Abstract
Background
Though pharmacokinetic studies suggest accelerated biologic drug clearance with increasing body weight, evidence of obesity’s impact on clinical outcomes in biologic-treated patients with ulcerative colitis (UC) is inconsistent.
Aims
We evaluated the impact of obesity on real world response to biologic therapy in patients with UC.
Methods
In a single-centre retrospective cohort study between 2011-16 of biologic-treated patients with UC, we evaluated treatment response by baseline body mass index (BMI). Primary outcome was treatment failure (composite outcome of IBD-related surgery/hospitalization or treatment modification including dose escalation, treatment discontinuation or addition of corticosteroids); secondary outcomes were risk of IBD-related surgery/hospitalization and endoscopic remission. We conducted multivariate Cox proportional hazard analyses to evaluate the independent impact of BMI on clinical outcomes. Stratified analysis by weight-based regimens (infliximab) or fixed-dosing regimens (adalimumab, golimumab, vedolizumab, certolizumab pegol) was performed.
Results
We included 160 biologic-treated UC patients (50% males, 55% on infliximab) with median (IQR) age 36y (26–52) and BMI 24.3kg/m2 (21.4–28.7). On multivariate analysis, each 1kg/m2 increase in BMI was associated with 4% increase in the risk of treatment failure (adjusted hazard ratio [aHR], 1.04 [95% CI, 1.00–1.08]), 8% increase in the risk of surgery/hospitalization (aHR, 1.08 [1.02–1.14],). The effect on treatment failure was seen in patients on weight-based dosing regimens or fixed-dose therapies.
Conclusion
BMI is independently associated with increased risk of treatment failure in biologic-treated patients with UC, independent of dosing regimen.
Keywords: Obesity, treatment failure, tumour necrosis factor antagonists, anti-integrin agents
INTRODUCTION
The prevalence of obesity and inflammatory bowel disease (IBD) is increasing in parallel; approximately 15-40% of patients with IBD are obese and up to 66% are overweight.(1–4) Obesity has been associated with increased risk of developing Crohn’s disease, but not ulcerative colitis (UC).(5, 6) Obesity has been variably associated with IBD phenotype, with some studies suggesting milder disease and others suggesting lower prevalence of remission in cross-sectional studies.(7, 8) Longitudinal studies have suggested that obesity is associated with inferior quality of life, higher burden of hospitalization and healthcare utilization, and higher risk of relapse; among patients who undergo surgery, obesity has been associated with increased risk of short-term complications.(2–4, 8)
Population pharmacokinetic studies of biologic agents have consistently shown that higher body weight is associated with increased drug clearance and lower trough concentrations.(9–11) However, clinical studies of obesity’s impact on response to biologic agents in patients with IBD have been sparse and conflicting. In their cohort of 261 infliximab-treated patients, Billiet and colleagues observed that each unit increase in body mass index (BMI) was associated with 6% higher risk of treatment failure only on univariate analysis, but not on multivariate analysis.(12) In contrast, Bultman and colleagues observed that obesity (BMI ≥ 30 kg/m2) is associated with increased need for adalimumab dose escalation.(13) Most of the studies have been performed in patients with Crohn’s disease, with only a single 24 patient study in infliximab-treated patients with UC.(14) Given higher drug clearance and faecal wasting in patients with severe UC compared to Crohn’s disease, obesity may be more relevant in biologic-treated patients with UC.(15, 16)
Hence, we conducted a retrospective cohort study to evaluate the impact of obesity on response to biologic therapy in patients with UC. We hypothesized that higher BMI is associated with an increased risk of treatment failure in biologic-treated patients with UC, particularly in a subset of patients treated with fixed-dose regimens.
METHODS
Study design
We performed a retrospective cohort study in biologic-treated patients with UC seen and followed at University of California San Diego (UCSD). Patients were included if they had UC, were new users of a biologic agent (anti-tumour necrosis factor-α [TNF] agent such as infliximab, adalimumab or golimumab, or anti-integrin agent, vedolizumab) between 1/1/2011 and 12/31/2016, were followed at UCSD for at least 6 months, and had a BMI recorded within 3 months of start of biologic therapy. Patients were excluded if they: (a) had Crohn’s disease or indeterminate colitis, (b) were not treated with biologic agents, (c) were followed at UCSD for <6 months, (d) were underweight with BMI <18.5 kg/m2 at time of cohort entry, (e) were pregnant, or (f) had already undergone colectomy prior to starting biologic therapy. Prevalent users of biologic agents (i.e., patients who were already on a biologic agent at time of study start date) were also excluded to minimize immortal time bias. This study was approved by the UCSD Institutional Review Board (IRB #160967).
