Abstract
Androgen signaling is essential for prostate development, morphogenesis, and regeneration. Emerging evidence indicates that Wnt/β-catenin signaling also contributes to prostate development specifically through regulation of cell fate determination. Prostatic Axin2-expressing cells are able to respond to Wnt signals and possess the progenitor properties to regenerate prostatic epithelium. Despite critical roles of both signaling pathways, the biological significance of androgen receptor (AR) in Axin2-expressing Wnt-responsive cells remains largely unexplored. In this study, we investigated this important question using a series of newly generated mouse models. Deletion of Ar in embryonic Axin2-expressing cells impaired early prostate development in both ex vivo and tissue implantation experiments. When Ar expression was deleted in prostatic Axin2-expressing cells at pre-puberty stages, it results in smaller and underdeveloped prostates. A subpopulation of Axin2 expressing cells in prostate epithelium is resistant to castration and, following androgen supplementation, is capable to expand to prostatic luminal cells. Deletion of Ar in these Axin2-expressing cells reduces their regenerative ability. These lines of evidence demonstrate an indispensable role for the Ar in Wnt-responsive cells during the course of prostate development, morphogenesis, and regeneration, which also imply an underlying interaction between the androgen and Wnt signaling pathways in the mouse prostate.
Keywords: Androgen receptor, Wnt signaling, β-catenin, Prostate development
Graphical abstract
INTRODUCTION
Androgen signaling is mediated through the androgen receptor (AR) and its ligands, testosterone and 5α-dihydrotestosterone (DHT) [1, 2]. The AR is a member of the nuclear hormone receptor superfamily [3], and plays a decisive role in prostate development, maturation, and tumorigenesis [1, 2]. Mouse prostatic development initiates at embryonic day 17.5 (E17.5) from the urogenital sinus (UGS) in response to rising levels of testicular androgens [4]. After birth, the unbranched buds and ducts in the ventral, dorsal, and lateral prostatic lobes grow peripherally to give rise to lobe-specific ductal branching patterns [4, 5]. Morphogenesis of the entire prostatic complex in mice is completed between postnatal days 15 and 30 (P15-30) [5]. Circulating androgens levels rise during puberty (P25-40), resulting in continued prostate growth and maturation [5, 6]. Prostatic regeneration through repeated cycles of androgen deprivation and replacement further demonstrate that androgen signaling is essential for prostatic regeneration and growth [4, 7].
Recent studies demonstrate that the canonical Wnt signaling pathway, whose effects are mainly mediated through β-catenin, plays a critical role in prostate development, morphogenesis, and organogenesis [8–11]. Activation of Wnt/β-catenin signaling in prostatic epithelium is necessary for the development of the prostate [9, 10, 12]. Deletion of the ctnnb1 gene in the mouse prostate at embryonic stages results in inhibition of prostatic development [10]. Moreover, Axin2, a well-established direct transcriptional target of β-catenin and a component of the destruction complex in the negative feedback loop of the canonical Wnt signaling pathways, is expressed in both embryonic and adult prostate [13, 14]. In embryonic stages, Axin2 expressing cells can be observed in both the urogenital sinus mesenchyme (UGM) and the urogenital sinus epithelium (UGE), while its expression is limited to the luminal epithelium in adult stages [9, 10, 19]. Recent studies have shown that Axin2-expressing cells have stem/progenitor cell properties in a variety of mouse tissues, including the prostate [15–19]. Moreover, deletion of β-catenin or expression of stabilized β-catenin in prostatic Axin2-expressing epithelial cells results in abnormal prostate development or oncogenic transformation, respectively [19]. In addition, the direct interaction between the AR and β-catenin has also been observed in prostate cancer cells [20–22].
Although emerging evidence has shown that both the androgen and Wnt signaling pathways play a critical role in prostate development and tumorigenesis, it is unclear how Wnt signaling pathways specifically control cell fate commitment and determination during the course of prostate development, maturation, and regeneration. Specifically, it is also unknown the role of androgen signaling in regulating prostatic Wnt responsive progenitor cells. In this study, we directly assess these important questions using relevant mouse models and other experimental approaches. Our results reveal that androgen signaling plays an essential role in Wnt/β-catenin mediated prostate cell fate commitment, implicating a molecular mechanism underlying the regulatory roles of androgen and Wnt signaling pathways in prostate development and regeneration.
MATERIALS AND METHODS
Mouse mating and genotyping
Axin2CreERT2 and RosamTmG mice were obtained from Jackson Laboratories; stock 18867 and stock 7676) [18, 23]. Arflox/Y mice were obtained from Dr. Guido Verhoeven [24]. All animal procedures were performed based on animal care guidelines approved by the Institutional Animal Care and Use Committee at Beckman Research Institute and City of Hope.
Mouse procedures
Castration of adult male mice was performed as described previously [25]. For androgen supplementation, testosterone pellets (12.5 mg, Innovative Research of America) were placed subcutaneously in castrated mice. To elicit genetic recombination mice received a single intraperitoneal injection of 4 mg/25 g body weight tamoxifen (Sigma) suspended in corn oil, corresponding to a total dose of 1 mg for prepubescent mice (injected at P14) and 4 mg of tamoxifen for adult mice (injected at P84). To label Axin2-expressing cells in embryos, pregnant mothers received a single intraperitoneal injection at E13.5, totaling 1mg/25g body weight.
Histology and immunostaining
Prostatic tissues were fixed in 10% neutral-buffered formalin, and processed into paraffin. Five micron serial sections were cut and processed from xylene to water through a decreasing ethanol gradient. For histology, hematoxylin-eosin staining was performed as reported earlier [26, 27]. For immunohistochemistry, slides were treated by boiling in 0.01M Citrate buffer (pH 6.0) for antigen retrieval, incubated in 0.3% H2O2 for 15 min, blocked in 5% normal goat serum for 30 min, and incubated with primary antibodies diluted in 1% normal goat serum at 4°C overnight. Slides were incubated with biotinylated secondary antibodies for 1 hour then with horseradish peroxidase streptavidin (SA-5004, Vector Laboratories) for 30 minutes and visualized by DAB kit (SK-4100, Vector Laboratories). Slides were counterstained with 5% (w/v) Harris Hematoxylin, and coverslips were mounted with Permount Mounting Medium (SP15-500, Fisher Scientific).
For immunofluorescent staining and detection of mTmG signals, mouse tissues were fixed in 4% PFA at 4°C overnight, cryoprotected in 30% sucrose at 4°C overnight, and embedded in OCT (Tissue-Tek). Five micron sections were obtained using a Leica cryostat, and slides were washed three times with PBS. For detection of membrane-bound Tomato (mT) and membrane-bound green fluorescent protein (mGFP) signals, slides were directly mounted with VECTASHIELD Mounting Medium with DAPI (H-1200, Vector Laboratories). For immunofluorescence staining, slides were treated for antigen retrieval as described above. Tissues were then blocked in 5% normal goat serum for 30 min, and incubated with primary antibodies diluted in 1% normal goat serum at 4°C overnight. Slides were washed in PBS then incubated with fluorescent-conjugated secondary antibodies for 1 hour, and then mounted as described above.
