Abstract
Sarcopenia, the loss of muscle mass and function, is a common feature of aging and impacts on individual health and quality of life. Several cellular mechanisms have been involved in the pathogenesis of this syndrome, including mitochondrial dysfunction, altered apoptotic and autophagic signaling, and, more recently, trace metal dyshomeostasis. Calorie restriction (CR) without malnutrition has been shown to ameliorate the age-related loss of muscle mass in a variety a species. Mechanisms of protection span from preservation of mitochondrial functional and structural integrity to mitochondrial biogenesis, reduction of oxidative stress, and favorable modulation of apoptotic and autophagic signaling pathways. Importantly, preliminary evidence indicates that moderate CR may promote muscle mitochondrial biogenesis in middle-aged human subjects. Further research is warranted to investigate whether CR may represent a safe and efficient strategy to delay the onset and mitigate the progression of sarcopenia in older adults.
Keywords: mitochondria, oxidative stress, apoptosis, autophagy, iron
1. Introduction
The age-related loss of muscle mass and function, referred to as sarcopenia, is a universal characteristic of the aging process, documented in several species, from worms to humans [1]. In older individuals, compromised muscle function is highly predictive of falls [2], disability [3], and all-cause mortality [4]. Moreover, mobility disability resulting from muscle loss is associated with poor quality of life and increased social and health care needs in older adults [5]. The age-dependent loss of muscle mass is often accompanied by a parallel gain in fat mass, leading to a phenotype called “sarcopenic obesity” [6]. The coexistence of these conditions is thought to promote a vicious cycle in which the decline in muscle mass reduces resting metabolic rate and physical activity, leading to increased deposition of adipose tissue [6]. The accumulation of fat mass, in turn, accelerates the loss of muscle mass via the secretion of catabolic cytokines (i.e., TNF-α) and insulin resistance.
The etiology of sarcopenia is complex and characterized by the contribution of multiple factors, including loss of α-motor neurons [7], increased contraction-induced injury [8], impaired satellite cell function [9], altered hormonal status (e.g., decline of growth hormone and testosterone levels) [10], increased production of catabolic cytokines [11], inadequate nutrition [12], and decreased physical activity [10].
Histologically, the aged muscle is characterized by a decline of both the number and size of muscle fibers, with a preferential loss of type II (fast-twitch) fibers [13]. Increased fiber size variability and accumulation of nongrouping, scattered, and angulated fibers have also been described in old rodent muscles [14]. In addition, advanced age is associated with increases in the extracellular space and deposition of protein aggregates within the interstitial matrix [15]. The subsequent disruption of muscle architecture contributes to muscle fatigability and decreased force production observed with age [16]. Studies have shown that sarcopenic changes may be the consequence of accumulating oxidative damage to muscle constituents [17,18], which is thought to stem from altered mitochondrial function [19]. With regards to the actual mechanism responsible for the loss of muscle fibers, several reports have indicated that it may reside in a defective regulation of myocyte apoptotic signaling, as evidenced by the increased occurrence of myonuclear apoptotic DNA fragmentation in aged muscles [17,20–25]. In this context, it is noteworthy that mitochondria, besides their role as energy suppliers, are also centrally involved in the regulation of the apoptotic program [17].
Calorie restriction (CR) without malnutrition is currently considered the most powerful anti-aging intervention, owing to its ability of extending both mean and maximum lifespan in a variety of species. With regards to skeletal muscle, consistent evidence has shown that CR is able to retard the onset and impede the progression of sarcopenia by acting at several critical control points, ranging from mitochondrial function to oxidative stress, muscle architecture, and myonuclear apoptosis. In addition, recent experimental evidence from our laboratory suggests that CR might positively modulate autophagy in myocytes, a cellular housekeeping process that becomes dysfunctional over the course of aging (unpublished results).
