Summary
Non-obstructive azoospermia (NOA) patients present with high levels of serum FSH. At the protein level, the etiology and pathways underlying different subtypes of NOA is unclear. The aim was to evaluate quantitatively differences in proteomic profiles of NOA patients presenting with normal serum FSH and large testicular volume and high serum FSH and small testicular volume. The study comprised of 14 non-obstructive azoospermic men (N= 4; normal FSH and normal testicular volume and N=10; high FSH and small testicular volume) and 7 normozoospermic men. Proteomic analysis was done using LC- MS. GSTM3 and PGK2 were less abundant in the normal and high FSH group compared to controls. HSPA4L and HSPA4 were exclusively present in control group whereas HSP90AB1, HSPA1B, HSP90AA1 and HSPA2 were less abundant and exclusive to the normal and high FSH group. We have identified 6 heat shock proteins that may have a role in the pathology of NOA. FSH and testicular volume by itself are not good markers of NOA. The inverse association of GSTM3 and PGK2 regulation with FSH levels along with 12 proteins exclusively in NOA groups suggests further evaluation of their predictive potential in a larger cohort of patients.
Keywords: Male, infertility, non-obstructive azoospermia, FSH, proteomics
Introduction
Azoospermia, a condition described as the complete absence of sperm cells in the semen, is an invariable cause of male infertility and affects 10–15% of all infertile men (Gudeloglu and Parekattil, 2013; Cocuzza et al., 2013). Recent epidemiological studies estimate that approximately 1% of the general male population at reproductive age present azoospermia, suggesting that approximately 10 million men worldwide are azoospermic (Cocuzza et al., 2013).
In clinical proceedings, azoospermia can be classified as obstructive (OA) or non-obstructive (NOA) (Gudeloglu and Parekattil, 2013). Commonly, OA accounts for 15–20% of azoospermic men and its causes relate to mechanical blockage in the upper or lower reproductive tract (epididymis, vas deferens, seminal vesicles or ejaculatory ducts), which prevents sperm cells from reaching the urethral meatus (Baker and Sabanegh, 2013). It results from vassal or epididymal pathology and congenital abnormalities. Physiological outcomes of OA are similar to those seen after vasectomy. NOA, in turn, is characterized by severely impaired or non-existent spermatogenesis (Palermo et al., 2014). The pathophysiology of NOA is rather heterogeneous and a variety of conditions may be related to its development, including genetic/congenital abnormalities, post-infectious issues, varicocele, trauma, endocrine disorders, exposure to gonadotoxins, Sertoli cell-only (SCO) syndrome, maturation arrest and idiopathic causes (Esteves and Agarwal, 2013; Esteves, 2015). Even though NOA might indicate impaired spermatogenesis in the entire testis, in 50–60% of cases, sperm cells can still be produced in some islets (Esteves, 2015). Therefore, regardless of its type, azoospermia does not imply sterility, as previously assumed (Esteves and Agarwal, 2013). Invasive procedures such as testicular histology with surgical exploration of the genital tract is the only method for a differential diagnosis of azoospermia. Pregnancies can be achieved using testicular sperm extracted from azoospermic men using assisted reproductive techniques such as intracytoplasmic sperm injection (ICSI) (Vloeberghs et al., 2015).Testicular sperm extraction (TESE) and testicular sperm aspiration (TESA) are the most common procedures for obtaining sperm cells from azoospermic patients (Rajfer, 2006). Generally, TESE/TESA are successful in retrieving sperm almost 100% of OA cases (Tournaye et al., 1997).
In the population of NOA patients, however, the possibility of finding sperm cells is only about 50%, and therefore, random sampling of the testis does not reflect histopathology of NOA accurately and NOA patients are submitted to multiple testicular biopsies (Tournaye et al., 1997). The disadvantage of such invasive procedures is that the removal of large fragments of testicular tissue may endanger, in a transient or permanent fashion, the androgen production, possibly leading to severe hypogonadism (Esteves et al., 2015). Evaluation of non-invasive preoperative markers have been suggested in order to accurately access the histopathological subtypes of NOA, and to predict the outcomes of TESE/TESA. Evaluation of the serum levels of follicle stimulating hormone (FSH) and the testicular volume have been incorporated in clinical routine (Bernie et al., 2013). Predictive power of these markers is rather poor; however, they continue to be recommended (Tournaye et al., 1996).
Drabovich et al. (2013) compared seminal plasma obtained from NOA, OA and men presenting with normal spermatogenesis and identified ECM1 and TEX 101 as clinical biomarkers for azoospermia. These markers have the potential to distinguish between OA and NOA, improve the confidence of NOA diagnosis, eliminate most of the diagnostic testicular biopsies and TESE/TESA procedures for patients with SCO, and thus facilitate prediction of the outcome of sperm cell retrieval procedures for ART. Furthermore, a simple and promising two-marker decision tree for the noninvasive differential diagnosis of NOA subtypes was proposed that can potentially be incorporated in clinical routine (Drabovich et al., 2013).
