Abstract
In this review we compile results cited in reliable journals that show a ratio for the use of pulsed electromagnetic fields (PEMF) in therapy, indeed. This is true especially for chronically inflamed joints. Furthermore, we try to link this therapeutic approach to the molecular background of chronic inflammation and arthritis. At first we start with the clinical outcome of PEMF therapy. Then, we look for possible triggers and an electromagnetic counterpart that is endogenously inherent in cell biology and in the tissues of interest. Finally, we want to investigate causal molecular and cellular mechanisms of possible PEMF actions. It shows that there are endogenous mechanisms, indeed, which can act as triggers for PEMF like the resting membrane potential as well as resonance mechanisms in charged moieties like membrane transporters. Especially voltage-gated calcium channels can be triggered. These may lead into specific signaling pathways and also may elicit nitric oxide as well as moderate radical reactions, which can ultimately lead to e.g. NFκB-like reactions. Concerted in the right way, these reactions can cause a kind of cell protection and ultimately lead to a dampening of inflammatory signals like interleukins.
Keywords: PEMF, arthritis, endogenous electric, molecular mechanisms, cell biology
Introduction
This review will concentrate on pulsed electromagnetic field (PEMF) therapy in arthritis and on possible mechanisms linking PEMF with endogenous electric phenomena on cell- and molecular biological level.
In degenerative processes of joints, like in arthritis, interleukin (IL)-1β and tumor necrosis factor TNF-α represent two key mediators. These signaling factors are synthesized and secreted by monocytes, macrophages as well as in the local tissues [1-4]. Many other clinical, in vivo and in vitro studies show a massive secretion of IL-1β and TNF-α in these degenerative diseases [5-8]. Miller et al. [4] report that such pro-inflammatory factors in turn stimulate catabolic degradation of collagen matrix via matrix metalloproteinases 2 (MMP2) and 13 (MMP13) [9,10] also linked to painful disc degeneration [11]. In this context, a diminished water binding capacity of the collagen matrix is discussed. MMPs also activate NF-κB [12] signaling to promote IL-6 [13] and IL-17 [14] expression. By this, MMPs are driving a vicious cycle. Regarding PEMF many studies report that this external stimulation is followed by complex biological reactions meditated by different signaling pathways [3]. In osteoarthritic situations, many positive effects of PEMF therapy were observed [15-19].
In the present review we want to look into the literature if there are a) really positive clinical outcomes after PEMF therapy; b) screen experimental studies dealing with PEMF and musculoskeletal cells and tissue, especially regarding interleukins and TNF-α and c) search for an electromagnetic intrinsic counterpart and trigger for PEMF in cells and tissues. Finally, we want d) to look for causal molecular and cellular mechanisms of possible PEMF actions.
Results and discussion
Clinical data
Many papers report that PEMF as FDA - approved therapy is effective for treating pseudoartrosis, diabetes mellitus induced complications, delayed wound healing, pain and neurodegenerative disorders [20-28]. In the clinic, this therapy has positive effects for the regeneration of musculoskeletal tissues such as cartilage, bone, tendon and ligament [29-34]. Ryang We et al. [18] found a significant beneficial effect of PEMF on WOMAC pain scores at 1 month compared with a sham treatment (see [35]). In addition, a recent study of our group revealed a significant and relevant improvement in pain category of the WOMAC questionnaire, and significant improvements in mobility, daily activity score as well as global score during treatment of acute osteoarthritis of knee joint (severity level 2-4 according to ACR criteria).
PEMF therapy option is of particular relevance due to its effect on pain in patients. This is important when the patients suffer from intolerance to chronic and high doses of e.g. non- steroidal anti-rheumatic drugs. Due to pain reduction, mobility and ability to perform daily activities were improved. In consequence, this is beneficial for both passive physical movement and for physical training performed by the patient [36]. In addition, several recent studies showed again the effectiveness of the PEMF treatment in clinical assessment of arthritis and neuropathy [37-40]. On the other hand, transcutaneous electro stimulation by electrodes for therapy of knee osteoarthritis is reported to be not effective for pain relief [41].
Experimental in vivo and in vitro data
Regarding bone density, PEMF therapy increases osteoblast activity but significantly reduces osteoclast formation [42-44]. Osteogenic differentiation is enhanced in MSCs by PEMF if the cells are pre-committed [45]. Also, MSCs derived from adipocytes differentiate faster and more expressed if they are cultured in a medium favoring osteogenic differentiation. What is more, Zhai et al. [46] could show that PEMF stimulation (38 Hz, 2 mT) for 2 h per day enhanced osteoblastic functions through amelioration of the cytoskeletal organization; increased proliferation-related gene expressions as well as upregulated gene and protein expressions of collagen type 1 of the Runt-related transcription factor 2 and of Wnt/β-catenin signaling [46]. Furthermore, a cell protective effect was found via the activation of the PI3K/Akt/Bad signaling pathway. In guinea pigs, Veronesi et al. could show that PEMF (75 Hz) dampened all symptoms of knee osteoarthritis [47].
Furthermore, PEMF can lead to chondroprotective effects on joint cartilage in animal models [32,48-55].
A more indirect indicator is the positive effect of PEMF on angiogenesis by enhanced production of fibroblast growth factor beta-2 [56]. Since angiogenesis is a process critical for successful healing, this represents also an important aspect for therapy. In the case of cultured tendon fibroblasts, following PEMF exposition, de Girolamo et al. [57] among others, established increased collagen I expression and increase of anti-inflammatory prostaglandins, and a huge rise in the Vascular Endothelial Growth Factor (VEGF)-A-mRNA transcription. Thus, these findings indicate a tendency towards proliferation and increase in vascular density.
In cell lines (murine osteosarcoma, [58] PEMF can increase proliferation rates as well as in osteoblasts [44,59] and in chondrocytes [60] the stimulatory effect of PEMF on osteoblast proliferation and differentiation is accompanied by an increase in nitric oxide (NO) synthesis [61]. It is known that in addition to its vasodilatory effect, NO exerts many important functions on the vascular wall like inhibiting apoptosis [62]; regulating cell migration and angiogenesis [63] - and importantly, suppressing the inflammatory response induced by cytokines [64]. Our own group could demonstrate stimulated increases in NO production in HUVEC cultures. These experiments could also explain the stimulation of peripheral blood flow observed in vivo in forearms and hands of volunteers observed in a concomitant study in this paper (see [65]).
Some recent clinical and experimental studies report effects of PEMF on interleukin IL-1β (IL1β) levels, too. Here, Boopalan et al. [66] and Ongaro et al. [55] could show that IL1β is reduced by PEMF. What is more, gene expression in members of the Transforming Growth Factor (TGF-β) family is enhanced by PEMF [67] and local expression of TGF-β results in improved bone fracture healing [68]. In this turn, proliferation, differentiation and synthesis of cartilage matrix proteins [48,69] are enhanced by PEMF.