Data Abstraction
A single reviewer (SK) abstracted data through medical record review using a piloted data abstraction form, with constant feedback from a second gastroenterologist reviewer (SS). Besides exposure and outcomes (as detailed below), data on the following variables were abstracted: (a) patient characteristics – age, sex, smoking status, and BMI at time of starting new biologic therapy; (b) disease characteristics – disease extent, disease duration, endoscopic disease activity at time of starting biologic agent classified by Mayo endoscopy score, laboratory variables including haemoglobin, erythrocyte sedimentation rate, albumin and C-reactive protein at time of starting biologic agent, prior hospitalization within 1 year of cohort entry; (c) treatment characteristics – current index biologic agent, prior use of immunomodulators and other biologic agents, prior use of corticosteroids within the past 1 year, concurrent therapy with immunomodulators and/or corticosteroids; and (d) outcomes: date of IBD-related surgery, hospitalization, dose escalation, treatment discontinuation and addition of corticosteroids, and endoscopic re-assessment after starting biologic therapy.
Exposure
The primary predictor variable was BMI as a continuous variable. We evaluated the association between each 1 kg/m2 increase in BMI and clinical outcomes.
Outcomes
Primary outcome of interest was time to treatment failure, a composite outcome of IBD-related surgery, hospitalization, or treatment modification (including index biologic dose escalation, drug discontinuation, or addition/continuation of corticosteroids after 3 months of starting index biologic therapy). Secondary outcomes of interest were: time to IBD-related surgery (ileal pouch anal anastomosis, ileorectal anastomosis, or colectomy with end ileostomy), time to IBD-related hospitalization (primary discharge diagnosis of UC flare), or achieving endoscopic remission (Mayo endoscopy sub-score of 0 or 1, based on review of endoscopy reports performed by study IBD specialists) within 1 year of starting biologic therapy. Patients were followed from time of starting new biologic therapy until occurrence of study outcome or definitive colectomy surgery (all other outcomes were considered independent of other), loss to follow-up, treatment interruption for >6 months, or study completion (12/31/2016).
Additionally, we performed post-hoc analysis evaluating the association between biologic trough concentration and body mass index. At our centre, routine proactive therapeutic drug monitoring (TDM) is not performed for all patients with quiescent IBD; instead, providers variably perform reactive TDM in patients failing therapy, and in selected patients with quiescent disease.
Statistical Analysis
We performed univariate time-to-event analysis or logistic regression (for endoscopic remission outcome) to evaluate the association between BMI and clinical outcomes. To evaluate the independent effect of BMI on response to biologic therapy, we performed multivariate Cox proportional hazard analyses with a combination of backward variable selection (p<0.15 in univariate analysis among age, sex, BMI, disease duration, disease extent, C-reactive protein, prior and concomitant use of steroids and/or immunomodulators) and inclusion of clinically relevant variables (albumin [≥3.5g/dl vs. <3.5g/dl], prior anti-TNF failure for all outcomes; in addition, for hospitalization outcome, prior hospitalization within 1 year of cohort entry). Proportional hazard assumption was met based on graphical evaluation and examining weighted residuals.(17) Association between biologic trough concentration and BMI was examined using Pearson’s correlation coefficient.
To evaluate whether BMI may differentially impact response to weight-based dosing regimens vs. fixed-dose therapies, we performed stratified analysis by type of index biologic agent, evaluating the impact of BMI on clinical outcomes in each stratum. Our sample was a convenient consecutive sample, starting from the time the IBD centre was established at our hospital. No formal sample size assessment was performed.