FSH is traditionally used as a marker of azoospermia. 25–35% of NOA patients present with high levels of serum FSH (Gudeloglu and Parekattil, 2013). At the protein level, our understanding of the etiology and pathways underlying different subtypes of NOA remain unclear. In the present study, we used mass spectrometry (MS)-based proteomics to, quantitatively, investigate the seminal plasma of different NOA patients. The aim of this study was to compare proteomic profiles in nonobstructive azoospermia patients with 1) normal serum levels of FSH and normal testicular volume and 2) high serum levels of FSH and small testicular volume with normozoospermic controls.
Materials and Methods
Study Design and Subjects
Institutional Review Board approval was obtained from Cleveland Clinic Research Ethics Committee, and each participant included in this study signed a written informed consent. All patients provided detailed information regarding their medical history, and laboratorial tests were performed to access the: (i) testicular volume (of both sides), (ii) hormonal status (testosterone, FSH, and LH), (iii) karyotype, and (iv) microdeletion of the azoospermia factor (AZF) region in Y chromosome. 14 non-azoospermic patients and 7 normozoospermic controls were included in this study. Patients aged from 20–45 years were diagnosed as azoospermic after confirmation of complete absence of sperm by the cytospin and nuclear fast red-picroindigocarmine (NFPIC) staining described below.
Semen Collection and Analysis
After liquefaction (20 min at 37°C), seminal parameters (volume, total sperm count, concentration, sperm motility, round cells) were manually evaluated according to the WHO 5th edition guidelines (WHO, 2010).
Diagnostic Confirmation of Azoospermia
After the initial semen analysis, if no sperm cells were observed in a wet smear, samples were centrifuged using the cytospin method as described earlier (Hendin et al., 1998). The cytopellet was examined with nuclear fast red-picroindigocarmine (NFPIC) stain to assess the presence or absence of sperm cells microscopically in the specimen. A control slide was also prepared to assess stain quality. Sperm heads stained red, whereas tails stained green. A second sample was examined 2 weeks later to confirm the diagnosis of azoospermia.
Characterization of NOA and Study Groups
FSH levels in serum were measured on at least two occasions for each patient. In order to distinguish between OA and NOA, FSH levels and testicular volume were used and testicular biopsy results were used where the data was available. The accurate characterization of NOA was made through the use of the algorithm proposed by Cocuzza et al. (Cocuzza et al., 2013) and based on the results from general physical, andrological and laboratory examination.
Accordingly, all 14 azoospermic patients included in this study presented NOA. Schoor et al. found that 96% of men with obstructive azoospermia had a FSH of less than 7.6 mIU/ml or testicular long axis greater than 4.6 cm (Schoor et al., 2002). We do not measure testis long axis at our institution but use testicular volume as estimated during physical exam instead. “Normal” testicular volume is difficult to determine, but many studies use greater than 18–20 cm3 as the cutoff for normal testicular volume (Cocuzza et al., 2013).
These patients were then, divided into two sub-groups according to i) levels of FSH ii) testicular volume and iii) abnormal spermatogenesis (evaluated in the testicular biopsy) (see Table 1). Thus, the first sub-group (hereafter high FSH) included 10 NOA patients presenting with high FSH levels (>7.6 mIU/mL; average 24.7 mIU/mL; range 16.7–30.6 mIU/mL) OR small testicular volume (< 18 cm3; average 10.4 cm3; range 4–16 cm3), and abnormal spermatogenesis. The second sub-group (hereafter normal FSH) included 4 NOA patients presenting normal FSH levels (6–8.6 mIU/mL; average 5.7 mIU/mL) OR normal testicular volume (> 18 cm3, average 18.7cm3; range 16–20 cm3) and abnormal spermatogenesis.
Table 1.
