Abstract
Decoding how tissue properties emerge across multiple spatial and temporal scales from the integration of local signals is a grand challenge in quantitative biology. For example, the collective behavior of epithelial cells is critical for shaping developing embryos. Understanding how epithelial cells interpret a diverse range of local signals to coordinate tissue-level processes requires a systems-level understanding of development. Integration of multiple signaling pathways that specify cell signaling information requires second messengers such as calcium ions. Increasingly, specific roles have been uncovered for calcium signaling throughout development. Calcium signaling regulates many processes including division, migration, death, and differentiation. However, the pleiotropic and ubiquitous nature of calcium signaling implies that many additional functions remain to be discovered. Here we review a selection of recent studies to highlight important insights into how multiple signals are transduced by calcium transients in developing epithelial tissues. Quantitative imaging and computational modeling have provided important insights into how calcium signaling integration occurs. Reverse-engineering the conserved features of signal integration mediated by calcium signaling will enable novel approaches in regenerative medicine and synthetic control of morphogenesis.
Keywords: developmental biology, morphogenesis, bow-tie network, cell signaling, information processing, communication, collective behavior, computational modeling
1. Introduction
Organ development requires extensive coordination among and within heterogeneous cell populations to generate complex organ morphologies [1–3]. During morphogenesis, epithelial cells must process a large range of environmental signals to implement the genetic program that drives development. To do so, a sophisticated cell communication network processes a broad range of inputs to compute output responses [3,4]. As a second messenger, the calcium ion (Ca2+) is a central integrator embedded in this network (Figure 1A, [5,6]). However, the central role and pleiotropic functions of Ca2+ signaling make it difficult to assign mechanistic roles to specific Ca2+ signaling dynamics. The recent development of genetically encoded Ca2+ indicators such as the fluorogenic GCaMP sensors have begun to reveal new roles for Ca2+ signaling in epithelial development [7,8]. Due to nonlinear and stochastic dynamics, computational modeling is essential for decoding Ca2+ signals in developing tissues as reviewed in [9–11].
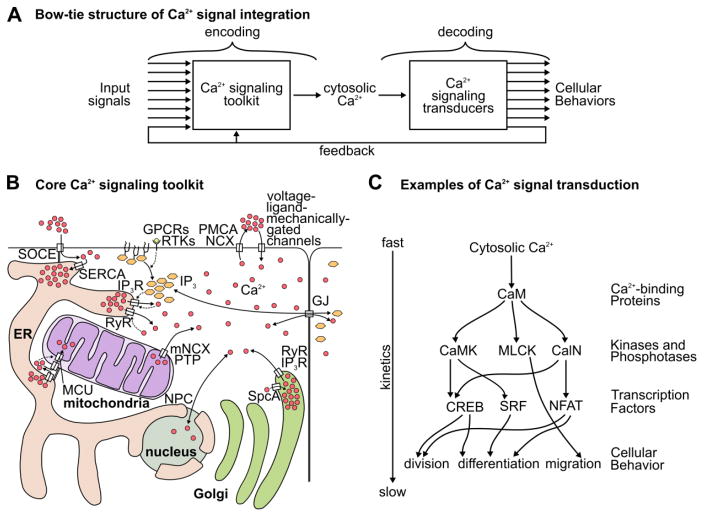
Figure 1. The transport and activity of Ca2+ in signal integration.
(A) General network structure of Ca2+ signaling. (B) Molecular diagram of cellular Ca2+ transport. Solid arrows represent transport. Dashed arrows represent regulatory interactions. (C) Ca2+ binds to Ca2+-binding proteins, which can buffer Ca2+ concentrations and activate enzymatic targets and transcription factors. Activation of kinases and phosphatases can affect the cell rapidly. Altering cell fate through transcription factors takes longer because the timescale of transcriptional machinery is slower. Ca2+ impacts many cellular functions including division, growth, death, differentiation, and migration. Arrows represent activating or inhibitory regulations. Specific components of the calcium signaling machinery are described in the text.
This review is structured as follows. First, the Ca2+ signaling network is described, with emphasis on how the core Ca2+ toolkit encodes dynamic Ca2+ signals, how downstream transduction machinery decodes these signals, and general principles of the network structure of Ca2+ signaling. Second, several biological examples of decoding and encoding of Ca2+ dynamics in epithelial development are described. Finally, the review closes with a summary and an overview of unanswered questions for Ca2+ signaling in epithelia. Many of these questions are applicable to other second messengers and for other tissue types.
2. Ca2+ signaling: ubiquitous, pleiotropic, yet specific
The molecular mechanisms defining the core Ca2+ signaling toolkit in cells are well established and ubiquitous across cell types (a few representative reviews include [12–16]). Yet, cellular behaviors in response to Ca2+ signaling are often cell type specific. This is in part because the encoding of information through Ca2+ dynamics depends on the signal transduction genes expressed (Figure 1A–B). Ca2+ is downstream of many chemical, mechanical, and electrical inputs, and transduces these inputs into a dynamic signal: the concentration of cytosolic Ca2+. This dynamic signal encodes more information than a static signal, and a large network of genes interprets this information, allowing Ca2+ to affect many diverse processes (Figure 1B–C).
Transmission of Ca2+ signaling information between compartments in the cell or between cells occurs through the control of fluxes by Ca2+ channels. Several organelles serve as Ca2+ ion reservoirs that can be regulated to amplify signals (Figure 1B). Decoding Ca2+ information requires downstream proteins with protein domains that reversibly bind to Ca2+ as well as proteins that are regulated by Ca2+-binding proteins (Figure 1C). Cellular processes that are influenced by Ca2+ signaling are diverse and include cell growth, division, death, differentiation, migration, cytoskeletal mechanics, and protein secretion (reviewed in, among others, [17–19]). Here we briefly discuss these components and their functions.
2.1. Molecular components of the Ca2+ signaling toolkit
The cytosol must maintain low concentrations of Ca2+ (~0.1 μM [12,13]) to avoid toxicity. The mitochondrial matrix is maintained at slightly higher levels (~0.1–2.0 μM [20]). Conversely, the extracellular fluid, other organelles, and the space between the inner and outer mitochondrial membranes are generally maintained at high Ca2+ concentrations (~50–1000 μM [12,13]). Channels permit diffusion of Ca2+ ions down a concentration gradient, and can be opened to induce a rapid and efficient Ca2+ response [13,21–23]. Mechanically-gated [24,25], electrically-gated [26], and receptor-operated channels [14,27–30] all contribute to the wide range of stimuli that can regulate Ca2+ concentrations in the cell. For example, extrinsic tensile forces on epithelia causes the stretch-activated Ca2+ channel Piezo to induce a Ca2+ response, causing extracellular signal-regulated kinase (ERK) phosphorylation, which leads to cell division [31]. Two additional classes of membrane-bound ligand receptors can activate Ca2+ signaling: G-protein-coupled receptors (GPCRs) and receptor tyrosine kinase (RTKs), which stimulate release of Ca2+ from the ER stores through phospholipase C (PLC) activation of inositol trisphosphate receptors (IP3Rs).
The cell actively sequesters Ca2+ into organelles and across the plasma membrane to maintain a low Ca2+ concentration in the cytosol (Figure 1B). This role is fulfilled by various Ca2+-ATPases, which are high-affinity, low-capacity pumps. The high affinity of Ca2+-ATPases ensures the pumps are constitutively active. A low capacity results in a long time-scale required for significantly changing cytosolic Ca2+ concentrations. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA, [32]), plasma membrane Ca2+ ATPase (PMCA, [33]), and secretory pathway Ca2+ ATPase (SpcA, [34]) maintain a gradient of Ca2+ across the ER, plasma membrane, and Golgi apparatus membranes respectively. When the concentration of Ca2+ in the cytosol spikes beyond the limits of what the Ca2+-ATPase pumps can efficiently remove, the dormant capacity of low-affinity, high-capacity pumps such as the Na+/Ca2+ exchanger (NCX, [33]) jump into action to rapidly return the cytosol to basal levels. The mitochondria has an analogous mitochondrial Na+/Ca2+ exchanger (mNCX, [35]) as its primary Ca2+-extrusion pump. Additionally, stress can cause the permeability transition pore (PTP) complex to assemble, leading to a rapid equilibration of cytosolic and mitochondrial Ca2+ concentration, frequently leading to cell death [36]. Together, these molecular components maintain a low Ca2+ concentration in the cytosol and mitochondrial matrix, and a high concentration in other organelles.