All hypothesis testing was performed using a two-sided p-value with a statistical significance threshold <0.05. All statistical analyses were performed with Stata MP (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
RESULTS
Patient characteristics
Table 1 shows the baseline characteristics of 160 patients with UC who formed the study cohort. Median age of cohort was 36.0y (IQR, 26.0–51.8), with 80 males and 80 females. Median BMI was 24.3 kg/m2 (IQR, 21.4–28.7), and 18.1% were obese (class I obese, with BMI 30.0–34.9 kg/m2: 8.7%; class II obesity with BMI 35.0–39.9 kg/m2: 3.1%; class III obesity with BMI ≥40.0 kg/m2: 6.3%). Approximately 61% patients had pancolitis, and 53% had severely active endoscopic disease. Approximately 73% patients were biologic-naïve at time of cohort entry, 55.0% treated with infliximab (weight-based therapy) and 18.8% were treated with vedolizumab. About 53% and 52% were concomitantly on immunomodulators and corticosteroids, respectively, and 81% had used corticosteroids in the last 1 year prior to initiation of biologic therapy. Over a median follow-up of 2 years after starting biologic therapy, 110 patients experienced treatment failure, 23 patients underwent surgery, and 41 patients experienced IBD-related hospitalization. All patients with ‘treatment failure’ underwent treatment modification as the primary reason for treatment failure; within treatment modification, initiation of corticosteroids was the most common intervention. These have been detailed in eTable 1.
Table 1.
Baseline characteristics of biologic-treated patients with ulcerative colitis included in cohort
| Baseline characteristics | Normal BMI (18.5-24.9kg/m2) |
Overweight (25.0-29.9kg/m2) |
Obese (≥30kg/m2) |
|---|---|---|---|
|
| |||
| Number of patients | 90 (56.3%) | 41 (25.6%) | 29 (18.1%) |
|
| |||
| PATIENT CHARACTERISTICS | |||
|
| |||
| Age at cohort entry, in years (median, IQR) | 31.0 (24.0-45.8) | 43.0 (31.5-53.0) | 40.0 (29.0-54.5) |
|
| |||
| Gender – Males/Females | 36/54 | 28/13 | 16/13 |
|
| |||
| Follow up, months (median, IQR) | 22.0 (14.5-36.7) | 28.5 (19.6-34.3) | 20.8 (14.1-31.7) |
|
| |||
| Body mass index (kg/m2) (median, IQR) | 21.6 (20.4-23.2) | 27.4 (26.0-28.7) | 35.2 (32.2-40.9) |
|
| |||
| Smoking status (%) | |||
| • Current smokers | 4 (4.4) | 2 (4.9) | 2 (6.9) |
| • Recent past smoker (<1 y from cohort entry) | 1 (1.1) | 0 (0) | 1 (3.4) |
| • Former smokers | 14 (15.6) | 16 (39.0) | 9 (31.0) |
| • Never smoker | 71 (78.9) | 23 (56.1) | 17 (58.6) |
|
| |||
| DISEASE CHARACTERISTICS | |||
|
| |||
| Disease duration at cohort entry, in years (median, IQR) | 4.0 (1.0-8.0) | 5.0 (2.0-11.5) | 4.0 (1.0-7.5) |
|
| |||
| Disease extent, N (%) | |||
| • Extensive colitis | 59 (65.6) | 19 (46.3) | 20 (69.0) |