Ex vivo culture and manipulation
Female mice were mated overnight. The following day was considered as E0.5 if a vaginal plug was detected. The urogenital sinuses (UGS) were dissected at E14.5 and placed on 0.4 μM Millicell-CM filters (Millipore) in 6-well tissue culture plates. Culture media consisted of DMEM-F12 (1:1) media, supplemented with nonessential amino acids, 1% insulin-transferrin-selectin (ITS), L-glutamine, Penicillin/Streptomycin, and 1×10−8 M dihydrotestosterone (DHT). 2 μM of (Z)-4-Hydroxytamoxifen was added to the media to activate Axin2CreER. Media was changed every 2 days, and the UGS explants were cultured for 7 days and then processed for histological and immunofluorescent analyses.
Antibodies
The following primary antibodies were used: anti-GFP (Rabbit, 1:1,000, A11122, Molecular Probes), anti-GFP (mouse, 1:300, 2955, Cell Signaling), anti-GFP (Chicken, 1:2000, ab13970, Abcam), anti-K5 (rabbit, 1:3000, PRB-160P, Covance), anti-K8 (mouse, 1:2500, MMS-162P, Covance), anti-p63 (mouse, 1:200, sc-8431, Santa Cruz), anti-Ki67 (mouse, 1:1000, NCL-ki67, Novacastra), anti-E-cadherin (mouse, 1:300, c20820, BD Transduction Laboratories), anti-androgen receptor (rabbit, 1:500, sc-816, Santa Cruz). Biotinylated anti-rabbit or anti-mouse secondary antibodies (BA-1000 or BA-9200, Vector Laboratories) were used for immunohistochemistry experiments. For immunofluorescence studies AlexaFluor-conjugated anti-rabbit, anti-mouse or anti-chicken antibodies (Molecular Probes) were used.
Microscope image acquisition
Images of H&E and immunohistochemistry were acquired on an Axio Lab. A1 microscope using 10x and 40x Zeiss A-Plan objectives with a Canon EOS 1000D camera and using Axiovision software (Carl Zeiss). Images of immunofluorescent staining and mTmG signals and were acquired on an Nikon ECLIPSE E800 epi-fluorescence microscope using 20x and 40x Nikon Plan Fluor objectives with an QImaging RETIGA EXi camera and using QCapture software (QImaging). Epithelial and mesenchymal cell numbers were counted manually using 40x photomicrographs as described in Supplementary Tables S1-S5. Statistical analyses were performed using 2-tailed Student’s t test or 2-way ANOVA.
RESULTS
Distinctive co-localization of the AR and Axin 2 in the urogenital sinus of embryonic mice
Expression of Axin2 has been observed in mouse UGE [10]. Using Axin2CreERT2/+;RosamTmG/+ mice [18] (Fig. 1A), we assessed Axin2 expression at embryonic stages during the course of early urogenital development in mice (Fig. 1B). Prostatic buds emerge from mouse UGE at E16.5 to E17.5 [4]. To evaluate Axin2 expression, we injected tamoxifen (TM) at E13.5 and examined induced membrane-bound green fluorescent protein (mGFP) expression at E15.5 and 17.5 (Fig. 1C). We observed TM-induced GFP expression at E15.5 mainly in urogenital sinus mesenchyme (UGM) in close apposition to the adjoining UGE (Fig. 1C1-2). At E17.5, Axin2 expressing cells were detected in both UGM and UGE (Fig. 1C3, Table S1), especially, in the newly formed prostatic epithelial buds (arrow heads, Fig. 1C4). Since Axin2 has been well-established as a downstream target of Wnt/β-catenin signaling pathways [13, 14], the above observation implicates the activation of Wnt/β-catenin signaling in both prostatic epithelium and mesenchyme at prostate embryonic development. In this study, we also examine the expression of Lef-1 and CyclinD1, two bona fide Wnt/β-catenin downstream targets in Axin2-expressing cells. We observed the co-expression of mGFP with Lef-1 or CyclinD1 in Axin2-expressing cells, which provides an additional line of evidence for the responsiveness of Axin2-expressing cells to Wnt/β-catenin signaling (Supplemental Figure 1).
Figure 1. Temporal analysis of Axin2 and Ar expression during early development of mouse prostate.
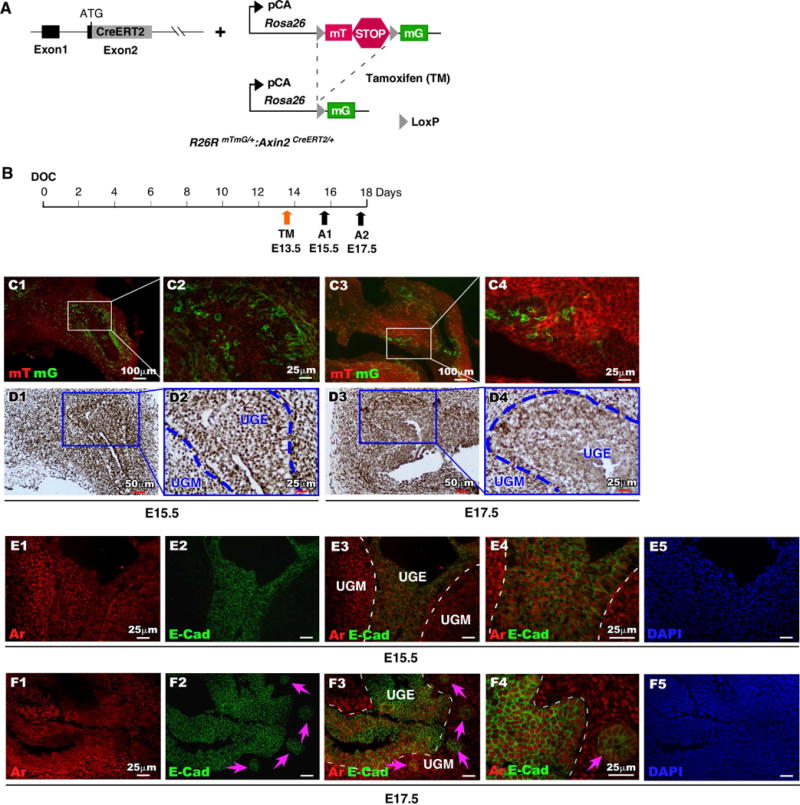
(A): Illustration of transgenic alleles present in R26RmTmG/+:Axin2CreERT2/+ mice. (B): Schematic depicting experimental timeline for labeling and analyzing UGS tissues during embryonic stage. (C): mTomato (red) or mGFP (green) expression in frozen tissue sections of male UGS from E15.5 and E17.5. (D): Ar expression (brown) in male UGS tissues from both E15.5 and E17.5. (E-F): Localization of Ar (red) and E-cadherin (green) in male UGS tissues isolated at E15.5 and E17.5.
