Summary
Non-muscle myosin heavy chain IIA (NMMHC IIA) has been shown to be involved in thrombus formation and inflammatory microparticle release in endothelial cells. However, the role of NMMHC IIA in regulating the expression of tissue factor (TF) and deep venous thrombosis remains to be elucidated. In the present study, endothelial cells were stimulated with tumour necrosis factor-α (TNF-α) to induce TF expression. Pretreatment with the NMMHC II inhibitor blebbistatin suppressed the mRNA and protein expressions as well as the procoagulant activity of TF in a dose-dependent manner. Blebbistatin enhanced Akt and GSK3β phosphorylation and inhibited NF-κB p65 nuclear translocation and IκB□ degradation. These observations were similar to the effect of CHIR99021, a GSK3β inhibitor. TF downregulation by blebbistatin was antagonised by the PI3K inhibitor, wortmannin. Furthermore, siRNA knockdown of NMMHC IIA, but not IIB or IIC, inhibited TF expression, activated Akt/GSK3β and suppressed NF-κB signalling pathways, whereas the overexpression of NMMHC IIA increased TF expression. The binding of NMMHC IIA and TNF receptor 2 mediated signal internalisation in TNF-α-stimulated endothelial cells. Importantly, blebbistatin decreased endothelium NMMHC IIA and TF expression, deactivated GSK3β by inducing its phosphorylation, suppressed p65 nuclear translocation, and inhibited thrombus formation in a mouse deep venous thrombosis model. Our findings provide solid evidence that inhibition of NMMHC II, most likely NMMHC IIA, impedes TF expression and venous thrombosis via Akt/GSK3β-NF-κB signalling pathways in the endothelium both in vitro and in vivo. NMMHC IIA might be a potential novel target for the treatment of thrombotic disorders.
Keywords: NMMHC IIA, venous thrombosis, tissue factor, endothelium, signalling transduction
Introduction
Deep venous thrombosis (DVT), the formation of a blood clot within a deep vein, is associated with various adverse outcomes (e.g. pulmonary embolism [PE], chronic venous insufficiency [CVI], and the postthrombotic syndrome [PTS]) (1). DVT is one of the leading causes of morbidity and mortality worldwide (2, 3). Many risk factors related to venous thrombosis have been identified, including altered blood flow, activation of the endothelium, and increased blood coagulation (4). However, the precise cellular mechanisms that trigger clotting in the venous system have not been fully elucidated (2, 5). It has been reported that the endothelial lining of the blood vessels plays a critical role in regulating thrombosis by preventing the attachment of inflammatory cells to the surface (6). Under normal conditions, endothelial cells show anti-coagulant and anti-inflammatory properties. While in an activated state, these cells exhibit procoagulant and proinflammatory activities. This transformation is primarily mediated by the gene expression of procoagulant molecules, such as tissue factor (TF), and adhesion molecules, such as vascular cell adhesion molecule-1 (7).
The full-length TF (aka coagulation factor III) is a transmembrane glycoprotein that is responsible for initiating the coagulation cascade upon tissue damage (8–10). Under pathological conditions, vascular endothelial cells and monocytes are two major cell types that release TF to trigger the formation of venous clots (2, 11). In the TF-initiated extrinsic coagulation pathway, prothrombin is converted into thrombin, which then causes subsequent cellular responses including fibrin formation, platelet activation, and thrombus formation (5). It has been proposed that the inhibition of TF may represent an effective strategy for the treatment of DVT (11).
In response to various inflammatory stimuli such as tumour necrosis factor (TNF)-α, interleukin-1 and bacterial lipopolysaccharide, the expression of TF is primarily regulated by the nuclear factor (NF)-κB, the phosphoinositide 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) signalling pathways (8, 12–14). Activation of the NF-κB and MAPK signalling pathways promotes TF expression, whereas the PI3K/Akt pathway negatively regulates TF expression through GSK3β (7, 15). The activated PI3K/Akt pathway also directly or indirectly inactivates the MAPK and NF-κB pathways by negatively regulating their upstream kinases (13, 16). Modulation of these inter-related signalling pathways and inhibition of TF expression or activity may exert therapeutic effects against venous thrombosis.
Non-muscular myosin II is an actin-based motor protein that is essential to cell adhesion, migration, proliferation and differentiation (17–20). The holoenzyme consists of two identical heavy chains and two sets of light chains. The non-muscular myosin heavy chains (NMMHCs) are encoded by three genes, MYH9, MYH10, and MYH14, which generate three different non-muscular myosin II isoforms, i. e. IIA, IIB, and IIC, respectively (21). Previous studies suggest that NMMHC IIA deficiency or inhibition can reduce platelet aggregation and arterial thrombus formation (17, 22, 23). In TNF-α-stimulated endothelial cells, myosin II regulates the expression of various proinflammatory factors (24). In addition, myosin II mediates the release of proinflammatory microparticles and upregulates E-selectin, intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and TF in endothelial cells activated by anti-β2GPI antibodies (25). However, the direct evidence of the underlying mechanism of NMMHC IIA in modulating TF expression in endothelial cells and its involvement in DVT remains unclear.
In the present study, we demonstrated that inhibiting NMMHC II ATPase activity or suppressing NMMHC IIA expression decreased TNF-α-induced TF expression by modulating GSK3β and NF-κB signalling pathways in endothelial cells. We uncovered that NMMHC IIA overexpression increased TF expression in 293T cells and the interaction of NMMHC IIA with tumor necrosis factor receptor 2 (TNFR2) in TNF-α-stimulated endothelial cells. Furthermore, NMMHC II inhibition suppressed thrombus formation in a mouse model of DVT. Both the in vitro and in vivo findings point to a key role of NMMHC IIA in TF expression and venous thrombus formation.
Materials and methods
Reagents
Dulbecco’s modified Eagle medium (DMEM) was purchased from GIBCO/BRL (Life Technologies, Carlsbad, CA, USA). Prothrombin complex was purchased from Hualan Bioengineering Company (Xinxiang, China). Factor Xa chromogenic substrate, human TNF-α and blebbistatin were purchased from Sigma-Aldrich (St. Louis, MO, USA). CHIR99021 was purchased from Selleckchem (Houston, TX, USA). Antibodies against human TF and mouse TF (for western blotting, full length) were purchased from R&D Systems (Minneapolis, MN, USA). An antibody against TF (for immunohistochemistry) was purchased from Epitomics (Burlingame, CA, USA). An antibody against GAPDH was purchased from KangChen Bio-tech (Shanghai, China). Antibodies against myosin IIA, myosin IIB, myosin IIC, p65, phospho-p65, IκBα, phospho-IκBα, Akt, phospho-Akt, GSK3β, and phospho-GSK3β were obtained from Cell Signalling Technology (Boston, MA, USA). Wortmannin and 4’,6– diamidino-2-phenylindole (DAPI) were purchased from Beyotime biotechnology (Shanghai, China). Antibodies against PI3K, and phospho-PI3K and horseradish peroxidase (HRP)-conjugated secondary antibody were purchased from Bioworld Technology (St. Louis Park, MN, USA). RIPA lysis buffer, protease inhibitor, a first-strand cDNA synthesis kit, ExFect Transfection Reagent and enhanced chemiluminescence (ECL) reagent were purchased from Vazyme Biotech (Nanjing, China). SYBR Green Master Mix was purchased from Bio-Rad (Hercules, CA, USA). Alexa Fluor® 488 Donkey Anti-Goat IgG (H+L), Alexa Fluor® 594 Donkey Anti-Goat IgG (H+L) antibodies and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA), and all other regents used in this study were of the highest purity commercially available.
Cell culture
The EA.hy926 endothelial cells and HEK 293T cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and were cultured in DMEM supplemented with 10 % fetal bovine serum (ScienCell, San Diego, CA, USA) at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. For all experiments, cells were plated at an appropriate density according to the experimental design and were grown to 80–90 % confluence before experimental use. The primary HUVECs were purchased from ScienCell (Catalog Number: 8000) and cultured as per the manufacturer’s instructions. Primary HUVECs at passages 2–4 were used for the experiments.