A recent quantitative study of organelle distribution in fibroblast-like cells has revealed the extent to which the ER colocalizes with other organelles [37]. In particular, 25% the volume of mitochondria was in contact with ER volume [37]. These connections are stabilized by the mitofusin proteins (MFN, [38]) and facilitate Ca2+ transfer from the ER to the mitochondria. Ca2+ released at these sites enters the mitochondrial lumen through the voltage-dependent anion channel (VDAC, [39]) and the low-affinity mitochondrial uniporter complex (MCU, [40]).
When the nuclear pore complex (NPC) is open, Ca2+ freely diffuses between the cytosol and the nucleus. The degree to which cells regulate the concentration of Ca2+ in the nucleus is debated; however nuclear Ca2+ has been shown to affect transcription [41–43], and nuclear Ca2+ oscillations have also been implicated in cell division [44]. The NPC can undergo conformational changes in response to Ca2+ concentration [45], and can close, which can result in differential Ca2+ concentration in the nucleus and the cytosol.
Many proteins contain a Ca2+-binding domain. Ca2+ binding to an EF-hand or C2 domain causes proteins to alter their structure and therefore function [46,47]. Calmodulin (CaM [48]) is a key protein that transduces changes in Ca2+ concentration to downstream target proteins (Figure 1C). Additionally, proteins such as calbindin (Calb) buffer Ca2+ concentration. Ca2+ buffering proteins alter the dynamics of free cytosolic Ca2+ and slow down Ca2+ diffusion. CaM and similar effectors can result in specific and rapid activation of kinases and phosphatases including CaM kinase (CaMK [49,50]), myosin light chain kinase (MLCK [51]), and calcineurin (CalN [50,52]), and transcription factors such as nuclear factor of activated T-cells (NFAT [53]), serum response factor (SRF [54,55]), and cAMP response element-binding protein (CREB [56,57]). These kinases and transcription factors decode Ca2+ signals and enact the relevant outcomes including cell death [58], proliferation [43,59–61], migration [62,63], secretion [64,65], and differentiation [66–68].
2.2. Spatial and dynamical properties of Ca2+ signaling
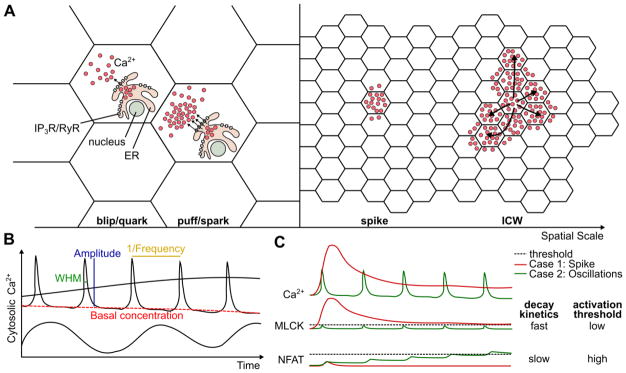
The buffering of Ca2+ leads to subcellular compartmentalization of Ca2+ responses [69,70]. Ca2+ dynamics can occur on the order of less than a micron (reviewed in [13,71,72]). The two smallest scales of Ca2+ signaling that have been described are blips (also referred to as quarks), and puffs (also referred to as sparks) (Figure 2A). Blips are caused by the opening of an individual membrane-bound channel, while puffs are caused by multiple adjacent channels opening in concert. Generation of Ca2+ signals can be subcellularly-localized due to sparsity of ligand-gated Ca2+ channels such as IP3R and RyR [13,72,73], especially near the nuclear pore complex [74]. One functional implication of subcellular compartmentalization of Ca2+ signals is that transcriptional regulation by Ca2+ may be localized to the nucleus and may be independent from signaling events at the cell membrane [71,75]. Another scale of Ca2+ signaling are spikes, which are large enough to excite most of the channels in the cell [66,76–79]. Ca2+ signals of sufficiently-high magnitude can propagate to neighboring cells through gap junctional diffusion or paracrine signaling, resulting in intercellular Ca2+ transients or waves. This wide range of spatial scales greatly increases the design-space for Ca2+ to impact the behavior of the whole system.
Figure 2. Spatial and temporal properties of Ca2+ dynamics.
(A) Cartoon of four spatial scales of Ca2+ dynamics. Blips involve only one receptor activated channel. Puffs involve more than one receptor-activated channel (IP3R or RyR). In a spike, the entire cell is active, and Ca2+ may spread to adjacent cells. An intercellular Ca2+ wave (ICW) involves multiple full-cell responses that are chained together, resulting in a self-sustaining transient of Ca2+ signaling across an entire tissue. Arrows represent the diffusion of the Ca2+ signal dynamics. (B) Several examples of Ca2+ signals. Shown is a slow change in Ca2+ concentration, a sinusoidal Ca2+ oscillation, and Ca2+ oscillation spikes. An oscillatory signal can contain more information than a static signal including oscillation Frequency, Amplitude, Basal concentration, and Width at Half Max (WHM). (C) Example of how a dynamic signal can be decoded to activate one of two different targets. In this case, the red signal is a non-oscillatory sustained increase in basal Ca2+ concentration, and the green signal is an oscillatory signal with high frequency and low amplitude and WHM. MLCK has a low activation threshold and can detect the change in basal concentration caused by the red signal. NFAT has a slow decay rate and can accumulate after many individual Ca2+ oscillations.
Ca2+ oscillations contain far more information than the static concentrations of non-oscillatory signals through the amplitude, frequency, shape, and basal concentration, which can all be decoded by independent signaling pathways (Figure 2B). Ca2+ response times range from the order of nanoseconds or microseconds during membrane damage [80], seconds in response to mechanical or wounding stimuli [81–85], and on longer time-scales in response to changes in basal Ca2+ concentrations [62].
Oscillations in the cellular concentration of a species occur when the rate of production and decay rapidly shift to adapt to changes in inputs to restore homeostasis [4,86]. The requirement for every cell to control cytosolic Ca2+ leads to an increased tendency for oscillations to develop [86]. Ca2+ oscillations emerge as temporary increases in cytosolic Ca2+ inevitably lead to a strong push towards homeostasis, a feature common to other oscillatory phenomena [86]. Ca2+ channels are often inactivated by the resulting Ca2+ response, or close naturally over time allowing the cell to return to a basal Ca2+ level. The dynamic nature of these oscillations allows cytosolic Ca2+ to encode multiple signals.
One mechanism for this is demonstrated in Figure 2C, which shows how “ratcheting” and “non-ratcheting” mechanisms can be used to discriminate between high basal-concentration and high frequency signals [62]. Slow responses to Ca2+ signaling will integrate the signal over time, converting stationary but highly-dynamic Ca2+ signals into a first-order signal with a static set-point (Figure 2C). If the response is fast and insensitive to small perturbations in the signal, it will convert the signal into a spike train, which is a rapid series of discrete spikes similar to a neuronal action potential ([87], Figure 2C). The downstream transcription factor NFAT integrates the frequency of oscillatory Ca2+ signals, but not the amplitude [88]. NFκB is sensitive to infrequent Ca2+ oscillations that NFAT cannot detect [88,89]. A field of cells that would oscillate with different frequencies when cultured independently can be induced to undergo coordinated oscillations through the oscillation of a small population of “pacemaker cells” [90,91]. This is similar to applying a MAX operation to a pool of Ca2+ oscillation frequencies, in which the fastest-oscillating cells tend to set the pace for oscillations within the tissue, but also tend to slow down slightly. This adds an additional layer of spatial and population-level complexity to signal integration.
Ca2+ oscillations have been modeled extensively, both in excitable [92,93] and non-excitable [94,95] systems. Modeling has uncovered universal principles and archetypical motifs of Ca2+ signaling. One such work is the distinction between Class I and Class II models of IP3R-mediated Ca2+ oscillations, whereby IP3 is stable in the former and IP3 oscillates in the latter [9]. Additionally, extensive work has been done on the encoding and decoding of the frequency of Ca2+ oscillations [96–98]. These models have been reviewed ([10,11,99–101]) and are briefly summarized here.
