prominent works are briefly listed in Table 2.
Table 2.
Physiological age-related changes. The variables measured, as well as the affected tissue(s)/organ(s) are also indicated.
| Measured variable | Tissue/Organ | Observations | Refs. |
|---|---|---|---|
| Function of epithelial barriers | Lung; oral cavity; pharynx; esophagus; stomach; intestine; epidermis | Decreased epithelial barrier function associated with increased pathogenic invasion of mucosal tissues | Weiskopf et al. (2009) |
| Expiratory volume | Lung | Lung forced expiratory volume decreases with | Klocke(1977) |
| Modifications to proteins and membrane components | Lenses | Increased incidence of presbyopia and age-related nuclear cataract | Truscott and Zhu (2010) |
| Rates of neuronal and astroglial tricarboxylic acid cycles and neuroglia glutamate–glutamine cycling | Brain l (mitochondria) | Neuronal mitochondrial metabolism and glutamate-glutamine cycle decreased in elderly (∼30%) | Boumezbeur et al. (2010) |
| Protein level | Arterial intima | Collagen content of human arterial intima shows an average increase of 100%. Accompanied by a large increase in intimal embrittlement. | Johnson et al. (1986) |
| Cell proliferation | Increase in intima cell proliferation. | Chisolm and Steinberg (2000) | |
| Prevalence of arteriosclerosis | Artery | Arteriosclerosis incidence increases with age | Wilkinson and McEniery (2012) |
| Cholesterol level | Plasma | Incidence increases in age brackets 45–64 and 65–74 | CDC, (2015) |
| Hematopoietic bone marrow volume | Bone marrow | Volume of hematopoietic bone marrow decreases with age | Sharma et al. (2009) |
| Clonal mosaic abnormalities | Blood; oral cavity | Detectable clonal mosaic events increased with age | Jacobs et al. (2012) |
| White matter volume | White matter | White matter volume decreases in the individuals aged 59–85 | Resnick et al. (2007) |
| Atrophy of hypodermal layer | Skin (subcutaneous) | Hypodermal layer suffers atrophy with age | Arking (2006) |
Nonetheless, one must consider such findings with a grain of salt. For example, though it has been described that there is a positive correlation between the atrophy of the hypodermal layer with age (Arking, 2006), it should be underlined that this is a regional change and usually affects the face and back of hands, but not the waist and/or thighs, which may be related to exposure. Another example is that of the somewhat widespread belief that there is a global neuron loss with age. In fact, the difference in total neuron number over the age range of 20–90 years is less than 10% (Pakkenberg et al., 2003; Pannese, 2011), though some morphological alterations do take place, such as significant decrease loss of synapses (Mostany et al., 2013), axon demyelination (Adamo, 2014) or loss of dendritic spines (Dickstein et al., 2013).
(3) Pathological changes
What's in a name? Contrary to what one might expect, this question is more closely related to pathological age-related changes than expected. In fact, pathological changes are not always readily and easily identified and what distinguishes them from “normal” age-related changes is, hence, somewhat elusive. For example, mild changes in neurologic functions occur with aging, though these do not substantially interfere with everyday activities, unless disease intervenes (Morris and McManus, 1991). However, there are macroscopic changes in the aging brain that are almost universally seen, such as the thickening of the arachnoid, increased ventricular volume and variable degrees of cortical and white matter atrophy have also been reported (Donahue, 2012).
Additionally, some of the data reported should be critically appraised. For example, Banks et al. (2009) reported a positive correlation of hip fracture incidence with age among postmenopausal women, though this is – in our view – not surprising, considering that movement, coordination and visual impairment significantly increase with age, inevitably leading to more falls and collisions, which, in turn, ultimately lead to hip fractures (as well as other fractures).
Consequently, most of the age-related pathological changes are not a result of single-measurements, but rather of continuous observation and serial incidence reports. In Table 3, some of these incidences of pathological age-related changes are listed.
Table 3.
Pathological age-related changes.
| Measured variable | Tissue/Organ | Observations | Refs. |
|---|---|---|---|
| Cancer incidence | Multiple | Morbidity per 100,000 was >370 times in 85 years old than individuals aged 18–24. | CDC (2006) |
| Incidence of acute rheumatic fever and chronic rheumatic heart diseases | Heart; skin; brain | Morbidity per 100,000 was 165 times higher in 85 years old than individuals aged 25–44. | |
| Coronary artery disease incidence | Heart; artery | Prevalence of coronary artery disease increases markedly with age | CDC (2006), Odden et al. (2011) |
| Chronic obstructive pulmonary disease (COPD) and small airway obstruction incidence | Lung | COPD increases with age, as well as small airway obstruction. | Sharma et al. (2009) |
| Incidence of prebyscusis (hearing loss) | Cochlea (inner ear) | Positive correlation of prebyscusis with age | Albert and Knoefel (2011) |
| Renal arteriosclerosis incidence | Kidney | Renal arteriosclerosis increase with age | Bolignano et al. (2014), Glassock and Rule (2012) |
| Gastroesophageal reflux disease incidence | Esophagus; stomach | Incidence and severity of gastroesophageal reflux disease increases with age, particularly after 50 | Becher and Dent (2011) |
| Asthma incidence | Lung | Morbidity per 100,000 was >40 times higher in 85 years old than individuals aged 18–24 | CDC (2006) |
| Clinical presentation and pathological staging in colorectal cancer | Colon | Older patients exhibited lower frequency of abdominal pain; time from onset to diagnosis and pathological staging were similar | Paganini Piazzolla et al. (2015) |
(4) Psychological changes
Discussing the psychology of aging inevitably leads, albeit ever so slightly, to sociological considerations (Tischler, 2013). Although concrete analyses can be performed, such as measurements of cognitive deficits and alterations in sleep patterns, psychological age-related changes are intimately interweaved with the dynamics of stress and coping mechanisms during aging. In other words, as eloquently put by a personal connection, “elderly must learn to age”. Western societies tend to show a mixed feeling towards the elderly. While generally appreciated, there is a pop culture that is youth-oriented, we look to preserve our younger self, resorting to a wide variety of hyped age-delaying crèmes, and, when picturing older people, we often think of people who are physically and/or mentally slower and TV programs do little to contradict such stereotypes (Lee et al., 2007). Yet, there are definite alterations that we can evaluate, such as those observed in the functional neuroanatomy that induce alterations in overt speech production (Soros et al., 2011). In Table 4, some of these quantifiable variations are listed.
Table 4.
Psychological age-related changes.
| Measured variable | Observations | Refs. |
|---|---|---|
| Speech production | Speech production problems and reduced speech rate increased with aging | Soros et al. (2011) |
| Alterations in sleep patterns | Older individuals reported higher number of awakenings and modifications in sleep duration. | Crowley (2011), Feinberg et al. (1967) |
| Long-term depression | Individuals age 65+ showed increased incidence of depression | Roblin (2015) |
| Cognitive decline | Cognitive decline was found to be almost universal in the general elderly population and increases with age. | Park et al. (2003), Schönknecht et al. (2005) |
| Cognitive processing speed | Processing speed decreases with age | Eckert (2011) |
| Cognitive executive functions | Executive functions (e.g., planning), decreased with age | Glisky (2007) |
| Subjective memory | Normal aging found to be accompanied by memory impairment | Gazzaley et al. (2005) |
| Visual memory | Interaction of deficits in inhibition and processing speed was found to contribute to age-related cognitive impairment | Gazzaley et al. (2008) |
| Verbal memory | Age-related differences were found in 8 verbal span tasks | Bopp and Verhaeghen (2005) |
| Long-term potentiation | Greater and longer stimulation was necessary for long-term potentiation in older subjects | Kumar (2011) |
5. Aging therapies—cure aging or die trying?
Is aging a disease? Aging is a process characterized by numerous pathologies, the sum of which inevitably leads to death and its biology by loss of homeostasis and the accumulation of molecular damage (Vijg and de Grey, 2014). Yet, if disease is defined as a disorder or abnormality of structure or function (Scully, 2004), than certainly aging is not a disease, as everyone suffers from it, though aging and disease often overlap. Hence, the question shifts towards should we cure aging? Opinions diverge (e.g., (Aledo and Blanco, 2015; Anton et al., 2005; Baars, 2012; Caplan, 2005; de Magalhaes, 2014; de Magalhães, 2013; Vijg and de Grey, 2014)), and commonly described fears include concerns about overpopulation and inequality, economic collapse due to healthcare and the idea that aging is natural and should not be tampered with (de Magalhaes, 2014; de Magalhães, 2013). Advocates of life-extension research state that curing aging is not scientifically implausible and we may soon reach the “longevity escape velocity” (de Grey, 2004), a stage of medical progress that will result in delaying aging-related degeneration and death to such an extent that there is time to carry out research seeking more effective therapies later on (Vijg and de Grey, 2014) and dispute the alarms raised by others by noting the failed predictions of Malthus regarding the disasters due to overpopulation (Sethe and de Magalhães, 2013; Trewavas, 2002). No matter where one stands in respect to the pursuit of an increasingly longer life, there is no disagreement about the necessity of fighting age-related illnesses and comorbidities (Longo et al., 2015). Nonetheless, ultimately, finding a “cure” for aging is certainly a matter of personal belief.
As detailed in the following paragraphs, the technological advances aiming at the explicit purpose of curing aging, much as we would cure a disease, is essentially non-existent, though great endeavors are being undertaken to prolong an healthy life, whether we agree with it or not. However, the research is very much in its infancy and the road to longevity is still long. Moreover, considering the multifactorial nature of the aging process, it is not likely that there will be a silver bullet for aging.
(1) Caloric restriction
Contrary to what pharmaceutical companies would have you believe, there is still no way to delay aging, even faintly, and the long searched Fountain of Youth (Grene, 2010) remains elusive to this day. Yet, some of the effects of aging can be delayed. For example, skin aging can be minimized by reducing exposure to the sun (Kimlin and Guo, 2012) and it has been known since the 1930s that restricting calories (caloric restriction, CR) can extend life-span in laboratory animals (McCay, 1935). Some have postulated that this is due to an increased formation of free radicals within the mitochondria, which causes a secondary induction of increased antioxidant defense capacity (Shimokawa and Trindade, 2010), while others suggest that the limited availability of nutrients forces the metabolism to undergo optimization (de Magalhães, 2013). Considering observations made in mice, others believe that the genetic program maybe “slowed down”, thus indirectly affecting aging (de Magalhaes and Church, 2005). Additionally, because CR also induces various alterations, both at the hormone (Kim et al., 2015; Masoro et al., 1992) and at the proteome level (Baumeier et al., 2015), caloric restriction is recognized as the sole therapy capable of potentially delaying aging. In Fig. 10, simplified views of the complex metabolic pathways that regulate mammalian longevity are highlighted.
Fig. 10.
