Abstract
Objective
We examined whether greater depressive symptoms were associated with domain-specific cognitive performance, change in cognition, and MRI markers of brain atrophy and subclinical cerebrovascular disease in a diverse sample of older adults from the Northern Manhattan Study.
Methods
Data were analyzed from the Northern Manhattan Study, a prospective cohort study of mostly Caribbean Hispanic, stroke-free, older adults. A total of 1,111 participants had baseline measures of depressive symptoms, measured as the Center of Epidemiological Studies–Depression Scale, MRI markers, and cognitive function. A Center of Epidemiological Studies–Depression score ≥16 was considered indicative of greater depressive symptoms. Multivariable linear and logistic regression models were used to examine the associations of interest.
Results
At baseline, 22% of participants had greater depressive symptoms. Greater depressive symptoms were significantly associated with worse baseline episodic memory in models adjusted for sociodemographic, vascular risk factor, behavioral, and antidepressive medication variables (β [95% confidence interval] = −0.21 [−0.33 to −0.10], p = 0.0003). Greater depressive symptoms were also associated with smaller cerebral parenchymal fraction (β [95% confidence interval] = −0.56 [−1.05 to −0.07], p = 0.02) and increased odds of subclinical brain infarcts (odds ratio [95% confidence interval] = 1.55 [1.00–2.42], p = 0.05), after adjustment for sociodemographic, behavioral, and vascular risk factor variables. Greater depressive symptoms were not significantly associated with white matter hyperintensity volume, hippocampal volume, or change in cognition over an average of 5 years. Results were unchanged when stabilized inverse probability weights were applied to address selective attrition during the study period.
Conclusions
In this sample of mostly Caribbean Hispanic, stroke-free, older adults, greater depressive symptoms were associated with worse episodic memory, smaller cerebral volume, and silent infarcts.
As many as 25% of community-living, older adults experience depressive symptoms.1 Evidence suggests that greater depressive symptoms and cognitive dysfunction are strongly interrelated,2 and both result in poorer quality of life.2,3 Since depression is amenable to treatment, greater depressive symptoms are an attractive target for the prevention or attenuation of cognitive decline in older adults. Thus, it is critical to gain a better understanding of the relationships among greater depressive symptoms, cognitive function, and neural correlates of cognition.
While most studies suggest that greater depressive symptoms increase the risk of cognitive decline,4–6 other studies suggest otherwise.7,8 Brain imaging studies can complement our understanding of this relationship. For example, previous reports have shown robust associations between greater depressive symptoms and greater hippocampal atrophy and increased white matter changes,9,10 both of which are related to poorer cognition.11
Most studies of greater depressive symptoms and brain health have been conducted in non-Hispanic black and white populations, and as such, the prevalence and significance in Hispanic/Latino populations remain unclear. These associations remain underexplored in Hispanic populations, despite the increased risk of cognitive impairment in this large subgroup.12
The Northern Manhattan Study (NOMAS) enrolled 3,298 participants, 52% of whom were Caribbean Hispanic individuals, with rich data collection including in-depth neuropsychological assessment and brain imaging. We used data from NOMAS to examine the associations of baseline greater depressive symptoms with markers of brain aging, cognitive function, and cognitive change in a racially and ethnically diverse urban cohort of older adults. In sensitivity analyses, we addressed the potential influence of confounding and selective attrition during the study period on our findings using propensity score matching and stabilized inverse probability weights.
Methods
Study population
NOMAS consists of a longitudinal, population-based cohort of adults living in the Northern Manhattan area. NOMAS enrolled 3,298 participants at baseline, between 1993 and 2001, via random digit dialing using dual-frame sampling to identify published and unpublished phone numbers in Northern Manhattan. Details of the study have been described elsewhere.13 Briefly, the eligibility criteria included the following: no diagnosis of stroke, 40 years of age or older, and residents of Northern Manhattan for >3 months in a household with a telephone. Between 2003 and 2008 (this study's baseline), 1,290 NOMAS participants were enrolled in the MRI substudy to undergo brain MRI and neuropsychological assessment, including 199 household members who met the above criteria. The following criteria were included: still clinically stroke-free, 50 years of age or older, and no contraindications to MRI.
Standard protocol approvals, registrations, and patient consents
All participants provided written informed consent. The study was approved by the institutional review boards at the University of Miami and Columbia University.
