Abstract
Gene-mediated cytotoxic immunotherapy (GMCI) is an immune strategy implemented through local delivery of an adenovirus-based vector expressing the thymidine kinase gene (aglatimagene besadenovec, AdV-tk) followed by anti-herpetic prodrug valacyclovir. A phase I dose escalation trial of GMCI followed by chemotherapy was conducted in patients with malignant pleural effusion (MPE). AdV-tk was administered intrapleurally (IP) in three cohorts at a dose of 1 × 1012 to 1013 vector particles. Primary endpoint was safety; secondary endpoints included response rate, progression-free survival, and overall survival. Nineteen patients were enrolled: median age 67 years; 14 with malignant mesothelioma, 4 non-small-cell lung cancer (NSCLC), and 1 breast cancer. There were no dose limiting toxicities. All 3 patients in cohort 2 experienced transient cytokine release syndrome (CRS). Addition of celecoxib in cohort 3 reduced the incidence and severity of CRS (none > grade 2). Three patients are alive (23–33 months after GMCI), and 3 of 4 NSCLC patients had prolonged disease stabilization; one is alive 29 months after GMCI, 3.6 years after initial diagnosis. GMCI was safe and well tolerated in combination with chemotherapy in patients with MPE and showed encouraging response. Further studies are warranted to determine efficacy.
Keywords: intrapleural gene-mediated cytotoxic immunotherapy, malignant pleural effusion, immunotherapy
Aggarwal et al. report results from a phase I trial of intrapleural gene-mediated cytotoxic immunotherapy using an adenovirus-based vector. This approach in combination with chemotherapy was feasible and safe in patients with malignant pleural effusion and showed encouraging anti-tumor responses. Further studies are warranted to determine efficacy.
Introduction
Patients with a variety of malignancies can develop malignant pleural effusion (MPE), with usually poor prognosis.1, 2 The accessibility of pleural fluid in the thoracic cavity facilitates directed administration of therapeutic agents and subsequent analysis of treatment effects, making patients with MPE an attractive model to study gene transfer approaches. One such approach is gene-mediated cytotoxic immunotherapy (GMCI),3 which generates a systemic tumor vaccine effect through local delivery of an adenoviral vector, aglatimagene besadenovec (AdV-tk), expressing the herpes simplex virus thymidine kinase gene followed by an anti-herpetic prodrug, such as valacyclovir.
Although intratumoral injections of a non-replicating adenovirus induce some inflammation, the hypothesized mechanism of GMCI involves both thymidine-kinase-specific cytotoxic and immune stimulatory properties. The cytotoxic effect is mediated by phosphorylation of the anti-herpetic prodrug, which generates nucleotide analogs that lead to termination of tumor DNA repair or replication and consequently cell death by necrosis and apoptosis.4, 5 The prodrug is administered systemically but cytotoxicity is localized to the tumor due to the local administration of AdV-tk. Alteration of the tumor microenvironment, induction of “danger” signals, and pro-inflammatory cytokines, such as interleukin-2 (IL-2), IL-12, and IFN-gamma, create a hyperimmunogenic microenvironment that stimulates antigen-presenting cell maturation and CD8+ T cell expansion.3, 6 This immune effect is critical for effectiveness as evidenced by increased efficacy of this approach in immune-competent compared with immune-deficient models.7, 8 Evidence of tumor cell necrosis, apoptosis, and intratumoral T cell infiltration have been observed in phase I and II trials using this approach.9, 10, 11
