Abstract
Methylmercury (MeHg) is a ubiquitous environmental contaminant and neurotoxin, particularly hazardous to developing and young individuals. MeHg neurotoxicity during early development has been shown to be sex-dependent via disturbances in redox homeostasis, a key event mediating MeHg neurotoxicity. Therefore, we investigated if MeHg-induced changes in key systems of antioxidant defense are sex-dependent. C57BL/6J mice were exposed to MeHg during the gestational and lactational periods, modeling human prenatal and neonatal exposure routes. Dams were exposed to 5 ppm MeHg via drinking water from early gestational period until postnatal day 21 (PND21). On PND21 a pair of siblings (a female and a male) from multiple (5–6) litters were euthanized and tissue samples were taken for analysis. Cytoplasmic and nuclear extracts were isolated from fresh cerebrum and cerebellum and used to determine thioredoxin (Trx) and glutathione (GSH) levels, as well as thioredoxin reductase (TrxR) and glutathione peroxidase (GPx) activities. The remaining tissue was used for mRNA analysis. MeHg-induced antioxidant response was not uniform for all the analyzed antioxidant molecules, and sexual dimorphism in response to MeHg treatment was evident for TrxR, Trx and GPx. The pattern of response, namely a decrease in males and an increase in females, may impart differential and sex-specific susceptibility to MeHg. GSH levels were unchanged in MeHg treated animals and irrespective of sex. Trx was reduced only in nuclear extracts from male cerebella, exemplifying a structure-specific response. Results from the gene expression analysis suggest posttranscriptional mechanism of sex-specific regulation of the antioxidant response upon MeHg treatment. The study demonstrates for the first time sex-and structure-specific changes in the response of the thioredoxin system to MeHg neurotoxicity and suggests that these differences in antioxidant responses might impart differential susceptibility to developmental MeHg exposure.
Keywords: Methylmercury, Sex, Thioredoxin, Thioredoxin reductase, Glutathione peroxidase, Glutathione
1. Introduction
Methylmercury (MeHg) is an environmental pollutant that targets the central nervous system (CNS) and causes severe neurological deficits (Bisen-Hersha et al., 2014; Fischer et al., 2008; Manfroi et al., 2004; Sanfeliu et al., 2003). This is particularly true for newborn and young individuals, which are more susceptible to the toxin due to undeveloped blood-brain barrier (BBB) and lower excretion capacity (Fischer et al., 2008; Manfroi et al., 2004). Targeting the brain by MeHg during early periods of development, when critical processes, such as cell division and neuronal migration take place, leads to irreversible damage, as shown in numerous epidemiological (Llop et al., 2013) and experimental studies (Fischer et al., 2008; Gimenez-Llort et al., 2001; Manfroi et al., 2004). It is noteworthy that sexual dimorphism in response to developmental MeHg exposures has been reported, with males showing increased susceptibility to MeHg than females in behavioral evaluations (Björklund et al., 2007; Gimenez-Llort et al., 2001; Llop et al., 2013; Rossi et al., 1997). However, because of scarce biochemical data, the mechanisms underlying these differences have yet to be elucidated.
Oxidative stress and disrupted antioxidant protection is a key event in MeHg neurotoxicity. It is believed to be a consequence of MeHg interacting with nucleophilic groups, especially sulfhydryl and selenohydryl groups present in numerous antioxidant molecules (Farina et al., 2011; Farina et al., 2013). The thioredoxin (Trx) and glutathione (GSH) systems are major antioxidant systems targeted by MeHg (Farina et al., 2011; Farina et al., 2013). The Trx system consists of two major isoforms of Trx and Trx-regenerating selenoproteins – thioredoxin reductases (TrxR); Trx1/TrxR1 is present in both cytoplasm and nuclei, and Trx2/TrxR2 is localized in mitochondria (Aon-Bertolino et al., 2011; Lu and Holmgren, 2014; Silva-Adaya et al., 2014). The Trx system is expressed in the CNS, with higher levels of Trx in neurons and TrxR in astrocytes (Lippoldt et al., 1995; Rozell et al., 1985; Rubartelli et al., 1992; Rybnikova et al., 2000; Silva-Adaya et al., 2014), indicating on important role of Trx in neuronal differentiation and regeneration (Endoh et al., 1993; Masutani et al., 2004). The system provides the reducing equivalents for thioredoxin-dependent peroxiredoxins (Prx), which can efficiently remove reactive oxygen species (ROS). Moreover, Trx system regulates the activities of numerous oxidative-sensitive molecules, such as ribonucleotide reductases, methionine sulfoxide reductases, transcription factors, caspases and kinases, and is involved in the repair of oxidized proteins (Lu and Holmgren, 2014; Silva-Adaya et al., 2014). The redox homeostasis is also maintained by system using glutathione (GSH), the most abundant low molecular thiol present in various cellular compartments (Conrad et al., 2013; Go and Jones, 2010). Oxidized glutathione (GSSG) is reduced back to its reduced form (GSH) by glutathione reductase (GR) and utilized by antioxidant enzymes, such as glutathione peroxidases (GPx) and glutaredoxins (Grx). It is noteworthy that both the GSH and Trx antioxidant systems act cooperatively in the homeostatic maintenance of the redox state in cells (Conrad et al., 2013; Go and Jones, 2010).
The antioxidant response to MeHg neurotoxicity has been studied in various experimental models, showing disruption of both the Trx and GSH systems (Farina et al., 2011; Farina et al., 2013). However, the preponderance of studies, conducted primarily in males, overlooks the fact that the redox system is differentially regulated in males vs. females (Benner et al., 2013; Borrás et al., 2003; Kenchappa et al., 2004; Malorni et al., 2007; Marotti et al., 2010). For example, sex hormones have been shown to affect both oxidative stress-promoting molecules and endogenous antioxidant, most likely through transcriptional and post-transcriptional regulation (Benner et al., 2013; Ejima et al., 1999; Kenchappa et al., 2004; Marotti et al., 2010). The same studies suggest more efficient antioxidant protection in females vs. males (Benner et al., 2013; Ejima et al., 1999; Kenchappa et al., 2004; Marotti et al., 2010). In fact, one study demonstrated positive effect of the female hormone, 17-β-estradiol, in suppressing MeHg-induced neurotoxicity in adult male mice (Malagutti et al., 2009). These observations led us to hypothesize that the relative resistance of young females to MeHg neurotoxicity might be secondary to differential regulation of their antioxidant systems. Since Trx was previously shown to be upregulated by female hormones (Ejima et al., 1999; Lee et al., 2003), we focused herein predominantly on the Trx system.