Measurement of TF procoagulant activity
The TF procoagulant activities of the cell lysates, cell surface and mouse plasma were measured by a chromogenic assay as previously reported (26). The cell lysates were frozen and thawed three times before they were used in the assay. The cells were washed twice and incubated with fresh medium before TF activity on the cell surface was assayed. Mouse plasma was prepared by centrifugation at 3,000×g for 15 minutes (min), and stored at −70 °C before use. Briefly, the samples (45 µl) were incubated with a reagent mixture (5 µl, pH 7.3) containing 10 g/l prothrombin complex and 100 mM CaCl2 in a 96-well plate. After incubation at 37 °C for 15 min, 50 µl of factor Xa chromogenic substrate (0.5 mM) containing 100 mM EDTA (pH 8.4) was added. The reaction was incubated at 37 °C for 5 min, and the absorbance was measured at 405 nm. TF activity of the model group was set as 100 %.
Western blotting analysis
Western blotting analyses were performed as described previously. After washing with ice-cold phosphate-buffered saline (PBS), the cells or inferior vena cava (IVC) vessels were lysed with RIPA lysis buffer supplemented with protease inhibitor. Equal amounts of proteins were loaded into a 10–12.5 % SDS-PAGE and transferred to PVDF membranes (Millipore Corporation, Billerica, MA, USA) by electroblotting. After blocking with 5 % non-fat milk (BD Biosciences, San Jose, CA, USA) for 2 hours (h), PVDF membranes were probed overnight at 4 °C with primary antibodies against TF, GAPDH, myosin IIA, p65, phospho-p65, IκBα, phospho-IκBα, Akt, phospho-Akt, GSK3β, phospho-GSK3β, PI3K, and phospho-PI3K (dilution 1:800, 1:8000, 1:1000, 1:1000, 1:600, 1:800, 1:600, 1:1000, 1:600, 1:1000, 1:1000, 1:600, and 1:600, respectively). The blots were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (dilution 1:10,000; Bioworld Technology) and developed with ECL reagent. The immunoreactive bands were visualised using the ChemiDoc™ MP System (Bio-Rad) and analysed using the Image Lab™ Software (version 4.1, Bio-Rad).
Flow cytometry
Flow cytometry analysis was performed as previously described. Trypsin-digested cells were washed with PBS and stained with CD142 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) for surface antigen analysis. The cells were sorted using a FACSCalibur flow cytometer (BD Biosciences). Data were analysed using FlowJo software (Tree Star Inc., San Carlos, CA, USA).
Immunofluorescence analysis
To determine the NF-κB p65 cytoplasmic-nuclear translocation after drug treatment, the EA.hy926 cells were cultured on glass coverslips. After pre-incubated with blebbistatin for 1 h, the cells were exposed to TNF-α for an additional 30 min. The cells were then fixed, permeabilised and incubated with anti-NF-kB p65 primary antibody, followed by incubation with an Alexa Fluor® 488 conjugated Donkey Anti-Goat IgG (H+L) antibody and DAPI. The pictures were taken using an Olympus Confocal Laser Scanning Microscope (Olympus, Nagano, Japan). Analyses of NF-kB p65 cytoplasmic–nuclear translocation were accomplished using the Image-Pro Plus 6.0 software.
Real-time quantitative polymerase chain reaction (qPCR)
Total RNAs were extracted from cells or frozen tissues with Trizol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesised with a first-strand cDNA synthesis kit (Vazyme Biotech, Nanjing, China) according to the manufacturer□s instruction. Real-time quantitative PCR was performed using Bio-Rad CFX Manager 3.0 system and SYBR Green Master Mix (Bio-Rad). The relative amount of each gene in each sample was estimated using the ΔΔCT method. The following primers were used: hTF forward, GAACCCAAACCCGTCAAT; hTF reverse, TCTCATACAGAGGCTCCC; mTF forward, ACAAGCTATGATTTTCTCCAGG; mTF reverse, AAACTCTTCCATTGCTCGGT; hMYH9 forward, AGGGCACTGTCAAGTCCAA; hMYH9 reverse, CCTTCAGCTTCTTCTCGGTC; mMYH9 forward, AGCGTGAGCTGGAAGATGC; mMYH9 reverse, GCGAGTCACGACAAATGGC; h18S forward, AAACGGCTACCACATCCAAG; h18S reverse, CCTCCAATGGATCCTCGTTA; m18S forward, TGTGATGCCCTTAGATGTCC and m18S reverse, TGGGGTTCAACGGGTTAC.
Plasmid and siRNA transfection
The plasmid of NMMHC IIA (EX-T1335-M98) and negative control plasmid (EX-NEG-M98) were purchased from the FulenGen Company (Guangzhou, China). Plasmid was transfected into cells using the Endofectin Transfection Reagent (FulenGen, Guangzhou, China). Before transient transfection, the HEK 293T cells were seeded onto six-well plates (4×105) and cultured for 24 h. They were then transfected at 70–80 % confluence with 3 µg of plasmid using 9 µl the Endofectin reagent. After 48 h, the cells were harvested, and the whole-cell extracts were assayed in subsequent experiments.
MYH9 siRNA, and control non-specific siRNA were designed and synthesised by Biomics Biotechnologies (Nantong, Jiangsu, China). siRNA transfection was performed using ExFect Transfection Reagent (Vazyme Biotech, Nanjing, China) instructions. The MYH9 sequences were: forward, 5-GAGGCAAUGAUCACUGACUdTdT-3 and reverse, 5-AGUCAGUGAUCAUUGCCUCdTdT-3. To assess the MYH9 siRNA efficiency, total cell lysates were subjected to SDS–PAGE for immunoblotting analysis with GAPDH (Kangchen Bio-tech, Shanghai, China) as a reference.
Co-immunoprecipitation
The primary HUVECs were lysed in a RIPA lysis buffer containing protease inhibitor. Anti-NMMHC IIA antibody (Abcam, Cambridge, UK), together with protein A/G agarose, was used to immunoprecipitate TNFR2 and associated proteins. Proteins were resolved by SDS-PAGE and detected by western blotting analysis.
Proximity ligation assays (PLA)
To demonstrate a complex between native TNFR2 and NMMHC IIA in the primary HUVECs, a PLA kit (Sigma-Aldrich) was used as per manufacturer protocols (27). Primary antibodies to TNFR2 and NMMHC IIA were used, and secondary antibodies were conjugated to oligonucleotides for ligation and subsequent rolling circle amplification.
Inferior vena cava ligation (IVCL)
C57BL/6J mice (22–25 g) were purchased from Model Animal Research Centre of Yangzhou University (Yangzhou, Jiangsu, China). The animals were housed in a standard vivarium with free access to food and water. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Briefly, mice were anesthetised, and a midline laparotomy was performed. The small bowel was exteriorised and placed onto a moistened gauze pad next to the animal. The infrarenal IVC was identified, and all side branches were ligated with nonreactive 7–0 Prolene suture. Posterior venous branches were cauterised. A 7–0 Prolene suture was tied down on the IVC, caudally to the left renal vein (28, 29). After surgery, peritoneum and skin were closed by monofilament suture. Two days later, plasma and the thrombosed IVC were collected for analysis.
Histology and immunohistochemistry
The excised vessels were dehydrated with 40 % sucrose, embedded in OTC, and frozen at −70 °C. The IVC was sectioned into slices of 10 µm thickness with a cryotome (Leica, Mannheim, Germany). Specimens were washed in PBS and stained with hematoxylin and eosin (H&E). For immunohistochemical staining, slides were incubated with primary antibodies against TF, CD31, p65, phospho-GSK3β and myosin IIA at 4 °C overnight (dilution 1:100, 1:100, 1:50 1:300 and 1:50, respectively). Alexa Fluor® 488-conjugated Donkey Anti-Goat IgG (H+L) antibody (1:600) and Alexa Fluor® 594-conjugated Donkey Anti-Goat IgG (H+L) antibody (1:800) were used as secondary antibodies (Invitrogen, Carlsbad, CA, USA). The nuclei were stained with DAPI. Pathological changes in IVC were observed under fluorescence microscopy (Leica).
Statistical analysis
All data were expressed as the means ± SEM from at least three independent experiments. The data were analysed by a two-tailed Student’s t-test (two groups) or two-way analysis of variance (ANOVA) followed by Bonferroni’s test (three or more groups). Differences were considered significant with a p-value of less than 0.05.