Ascertainment of depressive symptoms: Predictor of interest
At the time of MRI assessment (this study's baseline), the Center for Epidemiologic Studies–Depression (CES-D) scale was administered to participants of the MRI substudy in their preferred language (English or Spanish).14 Briefly, the CES-D is a 20-item scale (range 0–60) that measures depressive symptoms in 9 domains as defined by the American Psychiatric Association DSM-V. Participants with a score of ≥16 were considered to have greater depressive symptoms.15 Thus, the predictor of interest was treated as a binary variable (greater depressive symptoms vs no greater depressive symptoms).
Assessment of domain-specific cognitive function and change: Outcome of interest
At the time of MRI assessment, participants underwent a neuropsychological assessment in English or Spanish administered by a trained, bilingual research assistant. Consistent with previous NOMAS analyses,16 cognitive function was analyzed as z scores. Briefly, exploratory factor analysis and a literature review were used to group tests into 4 cognitive domains. Executive function comprised the Color Trails forms 1 and 217 (difference in time to complete) and the sum of Odd-Man-Out subtests 2 and 4.18 Episodic memory comprised scores from 3 subtests from the Verbal Learning Test: list learning total, delayed recall, and delayed recognition.16 Semantic memory comprised a picture naming (modified Boston Naming) test,19 category fluency (Animal Naming),20 and phonemic fluency test (C, F, L in English speakers and F, A, S in Spanish speakers).20 Processing speed comprised the Grooved Pegboard task in the nondominant hand21 and the Color Trails test form 1.17 Raw test scores were standardized using the means and SDs from the baseline sample. Based on the domain groupings above, these test-specific z scores were averaged to obtain a domain-specific z score.
Participants were reexamined an average of 5 years later using a similar neuropsychological assessment as in baseline. Raw test scores from the second neuropsychological assessment were standardized using the baseline means and SDs. Change in domain-specific cognitive performance was computed as the difference between time 2 and time 1 z scores.
Brain MRI markers: Outcome of interest
White matter hyperintensity volume, total intracranial volume, and total cerebral volume (global brain MRI markers)
Brain MRI was performed on a 1.5T MRI system (Philips Medical Systems, Best, the Netherlands) at Columbia University Medical Center. Quantification of global brain MRI markers has been previously described.22 Briefly, brain MRIs were sent to collaborators at the University of California, Davis, for analysis using a custom-designed image analysis package (QUANTA 6.2 using a Sun Microsystems Ultra 5 workstation). Nonbrain elements were removed manually using an operator-guided tracing of the dura mater within the cranial vault, including the middle cranial fossa but above the posterior fossa and cerebellum, to define total intracranial volume (TIV). Total cerebral volume was calculated from the T1 segmentation process as a sum of whole brain volume voxels. A segmentation threshold for white matter hyperintensity volume (WMHV) was determined a priori as 3.5 SDs in pixel intensity above the mean of the fitted distribution of brain parenchyma. All analyses were performed blind to participant identification or risk factor information. In this analysis, total cerebral volume and WMHV were expressed as a percent of TIV to account for differences in head size. Total cerebral volume expressed as a percent of TIV is labeled as the cerebral parenchymal fraction.
Hippocampal volume
Hippocampal volumes were estimated with the publically available FreeSurfer image analysis suite, version 5.1 (surfer.nmr.mgh.harvard.edu),23 after T1-weighted MRIs underwent motion correction, skull stripping, and transformations into Talairach space. Images then underwent identification of gray/white matter boundaries, automated topology correction, and surface deformation.24 In this analysis, hippocampal volume is expressed as a percent of TIV to account for differences in head size.
