TO THE EDITOR:
Mobilized hematopoietic stem and progenitor cells (HSPCs) collected from the peripheral blood are the most common source of HSPC used for stem cell transplantation. Granulocyte colony-stimulating factor (G-CSF) is the most common agent used for stem cell mobilization. Unfortunately, 5% to 30% of patients fail to mobilize sufficient stem cells for autologous stem cell transplantation in response to G-CSF.1,2 Although other modalities, including adding plerixafor to G-CSF or chemomobilization, have been used to enhance mobilization, collection of adequate numbers of HSPC remains a major problem. Therefore, new strategies are needed to optimize stem cell mobilization.1 We previously reported that bortezomib directly and rapidly mobilizes HSPC in mice by modulating the VLA-4/VCAM-1 axis.3 A phase 1 clinical trial of bortezomib plus G-CSF for stem cell mobilization in patients with MM was completed at Washington University in St. Louis (NCT02220608). Preliminary results from this phase 1 trial suggest that administration of bortezomib at the time of peak G-CSF mobilization is safe and well tolerated and enhances G-CSF–induced stem cell mobilization.4 Ixazomib is a next-generation small-molecule proteasome inhibitor that has several potential advantages over bortezomib, including oral route of administration and lower risk of peripheral neuropathy.5 Here we studied the effect of ixazomib on stem cell mobilization in mice.
Ixazomib (Ninlaro) was the first oral proteasome inhibitor approved by the US Food and Drug Administration for the treatment of multiple myeloma in combination with lenalidomide and dexamethasone in patients who have received at least 1 prior therapy.5 Ixazomib is a potent, reversible, and specific inhibitor of the 20S proteasome, which has potentially superior penetration in tissues compared with bortezomib because of its shorter half-life of dissociation from the 20S proteasome, resulting in less trapping in circulating red blood cells, which have high concentrations of proteasomes.5-7 Preclinical studies of ixazomib have used oral and IV dose administration.7 To investigate the ability of ixazomib to mobilize HSPC, we treated mice with a single dose of: (1) ixazomib (IV, 2 mg/kg), (2) ixazomib (IV, 4 mg/kg), (3) ixazomib (IV, 8 mg/kg), (4) ixazomib (IV, 14 mg/kg, the maximal tolerated dose in mice), and (5) vehicle (ixazomib diluent, 2-hydroxypropyl-β-cyclodextrin) IV. Mice were analyzed for peripheral blood colony forming unit-cells (PB CFU-Cs) at baseline, and 12, 15, 24, and 48 hours after dosing. We subsequently compared the effects of IV vs oral gavage (o.g.) administration of ixazomib on HSPC mobilization in mice. Additionally, to compare the ability of ixazomib and bortezomib to mobilize HSPC, we treated mice with a single dose of: (1) ixazomib (o.g., 8 mg/kg), (2) bortezomib (IV, 0.8 mg/kg), and (3) control diluent for ixazomib. Blood was harvested at baseline, and 12, 15, and 24 hours after ixazomib or bortezomib administration. We also hypothesized that ixazomib, similar to bortezomib, will augment G-CSF and plerixafor mobilization effect. To test this hypothesis, we compared the mobilization effect of G-CSF only with G-CSF + ixazomib and plerixafor only with plerixafor + ixazomib. The G-CSF + ixazomib group received G-CSF (250 µg/kg/day subcutaneously [SC]) on days 1, 2, 3, and 4, and ixazomib on day 4. Blood was harvested on day 5, 12 hours after day 4 ixazomib administration. The G-CSF control group received G-CSF on days 1, 2, 3, and 4. Blood was harvested on day 5. The plerixafor + ixazomib group received ixazomib (o.g., 8 mg/kg), at baseline and plerixafor (5 mg/kg SC) 12 hours later. The plerixafor group received plerixafor (5 mg/kg SC). Blood was harvested 1 to 3 hours after plerixafor administration in both groups. Both DBA mice and BALB-C mice were used in these experiments. Harvested peripheral blood was plated on MethoCult media (Stem Cell Technologies) for CFU-C enumeration.