Models for single-cell cytosolic Ca2+ concentration generally rely on three classes of fluxes: channel, pump, and leak (Figure 3A, [94]). These fluxes can occur across the plasma membrane or the membranes of organelles such as the ER. The molecular components involved in each of these fluxes are described in section 2.1. Release of Ca2+ into the cytosol through Ca2+ channels incorporates case-specific flux equations, and is often regulated by signaling from IP3, ATP, or Ca2+ (Figure 3A). Ca2+ pumping out of the cytosol is assumed to occur in a concentration-dependent manner. Another common assumption is that Ca2+ leaks from high concentration compartments into the cytosol at a constant rate. Tissue-scale models generally couple individual cells through extracellular diffusion across cells, jump boundary conditions between juxtaposed cell membranes, or homogenization of the tissue domain to model “effective” diffusion (Figure 3B). Common mechanisms include flux of Ca2+ or IP3 through gap junctions [102–104] or release of ligands such as ATP into the extracellular space [29,103,105]. Recent models coupling Ca2+ signaling and stress fiber formation can also lead to models for propagation of mechanical waves [106]. These modeling efforts explain how differential expression of Ca2+ toolkit components can result in different Ca2+ dynamics from the same input signal. The delicate balance of the three cellular fluxes and cell-cell communication can be summarized in bifurcation diagrams (Figure 2C). Bifurcation diagrams offers insight into movement of the system equilibrium to and from states of dynamic instability (Figure 2C).
Figure 3. Modeling Ca2+ dynamics in non-excitable epithelial tissues.
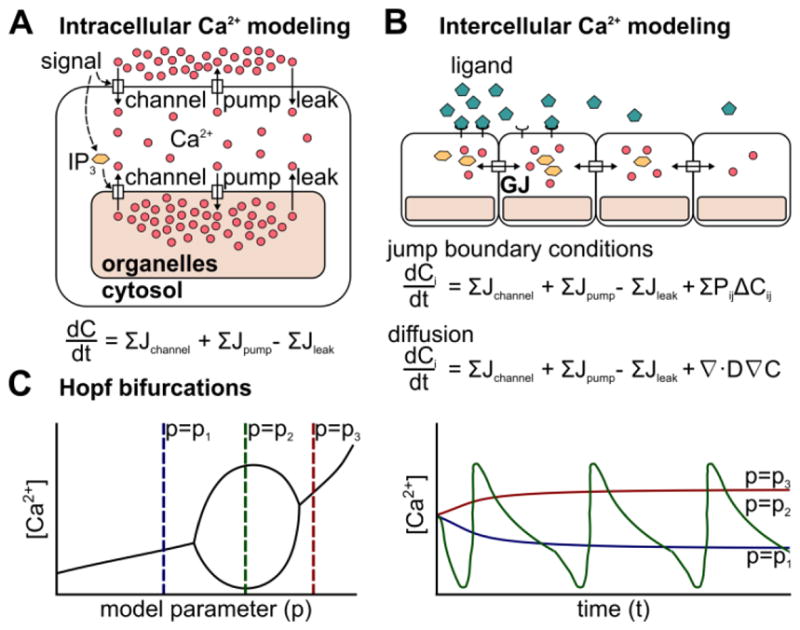
(A) Cartoon representation of molecular sources of various flux terms. General equation for chemical balance arising from molecular components of Ca2+ signaling toolkit. (B) Cartoon representation of Ca2+ flux through an epithelial sheet. General equation for chemical balance arising from cell-cell interactions. Pij represents the relative permeability of cells i and j, and is 0 when cells are not in contact. (C) Cartoon representation of Hopf-bifurcation in the parameter domain (p) and in the time domain (t). Oscillations arise for a specific range of model parameters.
Recently, stochastic models have been used to explore the kinetics of IP3R [107,108]. IP3R shifts stochastically between a highly-active “drive” and mainly inactive “park” mode. This model involves 10 rate constants, of which 2 depend on Ca2+, IP3, and ATP concentration [107]. Because ligand concentration is only important for switching between drive and park mode, this model updates the prior hypothesis that Ca2+ inhibits IP3R slowly over time. However, these mechanistic differences do not affect previous models as the overall temporal kinetics of the two cases are similar [9]. Other epithelial systems with recent advances in modeling Ca2+ waves include retinal pigment epithelium [109], urothelial cells [110], and the developing cochlea [111]. Additionally, experimentally-developed models have been proposed for coupled transport of Ca2+ and actomyosin after wounding that will require the development of computational models to test [84].
2.3. Information flow through the Ca2+ signaling network
Information in biological systems is frequently processed through signal transduction networks that include bow-tie motifs. In a bow-tie motif, many inputs are integrated into a specialized processing system with very few components, forming a knot, and regulate many targets (Figure 1A, [6,112–115]). Examples of bow-tie networks in biology include metabolism of key nutrients [112,116], and the immune response [117]. By relying on few components to perform many functions, the system becomes vulnerable to perturbations to those key components. Despite this fragility, bow-tie networks are able to react faster and more efficiently than networks with more central nodes [113].
Second messengers like Ca2+ are suited for a role as nodes within the central knot as their homeostasis is critical for cell survival. Therefore a great energetic investment is already made to regulate their concentrations [12,13,32,118]. This property improves the speed and efficiency by which a decision can be reached, and “consensus forming,” in which multiple competing inputs are combined into a single central message [6,113–115], (Figure 1A). Characterizing bow-tie networks that rely on second messengers offers valuable insight into how cells perform complex collective behaviors despite limited and local information. The problem of collective cell behavior mirrors the problem solved by “swarm intelligence,” wherein a system has many individuals interacting by simple rules, to self-organize into complex higher-order behaviors [119]. Ca2+ signaling contributes to the regulation of collective behaviors such as morphogenesis, regeneration, and wound healing by regulating cellular processes like division, death, migration, secretion, and differentiation. Compared with the great wealth of studies modeling the encoding of Ca2+ oscillations, there has been considerably less effort on the mechanisms behind the decoding of Ca2+ oscillations. Recent modeling efforts have yielded insight on how specific transcription factors decode frequency or amplitude of Ca2+ oscillations [96,120,121]. For example, shorter trains of oscillatory signals may target proteins more specifically than longer trains [122]. Much integrative modeling work remains to be done to connect both the coding and decoding modules of Ca2+ bow-tie network within the context of collective cell decision making that occurs during epithelial morphogenesis and homeostasis.
3. Ca2+ signaling during epithelial development
Development occurs over multiple spatial and temporal scales. Embryonic morphogenesis in many animal systems takes place over the course of many hours, and yet critical steps in gastrulation occur on the order of minutes. Precise coordination of adjacent cells is required for tissue-scale morphogenesis, despite individual cells only interacting with their immediate neighbors. Morphogen signaling through diffusible ligands is the primary mechanism by which cells coordinate throughout development [1–3,79,123]. Morphogen-based receptors can stimulate Ca2+ fluxes into cells (Figure 1B). The presence or absence of Ca2+ toolkit components and Ca2+ signal transducers allows for high specificity in the Ca2+ response to morphogen signals [13]. Several specific roles for Ca2+ regulation of morphogenesis have been proposed and have been reviewed in depth [17,123]. Here we review a selection of recent discoveries in which Ca2+ encodes diverse inputs to guide epithelial development.
3.1. Ca2+ dynamics encode growth factor concentration to coordinate tubulogenesis
Endothelia are specialized epithelial tissues that develop into vessels or tubes. Endothelial cells must reliably and irreversibly commit to specific cell behaviors that are needed to form a tube [124–126]. The growing tube is led by a tip cell. Adjacent cells referred to as stalk cells divide to increase the length of the tube (Figure 4A, [62,127,128]). A central regulator of tube formation is vascular endothelial growth factor (VEGF), which can stimulate cells to divide through NFAT signaling, or to migrate through MLCK, but both behaviors are mutually exclusive [62,124]. Ca2+ is the second messenger mediating these responses [62]. To determine how endothelial cells stimulated with VEGF choose between these two fates, Noren et. al. treated porcine aortic and human umbilical vein endothelial cells with VEGF and observed the resulting Ca2+ dynamics (Figure 4A, [62]). VEGF is required to stimulate a Ca2+ response. However, addition of VEGF at low concentrations led to populations of cells exhibiting rapid Ca2+ oscillations, and at higher concentrations led to cells exhibiting one large peak, followed by a return to basal Ca2+ concentration. These findings were confirmed in vivo by showing that high-frequency oscillations in zebrafish tip cells reduced tip migration, and was correlated with division in stalk cells [62].