Testicular volume and FSH levels with biopsy findings in NOA +High FSH and NOA + normal FSH group of azoospermic patients
| Subject | FSH | Testicular volume (cm3) |
Testicular biopsy findings |
|||
|---|---|---|---|---|---|---|
| Left | Right | Sertoli- cell only |
Presence of Sperm |
|||
| Yes | No | |||||
| NOA+ High FSH | ||||||
| 1 | 26.1 | 10 | 10 | x | x | |
| 2 | 16.7 | 14 | 14 | x | x | |
| 3 | 28.8 | <4 | <4 | NBD | NBD | |
| 4 | 29 | 6 | 6 | x | x | |
| 5 | 25.3 | 14 | 14 | NBD | NBD | |
| 6 | 25.5 | 14 | 14 | x | x | |
| 7 | 30.6 | 6 | 6 | NBD | NBD | |
| 8 | 17 | 16 | 16 | x | x | |
| 9 | 18.7 | 16 | 16 | x | x | |
| 10 | 28.9 | 4 | 4 | x | x | |
| NOA+ Normal FSH | ||||||
| 1 | 8.6 | NA | NA | NBD | NBD | |
| 2 | 2.2 | 20 | 20 | NBD | x | |
| 3 | 6 | 20 | 20 | NBD | x | |
| 4 | 6.1 | 16 | 16 | NBD | NBD | |
NA = Not available; NBD = No Biopsy data
Seminal Plasma Preparation and SDS-PAGE
After semen analysis, all samples were centrifuged at 4,000g for 7 min. at 4°C. The clear seminal plasma was supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN) followed by ultracentrifugation at 4°C, 105,000×g for 45 min. The supernatant was recovered and protein concentration was determined with the bicinchoninic acid assay (BCA) (Thermo Scientific, Rockford, IL). Aliquots containing 25 µg of total protein were separated from each pool, mixed with standard sodium dodecyl sulfate (SDS) buffer, boiled and proteins separated using gel electrophoresis (SDS-PAGE). Briefly, samples were applied in a 12.5% Tris-HCl gel (Bio-Rad, Hercules, CA). Subsequently, the protein containing-gel was placed in a Bio-Rad PowerPac Electrophoresis system (Bio-Rad, Hercules, CA) and electrophoresis was then performed using Tris/Glycine/SDS (TGS buffer) for 35 min at 150V. The short gel was fixed for 30 min in 50% (v/v) ethanol, 10% (v/v) acetic acid solution, washed with water thoroughly and stained with GelCode Blue (Thermo Fisher Scientific, Rockford, IL). The protein bands were excised and cut into five smaller pieces; this procedure was followed by in-gel digestion using standard protocol as proposed by Shevchenko et al. (1996). Gel pieces were initially destained by washing with acetonitrile and subjected to reduction with 50mM DTT (in 100mM ammonium bicarbonate) and alkylation with 150mM Iodoacetamide (in 100mM ammonium bicarbonate). Reduction and alkylation steps were performed at room temperature for 1 hour. The in-gel digestion step was carried out using 5 µL Sequencing Grade Modified Trypsin (10 ng/µL trypsin in 50 mM ammonium bicarbonate) (Promega, Wisconsin, WI).
LC-MS/MS Analysis and Protein Identification
The LC-MS/MS analysis was performed by coupling a Dionex Ultimate 3000 HPLC system to a LTQ-Orbitrap Elite (Thermo Scientific, San Jose, CA). Five µL of recovered peptides were injected for each analysis, and each sample was injected 3 times. Peptides were delivered to an Acclaim PepMap 100 C18 (15 cm × 75 µm internal diameter, 2 µm, 100 Å) reversed phase capillary chromatography column (Thermo Scientific, Rockford, IL). Five µL of the extract was injected and eluted by an acetonitrile/0.1% formic acid gradient at a flow rate of 0.25 µL/ min were introduced into the source of the mass spectrometer on-line. The nanoelectrospray ion source was operated at 2.5 kV and the Orbitrap Elite was operated in data dependent mode (DDA), automatically switching between MS and MS2. Peptide identifications were accepted if they could be established at > 95.0% probability by the Peptide Prophet algorithm with Scaffold delta-mass correction. Protein identifications were accepted if they could be established at > 99.0% probability to achieve a false detection rate (FDR) < 1.0% and contained at least 2 identified peptides.
Relative Quantification and 203-Analysis
The total number of mass spectra that matched peptides to a particular protein (termed Spectral counts) was used to access the regulation of proteins in the complex mixture. Normalization of spectral counts using the NSAF (normalized spectral abundance factor) (NSAF= SC/ (ΣSC*protein length) approach was applied prior to relative protein quantification (Zhang et al., 2010; Zybailov et al., 2005). Appropriate filters were used to identify differentially expressed proteins (hereafter DEP) that were dependent on the overall regulation of the proteins. Normalized ratios were calculated to access the proteins showing differential regulation and statistical significance was assessed using two-tailed statistical tests. The NSAF values were used to access the ratios (fold-change) among the three groups (normal FSH/Control, high FSH/Control and normal FSH/high FSH), which were further normalized by log2 transformation. By comparing all identified proteins between these groups, it was possible to observe the presence of group-exclusively identified proteins. These proteins were filtered and only those presenting spectra collected in three out of three MS runs were considered for further data analysis. The criteria for classifying DEPs into upregulated or downregulated protein(s) was based on the log transformed ratios >0 (upregulated) and <0 (downregulated).
Functional Interpretation
Functional interpretation of the results analysis was done using publicly available software packages such as the Gene Ontology (GO) Term Finder (Boyle et al., 2004), UNIPROT and Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://david.niaid.nih.gov) to identify the differentially affected processes, pathways, interactions and cellular distribution of the proteins.
Results
The FSH levels, testicular volume and biopsy findings where available are shown in Table 1. A total number of 542 individual proteins were detected.
Label-free Quantitation by Spectral Counting and Group Comparisons
The relative quantity of proteins was determined by comparing its spectral counts number. The numerical values used in the quantitation correspond to the NSAF. The total spectral counts collected in this study ranged from 15698 to 20715, and their distribution according to group can be observed in Figure 1A. Moreover, the spectral count comparison of both NOA groups with the control group made it possible to calculate the ratios (normal FSH/control or high FSH/control) for each individual protein. According to these ratios, proteins were then divided into four categories: i) downregulated in normal FSH, ii) upregulated in normal FSH, iii) downregulated in high FSH and (iv) upregulated in high FSH.