Presence/absence of subclinical brain infarcts
Determination of subclinical brain infarcts has been previously described.25 Briefly, a superimposed image of the subtraction, proton density, and T2-weighted images at 3 times magnified view was used to assist in the interpretation of lesion characteristics. Infarcts were counted for total number and characterized by location (cortical, subcortical, and specific region) and size (small: <1 cm; large: ≥1 cm). Two raters were used to determine the presence of infarcts, and agreement among them has been generally good (previously published κ values: 0.73–0.90).26
Covariate measurement
At the time of MRI assessment, in-person examinations were performed by trained bilingual research assistants and physicians. Examinations included medical interviews based on the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System and ascertainment of fasting blood samples for glucose and lipid panels. Race/ethnicity was self-reported by the participant. Moderate alcohol consumption was defined as current drinking between 1 drink per month and 2 drinks per day vs other.27 Leisure-time physical activity was defined as any recreational activity in the prior 2 weeks vs none.28 Hypertension was defined as having a blood pressure of ≥140/90 mm Hg, which was based on the average of 2 measurements with a mercury sphygmomanometer, or self-reported antihypertensive medication use. Type 2 diabetes was defined as having a blood glucose level of ≥126 mg/dL or self-reported diabetes medication use. History of cardiac disease included any history of angina, myocardial infarction, congestive heart failure, coronary artery disease, atrial fibrillation, or valvular heart disease. Use of antidepressive medication was self-reported.
Statistical analysis
Of the 1,290 participants in the NOMAS MRI substudy, our final analytical sample included a maximum of 1,111 participants who had baseline measures of depressive symptoms, MRI markers, and cognitive function. For the longitudinal analysis exploring 5-year cognitive change, our analytical sample included a total of 858 participants who also had cognitive function assessed at the 5-year follow-up.
We first assessed participants' baseline characteristics across categories of greater depressive symptoms. Differences in means and proportions of those characteristics were tested using 2-sample, 2-sided t tests and χ2 tests, respectively.
We used multivariable linear regression models to examine the cross-sectional associations of greater depressive symptoms with domain-specific cognitive performance and brain MRI markers at baseline, except for presence/absence of subclinical brain infarct for which we used logistic regressions. Similarly, we used linear regression models to examine the association between greater depressive symptoms and change in domain-specific cognitive performance over an average of 5 years. Model 1 was unadjusted. In model 2, we adjusted for sociodemographic confounders (age, sex, race/ethnicity, and years of education). In model 3, we additionally adjusted for baseline behavioral and cardiovascular risk factors (smoking status, alcohol consumption, physical activity, cardiac disease history, hypertension, and type 2 diabetes). In model 4, we further adjusted for antidepressive medication use. Covariates were chosen a priori based on the literature as well as their relationships with the predictors and outcomes of interest. For the outcome of change in cognition, baseline cognitive function was added as a covariate to each model. Alpha was set at 5%, and results are presented as β coefficients or odds ratios with 95% confidence intervals (CIs).
In post hoc analyses, we also examined whether the associations of greater depressive symptoms with cognition and brain MRI markers varied by sex, race/ethnicity, and antidepressive medication use by including appropriate multiplicative interaction terms in sociodemographic-adjusted models. Interactions were considered nonsignificant at an α level of 10%.
To examine the influence of potential confounding, we computed for each participant a propensity score for greater depressive symptoms using the same covariates from the multivariable adjusted models above. We then reran our analyses, (1) adjusting for the propensity score, and (2) using the “greedy digit matching algorithm” to generate a 1:1 propensity score–matched sample of those with and without greater depressive symptoms29 (tables e-1 and e-2, links.lww.com/WNL/A507). We also compared covariate balance in the matched sample (table e-3).