2. Materials and methods
2.1. MeHg treatment
All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals. Adult, twelve-weeks-old C57BL/6J mice were maintained at standard conditions with free access to food and water. Eight groups, each containing one male and two females, were kept together for ten days. Next, pregnant females were separated and randomly assigned to the control or treatment groups (eight mice in each). The control group received drinking water and the treated group received water with 5 ppm methylmercury (II) chloride (MeHg) (Sigma-Aldrich, #442534), which produces exposure of about 400 μg/kg/day (Newland and Reile, 1999). Fresh MeHg solutions were replaced weekly, made from 50 ppm stock. Water bottles were not available for pups, so their exposure was only via maternal milk. Treatment was started immediately after dams’ assignment into groups and carried out until postnatal day 21 (PND21). Next, the pups were sacrificed by decapitation preceded with isoflurane anesthesia. Samples from cerebrum and cerebellum were isolated on wet ice and used for extraction or frozen at −80 °C. Samples of siblings, female and male from multiple litters, were used for the analysis. All the pups from two of the control group and one of the treated group died. Moreover, same-sex siblings were inherent to three litters; therefore, the final number of analyzed samples was 5–6: 5 males and 6 females from control litters and 6 males and 6 females from treated litters. No sex-specific effect was observed in pups’ survival, at PND21 an average% of male pups in litter was 48,19 (±26,98) for control and 51,69 (±32,79) for MeHg-treated group.
2.2. Nuclear and cytoplasmic extracts isolation
Nuclear and cytoplasmic extracts were isolated from 80 mg of fresh tissues by gentle lysis and rapid centrifugation with NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (Thermo Scientific, #78835), according to the manufacturers’ protocol, and frozen at −80 °C for further analysis.
2.3. Biochemical determinations
TrxR activity was measured using a Kit for Assay of Mammalian Thioredoxin Reductase (Cayman –IMCO Corp., #FkTRXR-03), according to the manufacturer’s protocol. Approximately 10 μg of protein extract was used in a reaction mixture. The results were compared with standard curve for active TrxR (in nM) and presented as nmol/mg of total protein.
Reduced Trx level was measured using a Kit for Assay of Thioredoxin (Cayman –IMCO Corp., #FkTRX-02-V2), according to the manufacturer’s protocol. Approximately10 μg of protein extract was used in a reaction mixture. The results were compared with standard curve for active Trx (in ng) and presented as μg/mg of total protein.
GPx activity was measured using a Glutathione Peroxidase Assay Kit (Cayman, #703102), according to the manufacturer’s protocol. Approximately 50 μg of protein extract was used in a reaction mixture. The results were calculated as nmol/(min · mg of total protein).
Total glutathione (GSH) level was measured using method based on enzymatic recycling reaction (Baker et al., 1990), performed as described previously in (Caito and Aschner, 2015). Diluted 5x extract was used in a reaction mixture. The results were compared with standard curve for reduced GSH and calculated as nmol/mg of total protein.
All results were standardized to total protein content, determined with the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, #23225), according to the manufacturer’s protocol.
2.4. Gene expression assay
Gene expression was evaluated with quantitative real time PCR method (qRT- PCR). Total RNA was isolated from 50 mg of frozen tissue using the standard protocol for Trizol Reagent (Ambion, #10296028) as was described previously (Livak and Schmittgen, 2001). The quantity and purity of RNA were measured and we used samples with OD260/OD280 > 1.7. To remove genomic DNA, a Recombinant DNase I (Ambion, #AM2235) was used according to the method described by a producer. Complement DNA was synthesized from 1 μg of RNA with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, #4368814) according to the manufacturer’s protocol. The level of gene expression was measured using TaqMan Gene Expression Master Mix (Applied Biosystems, #4369016) with 1 μl cDNA in 10 μl of reaction volume. The assay IDs were: Mm00726847_s1 (Trx1), Mm00443675_m1 (TrxR1), Mm00656767_g1 (GPx1), Mm02619580_m1 (β-actin – internal control). Change in gene expression was determined by the 2−ΔΔCt method (Chomczynski and Sacchi, 1987) and expressed as relative fold change to female control cerebral level.
2.5. Statistical analysis
The results were expressed as mean ± SD. Data were analyzed by three-way ANOVA followed by Tukey’s multiple comparisons tests, to compare the mean values of various experimental groups, p <0.05 was considered statistically significant. The statistical analyses were performed using SPSS 24 and Graph Pad Prism 6 software.
3. Results
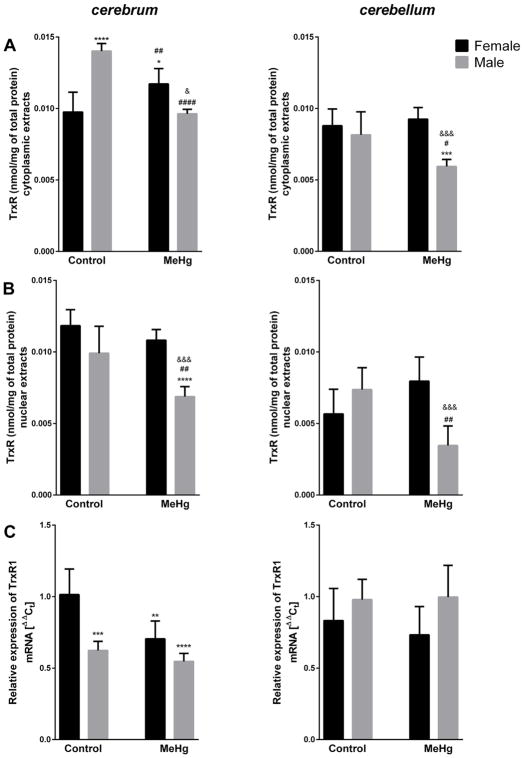
Three-way ANOVA analysis revealed that MeHg affects the antioxidant system in young mice in a sex- and structure-specific manner (Table 1). Third order interaction (brain structure × sex × MeHg) was significant for cytoplasmic TrxR activity (F = 6.459, p = 0.015) (Fig. 1A). MeHg exposure led to substantial decreases in TrxR activity in cytoplasmic extracts in male mice cerebrum (31%) and cerebellum (27%), whereas in female mice we observed a significant increase (20%) in TrxR activity in cerebrum and absent changes in cerebellum. Moreover, control levels of active TrxR were significantly higher in cytoplasmic extracts from cerebrum of males vs. females (Fig. 1A). Similarly, a brain structure × sex × MeHg interaction was significant for nuclear TrxR activity (F = 7.622, p = 0.009) (Fig. 1B). MeHg inhibited the nuclear TrxR in males (31% decrease in cerebrum and 53% in cerebellum), but did not modify the enzyme in females. Furthermore, the TrxR activity was lower in cerebellum than cerebrum (Fig. 1B). Brain structure × sex interaction effect (F = 21.848, p <0.001) was noted for mRNA coding for TrxR1, a major form of TrxR present in cytoplasm and nuclei. Gene expression was lower in cerebrum of untreated males than females, with opposite, yet not significant effect in the cerebellum, and MeHg downregulated TrxR1 mRNA in female cerebrum only (Fig. 1C).