| • Left-sided colitis | 30 (33.3) | 22 (53.7) | 9 (31.0) |
| • Proctitis | 1 (1.1) | 0 (0) | 0 (0) |
|
| |||
| Disease severity* (%) | |||
| • Mayo score 0 | 0 (0) | 1 (4.3) | 0 (0) |
| • Mayo score 1 | 10 (18.5) | 0 (0) | 0 (0) |
| • Mayo score 2 | 13 (24.1) | 6 (26.1) | 8 (53.3) |
| • Mayo score 3 | 31 (57.4) | 16 (69.6) | 7 (46.7) |
|
| |||
| Prior IBD hospitalization <1 y from cohort entry (%) | 33/90 (36.7) | 8/41 (19.5) | 3/29 (10.3) |
|
| |||
| TREATMENT CHARACTERISTICS | |||
|
| |||
| Anti-TNF taken at cohort entry (%) | |||
| • Infliximab | 49 (54.4) | 23 (56.1) | 16 (55.2) |
| • Adalimumab | 19 (21.1) | 5 (12.2) | 7 (24.1) |
| • Golimumab | 6 (6.7) | 4 (9.8) | 0 (0) |
| • Certolizumab pegol | 0 (0.0) | 1 (2.4) | 0 (0) |
| • Vedolizumab | 16 (17.8) | 8 (19.5) | 6 (20.7) |
|
| |||
| # of prior anti-TNF failures | |||
| • 0 | 71 (78.9) | 29 (70.7) | 17 (58.6) |
| • 1 | 16 (17.8) | 8 (19.5) | 10 (34.5) |
| • 2 | 2 (2.2) | 4 (9.8) | 2 (6.9) |
| • 3 | 1 (1.1) | 0 (0) | 0 (0) |
|
| |||
| Prior steroid use <1 year from cohort entry (%) | 74 (82.2) | 34 (82.9) | 22 (75.9) |
|
| |||
| Steroid use at cohort entry (%) | 46 (51.1) | 22 (53.7) | 15 (51.7) |
|
| |||
| Prior use of immunomodulators (%) | 47 (52.2) | 20 (48.8) | 18 (62.1) |
|
| |||
| Immunomodulator use at cohort entry (%) | |||
| • Azathioprine | 30 (63.8) | 17 (65.4) | 7 (43.75) |
| • 6-mercaptopurine | 11 (23.4) | 8 (30.8) | 7 (43.75) |
| • Methotrexate | 6 (12.8) | 1 (3.8) | 2 (12.5) |
|
| |||
| C-reactive protein, g/L (median, IQR) | 0.002 (0.001-0.013) | 0.007 (0.003-0.027) | 0.003 (0.002-0.006) |
|
| |||
| Albumin, g/L (median, IQR) | 40.0 (37.0-43.0) | 41.0 (37.0-42.3) | 41.0 (39.0-44.0) |
|
| |||
| Hemoglobin, g/L (mean, SD) | 120 (21) | 125 (22) | 126 (20) |
|
| |||
| Erythrocyte sedimentation rate, mm/hr (median, IQR) | 15.5 (6.0-30.0) | 18.5 (6.0-42.3) | 11.0 (7.0-18.8) |
Data available for 92/160 patients [54 patients with normal BMI, 23 overweight patients, 15 obese patients]
[Abbreviations: BMI=Body mass index, IQR=Interquartile range, IBD=Inflammatory bowel disease, TNF=Tumour necrosis factor]
Primary Outcome
On multivariate analysis, each 1 kg/m2 increase in BMI was associated with a 4% higher risk of treatment failure (aHR, 1.04; 95% CI, 1.00–1.08, p=0.029). This effect was similar in patients treated with weight-based therapy (aHR, 1.05; 95% CI, 1.00–1.10, p=0.050) and in patients treated with fixed dose therapy (aHR, 1.05; 95% CI, 0.99–1.10, p=0.106) (Figure 1). Besides BMI, low albumin and shorter disease duration were associated with treatment failure (Table 2).
Figure 1.
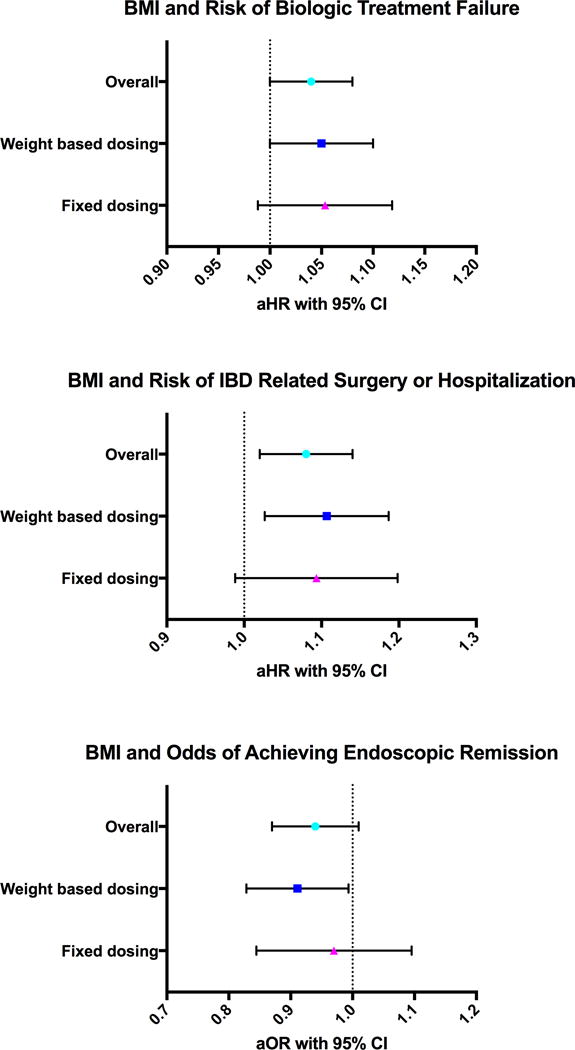
Association between BMI and risk of treatment failure, risk of IBD-related surgery or hospitalization, and odds of achieving endoscopic remission
Table 2.