Furthermore, to examine the influence of differential attrition over the 5-year follow-up on our longitudinal findings, we computed stabilized inverse probability-of-continuation weights30 and applied them to the analysis of greater depressive symptoms and 5-year cognitive change (table e-4, links.lww.com/WNL/A507). All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
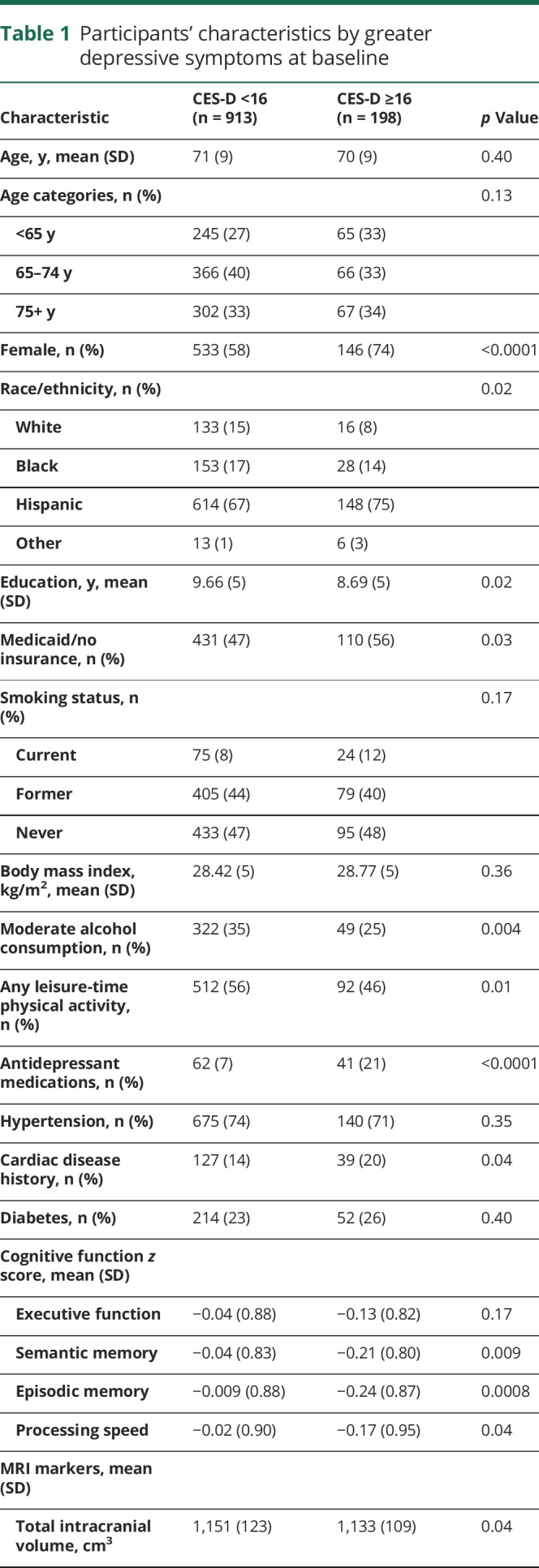
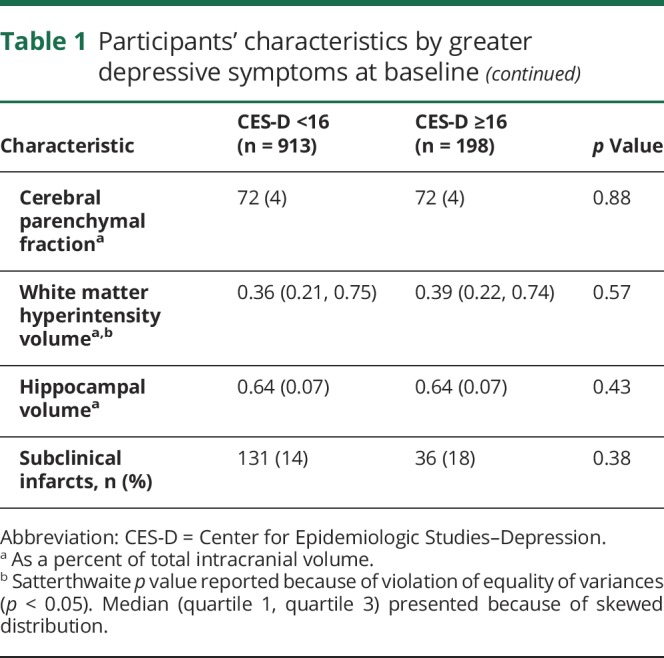
In table 1, we present participant characteristics at baseline across categories of greater depressive symptom status. At baseline, 22% of participants had greater depressive symptoms. Participants with greater depressive symptoms were significantly more likely to be female, Hispanic, uninsured or on Medicaid, taking antidepressant medications, and have lower mean years of education. Participants with greater depressive symptoms were less likely to report moderate alcohol use or to be engaged in any leisure-time physical activity. Participants with greater depressive symptoms had smaller intracranial volume and performed significantly worse on all cognitive tests at baseline, except executive function.
Table 1.