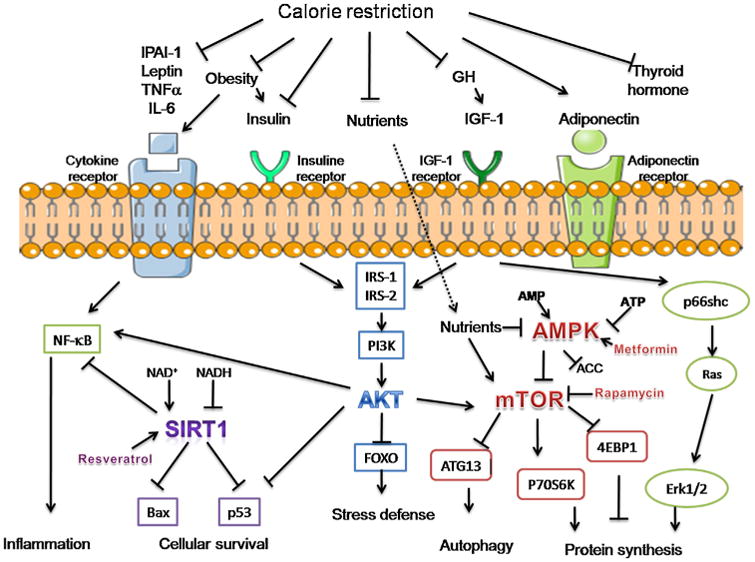
Main metabolic pathways involved in the regulation of mammalian longevity and affected by CR. These include reduced cytokine levels, adiposity, thyroid hormone levels, IIS signaling and increased adiponectin. CR engages multiple downstream cellular pathways, including SIRT1 activation, insulin/IGF-1/phosphatidylinositol 3-kinase (PI3 K)/Akt signaling, as well as AMPK/mTOR and extracellular signal-regulated kinase 1/2 (Erk1/2) signaling. Ultimately, the collective response is believed to lead to the promotion of longevity through activation of stress defense mechanisms, autophagy and survival pathways, with concomitant attenuation of pro-inflammation mediators and cell growth. Pharmacologic approaches, such as those involving the use of rapamycin, metformin or resveratrol are believed to exert an analogous effect via the mechanisms shown. Arrows indicate the directional stimulatory effect and blunt-ended line an inhibitory one. IL-6 stands for interleukin-6, TNFα for tumor necrosis factor-α, NF-κB for nuclear factor-κB, IRS-1 for insulin receptor substrate-1 and PAI-1 for plasminogen activator inhibitor 1. Adapted from (Barzilai et al., 2012).
(2) Stem cell therapies
There has been a continuous and widespread buzz over stem cells in the general public and this notoriety is thoroughly deserved. These cells have been demonstrated to be a viable solution to health issues ranging from blindness (Nazari et al., 2015) and nerve regeneration (Faroni et al., 2013) to liver restoration (Christ et al., 2015), as well as potential therapies in movement disorders (Mochizuki et al., 2014) and other age-related illnesses, namely, muscular dystrophies (Bose and Shenoy, 2016) and skin deterioration (Peng et al., 2015). It is then not surprising that stem cells have been touted as potential treatments for the diseases of aging and for rejuvenation. Recently, Liu and co-workers reported the use of platelet rich plasma for the recovery of stem cell senescence in SAMP8 mice (Liu et al., 2014) and postulated that rejuvenation of lineage could be achieved through the transplantation of restored stem cells in aged individuals, which could be applied in the treatment of age-related ailments. Experimental studies have also suggested that CR exerts its effect over stem cell dynamics and viability, by enhancing the preservation of a more durable population in the diverse stem cell niches of body tissues (reviewed elsewhere (Mazzoccoli et al., 2014)). Nonetheless, there is no direct evidence that stem cell-based anti-aging therapies will work, and, before such treatments are available, it is necessary to fully understand the mechanisms of action. Though depletion of stem cells are considered to play a role in aging, it remains largely unknown whether decline in stem cell function during aging influences longevity (Signer Robert and Morrison Sean, 2013) and the exact comprehension of the mechanisms are still vague (Oh et al., 2014), though, in somatic stem cells, it has been suggested that mitochondrial metabolism is an important regulator in aging (Ahlqvist et al., 2015). Additionally, numerous technical challenges remain. Harvesting and/or preparing stem cells remains an uncertain and laborious process (de Magalhães, 2013) and, in the case of induced pluripotent stem cells, there is the need to take pause and ascertain whether subtle differences between these and embryonic stem cells may affect both their research applications and therapeutic potential (Robinton and Daley, 2012). Stem cells applications are very much in their infancy and there is the need to investigate further, namely, at the tissue-specific level, where variations in mechanisms and signaling pathways may yield significant exceptions in delaying the aging process.
(3) Breaking AGEs
Intervention studies have clearly demonstrated that high intake of AGEs positively correlates with tissue damage and that it can be prevented by dietary AGEs restriction (Feng et al., 2007; Poulsen et al., 2013; Van Puyvelde et al., 2014). This is further evidenced by the low-calorie intake described in numerous studies of centenarians (Martin et al., 2013; Redman and Ravussin, 2011; Weiss and Fontana, 2011). Whether the low-calorie diet itself or the AGEs content could affect aging has also been studied and, in animal models, the high levels of AGEs in the CR-high diet were shown to compete with the benefits of CR, though the mechanism remained uncertain (Cai et al., 2008).
Numerous pharmacological agents have also been studied as blockers of the crosslinking reactions leading to AGEs, or as blockers of their actions, such as aminoguanidine (Thornalley, 2003), benfotiamine (Stirban et al., 2006), aspirin (Urios et al., 2007), metformin (Ishibashi et al., 2012) and inhibitors of the renin-angiotensin system (Zhenda et al., 2014). Among these compounds, ALT-711 has received much of the public attention as the next-generation anti-aging product. It acts by catalytically breaking AGE crosslinks and research has highlighted its potential in alleviating numerous age-related conditions, such as heart failure (Little et al., 2005), diabetic nephropathy (Thallas-Bonke et al., 2004), type II diabetes (Freidja et al., 2012) and age-associated ventricular and vascular stiffness (Steppan et al., 2012), among others.
Nonetheless, despite the extensive research carried out, and although some of these agents are in preclinical trials, the full effects and side-effects of these drugs are still unknown and it could be a long time before any of these compounds emerge as safe and efficient agents with therapeutic actions against AGES and/or their effects (Luevano-Contreras and Chapman-Novakofski, 2010).
More recently, exercise has been described as a promising venue for the amelioration of the effects of AGEs. These reports, however, are sparse, and the direction of causality between exercise tolerance and AGEs is not always clear. For example, Hartog et al. (2011) describe that breaking AGEs yields positive effects over exercise tolerance and cardiac function, but Delbin et al. (2012) postulate that the exercise itself can lead to a decrease in AGEs and, consequently, improve vascular responsiveness. As such, the interaction mechanism remains unclear, though it certainly exists and further research is required.
(4) Hormonal therapies
On the heels of the realization that patients with GH and IGF-1 deficiencies exhibit signs of early aging (Anisimov and Bartke, 2013; Vanhooren and Libert, 2013), growth hormone began being used as an anti-aging treatment and there are some evidences suggesting that human GH has beneficial effects in the elderly (Taub et al., 2010) and hGH supplements have been implicated in muscle mass and libido increase, as well as strengthening of the immune system (de Magalhães, 2013). Alas, similarly to many other anti-aging products, it failed to live up to the expectations, partially due to its negative side-effects, such as alterations in body composition and metabolism (Carroll et al., 1998), high blood and intracranial pressure (Malozowski et al., 1993) and diabetes (Lewis et al., 2013). There are also concerns as to whether hGH could stimulate cancer, particularly in patients with existing malignant or pre-malignant tumors (Clayton et al., 2011). As such, the general consensus is that its use as an anti-aging therapeutic agent is imprudent (Liu et al., 2007). More research is needed to evaluate any possible deleterious effects and ensure its safe use as a therapeutic agent.
(5) Antioxidants
In order to fight ROS Eqs. (1)–(4) and their effects over lipids (Sharma et al., 2012), proteins (Youle and Van Der Bliek, 2012) and nucleic acids (Ray et al., 2012), cells exhibit an array of endogenous antioxidant systems, further amplified by an input from co-factors and by the ingestion of exogenous antioxidants (Rahman, 2007). Many of these can either be synthesized or extracted and subsequently purified and then sold (de Magalhães, 2013). The most common antioxidants include vitamins A, C and E, as well as the coenzyme Q10, the latter extensively advertised in face creams (Prahl et al., 2008), but also found to be effective in preserving mitochondrial respiratory function in aged rat skeletal (Sugiyama et al., 1995) and cardiac muscles (Park and Prolla, 2005). However, some studies have revealed that antioxidants do not delay the aging process per se, but rather contribute to increase longevity (Holloszy, 1998). Vitamin C, for example, has proven to be ineffective at prolonging life-span in mice, partly because any positive benefits were offset by compensatory reductions in endogenous protection mechanisms, ultimately resulting in no net reduction of the accumulated oxidative damage (Selman et al., 2006). Despite these data, antioxidants are repeatedly hailed as miracle cures against aging, and are often found in dietary supplements (Bailey et al., 2013; Wolfe and Liu, 2007). The increased commercialization of these products should be worrisome, as not only large cohort studies have shown that dietary supplements do not affect mortality either positively or negatively (Park et al., 2011), but have also been proven to be involved in the accelerated cancer development in mice (Sayin et al., 2014). Moreover, high-dose antioxidant supplements may in fact do more harm than good (Bjelakovic et al., 2004, 2008; Combet and Buckton, 2014), partly due to the fact that, as previously mentioned, low levels of ROS may be beneficial and may have a positive role in life-span (Lee et al., 2010). Therefore, although low-dose mixtures of antioxidants can sometimes have a beneficial effect (Gutteridge and Halliwell, 2010), it reflects mostly (if not only) in those members of populations whose diet and lifestyle result in micronutrients deficiencies (Shenkin, 2013).
Overall, there is little evidence that antioxidants have the power to delay aging and these are perhaps more suited to be used in alternative applications, such as functional ingredients in food systems to reduce oxidative changes (Samaranayaka and Li-Chan, 2011) and “cosmoceuticals” (Bogdan Allemann and Baumann, 2008). The intake of antioxidants should, hence, occur when, and only when, supplemented in our diet, not tablets or pills (Bjelakovic et al., 2014).
(6) Telomere-based therapies
If telomere extension can increase cell proliferative capacity in vitro (Ramunas et al., 2015) and account for the reversal of tissue degeneration in mice (Jaskelioff et al., 2011), than there is the possibility of being used to attenuate the rate of aging. That is certainly the core concept behind the commercialization, by some companies, of telomere measurement kits (Wolinsky, 2011), aimed at estimating the biological age of individuals and, to some extent, the risk of developing telomere shortening-associated diseases, such as atherosclerosis (Samani et al., 2001), coronary heart diseases (Ogami et al., 2004) and liver cirrhosis (Wiemann et al., 2002). Nonetheless, despite the media hype (Geddes and Macrae, 2015; Knight, 2015; Pollack, 2011), you might be better off looking at a calendar, as there is little evidence to support the claim that telomere measurement provides a better estimate of biological age than chronological age (de Magalhães, 2013). Pharmaceutical companies are, however, making efforts in developing telomerase-based therapies. One natural telomerase activator product, TA-65®, is already available (Harley et al., 2011) and, although it has failed to prolong life-span, it has yielded apparent positive immune remodeling and beneficial effects over metabolic, bone, and cardiovascular health (Harley et al., 2013).