Expression of Ar protein has been observed as early as at E13 in mouse UGM prior to prostatic bud formation [28, 29], suggesting that Ar is likely to be expressed in Axin2-expressing Wnt-responsive cells that are found in the UGM. Using immunohistochemical approaches, we assessed Ar expression, and observed uniform nuclear staining of Ar protein in both UGM and UGE areas at E15.5 and E17.5 (Fig. 1D1-4). We further evaluated the cellular identity of Ar positive cells using co-immunofluorescence (co-IF) analyses. As shown in Figure 1E2 and F2, E-cadherin expression appears within UGE areas of both E15.5 and E17.5 mice (Fig. 1E-F). At E17.5 E-cadherin staining was observed in epithelial cells of the newly formed prostatic buds (arrow heads, Fig. 1F2-4) co-expressing Ar (Fig. 1F3 and 1F4). Further analyses show that a subset of these Ar-expressing epithelial cells is also immunoreactive for Ck8, p63, and Ki67 (Supplemental Figure 2). Intriguingly, a portion of E-cadherin-positive epithelial cells in the UGS at E15.5 and E17.5 were also membrane-bound GFP positive, indicating their Wnt-responsive cellular identities (compare Fig. 1C3-4, and 1F3-4). Taken together, this data suggests an interaction between androgen and Wnt/β-catenin signaling pathways during the course of embryonic prostatic development. To explore this idea, we directly examined the co-localization of Ar and GFP expression within the UGM at E15.5 (Fig. 2A4-5), in the closely adjoining UGE (Fig. 2B1-4), and in newly formed prostatic buds (arrow heads, Fig. 2B5) at E17.5. The expansion of Ar and GFP double positive cells from the UGM into both the UGE and UGM between E15.5-17.5 suggests an important role for Wnt/β-catenin signaling in androgen-induced UGE formation and prostatic bud initiation. Since Axin2-expressing epithelial cells have been shown to possess progenitor cell properties [19], we further evaluated the Ar and GFP double-positive cells in both E15.5 and 17.5 tissues using triple immunofluorescence approaches. At E15.5, the majority of Ar and GFP double positive cells are localized in the UGM (Fig. 2C4), but a small minority also appears in the UGE (Fig. 2C4). Double positive epithelial cells were immunoreactive to antibodies against E-cadherin (Arrowheads, Fig. 2C4-5), Ck8 (Fig. 2D4-5), p63 (Fig. 2E4-5), and is some cases also to Ki67 (Fig. 2F4-5). At E17.5, Ar and GFP positive UGM cells are mainly localized in the areas adjacent to the UGE and in the newly formed prostatic buds (Fig. 2G4-5), which appear immunoresponsive to the both Ck8 and p63 antibodies (Arrowheads, Fig. 2H4-5, 2I4-5) as well as Ki67 antibody (Arrowheads, Fig. 2J4-5). Our observation of endogenous Ar expression in Axin2 positive cells in the UGS is interesting, and it suggests a biological role of androgen signaling in Wnt/β-catenin-mediated prostatic differentiation and development.
Figure 2. Expression of Ar in Axin2-expressing cells during early development of mouse prostate.
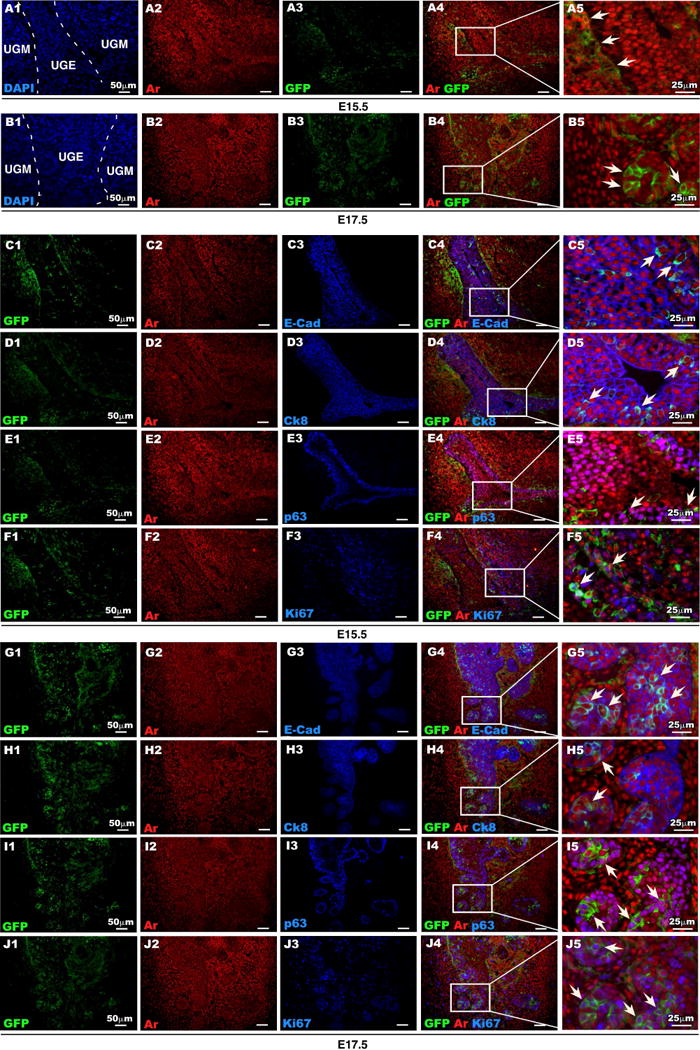
(A-B): Ar (red) and GFP (green) expression in male UGS isolated at E15.5 and E17.5. (C-F): Colocalization of Ar (red) and GFP (green) with E-cad, CK8, p63 or Ki67 (blue) in UGS tissue isolated at E15.5. (G-J): Colocalization of Ar (red) and GFP (green) with E-cad, CK8, p63 or Ki67 (blue) in UGS tissue isolated at E17.5.
Deletion of Ar expression in Axin2 positive cells impairs embryonic prostate development
Currently, it is still unclear how Wnt signaling regulates epithelial cell fate commitment and determination during the course of prostate development and organogenesis. To directly assess the role of the AR in prostatic Wnt-responsive cells during prostate development, maturation, and regeneration, we generated R26RmTmG/+:Arfloxed/Y:Axin2CreERT2/+ (R26RmTmG/+:Arf/Y:Axin2CreERT2/+) compound mice, in which TM-induced Cre activity results in conditional deletion of the Ar and induced expression of GFP in Axin2 expressing cells (Fig. 3A). We did not observe abnormalities in embryonic prostatic development in the above fetuses (data not shown). We then isolated UGS tissues from E14.5 male R26RmTmG/+:Arf/Y:Axin2CreERT2/+ and control R26RmTmG/+:Axin2CreERT2/+ fetuses, and cultured them with 2 μM of (Z)-4-Hydroxytamoxifen (Fig. 3B). After seven days of ex vivo culture, sizes of UGS tissues from R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice appeared smaller than ones from R26RmTmG/+:Axin2CreERT2/+ controls (n=4), respectively. The representative images are shown in Figure 3C1-2 and 3D1-2. Histologically, we observed glandular structures in the center of UGS of R26RmTmG/+:Axin2CreERT2/+ controls, which is very similar to normal prostatic budding formation in UGS in vivo, but not in R26RmTmG/+:Arf/Y:Axin2CreERT2/+ fetuses (Fig. 3E1-4). Analysis of E-cadherin expression in frozen sections revealed more prostatic budding in cultured UGS tissues of R26RmTmG/+:Axin2CreERT2/+ controls than of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ fetuses (Fig. 3F1-4). This provides the first line of evidence that demonstrates the decisive role of Ar in Axin2 expressing UGE cells in regulating prostatic bud formation and early prostatic development. We then preformed co-IF approaches to assess Ar expression in mouse UGE cells. As shown in Fig. 3G1-2 and 3I, almost all of E-cadherin-positive UGE cells are Ar positive in R26RmTmG/+:Axin2CreERT2/+ controls. In contrast, only a few E-cadherin and Ar positive cells were observed in UGE from R26RmTmG/+:Arf/Y:Axin2CreERT2/+ fetuses (Fig. 3G3-4, and 3I). Accordingly, there is a significant reduction of Ki67 positive cells in UGE of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ in comparison with UGE cells of R26RmTmG/+:Axin2CreERT2/+ controls (Fig. 3H1-4 and 3I, Table S2). Taken together, our results show that deletion of Ar expression in Axin2 positive cells at an embryonic stage reduces prostatic epithelial cell proliferation and impairs prostatic gland formation.