Participants' characteristics by greater depressive symptoms at baseline
In table 2, we present the cross-sectional associations between greater depressive symptoms and domain-specific cognitive function at baseline. Presence of greater depressive symptoms was significantly associated with worse semantic memory, episodic memory, and processing speed in unadjusted models (table 2, model 1). In sociodemographic-adjusted models 2, presence of greater depressive symptoms was only associated with worse episodic memory. This association remained significant, and effect estimates remained stable after adjustment for behavioral and vascular risk factors, and further adjustment for use of antidepressive medication (β [95% CI] = −0.21 [−0.33 to −0.10], p = 0.0003). Associations of greater depressive symptoms with semantic memory or processing speed were no longer significant and largely attenuated after adjustment for sociodemographics, behavioral and vascular risk factors, and antidepressive medication use. Furthermore, we found no significant longitudinal associations between greater depressive symptoms and cognitive change in any domain (p > 0.05; table 3), and we did not detect any significant interactions with sex, race/ethnicity, or antidepressant medication use for the associations with baseline cognition or cognitive change (p > 0.10).
Table 2.
Cross-sectional associations between greater depressive symptoms and domain-specific cognitive performance at baseline, from multivariable linear regression models
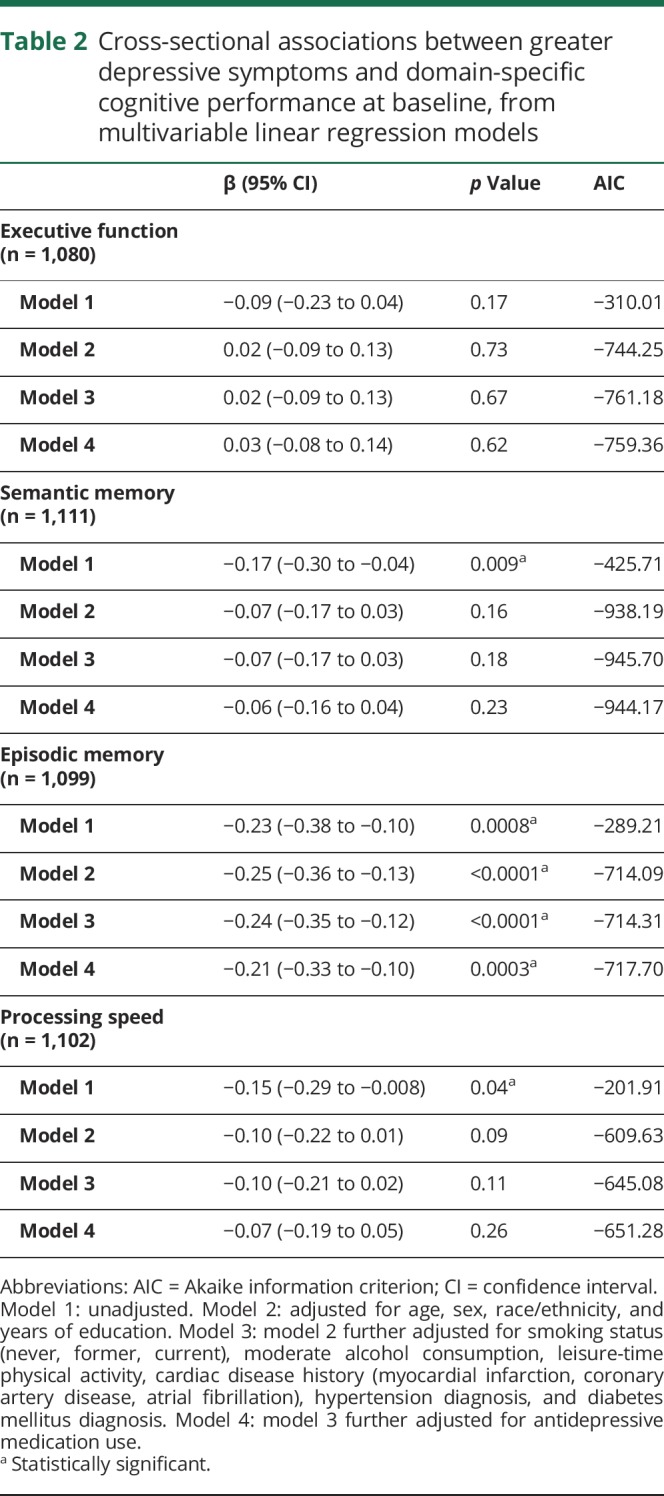
Table 3.