However, conflicting evidence (Cheung et al., 2014; Effros, 1998; Holliday, 2014; Toda et al., 1998) and the realization that mice over-expressing telomerase do not live longer (de Magalhães and Toussaint, 2004) are powerful reasons to take pause regarding such therapies. Moreover, telomerase expression has long been linked to promotion of tumor growth and cell proliferation (Peterson et al., 2015), and, therefore, there is the justified fear that the use of telomerase activators may increase cancer development risk.
(7) Therapies to come
There are various approaches that have yielded promising initial results in delaying aging.
The use of rapamycin is one such approach. This is an immunosuppressant, commonly used to prevent organ rejection (Dumont and Su, 1996). Rapamycin has been demonstrated to extend maximal life-span in mammalian species, but it is not clear whether the drug slows mammalian aging or if it has isolated effects on longevity by suppressing cancers, which is the main cause of death in mouse strains (Ehninger et al., 2014) and it has been shown to extend murine life-span, tough exhibiting limited effects on aging (Neff et al., 2013). Rapamycin works by inhibiting a complex pathway called the target of rapamycin (TOR), and, more specifically, over the mammalian target of rapamycin (mTOR), a kinase at a key signaling node that aggregates and integrates information regarding extracellular growth factor stimulation, nutrient availability and energy supplies (Ehninger et al., 2014) (Fig. 10). This compound shows, nonetheless, serious side effects, such as nephrotoxicity (Murgia et al., 1996), thrombocytopenia (decrease of platelets) (Sacks, 1999) and hyperdyslipidemia (elevated levels of lipids) (Stallone et al., 2009). Consequently, different laboratories and companies are currently targeting more specific downstream nodes of this pathway, in order to develop anti-aging drugs without the side-effects of rapamycin (de Magalhaes et al., 2012).
The klotho gene, which codes for one membrane protein and one secreted transcript that acts as a circulating hormone, appears to influence aging, as mutations in this gene have resulted in accelerated aging in mice, as well as low-level expression (Kuroo et al., 1997). Overexpression of klotho, in turn, extended life-span by about 30% (Kurosu et al., 2005). The action mechanism of this gene remains largely unknown, but evidences point to the insulin/IGF-1 signaling pathways and may also be involved in calcium metabolism and in a vitamin D endocrine system (Tsujikawa et al., 2003). Additionally, resveratrol has also been described as an inducer of klotho expression (Hsu et al., 2014). These data make the involvement of the klotho gene in aging rather plausible, though more work is necessary to confirm this claim and elucidate the mechanisms involved in this process, for this and other genes which have been implicated in the aging process (ElSharawy et al., 2012; Hackl et al., 2010; Klement et al., 2012; Zhong et al., 2015). Such extensive knowledge would allow for effective gene therapies, based on the modulation of these aging-related genes and, hence, extend life-span.
Supplementation with precursors of the oxidized form of cellular nicotinamide adenine dinucleotide (NAD+) has also been demonstrated to extend life-span and to rescue premature aging phenotypes in both nematodes (Fang Evandro et al., 2014) and mice (Scheibye-Knudsen et al., 2014; Zhang et al., 2016). Hence, strategies aiming at the conservation of cellular NAD+ may result in improvements in mammalian life-span, though it remains to be seen whether NAD+ precursor supplementation will, in effect, yield overall health benefits in aging human populations.
Perhaps the most futuristic anti-aging therapy – at least, in our collective imagination – is nanotechnology, which may be due, in part, to the book in which the term was coined, Engines of Creation (Drexler, 1996), immediately evoking images of tiny, highly complex nano-machines, or nanobots, sometimes also referred to as nanites. Nanotechnology holds many promises and expectations in a wide range of applications. Nonetheless, thus far, the biomedical applications, including the nanotech fight against aging, entail a level of technological advancements that are certainly within our reach, though not yet available. The first steps into this brave new world have been taken and, recently, an intelligent system has been devised that lays the foundations for the future development of new therapies against aging. This nanodevice consists of capped silica nanoparticles that can selectively release drugs in aged human cells (Agostini et al., 2012) and has enormous potential in the treatment of a myriad of diseases, namely, cancer or Alzheimer's. Hence, there is a promise that nanostructures of the like will be able to drive chemical reactions that are capable of slowing down or even reverse senescence, by reversing the chemical reactions and damage that take place with aging. Soon.
6. Conclusions
Biological aging, termed senescence, is one of the most complex biological processes. Theories of aging are generally classified as either program theories or damage theories. More recently, combined theories, in which the aging process is considered at a more comprehensive and global degree, have emerged, but definitive evidences are still elusive.
The complexity of the aging process has led to the realization that an integrative approach is necessary to better understand the mechanisms of aging. In this regard, omics – genomics, transcriptomics, proteomics, lipidomics and metabolomics – can play a pivotal role in the elucidation of the complex, interconnected changes that take place at the different levels of the biological hierarchy during aging, though the current knowledge of these molecular interactions is still very limited.
Senescence is not the inevitable fate of all organisms and it can be delayed. In the last few decades, there has been an increase of in evidences showing that aging is not an irreversible process. Additionally, we are now privy to a multitude of mechanisms that allow considerable life-span extensions.
Most of the reported life-extension mechanisms have been observed in simpler organisms and these have still to be demonstrated as viable anti-aging therapies in humans. Additionally, these do not curtail one of the hallmarks of aging, cognitive impairment.(5) Research into aging is blooming but biogerontologists must be aware of this interconnectivity that, not seldom, obfuscates the primary cause(s) of aging and greatly limits the ability to reach valid and definitive conclusions.
Acknowledgments
This work was supported by national funds through FCT/MEC (PIDDAC) under project IF/00407/2013/CP1162/CT0023. Thanks are also due, for the financial support to CESAM (UID/AMB/50017), to Portuguese Science Foundation (FCT) FCT/MEC through national funds, and the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. This work was also funded by FCT through SFRH/BPD/102452/2014 under POCH funds, co-financed by the European Social Fund and Portuguese National Funds from MEC.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Abdelmegeed MA, Choi Y, Ha SK, Song BJ. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic Biol Med. 2016;91:188–202. doi: 10.1016/j.freeradbiomed.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo AM. Nutritional factors and aging in demyelinating diseases. Genes Nutr. 2014;9:360. doi: 10.1007/s12263-013-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afanas'ev I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis. 2014;5:52–62. doi: 10.14336/AD.2014.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini A, Mondragón L, Bernardos A, Martínez-Máñez R, Marcos MD, Sancenón F, Soto J, Costero A, Manguan-García C, Perona R, Moreno-Torres M, Aparicio-Sanchis R, Murguía JR. Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew Chem Int Ed. 2012;51:10556–10560. doi: 10.1002/anie.201204663. [DOI] [PubMed] [Google Scholar]
- Ahlqvist KJ, Suomalainen A, Hämäläinen RH. Stem cells, mitochondria and aging. Biochim Biophysi Acta(BBA)—Bioenerg. 2015;1847:1380–1386. doi: 10.1016/j.bbabio.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diab Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Albert ML, Knoefel JE. Clinical Neurology of Aging. OUP; USA: 2011. [Google Scholar]
- Aledo JC, Blanco JM. Aging is neither a failure nor an achievement of natural selection. Curr Aging Sci. 2015 [PubMed] [Google Scholar]
- Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009;276:5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amidi F, French BA, Chung D, Halsted CH, Medici V, French SW. M-30 and 4HNE are sequestered in different aggresomesin the same hepatocytes. Exp Mol Pathol. 2007;83:296–300. doi: 10.1016/j.yexmp.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87:201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton B, Vitetta L, Cortizo F, Sali A. Can we delay aging? The biology and science of aging. Ann N Y Acad Sci. 2005;1057:525–535. doi: 10.1196/annals.1356.040. [DOI] [PubMed] [Google Scholar]
- Arking R. Biology of Aging: Observations and Principles. Oxford University Press; 2006. [Google Scholar]
- Artal-Sanz M, Tavernarakis N. Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C [thinsp]elegans. Nature. 2009;461:793–797. doi: 10.1038/nature08466. [DOI] [PubMed] [Google Scholar]
- Austad SN. Is aging programed? Aging Cell. 2004;3:249–251. doi: 10.1111/j.1474-9728.2004.00112.x. [DOI] [PubMed] [Google Scholar]
- Azemin MZC, Kumar DK, Wong TY, Wang JJ, Mitchell P, Kawasaki R, Wu H. Age-related rarefaction in the fractal dimension of retinal vessel. Neurobiol Aging. 2012;33:194.e191–194.e194. doi: 10.1016/j.neurobiolaging.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Baars J. Aging and the Art of Living. Johns Hopkins University Press; 2012. [Google Scholar]
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Int Med. 2013;173:355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- Baltes BB, Rudolph CW, Bal AC. The Oxford Handbook of Work and Aging. OUP (Oxford Univ. Press); USA: 2012. A review of aging theories and modern work perspectives; pp. 117–136. [Google Scholar]
- Banito A, Lowe Scott W. A new development in senescence. Cell. 2013;155:977–978. doi: 10.1016/j.cell.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks E, Reeves GK, Beral V, Balkwill A, Liu B, Roddam A. Hip fracture incidence in relation to age, menopausal status, and age at menopause: prospective analysis. PLoS Med. 2009;6:e1000181. doi: 10.1371/journal.pmed.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri M, Bonafè M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal. 2013;19:1420–1445. doi: 10.1089/ars.2012.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeier C, Kaiser D, Heeren J, Scheja L, John C, Weise C, Eravci M, Lagerpusch M, Schulze G, Joost HG, Schwenk RW, Schürmann A. Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim Biophys Acta (BBA)—Mol Cell Biol Lipids. 2015;1851:566–576. doi: 10.1016/j.bbalip.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Becher A, Dent J. Systematic review: ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol Ther. 2011;33:442–454. doi: 10.1111/j.1365-2036.2010.04542.x. [DOI] [PubMed] [Google Scholar]