Table 1.
Three-way ANOVA analysis of brain structure, MeHg and sex on antioxidant response in young mice.
| Factor | TrxR cytoplasmic extracts |
TrxR nuclear extracts |
TrxR1 mRNA |
Trx cytoplasmic extracts |
Trx nuclear extracts |
Trx1 mRNA |
GPx cytoplasmic extracts |
GPx1 mRNA |
GSH cytoplasmic extracts |
|---|---|---|---|---|---|---|---|---|---|
| brain structure | F = 95.329 | F = 78.178 | F = 10.092 | F = 0.968 | F = 189.337 | F = 101.764 | F = 2.213 | F = 23.484 | F = 0.865 |
| p <0.001 | p <0.001 | p = 0.003 | p = 0.332 | p <0.001 | p <0.001 | p = 0.146 | p <0.001 | p = 0.359 | |
| MeHg | F = 12.579 | F = 13.427 | F = 5.222 | F = 0.041 | F = 0.175 | F = 3.242 | F = 0.264 | F = 1.568 | F = 3.684 |
| p = 0.001 | p = 0.01 | p = 0.029 | p = 0.841 | p = 0.678 | p = 0.081 | p = 0.611 | p = 0.219 | p = 0.063 | |
| sex | F = 1.146 | F = 25.071 | F = 0.449 | F = 7.487 | F = 2.053 | F = 7.325 | F = 0.912 | F = 9.255 | F = 1.549 |
| p = 0.292 | p <0.001 | p = 0.507 | p = 0.01 | p = 0.161 | p = 0.011 | p = 0.346 | p = 0.004 | p = 0.221 | |
| brain structure × MeHg | F = 0.692 | F = 1.534 | F = 2.194 | F = 0.337 | F = 1.616 | F = 7.498 | F = 0.22 | F = 1.041 | F = 0.128 |
| p = 0.411 | p = 0.224 | p = 0.148 | p = 0.565 | p = 0.212 | p = 0.01 | p = 0.884 | p = 0.300 | p = 0.723 | |
| brain structure × sex | F = 25.481 | F = 4.402 | F = 21.848 | F = 7.849 | F = 1.584 | F = 4.971 | F = 0.176 | F = 13.324 | F = 1.262 |
| p <0.001 | p = 0.043 | p <0.001 | p = 0.008 | p = 0.216 | p = 0.033 | p = 0.677 | p = 0.001 | p = 0.268 | |
| sex × MeHg | F = 44.809 | F = 27.147 | F = 2.905 | F = 0.222 | F = 0.340 | F = 64.361 | F = 8.575 | F = 1.029 | F = 3.515 |
| p <0.001 | p <0.001 | p = 0.097 | p = 0.641 | p = 0.563 | p <0.001 | p = 0.006 | p = 0.317 | p = 0.069 | |
| brain structure × sex × MeHg | F = 6.459 | F = 7.622 | F = 0.317 | F = 0.556 | F = 0.049 | F = 35.53 | F = 0.121 | F = 0.351 | F = 2.451 |
| p = 0.015 | p = 0.009 | p = 0.577 | p = 0.461 | p = 0.825 | p <0.001 | p = 0.730 | p = 0.557 | p = 0.126 |
Fig. 1.
Effect of MeHg exposure on thioredoxin reductase (TrxR) activity in cytoplasmic (A) and nuclear (B) extracts and relative expression of mRNA coding thioredoxin reductase 1 (TrxR1) (C) in cerebrum and cerebellum of young female and male mice; n = 5–6; **p <0.01, ***p <0.005, ****p <0.001 vs. Female Control, #p <0.05, ##p<0.001, ####p <0.001 vs. Male Control, &p <0.05, &&&p <0.005 vs. Female MeHg.
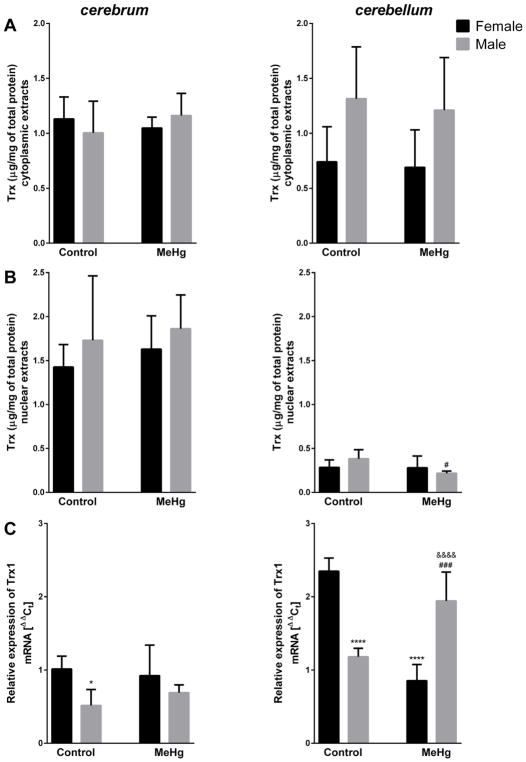
Brain structure × sex interaction was significant for cytoplasmic thioredoxin (Trx) levels (F = 7.849, p = 0.008) showed a trend for higher levels in male vs. female cerebellum (Fig. 2A). For the nuclear pool, significant main effect of brain structure was revealed (F = 189.33, p <0.001), with lower levels of Trx in cerebellum (Fig. 2B). Moreover, the MeHg-induced decrease (43%) in nuclear Trx in male cerebellum was significant (p <0.05) in post–hoc Tukey’s test (Fig. 2B). For expression of mRNA coding Trx1, a significant brain structure × sex × MeHg interaction effect was noted (F = 35.53, p <0.001) – for untreated animals the expression was higher in cerebellum than cerebrum, and lower in males than females. Furthermore, MeHg exposure downregulated cerebellar mRNA coding Trx1 in females and upregulated its levels in males (Fig. 2C).