Caliskan et al. [70] studied especially the effects of IL-1β and TNF. They concentrated on the effects of PEMF on the MMP-9 and TIMP-1 production in chondrosarcoma cells stimulated with low and high doses of IL-1β. In sum, this study could reveal that PEMF treatment suppressed IL-1β-mediated up-regulation of MMP-9 protein levels. In primary rat nucleus pulposus cells, Zou et al. [3] found that the levels of IL-1β and TNF-α secreted into the culture media were significantly reduced in an intensity-dependent manner by low-frequency PEMF stimulation.
Miller et al. [4] exposed human annulus fibrosus and nucleus pulposus cells to IL-1α and stimulated by PEMF for 4 hours daily for up to 7 days. They found that PEMF treatment lessened the IL-1α-induced upregulation of genes expressed in degenerated intervertebral disc cells. After 4 days, PEMF tended to reduce IL-1α-associated gene expression of IL-6 in nucleus pulposus cells and MMP13 in annulus fibrosus cells. Additionally, PEMF treatment significantly diminished IL-1α-induced gene expression of IL-17A and MMP2 in nucleus pulposus cells and NFκB in annulus fibrosus cells.
Tang et al. [71] used a GFP reporter system driven by IL-6 promoter to visualize the PEMF treatment effect on IL-6 transcription in single living cells. IL-6-MS2 reporter-labeled cells were treated with IL-1α to mimic the in situ inflammatory environment of degenerative disc while simultaneously exposed to PEMF continuously for 4 h. The authors could show in live cell imaging that the pro-inflammatory factor IL-1α significantly promoted IL-6 transcription over time. Imaging and PCR data demonstrated that the inductive effect of IL-1α on IL-6 expression could be significantly inhibited by PEMF treatment in a time-dependent manner. The authors [71] conclude that PEMF may have a role in the clinical management of patients with chronic low back pain. The above mentioned positive effects of PEMF on molecular biological pathways motivate again for a search for an electromagnetic intrinsic counterpart and trigger for PEMF in cells and tissues.
Endogenous electromagnetic counterpart in cells and tissues
Indeed, electromagnetic fields (EMF) are produced endogenously within an organism. Many EMF - rhythms are present in the nervous system, in the musculoskeletal system and within all connective tissue. Like in this kind of tissue, mechanical deformation also of bone causes piezoelectricity. Furthermore, bending strain couples to permanent dipoles in collagen molecules [72,73]. Frequencies from 5 to 30 Hz were found during postural muscle activity (quiet standing) and of 10 Hz during walking [74]. So, everything in living systems is in motion and by changing EF also magnetic fields are associated. That means EMF and PEMF arise from movements of muscles, tendons, etc. In body fluids, streaming potentials can arise; this means the electric potential difference between a liquid and a capillary, a diaphragm, or a porous solid [42]. All this is also additional information from cell to cell and within the tissue.
At the dimensions of a single cell, microdomains of ion channels and transporters are distributed in a pattern across the entire two-dimensional surface of a cell. This pattern, too, can encode an enormous amount of information [75]. What is more, these ion pumps normally do not maintain the same level of work over time. A characteristic pattern of fluctuation in activity, can add specific rhythms to the spatial patterns. Channel clustering, especially in cell protrusions is very important - this information came from experiments of Kindzelskii and Petty [76] who showed that in neutrophils this phenomenon can significantly lower the signal-noise ratio (see above). At the lamellipodia, store operated Ca2+ channels are clustered and inhibition of these channels abolished the migration response of these cells. It seems likely that these store-operated channels are part of plasma membrane proteins, which can be affected by weak electric fields (EF). In addition, several of such channels are members of the transient receptor potential-like (TRP) family of gene products. Among these proteins, TRP1 is a lipid raft-associated protein [77,78]. These clusters of receptors will by drawn by the charge difference of a putative electrode or charge gradient and the charged receptor in a kind of micro-iontophoresis [79]. Kindzelskii and Petty [76] again, could show that clusters of such proteins enhance the sensitivity for EF detection and that a discontinuous cell geometry with clustered “receptors” favors EF detection whereas spherical cells with equal distribution of receptors are relative insensitive. The number of the clustered “receptors” can amount to 106 in clusters of µm size. Thus, an estimated signal/noise ratio of at least a factor of 30 can result.
And, again, if the EF come rhythmic in a resonance frequency of the receptor - “antennae”, than it is clear that this elicits a more expressed effect [80] (Figure 1).
Figure 1.
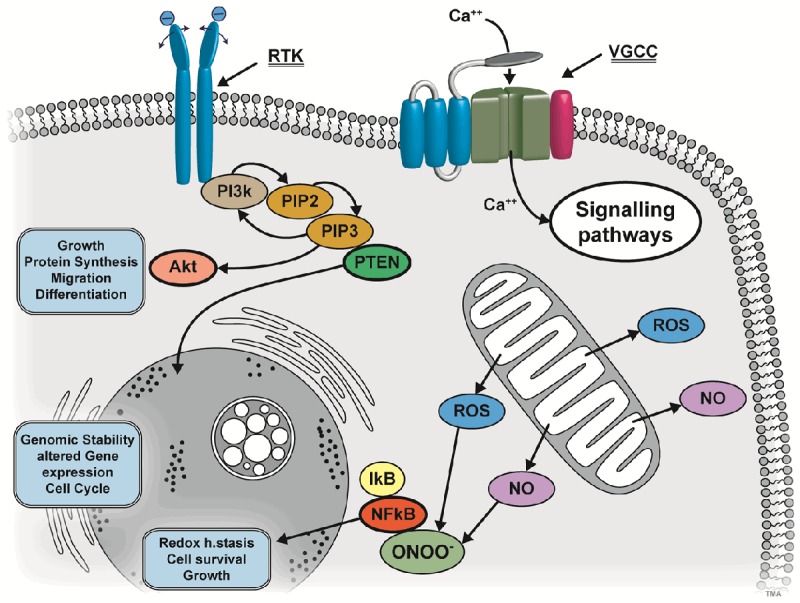
Different ways of PEMF-coupling to molecular biology of the cell: Upper left: ligands with polar moieties can go into resonance with PEMF-frequencies. Downstream events are elicited e.g. via receptor tyrosine kinases (RTK) PIP2 (Phosphatidylinositol 4,5-biphosphate), PIP3 (Phosphatidylinositol 3,4,5-triphosphate) and lipid Phosphatase PTEN (Phosphatase and Tensin homolog). PIP3 can signal further via Akt and Akt itself is the center of many other signaling pathways. Thus, many functional ways can be accessed by these signaling cascades. Upper right: voltage-gated calcium channels (VGCCs) can be addressed directly by PEMF. The Ca++ stream into the cell can act on many other pathways and organelles. Bottom right: PEMF can also act by its magnetic component on radical production and in medium with oxygen also to radical oxygen species (ROS). Further, by spin triplet reorientation also a directional component can be induced. Nitric oxygen (NO) can also be released from mitochondria by PEMF and by radical production. NO and ROS in turn can also react to peroxynitride (ONOO-). This in turn will activate IκB and NFκB and this can elicit in “moderate” amounts cell reactions which lead to a kind of “pre-conditioning” and protection.