- Bengtson VL, Rice CJ, Johnson ML. Handbook of Theories of Aging. McMaster University; 1999. Are theories of aging important? Models and explanations in gerontology at the turn of the century; pp. 3–20. [Google Scholar]
- Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–1177. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Biliński T, Zadrąg-Tęcza R, Bartosz G. Hypertrophy hypothesis as an alternative explanation of the phenomenon of replicative aging of yeast. FEMS Yeast Res. 2012;12:97–101. doi: 10.1111/j.1567-1364.2011.00759.x. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Systematic review: primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Aliment Pharmacol Ther. 2008;28:689–703. doi: 10.1111/j.1365-2036.2008.03785.x. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud C. Antioxidant supplements and mortality. Curr Opin Clin Nutr Metab Care. 2014;17:40–44. doi: 10.1097/MCO.0000000000000009. [DOI] [PubMed] [Google Scholar]
- Bjorksten J. The crosslinkage theory of aging. J Am Geriatr Soc. 1968;16:408–427. doi: 10.1111/j.1532-5415.1968.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging is not programmed. ABBV Cell Cycle. 2013;12:3736–3742. doi: 10.4161/cc.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Bogdan Allemann I, Baumann L. Antioxidants used in skin care formulations. Skin Ther Lett. 2008;13:5–9. [PubMed] [Google Scholar]
- Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C. The aging kidney revisited: a systematic review. Ageing Res Rev. 2014;14:65–80. doi: 10.1016/j.arr.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory span: a meta-analysis. J Gerontol Ser B: Psychol Sci Soc Sci. 2005;60:223–P233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Bose B, Shenoy PS. Aging induced loss of stemness with concomitant gain of myogenic properties of a pure population of CD34+/CD45− muscle derived stem cells. Int J Biochem Cell Biol. 2016;70:1–12. doi: 10.1016/j.biocel.2015.10.009. http://dx.doi.org/10.1016/j.biocel.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135:434–440. [PMC free article] [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakefield PM, Gems D, Cowen T, Christensen K, Grubeck-Loebenstein B, Keller L, Oeppen J, Rodriguez-Pena A, Stazi MA, Tatar M, Westendorp RGJ. What are the effects of maternal and pre-adult environments on ageing in humans, and are there lessons from animal models? Mech Ageing Dev. 2005;126:431–438. doi: 10.1016/j.mad.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Brosh RM., Jr DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer. 2013;13:542–558. doi: 10.1038/nrc3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownborg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the aging process. Nature. 1996;384:33–33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention, Cf.D.Ca, editor. CDC. Mortality by underlying and multiple cause, ages 18+: US, 1981–2006. Center for Disease Control and Prevention; 2006. [Google Scholar]
- CDC, editor. CDC. Cholesterol Level, ages 20+: US, 1988–2012. Centers for Disease Control and Prevention; 2015. [Google Scholar]
- Cabreiro F, Ackerman D, Doonan R, Araiz C, Back P, Papp D, Braeckman BP, Gems D. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic Biol Med. 2011;51:1575–1582. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, Vlassara H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173:327–336. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AL. Death as an unnatural process. Why is it wrong to seek a cure for aging? EMBO reports 6 Spec No, S72-75. 2005 doi: 10.1038/sj.embor.7400435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll PV, Committee: E.R.C. t. m. o.G.H.R.S.S. Bengtsson BÅ, Carlsson L, Christiansen JS, Clemmons D, Hintz R, Ho K, Laron Z, Sizonenko P, Sönksen PH, Tanaka T, Thorner M. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. J Clin Endocrinol Metab. 1998;83:382–395. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- Cary H, Davis H, Burges G. The Works of Plato: The Laws. H.G. Bohn; 1852. [Google Scholar]
- Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- Cevenini E, Bellavista E, Tieri P, Castellani G, Lescai F, Francesconi M, Mishto M, Santoro A, Valensin S, Salvioli S, Capri M, Zaikin A, Monti D, de Magalhaes JP, Franceschi C. Systems biology and longevity: an emerging approach to identify innovative anti-aging targets and strategies. Curr Pharm Des. 2010;16:802–813. doi: 10.2174/138161210790883660. [DOI] [PubMed] [Google Scholar]
- Chesser B. Senescence in Humans. M L Books International; 2015. [Google Scholar]
- Cheung HH, Liu X, Canterel-Thouennon L, Li L, Edmonson C, Rennert OM. Telomerase protects Werner syndrome lineage-specific stem cells from premature aging. Stem Cell Rep. 2014;2:534–546. doi: 10.1016/j.stemcr.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- Chondrogianni N, Gonos ES. Proteasome activation as a novel antiaging strategy. IUBMB Life. 2008;60:651–655. doi: 10.1002/iub.99. [DOI] [PubMed] [Google Scholar]
- Chondrogianni N, Petropoulos I, Franceschi C, Friguet B, Gonos ES. Fibroblast cultures from healthy centenarians have an active proteasome. Exp Gerontol. 2000;35:721–728. doi: 10.1016/s0531-5565(00)00137-6. [DOI] [PubMed] [Google Scholar]
- Christ B, Brückner S, Winkler S. The therapeutic promise of mesenchymal stem cells for liver restoration. Trends Mol Med. 2015;21(November (11)):673–686. doi: 10.1016/j.molmed.2015.09.004. http://dx.doi.org/10.1016/j.molmed.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Clarke S. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–285. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- Combet E, Buckton C. Micronutrient deficiencies, vitamin pills and nutritional supplements. Medicine (U K) 2014;43:66–72. [Google Scholar]
- Craig T, Smelick C, Tacutu R, Wuttke D, Wood SH, Stanley H, Janssens G, Savitskaya E, Moskalev A, Arking R, de Magalhães JP. The digital ageing atlas: integrating the diversity of age-related changes into a unified resource. Nucleic Acids Res. 2015;43:D873–D878. doi: 10.1093/nar/gku843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: amicroarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21:41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2011;2012 doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG. The dysdifferentiative hypothesis of mammalian aging and longevity. In: Jacobini E, editor. The Aging Brain. Raven Press; New York: 1982. [Google Scholar]
- da Costa JP, Rocha-Santos T, Duarte AC. Analytical tools to assess aging in humans: the rise of Geri-Omics. TrAC Trends Anal Chem. 2016;80:204–212. http://dx.doi.org/10.1016/j.trac.2015.09.011. [Google Scholar]
- de Grey ADNJ. Escape velocity: why the prospect of extreme human life extension matters now. PLoS Biol. 2004;2:e187. [Google Scholar]
- de Magalhães JP, Toussaint O. Telomeres and telomerase: a modern fountain of youth? Rejuvenation Res. 2004;7:126–133. doi: 10.1089/1549168041553044. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP. Open-minded scepticism: inferring the causal mechanisms of human ageing from genetic perturbations. Ageing Res Rev. 2005;4:1–22. doi: 10.1016/j.arr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP. senescence.info. Senescence, UK: 2013. [Google Scholar]
- de Magalhaes JP, Church GM. Genomes optimize reproduction: aging as a consequence of the developmental program. Physiol (Bethesda Md) 2005;20:252–259. doi: 10.1152/physiol.00010.2005. [DOI] [PubMed] [Google Scholar]
- de Magalhaes JP, Wuttke D, Wood SH, Plank M, Vora C. Genome-environment interactions that modulate aging: powerful targets for drug discovery. Pharmacol Rev. 2012;64:88–101. doi: 10.1124/pr.110.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhaes JP. The scientific quest for lasting youth: prospects for curing aging. Rejuvenation Res. 2014;17:458–467. doi: 10.1089/rej.2014.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DC. Aging and the aggregating proteome. Front Genet. 2012;3 doi: 10.3389/fgene.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loof A, De Haes W, Boerjan B, Schoofs L. The fading electricity theory of ageing: the missing biophysical principle? Ageing Res Rev. 2013;12:58–66. doi: 10.1016/j.arr.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Delbin MA, Davel A, Couto GK, de Araujo GG, Rossoni LV, Antunes E, Zanesco A. Interaction between advanced glycation end products formation and vascular responses in femoral and coronary arteries from exercised diabetic rats. PLoS One. 2012;7:e53318. doi: 10.1371/journal.pone.0053318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein D, Weaver C, Luebke J, Hof P. Dendritic spine changes associated with normal aging. Neuroscience. 2013;251:21–32. doi: 10.1016/j.neuroscience.2012.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M. Oxidative damage to DNA in mammalian chromatin. Mutat Res /DNAging. 1992;275:331–342. doi: 10.1016/0921-8734(92)90036-o. [DOI] [PubMed] [Google Scholar]
- Dollemore D. Aging NIo Aging Under the Microscope: A Biological Quest. National Institutes of Health, National Institute on Aging, Office of Communications and Public Liaison; 2002. [Google Scholar]
- Donahue JE. Normal and pathological changes with age in the brain. Medicine and health, Rhode Island. 2012;95:75–76. [PubMed] [Google Scholar]
- Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J Cell Biol. 2010;190:719–729. doi: 10.1083/jcb.201005144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler KE. Engines of Creation. Fourth Estate 1996 [Google Scholar]
- Dumont FJ, Su Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1996;58:373–395. doi: 10.1016/0024-3205(95)02233-3. [DOI] [PubMed] [Google Scholar]
- Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Replicative senescence in the immune system: impact of the hayflick limit on T-Cell function in the elderly. Am J Hum Genet. 1998;62:1003–1007. doi: 10.1086/301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Roy Walford and the immunologic theory of aging. Immun Ageing. 2005;2:7. doi: 10.1186/1742-4933-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71:4325–4346. doi: 10.1007/s00018-014-1677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSharawy A, Keller A, Flachsbart F, Wendschlag A, Jacobs G, Kefer N, Brefort T, Leidinger P, Backes C, Meese E, Schreiber S, Rosenstiel P, Franke A, Nebel A. Genome-wide miRNA signatures of human longevity. Aging Cell. 2012;11:607–616. doi: 10.1111/j.1474-9726.2012.00824.x. [DOI] [PubMed] [Google Scholar]
- Elewa R, Zouboulis CC. Molecular mechanisms of action of topical antiaging compounds. J Egypt Women's Dermatol Soc. 2014;11:73–78. [Google Scholar]
- Fang Evandro F, Scheibye-Knudsen M, Brace Lear E, Kassahun H, SenGupta T, Nilsen H, Mitchell James R, Croteau Deborah L, Bohr Vilhelm A. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reduction. Cell. 2014;157:882–896. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroni A, Terenghi G, Reid AJ. Chapter five—adipose-derived stem cells and nerve regeneration: promises and pitfalls. In: Stefano Geuna IPPT, Bruno B, editors. International Review of Neurobiology. Academic Press; 2013. pp. 121–136. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Koresko RL, Heller N. EEG sleep patterns as a function of normal and pathological aging in man. J Psychiatr Res. 1967;5:107–144. doi: 10.1016/0022-3956(67)90027-1. [DOI] [PubMed] [Google Scholar]