Figure 3. Ar is required in Axin2-expressing cells during prostatic bud formation of ex vivo cultured UGS.
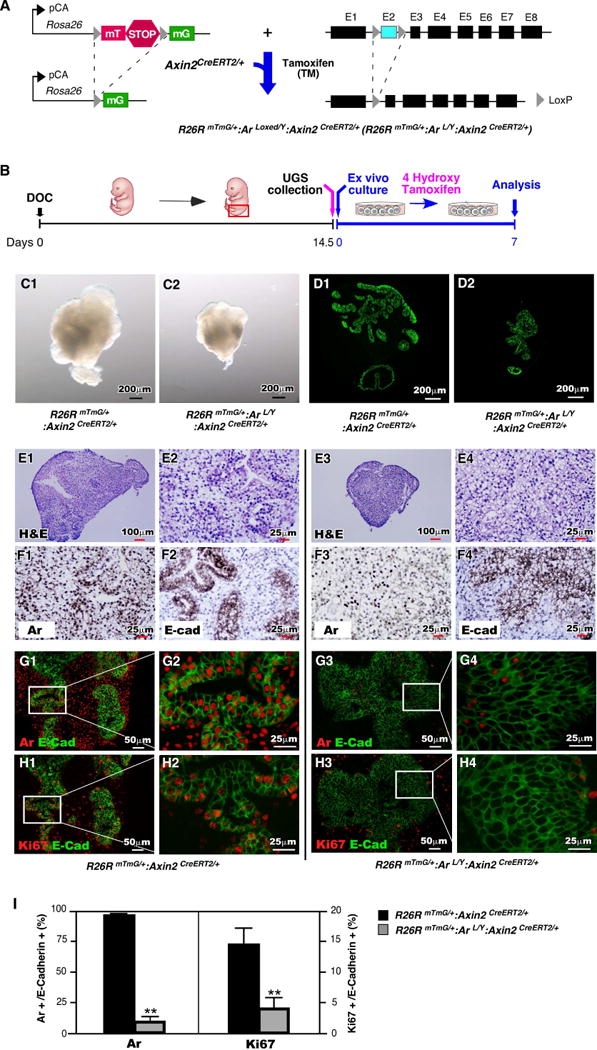
(A): Illustration of floxed alleles present in R26RmTmG/+:Arf/Y+:Axin2CreERT2/+mice. (B): Diagram depicting experimental timeline for ex vivo culture of UGS tissues. (C): Gross images of ex vivo cultured UGS tissues isolated from R26RmTmG/+:Axin2CreERT2/+ or R26RmTmG/+:Arf/Y+:Axin2CreERT2/+ mice. (D): E-cadherin expression and localization on sections of ex vivo cultured UGS tissues. (E): H&E stained sections of ex vivo cultured UGS tissues. (F): Ar or E-cadherin (brown) expression pattern in sections of UGS tissues following 7 days of ex vivo culture. (G-H): Colocalization of E-cadherin (green) with Ar or Ki67 (red) in UGS cultured for 7 days ex vivo. (I): Percentage of Ar-expressing cells or Ki67-expressing cells present in urogenital epithelium. ** P<0.01.
To further assess the role of Ar in Axin2 positive cells during the course of prostatic development, we took another approach by implanting UGS tissues isolated from both R26RmTmG/+:Arf/Y:Axin2CreERT2/+ and R26RmTmG/+:Axin2CreERT2/+ fetuses under kidney capsule (Fig. 4A). After 8 weeks of implantation, UGS grafts isolated from R26RmTmG/+:Arf/Y:Axin2CreERT2/+ were smaller in size and weighed less than ones from R26RmTmG/+:Axin2CreERT2/+ controls, (see the representative images in Fig. 4B1-B2 and 4C). In co-IF experiments, we observed much more AR positive prostatic epithelial cells in the grafts of R26RmTmG/+:Axin2CreERT2/+ controls (Fig. 4E1-2) than those of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice (Fig. 4E3-4). As a consequence, Ar deleted grafts also showed less Ki67 expressing cells than controls (Fig. 4F1-4, Table S3). Intriguingly, we observed smaller and abnormal acini with flattened epithelial cells in prostatic glands of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ grafts (Arrows, Fig. 4H1-4), suggesting an impaired prostate development phenotype. We only observed a minor subset of the GFP-positive epithelial cells appeared flattened. These flattened cells are also immunoreactive to Ck8 but not Ck5 antibody (Supplemental Figure 3), suggesting they are of luminal and not basal origin. These data are consistent with the results of ex vivo UGS cultures and implicate conditional deletion of Ar expression in Axin2-expressing epithelial cells impairs embryonic prostate development.
Figure 4. Deletion of Ar in Axin2 expressing cells during embryonic stage impairs prostate gland formation in renal capsule of SCID mice.