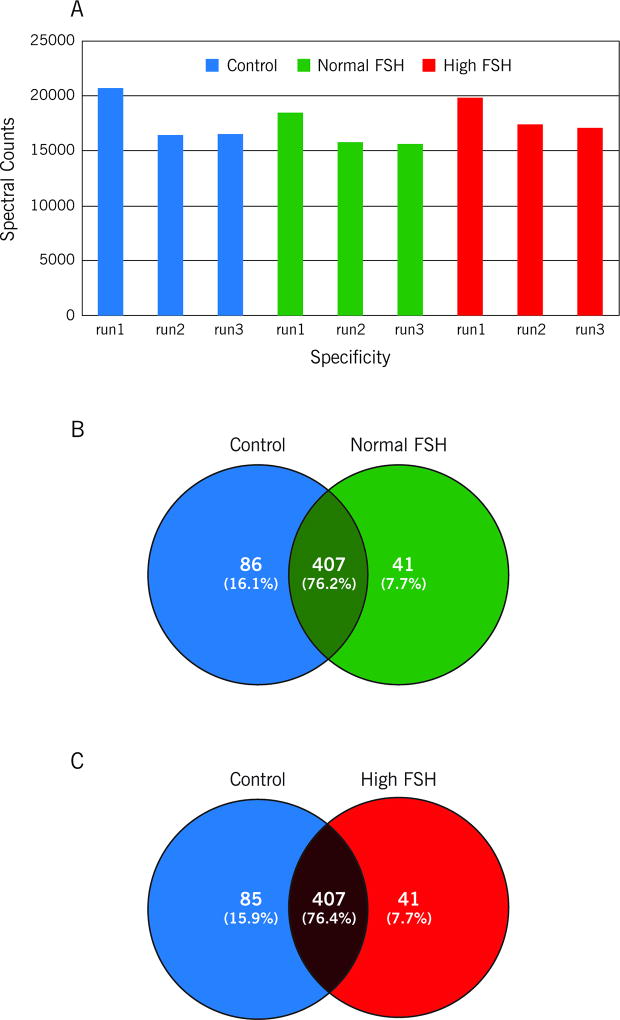
Figure 1.
The relative quantity of the seminal plasma proteins was determined by comparing the number of spectral counts, used to identify each protein in A: control, normal FSH and high FSH group. The total spectral counts ranged from 15698 to 20715, B: Venn diagram showing the overlap between the proteins identified in the control and normal FSH groups, C: Venn diagram showing the overlap between the proteins identified in the control and high FSH groups.
Normal FSH versus Control group
The comparison of identified proteins between normal FSH and control groups showed 407 proteins in common (Figure1B and Supplemental Table S1). According to their NSAF ratios (or fold-change), proteins were divided into two categories: downregulated in normal FSH (ratio<0) and upregulated in normal FSH (ratio>0).
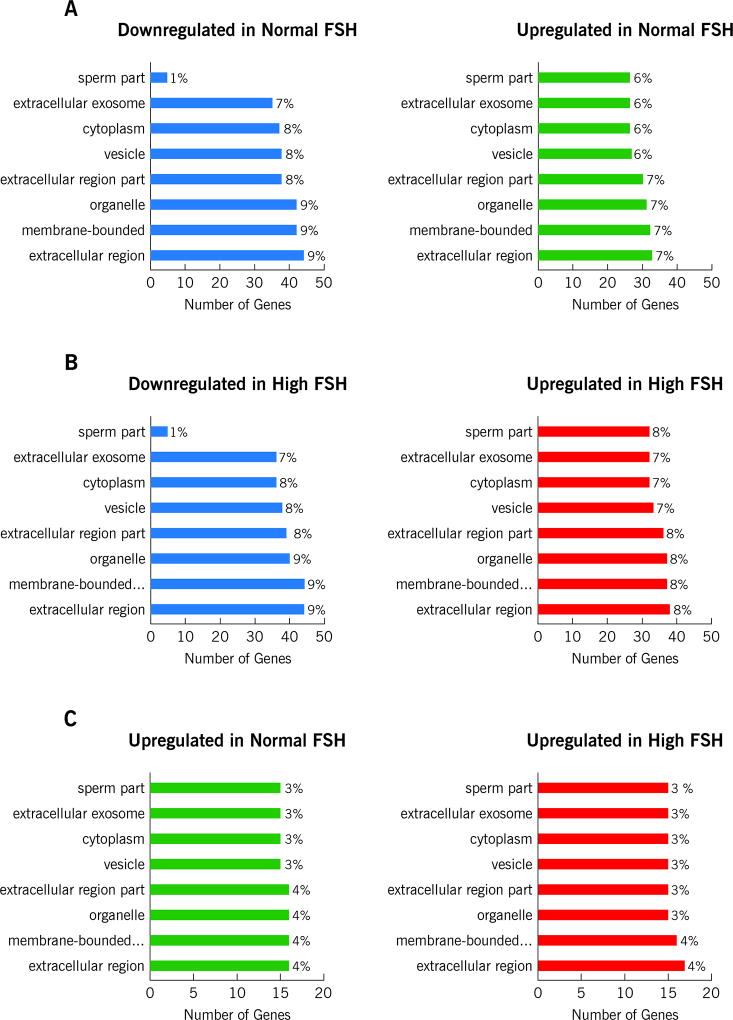
Functional interpretation of this differential proteome was done by GO enrichment analysis and revealed that the majority of the upregulated proteins (~33%) belong to the extracellular region (Figure 2A). We observed the upregulation of common seminal plasma proteins such as Annexins secreted by the prostate, MUC6 by the bulbourethral glands, and Serpins by the seminal vesicles. Interestingly, although ~41% of downregulated proteins belong to the extracellular region, about 3.5% were annotated as cellular components of sperm cells (Figure 2A). Thus, the downregulation of ACR, ACRBP, GSTM3, PGK2, TEX101 and TXNDC2 was observed. The proteins PGK2, GSTM3 and TXNDC2 were annotated as part of the sperm flagellum and, therefore, implicated in cellular motility.