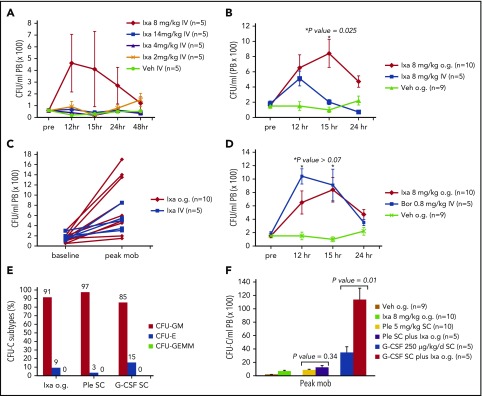
When ixazomib was administered IV to mice, an 8 mg/kg dose showed a trend toward higher mobilization, although it was not statistically significant (Figure 1A). Mean PB CFU-C at 12 hours after administration of vehicle and ixazomib at 2, 4, 8, and 14 mg/kg were 20/mL (standard error [SE], 12/mL), 90/mL (SE, 45/mL), 40/mL (SE, 18/mL), 460/mL (SE, 243/mL), and 70/mL (SE, 25/mL), respectively. Based on this experiment, the 8 mg/kg IV dose was used for subsequent experiments for comparison with orally dosed ixazomib. Ixazomib dosed at 8 mg/kg o.g. mobilized more HSPC than ixazomib dosed at 8 mg/kg IV (Figure 1B-C). A trend toward higher mobilization was noted at 12 hours, but became significant at 15 hours (P = .025). Compared with vehicle alone, a single oral dose of ixazomib at 8 mg/kg resulted in a significant rise (∼fivefold over baseline) in PB CFU-C 12 to 15 hours after administration (mean peak CFU-C, 132/mL; SE, 38/mL vs 713/mL; SE, 123/mL after vehicle and ixazomib administration respectively; P = .0003; Figure 1B). The majority of CFU-C mobilized by ixazomib were CFU-granulocyte and monocyte (Figure 1E).The magnitude and kinetics of HSPC mobilization in the single ixazomib oral gavage group were very similar to a single dose of IV bortezomib (Figure 1D; P > .07). There was no statistically significant difference in peak HSPC mobilization between the ixazomib (12 hours after administration) and plerixafor groups (1 to 3 hours after plerixafor administration) (Figure 1F; mean peak of 650/mL; SE, 171/mL vs 810/mL; SE, 180/mL, respectively; P = .518), suggesting that ixazomib is a potent HSPC mobilizing agent with intermediate mobilization kinetics compared with plerixafor (1 to 3 hours) and G-CSF (5 to 6 days) and with indistinguishable kinetics from IV bortezomib (12 to 15 hours). In G-CSF + ixazomib experiments, ixazomib administration on day 4 of G-CSF resulted in significant augmentation in PB CFU-C counts compared with G-CSF alone (Figure 1F), but in plerixafor + ixazomib experiments, addition of ixazomib resulted in a trend toward higher mobilization compared with plerixafor alone that was not statistically significant (Figure 1F).
Figure 1.
Mobilization of HSPC by ixazomib in mice. Mice were analyzed for peripheral blood (PB) CFU-C at various time points. (A) Mice were treated with vehicle (Veh) or ixazomib (Ixa) IV at 2, 4 , 8, or 14 mg/kg. Mice were analyzed for PB CFU-C at baseline and 12, 15, 24, and 48 hours (hr; n = 5 mice/group). (B) Mice were treated with IV ixazomib (n = 5) vs o.g. of ixazomib (n = 10). Mice were analyzed for PB CFU-C at baseline and 12, 15, and 24 hours (P = .025 comparing oral vs IV ixazomib; P = .0003 comparing oral ixazomib vs Veh). (C) Peak mobilization of each mouse treated with ixazomib o.g. vs IV. (D) Ixa o.g. vs bortezomib (Bor) HSPC mobilization. PB CFU-C were analyzed at baseline and 12, 15, and 24 hours after ixazomib and bortezomib (*P > .07 comparing peak oral ixazomib mobilization vs peak IV bortezomib mobilization). (E) CFU-C subtypes after ixazomib o.g. plerixafor SC, and G-CSF SC, CFU-erythroid (CFU-E), CFU-granulocyte and monocyte (CFU-GM), and CFU-granulocyte and erythrocyte and monocyte, and megakaryocyte (CFU-GEMM). (F) Mobilization of HSPC in response to ixazomib in combination with G-CSF and plerixafor. Mean PB CFU-C in G-CSF + ixazomib vs GCSF alone: 11 320/mL; SE, 1760/mL vs 3420/mL; SE, 915/mL, respectively; P = .01. Mean PB CFU-C in plerixafor plus ixazomib vs plerixafor: 1190/mL; SE, 361/mL vs 810/mL; SE, 180/mL, respectively; P = .34. Data are mean ± SE.