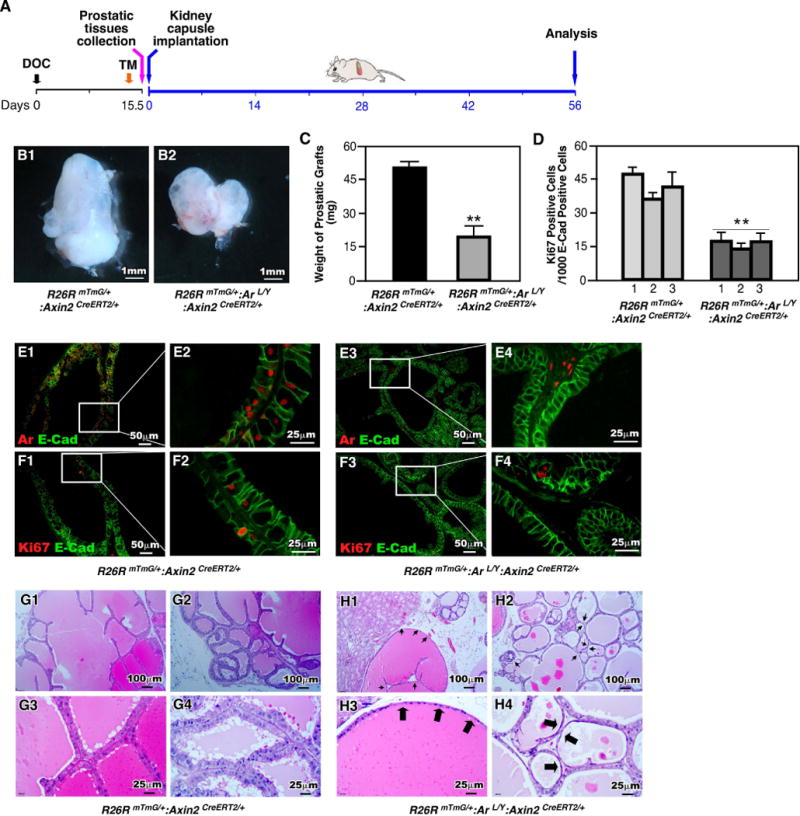
(A): Schematic demonstrating experimental timeline including activation of Axin2CreER, UGS collection, renal capsule transplantation and analysis. (B): Gross images of xenografts from UGS tissue isolated from R26RmTmG/+:Axin2CreERT2/+ or R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice. (C): Graphical representation of xenograft weight from grown from mice of each genotype. ** P<0.01. (D): Quantification of Ki67 positive cells in the epithelial cellsfrom xenografts of each genotype. ** P<0.01. (E-F): Colocalization of E-cadherin (green) with Ar or Ki67 (red) in R26RmTmG/+:Axin2CreERT2/+ and R26RmTmG/+:Arf/Y:Axin2CreERT2/+ xenografts. (G-H): Representative H&E staining of tissue sections from xenografts of each genotype.
Deletion of Ar expression in prepubescent Axin2-expressing cells negatively regulates prostate maturation and morphogenesis
Morphogenesis of the entire prostatic complex in mice is completed between postnatal days 15 to 30 (P15-30) and is tightly regulated by the androgen signaling pathway [4, 5]. Additionally, it has been shown that during this period Axin2-expressing cells expand to form the majority of luminal compartment in the mature prostate [19]. Thus, we next examined the effect of Ar deletion in prepubescent Axin2-expressing cells during expansion of the luminal lineages at puberty. We injected TM into both R26RmTmG/+:Arf/Y:Axin2CreERT2/+ and R26RmTmG/+:Axin2CreERT2/+ mice at postnatal day 14, P14 (Fig. 5A). Analysis of those mice after six weeks, at P56, showed smaller and underdeveloped prostates in mutant R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice in comparison to R26RmTmG/+:Axin2CreERT2/+ controls (Fig. 5B1 and 5B3). Specifically, anterior prostate (AP) and dorsolateral prostate (D/LP) of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ lobes are much smaller in size than those in controls (Fig. 5B2 versus B4, blue and pink arrowheads). The prostate weights of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice are also less than those of R26RmTmG/+:Axin2CreERT2/+ controls (Fig. 5C). Ar expression was significantly reduced in prostatic glands of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice in comparison to those of controls (Fig. 5E1-4). Less Ki67 positive epithelial cells appear in prostatic tissues isolated from R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice than those from R26RmTmG/+:Axin2CreERT2/+ controls (Fig. 5D, and F1-F4, Table S4). As expected, normal prostatic glandular architecture was present in R26RmTmG/+:Axin2CreERT2/+ control mice (Fig. 5G1-6). In contrast, pathological changes featured with scattered foci of marked glandular attenuation was observed in prostatic lobes of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice (arrows, Fig. 5H1-6). IHC analyses showed most of the flattened cells are immune-reactive to Ck8, but not Ar and Ck5 antibody (data not shown). These lines of evidence demonstrate that conditional deletion of Ar expression in prepubescent Axin2-expressing cells impairs prostate maturation and morphogenesis.
Figure 5. Ar is required in prepubescent Axin2-expressing cells for prostate development.
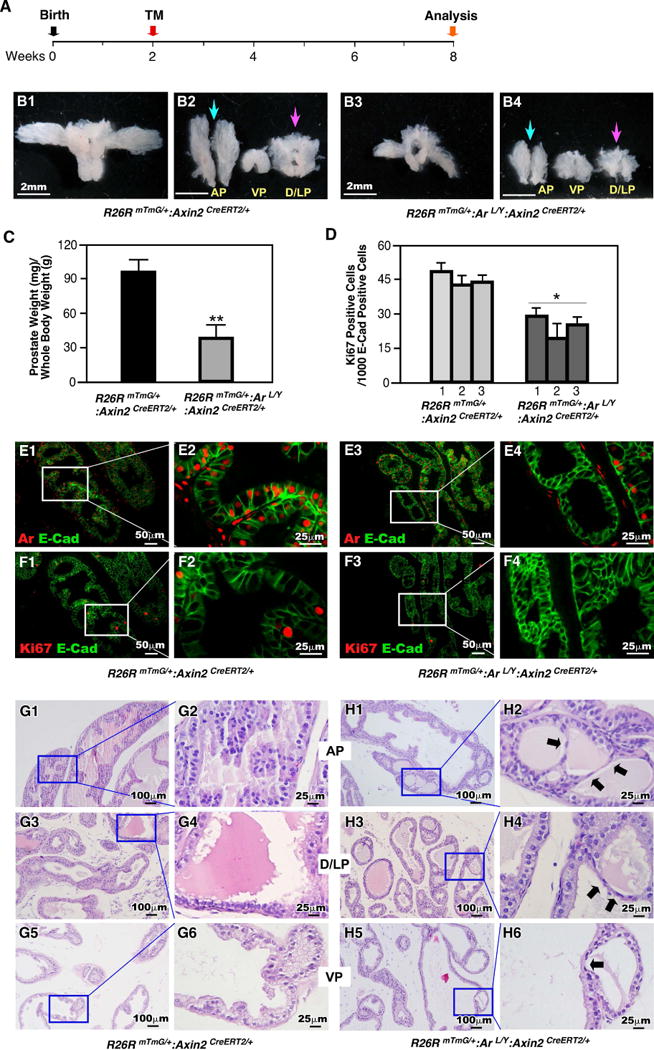
(A): Illustration depicting experimental timeline of Axin2CreER activation (TM injection) and tissue analysis. (B): Gross images of prostates isolated at P56 from R26RmTmG/+:Axin2CreERT2/+ or R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice. (C): Graphical representation of the ratio of prostate wet weight/body weight. ** P<0.01. (D): Quantification of Ki67 positive cells per 1000 epithelial cells (E-cadherin positive) from prostate tissues of each genotype. * P<0.05. (E-F): Colocalization of E-cadherin (green) with Ar or Ki67 (red) in prostates isolated from R26RmTmG/+:Axin2CreERT2/+ or R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice. (G-H): H&E staining of tissue sections from prostates of each genotype.
