Abstract
Minimal residual disease (MRD) after initial therapy is integral to risk stratification in B- and T-precursor acute lymphoblastic leukemia (B-ALL, T-ALL). While MRD determines depth of remission, remission remains defined by morphology. We determined the outcomes of children with discordant assessments of remission by morphology vs. flow cytometry using patients age 1–30.99 years enrolled on Children’s Oncology Group ALL trials who underwent bone marrow assessment at the end of induction (N=9 350). Morphologic response was assessed locally as M1 (<5% lymphoblasts; remission), M2 (5–25%), or M3 (>25%). MRD was centrally measured by flow cytometry. 19.8% of patients with M2/M3 morphology had MRD<5%. M1 with MRD≥5% was less common in B-ALL (0.9%) than T-ALL (6.9%; p<0.0001). In B-ALL, M1/MRD≥5% was associated with superior 5-year event-free survival (EFS) than M2/MRD≥5% (59.1%±6.5% vs. 39.1%±7.9%; p=0.009), but was inferior to M1/MRD<5% (87.1%±0.4%; p<0.0001). MRD levels were higher in M2/MRD≥5% than M1/MRD≥5% patients. In T-ALL, EFS was not significantly different between M1/MRD≥5% and M2/MRD ≥5%. Patients with morphologic remission but MRD ≥5% have outcomes similar to those who fail to achieve morphological remission, and significantly inferior to those with M1 marrows and concordant MRD, suggesting that flow cytometry should augment the definition of remission in ALL.
INTRODUCTION
Cure rates in childhood acute lymphoblastic leukemia (ALL) have dramatically improved, in part due to increasingly comprehensive risk stratification and consequent assignment of therapy.1 The presence of bone marrow (BM) minimal residual disease (MRD) after initial leukemia therapy has proven to be the single strongest adverse prognosticator.2–7 MRD results are therefore a critical component of modern risk stratification. Intensification of therapy for patients with positive MRD improves outcomes for this population.8
While MRD, measured by either flow cytometry or PCR-based techniques, is used to assess the depth of remission, remission itself continues to be defined by morphology (<5% lymphoblasts) both in clinical practice and in clinical trials.9 This is despite known difficulty in morphologically distinguishing malignant lymphoblasts from non-malignant regenerating cells (hematogones) in BM after chemotherapy exposure.10, 11 Individual cases of discordance between remission status as assessed by morphology versus by flow cytometry present challenges to treating physicians and to the conduct of clinical trials.
In a recent report from the UKALL 2003 trial, patients in morphological remission at the end of Induction therapy (EOI), but with PCR-based MRD indicating ≥5% residual lymphoblasts had poor outcomes, similar to those of patients with morphologic induction failure (M2/M3).12 Based on these findings, the UK group has proposed a new definition of induction failure: ≥5% residual blasts, measured by either morphology or PCR. Their findings have not yet been verified in multi-site cooperative group studies using different treatments and different MRD measurement techniques.
The objective of this study was therefore to determine the outcome of children, adolescents, and young adults with discordant assessments of remission by morphology versus by flow cytometry at the EOI. In doing so, we also aimed to determine the extent to which morphologic assessment of remission contributes to risk assessment in modern ALL risk stratification.