Adenoviral vectors are well suited for the large volumes and high titers required for intrapleural (IP) gene transfer based on their ease of production and stability. The lack of viral integration and high immunogenicity of adenoviral vectors are additional advantages for this approach. Our group has previously studied the use of IP adenoviral gene therapy for patients with malignant pleural mesothelioma, including vectors expressing HSV-tk, interferon alpha, and interferon beta.12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Adenoviral vectors were generally well tolerated in these trials; gene transfer into tumors was detected, albeit with limited distribution. Anti-tumor humoral immune responses were noted among subjects treated on these trials along with evidence of clinical responses, including two very long-term survivors (8.5 years and >15 years).20 Additionally, intraperitoneal delivery of up to 2 × 1013 vector particles followed by oral valacyclovir has also been evaluated in patients with stage IIIc ovarian cancer without any emergent dose-limiting toxicity (DLT).22
Clinical trials with GMCI have shown encouraging results with evidence of biologic and clinical activity in multiple solid tumors, including prostate, pancreatic cancer, and malignant glioma.9, 10, 11, 23, 24 Preclinical studies have indicated sensitivity and improved tumor responses of mesothelioma, lung, ovarian, and breast cancer cell lines with GMCI followed by chemotherapy.12, 25, 26, 27, 28 Increased efficacy after receipt of chemotherapy was shown to be due at least in part to induction of antigen-specific memory T cells, increase in number and activity of intratumoral cytotoxic CD 8+ T cells, improved leukocyte trafficking into tumor sites, and augmentation of pro-inflammatory cytokine changes in the tumor microenviroment.29 These observations support the hypothesis that a “priming” effect of GMCI could be “boosted” using chemotherapy.29 Based on preclinical evidence of synergy with sequential chemotherapy, we designed a dose escalation study of AdV-tk accompanied by valacyclovir and then followed by standard of care chemotherapy to evaluate the safety and potential for clinical benefit in patients with MPE.
Results
Patient Demographics
Twenty patients were enrolled between October 2013 and August 2015, and 19 completed therapy: 3 patients in cohort 1 (1 × 1012 vector particles); 3 patients in cohort 2 (1 × 1013 vector particles); and 14 patients in cohort 3 (1 × 1013 vector particles plus celecoxib; 14 enrolled, 13 treated). One patient withdrew before receiving treatment due to ineligibility on day 0. Median age was 67 years (range 41–89). Fourteen patients had malignant mesothelioma (9 epithelioid, 3 sarcomatoid, and 2 biphasic histology), 4 non-small-cell lung cancer (NSCLC), and 1 breast cancer (Table 1). All 19 patients were able to receive standard of care therapy post-vector treatment (Table 2). Seventeen patients received chemotherapy, one patient (epithelioid malignant mesothelioma) received palliative radiation therapy alone, and one patient with breast cancer received hormonal therapy. Eight patients received this therapy as first-line treatment. The remaining 11 patients that received therapy in the 2nd or 3rd line setting had progressive disease at the time of enrollment.
Table 1.
Patient Demographics
| Patient Characteristics | Diagnosis |
||||
|---|---|---|---|---|---|
| Malignant Mesothelioma | NSCLC | Breast Cancer | Total | ||
| Number of patients | 14 | 4 | 1 | 19 | |
| Median age in years (range) | 69.5 (64–89) | 62.5 (41–84) | 61 | 67 (41–89) | |
| Cohort | 1 (1 × 1012 vector particles) | 2 | 1 | – | 3 |
| 2 (1 × 1013 vector particles) | 3 | – | – | 3 | |
| 3 (1 × 1013 vector particles + celecoxib) | 9 | 3 | 1 | 13 | |
| Histology | epithelioid | 9 | – | – | 9 |
| sarcomatoid | 3 | – | – | 3 | |
| biphasic | 2 | – | – | 2 | |
| adenocarcinoma | – | 4 | 1 | 5 | |
| ECOG | 0 | 4 | – | 1 | 5 |
| 1 | 10 | 4 | – | 14 | |
| Line of treatment | 1st | 7 | 1 | – | 8 |
| 2nd | 4 | 2 | 1 | 7 | |
| 3rd | 3 | 1 | – | 4 | |
Table 2.