The regenerative ability of Axin2 expression cells relies on Ar-mediated signaling events
Axin2 expressing prostatic epithelial cells have been shown to possess luminal cell properties and have ability to generate prostatic luminal cells [19]. To explore the role of the Ar in Axin2 expressing cells, we assessed the ability of castration-resistant Axin2 expressing cells in prostate regeneration. Both R26RmTmG/+:Arf/Y:Axin2CreERT2/+ and R26RmTmG/+:Axin2CreERT2/+ mice were castrated at 8 weeks, administered TM at 12 weeks, and then analyzed at 13 weeks of age (Fig. 6A). Histological analyses showed regressed glands in all prostatic lobes of castrated mice in both genotypes (data not shown). Membrane GFP (mGFP) positive, Axin2 expressing cells, were present in all prostatic lobes of both R26RmTmG/+:Arf/Y:Axin2CreERT2/+ and R26RmTmG/+:Axin2CreERT2/+ mice (Fig. 6B1-3 and D1-3). We observed that most mGFP positive Axin2-expressing cells are immunoreactive to Ck8 antibody as we reported earlier [19]. Following androgen supplementation we tracked the fate of these castration-resistant Axin2 expressing cells through androgen-mediated prostatic regeneration (Fig. 6A). After regeneration, a significant increase of mGFP-positive luminal cells was observed in prostatic lobes in R26RmTmG/+:Axin2CreERT2/+ mice (Fig. 6B1-3, 6C1-3, and 6F). In contrast, only slight expansion of mGFP-positive cells revealed in prostatic tissues of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice (Fig. 6D1-3, E1-3, and 6F, Table S5). Interestingly, we noticed that the majority of mGFP positive cells in regenerated prostatic tissues of R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice showed Ar positive staining, indicating the requirement of Ar in Axin2 expressing cell mediated regeneration (Arrowheads, Fig. 6G4). Thus, these lines of evidence demonstrate that deletion of Ar expression in Axin2 expressing cells significantly reduces the regenerative ability of those Wnt/β-catenin-responsive cells.
Figure 6. Ar is required for the regenerative potential of castration-resistant Axin2-expressing cells.
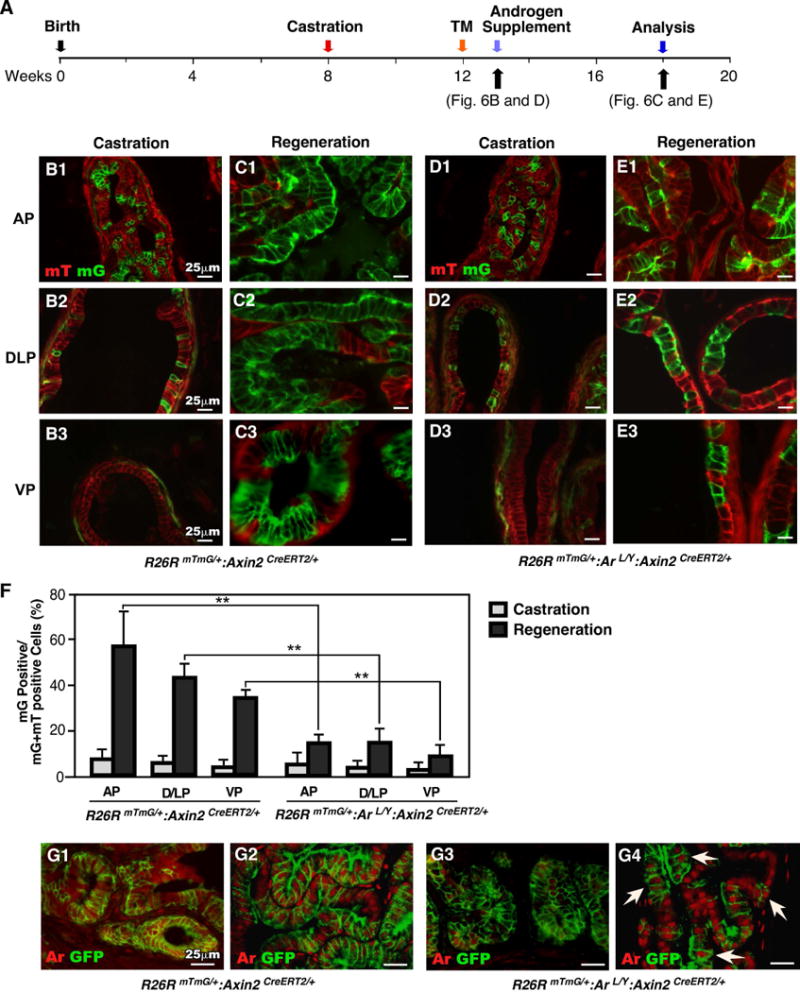
(A): Diagram of experimental design describing timelines for castration, activation of Axin2CreER (TM injection), androgen supplementation (regeneration) and analysis. (B-E): mTmG analysis of both castrated and regenerated prostate tissues from mice of different genotypes. (F): Graphical quantification of the ratio of mG positive cells per total cells in different lobes of both castrated and regenerated prostates. ** P<0.01. (G) Colocalization of GFP (green) and Ar (red) in frozen sections of both castrated and regenerated prostate tissues from R26RmTmG/+:Axin2CreERT2/+ and R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice.
DISCUSSION
Mouse prostate develops from the UGS at embryonic day 16.5-17.5. During this short period, the cells in epithelial layer outgrow into surrounding mesenchyme to form prostatic buds [4]. The process of prostatic bud formation requires interaction between the UGE and the UGM. It has been demonstrated that androgen signaling is essential for prostate early development. Androgen actions upon UGS are mediated solely through the AR. Mutation of the Ar in Tfm mice results in a complete absence of prostate development, demonstrating the indispensable role of the Ar in prostate development [30]. Canonical Wnt signaling pathways that are mainly mediated through β-catenin play a critical role in prostate development. Deletion of β-catenin in the UGE impairs prostatic bud formation in vivo and ex vivo [9, 10, 12]. Expression of Axin2, a direct downstream target of Wnt signaling, occurs at E15.5 and was induced by the presence of androgen within the UGE [10]. Emerging evidence has shown that Axin2 expressing cells function as Wnt responsive cells that possess progenitor properties during the course of prostate development and regeneration [19]. In this study, we directly addressed the biological significance of the AR in Axin2-expressing cells in regulating prostatic cell fate commitment and differentiation during prostate development, maturation, and regeneration.
Earlier studies have demonstrated Ar expression in the UGM during prostatic buds formation as early as E17 [29, 31]. Using both IHC and IF approaches, we detected extensive Ar expression in both UGE and UGM at or before E15.5, before bud formation. Our observation is consistent with the recent study that demonstrated Ar in the UGE at E14 [32]. Nuclear staining of Ar in both the UGE and UGM further indicates androgen-mediated nuclear translocation of Ar in UGS tissues. Intriguingly, we observed co-expression of Ar and Axin2 in UGE starting at E15.5, suggesting a possible crosstalk between androgen/Ar and wnt/β-catenin signaling pathways during early prostate development. In addition, both Axin2 activated GFP expression and Ar expression were co-expressed in newly formed prostatic buds at E17.5. These data provide the first line of evidence demonstrating that both androgen and Wnt signaling pathways are active in prostatic bud formation. To directly assess the role of Ar in Axin2 expressing cells, we generated R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice, which exhibited conditional deletion of Ar and simultaneous expression of GFP in Wnt responsive cells. We did not observe notable defects in prostatic development in the above mice when TM was administrated at E13.5. However, deletion of Ar expression in Axin2 expressing cells elicited impaired prostatic bud formation in both ex vivo culture and kidney capsule transplantation experiments. Using co-IF approaches, we further demonstrated a link between the deletion of Ar expression in Axin2-expressing cells and the above defect phenotypes. Several factors may contribute to the discrepancy that we observed in vivo versus ex vivo and implantation experiments. For example, the dosage of TM used to inject R26RmTmG/+:Arf/Y:Axin2CreERT2/+ female mice is relatively lower that the one that was used in the ex vivo culture approaches. Thus, we observed much greater recombination effects from the later than the former. Moreover, additional factors may exist in vivo and be able to compensate the effect of Ar deletion in a physiological context.