Results
The NMMHC II inhibitor, blebbistatin, suppresses TNF-α-induced TF expression and procoagulant activity in endothelial cells
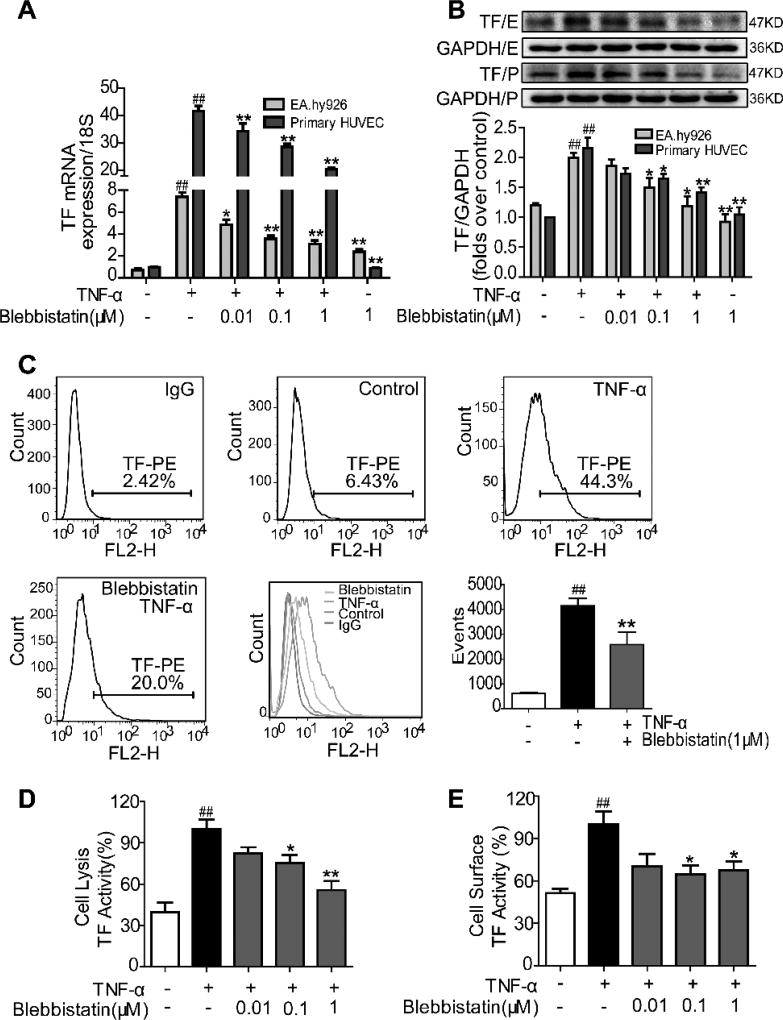
To determine whether NMMHC II regulates TF expression in activated endothelial cells, we treated the cells with blebbistatin, a myosin II inhibitor (30, 31). As observed in Figure 1 A–B, pretreatment of blebbistatin down-regulated TNF-α-stimulated TF mRNA and protein expression in EA.hy926 endothelial cells and the primary HUVECs. Flow cytometry analysis also showed that blebbistatin decreased TF protein expression in endothelial cells stimulated with TNF-α (Figure 1 C), further confirming the inhibitory effect of blebbistatin on TF expression. Consistent with the altered TF expression, blebbistatin treatment also functionally decreased the clotting activity of TF induced by TNF-α both in the total cell lysate and on the cell surface (Figure 1 D–E). Together, these data demonstrate that NMMHC II inhibition suppressed TNF-α-induced TF expression and procoagulant activity in endothelial cells.
Figure 1. Blebbistatin suppressed TNF-α-induced TF expression and procoagulant activity in endothelial cells.
EA.hy926 endothelial cells (E) and the primary HUVECs (P) were pretreated with blebbistatin for 1 h at various concentrations, followed by TNF-α (10 ng/ml) stimulation for 5 h. A) TF mRNA expression levels after TNF-α exposure in the presence or absence of blebbistatin at 0.01–1 µM. B–C) TF protein expression levels were analysed by western blotting and flow cytometry. D–E) TF procoagulant activities of cell lysates and cell surfaces were examined with a chromogenic assay. Data were shown as the means ± SEM of three independent experiments. ##P< 0.01 vs the unstimulated group; *P< 0.05, **P< 0.01 vs TNF-α alone group.
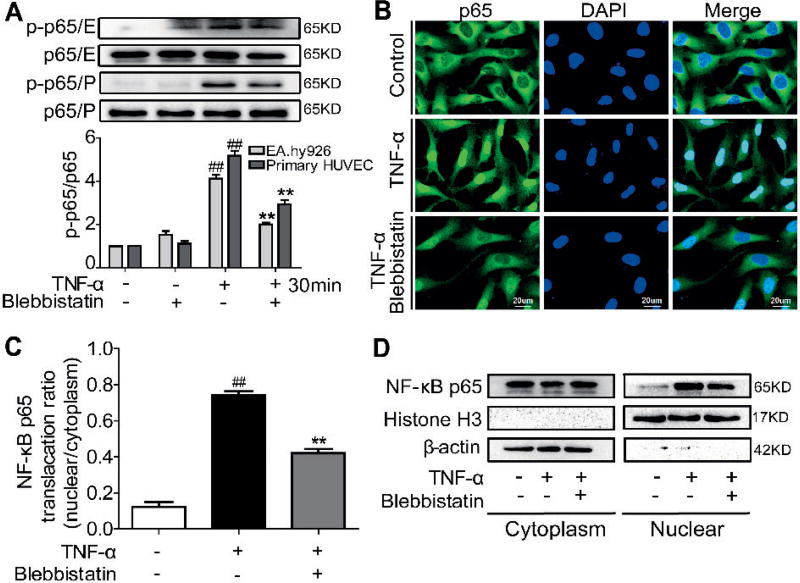
Blebbistatin inhibits TNF-α-activated NF-κB signalling pathway and NF-κB p65 nuclear translocation in the endothelial cells
NF-κB activation is mediated through NF-κB p65 nuclear translocation and then binds to the κB binding site in the TF promoter (7). Therefore, we next determined whether NMMHC II inhibition could affect the TNF-α-induced NF-κB activation in endothelial cells. As shown in Suppl. Figure 1A–B (available online at www.thrombosis-online.com), TNF-α challenge induced IκBα phosphorylation with IκBα degradation, but these alterations were reversed by treatment with blebbistatin. As a result, we observed that TNF-α-induced NF-κB p65 phosphorylation was also inhibited by blebbistatin in EA.hy926 endothelial cells and the primary HUVECs (Figure 2 A and Suppl. Figure 1C, available online at www.thrombosis-online.com). Immunocytochemistry analysis also demonstrated that blebbistatin suppressed the p65 translocation into the nuclei in response to TNF-α exposure (Figure 2 B–C). Figure 2 D further showed that blebbistatin reversed TNF-α-induced cytoplasmic p65 protein decrease and nucleic p65 protein increase. Taken together, these findings showed that NMMHC II inhibition by blebbistatin attenuated TNF-α-induced NF-κB activation.
Figure 2. Blebbistatin downregulated the NF-κB pathway and inhibited NF-κB p65 nuclear translocation induced by TNF-α in the endothelial cells.
EA.hy926 endothelial cells (E) and the primary HUVECs (P) were treated with TNF-α (10 ng/ml) with or without blebbistatin (1 µM) for the indicated time. A) Total and phosphorylated p65 were examined by western blotting. #P< 0.05, ##P< 0.01 vs the unstimulated group at 0 min; *P< 0.05, **P< 0.01 □s TNF-α stimulated group at certain time. B) Immunofluorescent staining of NF-κB p65 in endothelial cells after treatment with 1 µM blebbistatin for 1 h, followed by 30 min stimulation of TNF-α (10 ng/ml) or vehicle. Scale bar, 20 µm. Representative images from three individual experiments with similar results were shown. C) Quantitative analysis of cytoplasmic and nuclear fractions of NF-κB p65 using the Image-Pro Plus 6.0 software (means ± SEM). ##P< 0.01 vs the unstimulated group; **P< 0.01 vs TNF-α stimulation group. D) NF-κB p65 expression in the cytoplasm and nucleus of blebbistatin–treated endothelial cells. Cells were pretreated with blebbistatin (1 µM) for 1 h and stimulated with TNF-α (10 ng/ml) for 30 min. Proteins were extracted from the cytoplasm and nucleus, with β-actin and histone H3 as the internal standards.