Fig. 2.
Effect of MeHg exposure on reduced thioredoxin (Trx) level in cytoplasmic (A) and nuclear (B) extracts and relative expression of mRNA coding thioredoxin 1 (Trx1) (C) in cerebrum and cerebellum of young female and male mice; n = 5–6; *p <0.05, ****p <0.001 vs. Female Control; #p <0.05, ###p <0.005 vs. Male Control; &&&&p <0.001 vs. Female MeHg.
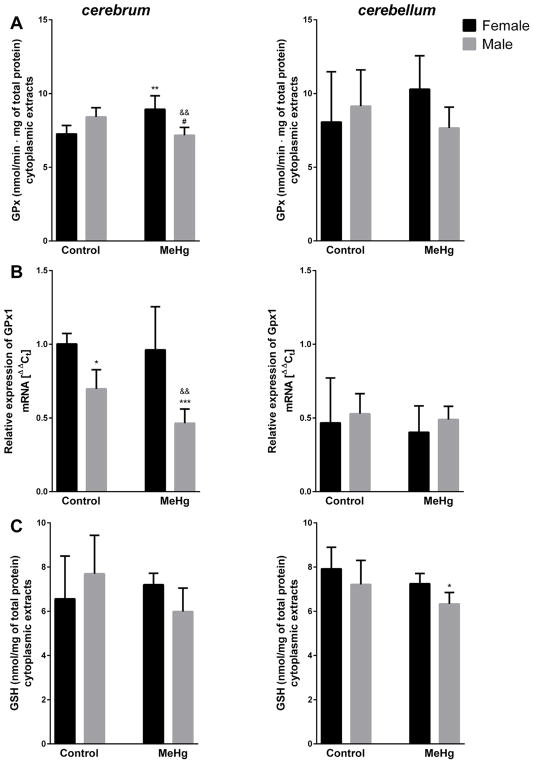
Significant sex × MeHg interaction was revealed for glutathione peroxidase (GPx) activity (F = 8.575, p = 0.006) (Fig. 3A), showing reduced activity in male (15%) and elevated activity in female (23%) cytoplasmic extracts from cerebrum of MeHg-exposed mice. Analogously, yet a statistically non-significant trend was observed in the cerebellum of these mice (Fig. 3A). Brain structure × sex interaction for GPx1 mRNA expression (F = 13.324, p = 0.001) was reflected by lower expression in cerebellum than cerebrum of untreated mice and preferential male susceptibility, revealed in cerebral MeHg-induced decrease of mRNA (Fig. 3B). Moreover, the lower expression of GPx1 was detected in cerebrum of males vs. females (Fig. 3B). Total glutathione (GSH) levels in cytoplasmic extract were unaffected by MeHg treatment, in both groups and in both brain structures (Fig. 3C).
Fig. 3.
Effect MeHg exposure on glutathione peroxidase (GPx) activity (A) and total glutathione (GSH) levels (C) in cytoplasmic extracts, and relative expression of mRNA coding glutathione peroxidase 1 (GPx1) (B) in cerebrum and cerebellum of young female and male mice; n = 5–6; *p <0.05, **p <0.01, ***p <0.005 vs. Female Control; #p <0.05 vs. Male Control; &&p <0.01 vs. Female MeHg.
4. Discussion
The present study demonstrates, for the first time, sex- and structure –specific changes in the Trx system in response to MeHg exposure.
The pattern indicates that antioxidants are increased in female brains, yet decreased in male brains in response to MeHg. This observation is consistent with higher susceptibility to MeHg in exposed males (Björklund et al., 2007; Gimenez-Llort et al., 2001; Rossi et al., 1997). MeHg decreased brain TrxR activity in male, but not in female mice (Fig. 1A). Notably, a slight increase in TrxR activity was induced by MeHg in cytoplasmic extracts derived from female cerebrum (Fig. 1A). Similar pattern was found for GPx activity, especially in the cerebrum (Fig. 3A). Results from males corroborate earlier observations, showing MeHg-induced TrxR and GPx inhibition in numerous experimental models (Branco et al., 2011; Branco et al., 2012a, 2012b; Branco et al., 2014; Dalla Corte et al., 2013; Farina et al., 2009; Franco et al., 2009; Wagner et al., 2010). The effect of MeHg on antioxidant systems in females has yet to be systematically evaluated, with no studies identified on its effect on the Trx system, and only one study showing unchanged murine cerebellar GPx activity in MeHg-exposed females and a decrease in MeHg-exposed males (Malagutti et al., 2009). The reason for the sexual dimorphism in antioxidant responses to MeHg is unclear. Accordingly, we posit several scenarios which might contribute to this effect: sex-specific MeHg distribution and/or metabolism, regulation of gene expression and selenium (Se) bioavailability.
Sex-specific distribution and metabolism of mercurials was reported both in humans (Miettinen, 1973) and experimental models (Hirayama et al., 1987; Thomas et al., 1986; Thomas et al., 1987), indicating more efficient excretion of the metal in females vs. males. Thus, reduced MeHg levels in females might result in higher antioxidant activities compared with males, as corroborated herein. Nevertheless, we failed to note differences in Hg levels between males and females, with equally elevated Hg levels detected in both sexes, (data not published). Accordingly, direct interaction between MeHg and antioxidants is unlikely in contributing to the observed antioxidants’ regulation. On the other hand, the poor correspondance in antioxidants’ mRNA expression and their activities suggests the involvement of posttranscriptional mechanisms. However, the participation of other isoforms, especially for GPx (Conrad et al., 2013), in enzymatic activity can not be excluded, but it is not plausible for TrxR, with only one known cytosolic/nuclear isoform, TrxR1 (Lu and Holmgren, 2014). Our results are consistent with differences in Se bioavailability, which might account for the sex-specific response in antioxidant levels in response to MeHg exposure, as discussed below.