MATERIALS AND METHODS
Between 2004 and 2014, children, adolescents, and young adults with newly diagnosed ALL were enrolled onto one of several Children’s Oncology Group (COG) clinical trials for newly diagnosed ALL. After enrollment on a common classification protocol, AALL03B1, patients enrolled on AALL0331 [NCI standard risk (SR) B-lymphoblastic leukemia (B-ALL), age >1 year and <10 years and initial WBC <50,000/µL; 2005–2010], AALL0232 [NCI high risk (HR) B-ALL, age 10–30 years or initial WBC ≥50,000/µL and any age; 2004–2011], or AALL0434 [T lymphoblastic leukemia (T-ALL), age 1–30 years; 2007–2014]. All patients who were evaluable for morphological and MRD response assessment at the EOI were included in this study. All studies were approved by the NCI and by the institutional review board of each participating center. Participating patients and/or a parent or guardian provided informed consent. Details of each clinical trial, including chemotherapy regimens and randomized treatment interventions are described elsewhere, and/or have been previously published (AALL03B1 – NCT00482352; AALL08B1 – NCT01142427; AALL0331 – NCT00103285; AALL0232 – NCT00075725; AALL0434 – NCT00408005).3, 13, 14 Induction therapy with either three (AALL0331) or four drugs (AALL0232, AALL0434) was followed by post induction therapy, the intensity of which was determined by risk status, which included early response measures.15
Morphologic assessments of EOI BM aspirates were performed by local institutions without central review and categorized as M1 (<5% lymphoblasts) vs. M2 (5–25%) vs. M3 (>25%). COG protocols did not mandate specific techniques for either conducting bone marrow aspirates or evaluating morphology, leaving this to local clinical practice. Throughout the study period, flow cytometric assessment of MRD for patients with B-ALL was measured at one of two central reference laboratories using 6-color flow according to a previously described methodology.3 Samples were stained with 2 different 6-color antibody combinations (CD20-FITC/CD10-PE/CD38-PerCPCy5.5/CD58-APC/CD19-PECy7/CD45-APCH7 and CD9/CD13/33/CD34/CD10/CD19/CD45). A third tube contained SYTO-16 to identify all nucleated cells. CD19 in this third tube was used to express B cells as a percent of all nucleated cells; MRD identified in either of the two test tubes was expressed as a percent of B cells and the third tube used to calculate MRD as a percent of nucleated cells. Mononuclear cells were estimated on a display of CD45/SSC to exclude granulocytes; MRD was ultimately expressed as a percentage of mononuclear cells. For patients with T-ALL, flow cytometric assessment was performed at a single site using a previously described methodology modified by replacement of CD48 for CD2 and use of denominator tube containing a nucleic acid binding dye to allow direct enumeration of nucleated cells.16, 17
SR and HR B-ALL patients with EOI M3 BM were considered induction failures and they were taken off protocol therapy post-induction. An M3 marrow at EOI was considered to be an event in primary analyses. Patients with either flow indicating ≥5% blasts (flow ≥5%) or M2 morphology at the EOI were assigned to receive 2 further weeks of extended induction therapy. After extended induction, if there was persistent MRD ≥1% or M2/M3 morphology, patients were taken off protocol therapy and also considered an induction failure/event in primary analyses. In contrast, patients with T-ALL on AALL0434 were eligible to continue on protocol therapy regardless of marrow morphology or MRD at EOI. Patients with M2/M3 marrows at EOI were non-randomly assigned to a treatment arm that included nelarabine, and were only taken off protocol therapy if they had persistent M2/M3 morphological disease at the end of consolidation (about week 13 of therapy).18
Possible prognostic variables included age at diagnosis, gender, presenting WBC, and lineage (B-ALL vs. T-ALL). Among patients with B-ALL, favorable cytogenetics included ETV6-RUNX1 fusion, or simultaneous trisomies of chromosomes 4, 10 and 17, while unfavorable cytogenetics included hypodiploidy with modal chromosome number <44, intrachromosomal amplification of chromosome 21 (iAMP21), KMT2A (MLL) rearrangements, and BCR-ABL1 fusion.3, 15, 19 The remainder of B-ALL patients were classified as having neutral cytogenetics. T-ALL patients were categorized as having an early thymocyte precursor (ETP) phenotype if the immunophenotype showed absence of CD1a and CD8, positive for one or more myeloid/stem cell marker (CD34, CD117, HLADR, CD13, CD33), and negative or weak (<75%) expression of CD5 in a central reference laboratory.20
Statistical Methods
Proportions were compared between groups using the chi-squared test. Predictors of discordant marrows were determined using multivariate logistic regression analysis; variables significant at the p<0.1 level in univariate analyses were included in the multivariate model. Event-free survival (EFS) was defined as time from study enrolment to first event (relapse, remission death, or the development of a second malignant neoplasm) or last follow-up in subjects who were event-free. Overall survival (OS) was defined as the time from study enrolment to death from any cause or date of last follow-up if alive. Induction failure was defined as an M3 marrow at EOI; analyses involving such patients were restricted to OS given a lack of data on second events. Survival rates were estimated using the Kaplan-Meier method with standard errors of Peto et al.21, 22 Survival curves were compared using the log-rank test. Exact MRD levels were compared between groups using the Mann-Whitney U. Cox proportional-hazards models were used for multivariate analysis of outcomes; in these models, MRD was treated as a continuous variable but log-transformed given its skewed distribution. Given the objective of determining the prognostic impact of increasing levels of MRD (as a continuous variable), multivariate models of outcome were restricted to patients with a significant burden of MRD (>0.1%). All analyses were performed using SAS® software version 9.4; SAS Institute, Cary, NC). All graphics were generated using R (http://www.R-project.org, version 2.13.1).