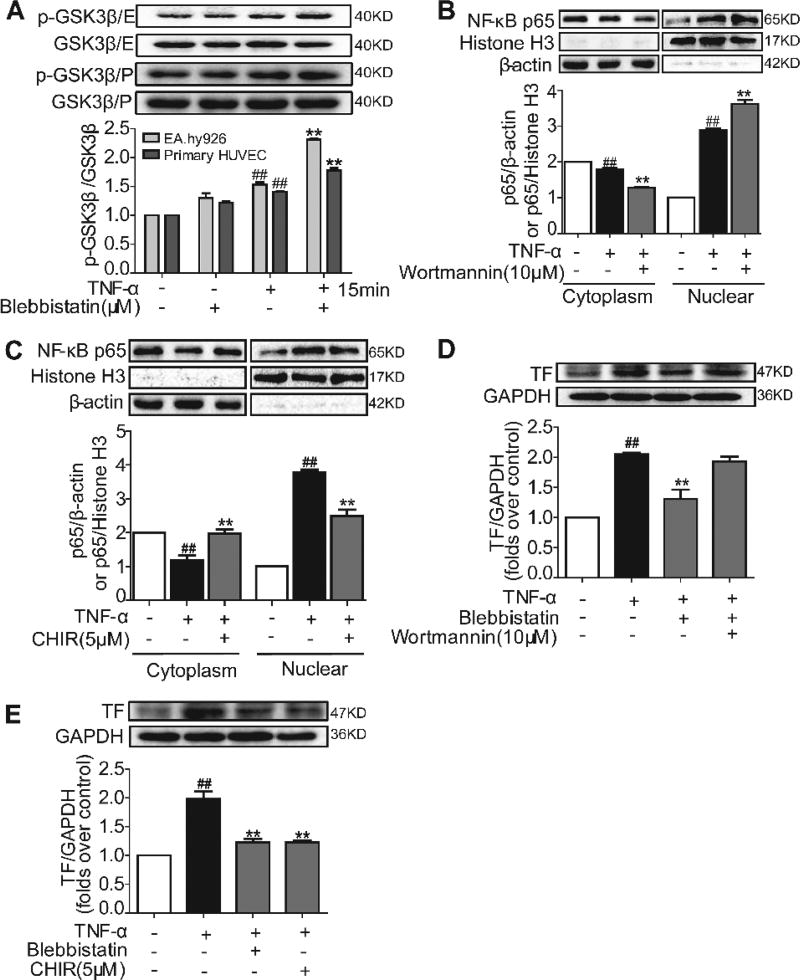
Blebbistatin activates the Akt/GSK3β signalling pathway and inhibits NF-κB p65 nuclear translocation and TF expression in endothelial cells
The PI3K/Akt signalling pathway has been shown to negatively regulate TF expression (32–34). We then investigated whether blebbistatin could regulate TF by affecting the PI3K/Akt pathway. TNF-α initiated minor PI3K and Akt phosphorylation, whereas blebbistatin treatment further enhanced PI3K and Akt phosphorylation substantially in the presence of TNF-α (Suppl. Figure 1D–E, available online at www.thrombosis-online.com). Because GSK3β is a major downstream regulator of Akt, we further observed that GSK3β activity in EA.hy926 endothelial cells and the primary HU-VECs were subjected to TNF-α exposure. Although TNF-α stimulation mildly increased GSK3β phosphorylation, blebbistatin treatment significantly increased GSK3β phosphorylation, and the phosphorylation remained at a higher level (Figure 3 A and Suppl. Figure 1F, available online at www.thrombosis-online.com). This result indicated that blebbistatin inhibited GSK3β activation by inducing its phosphorylation. GSK3β activation has been shown to be required for the regulation of NF-κB activation (8, 35). To determine whether this action is also present in the endothelial cells, we examined TNF-α-induced NF-κB p65 translocation in the presence of the PI3K inhibitor wortmannin or the GSK3β inhibitor CHIR99021. Blocking PI3K signalling by wortmannin enhanced TNF-α-induced NF-κB p65 translocation (Figure 3 B), which is indicative of the inhibitory role of PI3K in NF-κB activation. In contrast, CHIR99021 blocked the TNF-α-induced NF-κB p65 translocation from the cytosol to the nuclei (Figure 3 C), further confirming the role of GSK3β signalling in the promotion of NF-κB activation in endothelial cells. Consistent with these results, wortmannin blocked the inhibitory action of blebbistatin and CHIR99021 reduced TF induction expression (Figure 3 D–E), suggesting that blebbistatin inhibited TF expression through PI3K/Akt signalling with suppression of GSK3β activity.
Figure 3. Blebbistatin activated the Akt/GSK3β signalling pathway and inhibited NF-κB p65 nuclear translocation and TF expression induced by TNF-α in the endothelial cells.
EA.hy926 endothelial cells (E) and the primary HUVECs (P) were treated with either blebbistatin (1 µM) or TNF-α (10 ng/ml) and blebbistatin (1 µM) for different periods and lysed. A) GSK3β and phosphorylated GSK3β were examined by western blotting. #P< 0.05, ##P< 0.01 vs the unstimulated group at 0 min; *P< 0.05, **P< 0.01 vs TNF-α model group at certain time. B–C) NF-κB p65 protein expression in the cytoplasm and nucleus of wortmannin and CHIR99021–treated endothelial cells. Cells were, respectively pretreated with 10 µM wortmannin and 5 µM CHIR99021 for 1 h and stimulated with TNF-α (10 ng/ml) for 30 min. Proteins were extracted from the cytoplasm and nucleus, with β-actin and Histone H3, respectively, as the internal standards. D–E) TF in endothelial cell was examined by western blotting at 5 h after treatment with TNF-α (10 ng/ml) or TNF-α and blebbistatin (1 µM) or TNF-α and blebbistatin with wortmannin (10 µM) or TNF-α and CHIR99021 (5 µM). Data are shown as the means ± SEM of three independent experiments. ##P< 0.01 vs the unstimulated group; **P< 0.01 vs TNF-α stimulated group.
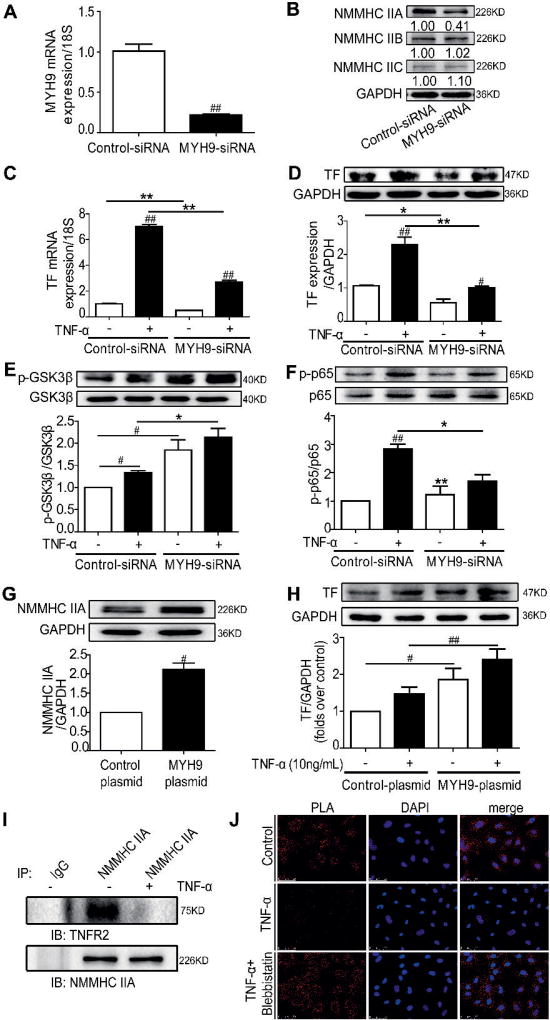
NMMHC IIA modulates TF expression and PI3K/Akt-NF-κB signalling pathways
Blebbistatin is an inhibitor of myosin II that includes three subtypes: NMMHC IIA, IIB and IIC. NMMHC IIA encoded by MYH9 has been shown to modulate platelet function and artery thrombosis (17–20). We performed a series of experiments to examine whether NMMHC IIA was the major subtype of myosin responsible for controlling TNF-α induced TF expression. NMMHC IIA was successfully knocked down by a siRNA specific target MYH9, without any effect on the expression levels of other myosin subtypes in the endothelial cells (Figure 4 A–B). MYH9 knock-down significantly decreased both the basal and TNF-α-induced TF mRNA and protein expression (Figure 4 C–D). Furthermore, NMMHC IIA knock-down facilitated PI3K/Akt activation and GSK3β phosphorylation (deactivation) with or without TNF-α stimulation (Figure 4 E and Suppl. Figure 2A–B, available online at www.thrombosis-online.com), an effect similar to the NMMHC II inhibitor blebbistatin. Meanwhile, NF-κB signalling was significantly inhibited by NMMHC IIA silencing (Figure 4 F and Suppl. Figure 2C–D, available online at www.thrombosis-online.com). These findings indicate that NMMHC IIA was the major myosin subtype in regulating PI3K/Akt-NF-κB pathways and TF expression in the activated endothelial cells.