A less specific effect of MeHg, e.g. a decrease on reduced Trx levels in male nuclear extracts from cerebellum only (Fig. 2B) and no changes in the total GSH level (Fig. 3C), indicates that not all antioxidants are analogously affected by MeHg. This disparity might result from a differential affinity of mercurials for thiols vs. selenols (Carvalho et al., 2011; Farina et al., 2011; Farina et al., 2013; Khan and Wang, 2009; Sugiura et al., 1976). The interaction between MeHg and selenoproteins seems to be much more stable when compared to MeHg–thiol interactions, which tend to be more dynamic (Khan and Wang, 2009; Sugiura et al., 1976). Thiol groups are present in active sites of Trx, TrxR and GSH, whereas selenols (as a selenocysteine residue) occur in GPx and TrxR; accordingly, one would expect that the activity of the letter two molecules might be more potently inhibited than others. Indeed, numerous studies have demonstrated exceptional suppression of selenoproteins upon MeHg treatment (Branco et al., 2011; Branco et al., 2014; Carvalho et al., 2008; Carvalho et al., 2011; Dalla Corte et al., 2013; Farina et al., 2009; Franco et al., 2009; Wagner et al., 2010) with decreased TrxR activity (Branco et al., 2011; Branco et al., 2012a, 2012b; Branco et al., 2014; Carvalho et al., 2008; Dalla Corte et al., 2013; Wagner et al., 2010), and unchanged or increased activity in the non-selenoprotein homologue of TrxR, glutathione reductase (GR) (Farina et al., 2005; Lash and Zalups, 1996). MeHg treatment resulted in inhibition of GPx activity, but not GR activity in zebrafish brain (Branco et al., 2012a, 2012b), and decreased GPx, but increased GR acrivity in cerebellum of adult male mice (Malagutti et al., 2009). Different responses of non- vs. selenoproteins are consistent with MeHg’s interaction with Se as an important contributing factor to the neurotoxic pathomechanism of this organometal (Khan and Wang, 2009). Se levels are at 1:1 molar ratio with Hg in organisms exposed to this metal (Kosta et al., 1975; Soares et al., 2002). Strong MeHg binding to Se is believed to decrease the toxicity of MeHg, but also causes Hg accumulation in the brain (Glynn et al., 1993) and induces Se deficiency without an effect on total Se levels (Chang and Suber, 1982; Fredriksson et al., 1993; Nishikido et al., 1987; Watanabe et al., 1999). MeHg was shown to depress the activity of selenoproteins, without changing the total Se concentration (Fredriksson et al., 1993; Nishikido et al., 1987; Watanabe et al., 1999), likely due to reduced Se bioavailability. MeHg was also shown to induce in vitro Se deficiency, resulting in degradation of mRNA coding GPx1 and synthesis of aberrant TrxR1 (Usuki et al., 2011). The analogous response pattern for the selenoprotiens TrxR and GPx inherent to the present study suggests a role for Se bioavailability in mediating MeHg-induced changes in their activity, and sex-dependent Se metabolism might explain the sexual dimorphism of these changes. Sex-dependent differences in Se metabolism, especially distribution and incorporation to particular selenoproteins, are well documented, and data from both animals and humans (Benner et al., 2013; Pitts et al., 2015; Schomburg et al., 2007) have established that males are more susceptible than females to Se dyshomeostasis (Nishikido et al., 1987). Sex-specific expression of selenoproteins was detected in various mice tissues (brain not studied), yet Se levels in the two sexes were comparable (Riese et al., 2006). This study also demonstrated modulatory effect of sex hormones,17-β-estradiol and androgens, on Se metabolism, and showed high incompatibility between selenoproteins’ expression and activity (Riese et al., 2006). Incompatibility between expression and activity was also inherent to our study. As mentioned earlier, not all antioxidants were regulated in an analogous manner by MeHg: the level of active Trx remained unchanged in cytoplasmic extracts from cerebrum and cerebellum of both sexes exposed to MeHg, while there was a significant decrease in nuclear extracts form male cerebellum only. The mRNA levels for Trx1 did not reflect these changes (Fig. 2). Molecular differences, namely, lack of Se and, in turn, different characteristics of interaction with MeHg might account for these changes. It might be also due to the fact that Trx does not rely solely on TrxR, and might be regenerated by the GSH system (GSH, GR, and Grx) (Du et al., 2012; Lu and Holmgren, 2014). GR is often upregulated by mercurials, as demonstrated in the brain of MeHg-treated adult mice, both males and females (Malagutti et al., 2009). In our model, total GSH level remained unchanged upon MeHg treatment which suggest unaffected GSH system, however GPx activity was slightly changed (Fig. 3A, C).
In our study the effect of MeHg differ depending not only on sex, but also brain structure. Multifactorial ANOVA revealed significant second and third order interaction effects for analyzed antioxidants, especially TrxR (Table 1), indicating on a tightly specific regulation of these molecules. The major differences are manifested in less clear effect of MeHg on cerebellum than cerebrum, as demonstrated in cytoplasmic activities of TrxR (Fig. 1A) and GPx (Fig. 3A), or in selective effect of MeHg in cerebellum, as was shown for decreased nuclear Trx levels and changed Trx1 expression (Fig. 2B, C). Selective decrease of nuclear Trx might contribute to a higher susceptibility of the cerebellum in males exposed to MeHg. It was shown previously that cerebellar cells are selectively targeted by mercurials in vivo (Sanfeliu et al., 2003), with disrupted motor activity representing one of the most frequent, sex-specific consequences of MeHg-induced neurotoxicity (Dietrich et al., 2005; Fischer et al., 2008; Gimenez-Llort et al., 2001; Grandjean et al., 1997; Llop et al., 2013; Manfroi et al., 2004; Montgomery et al., 2008; Rossi et al., 1997; Sakamoto et al., 1993). Decrease in Trx level might contribute to MeHg-induced cerebellar mitotic arrest (Rodier et al., 1984), necrosis and apoptosis (Castoldi et al., 2000). Otherwise, decreased levels of nuclear Trx might be a consequence of cerebellar damage, namely MeHg-induced disruption of redox balance might affect posttranslational modifications to cysteine on Trx1, critical to its nuclear localization (Carilho Torrao et al., 2013).