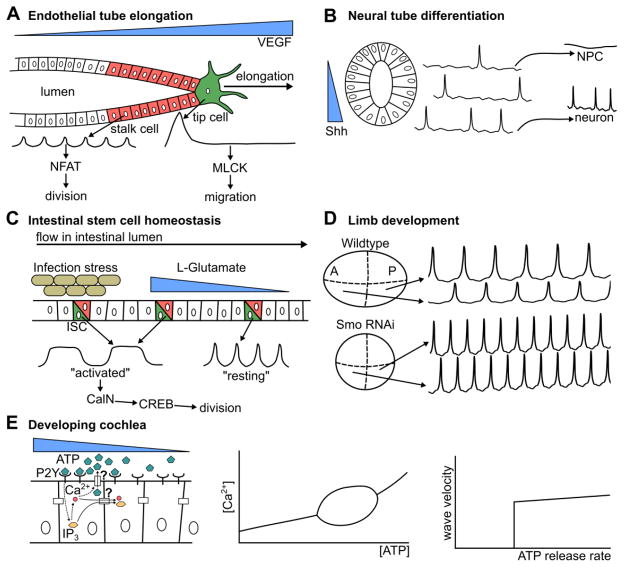
Figure 4. Ca2+ multiplexes morphogen and other ligand signals.
(A–E) Several examples where Ca2+ signaling interprets ligand concentrations and encodes information in dynamic features. (A) Ca2+ interprets VEGF signaling in porcine aortic endothelial cells. Cells closest to VEGF undergo a large, singular Ca2+ spike, which activates MLCK and induces migration. Cells further away undergo many smaller spikes, over time inducing NFAT activation and inducing proliferation. (B) In the Xenopus neural tube, Ca2+ spike frequency encodes Shh concentration and is correlated with neuronal fate specification. (C) High L-Glutamate or bacterial infection lead to longer, higher magnitude Ca2+ oscillations that can be interpreted by CREB through CalN and result in intestinal stem cell renewal. Low levels cause smaller spikes that do not induce cell division. ISC indicates intestinal stem cells. (D) Cells that secrete Hh exhibit higher oscillatory amplitudes in the developing wing disc. When Hh signaling is suppressed, the frequency and amplitude of Ca2+ oscillations are uniform and elevated in the entire disc. This indicates that multiple features of Ca2+ oscillations reflect the morphogen state of cells. A represents anterior, and P represents posterior compartment. (E) ATP induces Ca2+ waves across the developing cochlea. Mathematical modeling indicates that these oscillations primarily require ATP concentration to be within a specific range. The mechanism of propagation of the waves in the cochlea is paracrine signaling and not gap-junction mediated diffusion.
The authors propose that Ca2+ signaling functions to specify which of these fates is selected (Figure 4A, [62]). The two downstream pathways described in this work can be classified as “ratchet-like” and “non-ratcheting,” as described in Figure 2C. Ratchet-like mechanisms integrate many peaks because the signal transducer has a low decay rate and can accumulate over time as additional oscillations occur. Transducers in non-ratcheting mechanisms have a high decay rate, and reproduce the input signal with high fidelity, but are unable to maintain a “chemical memory” like ratchet-like pathways can. Many peaks of Ca2+ signaling are required to accumulate sufficient levels of activated NFAT to trigger downstream targets, making such a mechanism effective for filtering high-frequency oscillations. A non-ratcheting mechanism is unable to reach its activation threshold without a large increase in basal concentration as its downstream products rapidly decay. This makes non-ratcheting mechanisms effective in filtering short, high-magnitude signals. The cell utilizes the Ca2+ toolkit to robustly determine local VEGF concentration, specifying to the cell whether to express NFAT, activate MLCK, or exhibit no response in a mutually-exclusive manner [62]. These findings demonstrate that Ca2+ encodes VEGF concentration and is decoded to regulate both tip cell and stalk cell behavior during tube growth.
3.2. Ca2+ dynamics encode morphogen concentration to specify cell fate
The neural crest is the tissue from which neurons, glia, and other nervous system cell types are derived [129]. It is derived from the same embryonic tissue as epithelia—the ectoderm—and is initially comprised of tightly-adherent cells [129,130]. A gradient of Sonic Hedgehog (Shh) signaling directs the patterning of neuronal differentiation in the neural crest [77]. The development of the spinal cord in frogs is correlated with Ca2+ spike activity [66].
Recently, Belgacem and Borodinsky implicated a Ca2+-dependent form of non-canonical Type II Hh signaling in differentiation of neural crest cells (Figure 4B, [77,131]). The authors dissected the developing spinal cord and found a positive relationship between activation of Hh signaling through Shh ligand or Smoothened Agonist, and Ca2+ spike activity. The authors also demonstrated a decrease in GABAergic neuronal cell fates when decreasing Ca2+ signaling through voltage-gated channel blockers. Effectively, these spikes convert a noisy, continuous signal (Hh concentration) into a binary signal that specifies cell fates. Overall, these findings suggest that Ca2+ signaling encodes Shh concentration and is later decoded to differentiate neuronal crest cells into a GABAergic neuronal cell fate. A recent study has since found that Ca2+ signaling mediates the rearrangement of neural stem cells into rosettes in the neural tube, which is critical for neural development [132].
3.3. Ca2+ dynamics encode multiple inputs to coordinate epithelial stem cell division
Intestinal epithelial stem cells (ISCs) must continuously divide to maintain homeostasis in the intestine in response to metabolic conditions, stress, and Wnt signaling [133–136]. The rate of ISC proliferation is a critical factor in controlling the size and structure of the gut, as insufficient proliferation may cause a reduction in integrity and an increase in proliferation may cause hyperplasia or lead to tumorigenesis [133,136,137]. There are many inputs that an intestinal stem cell considers when deciding whether to divide including morphogenetic signals [138], tissue stress [139,140], and the nutritional state of the surrounding tissues [141,142]. For example, intestinal stem cells obtain metabolic information by binding of L-Glutamate to the metabotropic glutamate receptor (mGluR), a GPCR [134].
Deng et. al. recently showed Ca2+ functions as a key signal integrator to regulate proliferation in intestinal stem cells (Figure 4C [134]). They identified three “states” of Ca2+ oscillations that lead to different cell fates, including: 1. fast, low-amplitude oscillations (“resting”, 1–2 Hz), 2. slow, high amplitude oscillations (“activated”, 0.2–0.5 Hz), and 3. low amplitude, low frequency oscillations that were generally not observed in healthy tissues (“Ca2+ deficient”, less than 0.1 Hz). The authors found that the “activated” states led to proliferation specifically through activation of CREB by CalN and speculate that the role of the “resting” state is for cells to be able to rapidly adapt to environmental changes. They observed that glutamate stimulated cells to transition from a resting Ca2+ state to an activated state. Stimulation of the immune response with ecc15 bacterial infection also induced an “activated” Ca2+ waveform. This is consistent with evidence that bacterial infection increases division in intestinal stem cells [140]. A specific cell must make a consensus decision regarding proliferation despite receiving many potentially contradicting inputs. Ca2+ signaling provides a way for cells to encode these inputs into a single consensus signal that can then be decoded to regulate ICS homeostasis.
3.4. Ca2+ dynamics encode morphogen signal in multiple dynamic features in epithelia
Ca2+ signaling dynamics can also be spatially patterned in tissues, mirroring the patterns of morphogen signaling pathways. Spontaneous intercellular Ca2+ transients have recently been discovered in the developing butterfly and fly wing in vivo [78,143,144], and ex vivo in the presence of fly extract, a growth serum that contains a large range of active proteins [76,78,81,144,145]. Perturbation of Ca2+ signaling in the wing leads to a range of adult wing defects [78,144,146]. In the developing wing, the spatial range and frequency of these signaling events is decreased as the larvae ages. Ca2+ signaling dynamics can be investigated ex vivo by subjecting wing discs to different concentrations of fly extract. These yield qualitatively different types of Ca2+ signals in the wing disc, ranging from spikes, to long-range transients, to oscillatory waves, while absence of fly extract led to no Ca2+ oscillations, or rare, single-cell spikes [78]. The amplitudes of the Ca2+ oscillations ex vivo are higher in the posterior compartment of the pouch. Hh is secreted from the posterior compartment, but Hh signaling is not active in the posterior compartment (Figure 4D, [145,147]). Inhibition of the primary transducer of Hh signaling, Smoothened, in the pouch not only leads to an increase in oscillation frequency in the whole tissue, but also led to an increase in amplitude in the anterior compartment, leading to a uniform amplitude across the entire pouch [145]. These findings indicate that Ca2+ signaling exhibits spatial asymmetries downstream of morphogen signaling. Network inference methodologies may enable systems-level investigation of crosstalk between morphogen signaling and Ca2+ signaling dynamics in this tissue [148,149].