An additional enhancing of the sensitivity can be reached by coherence and cooperative interaction of receptors to receptors or channels to channels (the distance of individual channels normally being only about 7 nm). This coupling may take place via conformational mechanisms or via other coupling (electron tunneling or other quantum effects). All these mechanisms may further improve the signal amplification ([81,82] see [76]). Phase-matched EF in the presence of ion channel clusters caused e.g. myeloperoxidase (MPO) to traffic to the cell surface. As MPO participates in high amplitude metabolic oscillations, this suggests a link between the signaling apparatus and metabolic changes [76]. Thus, channel clustering plays an important role in EF detection and downstream responses.
At least, a very important factor for regulation of cell homeostasis is the level of the resting potential, generated on the cell membrane. What is more, recent studies imply that the resting potential is a key regulator of cell cycle as well as of proliferation. Depolarization of cell membrane potential by external changes in ion concentration inhibits G1/S progression of Schwann cells, astrocytes, fibroblasts and lymphocytes. This suggests that hyperpolarization should be important for initiating S-phase [83-85]. Many proteins are involved in this membrane potential triggered cell cycle control [85]. For G2/M transition, depolarization of the plasma membrane should be mandatory. In total a rhythmic change to hyperpolarization before DNA synthesis to longer depolarization during mitosis can be found as general pattern in tissue embryogenesis and regeneration [86-89].
Cell cycle can also determine cell fate in diseases, means depending on outside conditions, the resting membrane potential level can switch in a flip flop manner into different states - especially if the order between the cells is perturbed during a diseased state. This may happen also between larger groups of cells; because ion transmitting gap junctions exist as well as other ways to convey information. Nowadays computer-modeling studies arise, showing how groups of cells with altered membrane potential level behave compared to normal cells [80].
On the other hand, relatively few papers exist on how the resting potential in cells and group of cells is altered in pathogenesis, e.g. during inflammation. It is only known that inflammation causes a lowering of the threshold for action potentials [88]. Regarding inflammation- induced joint pain, Hatch et al., describe that hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are implicated [80].
In fact, the observation that the level of resting potential can switch from a diseased potential back to normal could be a very good argument for EMF/PEMF therapy [75]. Means, this therapy may trigger the tendency of the resting potential into the direction of a switch back from diseased to normal state.
To sum up the above-mentioned findings, a natural counterpart exits in the tissue environment for the ULF part of the EMF spectrum and for PEMF. And, one should keep in mind regarding time coordination that endogenous EFs precede most mechanical and biochemical processes in development, wound healing and regeneration.
Causal molecular and cellular mechanisms of PEMF
As already mentioned, to be effective, EMF-stimuli have to be coherent [90], presenting a train of regularly recurring signals. The stimuli must be present for a certain minimum duration [91]. “Windows” were found for certain frequencies at cell and molecular levels: for the brain [92-94] and also for non-neural cells [95,96]. In human granulocytes, Sontag and Dertinger [97] investigated the liberation of prostaglandin E2 (PGE2) during application of EMF of different frequencies: here “windows” at 6 and 16 Hz were found, where PGE was 200% above 0 Hz baseline. Beneath these “windows” (e.g. at 10 Hz) PGE was only slightly above the baseline.
Interestingly, PEMF pulsation frequencies and application profiles mostly have been “copied” from the above mentioned naturally occurring frequencies in order to give “healing signals” to the body. However, one has to consider that pulsing in near rectangular shape produces a spectrum of multiple frequencies [80].
The molecular mechanisms underlying the direct coupling of the electric field to the cells are largely unknown. PEMF are comprised of low energy photons and so the question arises how such low or non - thermal effect can act on cells and tissues.
Lever/antennae
Charged receptors or other kinds of ‘antennae’ outside the cell membrane should recognize EMF by their ability to resonate with respective EMF frequencies by appropriate dimensions of the moving parts that hold a charge on the free end. The resonance frequency thereby depends on the length of this lever (Figure 1). Induced surface charge movements on the membrane trigger then signaling pathways e.g. via a receptor tyrosine kinase [98,99] (Figure 1). This phenomenon is similar an electrophoretic mobility of charged molecules in the cell membrane exposed to a static EF. The induced charge movement would represent at least a modification of Coulombic forces on the outside of the cell [100,101] or a modification of the charge distribution on the attachment surface.
Proton channels
Regarding directionality in cell migration our group could show in experiments with DC EF electrode-stimulation that NaKA and NHE3 voltage sensitive channels on the cell membrane can act as directional sensors in EFinduced directional cell motility, indeed [102,103]. These channels act via PIP2 as a potential mediator and the cell membrane potential again is a regulatory cue (Figure 1). Using SaOS-2 and primary osteoblasts representing anode- and cathode-directed motility we show that active NHE3 is concentrated in membrane protrusions that are accompanied by proton fluxes at the leading edge of the cellular migration. This activity is required for the perception of direction. On the other hand NHE1 is homogenously localized throughout the surface membrane and is involved in directional migration. The resting potential as a result of NaKA activity has a regulatory function that maintains the persistent directionality by modulating the spatiotemporal changes between leading edge (hyperpolarized) and rear end (depolarized) in migrating cells.
Resting potential
For regenerative therapy the fact is important, that e.g. in human mesenchymal stem cells (hMSCs), cell differentiation is accompanied by a progressive hyperpolarization. Artificial depolarization holds these cells in an undifferentiated (stem cell-like) state, while artificial hyperpolarization accelerates differentiation [104]. For example, membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. In the next step of transduction from changes in resting membrane potential to intracellular mechanisms it is discussed an increase in Ca++ entry into the cell (see below) and a positive feedback loop between Ca++ entry and Ca++ dependent potassium channels [105]. In further signaling cascades till gene regulation, e.g. phosphatase and tensin homolog (PTEN) (see below) is involved as well as epigenetic regulators like histone decarboxylase (HDAC). Here, Levin [75] reports that the lipid phosphatase PTEN was found to be a component of an intrinsic voltage sensor [106]. PTEN negatively regulates the PI3K and Akt pathway by reducing the available amount of PtdIns [75,107,108] P3. Furthermore, genetic abrogation of pten enhanced ERK and Akt phosphorylation, and potentiated field-induced keratinocyte migration [75,109] (Figure 1).
Magnetic component
If we look at the influence of the magnetic component of PEMF it is known that ROS are characterized by very short intermediate spin triplets with one free triplet radical. Under the assumption that radicals will be produced, the direction of the magnetic field lines interferes with triplet orientation and by aligning of the free triplet radical along the field lines a directional information can happen. This information is used in the retina (radicals are produced here by blue light) of some bird species to orient along the weak field lines of earth’s magnetic field [110]. And, because the photoreceptors are aligned in perfect hemispheres, retinae are ideal antennas. In general, this triplet information again can be used by downstream signaling pathways (see above) to elicit the manifold cellular reactions.