Figure 4. Knockdown or overexpression of NMMHC IIA modulates TF expression and PI3K/Akt-NF-κB signalling pathways in vitro.
EA.hy926 endothelial cells were transfected with 100 nM of MYH9-siRNA or control-siRNA for 48 h. HEK 293T cells were transfected with MYH9-plasmid or control-plasmid for 48 h. A) Total cellular RNA was extracted, and MYH9 mRNA expression was analysed by qRT-PCR. B) Cell lysates were examined for NMMHC IIA, IIB and IIC expression by western blotting. C–D) Control and MYH9 knock-down cells were stimulated with 10 ng/ml TNF-α for 5 h, and TF expression was examined by qRT-PCR and western blotting. E–F) At 48 h post-transfection, endothelial cells were treated with 10 ng/ml TNF-α for 15 min or 30 min. Total and phosphorylated GSK3β, p65 were analysed by western blotting. G) HEK 293T cell lysates were examined for NMMHC IIA expression by western blotting. H) HEK 293T cell lysates were examined for TF expression by western blotting. I) Co-immunoprecipitation of NMMHC IIA and the TNFR2 from primary HU-VECs lysates. Cells were harvested, lysed, and immunoprecipitated with NMMHC IIA antibody. Immunocomplexes were separated by SDS-PAGE and immunoblotted with the indicated antibodies. Normal serum (IgG) was used as a negative control for immunoprecipitation. J) Confocal micrographs of primary HUVECs or primary HUVECs with blebbistatin (1 µM) in the presence or absence of 10 ng/ml TNF-α for 8 min, followed by PLA reaction for NMMHC IIA–TNFR2 interaction (red signal) and DAPI (blue) staining (n=3). Bar, 50 µm.
Furthermore, NMMHC IIA overexpression in 293T cells significantly increased both the basal and TNF-α-induced TF protein expression (Figure 4 G and H), which provided additional evidence for the correlation of NMMHC IIA and TF expression.
TNF-α stimulation disassociates basal TNFR2 and NMMHC IIA interaction in primary HUVEC cells
As previously documented (24), both myosin regulatory light chain (MRLC) and NMMHC II bound to TNFR2 and mediated signal internalisation in TNF-α-stimulated endothelial cells. To investigate the involvement of NMMHC IIA in the regulation of TNFR2 signalling, we examined the potential protein-protein interaction of NMMHCIIA and TNFR2 in untreated and TNF-α–stimulated primary HUVECs. As observed in Figure 4 I, TNFR2 was co-immunoprecipitated with NMMHCIIA in untreated cells but not under the TNF-α-stimulated conditions. Similar results were obtained by the Duolink Proximity ligation assay (Figure 4 J). These results suggest that TNF-α stimulation disrupts the basal interaction of the NMMHC IIA-TNFR2 interaction. The release of NMMHC IIA from TNFR2 may be critical for TNF-α-dependent signal activation.
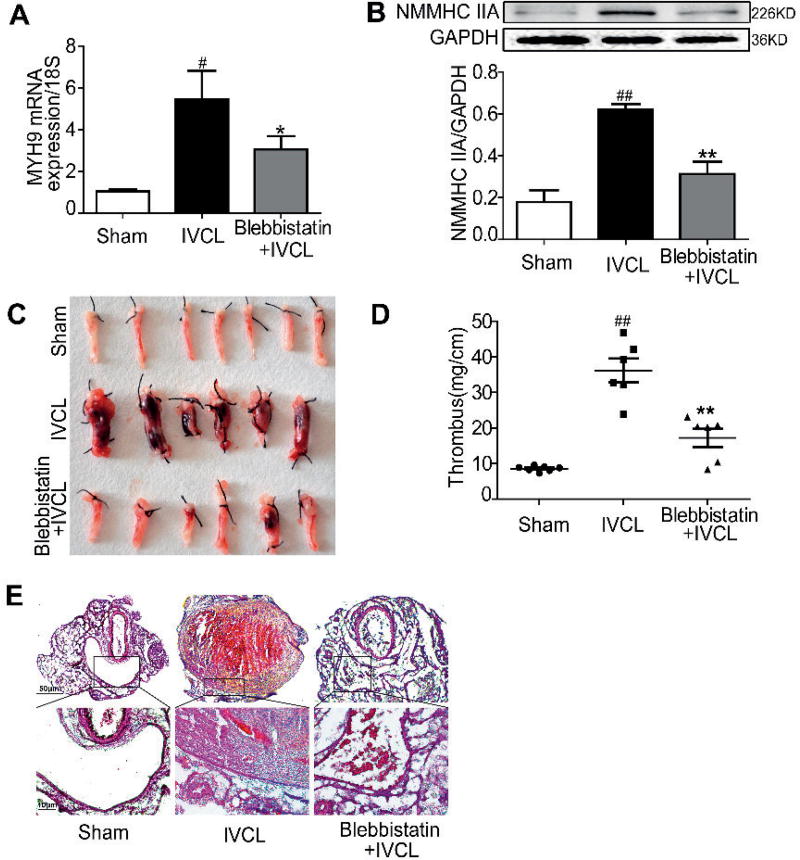
NMMHC IIA inhibition alleviates thrombus formation in a mouse model of DVT
Considering the role of TF in DVT (11), we examined whether NMMHC IIA played a role in the thrombus formation in a mouse model of DVT induced by IVC ligation (IVCL). As observed in Figure 5 A–B, compared with the sham-operated mice, the IVCs from IVCL mice expressed much higher mRNA and protein levels of NMMHC IIA, which were significantly reduced by blebbistatin. The response of blebbistatin on the expression of NMMHC IIA in the IVCL mice was further confirmed by immunofluorescence staining (Figure 6 D). More importantly, blebbistatin treatment reduced the mass of thrombus up to 65 % compared with IVCL group, thus demonstrating its inhibitory effect on thrombus formation (Figure 5 C–D). Morphological analysis revealed no thrombi formation in the sham-operated group (Figure 5 E, left panel). In the IVCL and blebbistatin treated groups, the thrombi consist mainly of fibrin, platelets, and erythrocytes (Figure 5 E). The endothelial surface of the IVC appeared intact in the sham-operated group. In contrast, an extensive fibrin network and cell aggregates were found on the IVC endothelial surface at 48 h after ligation, a phenotype suppressed by blebbistatin (Figure 5 E, right panel). Conversely, blebbistatin treatment alone had no obvious effect compared with the sham-operated group (Suppl. Figure 5, available online at www.thrombosis-online.com).
Figure 5. Inhibiton of NMMHC IIA by blebbistatin alleviated thrombus formation in a mouse model of DVT.
C57BL/6J mice (male, 22–25 g) were injected with vehicle (5 % ethanol in saline) or blebbistatin (1 mg/kg) intraperitoneally and then underwent IVCL to initiate thrombus formation in the IVC 1 h later. At 24 h after surgical procedure, mice were given blebbistatin once, and IVCs were collected for RNA extraction or processed for western blotting and histochemical analysis at 48 h after surgical procedure. ##P< 0.01 vs the sham group; **P< 0.01 vs IVCL group. A) MYH9 mRNA expression was analysed by qRT-PCR. B) IVC lysates were prepared and NMMHC IIA expression was analysed by western blotting. C–D) The thrombosed IVC was weighed and the length of the thrombus was measured. The size of the thrombus was quantified as mg/cm. Six or more representative thrombosed IVC from eight mice in each group and the quantitative data of thrombosed IVC are shown. E) Histological evaluation of the ligated IVC. Histological analysis: Representative photos of hematoxylin-eosin (HE) staining at 48 h after ligation (original magnification ×50 and ×400).