2. CR mitigates age-related mitochondrial functional decline and oxidative stress in skeletal muscle
Mitochondrial functional decline is considered as a central mechanism driving the aging process [19]. Given the high reliance of skeletal myocytes on ATP provision, the impact of mitochondrial functional loss is particularly pronounced in skeletal muscle, leading to impaired strength and endurance [26]. Past experimental evidence has indicated that mitochondrial oxidative phosphorylation capacity declines in muscles over the course of aging [27,28]. Notably, deletions and point-mutations in mitochondrial DNA (mtDNA) have been found to accumulate in aged muscles and to colocalize with electron transport chain (ETC) abnormalities and fiber atrophy [29–31]. MtDNA is particularly vulnerable to oxidative damage because of its proximity to the ETC (the main cellular source of oxidants) and the lack of protective histones [26]. Moreover, because of the compactness of mitochondrial genome (i.e., lack of intrones), each mutation is likely to affect gene integrity. Furthermore, the efficiency of the main pathway for the removal of small mtDNA base modifications (base excision repair, BER) declines at different levels with aging [32]. Mitochondrial production of reactive oxygen species (ROS) has been shown to increase in skeletal muscle over the course of aging [33], which may be responsible for the accumulation of mtDNA mutations. The relevance of mtDNA damage to sarcopenia is evidenced by decreased activity of complex I, and IV of the ETC observed in aged skeletal muscles of various species [29,30,34–36]. In contrast, content of complex II (succinate dehydrogenase), which is entirely encoded by nuclear DNA, increases in muscle fibers from old animals, probably as a result of compensatory upregulation of mitochondrial biogenesis. Notably, fibers harboring high levels of mtDNA deletions and ETC abnormalities often display morphological aberrations, including segmental atrophy, fiber splitting, and breakage [29–31,37]. Importantly, CR was shown to reduce the prevalence of mtDNA deletion mutations as well as the abundance of fiber displaying ETC abnormalities in old laboratory rodents [38,39].
Preservation of mitochondrial structural and functional integrity by CR is believed to result from the attenuation of oxidative damage promoted by the dietary intervention. In this context, it has been reported that CR reduces mitochondrial proton leak and ROS generation in skeletal muscle [27,40–42] and increases the expression of genes involved in ROS scavenging functions [43]. Additionally, it has been reported that CR may alter mitochondrial membrane fatty acid composition, making it more resistant to lipid peroxidation and less prone to proton leak [44,45]. Lass et al. [46] have also demonstrated that CR counteracts the age-associated increase in superoxide anion radical generation, lipid peroxidation, and mitochondrial protein damage in murine skeletal muscle. Moreover, Drew et al. [27] reported reduced levels of oxidative damage to mtDNA in the gastrocnemius muscle of old CR rats compared with ad libitum fed (AL) controls. Interestingly, in a very recent study, 6-month 25% CR increased skeletal muscle mitochondrial biogenesis and reduced DNA damage in healthy, middle-aged, overweight human subjects [47]. The increase in mitochondrial number may be interpreted as positive adaptation promoted by CR, as a larger mitochondrial mass imposes a reduced workload per unit mitochondria, thus limiting oxidant generation [48].
In conclusion, mitochondrial dysfunction appears as a prominent contributing factor to age-related muscle atrophy. CR limits the severity of mitochondrial alterations with age by inducing positive adaptations, which ultimately result in the maintenance of healthy mitochondria with high bioenergenetic capacity and reduced propensity toward oxidant production (Fig. 1).
Fig. 1.
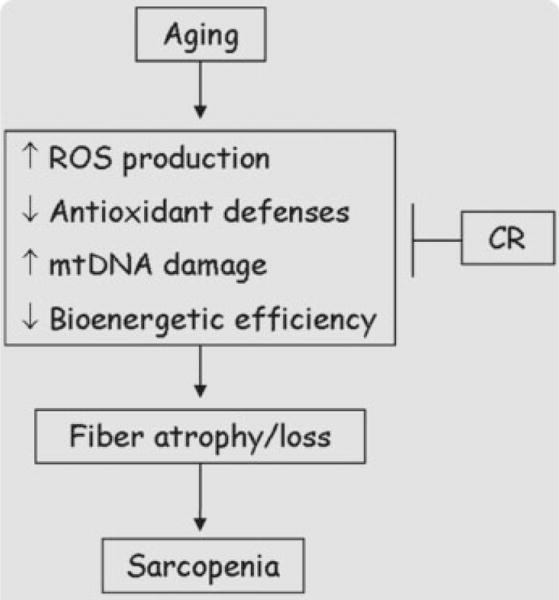
Age-related muscle mitochondrial dysfunction results from multiple contributing factors and plays a prominent role in the development of sarcopenia. Calorie restriction preserves mitochondrial function in the aged muscle, thus mitigating fiber atrophy and loss of muscle mass.
3. CR hinders muscle tissue iron accumulation with age
Iron (Fe) is an essential metal required for the proper functioning of many cellular processes including oxygen and electron transport, drug metabolism and steroid and DNA biosynthesis [49,50]. It is a cofactor for many enzymes because of its ability to fluctuate between the ferric (Fe3+, oxidized) and ferrous (reduced, Fe2+) state. Approximately 70% of total body Fe is associated with hemoglobin, with most of the remaining Fe stored in the liver as ferritin or as myoglobin in muscle cells. Fe deficiency is a high prevalent condition among older adults, contributing to the development of anemia and its detrimental consequences. However, evidence is accumulating indicating that age-related tissue Fe overload may be involved in the pathogenesis of several degenerative conditions, including Alzheimer’s disease [51], Parkinson’s disease [52], and possibly sarcopenia [18,53–56].