3.5. Modeling reveals the mechanism behind ICWs in the developing cochlea
The cochlea is the epithelial inner-ear organ responsible for converting mechanical signals caused by sound waves into electrical signals that can be transmitted to the brain [150]. The developing cochlea exhibits Ca2+ waves that are critical to development. In the cochlea, binding of ATP to purinergic (P2Y) receptors results in IP3R-mediated Ca2+ oscillations [151]. However, it is unknown whether the propagation of the resulting ICWs is due to diffusion of the resulting IP3 through gap junctions, or paracrine feedback through secretion of additional ATP by activated cells to facilitate activation of P2Y receptors in neighboring cells. This leads to two questions: 1. what is the mechanism by which the Ca2+ signal propagates in the cochlea, and 2. are IP3 oscillations required for the corresponding Ca2+ oscillations [111], (Figure 4E)?
Ceriani et. al. recently approached these questions using a mechanistic model incorporating generation of IP3 through P2Y signaling, IP3R-induced Ca2+ release, diffusion of ATP and IP3 [111]. The model predicted a Hopf bifurcation depending on the concentration of ATP outside of the stimulated cell (Figure 3C, 4E). This predicts that stable oscillations are obtained if and only if ATP is within a range of concentrations. This result was independent of IP3 oscillations, indicating that IP3 oscillations are not required for Ca2+ oscillations in the developing cochlea. This identifies the developing cochlea as a Class I system [9]. Additionally, the speed and extent of ICWs predicted by the model were consistent with those observed experimentally. The model showed that the wave propagation speed was bistable with respect to maximum rate of ATP release, either exhibiting a constant speed of around 10 μm/s, or no propagation at all. Conversely, IP3 diffusion through gap junctions did not affect wave propagation within physiological limits. This implicates ATP diffusion as the primary mechanism for ICW propagation in the developing cochlea over IP3 diffusion through gap junctions. Here, computational models played a critical role in understanding ICW formation in epithelial development.
4. Summary
Quantitative studies that map the encoding and decoding of Ca2+ signals during morphogenesis are beginning to crack the Ca2+ signaling code in epithelia. These encoding and decoding modules of Ca2+ signal transduction form a bow-tie network, which is critical for the role of Ca2+ as a signal integrator in epithelial development. In effect, Ca2+ signaling acts as an “embedded consultant” that helps inform cellular decisions. Differential expression of channels, pumps, and buffering proteins provide cell-type specificity in signals that are encoded by Ca2+ dynamics. The versatility of the Ca2+ toolkit allows many inputs to be encoded into a single dynamic signal (Figure 1A, [13]). Decoding Ca2+ dynamics through the bow-tie networks structure allows one signal to affect many downstream targets. There are many downstream proteins that decode Ca2+ signaling, including enzymes with fast dynamics on the scale of seconds. In this case, these proteins quickly respond to changes in Ca2+ binding kinetics. Transcription factors that act on the time scale of hours can integrate multiple peaks of Ca2+ oscillations through ratchet-like effects. Regardless of the variety of up-stream inputs, cytosolic Ca2+ encodes a single, albeit non-binary signal, allowing redundant or contradictory information to be removed in favor of consensus-building, which increases both the speed and efficiency of signal processing.
As demonstrated in several biological examples (Figure 4), Ca2+ signaling integrates information from multiple sources and can lead to different cell behaviors in developing epithelia. Recent mapping studies suggest that the developmental program generates spatiotemporal patterns of Ca2+ signal signatures, which include Ca2+ oscillation amplitude, frequency, and basal Ca2+ concentration. The broader implications of these signals remain to be discovered. However, like a consultant, Ca2+ is not always essential in the processes that it modulates. Depending on the context, Ca2+ oscillations could largely be a epiphenomenon or by-product of morphogenesis, provide primary features required for robust and efficient morphogenesis, or hint at the generation of a physiological-based memory system of morphogenetic patterns, necessary for wound healing and regeneration [152–154].
Many questions remain to be answered about the roles of Ca2+ as a signal integrator in developing epithelia and other tissue types. Ca2+ is necessary for morphogenesis, as no embryonic system has been found that can complete gastrulation in media devoid of Ca2+ [17]. Discerning the specific roles of Ca2+ in collective behavior within developing systems will require computational studies of tissue morphogenesis that couples chemical signaling with multi-scale descriptions of tissue morphogenesis [155–158]. Further, the generality of encoding/decoding modules across developmental systems needs to be characterized. The switch between division and migration in developing tubes has been observed in human and pig cells, as well as in zebrafish. Is this signaling motif universal, and if so, what other universal motifs rely on Ca2+ as a signal integrator? What specific morphogenetic unit operations rely on Ca2+ as a central integrator [159–161]? Part of the challenge in answering these questions is controlling the Ca2+ concentration of specific cells in developing epithelia. Optogenetic tools have recently been developed that will allow specific tissue regions, cells, or organelles to be targeted for Ca2+ release through optogenetic control of Ca2+ channels [121,162,163]. Further, control of downstream signal transducers including kinases, phosphatases, and transcription factors will allow a better understanding of the mechanisms involved in decoding Ca2+ signals. A better understanding of the role that Ca2+ plays in the integration of signals and the regulation of cellular processes will reveal systems-level insights into collective behavior in morphogenesis, wound healing, and regeneration. Future studies that create synthetic computational modules of Ca2+ signaling could be employed to control tissue mechanics synthetically or to embed sensors within developing tissues [164]. Finally, chemical-mechanical coupled models are needed to test develop theory linking Ca2+ signaling and actomyosin flow to cell mechanics.
Acknowledgments
The work in this manuscript was supported in part by NIH Grant R35GM124935, NSF Awards CBET-1403887, and CBET-1553826 and the Walther Cancer Foundation Interdisciplinary Interface Training Project (PB). The authors thank C. Narciso, Q. Wu, R. Paravitorghabeh, D. Soundarrajan, N. Kumar, and A. Lesko for feedback on early revisions of the manuscript. We apologize to authors that we did not cite due to space limitations.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Guillot C, Lecuit T. Mechanics of Epithelial Tissue Homeostasis and Morphogenesis. Science. 2013;340:1185–9. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 2.Paluch E, Heisenberg C-P. Biology and Physics of Cell Shape Changes in Development. Curr Biol. 2009;19:R790–9. doi: 10.1016/j.cub.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Lewis J. From Signals to Patterns: Space, Time, and Mathematics in Developmental Biology. Science. 2008;322:399–403. doi: 10.1126/science.1166154. [DOI] [PubMed] [Google Scholar]
- 4.Purvis JE, Lahav G. Encoding and Decoding Cellular Information through Signaling Dynamics. Cell. 2013;152:945–56. doi: 10.1016/j.cell.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb SE, Miller AL. Calcium signalling during embryonic development. Nat Rev Mol Cell Biol Lond. 2003;4:539–51. doi: 10.1038/nrm1149. [DOI] [PubMed] [Google Scholar]
- 6.Dodd AN, Kudla J, Sanders D. The Language of Calcium Signaling. Annu Rev Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 7.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mank M, Santos AF, Direnberger S, Mrsic-Flogel TD, Hofer SB, Stein V, Hendel T, Reiff DF, Levelt C, Borst A, Bonhoeffer T, Hübener M, Griesbeck O. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5:805–11. doi: 10.1038/nmeth.1243. [DOI] [PubMed] [Google Scholar]
- 9.Sneyd J, Han JM, Wang L, Chen J, Yang X, Tanimura A, Sanderson MJ, Kirk V, Yule DI. On the dynamical structure of calcium oscillations. Proc Natl Acad Sci. 2017 doi: 10.1073/pnas.1614613114. 201614613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rüdiger S. Stochastic models of intracellular calcium signals. Phys Rep. 2014;534:39–87. [Google Scholar]