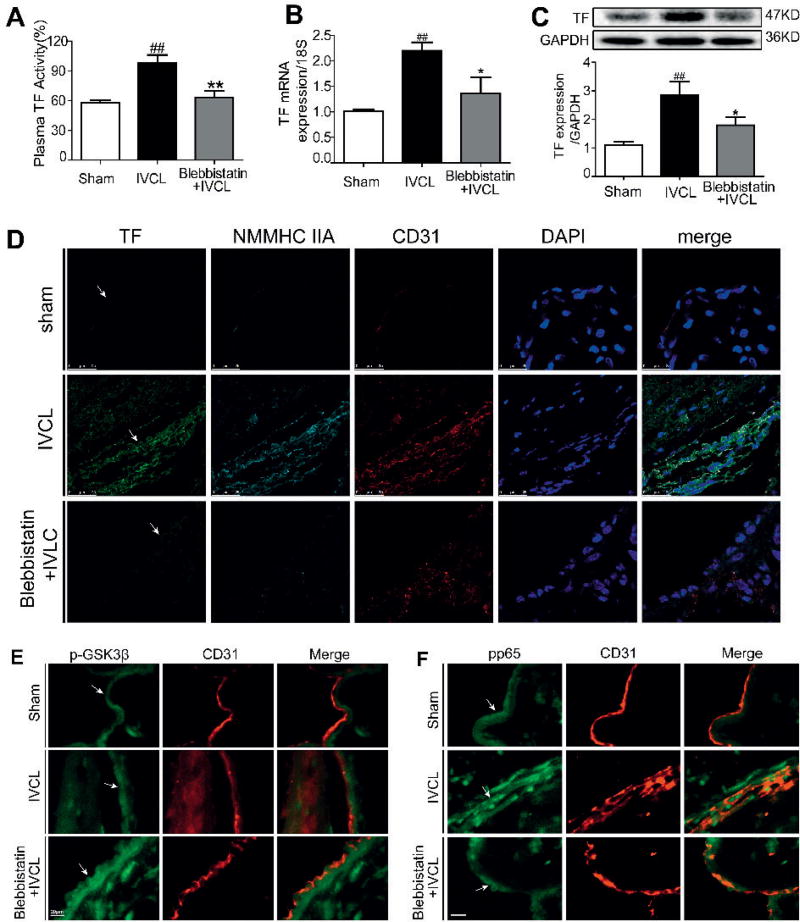
Figure 6. Blebbistatin inhibited TF activity and expression in DVT mice.
At 48 h after IVCL, plasma was collected and used to determine TF procoagulant activity and IVCs were harvested for RNA extraction or processed for western blotting and histochemical analysis. A) Plasma from mice was measured for TF procoagulant activity. B–C) TF mRNA and protein levels were analysed by qRT-PCR and western blotting. D) IVC sections were immunostained with a combination of anti-TF pAbs (green), anti-myosin IIA pAbs (indigotin), anti-CD31 pAbs (red) and DAPI (blue). Images were digitally merged. Representative results are shown. Bar, 25 µm. E–F) Double-colour immunofluorescence analysis of p-GSK3β (green) and CD31 (red), of p-p65 (green) and CD31(red) in the thrombi. IVC sections were immunostained with a combination of anti-p-GSK3β pAbs, anti-pp65 pAbs and anti-CD31 pAbs. Images were digitally merged. Representative results are shown. Bar, 20 µm (arrows indicate vessel wall). ##P< 0.01 vs sham group; *P< 0.05, **P< 0.01 vs IVCL group.
Blebbistatin inhibits TF activity and expression in DVT mice
To confirm the modulation of TF by NMMHC IIA, we then examined whether blebbistatin could inhibit TF expression and procoagulant activity in vivo. Figure 6 A showed that DVT mice had higher plasma TF procoagulant activity than did sham-operated animals. Furthermore, the TF mRNA level and protein expression (Figure 6 B–C) in IVC tissues from the DVT mice were significantly increased compared with those in the sham group. Blebbistatin treatment markedly reduced the TF expression and activity (Figure 6 B–C). Immunohistochemistry also demonstrated that in deep venous endothelium labelled by CD31, the IVCL group displayed higher expression levels of TF compared with the sham group. Blebbistatin treatment dramatically decreased the TF expression in deep venous endothelium (Figure 6 D).
Blebbistatin induces phosphorylation of GSK3β and suppresses NF-κB activation in DVT mice
Because NMMHC IIA inhibition could modulate the Akt/GSK3β and NF-κB pathways and suppress TF expression in vitro, we next tested whether these effects persist in vivo. The expression levels of phospho-GSK3β and phospho-p65 in IVC tissue were evaluated by immunofluorescence staining. As observed in Figure 6 E, IVCL significantly increased phospho-GSK3β (a deactivated form of GSK3β), suggesting that IVCL activated PI3K/Akt and suppressed GSK3β activity. Blebbistatin treatment further enhanced GSK3β phosphorylation (Figure 6 E), presumably by PI3K/Akt activation. Similar to the report that active GSK3β can enhance NF-κB/p65 (13), phospho-p65 was elevated in IVCL mice compared with those from the sham group. Alternatively, blebbistatin suppressed phospho-p65 overexpression in DVT mice (Figure 6 F). Taken together, these data suggested that NMMHC IIA inhibition by blebbistatin suppressed thrombus formation and TF expression by modulating Akt/GSK3β and NF-κB signalling pathways in vivo.
Discussion
Much evidence has indicated a key role of TF present on circulating monocytes and activated endothelium under inflammatory stimulation in the formation of DVT (2, 11–14). However, its upstream modulating molecules remain to be explored. Myosin II is an adenosine triphosphate-driven molecular motor protein that participates in many physiological and pathological functions (17–20). Recent studies have shown that mice with a platelet-specific deletion of NMMHC II A display a strong increase in bleeding time, absence of clot retraction and inhibition of artery thrombosis (17, 22), suggesting its regulatory role in thrombosis. However, the pathological importance of NMMHC II in DVT and its modulation in TF expression in TNF-α-activated endothelial cells have not been explored in the literature.
In the present study, we first use blebbistatin, an inhibitor of myosin II ATPase activity (30, 31), to explore the function of NMMHC II on the regulation of TF expression and DVT. The results in vitro and in vivo show that blebbistatin significantly inhibits TF expression and procoagulant activity (Figure 1 and Figure 6). The full-length TF protein is the main trigger of the coagulation cascade (33) and is considered the main source of procoagulant activity (36). It can catalyze the conversion of prothrombin to thrombin, which cleaves fibrinogen into fibrin, activates platelets, and finally induces clot formation. Our data present the first evidence that NMMHC II modulates TF and is involved in the coagulation and fibrinolytic pathways in endothelial cells.
Our findings demonstrate that inhibition of TF expression by blebbistatin in the endothelial cells occurs through the activation of the Akt/GSK3β signalling pathway and the inhibition of the NF-κB signalling pathway (Figure 3 and Figure 4). As documented, TF expression is primarily regulated by NF-κB, the phosphoinositide 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) signalling pathways (12–15). NF-κB is a homo- or heterodimeric complex of proteins, and the c-Rel/p65 heterodimer is critically involved in regulation of TF gene expression (37). In non-stimulated endothelial cells, NF-κB is localised in the cytoplasm as an inactive form bound to IκB. TNF-α stimulation induces NF-κB activity, thus leading to the dissociation and subsequent degradation of IκB proteins and allowing NF-κB dimers to enter the nucleus and induce TF gene expression (37). The PI3K/Akt pathway is a conserved family of signal transduction enzymes that participate in the regulation of cell proliferation and survival. A number of studies have demonstrated that PI3K/Akt is a negative-feedback regulator of TF expression (33). In response to TNF-α stimulation, activated endothelial cells have increased NF-κB-mediated TF expression and mildly enhanced activation of PI3K/Akt for survival (15). Our data (Figure 3) show that blebbistatin further activated the PI3K/Akt pathway, inactivated GSK3β by inducing its phosphorylation, and inhibited TF expression (8, 38). Furthermore, the inhibitory effect of blebbistatin on TF expression in endothelial cells is similar to the GSK3β inhibitor CHIR and can be partially reversed by the PI3K inhibitor wortmannin. Despite a few reports (39, 40), the relationship between PI3-kinase/Akt and NF-κB is not linear. However, our data support the regulation of PI3-kinase/Akt with NF-κB p65 activation (8, 13, 15) and present a possible explanation as to how NMMHC II modulates TF expression.