RESULTS
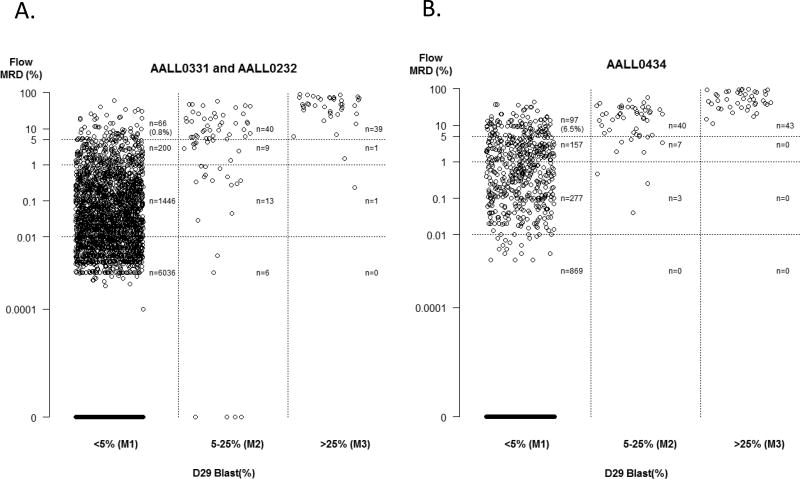
This report includes study data current as of December 31st 2015. Demographic characteristics of the study cohort, comprising 9 350 patients, are shown in Table 1. In total, 84.0% of patients had B-ALL (HR 30.0% and SR 54.0%) and 16.0% had T-ALL. EOI BM morphology and MRD results are shown in Table 2 and illustrated graphically in Figure 1. Morphology and flow results were concordant in the vast majority of children (N=9 111; 97.4%). 2 of 84 patients (2.4%) with M3 morphology had discordantly low MRD (i.e. <5%), while 38/118 (32.2%) with M2 morphology had discordantly low MRD. In total, of 202 patients with M2/M3 morphology, 40 (19.8%) had MRD <5%. Discordantly high flow cytometry results indicating a failure to achieve remission (i.e. blasts ≥5%) in the setting of M1 morphology was seen more frequently, and was significantly more common in patients with T-ALL as compared to B-ALL [97/1,493 (6.9%) vs. 66/7,857 (0.9%); p <0.0001].
Table 1.
Demographic characteristics of study cohort (N=9 350)
| N (%) | |
|---|---|
| Age at diagnosis | |
| <10 years | 6 797 (72.7) |
| ≥10 years | 2 553 (27.3) |
| Gender | |
| Male | 5 381 (57.6) |
| Female | 3 969 (42.4) |
| WBC at presentation | |
| <50 | 7 268 (77.7) |
| ≥50 | 2 082 (22.3) |
| Lineage | |
| B-ALL | 7 857 (84.0) |
| T-ALL | 1 493 (16.0) |
| CNS status | |
| CNS1 | 8 030 (85.9) |
| CNS2 | 1 083 (11.6) |
| CNS3 | 223 (2.4) |
| Therapeutic study | |
| AALL0331 | 5 049 (54.0) |
| AALL0232 | 2 808 (30.0) |
| AALL0434 | 1 493 (16.0) |
| Cytogenetics (B-ALL only) | |
| Favorable | 3 621 (46.1) |
| Neutral | 3 637 (46.3) |
| Unfavorable | 490 (6.2) |
| ETP (T-ALL only) | |
| Yes | 136 (9.1) |
| No | 862 (71.1) |
NS – central nervous system; ETP – early thymocyte precursor; WBC – white blood cell
Table 2.
Morphology and minimal residual disease assessments of end of Induction bone marrows, N(%)
| MRD - B-ALL | MRD – T-ALL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <5% | 5–<25% | ≥25% | Total | <5% | 5–<25% | ≥25% | Total | ||
| Morphology | M1 | 7 682 (97.8) | 59 (0.8) | 7 (0.1) | 7 748 (98.6) | 1 303 (87.3) | 88 (5.9) | 9 (0.6) | 1 400 (93.8) |
| M2 | 28 (0.4) | 29 (0.4) | 11 (0.1) | 68 (0.9) | 10 (0.7) | 25 (1.7) | 15 (1.0) | 50 (3.3) | |
| M3 | 2 (0.0) | 5 (0.1) | 34 (0.4) | 41 (0.5) | 0 (0.0) | 5 (0.3) | 38 (2.5) | 43 (2.9) | |
| Total | 7 712 (98.2) | 93 (1.2) | 52 (0.7) | 7 857 (100) | 1 313 (87.9) | 118 (7.9) | 62 (4.2) | 1 493 (100) | |
ALL – acute lymphoblastic leukemia; MRD – minimal residual disease
Figure 1.