Multivariate Cox proportional hazard analysis examining factors associated with treatment failure in biologic-treated patients with ulcerative colitis
| Variables | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Body mass index (per 1kg/m2 increase) | 1.043 | 1.004-1.082 | 0.029 |
| Prior prednisone use (yes vs. no) | 1.852 | 0.93-3.67 | 0.078 |
| Concomitant immunomodulators (yes vs. no) | 0.652 | 0.415-1.024 | 0.063 |
| Albumin (<3.5g/dl vs. ≥3.5g/dl) | 1.733 | 1.028-2.924 | 0.045 |
| Disease duration (per 1y) | 0.951 | 0.912-0.991 | 0.018 |
| Prior anti-TNF failure (yes vs. no) | 0.753 | 0.463-1.223 | 0.25 |
[Abbreviations: CI=confidence interval, TNF=tumour necrosis factor]
Secondary outcomes
IBD-related surgery and/or hospitalization
On multivariate analysis, each 1 kg/m2 increase in BMI was associated with 8% risk of IBD-related surgery or hospitalization (aHR, 1.08; 95% CI, 1.02–1.14, p=0.008). This negative effect was similar in patients treated with weight-based therapy (aHR, 1.10; 95% CI, 1.03–1.19, p=0.006) or in patients treated with fixed dose therapy (aHR, 1.09; 95% CI, 0.99–1.20, p=0.059) (Figure 1). Besides BMI, prior hospitalization within 1 year of cohort entry was also associated with 2.3 times higher risk of IBD-related surgery or hospitalization (aHR, 2.26; 95% CI, 1.01–5.05, p=0.047) (Table 3).
Table 3.
Multivariate Cox proportional hazard analysis examining factors associated with IBD-related surgery or hospitalization in biologic-treated patients with ulcerative colitis
| Variables | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Body mass index (per 1kg/m2 increase) | 1.078 | 1.020-1.138 | 0.008 |
| Prior prednisone use (yes vs. no) | 1.894 | 0.559-6.419 | 0.305 |
| Concomitant immunomodulators (yes vs. no) | 1.006 | 0.481-2.104 | 0.988 |
| Albumin (<3.5g/dl vs. ≥3.5g/dl) | 1.318 | 0.548-3.165 | 0.537 |
| Disease duration (per 1y) | 0.999 | 0.934-1.068 | 0.974 |
| Prior anti-TNF failure (yes vs. no) | 0.675 | 0.290-1.571 | 0.362 |
| Prior hospitalization (yes vs. no) | 2.261 | 1.012-5.053 | 0.047 |
[Abbreviations: CI=confidence interval, TNF=tumour necrosis factor]
Endoscopic Remission
Although not significant, each 1 kg/m2 increase in BMI was associated with 6% lower risk of achieving endoscopic remission (aOR, 0.94; 95% CI, 0.87–1.01, p=0.070), though it did not meet statistical significance. This negative effect was significant only in patients treated with weight-based therapy (aOR, 0.91; 95% CI, 0.83–0.99, p=0.035), but not in patients treated with fixed dose therapies (aOR, 0.96; 95% CI, 0.85–1.10, p=0.571) (Figure 1). Besides BMI, patients with longer disease duration were significantly more likely to achieve endoscopic remission (aOR, 1.12; 95% CI, 1.04–1.21, p=0.003) (Table 4).
Table 4.
Multivariate logistic regression analysis examining factors associated with achieving endoscopic remission in biologic-treated patients with ulcerative colitis
| Variables | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Body mass index (per 1kg/m2 increase) | 0.937 | 0.873-1.005 | 0.070 |
| Weight-based dosing (yes vs. no) | 2.201 | 0.884-5.480 | 0.090 |
| Albumin (<3.5g/dl vs. ≥3.5g/dl) | 3.110 | 0.930-11.13 | 0.065 |
| Disease duration (per 1y) | 1.121 | 1.040-1.209 | 0.003 |
| Prior anti-TNF failure (yes vs. no) | 1.118 | 0.405-3.085 | 0.829 |
[Abbreviations: CI=confidence interval, TNF=tumour necrosis factor]
Biologic trough concentration and body mass index
Overall, trough concentration during maintenance therapy was available for 51/88 patients treated with infliximab and 14/31 patients treated with adalimumab. A significant negative correlation was observed between BMI and adalimumab trough concentration (Pearson’s correlation coefficient = −0.58, p=0.03); none of the patients had undetectable adalimumab trough concentration. In contrast, we did not observe any significant association between BMI and infliximab trough concentration (Pearson’s correlation coefficient = 0.11, p=0.46); 10/51 patients had undetectable infliximab concentration.