Besides MeHg effect, some structural and/or sex-dependent differences were observed for the levels of analyzed antioxidants. Cerebellar levels of nuclear TrxR (Fig. 1B) and Trx (Fig. 2B) were generally lower, and cerebellar mRNA expression was higher for Trx1 (Fig. 2C) and slightly lower for GPx1 (Fig. 3B) when compared with cerebrum. QRT-PCR results from our study corroborated earlier observations, where immunohistochemical analysis revealed TrxR1 expression to be at the same level in cerebrum and cerebellum, and Trx1 to be particularly highly expressed in cerebellum of adult mice (Godoy et al., 2011). Analogously, GPx levels were previously shown to be lower in cerebellum than cerebrum of adult mice, while activity was comparable (Treupanier et al., 1996; Chen and Berry, 2003). Initial levels were also different between sexes – cytoplasmic TrxR activity was lower in cerebrum of female than male mice (Fig. 1A), and relative mRNA expression was higher in females vs. males for TrxR1 (Fig. 1C) and GPx1 (Fig. 3B) in cerebrum, and for Trx1 in cerebrum and cerebellum (Fig. 2C). The mRNA results are consistent with the literature, showing upregulation of various antioxidants, especially the Trx system, by female hormone estradiol in vitro (Ejima et al., 1999; Lee et al., 2003; Chen et al., 2010; Kenchappa et al., 2004). Moreover, Trx and TrxR expressions were shown to be higher in females than males when analyzed in midbrain and striatum of adult mice (Saeed et al., 2009), and the higher Trx protein level, but not reducing activity, was found in the caudate nucleus of female vs. male rats (Chen et al., 2010). GPx mRNA expression was increased in female blood upon estrogen therapy (Bellanti et al., 2013) and was higher in females vs. males in mitochondria isolated from murine liver (Borrás et al., 2003). Corroborating our data, no significant differences were observed in GPx activity between male and female mice cerebellum (cerebrum not studied) (Malagutti et al., 2009). The apparent disparity between expression and reducing activity of the analyzed antioxidants might be explained by various factors, namely posttranscriptional modifications, which might affect the protein level, redox status and cellular localization.
Taken together, we demonstrated that MeHg affects the antioxidant Trx and GSH systems in a sex-and structure-specific manner, and these changes are not associated with altered mRNA expression, but rather posttranscriptional mechanisms. The pattern of response suggests a possible role for Se metabolism in the observed changes.
Acknowledgments
This study was supported by the National Institutes of Health [grant numbers NIEHS R01ES07331 and NIEHS R01 ES020852].
Footnotes
Conflict of interest
None.
References
- Aon-Bertolino ML, Romero JI, Galeano P, Holubiec M, Badorrey MS, Saraceno GE, Hanschmann EM, Lillig CH, Capani F. Thioredoxin and glutaredoxin system proteins-immunolocalization in the rat central nervous system. Biochim Biophys Acta. 2011;1810:93–110. doi: 10.1016/j.bbagen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- Bellanti F, Matteo M, Rollo T, De Rosario F, Greco P, Vendemiale G, Serviddio G. Sex hormones modulate circulating antioxidant enzymes: Impact of estrogen therapy. Redox Biol. 2013;1:340–346. doi: 10.1016/j.redox.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner MJ, Settles ML, Murdoch GK, Hardy RW, Robison BD. Sex-specific transcriptional responses of the zebrafish (Danio rerio) brain selenoproteome to acute sodium selenite supplementation. Physiol Genom. 2013;45:653–666. doi: 10.1152/physiolgenomics.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisen-Hersha EB, Farina M, Barbosa F, Rocha JBT, Aschner M. Behavioral effects of developmental methylmercury drinking water exposure in rodents. J Trace Elem Med Biol. 2014;28:117–124. doi: 10.1016/j.jtemb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund O, Kahlström J, Salmi P, Ogren SO, Vahter M, Chen JF, Fredholm BB, Daré E. The effects of methylmercury on motor activity are sex- and age-dependent: and modulated by genetic deletion of adenosine receptors and caffeine administration. Toxicology. 2007;241:119–133. doi: 10.1016/j.tox.2007.08.092. [DOI] [PubMed] [Google Scholar]
- Borrás C, Sastre J, García-Sala D, Lloret A, Pallardó FV, Viña J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- Branco V, Canário J, Holmgren A, Carvalho C. Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Toxicol Appl Pharmacol. 2011;251:95–103. doi: 10.1016/j.taap.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Branco V, Canário J, Lu J, Holmgren A, Carvalho C. Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radic Biol Med. 2012a;52:781–793. doi: 10.1016/j.freeradbiomed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Branco V, Ramos P, Canário J, Lu J, Holmgren A, Carvalho C. Biomarkers of adverse response to mercury: histopathology versus thioredoxin reductase activity. J Biomed Biotechnol. 2012b;2012:359879. doi: 10.1155/2012/359879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco V, Godinho-Santos A, Gonçalves J, Lu J, Holmgren A, Carvalho C. Mitochondrial thioredoxin reductase inhibition, selenium status, and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds. Free Rad Biol Med. 2014;73:95–105. doi: 10.1016/j.freeradbiomed.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Caito SW, Aschner M. Quantification of glutathione in Caenorhabditis elegans. Curr Protoc Toxicol. 2015;64(6.18):1–6. doi: 10.1002/0471140856.tx0618s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carilho Torrao RB, Dias IH, Bennett SJ, Dunston CR, Griffiths HR. Healthy ageing and depletion of intracellular glutathione influences T cell membrane thioredoxin-1 levels and cytokine secretion. Chem Cent J. 2013;7:150. doi: 10.1186/1752-153X-7-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CML, Chew EH, Hashemy SI, Lu J, Holmgren A. Inhibition of the human thioredoxin system: a molecular mechanism of mercury toxicity. J Biol Chem. 2008;283:11913–11923. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- Carvalho CML, Lu J, Zhang X, Arnér ESJ, Holmgren A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning. FASEB J. 2011;25:370–381. doi: 10.1096/fj.10-157594. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Barni S, Turin I, Gandini C, Manzo L. Early acute necrosis, delayed apoptosis and cytoskeletal breakdown in cultured cerebellar granule neurons exposed to methylmercury. J Neurosci Res. 2000;59:775–787. doi: 10.1002/(SICI)1097-4547(20000315)59:6<775::AID-JNR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Chang LW, Suber R. Protective effect of selenium on methyl mercury toxicity: a possible mechanism. Bull Environ Contam Toxicol. 1982;29:285–289. doi: 10.1007/BF01706230. [DOI] [PubMed] [Google Scholar]