Associations between greater depressive symptoms and change in cognitive performance over 5 years, from multivariable linear regression models

We also examined the cross-sectional associations between greater depressive symptoms and brain MRI markers (table 4). Having greater depressive symptoms was significantly associated with smaller cerebral parenchymal fraction after adjustment for sociodemographic factors (model 2). This association remained significant and effect estimates remained stable after further adjustment for behavioral and vascular risk factors and antidepressive medication use (β [95% CI] = −0.56 [−1.05 to −0.07], p = 0.02). Presence of greater depressive symptoms was also significantly associated with greater odds of subclinical brain infarcts in models adjusted for sociodemographics (model 2). The association remained significant and odds ratios remained stable in models further adjusted for behavioral and vascular risk factors and antidepressant medication use (odds ratio [95% CI] = 1.55 [1.00–2.42], p = 0.05). There were no significant associations between greater depressive symptoms and WMHV or hippocampal volume (p > 0.05), and we did not detect any significant interactions with sex, race/ethnicity, or antidepressant medication use (p > 0.10).
Table 4.
Cross-sectional association between greater depressive symptoms and brain MRI markers at baseline, from multivariable linear and logistic regression models
In sensitivity analyses, results from the regression analyses of greater depressive symptoms with cognitive function and brain MRI markers at baseline were unchanged when we either adjusted for the propensity score or used the propensity score–matched sample (tables e-1 and e-2, links.lww.com/WNL/A507). Furthermore, in the propensity score–matched sample, there was proper covariate balance between those with and those without greater depressive symptoms (table e-3). Finally, when we accounted for selective attrition using the stabilized inverse probability weights, results from the longitudinal associations of greater depressive symptoms and cognitive change were unchanged (tables e-4 and e-5).
Discussion
In this diverse, stroke-free cohort of older adults, we found significant cross-sectional associations between greater depressive symptoms and episodic memory, but no evidence of association with change in cognition over an average of 5 years. Greater depressive symptoms were also significantly associated with smaller cerebral volumes and subclinical brain infarcts, a marker of cerebral small vessel disease. Although greater depressive symptoms may be concurrently associated with cognition and subclinical brain damage in older adults, greater depressive symptoms may not be associated with changes in cognition.
There are several mechanisms that may explain these associations. Several neurodegeneration-related processes, including chronic elevation of cortisol and β-amyloid plaque deposition, are hypothesized to contribute to depression pathogenesis.31 In addition, the “vascular depression hypothesis” posits that cerebrovascular disease contributes to the development of depression, though this relationship may be bidirectional.32,33 Disease mechanisms common to both depression and cognitive dysfunction may explain their consistent associations across cohorts, but the temporality of this association is still under debate.
Most studies have found that greater depressive symptoms are associated with cognitive decline,4–6 although there is some evidence to the contrary.7,8 A recent study from the WHICAP (Washington/Hamilton Heights Inwood Columbia Aging Project) using autoregressive latent trajectory models demonstrated that a higher baseline level of depressive symptoms was associated with a faster decline in episodic memory.5 These data are consistent with our domain-specific findings (i.e., episodic memory) but inconsistent with our null associations with cognitive change. There are several possible explanations for this result. Our sample of participants was stroke-free but not necessarily dementia-free at baseline, so it is highly likely that the sample represents a spectrum of cognitive performance. Therefore, greater depressive symptoms may not readily predict changes in cognition in this mixed sample. In addition, the measurement of cognitive change in our study was over a 5-year period, which may not be long enough to capture meaningful changes in cognition over time. Furthermore, while depressive symptoms have a dynamic nature that may also influence cognitive function,4 we were unable to examine this in our study because of the lack of repeated CES-D measures. Studies that examine trajectories of these phenomena may better illustrate the complex nature of this association. Finally, it is possible that greater depressive symptoms may manifest concurrently with cognitive impairment in this sample, indicating that depression-related pathology may not always precede cognitive decline.