DISCUSSION
In this retrospective study on the impact of BMI on treatment response and outcomes in 160 biologic-treated patients with UC, we made several key observations. First, we found that higher BMI is independently associated with an increased risk of treatment failure and IBD-related surgery and/or hospitalization, with each unit increase in BMI being associated with a 4-8% higher risk of adverse outcomes. This effect of BMI is independent of biologic dosing regimen, observed in patients treated with either weight-based dosed infliximab or other fixed dose therapies. Second, we also observed that higher BMI may be independently associated with failure to achieve endoscopic remission, though this outcome did not reach statistical significance. Our findings suggest that obesity is a negative prognostic factor in biologic-treated patients with UC and a treatment effect modifier that must be considered in clinical trial design and clinical practice.
Our findings build upon an evolving and conflicting body of evidence on the potential negative impact of obesity in patients with IBD, in particular its impact on treatment response to biologics.(18–20) While obesity has been consistently shown to negatively impact treatment response to anti-TNF agents in rheumatic diseases, this evidence has been inconsistent in patients with IBD. In a meta-analysis of 16 studies with 3,130 patients with IBD, we observed that obesity does not significantly influence treatment response to anti-TNF agents (odds of failing therapy, 1.20; 95% CI, 0.88–1.64).(21) However, there is considerable heterogeneity with differences in study design, obesity exposure categories, clinical outcomes, and variable adjustment for key confounding variables. Moreover, most of these studies have been conducted in patients with Crohn’s disease. It is possible that in patients with Crohn’s disease, local mesenteric creeping fat may play a more vital role in pathogenesis than systemic obesity.(22, 23) In contrast, there has been limited assessment of the impact of obesity on response to biologic agents in patients with UC. In a study of 24 patients with UC treated with infliximab, Harper and colleagues observed a 30% increase in risk of UC flare per unit increase in BMI, adjusting for prior surgery, steroid use, extra-intestinal manifestations, age, disease duration. However, due to the small sample size, this multivariate model was likely overfitted.(14) Subgroup analysis of ULTRA-2 trial of adalimumab in UC observed a non-significant 1.5 fold higher risk of failing to achieve clinical remission in patients weighing ≥70 kg vs. <70 kg.(24)
Obesity is recognized as a perpetual state of chronic low-grade inflammation, through systemic and paracrine increase in levels of cytokines, chemokines and adipokines, and is also associated with dysbiosis.(4, 22, 23) Obesity increases leptin secretion from adipocytes and resistin secretion from macrophages and leukocytes that increase levels of pro-inflammatory cytokines such as TNF, interleukin-1 and -6. Besides its direct impact on inflammation, obesity can also modify pharmacokinetics of biologic agents. Population pharmacokinetic studies of all approved biologic agents in IBD have identified high body weight as a risk factor associated with increased clearance of drug, resulting in shorter half-life and lower serum trough drug concentrations.(9–11) This effect might be related to rapid proteolysis and to a ‘TNF-sink’ phenomenon with higher inflammatory burden due to adipose tissue in patients with obesity. This may explain why patients with obesity treated even with weight-based regimens such as infliximab, had inferior response to therapy.