Figure 2.
GO enrichment analysis showing distribution of the proteins in A: downregulated and upregulated proteins in Normal FSH group B: downregulated and upregulated proteins in High FSH group and C: upregulated proteins in Normal and High FSH group.
While PGK2 and GSTM3 were observed to be 2.5-fold and 1.7-fold downregulated in the normal FSH group and TXNDC2 was exclusively identified in the control group. Likewise, ACR, ACRBP and TEX 101 were exclusively identified in the control group. Some proteins related directly to the testis and epididymis also presented differential regulation in our study. NPC2 and EDDM3B were less abundant in the normal FSH group, respectively. A group of six heat-shock proteins was also observed among the downregulated proteins in the normal FSH group. Detailed information, fold-changes and p-values for each of the aforementioned proteins are found in Supplemental Table S1.
High FSH versus Control group
The comparative results of high FSH and control groups were somewhat similar to the comparison of normal FSH and control. Thus, 407 proteins were commonly observed in both groups (Figure 1C; Supplementary Table S2). Proteins were divided into two categories: downregulated in high FSH and upregulated in high FSH. The GO enrichment analysis revealed that the majority of the upregulated proteins (~32%) belong to the extracellular region (Figure 2B). As observed in the normal FSH group, an over-regulation of ordinary seminal plasma proteins was revealed. Besides Annexins, MUC6 and Serpins, ACPP and KLK2, both secreted by the prostate, were also upregulated in the high FSH group. Among the downregulated proteins, it was possible to observe different sperm cells components proteins as in the normal FSH versus control comparison. Moreover, GSTM3 and PGK2 were, respectively, 3.7 and 3.1-fold downregulated in the high FSH group. The two epididymal proteins, NPC2 and EDDM3B, were also downregulated in the high FSH group. The same group of six heat-shock proteins, presenting down regulation in the normal FSH group, was observed. Detailed information, fold-changes and p-values for each of the aforementioned proteins are found in Supplementary Table S2.
Normal FSH versus High FSH
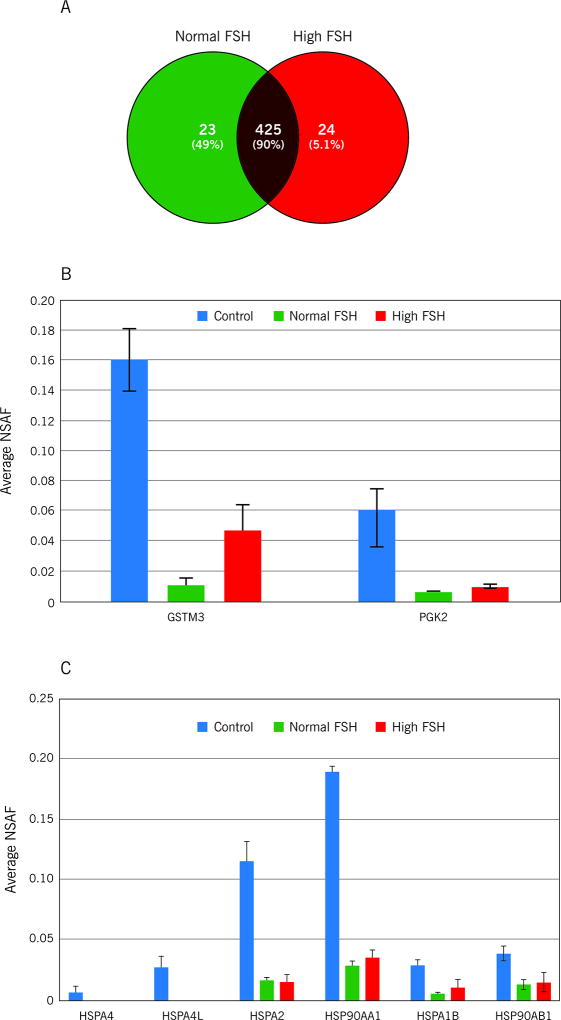
The quantitative comparison between normal FSH and high FSH groups showed 425 common proteins (Figure 2A; Supplementary Table S3). Among the exclusive identified proteins, 12 followed our criteria (spectra collected in three out of three MS runs). Thus APOE, CALML5, FABP5, PPIA, SERPINB3, TIAM1 and TFF3 were exclusively identified in the high FSH group. Furthermore, APEH, GPC4, MUC5B, MYH and TENM2 were exclusively identified in the normal FSH group. Based on fold-change, proteins were divided into two categories: upregulated in normal FSH and upregulated in high FSH.