- 11.Schuster S, Marhl M, Höfer T. Modelling of simple and complex calcium oscillations. Eur J Biochem. 2002;269:1333–55. doi: 10.1046/j.0014-2956.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 12.Clapham DE. Calcium Signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 15.Putney JW, Bird GSJ. The Inositol Phosphate-Calcium Signaling System in Nonexcitable Cells. Endocr Rev. 1993;14:610–31. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- 16.Foskett JK, White C, Cheung K-H, Mak D-OD. Inositol Trisphosphate Receptor Ca2+ Release Channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markova O, Lenne P-F. Calcium signaling in developing embryos: Focus on the regulation of cell shape changes and collective movements. Semin Cell Dev Biol. 2012;23:298–307. doi: 10.1016/j.semcdb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Elsholz F, Harteneck C, Muller W, Friedland K. Calcium - a central regulator of keratinocyte differentiation in health and disease. Eur J Dermatol. 2014;24:650–61. doi: 10.1684/ejd.2014.2452. [DOI] [PubMed] [Google Scholar]
- 19.Lansdown ABG. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10:271–85. doi: 10.1046/j.1524-475x.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- 20.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol-Cell Physiol. 1994;267:C313–39. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 21.Yamakage M, Namiki A. Calcium channels — basic aspects of their structure, function and gene encoding; anesthetic action on the channels — a reviewRevue: notions de base sur la structure, la fonction et l’encodage génétique des canaux calciques et action des anesthésiques sur ces canaux. Can J Anesth. 2002;49:151–64. doi: 10.1007/BF03020488. [DOI] [PubMed] [Google Scholar]
- 22.Verkhratsky A, Parpura V. Calcium signalling and calcium channels: Evolution and general principles. Eur J Pharmacol. 2014;739:1–3. doi: 10.1016/j.ejphar.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95:1383–436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol - Cell Physiol. 1992;262:C1083–8. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- 25.Franco-Obregón A, Lansman J. Calcium entry through stretch-in-activated ion channels in MDX myotubes. 1990;344 doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- 26.Catterall WA. Voltage-Gated Calcium Channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–3. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 28.Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- 29.Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 30.Striggow F, Ehrlich BE. Ligand-gated calcium channels inside and out. Curr Opin Cell Biol. 1996;8:490–5. doi: 10.1016/s0955-0674(96)80025-1. [DOI] [PubMed] [Google Scholar]
- 31.He L, Si G, Huang J, Samuel ADT, Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555:103–6. doi: 10.1038/nature25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Periasamy M, Huke S. SERCA Pump Level is a Critical Determinant of Ca2+ Homeostasis and Cardiac Contractility. J Mol Cell Cardiol. 2001;33:1053–63. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 33.Brini M, Carafoli E. The Plasma Membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wuytack F, Raeymaekers L, Missiaen L. PMR1/SPCA Ca2+ pumps and the role of the Golgi apparatus as a Ca2+ store. Pflüg Arch. 2003;446:148–53. doi: 10.1007/s00424-003-1011-5. [DOI] [PubMed] [Google Scholar]
- 35.Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen X, Madesh M, Houser SR, Elrod JW. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature. 2017;545:93–7. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–49. [PMC free article] [PubMed] [Google Scholar]
- 37.Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, Lippincott-Schwartz J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017;546:162–7. doi: 10.1038/nature22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 39.Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–24. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–4. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 41.Martins TV, Evans MJ, Wysham DB, Morris RJ. Nuclear pores enable sustained perinuclear calcium oscillations. BMC Syst Biol. 2016;10:55. doi: 10.1186/s12918-016-0289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J Cell Sci. 2009;122:2337–50. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- 43.Resende RR, Andrade LM, Oliveira AG, Guimarães ES, Guatimosim S, Leite MF. Nucleoplasmic calcium signaling and cell proliferation: calcium signaling in the nucleus. Cell Commun Signal. 2013;11:14. doi: 10.1186/1478-811X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng Y-C, Bennett AM, Nathanson MH. Nucleoplasmic Calcium Is Required for Cell Proliferation. J Biol Chem. 2007;282:17061–8. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoffler D, Fahrenkrog B, Aebi U. The nuclear pore complex: from molecular architecture to functional dynamics. Curr Opin Cell Biol. 1999;11:391–401. doi: 10.1016/S0955-0674(99)80055-6. [DOI] [PubMed] [Google Scholar]
- 46.Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem Sci. 1996;21:14–7. [PubMed] [Google Scholar]
- 47.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: Structural and functional diversity. Protein Sci. 1996;5:2375–90. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung WY. Calmodulin Plays a Pivotal Role in Cellular Regulation. Science. 1980;207:19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- 49.Koninck PD, Schulman H. Sensitivity of CaM Kinase II to the Frequency of Ca2+ Oscillations. Science. 1998;279:227–30. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 50.Saucerman JJ, Bers DM. Calmodulin Mediates Differential Sensitivity of CaMKII and Calcineurin to Local Ca2+ in Cardiac Myocytes. Biophys J. 2008;95:4597–612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hathaway DR, Adelstein RS. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci. 1979;76:1653–7. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci. 1979;76:6270–3. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain J, McCafffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 54.Miranti CK, Ginty DD, Huang G, Chatila T, Greenberg ME. Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca2+/calmodulin-dependent kinase. Mol Cell Biol. 1995;15:3672–84. doi: 10.1128/mcb.15.7.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. eScholarship. 2002;53:147–57. [PubMed] [Google Scholar]
- 56.Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–16. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheng M, Thompson MA, Greenberg ME. CREB: A Ca2(Plus)-Regulated Transcription Factor Phosphorylated by Calmodulin-Dependent Kinases. Sci Wash. 1991;252:1427. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 58.Ermak G, Davies KJA. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–21. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 59.Borowiec A-S, Bidaux G, Pigat N, Goffin V, Bernichtein S, Capiod T. Calcium channels, external calcium concentration and cell proliferation. Eur J Pharmacol. 2014;739:19–25. doi: 10.1016/j.ejphar.2013.10.072. [DOI] [PubMed] [Google Scholar]
- 60.El Boustany C, Katsogiannou M, Delcourt P, Dewailly E, Prevarskaya N, Borowiec A-S, Capiod T. Differential roles of STIM1, STIM2 and Orai1 in the control of cell proliferation and SOCE amplitude in HEK293 cells. Cell Calcium. 2010;47:350–9. doi: 10.1016/j.ceca.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Pinto MCX, Kihara AH, Goulart VAM, Tonelli FMP, Gomes KN, Ulrich H, Resende RR. Calcium signaling and cell proliferation. Cell Signal. 2015;27:2139–49. doi: 10.1016/j.cellsig.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Noren DP, Chou WH, Lee SH, Qutub AA, Warmflash A, Wagner DS, Popel AS, Levchenko A. Endothelial cells decode VEGF-mediated Ca2+ signaling patterns to produce distinct functional responses. Sci Signal. 2016;9:ra20–ra20. doi: 10.1126/scisignal.aad3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of Cell Migration by the Calcium-dependent Protease Calpain. J Biol Chem. 1997;272:32719–22. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 64.Jefferson KK, Smith MF, Bobak DA. Roles of Intracellular Calcium and NF-κB in the Clostridium difficile Toxin A-Induced Up-Regulation and Secretion of IL-8 from Human Monocytes. J Immunol. 1999;163:5183–91. [PubMed] [Google Scholar]
- 65.Rosay P, Davies SA, Yu Y, Sozen A, Kaiser K, Dow JA. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci. 1997;110:1683–92. doi: 10.1242/jcs.110.15.1683. [DOI] [PubMed] [Google Scholar]
- 66.Gu X, Olson E, Spitzer N. Spontaneous neurons calcium spikes and waves during early differentiation. 1994;14 doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–4. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamizu K, Matsunaga T, Katayama S, Kataoka H, Takayama N, Eto K, Nishikawa S-I, Yamashita JK. PKA/CREB signaling triggers initiation of endothelial and hematopoietic cell differentiation via Etv2 induction. Stem Cells Dayt Ohio. 2012;30:687–96. doi: 10.1002/stem.1041. [DOI] [PubMed] [Google Scholar]
- 69.Nathanson MH. Cellular and subcellular calcium signaling in gastrointestinal epithelium. Gastroenterology. 1994;106:1349–64. doi: 10.1016/0016-5085(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 70.Ashby MC, Tepikin AV. Polarized Calcium and Calmodulin Signaling in Secretory Epithelia. Physiol Rev. 2002;82:701–34. doi: 10.1152/physrev.00006.2002. [DOI] [PubMed] [Google Scholar]