Here, Ehnert et al. [111] could show that single exposure to ELF-PEMF induced ROS production in human osteoblasts, without reducing intracellular glutathione. Repetitive exposure to PEMF, however reduced ROS-levels, suggesting alterations in the cell’s antioxidative stress response. PEMF exposure also induced expression of GPX3, SOD2, CAT and GSR on mRNA, protein and enzyme activity level. The above-mentioned authors found that scavenging of radical species diminished the PEMF effect on osteoblasts function (AP activity and mineralization [111]. However, challenging with low amounts of H2O2 on the other hand improved the function. Thus, it is concluded that PEMF elicited non-toxic amounts of ROS. This might represent an interesting adjunct to conventional therapy supporting bone formation.
Direct action on voltage-gated calcium channels (VGCCs)
In a very comprehensive review regarding EMF - effect on biological tissue Pall [112] found out that the common denominator of many EMF-effect studies is a direct action on voltage-gated calcium channels (VGCCs) (Figure 1). This is normally accompanied by a rapid increase of Ca2+ [113-117]. The multiple reactions followed by an increase of Ca2+ include also the Ca2+/calmodulin dependent nitric oxide synthases like (neuronal-) nNOS, (endothelial-) eNOS and inducible NOS (iNOS); expressed in many cell types in response to cytokines and other agents to generate large amounts of NO ([118-121]. The NO produced can also react with radical induced superoxide to form peroxynitrite (ONOO-) (Figure 1). And indeed, EMF-studies exist, which show a concomitant rise of NO and ROS [122-125].
On the other hand, NO and ONOO- are often generated in excess during inflammatory and pathological conditions, contributing to associated toxic effects [126]. However, in physiological conditions - as signaling pathway - or after application of near infrared light (see [119]) a moderately generated amount of ROS, NO and ONOO- can occur, leading to a kind of preconditioning reaction of the cell which is beneficial and a shelter for subsequently released stress factors [119]. In detail, moderately generated ROS, NO and ONOO- lead to activation of IκB and NFκB which is then translocated into the nucleus and by this leads to altered gene expression causing cell survival, growth and proliferation and redox homeostasis [119] (see Figure 1). Thus, many of the above mentioned beneficial effects can be explained as secondary or tertiary effects of EMF or PEMF.
Regarding these presumed secondary and tertiary effects leading to preconditioning and cell survival it is interesting that PEMF was able to decrease the elevated levels of ER chaperons Grp94, PDI and the apoptosis marker CHOP in human liver carcinoma cell lines. Also cell viability was also improved by PEMF exposure. Thus, these results indicate that PEMF is able to alleviate ER stress (here induced by tunicamycin) [127]. The unfolded protein response (UPR) of the ER might also represent a kind of marker for preconditioning if this is a regulation after a short stress and can be compensated by the cell. If the cells in an inflamed zone have been functionally dissociated - like in inflammation or in an extreme stress, this UPR will lead into apoptosis (own results, submitted).
Let us go back to the situation using PEMF in therapy, then it is conceivable that cells can get some “orientation” again not only with regard of space but also with regard of timing (see above: [76]). Those space and time orientation cues give cells of inflamed zones a re-linking to the healthy tissue. Because the inflamed zone is in many aspects “decoupled” from the rest of the tissue - also by the cytokines released. Otherwise, older and stressed cells with no physiological orientation react with enhanced ROS production, pre-apoptotic signaling and signs of mitochondrial stress as well as other signs of energy - depletion [128].
So in sum, it may well be the case that by PEMF treatment of inflamed areas e.g. in osteoarthritis may switch the cells to a more healthy state. In this regard, new additional studies would be desirable also observing in vitro and vivo the resting potential of the stressed cells during and after PEMF.
Acknowledgements
The author thanks Torsten Schwalm and Kathrin Rienäcker for excellent technical assistance. Many thanks to the scientific staff and coworkers in the field of EMF and cell biology in our institute over the many years: Prof. T. Monsees, Dr. N. Ozkucur, Dr. C. Röhlecke, Dr. D. Wittig, Dr. S. Perike, Dr. U. Schumann, S. Bola, R. Blaesche, and W. Kandhavivorn.
Disclosure of conflict of interest
None.
References
- 1.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: roles of TNFalpha and IL-1beta in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–116. doi: 10.22203/ecm.v030a08. discussion 116-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Studer RK, Sowa GA, Vo NV, Kang JD. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976) 2008;33:2253–2259. doi: 10.1097/BRS.0b013e318182c35f. [DOI] [PubMed] [Google Scholar]
- 3.Zou J, Chen Y, Qian J, Yang H. Effect of a low-frequency pulsed electromagnetic field on expression and secretion of IL-1beta and TNFalpha in nucleus pulposus cells. J Int Med Res. 2017;45:462–470. doi: 10.1177/0300060516683077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller SL, Coughlin DG, Waldorff EI, Ryaby JT, Lotz JC. Pulsed electromagnetic field (PEMF) treatment reduces expression of genes associated with disc degeneration in human intervertebral disc cells. Spine J. 2016;16:770–776. doi: 10.1016/j.spinee.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Lencel P, Delplace S, Pilet P, Leterme D, Miellot F, Sourice S, Caudrillier A, Hardouin P, Guicheux J, Magne D. Cell-specific effects of TNF-alpha and IL-1beta on alkaline phosphatase: implication for syndesmophyte formation and vascular calcification. Lab Invest. 2011;91:1434–1442. doi: 10.1038/labinvest.2011.83. [DOI] [PubMed] [Google Scholar]
- 6.Gaspari S, Marcovecchio ML, Breda L, Chiarelli F. Growth in juvenile idiopathic arthritis: the role of inflammation. Clin Exp Rheumatol. 2011;29:104–110. [PubMed] [Google Scholar]
- 7.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN Jr. Autophagy in the degenerating human intervertebral disc: in vivo molecular and morphological evidence, and induction of autophagy in cultured annulus cells exposed to proinflammatory cytokines-implications for disc degeneration. Spine (Phila Pa 1976) 2015;40:773–782. doi: 10.1097/BRS.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 9.Rutges JP, Kummer JA, Oner FC, Verbout AJ, Castelein RJ, Roestenburg HJ, Dhert WJ, Creemers LB. Increased MMP-2 activity during intervertebral disc degeneration is correlated to MMP-14 levels. J Pathol. 2008;214:523–530. doi: 10.1002/path.2317. [DOI] [PubMed] [Google Scholar]