Morphology and minimal residual disease assessments of end of Induction bone marrows in children with A. B-precursor and B. T-precursor acute lymphoblastic leukemia. MRD – minimal residual disease
M1 Morphology with High MRD
Among patients with B-ALL and M1 morphology, age ≥10 years, initial WBC ≥50,000/µL, and either neutral or unfavorable cytogenetics were significantly associated with an increased risk of discordantly high MRD ≥5% in multivariable analysis (Table 3) despite having an M1 marrow. Among patients with T-ALL and M1 morphology, only those with an ETP phenotype were at significantly higher risk of discordantly high MRD. Of 217 participating study sites, the median patient enrolment per site was 33 (interquartile range 17–57). The incidence of discordantly high MRD did not differ significantly between low and high enrolment sites, whether defined by median or 25th percentile enrolment thresholds (data not shown).
Table 3.
Predicators of discordant MRD (≥5%) among patients with M1 end of induction bone marrows
| B-ALL | T-ALL | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| OR (95thCI) | P value | OR (95thCI) | P value | OR (95thCI) | P value | OR (95thCI) | P value | |
| Age at diagnosis | ||||||||
| <10 years | Ref | <0.0001 | Ref | 0.03 | Ref | 0.74 | - | - |
| ≥10 years | 3.1 (1.9–5.1) | 1.7 (1.1–2.8) | 1.1 (0.7–1.6) | - | - | |||
| Sex | ||||||||
| Male | Ref | 0.13 | - | - | Ref | 0.34 | - | - |
| Female | 0.7 (0.4–1.1) | - | - | 1.3 (0.8–2.0) | - | - | ||
| WBC at presentation | ||||||||
| <50 | Ref | <0.0001 | Ref | 0.005 | Ref | 0.47 | - | - |
| ≥50 | 3.1 (1.8–5.1) | 2.1 (1.3–3.6) | 0.9 (0.6–1.3) | - | - | |||
| Cytogenetics | ||||||||
| Favorable | Ref | Ref | ||||||
| Neutral | 15 (4.7–49) | 0.02 | 12 (3.6–39) | <0.0001 | ||||
| Unfavorable | 46 (14–157) | <0.0001 | 31 (8.9–109) | <0.0001 | ||||
| ETP | ||||||||
| No | n/a | n/a | n/a | n/a | Ref | <0.0001 | Ref | <0.0001 |
| Yes | n/a | n/a | n/a | n/a | 4.7 (2.7–8.1) | 4.7 (2.7–8.1) | ||
ALL – acute lymphoblastic leukemia; CI – confidence interval; ETP – early thymocyte precursor; OR – odds ratio; WBC – white blood cell
Bolded values are statistically significant at the p<0.05 level
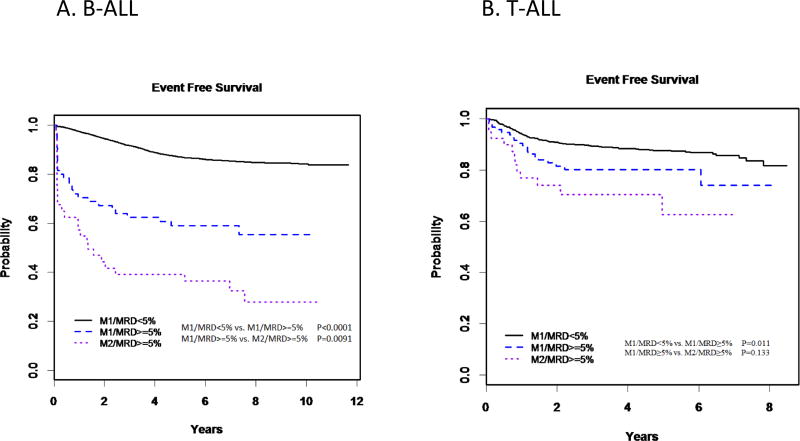
In children with B-ALL, those who were M1/MRD ≥5% had a significantly inferior 5-year EFS rate as compared to those concordantly assessed as being in remission (M1/MRD <5%) (59.1%±6.5% vs. 87.1%±0.4%, p=<0.0001), but superior to those concordantly assessed as not in remission (M2/MRD ≥5%; 39.1±7.9%, p=0.009) (Table 4, Figure 2). This was largely driven by NCI HR patients, as statistically different outcomes between the three groups were seen in children with HR ALL [concordant remission (80.0±0.9%) vs. M1/MRD ≥5% (44.9±8.3%; p<0.0001); M1/MRD ≥5% vs. concordant not in remission (29.0±8.2%; p=0.05)] but not in SR ALL [concordant remission (90.8±0.4%) vs. M1/MRD ≥5% (85.9±7.6%; p=0.25); M1/MRD ≥5% vs. concordant not in remission (76.2±15.2%; p=0.45)]. The same overall patterns were seen for OS (Table 4).