- 71.Berridge MJ. Calcium microdomains: Organization and function. Cell Calcium. 2006;40:405–12. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ambudkar IS. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium. 2014;55:297–305. doi: 10.1016/j.ceca.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erickson ES, Mooren OL, Moore D, Krogmeier JR, Dunn RC. The role of nuclear envelope calcium in modifying nuclear pore complex structureThis paper is one of a selection of papers published in this Special Issue, entitled The Nucleus: A Cell Within A Cell. Can J Physiol Pharmacol. 2006;84:309–18. doi: 10.1139/y05-109. [DOI] [PubMed] [Google Scholar]
- 75.Berridge MJ. Remodelling Ca2+ signalling systems and cardiac hypertrophy. Biochem Soc Trans. 2006;34:228–31. doi: 10.1042/BST20060228. [DOI] [PubMed] [Google Scholar]
- 76.Balaji R, Bielmeier C, Harz H, Bates J, Stadler C, Hildebrand A, Classen A-K. Calcium spikes, waves and oscillations in a large, patterned epithelial tissue. Sci Rep. 2017:7. doi: 10.1038/srep42786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belgacem YH, Borodinsky LN. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc Natl Acad Sci. 2011;108:4482–7. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Q, Brodskiy PA, Huizar FJ, Jangula JJ, Narciso C, Levis MK, Brito-Robinson T, Zartman JJ. In vivo relevance of intercellular calcium signaling in Drosophila wing development. bioRxiv. 2017 187401. [Google Scholar]
- 79.Markova O, Sénatore S, Chardès C, Lenne P-F. Calcium Spikes in Epithelium: study on Drosophila early embryos. Sci Rep. 2015;5:srep11379. doi: 10.1038/srep11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shannon EK, Stevens A, Edrington W, Zhao Y, Jayasinghe AK, Page-McCaw A, Hutson MS. Multiple Mechanisms Drive Calcium Signal Dynamics around Laser-Induced Epithelial Wounds. Biophys J. 2017;113:1623–35. doi: 10.1016/j.bpj.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Narciso CE, Contento NM, Storey TJ, Hoelzle DJ, Zartman JJ. Release of Applied Mechanical Loading Stimulates Intercellular Calcium Waves in Drosophila Wing Discs. Biophys J. 2017;113:491–501. doi: 10.1016/j.bpj.2017.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narciso C, Wu Q, Brodskiy P, Garston G, Baker R, Fletcher A, Zartman J. Patterning of wound-induced intercellular Ca 2+ flashes in a developing epithelium. Phys Biol. 2015;12:056005. doi: 10.1088/1478-3975/12/5/056005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wood W. Wound Healing: Calcium Flashes Illuminate Early Events. Curr Biol. 2012;22:R14–6. doi: 10.1016/j.cub.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Antunes M, Pereira T, Cordeiro JV, Almeida L, Jacinto A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J Cell Biol. 2013;202:365–79. doi: 10.1083/jcb.201211039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Justet C, Hernández JA, Torriglia A, Chifflet S. Fast calcium wave inhibits excessive apoptosis during epithelial wound healing. Cell Tissue Res. 2016;365:343–56. doi: 10.1007/s00441-016-2388-8. [DOI] [PubMed] [Google Scholar]
- 86.Cheong R, Levchenko A. Oscillatory signaling processes: the how, the why and the where. Curr Opin Genet Dev. 2010;20:665–9. doi: 10.1016/j.gde.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooling MT, Hunter P, Crampin EJ. Sensitivity of NFAT Cycling to Cytosolic Calcium Concentration: Implications for Hypertrophic Signals in Cardiac Myocytes. Biophys J. 2009;96:2095–104. doi: 10.1016/j.bpj.2008.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 90.Blasio BFD, Iversen J-G, Røttingen J-A. Intercellular calcium signalling in cultured renal epithelia: a theoretical study of synchronization mode and pacemaker activity. Eur Biophys J. 2004;33:657–70. doi: 10.1007/s00249-004-0409-0. [DOI] [PubMed] [Google Scholar]
- 91.Fridlyand LE, Tamarina N, Philipson LH. Bursting and calcium oscillations in pancreatic β-cells: specific pacemakers for specific mechanisms. Am J Physiol - Endocrinol Metab. 2010;299:E517–32. doi: 10.1152/ajpendo.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sala F, Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990;57:313–24. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider MF. Control of Calcium Release in Functioning Skeletal Muscle Fibers. Annu Rev Physiol. 1994;56:463–84. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- 94.Atri A, Amundson J, Clapham D, Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993;65:1727–39. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Höfer T. Model of Intercellular Calcium Oscillations in Hepatocytes: Synchronization of Heterogeneous Cells. Biophys J. 1999;77:1244–56. doi: 10.1016/S0006-3495(99)76976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tompa P, Töth-Boconádi R, Friedrich P. Frequency decoding of fast calcium oscillations by calpain. Cell Calcium. 2001;29:161–70. doi: 10.1054/ceca.2000.0179. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Q, Yi M, Xia K, Zhan M. Information propagation from IP3 to target protein: A combined model for encoding and decoding of Ca2+ signal. Phys Stat Mech Its Appl. 2009;388:4105–14. [Google Scholar]
- 98.Yi M, Zhao Q, Tang J, Wang C. A theoretical modeling for frequency modulation of Ca2+ signal on activation of MAPK cascade. Biophys Chem. 2011;157:33–42. doi: 10.1016/j.bpc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 99.Falcke M. Reading the patterns in living cells —the physics of ca2+ signaling. Adv Phys. 2004;53:255–440. [Google Scholar]
- 100.Dupont G, Sneyd J. Recent developments in models of calcium signalling. Curr Opin Syst Biol. 2017;3:15–22. [Google Scholar]
- 101.Smedler E, Uhlén P. Frequency decoding of calcium oscillations. Biochim Biophys Acta BBA - Gen Subj. 2014;1840:964–9. doi: 10.1016/j.bbagen.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 102.Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein RA, Hülser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–17. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jørgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and Gap Junction dependent Intercellular Calcium Signaling in Osteoblastic Cells. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dougoud M, Vinckenbosch L, Mazza C, Schwaller B, Pecze L. The Effect of Gap Junctional Coupling on the Spatiotemporal Patterns of Ca2+ Signals and the Harmonization of Ca2+-Related Cellular Responses. PLOS Comput Biol. 2016;12:e1005295. doi: 10.1371/journal.pcbi.1005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–4. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 106.Deshpande VS, McMeeking RM, Evans AG. A bio-chemo-mechanical model for cell contractility. Proc Natl Acad Sci. 2006;103:14015–20. doi: 10.1073/pnas.0605837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siekmann I, Wagner LE, Yule D, Crampin EJ, Sneyd J. A Kinetic Model for Type I and II IP3R Accounting for Mode Changes. Biophys J. 2012;103:658–68. doi: 10.1016/j.bpj.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cao P, Donovan G, Falcke M, Sneyd J. A Stochastic Model of Calcium Puffs Based on Single-Channel Data. Biophys J. 2013;105:1133–42. doi: 10.1016/j.bpj.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vainio I, Khamidakh AA, Paci M, Skottman H, Juuti-Uusitalo K, Hyttinen J, Nymark S. Computational Model of Ca2+ Wave Propagation in Human Retinal Pigment Epithelial ARPE-19 Cells. PLOS ONE. 2015;10:e0128434. doi: 10.1371/journal.pone.0128434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Appleby PA, Shabir S, Southgate J, Walker D. Cell-type-specific modelling of intracellular calcium signalling: a urothelial cell model. J R Soc Interface. 2013 doi: 10.1098/rsif.2013.0487. 10–20130487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ceriani F, Pozzan T, Mammano F. Critical role of ATP-induced ATP release for Ca2+ signaling in nonsensory cell networks of the developing cochlea. Proc Natl Acad Sci. 2016;113:E7194–201. doi: 10.1073/pnas.1616061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Csete M, Doyle J. Bow ties, metabolism and disease. Trends Biotechnol. 2004;22:446–50. doi: 10.1016/j.tibtech.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 113.Tieri P, Grignolio A, Zaikin A, Mishto M, Remondini D, Castellani GC, Franceschi C. Network, degeneracy and bow tie. Integrating paradigms and architectures to grasp the complexity of the immune system. Theor Biol Med Model. 2010;7:32. doi: 10.1186/1742-4682-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Friedlander T, Mayo AE, Tlusty T, Alon U. Evolution of bow-tie architectures in biology. PLOS Comput Biol. 2015;11:e1004055. doi: 10.1371/journal.pcbi.1004055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Polouliakh N, Nock R, Nielsen F, Kitano H. G-Protein Coupled Receptor Signaling Architecture of Mammalian Immune Cells. PLOS ONE. 2009;4:e4189. doi: 10.1371/journal.pone.0004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tanaka R. Scale-Rich Metabolic Networks. Phys Rev Lett. 2005;94:168101. doi: 10.1103/PhysRevLett.94.168101. [DOI] [PubMed] [Google Scholar]