- Feng JX, Hou FF, Liang M, Wang GB, Zhang X, Li HY, Xie D, Tian JW, Liu ZQ. Restricted intake of dietary advanced glycation end products retards renal progression in the remnant kidney model. Kidney Int. 2007 doi: 10.1038/sj.ki.5002162. [DOI] [PubMed] [Google Scholar]
- Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol. 2014;142:132–141. doi: 10.1016/j.jsbmb.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life Span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes JM, Yee LTL, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, Thomas MC, Burns WC, Deemer EK, Thorpe SR. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813–1823. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. preventive services task force. Ann Intern Med. 2013;159:824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- Freidja ML, Tarhouni K, Toutain B, Fassot C, Loufrani L, Henrion D. The AGE-Breaker ALT-711 restores high blood flow—dependent remodeling in mesenteric resistance arteries in a rat model of type 2 diabetes. Diabetes. 2012;61:1562–1572. doi: 10.2337/db11-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas AC, Andrade JC, Silva FM, Rocha-Santos TA, Duarte AC, Gomes AM. Antioxidative peptides: trends and perspectives for future research. Curr Med Chem. 2013;20:4575–4594. doi: 10.2174/09298673113209990147. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997;405:21–25. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes L, Macrae F. Why Stress of Divorce Could Make You Age More Quickly: Breakups, Bereavements and Unemployment can Make Body's Genetic Material Deteriorate Prematurely. The Daily Mail; UK: 2015. [Google Scholar]
- Gems D. An integrated theory of ageing in the nematode Caenorhabditis elegans. J Anat. 2000;197:521–528. doi: 10.1046/j.1469-7580.2000.19740521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilini D, Mari D, Castaldi D, Remondini D, Ogliari G, Ostan R, Bucci L, Sirchia SM, Tabano S, Cavagnini F, Monti D, Franceschi C, Di Blasio AM, Vitale G. Role of epigenetics in human aging and longevity: genome-wide DNA methylation profile in centenarians and centenarians' offspring. Age (Dordrecht Netherlands) 2013;35:1961–1973. doi: 10.1007/s11357-012-9463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012;82:270–277. doi: 10.1038/ki.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL. Changes in cognitive function in human aging. Brain aging: models, methods, and mechanisms. 2007:3–20. [Google Scholar]
- Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23:23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Goldsmith TC. On the programmed/non-programmed aging controversy. Biochem Mosc. 2012;77:729–732. doi: 10.1134/S000629791207005X. [DOI] [PubMed] [Google Scholar]
- Goldsmith TC. Arguments against non-programmed aging theories. Biochem Mosc. 2013;78:971–978. doi: 10.1134/S0006297913090022. [DOI] [PubMed] [Google Scholar]
- Goldsmith T. Modern evolutionary mechanics theories and resolving the programmed/non-programmed aging controversy. Biochem Mosc. 2014;79:1049–1055. doi: 10.1134/S000629791410006X. [DOI] [PubMed] [Google Scholar]
- Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- Grene D. The History. University of Chicago Press; 2010. [Google Scholar]
- Grimaud R, Ezraty B, Mitchell JK, Lafitte D, Briand C, Derrick PJ, Barras F. Repair of oxidized proteins identification of a new methionine sulfoxide reductase. J Biol Chem. 2001;276:48915–48920. doi: 10.1074/jbc.M105509200. [DOI] [PubMed] [Google Scholar]
- Grodstein F, O'Brien J, Kang JH, Dushkes R, Cook NR, Okereke O, Manson JE, Glynn RJ, Buring JE, Gaziano JM. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806–814. doi: 10.7326/0003-4819-159-12-201312170-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation toage. Hum Reprod (Oxf Engl) 2010;25:957–968. doi: 10.1093/humrep/deq014. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Guarente L. Aging research—where do we stand and where are we going? Cell. 2014;159:15–19. doi: 10.1016/j.cell.2014.08.041. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC, Halliwell B. Antioxidants: molecules, medicines, and myths. Biochem Biophys Res Commun. 2010;393:561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- Hackl M, Brunner S, Fortschegger K, Schreiner C, Micutkova L, Muck C, Laschober GT, Lepperdinger G, Sampson N, Berger P, Herndler-Brandstetter D, Wieser M, Kuhnel H, Strasser A, Rinnerthaler M, Breitenbach M, Mildner M, Eckhart L, Tschachler E, Trost A, Bauer JW, Papak C, Trajanoski Z, Scheideler M, Grillari-Voglauer R, Grubeck-Loebenstein B, Jansen-Durr P, Grillari J. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9:291–296. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman MR. Insulin-like growth factors and aging. Endocrinol Metab Clin N Am. 1987;16:995–1011. [PubMed] [Google Scholar]
- Hanes JW, Thal DM, Johnson KA. Incorporation and replication of 8-oxo-deoxyguanosine by the human mitochondrial DNA polymerase. J Biol Chem. 2006;281:36241–36248. doi: 10.1074/jbc.M607965200. [DOI] [PubMed] [Google Scholar]
- Harley CB, Liu W, Blasco M, Vera E, Andrews WH, Briggs LA, Raffaele JM. A natural product telomerase activator as part of a health maintenanceprogram. Rejuvenation Res. 2011;14:45–56. doi: 10.1089/rej.2010.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Liu W, Flom PL, Raffaele JM. A natural product telomerase activator as part of a health maintenance program: metabolic and cardiovascular response. Rejuvenation Res. 2013;16:386–395. doi: 10.1089/rej.2013.1430. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Free radical involvement in aging. Drugs Aging. 1993;3:60–80. doi: 10.2165/00002512-199303010-00006. [DOI] [PubMed] [Google Scholar]
- Hartog JW, Willemsen S, Veldhuisen DJ, Posma JL, Wijk LM, Hummel YM, Hillege HL, Voors AA. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur J Heart Fail. 2011;13:899–908. doi: 10.1093/eurjhf/hfr067. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington's disease. J Appl Physiol (Bethesda, Md: 1985) 1985;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hetz C, Glimcher LH. Protein homeostasis networks in physiology and disease. Curr Opin Cell Biol. 2011;23:123–125. doi: 10.1016/j.ceb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab. 2011;301:E779–E784. doi: 10.1152/ajpendo.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmborg J, Iachine I, Skytthe A, Vaupel J, McGue M, Koskenvuo M, Kaprio J, Pedersen N, Christensen K. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO. Effects of sex and age on the 24-h profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64:51–58. doi: 10.1210/jcem-64-1-51. [DOI] [PubMed] [Google Scholar]
- Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- Holliday R. The commitment of human cells to senescence. Aging: Facts Theor. 2014:1–7. doi: 10.1159/000358896. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Longevity of exercising male rats: effect of an antioxidant supplemented diet. Mech Ageing Dev. 1998;100:211–219. doi: 10.1016/s0047-6374(97)00140-1. [DOI] [PubMed] [Google Scholar]
- Hou L, Huang J, Green CD, Boyd-Kirkup J, Zhang W, Yu X, Gong W, Zhou B, Han JD. Systems biology in aging: linking the old and the young. Curr Genom. 2012;13:558–565. doi: 10.2174/138920212803251418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Huang SM, Chen A, Sun CY, Lin SH, Chen JS, Liu ST, Hsu YJ. Resveratrol increases anti-aging Klothogene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int J Biochem Cell Biol. 2014;53:361–371. doi: 10.1016/j.biocel.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495–2505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm Metab Res. 2012;44:891–895. doi: 10.1055/s-0032-1321878. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Matsui T, Yamagishi S. Olmesartan blocks advanced glycation end products-induced vcam-1 gene expression in mesangial cells by restoring angiotensin-converting enzyme 2 level. Horm Metab Res. 2014;46:379–383. doi: 10.1055/s-0033-1361114. [DOI] [PubMed] [Google Scholar]
- Jacobs KB, Yeager M, Zhou W, Wacholder S, Wang Z, Rodriguez-Santiago B, Hutchinson A, Deng X, Liu C, Horner MJ, Cullen M, Epstein CG, Burdett L, Dean MC, Chatterjee N, Sampson J, Chung CC, Kovaks J, Gapstur SM, Stevens VL, Teras LT, Gaudet MM, Albanes D, Weinstein SJ, Virtamo J, Taylor PR, Freedman ND, Abnet CC, Goldstein AM, Hu N, Yu K, Yuan JM, Liao L, Ding T, Qiao YL, Gao YT, Koh WP, Xiang YB, Tang ZZ, Fan JH, Aldrich MC, Amos C, Blot WJ, Bock CH, Gillanders EM, Harris CC, Haiman CA, Henderson BE, Kolonel LN, Le Marchand L, McNeill LH, Rybicki BA, Schwartz AG, Signorello LB, Spitz MR, Wiencke JK, Wrensch M, Wu X, Zanetti KA, Ziegler RG, Figueroa JD, Garcia-Closas M, Malats N, Marenne G, Prokunina-Olsson L, Baris D, Schwenn M, Johnson A, Landi MT, Goldin L, Consonni D, Bertazzi PA, Rotunno M, Rajaraman P, Andersson U, Beane Freeman LE, Berg CD, Buring JE, Butler MA, Carreon T, Feychting M, Ahlbom A, Gaziano JM, Giles GG, Hallmans G, Hankinson SE, Hartge P, Henriksson R, Inskip PD, Johansen C, Landgren A, McKean-Cowdin R, Michaud DS, Melin BS, Peters U, Ruder AM, Sesso HD, Severi G, Shu XO, Visvanathan K, et al. Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet. 2012;44:651–658. doi: 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz DF, Taipale M, Lindquist S. Protein homeostasis and the phenotypic manifestation of genetic diversity: principles and mechanisms. Annu Rev Genet. 2010;44:189–216. doi: 10.1146/annurev.genet.40.110405.090412. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams A, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, Horner JW, Maratos-Flier E, DePinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen DK, Bohr VA, Stevnsner T. DNA repair deficiency in neurodegeneration. Prog Neurobiol. 2011;94:166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K. Modern biological theories of aging. Aging Dis. 2010;1:72–74. [PMC free article] [PubMed] [Google Scholar]
- Johnson WT, Salanga G, Lee W, Marshall GA, Himelstein AL, Wall SJ, Horwitz O. Arterial intimal embrittlement: a possible factor in atherogenesis. Atherosclerosis. 1986;59:161–171. doi: 10.1016/0021-9150(86)90045-6. [DOI] [PubMed] [Google Scholar]
- Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Johnson TE. Increased life-span ofage-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249:908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- Jung T, Catalgol B, Grune T. The proteasomal system. Mol Asp Med. 2009;30:191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kaplan DT, Furman MI, Pincus SM, Ryan SM, Lipsitz LA, Goldberger AL. Aging and the complexity of cardiovascular dynamics. Biophys J. 1991;59:945–949. doi: 10.1016/S0006-3495(91)82309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kator K, Cristofalo V, Charpentier R, Cutler R. Dysdifferentiative nature of aging: passage number dependency of globin gene expression in normal human diploid cells grown in tissue culture. Gerontology. 1985;31:355–361. doi: 10.1159/000212724. [DOI] [PubMed] [Google Scholar]