Table 4.
5-year event free and overall survival among patients with concordant in remission, discordant, and concordant not in remission end of Induction bone marrows
| M1/MRD<5% | <- P value -> | M1/MRD≥5% | <- P value -> | M2/MRD≥5% | <- P value -> | M3 | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Event Free Survival | |||||||
|
| |||||||
| B-ALL, overall | 87.1±0.4 | <0.0001 | 59.1±6.5 | 0.009 | 39.1±7.9 | - | - |
| N=7 682 | N=66 | N=40 | |||||
|
| |||||||
| B-ALL, SR | 90.8±0.4 | 0.25 | 85.9±7.6 | 0.45 | 76.2±15.2 | - | - |
| N=5 000 | N=22 | N=9 | |||||
|
| |||||||
| B-ALL, HR | 80.0±0.9 | <0.0001 | 44.9±8.3 | 0.05 | 29.0±8.2 | - | - |
| N=2 682 | N=44 | N=31 | |||||
|
| |||||||
| T-ALL | 87.6±1.5 | 0.01 | 80.3±7.3 | 0.13 | 62.7±13.5 | - | - |
| N=1 303 | N=97 | N=40 | |||||
|
| |||||||
| Overall Survival | |||||||
|
| |||||||
| B-ALL, overall | 93.8±0.3 | <0.0001 | 77.2±5.6 | 0.01 | 59.0±8.9 | 0.36 | 43.4±8.9 |
| N=7 682 | N=66 | N=40 | N=41 | ||||
|
| |||||||
| B-ALL, SR | 96.6±0.3 | 0.24 | 95.5±4.6 | 0.75 | 88.9±12.1 | - | - |
| N=5 000 | N=22 | N=9 | |||||
|
| |||||||
| B-ALL, HR | 88.4±0.7 | <0.0001 | 66.9±8.3 | 0.06 | 51.4±10.4 | - | - |
| N=2 682 | N=44 | N=31 | |||||
|
| |||||||
| T-ALL | 91.9±1.3 | 0.005 | 83.4±6.8 | 0.34 | 76.7±12.3 | 0.24 | 63.5±11.1 |
| N=1 303 | N=97 | N=40 | N=43 | ||||
HR – high risk; MRD – minimal residual disease; SR – standard risk
Figure 2.
5-year event free survival among patients with concordant in remission, discordant, and concordant not in remission end of Induction bone marrows
In T-ALL, patients who were M1 and <5% MRD (concordant remission) had significantly better EFS and OS than either those who were discordant (M1/MRD ≥5%) or those concordantly not in remission (M2/MRD ≥5%). Between the latter two groups, discordant patients had superior EFS and OS, but these differences were not significant (5-year EFS 80.3±7.3% vs 62.7±13.5%, p=0.13; 5-year OS 83.4±6.8% vs. 76.7±12.3%, p=0.34). Interestingly, there was little difference in EFS or OS between B-ALL and T-ALL patients with concordant remission status (M1/MRD<5%), but T-ALL patients not in remission, whether identified by EOI morphology, MRD or both, had significantly better outcomes than equivalent B-ALL patients. Indeed, only 87.3% of T-ALL patients were M1/MRD<5%, as compared to 97.8% of B-ALL patients (Table 2; p<0.0001).
We hypothesized that the superior outcomes of B-lineage ALL patients with discordance compared to those concordantly not in remission were due to differences in MRD between the two populations, and thus we compared levels of MRD between these groups. Not surprisingly, MRD levels were higher in patients concordantly not in remission; this was largely driven by NCI HR patients (Table 5). In a regression model of B-ALL patients with M1 or M2 marrows and MRD>0.1%, both M1 vs. M2 morphologic status and MRD were significant predictors of EFS in univariate analysis. However, in multivariable analysis, the hazard associated with M2 status weakened, and was borderline statistically significant as an independent predictor of outcome after accounting for MRD status [hazard ratio (HR) 1.5, p=0.05; Table 6]. When the EOI percentage of blasts as determined by morphology was conceptualized as a continuous variable instead of dichotomized as M1 vs. M2, it was not a significant predictor of EFS in multivariable analysis (Supplemental Table 1).