- 117.Oda K, Kitano H. A comprehensive map of the toll-like receptor signaling network. Mol Syst Biol. 2006;2:2006. doi: 10.1038/msb4100057. 0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carafoli E. Intracellular Calcium Homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 119.McCarron JG, Lee MD, Wilson C. The Endothelium Solves Problems That Endothelial Cells Do Not Know Exist. Trends Pharmacol Sci. 2017;38:322–38. doi: 10.1016/j.tips.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–32. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hannanta-anan P, Chow BY. Optogenetic Control of Calcium Oscillation Waveform Defines NFAT as an Integrator of Calcium Load. Cell Syst. 2016;2:283–8. doi: 10.1016/j.cels.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marhl M, Perc M, Schuster S. A minimal model for decoding of time-limited Ca2+ oscillations. Biophys Chem. 2006;120:161–7. doi: 10.1016/j.bpc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 123.Slusarski DC, Pelegri F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol. 2007;307:1–13. doi: 10.1016/j.ydbio.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–7. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 125.Logsdon EA, Finley SD, Popel AS, Gabhann FM. A systems biology view of blood vessel growth and remodelling. J Cell Mol Med. 2014;18:1491–508. doi: 10.1111/jcmm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bazzazi H, Popel AS. Computational investigation of sphingosine kinase 1 (SphK1) and calcium dependent ERK1/2 activation downstream of VEGFR2 in endothelial cells. PLOS Comput Biol. 2017;13:e1005332. doi: 10.1371/journal.pcbi.1005332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jakobsson L, Franco C, Bentley K, Collins TR, Ponsioen B, Aspalter I, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. 2010;12 doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 129.Douarin NL, Kalcheim C. The Neural Crest. Cambridge University Press; 1999. [Google Scholar]
- 130.Duband JL, Monier F, Delannet M, Newgreen D. Epithelium-Mesenchyme Transition during Neural Crest Development. Cells Tissues Organs. 1995;154:63–78. doi: 10.1159/000147752. [DOI] [PubMed] [Google Scholar]
- 131.Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical Hedgehog Signaling. Vitam Horm. 2012;88:55–72. doi: 10.1016/B978-0-12-394622-5.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hříbková H, Grabiec M, Klemová D, Slaninová I, Sun Y-M. Calcium signaling mediates five types of cell morphological changes to form neural rosettes. J Cell Sci. 2018;131:jcs206896. doi: 10.1242/jcs.206896. [DOI] [PubMed] [Google Scholar]
- 133.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–64. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deng H, Gerencser AA, Jasper H. Signal integration by Ca2+ regulates intestinal stem cell activity. Nature. 2015;528:212–7. doi: 10.1038/nature16170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Casali A, Batlle E. Intestinal Stem Cells in Mammals and. Drosophila Cell Stem Cell. 2009;4:124–7. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 136.van der Flier LG, Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 137.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 138.He XC, Zhang J, Tong W-G, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt β-catenin signaling. Nat Genet. 2004;36:1117. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 139.Amcheslavsky A, Jiang J, Ip YT. Tissue Damage-Induced Intestinal Stem Cell Division in. Drosophila Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila Intestinal Response to Bacterial Infection: Activation of Host Defense and Stem Cell Proliferation. Cell Host Microbe. 2009;5:200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 141.O’Brien LE, Soliman SS, Li X, Bilder D. Altered Modes of Stem Cell Division Drive Adaptive Intestinal Growth. Cell. 2011;147:603–14. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McLeod CJ, Wang L, Wong C, Jones DL. Stem Cell Dynamics in Response to Nutrient Availability. Curr Biol. 2010;20:2100–5. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ohno Y, Otaki JM. Spontaneous long-range calcium waves in developing butterfly wings. BMC Dev Biol. 2015;15:1–13. doi: 10.1186/s12861-015-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Restrepo S, Basler K. Drosophila wing imaginal discs respond to mechanical injury via slow InsP3R-mediated intercellular calcium waves. Nat Commun. 2016;7:12450. doi: 10.1038/ncomms12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brodskiy PA, Wu Q, Huizar FJ, Soundarrajan DK, Narciso C, Levis M, Arredondo-Walsh N, Chen J, Liang P, Chen DZ, Zartman JJ. Intercellular calcium signaling is regulated by morphogens during Drosophila wing development. bioRxiv. 2017 doi: 10.1016/j.bpj.2019.01.007. 104745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eid J-P, Arias AM, Robertson H, Hime GR, Dziadek M. The DrosophilaSTIM1 orthologue, dSTIM, has roles in cell fate specification and tissue patterning. BMC Dev Biol. 2008;8:104. doi: 10.1186/1471-213X-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–14. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- 148.Lobo D, Levin M. Inferring Regulatory Networks from Experimental Morphological Phenotypes: A Computational Method Reverse-Engineers Planarian Regeneration. PLoS Comput Biol. 2015;11:e1004295. doi: 10.1371/journal.pcbi.1004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Uzkudun M, Marcon L, Sharpe J. Data-driven modelling of a gene regulatory network for cell fate decisions in the growing limb bud. Mol Syst Biol. 2015;11:815–815. doi: 10.15252/msb.20145882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Robles L, Ruggero MA. Mechanics of the Mammalian Cochlea. Physiol Rev. 2001;81:1305–52. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Res Rev. 2000;32:163–6. doi: 10.1016/s0165-0173(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 152.Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3527–36. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20:543–56. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Levin M, Martyniuk CJ. The bioelectric code: An ancient computational medium for dynamic control of growth and form. Biosystems. 2017 doi: 10.1016/j.biosystems.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fletcher AG, Osterfield M, Baker RE, Shvartsman SY. Vertex Models of Epithelial Morphogenesis. Biophys J. 2014;106:2291–304. doi: 10.1016/j.bpj.2013.11.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kursawe J, Brodskiy PA, Zartman JJ, Baker RE, Fletcher AG. Capabilities and Limitations of Tissue Size Control through Passive Mechanical Forces. PLoS Comput Biol. 2015;11:e1004679. doi: 10.1371/journal.pcbi.1004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aegerter-Wilmsen T, Heimlicher MB, Smith AC, de Reuille PB, Smith RS, Aegerter CM, Basler K. Integrating force-sensing and signaling pathways in a model for the regulation of wing imaginal disc size. Development. 2012;139:3221–31. doi: 10.1242/dev.082800. [DOI] [PubMed] [Google Scholar]
- 158.Bielmeier C, Alt S, Weichselberger V, La Fortezza M, Harz H, Jülicher F, Salbreux G, Classen A-K. Interface Contractility between Differently Fated Cells Drives Cell Elimination and Cyst Formation. Curr Biol. 2016;26:563–74. doi: 10.1016/j.cub.2015.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zartman JJ, Shvartsman SY. Unit Operations of Tissue Development: Epithelial Folding. Annu Rev Chem Biomol Eng. 2010;1:231–46. doi: 10.1146/annurev-chembioeng-073009-100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Davies JA, Cachat E. Synthetic biology meets tissue engineering. Biochem Soc Trans. 2016;44:696–701. doi: 10.1042/BST20150289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Davies JA. Synthetic morphology: prospects for engineered, self-constructing anatomies. J Anat. 2008;212:707–19. doi: 10.1111/j.1469-7580.2008.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kyung T, Lee S, Kim JE, Cho T, Park H, Jeong Y-M, Kim D, Shin A, Kim S, Baek J, Kim J, Kim NY, Woo D, Chae S, Kim C-H, Shin H-S, Han Y-M, Kim D, Heo WD. Optogenetic control of endogenous Ca2+ channels in vivo. Nat Biotechnol. 2015;33:1092. doi: 10.1038/nbt.3350. [DOI] [PubMed] [Google Scholar]
- 163.Sun L, Shay J, McLoed M, Roodhouse K, Chung SH, Clark CM, Pirri JK, Alkema MJ, Gabel CV. Neuronal Regeneration in C. elegans Requires Subcellular Calcium Release by Ryanodine Receptor Channels and Can Be Enhanced by Optogenetic Stimulation. J Neurosci. 2014;34:15947–56. doi: 10.1523/JNEUROSCI.4238-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barros MT. Ca2+-signaling-based molecular communication systems: Design and future research directions. Nano Commun Netw [Google Scholar]