Fe is a highly redox active metal capable of converting oxidant intermediates, such as hydrogen peroxide into harmful free radical species (e.g., hydroxyl radical). Additionally, Fe has been shown to catalyze the nitration of tyrosine residues resulting in protein damage [57]. Because of the potential toxicity of Fe, its homeostasis is strictly regulated by a multitude of sensors, transporters, and storage proteins [58].
Excess Fe has been associated with muscular atrophy due to disuse [18] and aging [18,54,55], likely through the exacerbation of oxidative damage to muscle constituents. The importance of Fe overload in muscle atrophy was first demonstrated by Kondo et al. [59], who reported mitigation of oxidative damage and muscle mass loss following administration of the Fe chelator deferoxamine to hind limb immobilized rats. Importantly, Hofer et al. [18] demonstrated that Fe accumulated in atrophied rather than normal fibers, suggesting a causal relation between Fe overload and loss of muscle mass. Furthermore, a recent study from our laboratory showed that advanced age was associated with increased Fe content within skeletal muscle mitochondria [56]. Notably, Fe accumulated to a higher extent in subsarcolemmal than in intermyofibrillar mitochondria and impacted on mtRNA oxidation and permeability transition pore (mPTP) opening susceptibility [56]. Importantly, mPTP opening can lead to cell death via necrosis or apoptosis [60]. Therefore, facilitation of permeability transition by Fe overload may represent a crucial mechanism underlying skeletal myocyte loss with age.
Evidence on the effects of CR on Fe accumulation with aging is scarce and conflicting. CR has been shown to mitigate Fe accumulation and oxidative damage in kidneys of aged rats [61]. Conversely, in the same study, old animals subjected to dietary restriction displayed higher Fe levels in liver and brain compared with age-matched ad libitum fed controls [61]. Further, CR was found to exacerbate the age-related accumulation of chelatable and non-heme Fe in mouse liver and kidney [62]. The same study reported no CR protection against age-dependent Fe accumulation in heart, striatum, hippocampus, midbrain, and cerebellum. Likewise, Borten et al. [63] showed that CR was unable to prevent age-related Fe accumulation in rat dorsal hippocampus of rats.
With regard to skeletal muscle, Xu et al. [54] recently reported an amelioration of age-associated accumulation of Fe and nucleic acid oxidative damage in the gastrocnemius muscle of CR rats. Interestingly, preservation of iron homeostasis by CR was positively correlated with forelimb grip strength, suggesting that Fe accumulation in aged muscle may contribute to the loss of function. The lack of a general consensus regarding the impact of CR on Fe homeostasis may result from tissue and species-specific effects of the dietary intervention. Additionally, the literature is void of studies investigating the effect of CR on Fe transport and storage mechanisms in skeletal muscle. In conclusion, the available evidence points toward a detrimental effect of age-related Fe accumulation in skeletal muscle, which appears to impact on both muscular mass and function. Mitigation of muscle Fe overload by CR may therefore be regarded as an additional means whereby the dietary intervention protects against sarcopenia.