We also found that greater depressive symptoms were associated with smaller cerebral volumes and increased odds of subclinical brain infarcts, but not with WMHV or hippocampal volume. Our results suggest that greater depressive symptoms may be related to general brain atrophy. Because brain atrophy may be due to vascular or neurodegenerative processes, greater depressive symptoms may affect brain health through either pathway. In the present study, greater depressive symptoms were associated with subclinical brain infarcts but not white matter hyperintensities. Since the etiology of white matter hyperintensities is heterogeneous,34 and subclinical brain infarcts are thought to share the same etiology as lacunar infarcts (i.e., ischemic due to hypertensive vasculopathy),35 these data imply that depressive symptoms may affect brain health through a vascular pathway, specifically via cerebral small vessel disease. Although not all subclinical brain infarcts are due to small vessel disease, the vast majority in the NOMAS cohort are small and subcortical in nature, consistent with small vessel disease.25 Furthermore, we did not find an association between greater depressive symptoms and hippocampal volume, a known marker of Alzheimer disease pathology.
In this study, there are several limitations worth noting. Because participants needed to be healthy enough to undergo an MRI, those in the MRI substudy were healthier than those in the larger cohort, which may have resulted in underestimating the true associations. We only had 2 repeated measurements of cognitive performance, limiting our ability to evaluate trajectories of cognitive decline. Repeated measures of neuropsychological data would help elucidate the association of greater depressive symptoms with trajectories of domain-specific cognition over time. Furthermore, the average time of follow-up between the 2 cognitive assessments was only 5 years, which may not be enough time to observe meaningful changes in cognition. The lack of repeated measures of depressive symptoms and information regarding symptom severity limits our ability to assess the association of dynamic changes in depressive symptoms with cognition. Although the sample of participants was stroke-free, clinical diagnoses of cognitive impairment and dementia adjudication were not conducted, thus limiting our ability to exclude participants with dementia and potentially resulting in a cognitively heterogeneous sample. While we adjusted for various potential confounders, there may still be residual confounding by other unmeasured covariates, such as drugs that are associated with cognitive function.
Despite these limitations, our study has several strengths. Few MRI cohorts include mostly Caribbean Hispanic participants, and thus, we are able to represent these populations in the literature. We had various brain MRI markers available, which enabled us to examine potentially different disease processes. We also had a comprehensive neuropsychological assessment with a variety of tests that allowed us to construct domain-specific scores. Finally, we addressed confounding and selective attrition by conducting sensitivity analyses using propensity score adjustment and matching as well as inverse probability-of-continuation weights.
The present study findings suggest that greater depressive symptoms are associated with episodic memory and markers of brain aging in a mostly Caribbean Hispanic sample. Our findings also suggest that greater depressive symptoms may affect cognition through a vascular pathway, at least in our cohort. Lack of significant findings regarding change in cognition may reflect a mixed cognitive sample as well as short follow-up time. Further studies are needed to elucidate the mechanisms between greater depressive symptoms and cognitive function in late life, especially in samples with repeated depressive symptoms and cognitive data.
Acknowledgment
The authors thank the NOMAS participants for their contributions to the study as well as data manager Janet DeRosa, MPH.
Glossary
- CES-D
Center for Epidemiologic Studies–Depression
- CI
confidence interval
- DSM-V
Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition)
- NOMAS
Northern Manhattan Study
- TIV
total intracranial volume
- WMHV
white matter hyperintensity volume
Author contributions
Adina Zeki Al Hazzouri: design of the study, analysis and interpretation of the data, drafting and revision of the manuscript for intellectual content. Michelle Caunca: design of the study, analysis and interpretation of the data, drafting and revision of the manuscript for intellectual content. Juan Carlos Nobrega: interpretation of the data, and drafting and revision of the manuscript for intellectual content. Tali Elfassy: interpretation of the data, and drafting and revision of the manuscript for intellectual content. Ying Kuen Cheung: interpretation of the data, and drafting and revision of the manuscript for intellectual content. Noam Alperin: interpretation of the data, and drafting and revision of the manuscript for intellectual content. Chuanhui Dong: interpretation of the data, and drafting and revision of the manuscript for intellectual content. Mitchell S.V. Elkind: interpretation of the data, and drafting and revision of the manuscript for intellectual content. Ralph L. Sacco: acquisition of data, interpretation of the data, and drafting and revision of the manuscript for intellectual content. Charles DeCarli: acquisition of data, interpretation of the data, and drafting and revision of the manuscript for intellectual content. Clinton B. Wright: acquisition of data, interpretation of the data, drafting and revision of the manuscript for intellectual content, and study supervision.
Study funding
This work was funded by grants from the NIH (R01 NS29993, K01 AG047273, F30 NS103462), the American Heart Association (AHA 17POST32490000), and the Evelyn F. McKnight Brain Institute.