- Chen J, Berry MJ. Selenium and selenoproteins in the brain and brain diseases. J Neurochem. 2003;86:1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- Chen TY, Tsai KL, Lee TY, Chiueh CC, Lee WS, Hsu C. Sex-specific role of thioredoxin in neuroprotection against iron-induced brain injury conferred by estradiol. Stroke. 2010;41:160–165. doi: 10.1161/STROKEAHA.109.562850. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conrad M, Schick J, Angeli JP. Glutathione and thioredoxin dependent systems in neurodegenerative disease: what can be learned from reverse genetics in mice. Neurochem Int. 2013;62:738–749. doi: 10.1016/j.neuint.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Dalla Corte CL, Wagner C, Sudati JH, Comparsi B, Leite GO, Busanello A, Soares FAA, Aschner M, Rocha JBT. Effects of diphenyl diselenide on methylmercury toxicity in rats. Bio Med Res Int. 2013;2013:983821. doi: 10.1155/2013/983821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M, Mantese CE, dos Anjos G, Souza DO, Farina M. Motor impairment induced by oral exposure to methylmercury in adult mice. Environ Toxicol Pharmacol. 2005;19:169–175. doi: 10.1016/j.etap.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Du Y, Zhang H, Lu J, Holmgren A. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J Biol Chem. 2012;287:38210–38219. doi: 10.1074/jbc.M112.392225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima K, Nanri H, Araki M, Uchida K, Kashimura M, Ikeda M. 17-β-Estradiol induces protein thiol/disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress. Eur J Endocrinol. 1999;140:608–613. doi: 10.1530/eje.0.1400608. [DOI] [PubMed] [Google Scholar]
- Endoh M, Kunishita T, Tabita T. Thioredoxin from activated macrophages as a trophic factor for central cholinergic neurons in vitro. Biochem Biophys Res Commun. 1993;192:760–765. doi: 10.1006/bbrc.1993.1479. [DOI] [PubMed] [Google Scholar]
- Farina M, Franco JL, Ribas CM, Meotti FC, Missau FC, Pizzolatti MG, Dafre AL, Santos AR. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol. 2005;57:1503–1508. doi: 10.1211/jpp.57.11.0017. [DOI] [PubMed] [Google Scholar]
- Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, Suñol C. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci. 2009;112:416–426. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JBT. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011;256:405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Avila DS, Rocha JBT, Aschner M. Metals, oxidative stress and neurodegeneration A focus on iron, manganese and mercury. Neurochem Int. 2013;62:575–594. doi: 10.1016/j.neuint.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Fredriksson A, Eriksson P. Coexposure of neonatal mice to a flame retardant PBDE 99(2,2′,4,4′,5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicol Sci. 2008;101:275–285. doi: 10.1093/toxsci/kfm271. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med. 2009;47:449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Gardlund AT, Bergman K, Oskarsson A, Ohlin B, Danielsson B, Archer T. Effects of maternal dietary supplementation with selenite on the postnatal development of rat offspring exposed to methyl mercury in utero. Pharmacol Toxicol. 1993;72:377–382. doi: 10.1111/j.1600-0773.1993.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Ahlbom E, Dare E, Vahter M, Ogren S, Ceccatelli S. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environ Toxicol Pharmacol. 2001;9:61–70. doi: 10.1016/s1382-6689(00)00060-0. [DOI] [PubMed] [Google Scholar]
- Glynn AW, Ilback NG, Brabencova D, Carlsson L, Enqvist EC, Netzel E, Oskarsson A. Influence of sodium selenite on 203Hg absorption, distribution, and elimination in male mice exposed to methyl 203Hg. Biol Trace Elem Res. 1993;39:91–107. doi: 10.1007/BF02783813. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. Redox control systems in the nucleus: mechanisms and functions. Antioxid Redox Signal. 2010;13:489–509. doi: 10.1089/ars.2009.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy JR, Funke M, Ackermann W, Haunhorst P, Oesteritz S, Capani F, Elsasser HP, Lillig CH. Redox atlas of the mouse Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2011;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Hirayama K, Yasutake A, Inoue M. Effect of sex hormones on the fate of methylmercury and on glutathione metabolism in mice. Biochem Pharmacol. 1987;36:1919–1924. doi: 10.1016/0006-2952(87)90489-8. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Diwakar L, Annepu J, Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J. 2004;18:1102–1104. doi: 10.1096/fj.03-1075fje. [DOI] [PubMed] [Google Scholar]
- Khan MAK, Wang F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem. 2009;28:1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- Kosta L, Byrne AR, Zelenko V. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature. 1975;254:238–239. doi: 10.1038/254238a0. [DOI] [PubMed] [Google Scholar]
- Lash LH, Zalups RK. Alterations in renal cellular glutathione metabolism after in vivo administration of a subtoxic dose of mercuric chloride. J Biochem Toxicol. 1996;11:1–9. doi: 10.1002/(SICI)1522-7146(1996)11:1<1::AID-JBT1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Lee SY, Andoh T, Murphy DL, Chiueh CC. 17-β-estradiol activates ICI 182,780-sensitive estrogen receptors and cyclic GMP-dependent thioredoxin expression for neuroprotection. FASEB J. 2003;17:947–948. doi: 10.1096/fj.02-0807fje. [DOI] [PubMed] [Google Scholar]