4. Modulation of skeletal muscle apoptotic signaling by CR
Growing evidence indicates that progressive elimination of myonuclei via an apoptosis-like process may represent a fundamental mechanism driving the onset and progression of sarcopenia [17,22,64–67]. Apoptosis is executed via specific signaling pathways, eventually leading to DNA fragmentation, nuclear condensation, proteolysis, membrane blebbing, and cell fragmentation, with formation of apoptotic bodies, which are then engulfed by macrophages or neighboring cells. Execution of apoptosis in skeletal muscle displays unique features, as myofibers are multinucleated. Therefore, apoptosis may result in the elimination of individual myonuclei (myonuclear apoptosis) and the relative portion of sarcoplasm, without demise of the entire fiber. With respect to the final executioner of cell death, two distinct pathways of apoptosis have been described, namely the caspase-independent and the caspase-dependent apoptosis. This latter pathway is carried out via sequential activation of cysteine-dependent, aspartate-specific proteases (caspases) [68]. The caspase-independent apoptotic pathway is executed via mitochondrial release of mediators (e.g., apoptosis inducing factor, AIF, and endonuclease G, EndoG) that are capable of directly producing DNA-fragmentation [69]. Mitochondria are considered a key center for the induction and regulation of apoptosis. Noticeably, mitochondria can induce apoptosis in both a caspase-dependent and independent manner [69]. Upon apoptotic stimuli, mitochondrial outer membrane permeabilization can occur, followed by release of cytochrome c, which initiates the intrinsic pathway of apoptosis. Once in the cytosol, cytochrome c promotes oligomerization of apoptosis protease activating factor-1 (Apaf-1) in the presence of ATP/dATP. The resulting apoptosome activates caspase-9, which in turn engages caspase-3. This latter is responsible for the proteolytic events and DNA fragmentation (via caspase-activated DNase, CAD). In addition, caspase-independent apoptogenic factors residing in the mitochondrial intermembrane space, such as AIF and EndoG, can be released into the cytosol, translocate to the nucleus and cleave DNA independent of caspase activation. Our laboratory has extensively investigated age-related changes in apoptotic signaling transduction pathways in skeletal muscle and their modulation by lifelong CR [23,70,71]. Our data indicate that CR is able to mitigate the majority of the apoptotic pathways involved in age-associated skeletal muscle loss. We reported that myocyte expression of procaspase-3 and cleaved caspase-3 as well as the extent of DNA fragmentation were elevated in the gastrocnemius muscle of aged rats and were significantly reduced by CR [23,70,71]. In addition, CR increased the cytosolic content of apoptosis repressor with a caspase recruitment domain (ARC) in the gastrocnemius muscle of old rodents [70]. Furthermore, expression levels of procaspase-12 were significantly lower in the gastrocnemius muscle of old CR rats compared with age-matched AL animals, indicating that CR also has the potential of attenuating sarcoplasmic reticulum stress-mediated apoptosis [70]. In the same study, we also reported a reduction in mitochondrial release of AIF in the plantaris muscle of CR rodents [70]. Recently, we found that CR also counteracted myocyte apoptosis induced by the death-receptor pathway triggered by TNF-α in aged rats [23,71]. Indeed, myocyte expression of TNF-α in the superficial vastus lateralis (SVL) [23] and gastrocnemius [71] muscles was increased in old rodents and prevented by the CR regimen. Furthermore, CR prevented the age-related elevation of cleaved caspase-8 levels, downstream of TNF-α [23,72]. As a result, apoptotic DNA fragmentation was significantly attenuated by the dietary restriction in the SVL and gastrocnemius muscles of old CR rats compared with age-matched AL controls.
Taken as a whole, our findings indicate that the proapoptotic environment taking place in aged skeletal muscle may be substantially attenuated by CR at several critical control points (Fig. 2). Additionally, preliminary data from our laboratory indicate that even mild CR (8%) might be effective in counteracting the age-related acceleration of myocyte apoptosis in rodents (unpublished results). Although mild CR may not maximize the potential benefits, this approach appears to be much more feasible for humans to maintain longterm.
Fig. 2.
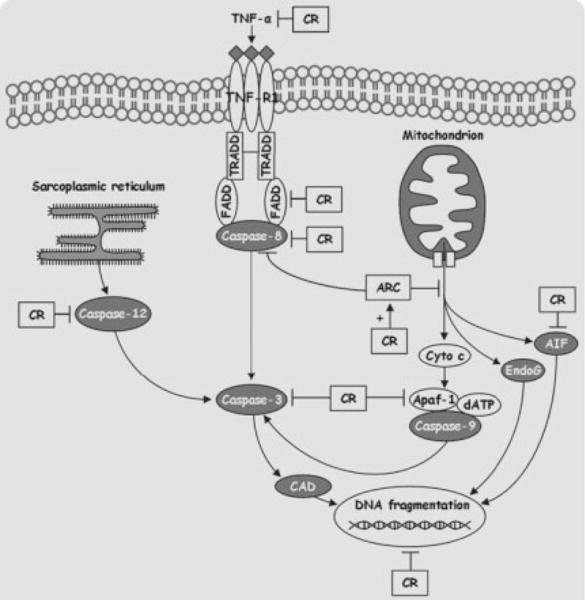
Overview of the apoptotic pathways involved in age-related myocyte elimination and their modulation by calorie restriction.