Patient Disease and Treatment Characteristics
| Case No. | Cohort | Age (years) | ECOG | Treatment Level | Diagnosis | Histology | Status | OS (Months) | PFS (Months) | RECIST Response | Post-vector Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1MP01P | 1 | 79 | 1 | 2nd line | MM | epithelioid | D | 5.9 | 3.8 | SD | gemcitabine |
| 1MP02P | 1 | 80 | 1 | 1st line | MM | sarcomatoid | D | 3.8 | 3.2 | PD | carboplatin/pemetrexed |
| 1MP03P | 1 | 84 | 1 | 2nd line | NSCLC | adenoca | D | 2.7 | 2.7 | SD | erlotinib/everolimus |
| 2MP01P | 2 | 67 | 1 | 3rd line | MM | epithelioid | D | 8.3 | 4.8 | PD | gemcitabine |
| 2MP02P | 2 | 64 | 0 | 3rd line | MM | epithelioid | D | 7.7 | 5.5 | PD | palliative RT |
| 2MP03P | 2 | 66 | 1 | 1st line | MM | epithelioid | D | 14.4 | 10.5 | PR | cisplatin/pemetrexed, RT |
| 2MP04P | 3 | 66 | 0 | 2nd line | MM | epithelioid | A | 33.4 | 8.2 | SD | carboplatin/gemcitabine, RT, vinorelbine, pembrolizumab, MDG-009 |
| 2MP05P | 3 | 61 | 0 | 2nd line | breast ca | adenoca | D | 13.6 | 6.2 | SD | anastrozole |
| 2MP06P | 3 | 67 | 1 | 1st line | MM | biphasic | D | 12.5 | 5.4 | NE | cisplatin/pemetrexed, RT, gemcitabine |
| 2MP07P | 3 | 41 | 1 | 2nd line | NSCLC | adenoca | A | 29.0 | 6.8 | SD | carboplatin/paclitaxel, nivolumab, gemcitabine/cisplatin, vinorelbine/Adriamycin, bevacizumab/vinorelbine |
| 2MP08P | 3 | 67 | 1 | 1st line | MM | sarcomatoid | D | 4.6 | 2.2 | PD | carboplatin/pemetrexed |
| 2MP09P | 3 | 78 | 1 | 2nd line | MM | sarcomatoid | D | 6.4 | 2.9 | PD | RT, gemcitabine |
| 2MP10P | 3 | 66 | 1 | 1st line | MM | epithelioid | D | 1.0 | 1.0 | NE | carboplatin/pemetrexed |
| 2MP11P | 3 | 72 | 1 | 1st line | MM | biphasic | D | 6.3 | 5.9 | SD | carboplatin/pemetrexed |
| 2MP12P | 3 | 71 | 1 | 3rd line | NSCLC | adenoca | D | 25.6 | 9.2 | PR | carboplatin/paclitaxel, nivolumab, Calithera study, RT |
| 2MP14P | 3 | 54 | 1 | 1st line | NSCLC | adenoca | D | 25.8 | 25.8 | PR | carboplatin/pemetrexed/bevacizumab |
| 2MP15P | 3 | 74 | 1 | 3rd line | MM | epithelioid | D | 17.2 | 7.5 | SD | gemcitabine, pembrolizumab |
| 2MP16P | 3 | 73 | 0 | 2nd line | MM | epithelioid | A | 22.9 | 22.9 | SD | carboplatin/pemetrexed |
| 2MP17P | 3 | 89 | 0 | 1st line | MM | epithelioid | D | 13.3 | 8.0 | PR | carboplatin/pemetrexed |
MM, malignant mesothelioma; RT, radiation therapy.
Safety
The study protocol was well tolerated without dose-limiting toxicities (DLTs) (Table 3). All three patients in cohort 1 experienced flu-like symptoms, which were grade 1 (low-grade fever, fatigue, and malaise on days 1–3 in one patient and fatigue starting on day 3 in another). All three patients in cohort 2 experienced transient symptoms of cytokine release syndrome (CRS), such as fever, nausea, and chills (grade 2) in the first 24 hr after vector administration that resolved within 3 days. The third patient in this cohort developed CRS with hypotension treated briefly with dopamine (grade 4), which resolved within 24 hr.
Table 3.