Our study is one of the largest studies evaluating the impact of BMI on treatment response to biologics in patients with UC, with systematic data collection and evaluation of patient-important outcomes. By limiting analyses to new users of biologics followed at our centre and by adjusting for key confounders, we were able to overcome potential limitations of a tertiary referral centre retrospective cohort study. However, our study has some limitations which merit attention. First, our data on association between BMI and biologic trough concentrations needs to be interpreted with caution, since routine biologic trough concentration assessments were not performed, and drug clearance estimates were not performed. Trough concentrations in our cohort were measured selectively and variably, in patients losing response to biologic therapy and do not accurately represent the association between BMI and biologic trough concentrations. Hence, we were only able to hypothesize why obesity may negatively impact treatment response based on systemic drug exposure. We also did not routinely measure thiopurine metabolite levels. Second, findings on subgroup analysis should be interpreted with caution. Within strata of weight-based and fixed dose therapies, though the summary estimates for all outcomes except endoscopic remission were very similar, overall results were sometimes borderline insignificant. These are likely a reflection of smaller sample size in subgroup analyses. Third, we were unable to evaluate the impact of obesity on achieving clinical remission or response based on validated disease activity indices in this retrospective study. Instead we relied on pragmatic clinically relevant outcomes including surgery, hospitalization or need for treatment modification, which may be subject to provider preferences. The secondary outcomes including IBD-related surgery and/or hospitalization as well as achieving endoscopic remission, however, are more robust with limited risk of bias. All endoscopies at our centre are performed by providers with clinical and research focus on IBD, which decreases risk of misinterpretation. Fourth, we were not able to study whether the association between BMI and response to biologics varied between anti-TNF agents and vedolizumab, due to a small number of patients on vedolizumab, most of whom had prior anti-TNF exposure. Finally, there is potential risk of considerable weight changes in patients with severe disease starting biologics, either weight loss due to severe disease activity or weight gain due to corticosteroid-dependent disease.(25)
Our findings have important clinical implications. In clinical practice, physicians may consider aggressive treatment and close proactive monitoring in patients with high BMI treated with biologic agents. Some potential changes include empirically using higher doses of anti-TNF therapy in overweight and obese patients, frequent therapeutic drug monitoring and/or use of combination therapy with immunomodulators to increase drug concentration and decrease risk of immunogenicity. Clinical trials should consider obesity as a potential effect modifier, and perform and report appropriate analyses. Finally, high BMI may offer a potential therapeutic intervention by directly targeting the obesity itself for treatment with a multi-disciplinary approach in IBD patients. Small RCTs and cohort studies in patients with psoriasis, psoriatic arthritis and rheumatoid arthritis have suggested a beneficial effect of intentional weight loss on treatment response to anti-TNF agents. (26–28)(22–24)
In conclusion, in a cohort of biologic-treated patients with UC, we observed that high BMI is independently associated with increased risk of treatment failure, including IBD-related surgery or hospitalization, and may be a lower risk of achieving endoscopic remission. These effects were seen in patients treated with weight-based dosing regimens as well as fixed dose agents. Prospective cohort studies and post-hoc analyses of RCTs with individual participant level data are warranted to confirm this association. If this effect is consistent, interventional studies targeting obesity should be explored for difficult-to-treat obese patients with UC.
Supplementary Material
Acknowledgments
Funding: This work was funded through a career development award to Siddharth Singh from the American College of Gastroenterology and the Crohn’s and Colitis Foundation. The publication (or project) described was partially supported by the National Institutes of Health, Grant TL1TR001443 of CTSA funding (to Soumya Kurnool). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures: Dr. Dulai has received research support, honorarium and travel support from Takeda, and research support from Pfizer. Dr. Zarrinpar received support from NIH K08 DK102902, AASLD Liver Scholar Award, and American Heart Association Beginning Grant-in-Aid (16BGIA27760160), and has consulted for Takeda, Herbalife, and Illumina. Dr. Boland received support from CCFA and UCSD KL2 (1KL2TR001444), has consulted for Abbvie, and has received research support from Takeda and Janssen. Dr. Vande Casteele has served as a consultant for Boehringer Ingelheim, Janssen, Takeda, and UCB Pharma. Dr. Grunvald has served as a consultant for Takeda, Orexigen, and Rhythm Pharmaceuticals. Dr. Sandborn has served as a consultant and received research funding from Janssen, Abbvie, UCB Pharma, Takeda, and Pfizer. Dr. Singh has received research support from Pfizer and AbbVie.