- 10.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 11.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 12.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-kappaB and MAP kinases. Eur Cell Mater. 2012;23:103–119. doi: 10.22203/ecm.v023a08. discussion 119-120. [DOI] [PubMed] [Google Scholar]
- 13.Noponen-Hietala N, Virtanen I, Karttunen R, Schwenke S, Jakkula E, Li H, Merikivi R, Barral S, Ott J, Karppinen J, Ala-Kokko L. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186–194. doi: 10.1016/j.pain.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Gabr MA, Jing L, Helbling AR, Sinclair SM, Allen KD, Shamji MF, Richardson WJ, Fitch RD, Setton LA, Chen J. Interleukin-17 synergizes with IFNgamma or TNFalpha to promote inflammatory mediator release and intercellular adhesion molecule-1 (ICAM-1) expression in human intervertebral disc cells. J Orthop Res. 2011;29:1–7. doi: 10.1002/jor.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falone S, Marchesi N, Osera C, Fassina L, Comincini S, Amadio M, Pascale A. Pulsed electromagnetic field (PEMF) prevents pro-oxidant effects of H2O2 in SK-N-BE(2) human neuroblastoma cells. Int J Radiat Biol. 2016;92:281–286. doi: 10.3109/09553002.2016.1150619. [DOI] [PubMed] [Google Scholar]
- 16.Iannitti T, Fistetto G, Esposito A, Rottigni V, Palmieri B. Pulsed electromagnetic field therapy for management of osteoarthritis-related pain, stiffness and physical function: clinical experience in the elderly. Clin Interv Aging. 2013;8:1289–1293. doi: 10.2147/CIA.S35926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadala M, Vallelunga A, Palmieri L, Palmieri B, Morales-Medina JC, Iannitti T. Mechanisms and therapeutic applications of electromagnetic therapy in Parkinson’s disease. Behav Brain Funct. 2015;11:26. doi: 10.1186/s12993-015-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryang We S, Koog YH, Jeong KI, Wi H. Effects of pulsed electromagnetic field on knee osteoarthritis: a systematic review. Rheumatology (Oxford) 2013;52:815–824. doi: 10.1093/rheumatology/kes063. [DOI] [PubMed] [Google Scholar]
- 19.Strauch B, Herman C, Dabb R, Ignarro LJ, Pilla AA. Evidence-based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet Surg J. 2009;29:135–143. doi: 10.1016/j.asj.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Cebrian JL, Gallego P, Frances A, Sanchez P, Manrique E, Marco F, Lopez-Duran L. Comparative study of the use of electromagnetic fields in patients with pseudoarthrosis of tibia treated by intramedullary nailing. Int Orthop. 2010;34:437–440. doi: 10.1007/s00264-009-0806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing D, Cai J, Shen G, Huang J, Li F, Li J, Lu L, Luo E, Xu Q. The preventive effects of pulsed electromagnetic fields on diabetic bone loss in streptozotocin-treated rats. Osteoporos Int. 2011;22:1885–1895. doi: 10.1007/s00198-010-1447-3. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Dong Y, Hou W, Ji Z, Zhi K, Yin Z, Wen H, Chen Y. Effects of PEMF on microcirculation and angiogenesis in a model of acute hindlimb ischemia in diabetic rats. Bioelectromagnetics. 2013;34:180–188. doi: 10.1002/bem.21755. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub MI, Herrmann DN, Smith AG, Backonja MM, Cole SP. Pulsed electromagnetic fields to reduce diabetic neuropathic pain and stimulate neuronal repair: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:1102–1109. doi: 10.1016/j.apmr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Canedo-Dorantes L, Garcia-Cantu R, Barrera R, Mendez-Ramirez I, Navarro VH, Serrano G. Healing of chronic arterial and venous leg ulcers through systemic effects of electromagnetic fields [corrected] . Arch Med Res. 2002;33:281–289. doi: 10.1016/s0188-4409(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 25.Roland D, Ferder M, Kothuru R, Faierman T, Strauch B. Effects of pulsed magnetic energy on a microsurgically transferred vessel. Plast Reconstr Surg. 2000;105:1371–1374. doi: 10.1097/00006534-200004040-00016. [DOI] [PubMed] [Google Scholar]
- 26.Goudarzi I, Hajizadeh S, Salmani ME, Abrari K. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats. Bioelectromagnetics. 2010;31:318–323. doi: 10.1002/bem.20567. [DOI] [PubMed] [Google Scholar]
- 27.Heden P, Pilla AA. Effects of pulsed electromagnetic fields on postoperative pain: a doubleblind randomized pilot study in breast augmentation patients. Aesthetic Plast Surg. 2008;32:660–666. doi: 10.1007/s00266-008-9169-z. [DOI] [PubMed] [Google Scholar]
- 28.Varani K, Vincenzi F, Targa M, Corciulo C, Fini M, Setti S, Cadossi R, Borea PA. Effect of pulsed electromagnetic field exposure on adenosine receptors in rat brain. Bioelectromagnetics. 2012;33:279–287. doi: 10.1002/bem.20704. [DOI] [PubMed] [Google Scholar]
- 29.van Bergen CJ, Blankevoort L, de Haan RJ, Sierevelt IN, Meuffels DE, d’Hooghe PR, Krips R, van Damme G, van Dijk CN. Pulsed electromagnetic fields after arthroscopic treatment for osteochondral defects of the talus: double-blind randomized controlled multicenter trial. BMC Musculoskelet Disord. 2009;10:83. doi: 10.1186/1471-2474-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannemann PF, van Wezenbeek MR, Kolkman KA, Twiss EL, Berghmans CH, Dirven PA, Brink PR, Poeze M. CT scan-evaluated outcome of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a randomised, multicentre, double-blind, placebocontrolled trial. Bone Joint J. 2014;96-B:1070–1076. doi: 10.1302/0301-620X.96B8.33767. [DOI] [PubMed] [Google Scholar]
- 31.Ongaro A, Pellati A, Bagheri L, Fortini C, Setti S, De Mattei M. Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells. Bioelectromagnetics. 2014;35:426–436. doi: 10.1002/bem.21862. [DOI] [PubMed] [Google Scholar]
- 32.Benazzo F, Cadossi M, Cavani F, Fini M, Giavaresi G, Setti S, Cadossi R, Giardino R. Cartilage repair with osteochondral autografts in sheep: effect of biophysical stimulation with pulsed electromagnetic fields. J Orthop Res. 2008;26:631–642. doi: 10.1002/jor.20530. [DOI] [PubMed] [Google Scholar]
- 33.de Girolamo L, Vigano M, Galliera E, Stanco D, Setti S, Marazzi MG, Thiebat G, Corsi Romanelli MM, Sansone V. In vitro functional response of human tendon cells to different dosages of low-frequency pulsed electromagnetic field. Knee Surg Sports Traumatol Arthrosc. 2015;23:3443–3453. doi: 10.1007/s00167-014-3143-x. [DOI] [PubMed] [Google Scholar]