- Kawagishi H, Finkel T. Unraveling the truth about antioxidants: ROS and disease: finding the right balance. Nat Med. 2014;20:711–713. doi: 10.1038/nm.3625. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Khrapko K, Bodyak N, Thilly WG, Van Orsouw NJ, Zhang X, Coller HA, Peris TT, Upton M, Vijg J, Wei JY. Cell-by-cell scanning of whole mitochondrial genomes in aged human heart reveals a significant fraction of myocytes with clonally expanded deletions. Nucleic Acids Res. 1999;27:2434–2441. doi: 10.1093/nar/27.11.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee Y, Kwak HB, Lawler JM. Lifelongwheel running exercise and mild caloric restriction attenuate nuclear EndoG in the aging plantaris muscle. Exp Gerontol. 2015;69:122–128. doi: 10.1016/j.exger.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimlin MG, Guo Y. Assessing the impacts of lifetime sun exposure on skin damage and skin aging using a non-invasive method. Sci Total Environ. 2012;425:35–41. doi: 10.1016/j.scitotenv.2012.02.080. [DOI] [PubMed] [Google Scholar]
- Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. 1990 doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood Thomas BL, Melov S. On the programmed/non-programmed nature of ageing within the life history. Curr Biol. 2011;21:R701–R707. doi: 10.1016/j.cub.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Systems biology of ageing and longevity. Philos Trans R Soc Lond Ser B Biol Sci. 2011;366:64–70. doi: 10.1098/rstb.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement K, Melle C, Murzik U, Diekmann S, Norgauer J, Hemmerich P. Accumulation of annexin A5 at the nuclear envelope is a biomarker of cellular aging. Mech Ageing Dev. 2012;133:508–522. doi: 10.1016/j.mad.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Klocke RA. Influence of aging in the lung. In: Finch CE, Hayflick L, editors. Handbook of the Biology of Aging. Van Nostrand Reinhold; New York: 1977. pp. 432–444. [Google Scholar]
- Knight M. Genetic Literacy Project. University of California Washington Center; Washington, DC: 2015. Buy Your Telomere Testing Kit Here! Evidence Based or Pseudo-science? [Google Scholar]
- Kohl M, Megger DA, Trippler M, Meckel H, Ahrens M, Bracht T, Weber F, Hoffmann AC, Baba HA, Sitek B, Schlaak JF, Meyer HE, Stephan C, Eisenacher M. A practical data processing workflow for multi-OMICS projects. Biochim Biophys Acta (BBA)—Proteins Proteom. 2014;1844:52–62. doi: 10.1016/j.bbapap.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBOJ. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzisnik C, Grguric S, Cvijovic K, Laron Z. Longevity of the hypopituitary patients from the Island Krk: a follow-up study. Pediatr Endocrinol Rev. 2010;7:357–362. [PubMed] [Google Scholar]
- Kriete A, Sokhansanj BA, Coppock DL, West GB. Systems approaches to the networks of aging. Ageing Res Rev. 2006;5:434–448. doi: 10.1016/j.arr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci U S A. 1995;92:258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Szklarczyk D, Pletscher-Frankild S, Blicher TH, von Mering C, Jensen LJ, Bork P. STITCH 4: integration of protein—chemical interactions with user data. Nucleic Acids Res. 2014;42:D401–D407. doi: 10.1093/nar/gkt1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. Long-term potentiation at CA3—CA1 hippocampal synapses with special emphasis on aging, disease, and stress. Front Aging Neurosci. 2011;3:7. doi: 10.3389/fnagi.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuroo M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123:990–995. doi: 10.1172/JCI64095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Carpenter B, Meyers LS. Representations of older adults in television advertisements. J Aging Stud. 2007;21:23–30. [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi G, Thomson J, Dobell B. Essays, Dialogues and Thoughts: (Operette Morali and Pensieri) of Giacomo Leopardi. G. Routledge & sonslimited; 1905. [Google Scholar]
- Lewis U, Singh R, Tutwiler G, Sigel M, Vander-Laan E, VanderLaan W. Human Growth Hormone: A Complex of Proteins1, Recent Progress inHormone Research: Proceedings of the 1979 Laurentian Hormone Conference. Academic Press; 2013. p. 477. [DOI] [PubMed] [Google Scholar]
- Little WC, Zile MR, Kitzman DW, Hundley WG, O'Brien TX. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- Liu HY, Huang CF, Lin TC, Tsai CY, Tina Chen SY, Liu A, Chen WH, Wei HJ, Wang MF, Williams DF, Deng WP. Delayed animal aging through the recovery of stem cell senescence by platelet rich plasma. Biomaterials. 2014;35:9767–9776. doi: 10.1016/j.biomaterials.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Lohwasser C, Neureiter D, Weigle B, Kirchner T, Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J Invest Dermatol. 2006;126:291–299. doi: 10.1038/sj.jid.5700070. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mitteldorf J, Skulachev VP. Programmed and altruistic ageing. Nat Rev Genet. 2005;6:866–872. doi: 10.1038/nrg1706. [DOI] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malozowski S, Tanner LA, Wysowski D, Fleming GA. Growth hormone, insulin-like growth factor I, and benign intracranial hypertension. N Engl J Med. 1993;329:665–666. doi: 10.1056/NEJM199308263290917. [DOI] [PubMed] [Google Scholar]
- Mangialasche F, Polidori MC, Monastero R, Ercolani S, Camarda C, Cecchetti R, Mecocci P. Biomarkers of oxidative and nitrosative damage in Alzheimer's disease and mild cognitive impairment. Ageing Res Rev. 2009;8:285–305. doi: 10.1016/j.arr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Mann K, Edsinger E. The Lottiagigantea shell matrix proteome: re-analysis including MaxQuant iBAQquantitation and phosphoproteome analysis. Proteome Sci. 2014;12:28. doi: 10.1186/1477-5956-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor B, Costa MD, Hu K, Newton E, Starobinets O, Kang HG, Peng C, Novak V, Lipsitz LA. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J Appl Physiol. 2010;109:1786–1791. doi: 10.1152/japplphysiol.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Hardy T, Tollefsbol T. Medicinal chemistry of the epigenetic diet and caloric restriction. Curr Med Chem. 2013;20:4050. doi: 10.2174/09298673113209990189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ, McCarter RJ, Katz MS, McMahan CA. Dietary restriction alters characteristics of glucose fuel use. J Gerontol. 1992;47:B202–208. doi: 10.1093/geronj/47.6.b202. [DOI] [PubMed] [Google Scholar]
- Matic I, Ahel I, Hay RT. Reanalysis of phosphoproteomics data uncovers ADP-ribosylation sites. Nat Methods. 2012;9:771–772. doi: 10.1038/nmeth.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Energy Metabolism and Lifespan Determination. Elsevier Science; 2003. [Google Scholar]
- Maurya PK, Noto C, Rizzo LB, Rios AC, Nunes SOV, Barbosa DS, Sethi S, Zeni M, Mansur RB, Maes M, Brietzke E. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;65:134–144. doi: 10.1016/j.pnpbp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G, Tevy MF, Borghesan M, Vergini MRD, Vinciguerra M. Caloric restriction and aging stem cells: the stick and the carrot? Exp Gerontol. 2014;50:137–148. doi: 10.1016/j.exger.2013.10.014. [DOI] [PubMed] [Google Scholar]
- McCay CM. Iodized salt a hundred years ago. Science. 1935;82:350–351. doi: 10.1126/science.82.2128.350-a. [DOI] [PubMed] [Google Scholar]
- Medawar PB. An Unsolved Problemof Biology. College; 1952. [Google Scholar]
- Medvedev ZA. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990;65:375–398. doi: 10.1111/j.1469-185x.1990.tb01428.x. [DOI] [PubMed] [Google Scholar]
- Meena J, Lenhard Rudolph K, Günes C. Telomere Dysfunction, chromosomal instability and cancer. Recent Results Cancer Res. 2015:61–79. doi: 10.1007/978-3-319-20291-4_3. [DOI] [PubMed] [Google Scholar]
- Meites J. Neuroendocrinology of Aging. Springer Science & Business Media; 2012. [Google Scholar]
- Meléndez A, Tallóczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- Miki H, Funato Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J Biochem. 2012;151:255–261. doi: 10.1093/jb/mvs006. [DOI] [PubMed] [Google Scholar]
- Miquel J. An integrated theory of aging as the result of mitochondrial-DNA mutation in differentiated cells. Arch Gerontol Geriatr. 1991;12:99–117. doi: 10.1016/0167-4943(91)90022-i. [DOI] [PubMed] [Google Scholar]
- Mishra S, Jha A, Dubey R. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma. 2011;248:565–577. doi: 10.1007/s00709-010-0210-0. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Choong CJ, Yasuda T. The promises of stem cells: stem cell therapy for movement disorders. Parkinsonism Relat Disord. 2014;20(Suppl. 1):S128–S131. doi: 10.1016/S1353-8020(13)70031-2. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Hulbert AJ, Buttemer WA. The long life of birds: the Rat-Pigeon comparison revisited. PLoS One. 2011;6:e24138. doi: 10.1371/journal.pone.0024138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Stress, aging, and neurodegenerative disease. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moronetti Mazzeo LE, Dersh D, Boccitto M, Kalb RG, Lamitina T. Stress and aging induce distinct polyQprotein aggregation states. Proc Natl Acad Sci U S A. 2012;109:10587–10592. doi: 10.1073/pnas.1108766109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, McManus DQ. The neurology of aging: normal versus pathologic change. Geriatrics. 1991;46:47–48. 51-44. [PubMed] [Google Scholar]
- Mostany R, Anstey JE, Crump KL, Maco B, Knott G, Portera-Cailliau C. Altered synaptic dynamics during normal brain aging. J Neurosci. 2013;33:4094–4104. doi: 10.1523/JNEUROSCI.4825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia MG, Jordan S, Kahan BD. The side effect profile of sirolimus: a phase I study in quiescent cyclosporine-prednisone-treated renal transplant patients. Kidney Int. 1996;49:209–216. doi: 10.1038/ki.1996.28. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Suzuki T, Ueda Y, Yamada S, Shoji H, Takeuchi M, Ueda S, Matsui T, Adachi H, Okuda S, Yamagishi Si. Circulating levels of advanced glycation end products (AGE) and interleukin-6 (IL-6) are independent determinants of serum asymmetric dimethylarginine (ADMA) levels in patients with septic shock. Pharmacol Res. 2009;60:515–518. doi: 10.1016/j.phrs.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, Humayun MS. Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res. 2015;48:1–39. doi: 10.1016/j.preteyeres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Höter SM, Moreth K, Prehn C, Puk O, Rácz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Höfler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D. Rapamycin extends murine lifespan but has limited effects on aging. The Journal of Clinical Investigation. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novella S, Dantas AP, Segarra G, Vidal-Gómez X, Mompeón A, Garabito M, Hermenegildo C, Medina P. Aging-related endothelial dysfunction in the aorta from female senescence-accelerated mice is associated with decreased nitric oxide synthase expression. Exp Gerontol. 2013;48:1329–1337. doi: 10.1016/j.exger.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Novo E, Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1:5. doi: 10.1186/1755-1536-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBOJ. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124:827–833 (e825). doi: 10.1016/j.amjmed.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol. 2004;24:546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Dean R, Chattopadhyay S, Cutler R. Dysdifferentiative nature of aging: age-dependent expression of MuLVand globin genes in thymus, liver and brain in the AKR mouse strain. Gerontology. 1985;31:362–372. doi: 10.1159/000212725. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Ostler E. In: Chemistry of Ageing. Ostler E, editor. Chemistry Central; 2012. [Google Scholar]