Table 5.
Exact levels of minimal residual disease in patients with discordant and concordantly not in remission end of Induction bone marrows, median (interquartile range)
| M1/MRD≥5% | M2/MRD≥5% | p-value | |
|---|---|---|---|
| B-precursor, overall | 8.2 (6.2–14.4) | 14.6 (7.1–25.9) | 0.02 |
| B-precursor, SR | 7.7 (6.6–12.6) | 8.3 (6.3–12.4) | 0.89 |
| B-precursor, HR | 8.6 (6.1–18.1) | 16 (9.0–27.3) | 0.02 |
| T-precursor, overall | 9.7 (7.1–13.6) | 21 (14.1–31.3) | 0.02 |
HR – high risk; MRD – minimal residual disease; SR – standard risk
Table 6.
Prognostic impact of morphologic and minimal residual disease assessment of end of Induction bone marrow upon 5-year event free survival among patients with B-ALL
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95thCI) | P value | HR (95thCI) | P value | |
| Morphologic assessment | ||||
| M1 | Ref | <0.0001 | Ref | 0.05 |
| M2 | 2.7 (1.9–3.8) | 1.5 (1.0–2.2) | ||
| MRD, per log percent | 1.3 (1.2–1.4) | <0.0001 | 1.3 (1.2–1.4) | <0.0001 |
ALL – acute lymphoblastic leukemia; CI – confidence interval; HR – hazards ratio; MRD – minimal residual disease
M2/M3 Morphology with Low MRD
Among patients with B-ALL, 30 (0.3%) patients had M2 or M3 morphology with low MRD (i.e. MRD<5%). These patients had a significantly inferior 5-year OS as compared to patients concordantly in remission (72.7±9.8% vs. 93.8±0.3%; p<0.0001) but also had significantly higher levels of MRD [median 0.5, interquartile range (IQR) 0.05–2.4 vs. median 0, IQR 0–0.005; p<0.0001]. By comparison, 5-year OS for B-ALL patients with M3 marrows was 43.4%±8.9%.
While the number of such patients stratified by MRD level is too small to perform detailed analyses, 17/20 (85%) of patients with M2/M3 marrows and MRD<1% were alive at last follow-up, vs. 4/10 (40%) of patients with M2/M3 marrows and MRD≥1%, suggesting that low levels of MRD may allow identification of a significant subset of M2/M3 patients that have an excellent outcome.
Among patients with T-ALL, there was no difference in OS between the two groups (M2/M3 with MRD<5% – 100%, vs. M1 with MRD<5% – 91.9±1.3%; p=0.41).
DISCUSSION
While morphology remains an integral part of the initial diagnosis and workup of acute leukemia,23 morphologic assessment of remission after chemotherapy exposure may be complicated by additional factors. First, malignant lymphoblasts may be difficult to accurately identify and enumerate morphologically, particularly when only present in small numbers and/or evenly scattered throughout the marrow.10 Second, lymphoblasts may be mistakenly identified as hematogones, which are morphologically similar to blasts, but are benign immature B-cell precursors that are present in increased numbers in regenerating marrow. Despite these limitations, to date all reported cooperative trial groups used morphology-based definitions of induction failure.4, 8, 9, 13, 24, 25
More sophisticated technologies to assess residual disease (flow cytometry or PCR) have more recently been incorporated into clinical trials and overcome many of the above limitations by accurately identifying small numbers of lymphoblasts and successfully distinguishing them from benign hematogones.26–28 While our analysis demonstrates that morphologic and flow cytometric assessment of remission was concordant in the vast majority of cases, the use of an MRD-based definition of remission is further supported by several findings of this analysis. First, we found that clinical characteristics associated with morphologic induction failure (older age, higher WBC at presentation, unfavorable cytogenetics, ETP phenotype in T-ALL)20 are also associated with MRD-defined induction failure in patients with M1 marrows. Second, we found that among patients with T-ALL and SR-ALL, the outcome of patients with M1/MRD≥5% was not statistically different from those concordantly not in remission, though this should be interpreted with caution, given the small number of patients in these groups. For patients with HR B-ALL, discordant patients experienced outcomes that, while poor, were superior to those concordantly not in remission. Higher levels of MRD in the latter mostly likely explain this difference in outcomes. Indeed, in our multivariable analysis of patients with MRD >0.1%, including MRD level as a continuous variable significantly weakened the impact of M2 vs. M1 morphology. Interestingly, similar results were recently demonstrated in childhood acute myeloid leukemia, where in a multivariable analysis morphologic response after induction chemotherapy was not significantly associated with EFS in multivariable analysis including flow MRD level, albeit dichotomized.29
Our results confirm many of the findings of O’Connor et al., who studied the impact of MRD as measured by real-time quantitative PCR among a smaller cohort of children with ALL treated on the UKALL 2003 trial.12 Despite the different MRD measurement techniques used, the incidence of discordantly high MRD is remarkably consistent between the two studies: 0.9% in B-ALL and 6.9% in T-ALL in our study as compared to 1.5% and 8.0% in the UK study.12 O’Connor et al. also found that among those with either morphology or MRD indicating ≥5% residual blasts at EOI, MRD level retained prognostic significance while morphology status did not.