- 34.Veronesi F, Fini M, Giavaresi G, Ongaro A, De Mattei M, Pellati A, Setti S, Tschon M. Experimentally induced cartilage degeneration treated by pulsed electromagnetic field stimulation; an in vitro study on bovine cartilage. BMC Musculoskelet Disord. 2015;16:308. doi: 10.1186/s12891-015-0760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuschech H, von Hehn U, Mikus E, Funk RH. Effects of PEMF on patients with osteoarthritis: results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics. 2015;36:576–585. doi: 10.1002/bem.21942. [DOI] [PubMed] [Google Scholar]
- 36.Rohde J. Die Gelenkschule. Manuelle Medizin. 2003;3:189–198. [Google Scholar]
- 37.Weintraub MI, Cole SP. A randomized controlled trial of the effects of a combination of static and dynamic magnetic fields on carpal tunnel syndrome. Pain Med. 2008;9:493–504. doi: 10.1111/j.1526-4637.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 38.Graak V, Chaudhary S, Bal BS, Sandhu JS. Evaluation of the efficacy of pulsed electromagnetic field in the management of patients with diabetic polyneuropathy. Int J Diabetes Dev Ctries. 2009;29:56–61. doi: 10.4103/0973-3930.53121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozguclu E, Cetin A, Cetin M, Calp E. Additional effect of pulsed electromagnetic field therapy on knee osteoarthritis treatment: a randomized, placebo-controlled study. Clin Rheumatol. 2010;29:927–931. doi: 10.1007/s10067-010-1453-z. [DOI] [PubMed] [Google Scholar]
- 40.Omar AS, Awadalla MA, El-Latif MA. Evaluation of pulsed electromagnetic field therapy in the management of patients with discogenic lumbar radiculopathy. Int J Rheum Dis. 2012;15:e101–108. doi: 10.1111/j.1756-185X.2012.01745.x. [DOI] [PubMed] [Google Scholar]
- 41.Rutjes AW, Nuesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, Brosseau L, Reichenbach S, Juni P. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst Rev. 2009:CD002823. doi: 10.1002/14651858.CD002823.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otter MW, McLeod KJ, Rubin CT. Effects of electromagnetic fields in experimental fracture repair. Clin Orthop Relat Res. 1998:S90–104. doi: 10.1097/00003086-199810001-00011. [DOI] [PubMed] [Google Scholar]
- 43.Hartig M, Joos U, Wiesmann HP. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur Biophys J. 2000;29:499–506. doi: 10.1007/s002490000100. [DOI] [PubMed] [Google Scholar]
- 44.Chang WH, Chen LT, Sun JS, Lin FH. Effect of pulse-burst electromagnetic field stimulation on osteoblast cell activities. Bioelectromagnetics. 2004;25:457–465. doi: 10.1002/bem.20016. [DOI] [PubMed] [Google Scholar]
- 45.Ferroni L, Tocco I, De Pieri A, Menarin M, Fermi E, Piattelli A, Gardin C, Zavan B. Pulsed magnetic therapy increases osteogenic differentiation of mesenchymal stem cells only if they are pre-committed. Life Sci. 2016;152:44–51. doi: 10.1016/j.lfs.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Zhai M, Jing D, Tong S, Wu Y, Wang P, Zeng Z, Shen G, Wang X, Xu Q, Luo E. Pulsed electromagnetic fields promote in vitro osteoblastogenesis through a Wnt/beta-catenin signalingassociated mechanism. Bioelectromagnetics. 2016 doi: 10.1002/bem.21961. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Veronesi F, Torricelli P, Giavaresi G, Sartori M, Cavani F, Setti S, Cadossi M, Ongaro A, Fini M. In vivo effect of two different pulsed electromagnetic field frequencies on osteoarthritis. J Orthop Res. 2014;32:677–685. doi: 10.1002/jor.22584. [DOI] [PubMed] [Google Scholar]
- 48.De Mattei M, Caruso A, Pezzetti F, Pellati A, Stabellini G, Sollazzo V, Traina GC. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res. 2001;42:269–279. doi: 10.3109/03008200109016841. [DOI] [PubMed] [Google Scholar]
- 49.De Mattei M, Fini M, Setti S, Ongaro A, Gemmati D, Stabellini G, Pellati A, Caruso A. Proteoglycan synthesis in bovine articular cartilage explants exposed to different low-frequency low-energy pulsed electromagnetic fields. Osteoarthritis Cartilage. 2007;15:163–168. doi: 10.1016/j.joca.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 50.De Mattei M, Pasello M, Pellati A, Stabellini G, Massari L, Gemmati D, Caruso A. Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connect Tissue Res. 2003;44:154–159. [PubMed] [Google Scholar]
- 51.Ciombor DM, Aaron RK, Wang S, Simon B. Modification of osteoarthritis by pulsed electromagnetic field--a morphological study. Osteoarthritis Cartilage. 2003;11:455–462. doi: 10.1016/s1063-4584(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 52.Fini M, Giavaresi G, Torricelli P, Cavani F, Setti S, Cane V, Giardino R. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res. 2005;23:899–908. doi: 10.1016/j.orthres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Fini M, Torricelli P, Giavaresi G, Aldini NN, Cavani F, Setti S, Nicolini A, Carpi A, Giardino R. Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed Pharmacother. 2008;62:709–715. doi: 10.1016/j.biopha.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Nicolin V, Ponti C, Baldini G, Gibellini D, Bortul R, Zweyer M, Martinelli B, Narducci P. In vitro exposure of human chondrocytes to pulsed electromagnetic fields. Eur J Histochem. 2007;51:203–212. [PubMed] [Google Scholar]
- 55.Ongaro A, Pellati A, Masieri FF, Caruso A, Setti S, Cadossi R, Biscione R, Massari L, Fini M, De Mattei M. Chondroprotective effects of pulsed electromagnetic fields on human cartilage explants. Bioelectromagnetics. 2011;32:543–551. doi: 10.1002/bem.20663. [DOI] [PubMed] [Google Scholar]
- 56.Tepper OM, Callaghan MJ, Chang EI, Galiano RD, Bhatt KA, Baharestani S, Gan J, Simon B, Hopper RA, Levine JP, Gurtner GC. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004;18:1231–1233. doi: 10.1096/fj.03-0847fje. [DOI] [PubMed] [Google Scholar]
- 57.de Girolamo L, Stanco D, Galliera E, Vigano M, Colombini A, Setti S, Vianello E, Corsi Romanelli MM, Sansone V. Low frequency pulsed electromagnetic field affects proliferation, tissuespecific gene expression, and cytokines release of human tendon cells. Cell Biochem Biophys. 2013;66:697–708. doi: 10.1007/s12013-013-9514-y. [DOI] [PubMed] [Google Scholar]
- 58.Miyagi N, Sato K, Rong Y, Yamamura S, Katagiri H, Kobayashi K, Iwata H. Effects of PEMF on a murine osteosarcoma cell line: drugresistant (P-glycoprotein-positive) and nonresistant cells. Bioelectromagnetics. 2000;21:112–121. doi: 10.1002/(sici)1521-186x(200002)21:2<112::aid-bem6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 59.Li JK, Lin JC, Liu HC, Sun JS, Ruaan RC, Shih C, Chang WH. Comparison of ultrasound and electromagnetic field effects on osteoblast growth. Ultrasound Med Biol. 2006;32:769–775. doi: 10.1016/j.ultrasmedbio.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Lohmann C, Boyan B, Simon B, Schwartz Z. Pulsed electromagnetic fields have direct effects on growth plate chondrocytes. Osteologie. 2005;14:769–775. [Google Scholar]