- Lippoldt A, Padilla CA, Gerst H, Andbjer B, Richter E, Holmgren A, Fuxe K. Localization of thioredoxin in the rat brain and functional implications. J Neurosci. 1995;15:6747–6756. doi: 10.1523/JNEUROSCI.15-10-06747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311:3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- Malagutti KS, da Silva AP, Braga HC, Mitozo PA, Soares Dos Santos AR, Dafre AL, de Bem AF, Farina M. 17-β-estradiol decreases methylmercury-induced neurotoxicity in male mice. Environ Toxicol Pharmacol. 2009;27:293–297. doi: 10.1016/j.etap.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Malorni W, Campesi W, Straface E, Vella S, Franconi F. Redox features of the cell: a gender perspective. Antioxid Redox Signal. 2007;9:1779–1801. doi: 10.1089/ars.2007.1596. [DOI] [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JBT, Frizzo ME, Souza DO, Farina M. Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci. 2004;81:172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Marotti T, Sobočanec S, Mačak-Šafranko ž, Šarić A, Kušić B, Balog T. Sensitivity to oxidative stress: sex matters. Med Sci. 2010;35:59–68. [Google Scholar]
- Masutani H, Bai J, Kim YC, Yodoi J. Thioredoxin as a neurotrophic cofactor and an important regulator of neuroprotection. Mol Neurobiol. 2004;29:229–242. doi: 10.1385/MN:29:3:229. [DOI] [PubMed] [Google Scholar]
- Miettinen JK. Absorption and elimination of dietary (Hg++) and methylmercury in man. In: Miller MW, Clarkson TW, editors. Mercury, Mercurial, and Mercaptans. C.C. Thomas; Springfield, IL: 1973. pp. 233–246. [Google Scholar]
- Montgomery KS, Mackey J, Thuett K, Ginestra S, Bizon JL, Abbott LC. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behav Brain Res. 2008;191:55–61. doi: 10.1016/j.bbr.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicol Sci. 1999;50:106–116. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- Nishikido N, Furuyashiki K, Naganuma A, Suzuki T, Imura N. Maternal selenium deficiency enhances the fetolethal toxicity of methyl mercury. Toxicol Appl Pharmacol. 1987;88:322–328. doi: 10.1016/0041-008x(87)90207-9. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Kremer PM, Hashimoto AC, Torres DJ, Byrns CN, Williams CS, Berry MJ. Competition between the brain and testes under selenium-compromised conditions: insight into sex differences in selenium metabolism and risk of neurodevelopmental disease. J Neurosci. 2015;35:15326–15338. doi: 10.1523/JNEUROSCI.2724-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese C, Michaelis M, Mentrup B, Götz F, Köhrle J, Schweizer U, Schomburg L. Selenium-dependent pre- and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology. 2006;147:5883–5892. doi: 10.1210/en.2006-0689. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Aschner M, Sager PR. Mitotic arrest in the developing CNS after prenatal exposure to methylmercury. Neurobehav Toxicol Teratol. 1984;5:379–385. [PubMed] [Google Scholar]
- Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp Brain Res. 1997;117:428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- Rozell B, Hansson HA, Luthman M, Holmgren A. Immunohistochemical localization of thioredoxin and thioredoxin reductase in adult rats. Eur J Cell Biol. 1985;38:79–86. [PubMed] [Google Scholar]
- Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- Rybnikova E, Damdimopoulos AE, Gustafsson JA, Spyrou G, Pelto-Huikko M. Expression of novel antioxidant thioredoxin-2 in the rat brain. Eur J Neurosci. 2000;12:1669–1678. doi: 10.1046/j.1460-9568.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- Saeed U, Karunakaran S, Meka DP, Koumar RC, Ramakrishnan S, Joshi SD, Nidadavolu P, Ravindranath V. Redox activated MAP kinase death signaling cascade initiated by ASK1 is not activated in female mice following MPTP: novel mechanism of neuroprotection. Neurotox Res. 2009;16:116–126. doi: 10.1007/s12640-009-9058-5. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Nakano A, Kajiwara Y, Naruse I, Fujisaki T. Effects of methyl mercury in postnatal developing rats. Environ Res. 1993;61:43–50. doi: 10.1006/enrs.1993.1048. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastia J, Cristofol R, Rodriguez-Farre E. Neurotoxicity of organomercurial compounds. Neurotoxicol Res. 2003;5:283–305. doi: 10.1007/BF03033386. [DOI] [PubMed] [Google Scholar]
- Schomburg L, Riese C, Renko K, Schweizer U. Effect of age on sexually dimorphic selenoprotein expression in mice. Biol Chem. 2007;388:1035–1041. doi: 10.1515/BC.2007.128. [DOI] [PubMed] [Google Scholar]
- Silva-Adaya D, Gonsebatt ME, Guevara J. Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxid Med Cell Longev. 2014;2014:590808. doi: 10.1155/2014/590808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D, Sarkis J, Muller R, Brabo E, Santos E. Correlation between mercury and selenium concentrations in Indian hair from Rondjnia State Amazon region, Brazil. Sci Total Environ. 2002;287:155–161. doi: 10.1016/s0048-9697(01)01002-6. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Hojo Y, Tamai Y, Tanaka H. Letter: selenium protection against mercury toxicity. Binding of methylmercury by the selenohydryl-containing ligand. J Am Chem Soc. 1976;98:2339–2341. doi: 10.1021/ja00424a059. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Fisher HL, Sumler MR, Marcus AH, Mushak P, Hall LL. Sexual differences in the distribution and retention of organic and inorganic mercury in methyl mercury-treated rats. Environ Res. 1986;41:219–234. doi: 10.1016/s0013-9351(86)80184-0. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Fisher HL, Sumler MR, Mushak P, Hall LL. Sexual differences in the excretion of organic and inorganic mercury by methyl mercury-treated rats. Environ Res. 1987;43:203–216. doi: 10.1016/s0013-9351(87)80072-5. [DOI] [PubMed] [Google Scholar]
- Treupanier G, Furling D, Puymirat J, Mirault ME. Immunocytochemical localization of seleno-glutathione peroxidase in the adult mouse brain. Neuroscience. 1996;75:231–243. doi: 10.1016/0306-4522(96)00222-9. [DOI] [PubMed] [Google Scholar]
- Usuki F, Yamashita A, Fujimura M. Post-transcriptional defects of antioxidant selenoenzymes cause oxidative stress under methylmercury exposure. J Biol Chem. 2011;286:6641–6649. doi: 10.1074/jbc.M110.168872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Sudati JH, Nogueira CW, Rocha JBT. In vivo and in vitro inhibition of mice thioredoxin reductase by methylmercury. Biometals. 2010;23:1171–1177. doi: 10.1007/s10534-010-9367-4. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol. 1999;21:83–88. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]