- Paganini Piazzolla L, Medeiros de Almeida R, Nóbrega dos Santos AC, Goncalves de Oliveira P, Freitas da Silva E, Batista de Sousa J. Does aging influence clinical presentation and pathological staging in colorectal cancer? Eur Geriatr Med. 2015;6:433–436. [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521:525–528. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216:85–89. doi: 10.1007/s00429-011-0308-y. [DOI] [PubMed] [Google Scholar]
- Park CB, Larsson NG. Mitochondrial DNA mutations in disease and aging. J Cell Biol. 2011;193:809–818. doi: 10.1083/jcb.201010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Prolla TA. Gene expression profiling studies of aging in cardiac and skeletal muscles. Cardiovasc Res. 2005;66:205–212. doi: 10.1016/j.cardiores.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Park HL, O'Connell JE, Thomson RG. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry. 2003;18:1121–1134. doi: 10.1002/gps.1023. [DOI] [PubMed] [Google Scholar]
- Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol. 2011;173:906–914. doi: 10.1093/aje/kwq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CK, Mietus JE, Liu Y, Lee C, Hausdorff JM, Stanley HE, Goldberger AL, Lipsitz LA. Quantifying fractal dynamics of human respiration: age and gender effects. Ann Biomed Eng. 2002;30:683–692. doi: 10.1114/1.1481053. [DOI] [PubMed] [Google Scholar]
- Peng Y, Xuan M, Leung VYL, Cheng B. Stem cells and aberrant signaling of molecular systems in skin aging. Ageing Res Rev. 2015;19:8–21. doi: 10.1016/j.arr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Peppa M, Uribarri J, Vlassara H. Aging and glycoxidant stress. HORMONES-ATHENS. 2008;7:123. doi: 10.1007/BF03401503. [DOI] [PubMed] [Google Scholar]
- Peterson DR, Mok HOL, Au DWT. Modulation of telomerase activity in fish muscle by biological and environmental factors. Comp Biochem Physiol Part C: Toxicol Pharmacol. 2015;178:51–59. doi: 10.1016/j.cbpc.2015.09.004. http://dx.doi.org/10.1016/j.cbpc.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Fukui H, Wang X, Pinto M, Moraes CT. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J Neurosci. 2011;31:9895–9904. doi: 10.1523/JNEUROSCI.6223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrafita G, Keller MA, Ralser M. The impact of non-enzymatic reactions and enzyme promiscuity on cellular metabolism during (oxidative) stress conditions. Biomolecules. 2015;5:2101–2122. doi: 10.3390/biom5032101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M, Moraes CT. Mechanisms linking mtDNA damage and aging. Free Radic Biol Med. 2015;85:250–258. doi: 10.1016/j.freeradbiomed.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A. A Blood Test Offers Clues to Longevity. The New York Times; New York: 2011. [Google Scholar]
- Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Powell SR, Wang P, Divald A, Teichberg S, Haridas V, McCloskey TW, Davies KJ, Katzeff H. Aggregates of oxidized proteins (lipofuscin) induce apoptosis through proteasome inhibition and dysregulation of proapoptotic proteins. Free Radic Biol Med. 2005;38:1093–1101. doi: 10.1016/j.freeradbiomed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Prahl S, Kueper T, Biernoth T, Wöhrmann Y, Münster A, Fürstenau M, Schmidt M, Schulze C, Wittern KP, Wenck H. Aging skin is functionally anaerobic: importance of coenzyme Q10 for anti aging skin care. Biofactors. 2008;32:245–255. doi: 10.1002/biof.5520320129. [DOI] [PubMed] [Google Scholar]
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- Ramunas J, Yakubov E, Brady JJ, Corbel SY, Holbrook C, Brandt M, Stein J, Santiago JG, Cooke JP, Blau HM. Transient delivery of modified mRNA encoding TERT rapidly extends telomeres in human cells. FASEB J. 2015;29:1930–1939. doi: 10.1096/fj.14-259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SIS. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S, Minotti G, Cairo G. Iron regulatory proteins: from molecular mechanisms to drug development. Antioxid Redox Sign. 2010;13:1593–1616. doi: 10.1089/ars.2009.2983. [DOI] [PubMed] [Google Scholar]
- Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14:275–287. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Lamar M, Driscoll I. Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Ann N Y Acad Sci. 2007;1121:562–575. doi: 10.1196/annals.1401.027. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5:545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin J. Les dépressions du sujet âgé: du diagnostic à la prise en charge. NPG Neurol Psychiatr Gériatr. 2015;15:206–218. [Google Scholar]
- Ross PD, Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981;20:3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Ruan H, Tang XD, Chen ML, Joiner M, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A. 2002;99:2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D. Growth hormone, body composition, and aging. J Am Geriatr Soc. 1985;33:800–807. doi: 10.1111/j.1532-5415.1985.tb04195.x. [DOI] [PubMed] [Google Scholar]
- Rufián-Henares JA, Pastoriza S. Maillard reaction. In: Caballero B, Finglas PM, Toldrá F, editors. Encyclopedia of Food and Health. Academic Press; Oxford: 2016. pp. 593–600. [Google Scholar]
- Rytkönen KM, Wigren HK, Kostin A, Porkka-Heiskanen T, Kalinchuk AV. Nitric oxide mediated recovery sleep is attenuated with aging. Neurobiol Aging. 2010;31:2011–2019. doi: 10.1016/j.neurobiolaging.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Sacks SH. Rapamycin on trial. Nephrol Dial Transplant. 1999;14:2087–2089. doi: 10.1093/ndt/14.9.2087. [DOI] [PubMed] [Google Scholar]
- Sah C, Aridogan IA, Izol V, Erdogan S, Doran S. Effects of long-term administration of the antiaging hormone dehydroepiandrosterone sulfate on rat prostates and testes as androgen-dependent organs. Korean J Urol. 2013;54:199–203. doi: 10.4111/kju.2013.54.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Samaranayaka AGP, Li-Chan ECY. Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J Funct Foods. 2011;3:229–254. [Google Scholar]
- Sata N, Klonowski-Stumpe H, Han B, Häussinger D, Niederau C. Menadione induces both necrosis and apoptosis in rat pancreatic acinar AR4-2 J cells. Free Radic Biol Med. 1997;23:844–850. doi: 10.1016/s0891-5849(97)00064-6. [DOI] [PubMed] [Google Scholar]
- Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra215. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- Schönknecht P, Pantel J, Kruse A, Schröder J. Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. Am J Psychiatry. 2005;162(November(11)):2071–2077. doi: 10.1176/appi.ajp.162.11.2071. http://www.ncbi.nlm.nih.gov/pubmed/16263846. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Mitchell Sarah J, Fang Evandro F, Iyama T, Ward T, Wang J, Dunn Christopher A, Singh N, Veith S, Hasan-Olive MdM, Mangerich A, Wilson Mark A, Mattson Mark P, Bergersen Linda H, Cogger Victoria C, Warren A, Le Couteur David G, Moaddel R, Wilson DavidM, Iii, Croteau Deborah L, de Cabo R, Bohr Vilhelm A. A High-Fat Diet and NAD+ Activate Sirt1 to Rescue Premature Aging in Cockayne Syndrome. Cell Metabolism. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiene C, Fischer G. Enzymes that catalyse the restructuring of proteins. Curr Opin Struct Biol. 2000;10:40–45. doi: 10.1016/s0959-440x(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Schumann AY, Bartsch RP, Penzel T, Ivanov PC, Kantelhardt JW. Aging effects on cardiac and respiratory dynamics in healthy subjects across sleep stages. Sleep. 2010;33:943. doi: 10.1093/sleep/33.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully JL. What is a disease? EMBO Rep. 2004;5:650–653. doi: 10.1038/sj.embor.7400195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, McLaren JS, Meyer C, Duncan JS, Redman P, Collins AR, Duthie GG, Speakman JR. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech Ageing Dev. 2006;127:897–904. doi: 10.1016/j.mad.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Semsei I. On the nature of aging. Mech Ageing Dev. 2000;117:93–108. doi: 10.1016/s0047-6374(00)00147-0. [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethe S, de Magalhães JP. Ethical perspectives in biogerontology. In: Schermer M, Pinxten W, editors. Ethics, Health Policy and (Anti) Aging: Mixed Blessings. Springer; Netherlands: 2013. pp. 173–188. [Google Scholar]
- Shah K, Kumar RG, Verma S, Dubey R. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes ingrowing rice seedlings. Plant Sci. 2001;161:1135–1144. [Google Scholar]
- Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:573–580. doi: 10.1513/pats.200904-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012 [Google Scholar]
- Shenkin A. Micronutrient supplements: who needs them? A personal view. Nutr Bull. 2013;38:191–200. [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: minireview mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Trindade LS. Dietary restriction and aging in rodents: a current view on its molecular mechanisms. Aging Dis. 2010;1:89–107. [PMC free article] [PubMed] [Google Scholar]
- Signer Robert AJ, Morrison Sean J. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GM, Finley D, Vogel C. K63 polyubiquitination is a new modulator of the oxidative stress response. Nat Struct Mol Biol. 2015;22:116–123. doi: 10.1038/nsmb.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis1,2. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Soros P, Bose A, Sokoloff LG, Graham SJ, Stuss DT. Age-related changes in the functional neuroanatomy of overt speech production. Neurobiol Aging. 2011;32:1505–1513. doi: 10.1016/j.neurobiolaging.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer'sdisease. Neurobiol Aging. 2011;32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Grandaliano G, Gesualdo L. Management of side effects of sirolimus therapy. Transplantation. 2009;87:S23–S26. doi: 10.1097/TP.0b013e3181a05b7a. [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Garbe JC. Increasing cell culture population doublings for long-term growth of finite life span human cell cultures. Google Patents 2015 [Google Scholar]
- Stampfer M, Vrba L, Fuchs L, Brothman A, LaBarge M, Futscher B, Garbe J. Abstract B008: Efficient immortalization of normal human mammary epithelial cells using two pathologically relevant agents does not require gross genomic alterations. Mol Cancer Res. 2013a;11:B008–B008. [Google Scholar]
- Stampfer MR, LaBarge MA, Garbe JC. An Integrated Human Mammary Epithelial Cell Culture System for Studying Carcinogenesis and Aging Cell and Molecular Biology of Breast Cancer. Springer; 2013b. pp. 323–361. [Google Scholar]
- Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47:565–572. doi: 10.1016/j.exger.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J. Benfotiamine prevents macro-and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diab Care. 2006;29:2064–2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- Strehler BL. Elements of unified theory of aging: integration of alternative models. In: Platt D, editor. Alternstheorien. Schattauer Verlag; Stuttgart: 1976. pp. 5–36. [Google Scholar]
- Sugiyama S, Yamada K, Ozawa T. Preservation of mitochondrial respiratory function by coenzyme Q10 in aged rat skeletal muscle. Biochem Mol Biol Int. 1995;37:1111–1120. [PubMed] [Google Scholar]