Acute Adverse Events Deemed Related to Experimental Therapy
| Category | Event | Cohort 1 (n = 3) |
Cohort 2 (n = 3) |
Cohort 3 (n = 13) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Flu-like and CRS | flu-like symptoms | 3 (100%) | – | 1 (33%) | 1 (33%) | – | – | 5 (39%) | 1 (8%) | – | – |
| CRS | – | – | – | 2 (66%) | – | 1 (33%) | 4 (31%) | 3 (23%) | – | – | |
| Gastrointestinal disorders | diarrhea | – | – | – | – | – | – | 1 (8%) | – | – | – |
| dyspepsia | – | 1 (33%) | – | – | – | – | – | – | – | – | |
| GERD | – | – | – | 1 (33%) | – | – | – | – | – | – | |
| nausea | – | – | – | – | – | – | – | 2 (16%) | – | – | |
| vomiting | – | – | – | – | – | – | – | 1 (8%) | – | – | |
| weight loss | – | – | – | – | – | – | 1 (8%) | – | – | – | |
| Other | blurred vision | – | – | – | – | – | – | 1 (8%) | – | – | – |
| headache | – | – | – | – | – | – | – | 1 (8%) | – | – | |
| presyncope | – | – | – | – | – | – | – | 1 (8%) | – | – | |
| Respiratory | cough | – | 1 (33%) | – | – | – | – | 2 (16%) | – | – | – |
| dyspnea | – | – | – | – | – | – | 1 (8%) | 1 (8%) | – | – | |
Possibly, likely, or definitely related to experimental treatment. GERD, gastroesopahgeal reflux disease.
Because of this toxicity, the protocol was amended to include therapy with celecoxib as prophylactic treatment to the same vector dose of cohort 2 to reduce the risk of CRS. This was based on the known anti-inflammatory effects of celecoxib, experience in previous clinical trials using celecoxib to reduce CRS with intrapleural administration of adenoviral vectors,14 and preclinical research showing that cycloxegenase-2 (COX-2) inhibition augmented the effects of immunotherapy using Ad.IFN-β in mesothelioma.32 Celecoxib was administered at a dose of 400 mg orally twice per day starting 3 days before AdV-tk administration and continuing for 2 days after. This dose and schedule was chosen based on the previous experience in the Ad-IFNα2b trial.14 However, in this study, celecoxib was only continued for 2 days after vector administration, rather than 11, because CRS symptoms were noted to be transient (<48 hr).
Addition of prophylactic celecoxib in cohort 3 reduced the incidence and severity of CRS (Table 3) among the first 3 patients treated. Ten patients were then enrolled and treated in an expansion of cohort 3. Among all 13 patients in cohort 3, CRS grade 1 was reported in 31% and grade 2 in 23%. None of the patients in this cohort experienced >grade 2 CRS. Overall in the study, no DLT was identified, and there were no treatment-related deaths.
Laboratory abnormalities were generally grade 2 or less and similar across cohorts, with the exception of higher grade lymphopenia during the first week in cohorts 2 and 3, which was considered possibly related (Table S1). These events were transient and did not have any clinical consequences. A single instance of grade 4 lymphopenia, occurring 4 weeks after AdV-tk, was considered possibly related, with chemotherapy considered a contributing factor.
Response Evaluation
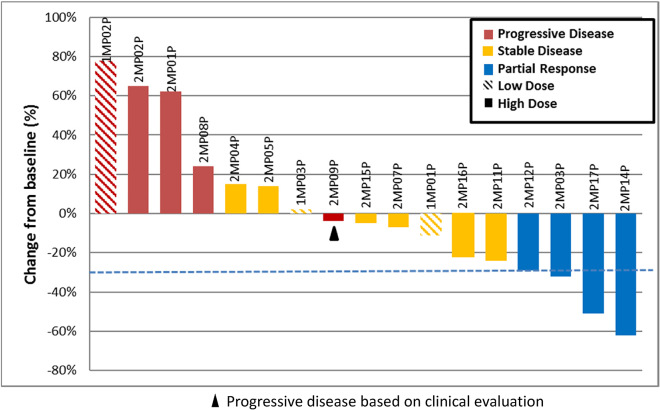
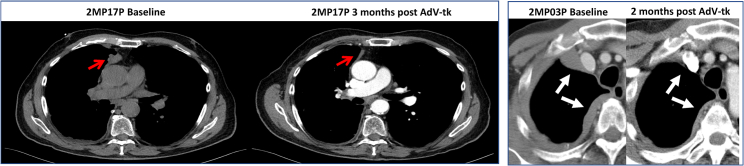
Response according to RECIST and modified RECIST was evaluable for 17 patients (Figure 1; Table 2). One patient (2MP06P) had a positron emission tomography (PET) computed tomography (CT) as baseline study, so formal RECIST measurements could not be performed (suboptimal comparison) and a second patient (2MP10P) died of progressive disease before the second restaging scan. For the seventeen patients with response evaluable disease, four patients had a partial response, all of which were in the high-vector dose group (29% response rate for evaluable high-dose patients), and two of these were patients with NSCLC (50% response rate for NSCLC). The patient (with malignant mesothelioma) that experienced transient grade 4 CRS (2MP04P) had a partial response that lasted for 8.6 months. Radiographic images of RECIST responses are shown in Figure 2. Response duration was 4–24 months for the 4 patients with partial response (PR). In addition, one patient with malignant mesothelioma (2MP04P) had a subsequent durable response to pembrolizumab that is ongoing after 12 months (Figure 3).