Majority of the upregulated proteins in the high FSH group belonged to the extracellular region. Among these, and according to fold-change values, BPIFB2, DSP, BCAM, DSG1, DSC1 presented greater regulation (Supplementary Table S3). The GO enrichment analysis also revealed that the majority of the upregulated proteins in the normal FSH group belong to the extracellular region. Surprisingly, GSTM3 and PGK2 were upregulated in the normal FSH group (Figure 3B). Based on the fold-change, S100A7, S100A8, CFI, DAG1, and LDHC were among the proteins presenting greater fold regulation in the normal FSH group (Supplementary Table S3).
Figure 3.
Venn diagram showing the overlap between the proteins identified in the A: normal FSH and high FSH groups, B: Average NSAF of GSTM3 and PGK2. Bar represents average ± standard deviation calculated for three MS runs, C: Average NSAF of heat-shock proteins (HSP90AB1, HSPA1B, HSP90AA1 and HSPA2, HSPA4L and HSPA4). Bar represents average ± standard deviation calculated for three MS runs.
Discussion
Seminal plasma proteome characterization of NOA patients
In the present study, we used MS-based proteomics to identify proteins in the seminal plasma from NOA patients, presenting different serum levels of FSH. Interestingly, the majority of proteins showing differential regulation in the normal FSH group also showed differential regulation in the high FSH group, demonstrating that there is no major difference, at the protein level, that could characterize either group.
To date, at least 20 heat shock proteins (HSPs) have been identified as being related to the spermatogenesis process (Ferlin et al., 2010). Spermatogenesis is accompanied by the regulation of different HSPs in the testes because different phases (including mitotic proliferation of spermatogonia, meiotic development of spermatocytes, post-meiotic development of spermatids and sperm cells maturation) represent conditions where dramatic transformations and cellular differentiation take place (Meinhardt et al., 1999). In normal cells, HSP 90 comprises about 20% of the total proteins, however in stressful conditions its levels can increase up to 10% with a concomitant increase in its activity. It also plays a role in degradation of unfolded proteins and therefore in maintaining protein homeostasis (Khurana and Bhattacharyya, 2015). In addition to the chaperone activity, HSP 90 plays an important role in regulating transcription factors. HSP90 and its co-chaperones are involved in modulating transcription activity by 1) altering the steady state availability of important transcription factors in response to physiological conditions, 2) modulating activity of certain epigenetic modifiers such as histone deacetylases or DNA methyl transferases in response to the changing environment and 3) participating in the removal of histones from the activator region and thereby turning on the gene regulation (Khurana and Bhattacharyya, 2015).
In our results, a group of four heat-shock proteins (HSP90AB1, HSPA1B, HSP90AA1 and HSPA2) were observed among the downregulated proteins in the NOA groups; moreover, HSPA4L and HSPA4 were exclusively identified in control samples (Figure 3C). This fact corroborates with the idea that low levels of HSPs regulation in spermatogonia might lead to a decreased level of protection, which could be involved in low spermatogenic efficiency, discussed earlier (Neuer et al., 2000; Meinhardt et al., 1999; Werner et al., 1997; Dix et al., 1996). Thus, considering that the HSPs regulation observed in the seminal plasma reflects their regulation in the testis, our results indicate that HSPs in NOA patients (regardless of serum levels of FSH) may be implicated in diminished spermatogenesis.
The GO enrichment analysis also revealed that among the downregulated proteins in both NOA groups, there were differences in the sperm cell components. ACR, ACRBP, TEX101 and TXNDC2 were exclusively identified in the control group. Using immunohistochemistry and immune-enrichment MS–based assays, Drabovich et al. demonstrated a differential regulation of TEX101 in distinct NOA subtypes (Drabovich et al., 2013). These authors reported that seminal concentrations of TEX101 are capable of differentiating Sertoli cell–only syndrome from other categories of NOA (Drabovich et al., 2013). Although TEX101 was annotated as a sperm cell protein in our results, immunohistochemistry has confirmed high regulation levels of TEX101 in spermatocytes and spermatids (Drabovich et al., 2013). The same was observed for GSTM3 and PGK2, and although both proteins were annotated as cellular components of sperm cells, their regulation in the testis has been demonstrated (Comstock et al., 2003; Saribek et al., 2006). The differential regulation of these proteins observed in our study indicates that although spermatogenesis may still be going on in the NOA groups, but somehow it is not as intense as in the control group.