- Suhasini AN, Brosh RM., Jr DNA helicases associated with genetic instability, cancer, and aging. Adv Exp Med Biol. 2013;767:123–144. doi: 10.1007/978-1-4614-5037-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Wang H, Zhu R, Tang K, Gong Q, Cui J, Cao Z, Liu Q. iPEAP: integrating multiple omics and genetic data for pathway enrichment analysis. Bioinformatics. 2014;30(5):737–739. doi: 10.1093/bioinformatics/btt576. http://www.ncbi.nlm.nih.gov/pubmed/24092766. [DOI] [PubMed] [Google Scholar]
- Surikow SY, Raman B, Licari J, Singh K, Nguyen TH, Horowitz JD. Evidence of nitrosative stress within hearts of patients dying of Tako-Tsubo cardiomyopathy. Int J Cardiol. 2015;189:112–114. doi: 10.1016/j.ijcard.2015.03.416. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yonekura H, Yamagishi Si, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-α through nuclear factor-(B, and by 17β-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–25790. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr Opin Pharmacol. 2010;10:408–424. doi: 10.1016/j.coph.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Johnson MJ. Physiology of Exercise and Healthy Aging. Human Kinetics 2008 [Google Scholar]
- Terrier P, Dériaz O. Kinematic variability, fractal dynamics and local dynamic stability of treadmill walking. J Neuroeng Rehabil. 2011;8:12. doi: 10.1186/1743-0003-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thallas-Bonke V, Lindschau C, Rizkalla B, Bach LA, Boner G, Meier M, Haller H, Cooper ME, Forbes JM. Attenuation of extracellular matrix accumulation in diabetic nephropathy by the advanced glycation end product cross-link breaker ALT-711 via a protein kinase Cadependent pathway. Diabetes. 2004;53:2921–2930. doi: 10.2337/diabetes.53.11.2921. [DOI] [PubMed] [Google Scholar]
- Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, Yongvanit P, Kawanishi S, Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2014;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Tischler H. Nelson Education, Series: Cengage Advantage Books. 11. Wadsworth Publishing; 2013. Cengage Advantage Books: Introduction to Sociology; p. 544. [Google Scholar]
- Toda T, Satoh M, Sugimoto M, Goto M, Furuichi Y, Kimura N. A comparative analysis of the proteins between the fibroblasts from Werner's syndrome patients and age-matched normal individuals using two-dimensional gel electrophoresis. Mech Ageing Dev. 1998;100:133–143. doi: 10.1016/s0047-6374(97)00131-0. [DOI] [PubMed] [Google Scholar]
- Tower J. Programmed cell death in aging. Ageing Res Rev. 2015a;23(Pt A):90–100. doi: 10.1016/j.arr.2015.04.002. http://www.ncbi.nlm.nih.gov/pubmed/25862945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. Superoxide dismutase (SOD) genes and aging indrosophila. In: Vaiserman AM, Moskalev AA, Pasyukova EG, editors. Life Extension. Springer International Publishing; 2015b. pp. 67–81. [Google Scholar]
- Tranah GJ, Yaffe K, Katzman SM, Lam ET, Pawlikowska L, Kwok PY, Schork NJ, Manini TM, Kritchevsky S, Thomas F, Newman AB, Harris TB, Coleman AL, Gorin MB, Helzner EP, Rowbotham MC, Browner WS, Cummings SR. Mitochondrial DNA heteroplasmy associations with neurosensory and mobility function in elderly adults. J Gerontol Ser A Biol Sci Med Sci. 2015;70:1418–1424. doi: 10.1093/gerona/glv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. Malthus foiled again and again. Nature. 2002;418:668–670. doi: 10.1038/nature01013. [DOI] [PubMed] [Google Scholar]
- Truscott RJW, Zhu X. Presbyopia and cataract: a question of heat and time. Prog Retin Eye Res. 2010;29:487–499. doi: 10.1016/j.preteyeres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol (Baltimore, Md) 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- Ünal E, Kinde B, Amon A. Gametogenesis eliminates age-induced cellular damage and resets life span in yeast. Science. 2011;332:1554–1557. doi: 10.1126/science.1204349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiumi F, Seki M, Furuichi Y. Helicases and human diseases. Front Genet. 2015;6:39. doi: 10.3389/fgene.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response oxidative stress, and aging. J Gerontol Ser A, Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urios P, Grigorova-Borsos AM, Sternberg M. Aspirin inhibits the formation of pentosidine, a cross-linking advanced glycation end product, in collagen. Diab Res Clin Pract. 2007;77:337–340. doi: 10.1016/j.diabres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Glass D, Spector TD. Omics technologies and the study of human ageing. Nat Rev Genet. 2013;14:601–607. doi: 10.1038/nrg3553. [DOI] [PubMed] [Google Scholar]
- Van Assche R, Broeckx V, Boonen K, Maes E, De Haes W, Schoofs L, Temmerman L. Integrating –Omics: systems biology as explored through C. elegans research. J Mol Biol. 2015;427:3441–3451. doi: 10.1016/j.jmb.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Van Puyvelde K, Mets T, Njemini R, Beyer I, Bautmans I. Effect of advanced glycation end product intake on inflammation and aging: a systematic review. Nutr Rev. 2014;72:638–650. doi: 10.1111/nure.12141. [DOI] [PubMed] [Google Scholar]
- Vanhooren V, Libert C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res Rev. 2013;12:8–21. doi: 10.1016/j.arr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Vasto S, Scapagnini G, Bulati M, Candore G, Castiglia L, Colonna-Romano G, Lio D, Nuzzo D, Pellicano M, Rizzo C, Ferrara N, Caruso C. Biomarkes of aging. Front Biosci (Sch Ed) 2010;2:392–402. doi: 10.2741/s72. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- Vina J, Salvador-Pascual A, Tarazona-Santabalbina FJ, Rodriguez-Mañas L, Gomez-Cabrera MC. Exercise training as a drug to treat age associated frailty. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.03.024. Available online: 25 March 2016. http://dx.doi.org/10.1016/j.freeradbiomed.2016.03.024. [DOI] [PubMed]
- Vijg J, de Grey ADNJ. Innovating aging: promises and pitfalls on the road to life extension. Gerontology. 2014;60:373–380. doi: 10.1159/000357670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Borras C, Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol. 2013;9:228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Bashton M, Kerrison ND, Chothia C, Teichmann SA. Structure, function and evolution of multidomain proteins. Curr Opin Struct Biol. 2004;14:208–216. doi: 10.1016/j.sbi.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Vogel C, Silva GM, Marcotte EM. Protein expression regulation under oxidative stress. Mol Cell Proteom: MCP(10, M111.009217) 2011 doi: 10.1074/mcp.M111.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitkun V, Zhitkovich A. Analysis of DNA-protein crosslinking activity of malondialdehyde in vitro. Mutat Res. 1999;424:97–106. doi: 10.1016/s0027-5107(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Wang L, Li YM, Lei L, Liu Y, Wang X, Ma KY, Chen ZY. Cranberry anthocyanin extract prolongs lifespan of fruit flies. Exp Gerontol. 2015;69:189–195. doi: 10.1016/j.exger.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Warner HR, Sprott RL, Schneider EL, Butler RN. Modern biological theories of aging 1987 [Google Scholar]
- Weinert BT, Timiras PS. Invited review: theories of aging. J Appl Physiol. 2003;95:1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- Weis B. Standardizing global gene expression analysis between laboratories and across platforms. Nat Methods. 2005;2:351–356. doi: 10.1038/nmeth754. [DOI] [PubMed] [Google Scholar]
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transplant Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Fontana L. Caloric restriction: powerful protection forthe aging heart and vasculature. Am J Physiol Heart. 2011;C301:H1205–H1219. doi: 10.1152/ajpheart.00685.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RK, Moshier E, Lubitz I, Schmeidler J, Godbold J, Cai W, Uribarri J, Vlassara H, Silverman JM, Beeri MS. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech Ageing Dev. 2014;140:10–12. doi: 10.1016/j.mad.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, McEniery CM. Arteriosclerosis: inevitable or self-inflicted? Hypertension. 2012;60:3–5. doi: 10.1161/HYPERTENSIONAHA.112.193029. [DOI] [PubMed] [Google Scholar]
- Williams SL, Mash DC, Züchner S, Moraes CT. Somatic mtDNA mutation spectra in the aging human putamen. 2013 doi: 10.1371/journal.pgen.1003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem. 2007;55:8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Testing time for telomeres: telomere length can tell us something about disease susceptibility and ageing, but are commercial tests ready for prime time? EMBO Rep. 2011;12:897–900. doi: 10.1038/embor.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Schwartz A, Vitartas C, Vittinghoff E, Satterfield S, Simonsick E, Launer L, Rosano C, Cauley J. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011;77:1351–1356. doi: 10.1212/WNL.0b013e3182315a56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Ogawa K, Ikei T, Udono M, Fujiki T, Katakura Y. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 2012;417:630–634. doi: 10.1016/j.bbrc.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Yin D, Chen K. The essential mechanisms of aging: irreparable damage accumulation of biochemical side-reactions. Exp Gerontol. 2005;40:455–465. doi: 10.1016/j.exger.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ylikallio E, Suomalainen A. Mechanisms of mitochondrial diseases. Ann Med. 2012;44:41–59. doi: 10.3109/07853890.2011.598547. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Van Der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelickson BR, Ballinger SW, Dell'Italia LJ, Zhang J, Darley-Usmar VM. Reactive oxygen and nitrogen species: interactions with mitochondria and pathophysiology. In: Lane WJLD, editor. Encyclopedia of Biological Chemistry. Academic Press; Waltham: 2013. pp. 17–22. [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. http://www.ncbi.nlm.nih.gov/pubmed/27127236. [DOI] [PubMed] [Google Scholar]
- Zhang H. Molecular signaling and genetic pathways of senescence: its role in tumorigenesis and aging. J Cell Physiol. 2007;210:567–574. doi: 10.1002/jcp.20919. [DOI] [PubMed] [Google Scholar]
- Zhenda Z, Cailian C, Ruimin D, Xiaoxian Q, Lin C. GW25-e3403 Advanced glycation end products upregulated the expression of angiopoietin-like protein 4 via activation the renin-angiotensin system in endothelials. J Am Coll Cardiol. 2014;64 [Google Scholar]
- Zheng CL, Ratnakar V, Gil Y, McWeeney SK. Use of semantic workflows to enhance transparency and reproducibility in clinical omics. Genome Med. 2015;7:73. doi: 10.1186/s13073-015-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Zhao J, Gu Yj, Zhao Yf, Zhao Yw, Fu GX. Differential levels of cathepsin B and L in serum between young and aged healthy people and their association with matrix metalloproteinase 2. Arch Gerontol Geriatr. 2015;61:285–288. doi: 10.1016/j.archger.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Zhou T, Sha J, Guo X. The need to revisit published data: a concept and framework for complementary proteomics. Proteomics. 2016;16:6–11. doi: 10.1002/pmic.201500170. [DOI] [PubMed] [Google Scholar]
- Zierer J, Menni C, Kastenmüller G, Spector TD. Integration of ‘omics’ data in aging research: from biomarkers to systems biology. Aging Cell. 2015;14:933–944. doi: 10.1111/acel.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zs-Nagy I. A membrane hypothesis of aging. J Theor Biol. 1978;75:189–195. doi: 10.1016/0022-5193(78)90230-8. [DOI] [PubMed] [Google Scholar]