Mouse prostatic ductal branching morphogenesis starts through the interaction between prostatic epithelial buds and mesenchymal pads [33], which is completed between postnatal days 15 and 30 (P15-30) [25]. During puberty between P25-40, circulating androgens levels raise, which further promotes prostate growth and maturation [6, 34]. It has been shown that the prepubescent Axin2 expressing epithelial cells have prostatic progenitor capabilities [19]. Thus, we injected TM at P14 to selectively delete Ar expression in prepubescent Axin2 expressing cells. Both the size and weight of prostates isolated from R26RmTmG/+:Arf/Y:Axin2CreERT2/+ and Arf/Y:Axin2CreERT2/+ only mice were significantly reduced as compared to their normal counterparts. The lack or reduced expression of both the Ar and Ki67 in underdeveloped prostate tissues further implicates an important role of Ar in Axin2 expressing cells in prostate maturation and morphogenesis during the period of pre-puberty. The function of the Ar in prostatic luminal cells has been assessed previously with different promoter-driven Cre strains [35–37]. Disruption of AR signaling in prostatic luminal cells appears to damage normal cellular barriers, increase apoptosis, and impair cellular maturity, which further induces inflammation and cell proliferation [35–37]. In this study, we observed prostatic atrophy and reduced cell proliferation when Ar expression was deleted in R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice. Specifically, flattened epithelial cells appeared in prostate glands of the above mice. IHC analyses showed that these cells arise from Ar-native Axin2-expressing cells and possess luminal characteristics, such as CK8 expression. While we did not observe any overt effect on the secretory functions of these cells, further study is needed to characterize the biological significance of these flattened luminal cells. Given the fact that prostatic luminal Axin2 expressing cells possess prostatic stem/progenitor properties, our data are consistent with the previous studies, and suggest that Ar expression in prepubescent Axin2 expressing cells is important for mouse prostate maturation and morphogenesis.
Prostatic stem/progenitor cells in adult mice are able to survive androgen withdrawal and to regenerate upon androgen supplementation [38]. It has been shown that a sub-population of Axin2-expressing cells are resistant to castration and able to regenerate to prostatic luminal cells upon androgen administration [19]. In this study, we performed a series of experiments to determine the role of Ar in Axin2 expressing cells during the course of prostate regeneration. We observed the existence of castration-resistant Axin2-expressing cells in all four prostatic lobes of both R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice and R26RmTmG/+:Axin2CreERT2/+ controls. In the presence of androgens, those cells are able to expand and regenerate prostatic luminal cells in R26RmTmG/+:Axin2CreERT2/+ controls, demonstrating their regenerative ability as prostatic progenitor epithelial cells. However, deletion of Ar expression in Axin2 expressing cells reduced and eliminated the regenerative ability in R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice. These data suggest an indispensable role of the Ar in Axin2-expressing cells in response to androgen mediated prostate regeneration. In this study, we have noted that the both mGFP expression and Ar deletion do not always simultaneously occur in TM-induced Axin2-expressing cells of the regenerative prostate tissues in R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice. Although we do not know the precise reasons for the mosaic Ar expression in the experiments, it is likely resulted by incomplete Cre-mediated recombination. Further studies are needed to further investigate the cellular identity of the Ar-positive Axin2-expressing cells in R26RmTmG/+:Arf/Y:Axin2CreERT2/+ mice. In addition, high dosages of TM or other systems may be used to fully delete the Ar in Axin2 expressing cells to better determine the effect of Ar in prostate regeneration.
Prostatic Axin2-expressing cells respond to Wnt/β-catenin signaling, possess progenitor properties, and are able to expand the luminal epithelial cell lineage during prostatic development, maturation, and regeneration. They can also survive androgen withdrawal and regenerate prostatic luminal epithelial cells following androgen replacement. As detailed in this study, we provide several lines of experimental evidence demonstrating an indispensable role of Ar in the cellular events regulated through Axin2-expressing cells. In addition, the results also suggest a new molecular mechanism for androgen signaling to regulate Wnt/β-catenin mediated prostate cell fate commitment and differentiation.
CONCLUSIONS
In this study, we demonstrated that deletion of Ar in embryonic Axin2-expressing cells impaired early prostate development in both ex vivo and tissue implantation experiments. We also shown that when Ar expression was deleted in prostatic Axin2 expressing cells at mouse pre-puberty, it results in smaller and underdeveloped prostates. Moreover, we observed that a subpopulation of Axin2 expressing prostatic epithelial cells is resistant to castration and, then following androgen supplementation, is capable to expand to prostatic luminal cells. Deletion of Ar in these Axin2-expressing cells reduces their regenerative ability. Our data indicate an indispensable role for the Ar in Wnt-responsive cells during the course of prostate development, morphogenesis, and regeneration.
Supplementary Material
Figure S1. Expression of Wnt downstream target genes in Axin2-expressing cells during early development of mouse prostate. (A) Colocalization of LEF-1 (red) and GFP (green) in UGS tissue isolated at E15.5. (B) Colocalization of CyclinD1 (red) and GFP (green) in UGS tissue isolated at E17.5.
Figure S2. Cellular properties of Ar-expressing cells in UGS at embryonic stage. (A): Colocalization of Ar (red) and CK8, p63 or Ki67 (green) in male UGS tissues isolated on E15.5. (B): Colocalization of Ar (red) and CK8, p63 or Ki67 (green) in male UGS tissues isolated on E17.5. Scale bars = 25μm.
Figure S3. Cellular properties of GFP-expressing cells in grafted prostatic tissues. (A) GFP (brown) expression in grafted prostatic tissues. (B) Ck8 (red) and Ck5 (green) expression in grafted prostatic tissues. Scale bars: 25μm.
SIGNIFICANT STATEMENT.
Androgen and Wnt signaling pathways are essential for prostate development and tumorigenesis. However, the molecular mechanisms underlying these pathways in regulating prostate development, maturation, and regeneration remains largely unexplored. In this study, we developed a series of novel and biologically relevant mouse models to assess the biological role of Ar in Wnt responsive cells at different stages during mouse prostate development. Using these mouse models, we demonstrated for the first time that Ar expression is indispensable in Axin2-expressing cells during prostate development and regeneration. Deletion of Ar in Axin2-expressing cells at embryonic or pre-pubescent stage impairs prostate early development and results in underdeveloped prostate, respectively. When Ar was deleted in castration-resistant Axin2-expressing cells, the regenerative ability of Axin2-expressing cells was decreased significantly.