- 61.Diniz P, Soejima K, Ito G. Nitric oxide mediates the effects of pulsed electromagnetic field stimulation on the osteoblast proliferation and differentiation. Nitric Oxide. 2002;7:18–23. doi: 10.1016/s1089-8603(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 62.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 63.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spiecker M, Peng HB, Liao JK. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IkappaBalpha. J Biol Chem. 1997;272:30969–30974. doi: 10.1074/jbc.272.49.30969. [DOI] [PubMed] [Google Scholar]
- 65.Funk RH, Knels L, Augstein A, Marquetant R, Dertinger HF. Potent stimulation of blood flow in fingers of volunteers after local shortterm treatment with low-frequency magnetic fields from a novel device. Evid Based Complement Alternat Med. 2014;2014:543564. doi: 10.1155/2014/543564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boopalan PR, Arumugam S, Livingston A, Mohanty M, Chittaranjan S. Pulsed electromagnetic field therapy results in healing of full thickness articular cartilage defect. Int Orthop. 2011;35:143–148. doi: 10.1007/s00264-010-0994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aaron RK, Ciombor DM. Pain in osteoarthritis. Med Health R I. 2004;87:205–209. [PubMed] [Google Scholar]
- 68.Boopalan PR, Daniel AJ, Chittaranjan SB. Managing skin necrosis and prosthesis subluxation after total knee arthroplasty. J Arthroplasty. 2009;24:322, e323–327. doi: 10.1016/j.arth.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res. 2002;20:40–50. doi: 10.1016/S0736-0266(01)00071-7. [DOI] [PubMed] [Google Scholar]
- 70.Caliskan SG, Bilgin MD, Kozaci LD. Effect of pulsed electromagnetic field on MMP-9 and TIMP-1 levels in chondrosarcoma cells stimulated with IL-1beta. Asian Pac J Cancer Prev. 2015;16:2701–2705. doi: 10.7314/apjcp.2015.16.7.2701. [DOI] [PubMed] [Google Scholar]
- 71.Tang X, Alliston T, Coughlin D, Miller S, Zhang N, Waldorff EI, Ryaby JT, Lotz JC. Dynamic imaging demonstrates that pulsed electromagnetic fields (PEMF) suppress IL-6 transcription in bovine nucleus pulposus cells. J Orthop Res. 2018;36:778–787. doi: 10.1002/jor.23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becker RO. The body electric: electromagnetism and the foundation of life. New York: William Morrow and Company; 1985. [Google Scholar]
- 73.Hastings GW, Mahmud FA. Electrical effects in bone. J Biomed Eng. 1988;10:515–521. doi: 10.1016/0141-5425(88)90109-4. [DOI] [PubMed] [Google Scholar]
- 74.Antonsson EK, Mann RW. The frequency content of gait. J Biomech. 1985;18:39–47. doi: 10.1016/0021-9290(85)90043-0. [DOI] [PubMed] [Google Scholar]
- 75.Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:261–270. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Kindzelskii AL, Petty HR. Ion channel clustering enhances weak electric field detection by neutrophils: apparent roles of SKF96365-sensitive cation channels and myeloperoxidase trafficking in cellular responses. Eur Biophys J. 2005;35:1–26. doi: 10.1007/s00249-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 77.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolinscaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 78.Trevino CL, Serrano CJ, Beltran C, Felix R, Darszon A. Identification of mouse trp homologs and lipid rafts from spermatogenic cells and sperm. FEBS Lett. 2001;509:119–125. doi: 10.1016/s0014-5793(01)03134-9. [DOI] [PubMed] [Google Scholar]
- 79.Poo M. In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng. 1981;10:245–276. doi: 10.1146/annurev.bb.10.060181.001333. [DOI] [PubMed] [Google Scholar]
- 80.Funk RH, Monsees T, Ozkucur N. Electromagnetic effects - from cell biology to medicine. Prog Histochem Cytochem. 2009;43:177–264. doi: 10.1016/j.proghi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Duke TA, Bray D. Heightened sensitivity of a lattice of membrane receptors. Proc Natl Acad Sci U S A. 1999;96:10104–10108. doi: 10.1073/pnas.96.18.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neumann E, Siemens PM, Toensing K. Electroporative fast pore-flickering of the annexin V-lipid surface complex, a novel gating concept for ion transport. Biophys Chem. 2000;86:203–220. doi: 10.1016/s0301-4622(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 83.Nuccitelli R. Ionic currents in morphogenesis. Experientia. 1988;44:657–666. doi: 10.1007/BF01941026. [DOI] [PubMed] [Google Scholar]
- 84.Borgens RB. Electric fields in vertebrate repair: natural and applied voltages in vertebrate regeneration and healing. A.R. Liss. 1989 [Google Scholar]
- 85.Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- 86.Borgens RB. Are limb development and limb regeneration both initiated by an integumentary wounding? A hypothesis. Differentiation. 1984;28:87–93. doi: 10.1111/j.1432-0436.1984.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 87.Borgens RB, McGinnis ME, Vanable JW Jr, Miles ES. Stump currents in regenerating salamanders and newts. J Exp Zool. 1984;231:249–256. doi: 10.1002/jez.1402310209. [DOI] [PubMed] [Google Scholar]
- 88.Cooper MS, Keller RE. Perpendicular orientation and directional migration of amphibian neural crest cells in dc electrical fields. Proc Natl Acad Sci U S A. 1984;81:160–164. doi: 10.1073/pnas.81.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lund EJ. The electrical polarity of Obelia and frog’s skin, and its reversible inhibition by cyanide, ether and chloroform. J Exp Zool. 1926;44:383–396. [Google Scholar]
- 90.Adey WR. Electromagnetics in biology and medicine. In: Matsumoto H, editor. Modern radio science. Oxford: Oxford University Press; 1993. [Google Scholar]
- 91.Litovitz TA, Krause D, Penafiel M, Elson EC, Mullins JM. The role of coherence time in the effect of microwaves on ornithine decarboxylase activity. Bioelectromagnetics. 1993;14:395–403. doi: 10.1002/bem.2250140502. [DOI] [PubMed] [Google Scholar]
- 92.Bawin SM, Kaczmarek LK, Adey WR. Effects of modulated VHF fields on the central nervous system. Ann N Y Acad Sci. 1975;247:74–81. doi: 10.1111/j.1749-6632.1975.tb35984.x. [DOI] [PubMed] [Google Scholar]
- 93.Blackman CF, Benane SG, House DE, Joines WT. Effects of ELF (1-120 Hz) and modulated (50 Hz) RF fields on the efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics. 1985;6:1–11. doi: 10.1002/bem.2250060102. [DOI] [PubMed] [Google Scholar]