5. CR stimulates autophagy: Possible implications for sarcopenia
Oxidative damage to lipids, proteins, and DNA, especially in postmitotic tissue of an aged organism, may be severe and ultimately lead to apoptotic or necrotic cell death. However, when the damage is less severe, autophagy-mediated cell survival may prevail [72]. Autophagy literally means “self-eating” and is a vital cellular process by which intracellular components are degraded within lysosomes [73,74]. There are three classifications of autophagy: (a) microautophagy, in which lysosomes directly take up cytosol, inclusions, and organelles for degradation; (b) chaperone-mediated autophagy, in which soluble proteins with a particular pentapeptide motif are recognized and transported across the lysosomal membrane for degradation; and (c) macroautophagy, in which a portion of cytoplasm including subcellular organelles is sequestered within a double membrane-bound vacuole that ultimately fuses with a lysosome [75]. Macroautophagy (subsequently referred to as autophagy) is the primary cellular pathway for degradation of long-lived proteins and organelles, and, importantly, the only mechanisms so far attributed to the degradation of dysfunctional and damaged mitochondria. It becomes apparent that autophagy is critical to overall cellular health, because in some postmitotic tissues, progressive accumulation of damaged intracellular components and potential lack of autophagic response eventually result in cell death and loss of tissue function. Accordingly, proper initiation and execution of autophagy have been associated with life-span extension in worms and flies [76–78]. A decline in autophagic activity during normal aging has been described for invertebrates and higher organisms [79–82], with the concomitant accumulation of damaged cellular components, such as undegradable lysosome-bound lipofuscin, protein aggregates, and damaged mitochondria [83]. However, it is the efficacy of autophagy within a specific tissue or organ that affects cellular homeostasis. Because the regulation and degree of autophagy are highly organ dependent [84], it seems reasonable to assume that age-related changes in autophagy are organ-specific as well. Although the autophagic activity in liver declines with age [81,82], data from our laboratory suggest that autophagy is maintained in heart and skeletal muscle of aged rats ([85] and unpublished data). Yet, the efficacy of autophagy in heart and skeletal muscle might not be sufficient to cope with the age-related magnitude of cellular damage. The consequences of autophagy dysregulation in skeletal muscle have mostly been studied with respect to myopathies, such as Pompe and Danon disease, in which myofiber morphology is altered and muscle function impaired [86,87]. However, the effect of age on the regulation of autophagy in skeletal muscle and the role of autophagy in sarcopenia have yet to be fully characterized.
Autophagy is a highly regulated process, with multiple signaling pathways controlling the induction as well as the formation and maturation of the autophagic vacuoles [88,89]. Autophagy is suppressed by amino acids and growth factors such as insulin [88,89]. However, dietary restriction is a potent inducer of autophagy in many species [84,85,90,91]. Autophagy may in fact play an important role in mediating CR’s beneficial effects, as, for instance, reduced activity of autophagy genes in C. elegans suppressed the lifespan extension promoted by inherent dietary restriction [77]. During CR, autophagy is induced via at least two of the signaling pathways: activation of phosphoinositide 3-kinase class III through complex formation with Beclin 1 and downregulation of the nutrient-sensor mitochondrial target of rapamycin (mTOR) (Fig. 3) [92–94]. Notably, mTOR deficiency extends lifespan in worms [95], but whether this effect is, at least in part, due to increased autophagy has yet to be confirmed.
Fig. 3.
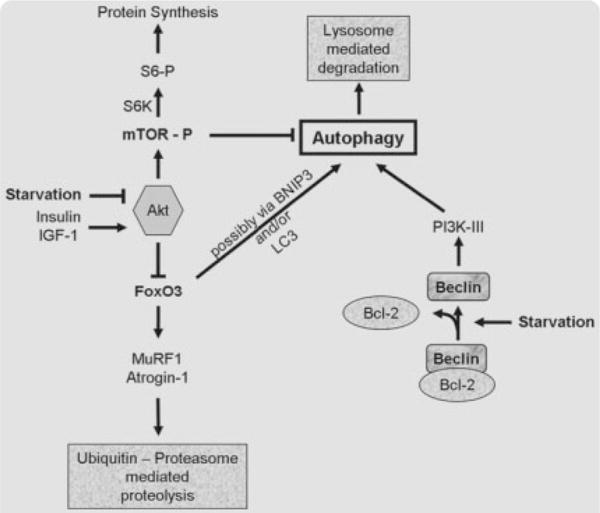
Regulatory pathways of autophagy. Autophagy is suppressed by amino acids and growth factors such as insulin, which act through protein kinase B (Akt/PKB). However, when cells are starved for amino acids (Starvation), autophagy is activated via at least two possible signaling pathways: mTor and class III phosphoinositide 3-kinase (PI3K-III). Beclin interacts with and presumably activates the PI3K-III thereby promoting autophagy. This interaction can be inhibited by Bcl2 or Bcl-XL, which directly interact with Beclin. Recently, FoxO3 transcription factor has been associated with increased ubiquitin-proteasome mediated proteolysis, as well as increased autophagy. FoxO is believed to stimulate autophagy via BNIP3 and LC3.