Figure 1.
Waterfall Plot of RECIST Tumor Responses
Figure 2.
Images of Two Patients with Partial Responses Post-AdV-tk
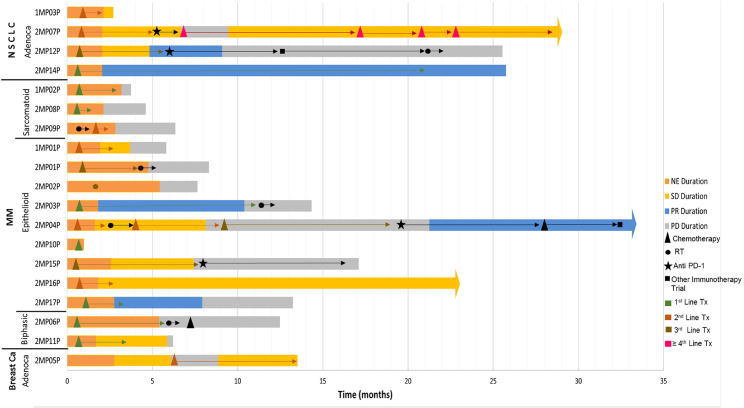
Figure 3.
Swimmer’s Plot of Follow-Up and Response to Therapy following AdV-tk
The pleural effusion improved or was stable after IP AdV-tk in all patients, except one patient with progressive disease who died at 1 month. The pleural catheter was able to be removed in 12 of 19 patients within 2 months (within 2 weeks for 7 patients), despite being in place prior to IP AdV-tk for an average of 4 months (range 12–840 days).
Three patients are alive and in active follow-up (one with NSCLC and 2 with malignant mesothelioma), with a range of follow-up duration of 23–33 months after IP AdV-tk. The other 16 patients have died of progressive disease. Figure 3 shows the disposition of all patients. For the 4 patients with NSCLC, median overall survival was 25.7 months post-AdV-tk. All three NSCLC patients in the high-dose group had prolonged disease stabilization and survived more than 2 years post-vector instillation. One patient who received AdV-tk therapy in the 2nd line setting following progression on platinum-based chemotherapy is still alive at 29 months, 3.6 years from initial diagnosis. Interestingly, two of the three NSCLC patients also received anti-programmed death receptor pathway (PD-1)-directed therapy after they progressed following GMCI. Of the two long-term survivors with malignant mesothelioma, one patient (2MP04P) also received anti PD-1 therapy and is currently alive at 33 months post-AdV-tk therapy.
Correlative Analyses
All patients were evaluated for baseline adenoviral-neutralizing antibody titers (Table S2). There was no apparent correlation between antibody titer and safety or tumor response. Changes in the expression of anti-tumor antibodies in the serum after treatment were evaluated by western blot for 13 patients. In 7 patients, there were no distinct changes in the number or intensity of anti-tumor immunoblot bands; in 4, there were increases in tumor bands, and in 2, there were mixed changes (some bands increasing and some decreasing). A representative blot showing increased bands in the NSCLC patient who is still alive is shown in Figure S1. Due to the small patient numbers and heterogeneous populations, significant differences in survival or in radiographic response rates could not be correlated with these humoral responses.