Two epididymal proteins, NPC2 and EDDM3B, were also downregulated in the NOA groups, when compared to control. It has been observed that NPC2 is decreased by 40% in seminal plasma from vasectomized men (Robinson et al., 1989). Additionally, our results suggest that NPC2 is, indeed a marker for NOA; however, it does not seem to be effective in distinguishing different NOA samples. Likewise, EDDM3B, seems to be a marker of NOA samples, but has no predictive value for normal FSH or high FSH samples.
Quantitative differences in the proteome of NOA patients presenting high and normal FSH
Interestingly, only ~25% of all identified proteins showed significant differential regulation between NOA groups (Drabovich et al., 2011; Lundgren et al., 2010). Although such similarity may be due to the nature of azoospermia, it is important to emphasize that in the present study we used spectral counting which is a semi quantitative method, and not remarkably sensitive for small changes, especially in downregulated proteins.
GO enrichment analysis was used to examine the origin of the upregulated proteins, and showed that, as expected, the majority of the proteins belong to the extracellular region, regardless of group. Among the extracellular molecules presenting greater regulation (based on spectral counting) in the high FSH group, proteins that play a role in innate immunity, such as BPIFB2, were observed. Similarly, the upregulation of CFI was evidenced in the normal FSH group. Moreover, adhesion molecules and components of intracellular junctions, like DSP, BCAM, DSG1, and DSC1 were found among the proteins that were upregulated in the high FSH group. Additionally, the epithelial S100A7, S100-A8, and DAG1 were found to be upregulated in the normal FSH group. It is known that immune/ epithelial cells are constantly secreting and shedding their content into the seminal plasma. These secretions, however, are dependent on the overall status of the male reproductive tract (i.e. infection, inflammation, etc.) and may, therefore, vary from patient to patient. (Meijer et al. 2012)
The testicular GSTM3 and PGK2, previously observed to be downregulated in NOA samples in contrast to the control group, showed upregulation in the normal FSH group when NOA groups were compared. Although their role in spermatogenesis is not fully elucidated, as discussed before, our results indicate that the testicular regulation of these proteins may be inversely proportional to FSH levels. In this scenario, both proteins may be possible candidates to distinguish NOA patients presenting with different levels of serum FSH. The limitations of our pilot study were 1) small sample size in NOA +Normal FSH subjects 2) We did not have testicular biopsies/ pathological diagnosis for all the patients, laboratory findings of Cytospin results where no sperm were seen and FSH levels and testicular volume were used along with testicular biopsies where available 3) We used the shotgun or label free proteomic approach in this study and 4) samples were pooled in the two study groups.
In conclusion, our results from this pilot study suggest that a group of six heat-shock proteins (HSP90AB1, HSPA1B, HSP90AA1 and HSPA2, HSPA4L and HSPA4) may be implicated in the pathophysiology of diminished spermatogenesis in NOA patients. We found no major difference in protein regulation that potentially characterizes NOA patients presenting low or normal serum levels of FSH. Although the regulation of testicular proteins GSTM3 and PGK2 may be useful in distinguishing low FSH and normal FSH groups, the predictive value of these proteins should be evaluated individually and/or in a larger cohort of patients. The same applies for the 12 proteins identified exclusively in either NOA group. More experimental data is necessary to validate this hypothesis, and shed some light on protein networks involved in NOA pathogenesis.
Supplementary Material
Acknowledgments
The authors are grateful to the Andrology Center technologists for scheduling the study subjects. Belinda Willard, Director, Proteomic Core Lab, Lerner Research Institute for providing assistance with proteomic analysis and Banu Gopalan, with Bioinformatics data analysis. The Orbitrap Elite mass spectrometer used in this study was purchased with funds from an NIH shared instrument grant 1S10RR031537-01 to Belinda Willard.
Funding
Financial support was provided by the American Center for Reproductive Medicine, Cleveland Clinic. Dr. Zhihong Cui’s visit was supported by a fellowship from the Chinese Government and the Institute of Toxicology, College of Preventive Medicine, Third Military Medical University, Chongqing, People’s Republic of China.