The autophagic response in skeletal muscle is clearly inducible by CR [84]. Furthermore, findings from our laboratory indicate that the autophagic response to CR persists even at old age (unpublished data). Although the beneficial effects of autophagy on longevity and cellular homeostasis have found broad support, it is yet unclear whether upregulation of autophagy is always beneficial in skeletal muscle. It needs to be identified whether a shift in the activity of regulatory proteins and autophagy-dependent degeneration of cellular components might contribute to disruption of myocyte function and muscle atrophy, or whether autophagic removal of cellular waste overall promotes myocyte health. Recent studies on mouse skeletal muscle [96–98] revealed a pivotal role for the forkhead transcription factor FoxO3 and its downstream targets atrogin 1 and MuRF1 in proteasome-associated skeletal muscle atrophy. Furthermore, Mammucari et al. [98] and Zhao et al. [99] demonstrated a link between FoxO3 and autophagy, possibly via disuse or fasting-induced transcription of BNIP3 and LC3, leading to unfavorable loss of muscle mass due to autophagic proteolysis. On the other hand, a recent study by Willcox et al. [100] identified FoxO3a genotype as associated with longevity in humans.
On the basis of our findings in aging rat heart [85] and skeletal muscle (unpublished data) and the emerging evidence for autophagy as essential for cellular homeostasis, we suggest that autophagy may be one mediator of the beneficial effects of CR on the attenuation of sarcopenia. However, more work is required to conclusively define the role of autophagy in age-related conditions such as sarcopenia. Once the role of autophagy in aging muscle is characterized, interventions to modulate this complex cellular process may represent a promising therapeutic strategy to counteract the detrimental accumulation of waste material in aging muscle.
6. Conclusions and future perspectives
CR has been consistently shown to attenuate the rate of functional decline and loss of muscle mass that occur with age. Importantly, CR has been recently reported to mitigate the severity of sarcopenia in non-human primates [101]. Experimental evidence indicates that these protective effects stem from the ability of CR to reduce the incidence of mitochondrial abnormalities, attenuate oxidative stress, maintain the proper functioning of autophagy, and counteract the age-related elevation of proapoptotic signaling in skeletal muscle. Importantly, moderate reduction in calorie intake appears to protect against mitochondrial functional decline also in human skeletal muscle. However, several reports suggest that an excessive CR in humans may be accompanied by a number of adverse effects, such as weakness, loss of libido, infertility, amenorrhea, osteoporosis, depression [102], which may limit its large-scale applicability to humans. However, recent data indicate that even a slight reduction in calorie intake (i.e., 8% restriction) combined with voluntary exercise may retain the ability of counteracting sarcopenic changes in old rodents [15]. Future studies will have to investigate whether CR, alone or in combination with physical exercise, may represent a safe and efficient strategy to delay the onset and mitigate the progression of sarcopenia in older adults.
Acknowledgments
This research was supported by grants to CL (NIA R01-AG17994 and AG21042) and by the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30 AG028740).