To further explore which subtype of NMMHC II is primarily involved in inhibiting TF expression through the Akt/GSK3β-NF-κB pathway, we then specifically knocked down NMMHC IIA with MYH9-siRNA in vitro, considering its function in endothelial activation and thrombosis (22–24). Our findings indicate that knocking down NMMHC IIA, but not IIB or IIC, significantly reduces TF protein and gene levels in TNF-α-stimulated endothelial cells. Furthermore, knocking down NMMHC IIA inactivated GSK3β by inducing its phosphorylation and suppressed the NF-κB signal pathway (Figure 4 E–F). Moreover, we have also shown that the overexpression of NMMHC IIA increased TF expression in HEK 293T tool cells, suggesting that both the biological function and the expression of NMMHC IIA are critical in mediating TF expression and related signalling pathways (Figure 4).
It has been reported that NMMHC II (or its subunits) participate in the trafficking and positive or negative regulation of multiple receptors, including the chemokine receptors CXCR4 and CXCR5, the multidrug resistance protein 1, the N-methyl-D-aspartate (NMDA) receptor, and TNF-α receptors (TNFRs) (24). TNFR2 is a proinflammatory and proatherogenic molecule (41, 42). Activation of TNFR2 displays prothrombotic activity in vivo, whereas stimulation of TNFR1 shows an antithrombotic effect (43). A previous study has shown that both the myosin regulatory light chain (MRLC) and NMMHC II bound to TNFR2 in endothelial cells (24). Under TNF-α stimulation, the ROCK1-mediated release of MRLC from TNFR2 was critical for TNF-α-dependent gene expression. However, the role of NMMHC II in TNFR2 signalling and target gene expression has not been investigated. In this paper, we give solid evidence for the binding and release of NMMHC IIA and TNFR2 in the primary HUVECs by co-immunoprecipitation and PLA assays (Figure 4 I–J). NMMHC IIA is likely located upstream of Akt/GSK3β-NF-κB based on its regulation of the signalling pathways verified in our present study.
Our current study further demonstrates that NMMHC IIA acts as a critical regulatory protein of TF expression in DVT. The IVCL model has been widely used to study venous thrombosis (28). In general, thrombus weights in mice are much larger after 48 h of ligation (28, 44). As previously documented (45), the rapid development of thrombosis after IVCL reflects a combination of stasis-induced vein wall injury and enhanced TF expression in endothelial cells and leukocytes, which was also demonstrated in our experiments (Figure 6 D and Suppl. Figure 3, available online at www.thrombosis-online.com). However, in the current study, we focused on the function of NMMHC IIA in the endothelial cells, but not leukocytes, on the pathogenesis of DVT in vitro and in vivo. Our present study gives the first evidence that blebbistatin inhibits plasma TF procoagulant activity, TF expression in CD31 positive-cells and thrombus mass in mice 48 h after IVCL, accompanied by phospho-GSK3β enhancement and phospho-p65 downregulation (Figure 6). We also confirmed that TF expression in the IVCL group was detected in endothelial cells adherent to the vein wall, where it was highly expressed with NMMHC IIA and phospho-p65 but did not have a significant influence on the other myosin subtypes in vivo, raising the possibility that NMMHC IIA-induced Akt/GSK3β-NF-κB pathway and TF expression play a role in DVT. It is a potential molecular mechanism in vivo that NMMHC IIA inhibits TF expression by modulating the Akt/GSK3β-NF-κB signalling pathways.
Of note, the myosin II ATPase inhibitor, blebbistatin, is commonly used as a tool to explore the function of NMMHC II (46). As documented (24, 47), the concentration in vitro and dosage in vivo of blebbistatin are usually 10–100 µM and 10 mg/kg, respectively. However, in our present study, blebbistatin has been found to modulate TF expression at 0.01–1 µM in activated endothelial cells in vitro and at 1 mg/kg in vivo. Blebbistatin alone did not exert obvious side effects on the normal endothelial cells and sham-operated mice. These findings indicate the safety and efficiency of NMMHC IIA as a potential target for DVT. However, given blebbistatin’s photosensitivity, phototoxicity and other side effects (48, 49), it might be very meaningful to screen for safer and/or low-cost inhibitors from natural products, in the future. In addition, blebbistatin suppresses both the NMMHC IIA function and the NMMHC IIA expression in vivo, suggesting that conditional NMMHC IIA-KO in endothelial cells may contribute to prevent DVT in mice, which requires further study.
Taken together, we use the NMMHC IIA inhibitor blebbistatin and MYH9-siRNA in the endothelial cells and overexpression of NMMHC IIA in tool cells to examine the function of NMMHC IIA on the pathogenesis of DVT in vitro and in vivo. Our findings uncover a key role of NMMHC IIA in modulating TF expression and activity by interacting with TNFR2 followed by modulating the Akt/GSK3β-NF-κB signalling pathways. These results provide compelling evidence of the role of NMMHC IIA in venous thrombosis and may lead to new strategies for the treatment of venous thrombosis and many other inflammation-related thrombotic diseases.
Supplementary Material
What is known about this topic?
The endothelial lining of blood vessels plays a critical role in regulating thrombosis by preventing procoagulant and proinflammatory activities.
Tissue factor (TF), a coagulation cascade promote, is primarily regulated by the NF-κB, the phosphoinositide 3-kinase (PI3K) and the mitogen-activated protein kinase (MAPK) signalling pathways.
Non-muscle myosin heavy chain IIA deficiency or inhibition can reduce platelet aggregation and arterial thrombus formation.
What does this paper add?
The study is the first to confirm that non-muscle myosin heavy chain IIA is involved in TF expression and deep venous thrombosis.
Inhibiting or knocking down non-muscle myosin heavy chain IIA decreases TF expression and thrombus formation mainly through the Akt/GSK3β-NF-κB signalling pathway.
Non-muscle myosin heavy chain IIA inhibition is a potential treatment strategy for many inflammation-related thrombotic diseases.
Acknowledgments
The authors thank Dr. Wentao Liu in Nanjing Medical University and Prof. Baolin Liu in China Pharmaceutical University for their critical comments and revision. We are also grateful to Chun Chu, Lu Zhang and Xingsheng Ren for their assistance in the animal experiments.
Financial support:
The present research was supported by the National Natural Science Foundation of China (No. 81274131), 2011’ Program for Excellent Scientific and Technological Innovation Team of Jiangsu Higher Education, a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Project Program of the State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. JKGZ201107).
Footnotes
Conflicts of interest
None declared.