- 94.Kolomytkin O, Yurinska M, Zharikov S. Response of brain receptor systems to microwave energy. In: Frey AH, editor. On the nature of electromagnetic field interactions with biological systems. Austin, TX: GR Landes; 1994. pp. 195–206. [Google Scholar]
- 95.Byus CV, Pieper SE, Adey WR. The effects of low-energy 60-Hz environmental electromagnetic fields upon the growth-related enzyme ornithine decarboxylase. Carcinogenesis. 1987;8:1385–1389. doi: 10.1093/carcin/8.10.1385. [DOI] [PubMed] [Google Scholar]
- 96.Walleczek J. Immune cell interactions with extremely low frequency magnetic fields: experimental verification and free radical machanisms. In: Frey AH, editor. On the nature of electromagnetic field interactions with biological systems. Austin TX: RG Landes; 1994. pp. 167–180. [Google Scholar]
- 97.Sontag W, Dertinger H. Response of cytosolic calcium, cyclic AMP, and cyclic GMP in dimethylsulfoxide-differentiated HL-60 cells to modulated low frequency electric currents. Bioelectromagnetics. 1998;19:452–458. [PubMed] [Google Scholar]
- 98.Fitzsimmons RJ, Baylink DJ. Growth factors and electromagnetic fields in bone. Clin Plast Surg. 1994;21:401–406. [PubMed] [Google Scholar]
- 99.Fitzsimmons RJ, Strong DD, Mohan S, Baylink DJ. Low-amplitude, low-frequency electric field-stimulated bone cell proliferation may in part be mediated by increased IGF-II release. J Cell Physiol. 1992;150:84–89. doi: 10.1002/jcp.1041500112. [DOI] [PubMed] [Google Scholar]
- 100.Otter MW, Porres L, McLeod KJ. An investigation of the Brownian ratchet in MC-3T3-E1 osteoblast-like cells using atomic force microscopy. Trans Soc Phys Regul Biol Med. 1996:16. [Google Scholar]
- 101.Otter MW, Rubin CT, McLeod KJ. Can the response of bone to extremely weak stimuli be explained by the Brownian ratchet? Ann Biomed Eng. 1997;25(Suppl 1) [Google Scholar]
- 102.Ozkucur N, Song B, Bola S, Zhang L, Reid B, Fu G, Funk RH, Zhao M. NHE3 phosphorylation via PKCeta marks the polarity and orientation of directionally migrating cells. Cell Mol Life Sci. 2014;71:4653–4663. doi: 10.1007/s00018-014-1632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ozkucur N, Perike S, Sharma P, Funk RH. Persistent directional cell migration requires ion transport proteins as direction sensors and membrane potential differences in order to maintain directedness. BMC Cell Biol. 2011;12:4. doi: 10.1186/1471-2121-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 107.Levin M. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell. 2014;25:3835–3850. doi: 10.1091/mbc.E13-12-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 109.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 110.Wiltschko R, Wiltschko W. Sensing magnetic directions in birds: radical pair processes involving cryptochrome. Biosensors (Basel) 2014;4:221–242. doi: 10.3390/bios4030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ehnert S, Fentz AK, Schreiner A, Birk J, Wilbrand B, Ziegler P, Reumann MK, Wang H, Falldorf K, Nussler AK. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of *O2(-) and H2O2. Sci Rep. 2017;7:14544. doi: 10.1038/s41598-017-14983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lisi A, Ledda M, Rosola E, Pozzi D, D’Emilia E, Giuliani L, Foletti A, Modesti A, Morris SJ, Grimaldi S. Extremely low frequency electromagnetic field exposure promotes differentiation of pituitary corticotrope-derived AtT20 D16V cells. Bioelectromagnetics. 2006;27:641–651. doi: 10.1002/bem.20255. [DOI] [PubMed] [Google Scholar]
- 114.Hojevik P, Sandblom J, Galt S, Hamnerius Y. Ca2+ ion transport through patch-clamped cells exposed to magnetic fields. Bioelectromagnetics. 1995;16:33–40. doi: 10.1002/bem.2250160109. [DOI] [PubMed] [Google Scholar]
- 115.Barbier E, Dufy B, Veyret B. Stimulation of Ca2+ influx in rat pituitary cells under exposure to a 50 Hz magnetic field. Bioelectromagnetics. 1996;17:303–311. doi: 10.1002/(SICI)1521-186X(1996)17:4<303::AID-BEM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 116.Craviso GL, Choe S, Chatterjee P, Chatterjee I, Vernier PT. Nanosecond electric pulses: a novel stimulus for triggering Ca2+ influx into chromaffin cells via voltage-gated Ca2+ channels. Cell Mol Neurobiol. 2010;30:1259–1265. doi: 10.1007/s10571-010-9573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rao VS, Titushkin IA, Moros EG, Pickard WF, Thatte HS, Cho MR. Nonthermal effects of radiofrequency-field exposure on calcium dynamics in stem cell-derived neuronal cells: elucidation of calcium pathways. Radiat Res. 2008;169:319–329. doi: 10.1667/RR1118.1. [DOI] [PubMed] [Google Scholar]
- 118.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beirne K, Rozanowska M, Votruba M. Photostimulation of mitochondria as a treatment for retinal neurodegeneration. Mitochondrion. 2017;36:85–95. doi: 10.1016/j.mito.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 120.McDonald LJ, Murad F. Nitric oxide and cyclic GMP signaling. Proc Soc Exp Biol Med. 1996;211:1–6. doi: 10.3181/00379727-211-43950a. [DOI] [PubMed] [Google Scholar]
- 121.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Esmekaya MA, Ozer C, Seyhan N. 900 MHz pulse-modulated radiofrequency radiation induces oxidative stress on heart, lung, testis and liver tissues. Gen Physiol Biophys. 2011;30:84–89. doi: 10.4149/gpb_2011_01_84. [DOI] [PubMed] [Google Scholar]
- 123.Aydin B, Akar A. Effects of a 900-MHz electromagnetic field on oxidative stress parameters in rat lymphoid organs, polymorphonuclear leukocytes and plasma. Arch Med Res. 2011;42:261–267. doi: 10.1016/j.arcmed.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Guler G, Turkozer Z, Tomruk A, Seyhan N. The protective effects of N-acetyl-L-cysteine and epigallocatechin-3-gallate on electric fieldinduced hepatic oxidative stress. Int J Radiat Biol. 2008;84:669–680. doi: 10.1080/09553000802241747. [DOI] [PubMed] [Google Scholar]
- 125.Guney M, Ozguner F, Oral B, Karahan N, Mungan T. 900 MHz radiofrequency-induced histopathologic changes and oxidative stress in rat endometrium: protection by vitamins E and C. Toxicol Ind Health. 2007;23:411–420. doi: 10.1177/0748233707080906. [DOI] [PubMed] [Google Scholar]
- 126.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 127.Keczan E, Keri G, Banhegyi G, Stiller I. Effect of pulsed electromagnetic fields on endoplasmic reticulum stress. J Physiol Pharmacol. 2016;67:769–775. [PubMed] [Google Scholar]
- 128.Gonzalez-Freire M, de Cabo R, Bernier M, Sollott SJ, Fabbri E, Navas P, Ferrucci L. Reconsidering the Role of Mitochondria in Aging. J Gerontol A Biol Sci Med Sci. 2015;70:1334–1342. doi: 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]