References
- 1.Fisher AL. Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc. 2004;52:1185–1190. doi: 10.1111/j.1532-5415.2004.52320.x. [DOI] [PubMed] [Google Scholar]
- 2.Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—the MINOS study. J Bone Miner Res. 2005;20:721–729. doi: 10.1359/JBMR.041230. [DOI] [PubMed] [Google Scholar]
- 3.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 4.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–M777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 6.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–888. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 7.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 8.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50:124–129. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- 9.Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2006;290:C609–C615. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- 10.Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 11.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 12.Dreyer HC, Volpi E. Role of protein and amino acids in the pathophysiology and treatment of sarcopenia. J Am Coll Nutr. 2005;24:140S–145S. doi: 10.1080/07315724.2005.10719455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 14.Usuki F, Yasutake A, Umehara F, Higuchi I. Beneficial effects of mild lifelong dietary restriction on skeletal muscle: prevention of age-related mitochondrial damage, morphological changes, and vulnerability to a chemical toxin. Acta Neuropathol. 2004;108:1–9. doi: 10.1007/s00401-004-0844-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress, and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43:317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payne AM, Dodd SL, Leeuwenburgh C. Life-long calorie restriction in Fischer 344 rats attenuates age-related loss in skeletal muscle-specific force and reduces extracellular space. J Appl Physiol. 2003;95:2554–2562. doi: 10.1152/japplphysiol.00758.2003. [DOI] [PubMed] [Google Scholar]
- 17.Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung L, Ng YC. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim Biophys Acta. 2006;1762:103–109. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 22.Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, Giovannini S, Leeuwenburgh C, Carter CS. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R558–R567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- 23.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 24.Pistilli EE, Siu PM, Alway SE. Molecular regulation of apoptosis in fast plantaris muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2006;61:245–255. doi: 10.1093/gerona/61.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005;288:C338–C349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- 26.Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 28.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- 30.Cao Z, Wanagat J, McKiernan SH, Aiken JM. Mitochondrial DNA deletion mutations are concomitant with ragged red regions of individual, aged muscle fibers: analysis by laser-capture microdissection. Nucleic Acids Res. 2001;29:4502–4508. doi: 10.1093/nar/29.21.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet. 2006;79:469–480. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissman L, de Souza-Pinto NC, Stevnsner T, Bohr VA. DNA repair, mitochondria, and neurodegeneration. Neuroscience. 2007;145:1318–1329. doi: 10.1016/j.neuroscience.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 33.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 34.Muller-Hocker J, Schneiderbanger K, Stefani FH, Kadenbach B. Progressive loss of cytochrome c oxidase in the human extraocular muscles in ageing—a cytochemical-immunohistochemical study. Mutat Res. 1992;275:115–124. doi: 10.1016/0921-8734(92)90016-i. [DOI] [PubMed] [Google Scholar]
- 35.Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11:573–581. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- 36.Lee CM, Lopez ME, Weindruch R, Aiken JM. Association of age-related mitochondrial abnormalities with skeletal muscle fiber atrophy. Free Radic Biol Med. 1998;25:964–972. doi: 10.1016/s0891-5849(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 37.Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles under-going sarcopenia. J Appl Physiol. 2002;92:2617–2624. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- 38.Lee CM, Aspnes LE, Chung SS, Weindruch R, Aiken JM. Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice. Ann NY Acad Sci. 1998;854:182–191. doi: 10.1111/j.1749-6632.1998.tb09901.x. [DOI] [PubMed] [Google Scholar]
- 39.Bua E, McKiernan SH, Aiken JM. Calorie restriction limits the generation but not the progression of mitochondrial abnormalities in aging skeletal muscle. FASEB J. 2004;18:582–584. doi: 10.1096/fj.03-0668fje. [DOI] [PubMed] [Google Scholar]
- 40.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab. 2004;286:E852–E861. doi: 10.1152/ajpendo.00367.2003. [DOI] [PubMed] [Google Scholar]
- 41.Lal SB, Ramsey JJ, Monemdjou S, Weindruch R, Harper ME. Effects of caloric restriction on skeletal muscle mitochondrial proton leak in aging rats. J Gerontol A Biol Sci Med Sci. 2001;56:B116–B122. doi: 10.1093/gerona/56.3.b116. [DOI] [PubMed] [Google Scholar]
- 42.Lambert AJ, Merry BJ. Effect of caloric restriction on mitochondrial reactive oxygen species production and bioenergetics: reversal by insulin. Am J Physiol Regul Integr Comp Physiol. 2004;286:R71–R79. doi: 10.1152/ajpregu.00341.2003. [DOI] [PubMed] [Google Scholar]
- 43.Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, Barazzoni R, Nair KS. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 2002;283:E38–E43. doi: 10.1152/ajpendo.00387.2001. [DOI] [PubMed] [Google Scholar]
- 44.Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J Gerontol A Biol Sci Med Sci. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- 45.Cefalu WT, Wang ZQ, Bell Farrow AD, Terry JG, Sonntag W, Waite M, Parks J. Chronic caloric restriction alters muscle membrane fatty acid content. Exp Gerontol. 2000;35:331–341. doi: 10.1016/s0531-5565(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 46.Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 49.Poulos TL, Li H, Raman CS. Heme-mediated oxygen activation in biology: cytochrome c oxidase and nitric oxide synthase. Curr Opin Chem Biol. 1999;3:131–137. doi: 10.1016/s1367-5931(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 50.Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Castellani RJ, Moreira PI, Liu G, Dobson J, Perry G, Smith MA, Zhu X. Iron: the Redox-active center of oxidative stress in Alzheimer disease. Neurochem Res. 2007;32:1640–1645. doi: 10.1007/s11064-007-9360-7. [DOI] [PubMed] [Google Scholar]