Footnotes
DR PARAMBIR S DULAI (Orcid ID: 0000-0002-9514-2321)
DR WILLIAM J SANDBORN (Orcid ID: 0000-0002-3314-7960)
DR SIDDHARTH SINGH (Orcid ID: 0000-0002-2640-7269)
- Study concept and design: SK, AZ, WJS, SS
- Acquisition of data: SK, SS
- Analysis and interpretation of data: SK, NHN, JP, SS
- Drafting of the manuscript: SK, SS
- Critical revision of the manuscript for important intellectual content: NHN, JP, PSD, BSB, NVC, EE, ELG, AZ, WJS
- Approval of the final manuscript: SK, NHN, JP, PSD, BSB, NVC, EE, ELG, AZ, WJS, SS
Guarantor of Article: Dr. Siddharth Singh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pringle PL, Stewart KO, Peloquin JM, et al. Body Mass Index, Genetic Susceptibility, and Risk of Complications among Individuals with Crohn’s Disease. Inflamm Bowel Dis. 2015;21:2304–2310. doi: 10.1097/MIB.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2857–2863. doi: 10.1097/MIB.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110–121. doi: 10.1038/nrgastro.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:361–368. doi: 10.1097/MIB.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SFM, Shaffer K, Singh AG, Prokop LJ, Grunvald E, Zarrinpar A, Sandborn WJ. Pre-Morbid Obesity is Associated with Increased Risk of Developing Immune-Mediated Inflammatory Diseases: a Systematic Review and Meta-Analysis. Gastroenterology. 2017;152:S976–S977. [Google Scholar]
- 7.Flores A, Burstein E, Cipher DJ, et al. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60:2436–2445. doi: 10.1007/s10620-015-3629-5. [DOI] [PubMed] [Google Scholar]
- 8.Jain ASS, Martin C, Sandler R, Sandborn WJ, Herfarth HH, Kappelman MD, Long MD. Obesity is Associated with Worse Disease Activity in Patients with Inflammatory Bowel Diseases: An Internet Based Cohort Study. Gastroenterology. 2017;152:S973–S974. [Google Scholar]
- 9.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Eckert D, Hyams JS, et al. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn’s disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis. 2015;21:783–792. doi: 10.1097/MIB.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 11.Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42:188–202. doi: 10.1111/apt.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billiet T, Cleynen I, Ballet V, et al. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: a 20-year single centre experience. Alimentary Pharmacology and Therapeutics. 2016;44:673–683. doi: 10.1111/apt.13754. [DOI] [PubMed] [Google Scholar]
- 13.Bultman E, de Haar C, van Liere-Baron A, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn’s disease patients. Aliment Pharmacol Ther. 2012;35:335–341. doi: 10.1111/j.1365-2036.2011.04946.x. [DOI] [PubMed] [Google Scholar]
- 14.Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118–2124. doi: 10.1097/MIB.0b013e31829cf401. [DOI] [PubMed] [Google Scholar]
- 15.Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology. 2017;153:835–857 e836. doi: 10.1053/j.gastro.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology. 2015;149:350–355 e352. doi: 10.1053/j.gastro.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Grambsch PTT. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:512–526. [Google Scholar]
- 18.Barre A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther. 2018 doi: 10.1111/apt.14550. [DOI] [PubMed] [Google Scholar]
- 19.Waljee AK, Liu B, Sauder K, et al. Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis. Aliment Pharmacol Ther. 2018;47:763–772. doi: 10.1111/apt.14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding NS, Malietzis G, Lung PFC, et al. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment Pharmacol Ther. 2017;46:883–891. doi: 10.1111/apt.14293. [DOI] [PubMed] [Google Scholar]
- 21.Singh SFA, Singh AG, Casteele NV, Zarrinpar A, Grunvald E, Curtis JR, Sandborn WJ. Obesity is Associated with Inferior Response to Anti-TNF Therapy in Immune-Mediated Inflammatory Diseases: A Systematic Review and Meta-Analysis. Gastroenterology. 2017;2017:S154. [Google Scholar]
- 22.Kredel L, Batra A, Siegmund B. Role of fat and adipokines in intestinal inflammation. Curr Opin Gastroenterol. 2014;30:559–565. doi: 10.1097/MOG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 23.Zulian A, Cancello R, Ruocco C, et al. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn’s disease. An in vivo and in vitro study. PLoS ONE. 2013;8:e78495. doi: 10.1371/journal.pone.0078495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265. e251–253. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Selinger CP, Parkes GC, Bassi A, et al. A multi-centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:964–973. doi: 10.1111/apt.14334. [DOI] [PubMed] [Google Scholar]
- 26.Di Minno MND, Peluso R, Iervolino S, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis. 2014;73:1157–1162. doi: 10.1136/annrheumdis-2012-202812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond) 2015;39:1197–1202. doi: 10.1038/ijo.2015.64. [DOI] [PubMed] [Google Scholar]
- 28.Sparks JA, Halperin F, Karlson JC, et al. Impact of Bariatric Surgery on Patients With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2015;67:1619–1626. doi: 10.1002/acr.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