Our ability to determine the significance of those with discordantly low MRD (i.e. M2/M3 morphology with MRD<5%) is limited by the small number of such patients and the inability to study EFS in this population due to B-ALL patients with M3 marrows being taken off study. However, while this population experienced an inferior OS as compared to those concordantly in remission, they were still superior as compared to the outcomes of children with M3 marrows. We believe this is again due to differential levels of MRD in the two populations; 17/20 (85%) of patients with M2/M3 marrows and MRD<1% were alive at last follow-up. This hypothesis is supported by O’Connor et al, who found that the six UK patients with M2 marrows but MRD<0.01% had an 5-year EFS of 100%.12 Future studies combining international cohorts may further clarify the outcomes of patients with discordantly low MRD.
While discordance between morphologic and MRD-based assessments of remission was rare, it is important to note that children with this discordance outnumbered children with M3 marrows and thus remain a clinically significant population. While most treatment regimens intensify therapy for patients with positive MRD, eligibility for salvage regimens and experimental agents is in many cases still dependent on morphology-based definitions of induction failure. This both limits clinical trial accrual and potentially restricts the ability of patients with morphologic remission but MRD≥5% to enroll on trials testing new agents.
Based on their findings, O’Connor et al. proposed a new definition of induction failure of ≥5% residual blasts as measured by either morphology or MRD.12 This definition is being used in the current UK trial. Our results in an independent cohort of over 9,000 patients and using a different MRD measurement technique support this new definition. The inferior outcomes of patients with M2/M3 morphology and MRD<5% as compared to those concordantly in remission prevents us from fully endorsing a definition of induction failure based only on MRD, despite the rarity of such patients and the likelihood of their inferior outcomes being due to higher levels of MRD. Of note, neither our study nor that of the UK group examined whether 5% lymphoblasts, regardless of how it is measured, is the most appropriate threshold for defining induction failure as opposed to higher or lower levels.
Strengths of the current study include central assessment of flow cytometry based MRD as well as the large cohort size, allowing us to analyze rare populations of discordant patients. Several limitations however also merit mention. First, morphological assessment of EOI BM was conducted at local institutions. While we cannot definitively separate the effect of morphologic vs. flow cytometric assessment of remission from that of local vs. central assessment, the similar incidence of discordance between low and high enrolment institutions suggests that lack of experience in assessing morphologic remission in smaller sites was not responsible for the study findings. Second and related, we do not know which “pull” of marrow was analyzed. Third, a denominator of “all cells” is traditionally used in morphologic assessment while “all mononuclear cells” was used in flow cytometric assessment in these studies, representing an additional potential source of discordance, though one that does not impact the overall conclusions or the clinical implications of our findings. Fourth, lack of prognostic impact of EOI morphology in T-ALL may be partially due to known slower response kinetics as compared to B-ALL and the essential prognostic contribution of later time point (end of consolidation) MRD.5 Higher incidence of discordance in T-ALL however indicates that greater difficulty in morphologic assessment likely also plays a contributory role. We were unable to examine how EOI response determined by morphology vs. flow cytometry impacted end of Consolidation response, or to study the incidence or impact of discordance in response assessments conducted at the end of Consolidation, a time point with known greater prognostic significance in T-ALL.5
In summary, our results support the idea of using both morphology and flow cytometry when assessing remission, and of considering patients with >5% blasts by flow cytometry as not having achieved remission, even if their marrows are M1 by morphology. This has consequences beyond simply MRD-based risk stratification as it may allow these very poor risk patients to have access to experimental therapy. The exact MRD cut off that will best identify this very poor risk group awaits further study.