- 52.Yantiri F, Andersen JK. The role of iron in Parkinson disease and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. IUBMB Life. 1999;48:139–141. doi: 10.1080/713803493. [DOI] [PubMed] [Google Scholar]
- 53.Altun M, Edstrom E, Spooner E, Flores-Moralez A, Bergman E, Tollet-Egnell P, Norstedt G, Kessler BM, Ulfhake B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve. 2007;36:223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- 54.Xu J, Knutson MD, Carter CS, Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoS ONE. 2008;3:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung SH, DeRuisseau LR, Kavazis AN, Deruisseau KC. Plantaris muscle of aged rats demonstrates iron accumulation and altered expression of iron regulation proteins. Exp Physiol. 2008;93:407–414. doi: 10.1113/expphysiol.2007.039453. [DOI] [PubMed] [Google Scholar]
- 56.Seo AY, Xu J, Servais S, Hofer T, Marzetti E, Wohlgemuth SE, Knutson MD, Chung HY, Leeuwenburgh C. Mitochondrial iron accumulation with age and functional consequences. Aging Cell. 2008;7:706–716. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bian K, Gao Z, Weisbrodt N, Murad F. The nature of heme/iron-induced protein tyrosine nitration. Proc Natl Acad Sci USA. 2003;100:5712–5717. doi: 10.1073/pnas.0931291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci. 2007;44:413–459. doi: 10.1080/10408360701428257. [DOI] [PubMed] [Google Scholar]
- 59.Kondo H, Miura M, Kodama J, Ahmed SM, Itokawa Y. Role of iron in oxidative stress in skeletal muscle atrophied by immobilization. Pflugers Arch. 1992;421:295–297. doi: 10.1007/BF00374844. [DOI] [PubMed] [Google Scholar]
- 60.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 61.Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech Ageing Dev. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 62.Sohal RS, Wennberg-Kirch E, Jaiswal K, Kwong LK, Forster MJ. Effect of age and caloric restriction on bleomycin-chelatable and nonheme iron in different tissues of C57BL/6 mice. Free Radic Biol Med. 1999;27:287–293. doi: 10.1016/s0891-5849(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 63.Borten O, Liberman A, Tuchweber B, Chevalier S, Ferland G, Schipper HM. Effects of dietary restriction and metal supplementation on the accumulation of iron-laden glial inclusions in the aging rat hippocampus. Biogerontology. 2004;5:81–88. doi: 10.1023/B:BGEN.0000025071.78517.3a. [DOI] [PubMed] [Google Scholar]
- 64.Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med. 2005;35:473–483. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- 65.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic Biol Med. 2008;44:160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 67.Pistilli EE, Jackson JR, Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis. 2006;11:2115–2126. doi: 10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 69.van GM, Festjens N, van LG, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–497. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- 70.Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, Quinn LS, Leeuwenburgh C. Changes in IL-15 expression and death-receptor apoptotic signalling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev In press. 2009 doi: 10.1016/J.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 73.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 74.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65–76. [PMC free article] [PubMed] [Google Scholar]
- 76.Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 77.Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- 78.Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 79.Terman A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology. 1995;41(Suppl 2):319–326. doi: 10.1159/000213753. [DOI] [PubMed] [Google Scholar]
- 80.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 81.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 82.Donati A, Cavallini G, Paradiso C, Vittorini S, Pollera M, Gori Z, Bergamini E. Age-related changes in the regulation of autophagic proteolysis in rat isolated hepatocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B288–B293. doi: 10.1093/gerona/56.7.b288. [DOI] [PubMed] [Google Scholar]
- 83.Terman A, Brunk UT. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- 84.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 86.Saftig P, Tanaka Y, Lullmann-Rauch R, von FK. Disease model: LAMP-2 enlightens Danon disease. Trends Mol Med. 2001;7:37–39. doi: 10.1016/s1471-4914(00)01868-2. [DOI] [PubMed] [Google Scholar]
- 87.Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E, Plotz PH, Raben N. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol. 2006;59:700–708. doi: 10.1002/ana.20807. [DOI] [PubMed] [Google Scholar]
- 88.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin. 2005;26:1421–1434. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 90.Del Roso A, Vittorini S, Cavallini G, Donati A, Gori Z, Masini M, Pollera M, Bergamini E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp Gerontol. 2003;38:519–527. doi: 10.1016/s0531-5565(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 91.Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macro-autophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57:203–208. doi: 10.1016/s0753-3322(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 92.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 93.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 94.Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide 3-kinase-Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J. 2003;376:577–586. doi: 10.1042/BJ20030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 96.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del PP, Burden SJ, Di LR, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg LA. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FoxO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127:1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]