Although we did not perform mechanistic studies in these experiments, we speculate that ixazomib mobilization effect is similar to bortezomib via modulation of VLA4/VCAM1 axis as we reported previously.3 Our experiments show that optimal ixazomib dose for mobilization in mice is 8 mg/kg, and the higher dose of 14 mg/kg has a reduced mobilization effect. It is possible that cytotoxic effects of ixazomib at 14 mg/kg neutralize its mobilization effect. CFU/GEMM are very rare multipotential progenitor cells in mobilized products. We did not see CFU-GEMM in these experiments and flow cytometry for detection of long- and short-term stem cells or early progenitors was not performed. The quest for a consistent, rapid, and robust HSPC mobilization regimen for patients undergoing both autologous and allogeneic stem cell transplant has remained an elusive goal. Although clinical reports in man have suggested that proteasome inhibitors have no deleterious effect on mobilization,4,8 no reports have yet shown that ixazomib directly mobilizes HSPC in mouse or man. In this report, we are the first to show that ixazomib directly and rapidly mobilizes HSPC in mice (with intermediate kinetics between that of plerixafor and G-CSF) and augments G-CSF mobilization effect. The potential tumor purging effect of ixazomib in addition to its mobilizing activity would be highly desirable in patients with multiple myeloma. The clinical role of ixazomib as a rapid mobilizing agent in man is yet to be determined.
Acknowledgments
J.F.D. is supported by grants from the National Institutes of Health, National Cancer Institute (U54 CA199092 [principal investigator: S. Achelifu], R01 CA152329-A1 [principal investigator: J.F.D.], and R35 CA210084-01A [principal investigator: J.F.D.]). A.G. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2 TR002346 (principle investigator: Victoria J. Fraser). M.P.R. is supported by the National Institutes of Health, National Cancer Institute (R50 CA211466). Takeda Pharmaceuticals provided ixazomib.
Authorship
Contribution: A.G., M.P.R., L.E., and J.F.D. designed and analyzed the experiments and wrote the paper; A.G., M.P.R., M.S.H., K.K., E.C., and J.K.R. performed the animal study; and all authors discussed the results and commented on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: jdipersi@wustl.edu.
References
- 1.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26(1):34-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43(3):181-195. [DOI] [PubMed] [Google Scholar]
- 3.Ghobadi A, Rettig MP, Cooper ML, et al. . Bortezomib is a rapid mobilizer of hematopoietic stem cells in mice via modulation of the VCAM-1/VLA-4 axis. Blood. 2014;124(17):2752-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghobadi A, Fiala MA, Uy GL, Stockerl-Goldstein K, Vij R, DiPersio JF. A phase I study of the safety and feasibility of bortezomib in combination with G-CSF for stem cell mobilization in patients with multiple myeloma [abstract]. Blood. 2016;128(22). Abstract 3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brayer J, Baz R. The potential of ixazomib, a second-generation proteasome inhibitor, in the treatment of multiple myeloma. Ther Adv Hematol. 2017;8(7):209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupperman E, Lee EC, Cao Y, et al. . Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer [published correction appears in Cancer Res. 2010;70(9):3853]. Cancer Res. 2010;70(5):1970-1980. [DOI] [PubMed] [Google Scholar]
- 7.Lee EC, Fitzgerald M, Bannerman B, et al. . Antitumor activity of the investigational proteasome inhibitor MLN9708 in mouse models of B-cell and plasma cell malignancies. Clin Cancer Res. 2011;17(23):7313-7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Giralt S, Stadtmauer EA, et al. ; International Myeloma Working Group. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114(9):1729-1735. [DOI] [PubMed] [Google Scholar]