Disclosure
A. Zeki Al Hazzouri, M. Caunca, J. Nobrega, T. Elfassy, Y.K. Cheung, N. Alperin, and C. Dong report no disclosures relevant to the manuscript. M. Elkind receives compensation for providing consultative services for BioTelemetry/CardioNet, BMS-Pfizer Partnership, Boehringer Ingelheim, Daiichi-Sankyo, Janssen Pharmaceuticals, and Sanofi-Regeneron Partnership; receives research support from diaDexus, Inc., Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, and the NIH/NINDS; has given expert legal opinions on behalf of Organon (NuvaRing and stroke litigation) and Hi-Tech; and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to stroke. R. Sacco receives private foundation support (American Heart Association Bugher Center) and pharma research support (Boehringer Ingelheim). C. DeCarli serves as a consultant for Novartis Pharmaceuticals. C. Wright receives royalties for 2 chapters on Vascular Dementia from UpToDate. Go to Neurology.org/N for full disclosures.
References
- 1.Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clin Geriatr Med 1992;8:235–251. [PubMed] [Google Scholar]
- 2.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003;58:249–265. [DOI] [PubMed] [Google Scholar]
- 3.McKenna MT, Michaud CM, Murray CJ, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med 2005;28:415–423. [DOI] [PubMed] [Google Scholar]
- 4.Zeki Al Hazzouri A, Vittinghoff E, Byers A, et al. Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci 2014;69:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahodne LB, Stern Y, Manly JJ. Depressive symptoms precede memory decline, but not vice versa, in non-demented older adults. J Am Geriatr Soc 2014;62:130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaup AR, Byers AL, Falvey C, et al. Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry 2016;73:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry 2006;63:153–160. [DOI] [PubMed] [Google Scholar]
- 8.Vinkers DJ, Gussekloo J, Stek ML, Westendorp RG, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ 2004;329:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 2004;161:1957–1966. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry 2008;79:619–624. [DOI] [PubMed] [Google Scholar]
- 11.Dong C, Nabizadeh N, Caunca M, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology 2015;85:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 14.Eaton W, Mutaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R). In: Maruish ME, editor.The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 3rd ed. Mahwah, NJ: Lawrence Erlbaum; 2004:363–377. [Google Scholar]
- 15.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 16.Siedlecki KL, Rundek T, Elkind MS, Sacco RL, Stern Y, Wright CB. Using contextual analyses to examine the meaning of neuropsychological variables across samples of English-speaking and Spanish-speaking older adults. J Int Neuropsychol Soc 2012;18:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test Professional Manual. Odessa: Psychological Assessment Resources; 1996. [Google Scholar]
- 18.Flowers KA, Robertson C. The effect of Parkinson's disease on the ability to maintain a mental set. J Neurol Neurosurg Psychiatry 1985;48:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanagan JL, Jackson ST. Test-retest reliability of three aphasia tests: performance of non-brain-damaged older adults. J Commun Disord 1997;30:33–42. [DOI] [PubMed] [Google Scholar]
- 20.Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: normal performance, validity and test-retest reliability. Br J Clin Psychol 2000;39(pt 2):181–191. [DOI] [PubMed] [Google Scholar]
- 21.Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. Madison: University of Madison Medical School; 1964. [Google Scholar]
- 22.Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 2008;39:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 24.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I: segmentation and surface reconstruction. Neuroimage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 25.Prabhakaran S, Wright CB, Yoshita M, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology 2008;70:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 27.Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke 2006;37:13–19. [DOI] [PubMed] [Google Scholar]
- 28.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke 1998;29:380–387. [DOI] [PubMed] [Google Scholar]
- 29.Parsons L. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute, Inc.; 2001.
- 30.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sierksma AS, van den Hove DL, Steinbusch HW, Prickaerts J. Major depression, cognitive dysfunction and Alzheimer's disease: is there a link? Eur J Pharmacol 2010;626:72–82. [DOI] [PubMed] [Google Scholar]
- 32.Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke 2012;43:32–37. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry 2008;79:619–624. [DOI] [PubMed] [Google Scholar]
- 34.Wardlaw JM, Valdes Hernandez MC, Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 2015;4:001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke 2008;39:2929–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.