Supplementary Material
Acknowledgments
Sources of Support: This study was supported by National Institutes of Health, National Cancer Institute grants (U10CA098543, U10CA098413, U10CA180886, and U10CA180899) and by St. Baldrick’s Foundation. In-kind support was also provided by Becton Dickinson Biosciences (San Jose, CA).
SG is supported by a young investigator grant from the Alex’s Lemonade Stand Foundation. MLL is the Benioff Chair of Childhood Health and the Deborah and Arthur Ablin Chair of Pediatric Molecular Oncology at the Benioff Children’s Hospitals, UCSF SF, CA. SPH is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics, Children's Hospital of Philadelphia
Footnotes
Conflict of Interest Statement: The authors declare no competing financial interests
References
- 1.Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol. 2015;33(27):2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111(12):5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borowitz MJ, Wood BL, Devidas M, Loh ML, Raetz EA, Salzer WL, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood. 2015;126(8):964–971. doi: 10.1182/blood-2015-03-633685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of teh AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 5.Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077–2084. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- 6.Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129(14):1913–1918. doi: 10.1182/blood-2016-12-725804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Velden VH, Corral L, Valsecchi MG, Jansen MW, De Lorenzo P, Cazzaniga G, et al. Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia. 2009;23(6):1073–1079. doi: 10.1038/leu.2009.17. [DOI] [PubMed] [Google Scholar]
- 8.Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15:809–818. doi: 10.1016/S1470-2045(14)70243-8. [DOI] [PubMed] [Google Scholar]
- 9.Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. New Engl J Med. 2012;366(15):1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreft A, Holtmann H, Schad A, Kirkpatrick CJ. Detection of residual leukemic blasts in adult patients with acute T-lymphoblastic leukemia using bone marrow trephine biopsies: Comparison of fluorescent immunohistochemistry with conventional cytologic and flow-cytometric analysis. Pathol Res Pract. 2010;206:560–564. doi: 10.1016/j.prp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Longacre TA, Foucar K, Crago S, Chen IM, Griffith B, Dressler L, et al. Hematogones: a multiparameter analysis of bone marrow precursor cells. Blood. 1989;73(2):543–552. [PubMed] [Google Scholar]
- 12.O'Connor D, Moorman AV, Wade R, Hancock J, Tan RMR, Bartram J, et al. Use of minimal residual disease assessment to redefine induction failure in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2017;35(6):660–667. doi: 10.1200/JCO.2016.69.6278. [DOI] [PubMed] [Google Scholar]
- 13.Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children's Oncology Group Study AALL0232. J Clin Oncol. 2016;34(20):2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter SS, Dunsmore KP, Devidas M, Eisenberg N, Asselin BL, Wood BL, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children's Oncology Group Study AALL0434. Pediatr Blood Cancer. 2015;62(7):1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109(3):926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. In: Czader M, editor. Hematologic Malignancies. Vol. 999. Springer Science and Business; New York: 2013. [DOI] [PubMed] [Google Scholar]
- 17.Roshal M, Fromm JR, Winter SS, Dunsmore KP, Wood BL. Immaturity associated antigens are lost during induction for T cell lymphoblastic leukemia: implications for minimal residual disease detection. Cytometry B Clin Cytom. 2010;78(3):139–145. doi: 10.1002/cyto.b.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winter SS, Dunsmore KP, Devidas M, Eisenberg N, Asselin BL, Wood BL, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children's Oncology Group Study AALL0434. Pediatr Blood Cancer. 2015;62:1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heerema NA, Carroll AJ, Devidas M, Loh ML, Borowitz MJ, Gastier-Foster JM, et al. Intrachromosomal amplification of chromosome 21 is associated with inferior outcomes in children with acute lymphoblastic leukemia treated in contemporary standard-risk Children’s Oncology Group Studies: A report from the Children’s Oncology Group. J Clin Oncol. 2013;31(27):3397–3402. doi: 10.1200/JCO.2013.49.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10(2):147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J. Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 24.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. New Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrooman LM, Sevenson KE, Supko JG, O'Brien J, Dahlberg SE, Asselin BL, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00–01. J Clin Oncol. 2013;31(9):1202–1210. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenna RW, LaBaron WT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- 27.Karawajew L, Dworzak M, Ratei R, Rhein P, Gaipa G, Buldini B, et al. Minimal residual disease analysis by eight-color flow cytometry in relapsed childhood acute lymphoblastic leukemia. Haematologica. 2015;100(7):935–944. doi: 10.3324/haematol.2014.116707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129(14):1913–1918. doi: 10.1182/blood-2016-12-725804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY, et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012;30(29):3625–3632. doi: 10.1200/JCO.2011.41.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.