Acknowledgments
This work was supported by NIH grants R01CA070297, R01CA151623, R01CA166894, R21CA190021, and R01DK104941
Footnotes
Author contributions: Yongfeng He: Conception and design, collection and assembly of data, and manuscript writing
Erika Hooker: Data analysis and interpretation and manuscript writing
Eun-Jeong Yu: Data analysis and interpretation
Huiqing Wu: Data analysis and interpretation
Gerald R. Cunha: Data analysis and interpretation and manuscript writing
Zijie Sun: Conception and design, collection and assembly of data, manuscript writing, and final approval of manuscript
DISCLOSURE OF POTENTIAL CONFLICTS of INTEREST: The authors indicate no potential conflicts of interests.
References
- 1.Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–138. doi: 10.1016/s0090-4295(02)01593-5. discussion 138-139. [DOI] [PubMed] [Google Scholar]
- 2.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Zhou ZX, Wong CI, Sar M, et al. The androgen receptor: an overview. Recent Progress Hormone Research. 1994;49:249–274. doi: 10.1016/b978-0-12-571149-4.50017-9. [DOI] [PubMed] [Google Scholar]
- 4.Cunha GR, Donjacour AA, Cooke PS, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 5.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi N, Sugimura Y, Kawamura J, et al. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod. 1991;45:308–321. doi: 10.1095/biolreprod45.2.308. [DOI] [PubMed] [Google Scholar]
- 7.Cunha GR, Donjacour AA, Sugimara Y. Stromal-epithelial interactions and heterogeneity of proliferative activity within the prostate. Biochem Cell Biol. 1986;64:608–614. doi: 10.1139/o86-084. [DOI] [PubMed] [Google Scholar]
- 8.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis JC, Thomsen MK, Taketo MM, et al. beta-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet. 2013;9:e1003180. doi: 10.1371/journal.pgen.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons BW, Hurley PJ, Huang Z, et al. Wnt signaling though beta-catenin is required for prostate lineage specification. Dev Biol. 2012;371:246–255. doi: 10.1016/j.ydbio.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ontiveros CS, Salm SN, Wilson EL. Axin2 expression identifies progenitor cells in the murine prostate. Prostate. 2008;68:1263–1272. doi: 10.1002/pros.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta V, Schmitz CT, Keil KP, et al. Beta-catenin (CTNNB1) induces Bmp expression in urogenital sinus epithelium and participates in prostatic bud initiation and patterning. Dev Biol. 2013;376:125–135. doi: 10.1016/j.ydbio.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan D, Wiesmann M, Rohan M, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jho EH, Zhang T, Domon C, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowman AN, van Amerongen R, Palmer TD, et al. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/beta-catenin-responsive neural stem cells. Proc Natl Acad Sci U S A. 2013;110:7324–7329. doi: 10.1073/pnas.1305411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardehali R, Ali SR, Inlay MA, et al. Prospective isolation of human embryonic stem cell-derived cardiovascular progenitors that integrate into human fetal heart tissue. Proc Natl Acad Sci U S A. 2013;110:3405–3410. doi: 10.1073/pnas.1220832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blauwkamp TA, Nigam S, Ardehali R, et al. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Johnson DT, Luong R, et al. Wnt/beta-Catenin-Responsive Cells in Prostatic Development and Regeneration. Stem Cells. 2015;33:3356–3367. doi: 10.1002/stem.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulholland DJ, Cheng H, Reid K, et al. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem. 2002;277:17933–17943. doi: 10.1074/jbc.M200135200. [DOI] [PubMed] [Google Scholar]
- 21.Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- 22.Yang F, Li X, Sharma M, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277:11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 23.Muzumdar MD, Tasic B, Miyamichi K, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 24.De Gendt K, Swinnen JV, Saunders PT, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugimura Y, Cunha GR, Bigsby RM. Androgenic induction of DNA synthesis in prostatic glands induced in the urothelium of testicular feminized (Tfm/Y) mice. Prostate. 1986;9:217–225. doi: 10.1002/pros.2990090302. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DT, Luong R, Lee SH, et al. Deletion of leucine zipper tumor suppressor 2 (lzts2) increases susceptibility to tumor development. J Biol Chem. 2013;288:3727–3738. doi: 10.1074/jbc.M112.417568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu C, Luong R, Zhuo M, et al. Conditional expression of the androgen receptor induces oncogenic transformation of the mouse prostate. J Biol Chem. 2011;286:33478–33488. doi: 10.1074/jbc.M111.269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda H, Chang C. Immunohistochemical and in-situ hybridization analysis of androgen receptor expression during the development of the mouse prostate gland. J Endocrinol. 1991;129:83–89. doi: 10.1677/joe.0.1290083. [DOI] [PubMed] [Google Scholar]
- 29.Shannon JM, Cunha GR. Autoradiographic localization of androgen binding in the developing mouse prostate. Prostate. 1983;4:367–373. doi: 10.1002/pros.2990040406. [DOI] [PubMed] [Google Scholar]
- 30.Cunha GR, Chung LW. Stromal-epithelial interactions-I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- 31.Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991;128:2867–2873. doi: 10.1210/endo-128-6-2867. [DOI] [PubMed] [Google Scholar]
- 32.Keil KP, Abler LL, Laporta J, et al. Androgen receptor DNA methylation regulates the timing and androgen sensitivity of mouse prostate ductal development. Dev Biol. 2014;396:237–245. doi: 10.1016/j.ydbio.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson AA, Timms BG, Barton L, et al. The role of smooth muscle in regulating prostatic induction. Development. 2002;129:1905–1912. doi: 10.1242/dev.129.8.1905. [DOI] [PubMed] [Google Scholar]
- 34.Staack A, Donjacour AA, Brody J, et al. Mouse urogenital development: a practical approach. Differentiation. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- 35.Simanainen U, Allan CM, Lim P, et al. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007;148:2264–2272. doi: 10.1210/en.2006-1223. [DOI] [PubMed] [Google Scholar]
- 36.Wu CT, Altuwaijri S, Ricke WA, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Kwon OJ, Henry G, et al. Non-Cell-Autonomous Regulation of Prostate Epithelial Homeostasis by Androgen Receptor. Mol Cell. 2016;63:976–989. doi: 10.1016/j.molcel.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of Wnt downstream target genes in Axin2-expressing cells during early development of mouse prostate. (A) Colocalization of LEF-1 (red) and GFP (green) in UGS tissue isolated at E15.5. (B) Colocalization of CyclinD1 (red) and GFP (green) in UGS tissue isolated at E17.5.
Figure S2. Cellular properties of Ar-expressing cells in UGS at embryonic stage. (A): Colocalization of Ar (red) and CK8, p63 or Ki67 (green) in male UGS tissues isolated on E15.5. (B): Colocalization of Ar (red) and CK8, p63 or Ki67 (green) in male UGS tissues isolated on E17.5. Scale bars = 25μm.
Figure S3. Cellular properties of GFP-expressing cells in grafted prostatic tissues. (A) GFP (brown) expression in grafted prostatic tissues. (B) Ck8 (red) and Ck5 (green) expression in grafted prostatic tissues. Scale bars: 25μm.