References
- 1.Baker K, Sabanegh E., Jr Obstructive azoospermia: reconstructive techniques and results. Clinics (Sao Paulo) 2013;68:61–73. doi: 10.6061/clinics/2013(Sup01)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernie AM, Ramasamy R, Schlegel PN. Predictive factors of successful microdissection testicular sperm extraction. Basic Clin Androl. 2013;23:5. doi: 10.1186/2051-4190-23-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Sherlock G. GO: TermFinder--open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo) 2013;68:15–26. doi: 10.6061/clinics/2013(Sup01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comstock KE, Johnson KJ, Rifenbery D, Henner WD. Isolation and analysis of the gene and cDNA for a human Mu class glutathione S-transferase, GSTM4. J Biol Chem. 1993;268:16958–16965. [PubMed] [Google Scholar]
- 6.Drabovich AP, Jarvi K, Diamandis EP. Verification of male infertility biomarkers in seminal plasma by multiplex selected reaction monitoring assay. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.004127. M110.004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Eddy EM. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci U S A. 1996;93:3264–3268. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drabovich AP, Dimitromanolakis A, Saraon P, Soosaipillai A, Batruch I, Mullen B, Diamandis EP. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci Transl Med. 2013;5:212ra160. doi: 10.1126/scitranslmed.3006260. [DOI] [PubMed] [Google Scholar]
- 9.Esteves SC, Agarwal A. The azoospermic male: current knowledge and future perspectives. Clinics (Sao Paulo) 2013;68:1–4. doi: 10.6061/clinics/2013(Sup01)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl. 2015;17:459–470. doi: 10.4103/1008-682X.148719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferlin A, Speltra E, Patassini C, Pati MA, Garolla A, Foresta C. Heat shock protein and heat shock factor regulation in sperm: relation to oligozoospermia and varicocele. J Urol. 2010;183:1248–1252. doi: 10.1016/j.juro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics (Sao Paulo) 2013;68:27–34. doi: 10.6061/clinics/2013(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendin BN, Patel B, Levin HS, Thomas AJ, Jr, Agarwal A. Identification of spermatozoa and round spermatids in the ejaculates of men with spermatogenic failure. Urology. 1998;51:816–819. doi: 10.1016/s0090-4295(98)00007-7. 1998. [DOI] [PubMed] [Google Scholar]
- 14.Khurana N, Bhattacharyya S. Hsp90, the Concertmaster: Tuning Transcription. Front Oncol. 2015;5:100. doi: 10.3389/fonc.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundgren DH, Hwang SI, Wu L, Han DK. Role of spectral counting in quantitative proteomics. Expert Rev Proteomics. 2010;7:39–53. doi: 10.1586/epr.09.69. [DOI] [PubMed] [Google Scholar]
- 16.Meijer B, Gearry RB, Day AS. The role of S100A12 as a systemic marker of inflammation. Int J Inflam. 2012;2012:907078. doi: 10.1155/2012/907078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinhardt A, Wilhelm B, Seitz J. Regulation of mitochondrial marker proteins during spermatogenesis. Hum Reprod Update. 1999;5:108–119. doi: 10.1093/humupd/5.2.108. [DOI] [PubMed] [Google Scholar]
- 18.Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6:149–159. doi: 10.1093/humupd/6.2.149. [DOI] [PubMed] [Google Scholar]
- 19.Palermo GD, Neri QV, Schlegel PN, Rosenwaks Z. Intracytoplasmic sperm injection (ICSI) in extreme cases of male infertility. PLoS One. 2014;9:e113671. doi: 10.1371/journal.pone.0113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajfer J. TESA or TESE: Which Is Better for Sperm Extraction? Rev Urol. 2006;8:171. [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson MO, McCarrey JR, Simon MI. Transcriptional regulatory regions of testis-specific PGK2 defined in transgenic mice. Proc Natl Acad Sci. 1989;86:8437–8441. doi: 10.1073/pnas.86.21.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saribek B, Jin Y, Saigo M, Eto K, Abe S. HSP90beta is involved in signaling prolactin-induced apoptosis in newt testis. Biochem Biophys Res Commun. 2006;349:1190–1197. doi: 10.1016/j.bbrc.2006.08.143. [DOI] [PubMed] [Google Scholar]
- 23.Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002;167:197–200. [PubMed] [Google Scholar]
- 24.Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F, Wilm M, Vorm O, Mann M. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci U S A. 1996;93:14440–14445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tournaye H, Verheyen G, Nagy P, Ubaldi F, Goossens A, Silber S, Van Steirteghem AC, Devroey P. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum Reprod. 1997;12:80–86. doi: 10.1093/humrep/12.1.80. [DOI] [PubMed] [Google Scholar]
- 26.Tournaye HJ, Liu J, Nagy Z, Camus M, Goossens A, Silber S, Devroey P. Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular sperm. Hum Reprod. 1996;11:127–132. doi: 10.1093/oxfordjournals.humrep.a019004. [DOI] [PubMed] [Google Scholar]
- 27.Vloeberghs V, Verheyen G, Haentjens P, Goossens A, Polyzos NP, Tournaye H. How successful is TESE-ICSI in couples with non-obstructive azoospermia? Hum Reprod. 2015;30:1790–1796. doi: 10.1093/humrep/dev139. [DOI] [PubMed] [Google Scholar]
- 28.Werner A, Meinhardt A, Seitz J, Bergmann M. Distribution of heat-shock protein 60 immunoreactivity in testes of infertile men. Cell Tissue Res. 1997;288:539–544. doi: 10.1007/s004410050839. [DOI] [PubMed] [Google Scholar]
- 29.WHO. WHO laboratory manual for the examination and processing of human semen. 5. Vol. 2010. WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 30.Zhang Y, Wen Z, Washburn MP. Florens L. Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Anal Chem. 2010;82:2272–2281. doi: 10.1021/ac9023999. [DOI] [PubMed] [Google Scholar]
- 31.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.