References
- 1.Dasari TW, Pappy R, Hennebry TA. Pharmacomechanical thrombolysis of acute and chronic symptomatic deep vein thrombosis: a systematic review of literature. Angiology. 2012;63:138–145. doi: 10.1177/0003319711410050. [DOI] [PubMed] [Google Scholar]
- 2.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122:2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz C, Engelmann B, Massberg S. Crossroads of coagulation and innate immunity: the case of deep vein thrombosis. J Thromb Haemost. 2013;11:233–241. doi: 10.1111/jth.12261. [DOI] [PubMed] [Google Scholar]
- 4.Reitsma PH, Versteeg HH, Middeldorp S. Mechanistic view of risk factors for venous thromboembolism. Arterioscler Thromb Vasc Biol. 2012;32:563–568. doi: 10.1161/ATVBAHA.111.242818. [DOI] [PubMed] [Google Scholar]
- 5.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol. 2011;73:515–525. doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des. 2009;15:1358–1372. doi: 10.2174/138161209787846702. [DOI] [PubMed] [Google Scholar]
- 7.Eto M, Kouroedov A, Cosentino F, et al. Glycogen synthase kinase-3 mediates endothelial cell activation by tumor necrosis factor-alpha. Circulation. 2005;112:1316–1322. doi: 10.1161/CIRCULATIONAHA.105.564112. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Wang J, Wang H, et al. Acadesine inhibits tissue factor induction and thrombus formation by activating the phosphoinositide 3-kinase/Akt signalling pathway. Arterioscler Thromb Vasc Biol. 2010;30:1000–1006. doi: 10.1161/ATVBAHA.110.203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YD, Ye BQ, Zheng SX, et al. NF-kappaB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J Biol Chem. 2009;284:4473–4483. doi: 10.1074/jbc.M806010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson GJ, Leis LA, Bach RR. Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty. Thromb Haemost. 2009;102:728–734. doi: 10.1160/TH09-04-0261. [DOI] [PubMed] [Google Scholar]
- 11.Ettelaie C, Collier ME, Mei MP, et al. Enhanced binding of tissue factor-micro-particles to collagen-IV and fibronectin leads to increased tissue factor activity in vitro. Thromb Haemost. 2013;109:61–71. doi: 10.1160/TH12-05-0279. [DOI] [PubMed] [Google Scholar]
- 12.Schabbauer G, Tencati M, Pedersen B, et al. PI3K–Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 13.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signalling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Oh JL, Ryu YS, et al. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16:126–128. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 15.Zhang WJ, Wei H, Hagen T, et al. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signalling pathway. Proc Natl Acad Sci USA. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M, Ge C, Zeng W, et al. Retinoic acid promotes proliferation of chicken primordial germ cells via activation of PI3K/Akt-mediated NF-kappaB signalling cascade. Cell Biol Int. 2012;36:705–712. doi: 10.1042/CBI20110542. [DOI] [PubMed] [Google Scholar]
- 17.Calaminus SD, Auger JM, McCarty OJ, et al. MyosinIIa contractility is required for maintenance of platelet structure during spreading on collagen and contributes to thrombus stability. J Thromb Haemost. 2007;5:2136–2145. doi: 10.1111/j.1538-7836.2007.02696.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, van Grunsven LA, Van Rossen E, et al. Blebbistatin inhibits contraction and accelerates migration in mouse hepatic stellate cells. Br J Pharmacol. 2010;159:304–315. doi: 10.1111/j.1476-5381.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maravillas-Montero JL, Santos-Argumedo L. The myosin family: unconventional roles of actin-dependent molecular motors in immune cells. J Leukoc Biol. 2012;91:35–46. doi: 10.1189/jlb.0711335. [DOI] [PubMed] [Google Scholar]
- 20.Lv Y, Lu S, Lu T, et al. Homology model of nonmuscle myosin heavy chain IIA and binding mode analysis with its inhibitor blebbistatin. J Mol Model. 2013;19:1801–1810. doi: 10.1007/s00894-012-1750-3. [DOI] [PubMed] [Google Scholar]
- 21.Clark K, Middelbeek J, Dorovkov MV, et al. The alpha-kinases TRPM6 and TRPM7, but not eEF-2 kinase, phosphorylate the assembly domain of myosin IIA, IIB and IIC. FEBS lett. 2008;582:2993–2997. doi: 10.1016/j.febslet.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 22.Léon C, Hechler B, Aleil B, et al. Megakaryocyte-restricted MYH9 inactivation dramatically affects hemostasis while preserving platelet aggregation and secretion. Blood. 2007;110:3183–3191. doi: 10.1182/blood-2007-03-080184. [DOI] [PubMed] [Google Scholar]
- 23.Ono A, Westein E, Hsiao S, et al. Identification of a fibrin-independent platelet contractile mechanism regulating primary hemostasis and thrombus growth. Blood. 2008;112:90–99. doi: 10.1182/blood-2007-12-127001. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekharan UM, Dechert L, Davidson UI, et al. Release of Nonmuscle Myosin II from the Cytosolic Domain of Tumor Necrosis Factor Receptor 2 Is Required for Target Gene Expression. Sci Signal. 2013;6:ra60. doi: 10.1126/scisignal.2003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betapudi V, Lominadze G, Hsi L, et al. Anti-beta2GPI antibodies stimulate endothelial cell microparticle release via a nonmuscle myosin II motor protein-dependent pathway. Blood. 2013;122:3808–3817. doi: 10.1182/blood-2013-03-490318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Q, Chen L, Gao M, et al. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-kappaB. Int immunopharmacol. 2012;12:88–93. doi: 10.1016/j.intimp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Gullberg M, Andersson A-C. Visualization and quantification of protein-protein interactions in cells and tissues. Nat Methods. 2010;7:641–647. [Google Scholar]
- 28.Diaz JA, Obi AT, Myers DD, Jr, et al. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:556–562. doi: 10.1161/ATVBAHA.111.244608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wrobleski SK, Farris DM, Diaz JA, et al. Mouse complete stasis model of inferior vena cava thrombosis. J Vis Exp. 2011:2738. doi: 10.3791/2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straight AF, Cheung A, Limouze J, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs M, Toth J, Hetenyi C, et al. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 32.Di Santo A, Amore C, Dell’Elba G, et al. Glycogen synthase kinase-3 negatively regulates tissue factor expression in monocytes interacting with activated platelets. J Thromb Haemost. 2011;9:1029–1039. doi: 10.1111/j.1538-7836.2011.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breitenstein A, Camici GG, Tanner FC. Tissue factor: beyond coagulation in the cardiovascular system. Clin Sci. 2010;118:159–172. doi: 10.1042/CS20080622. [DOI] [PubMed] [Google Scholar]
- 34.Eisenreich A, Malz R, Pepke W, et al. Role of the phosphatidylinositol 3-kinase/protein kinase B pathway in regulating alternative splicing of tissue factor mRNA in human endothelial cells. Circ J. 2009;73:1746–1752. doi: 10.1253/circj.cj-99-0225. [DOI] [PubMed] [Google Scholar]
- 35.Risbud MV, Fertala J, Vresilovic EJ, et al. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signalling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine. 2005;30:882–889. doi: 10.1097/01.brs.0000159096.11248.6d. [DOI] [PubMed] [Google Scholar]
- 36.Witkowski M, Weithauser A, Rauch U. Tissue Factor-a Link Between Vascular Procoagulability and Inflammation. Exp Clin Cardiol. 2014;20:1–7. [Google Scholar]
- 37.Sorensen BB, Rao LV, Tornehave D, et al. Antiapoptotic effect of coagulation factor VIIa. Blood. 2003;102:1708–1715. doi: 10.1182/blood-2003-01-0157. [DOI] [PubMed] [Google Scholar]
- 38.Meng F, Liu L, Chin PC, et al. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277:29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- 39.Molor-Erdene P, Okajima K, Isobe H, et al. Inhibition of lipopolysaccharide-induced tissue factor expression in monocytes by urinary trypsin inhibitor in vitro and in vivo. Thromb Haemost. 2005;94:136–145. doi: 10.1160/TH04-09-0577. [DOI] [PubMed] [Google Scholar]
- 40.Martin M, Rehani K, Jope RS, et al. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrasekharan UM, Siemionow M, Unsal M, et al. Tumor necrosis factor alpha (TNF-alpha) receptor-II is required for TNF-alpha-induced leukocyte-endothelial interaction in vivo. Blood. 2007;109:1938–1944. doi: 10.1182/blood-2006-05-020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandrasekharan UM. Decreased atherosclerosis in mice deficient in tumor necrosis factor-alpha receptor-II (p75) Arterioscler Thromb Vasc Biol. 2007;27:e16–17. doi: 10.1161/01.ATV.0000255551.33365.22. [DOI] [PubMed] [Google Scholar]
- 43.Pircher J, Merkle M, Wornle M, et al. Prothrombotic effects of tumor necrosis factor alpha in vivo are amplified by the absence of TNF-alpha receptor subtype 1 and require TNF-alpha receptor subtype 2. Arthritis Res Ther. 2012;14:R225. doi: 10.1186/ar4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz JA, Alvarado CM, Wrobleski SK, et al. The electrolytic inferior vena cava model (EIM) to study thrombogenesis and thrombus resolution with continuous blood flow in the mouse. Thromb Haemost. 2013;109:1158–1169. doi: 10.1160/TH12-09-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J, May L, Liao P, et al. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol. 2009;29:863–869. doi: 10.1161/ATVBAHA.109.185678. [DOI] [PubMed] [Google Scholar]
- 46.Mikulich A, Kavaliauskiene S, Juzenas P. Blebbistatin, a myosin inhibitor, is phototoxic to human cancer cells under exposure to blue light. Biochim Biophys Acta. 2012;1820:870–877. doi: 10.1016/j.bbagen.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Si J, Ge Y, Zhuang SG, et al. Inhibiting nonmuscle myosin II impedes inflammatory infiltration and ameliorates progressive renal disease. Lab Invest. 2010;90:448–458. doi: 10.1038/labinvest.2009.142. [DOI] [PubMed] [Google Scholar]
- 48.Swift LM, Asfour H, Posnack NG, et al. Properties of blebbistatin for cardiac optical mapping and other imaging applications. Eur J Physiol. 2012;464:503–512. doi: 10.1007/s00424-012-1147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolega J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun. 2004;320:1020–1025. doi: 10.1016/j.bbrc.2004.06.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.