Discussion
GMCI, an immune-stimulatory strategy implemented through local delivery of aglatimagene besadenovec (AdV-tk) followed by an anti-herpetic prodrug, has shown safety and encouraging results in various solid tumor clinical trials. Our group has previously assessed safety and feasibility of intrapleural delivery of various adenoviral vectors in patients with malignant mesothelioma. This study was designed to evaluate the safety and feasibility of intrapleural administration of increasing doses of AdV-tk followed by chemotherapy in patients with MPE from multiple histologies, including NSCLC. The rationale for this trial was to evaluate the feasibility of using GMCI to induce enhanced anti-tumor immune responses in these patients.
The study design stipulated dose escalation starting at 1 × 1012 vector particles per patient and escalating subsequent cohorts by one log. There were no significant adverse events in the first cohort. However, CRS was evident in all patients treated in cohort 2 (1 × 1013 vector particles per patient). Based on our earlier experience using COX-2 inhibitors in this setting to decrease inflammatory responses when delivering an AdIFNα2b vector, the protocol was amended to add a cohort maintaining the AdV-tk dose but adding celecoxib. Because the CRS observed with AdV-tk was of much shorter duration than what had been observed in the AdIFNα2b study, the celecoxib duration was shortened from 14 days in that protocol to 5 days in the current study. Symptoms of CRS were successfully mitigated with the use of prophylactic celecoxib. A total of 13 patients were successfully dosed in cohort 3 without additional >grade 2 CRS symptoms. It thus appears that this approach can be used in an outpatient setting.
Overall, intrapleural AdV-tk was well tolerated. Among all patients treated, flu-like symptoms were the most common adverse events. All patients were subsequently able to receive standard of care systemic therapy. Although immune responses were clinically evident, with the small number of evaluable patients, we were unable to correlate antibody responses with clinical responses or outcomes. Additionally, our study did not specifically evaluate induction of cellular immune responses, limiting our ability to correlate biologic immune response with clinical outcomes.
In terms of efficacy, despite the advanced stages of disease of the study population, the disease control rate (DCR) was 71%; eight of the 17 evaluable patients had stable disease and four had a partial response. All four patients with a PR received the higher 1 × 1013 vector particle dose. Interestingly, the last patient in cohort 2, who had a grade 4 CRS, was one of the patients with a PR. Three other responders all received the higher vector dose and prophylactic celecoxib therapy (cohort 3) and did not experience the same level of CRS. The observation of responses clustered in the patients who received higher doses of vector, with or without celecoxib, is hypothesis generating. The CRS symptoms may reflect the potent acute immune stimulation generated by the AdV-tk. The mitigation of the symptoms by celecoxib is an important safety enhancement of GMCI at these higher doses. The observation of objective responses in some of these patients suggests that celecoxib does not attenuate the beneficial immune response to GMCI. Additional studies will be required to further evaluate the potential impact of COX-2 inhibition in conjunction with GMCI.
Although it is difficult to interpret survival outcomes in a small phase 1, dose escalation study, especially one that enrolled patients with diverse histologies receiving different standard of care chemotherapy, the results presented here provide some encouraging observations. For example, all three patients with NSCLC in the high-dose groups had unexpectedly long disease stabilization post-AdV-tk instillation, with overall survival exceeding 2 years post-treatment. Even though it is impossible in a study, such as this one, to determine whether these responses are related to subsequent chemotherapy, vector administration, or the combination of the two, these early signals of efficacy and immune activation are encouraging.
Others have noted that some patients treated with immune therapies, such as checkpoint inhibitors, go on to manifest very slowly progressing disease, sometimes with surprisingly prolonged survival.33 It has been hypothesized that this phenomenon may reflect the delayed effects of immunotherapy, which may also make tumors more susceptible to subsequent therapies, including chemotherapy.34 This may be particularly pertinent for the combination of GMCI with anti-PD1 checkpoint inhibitors. AdV-tk has been shown to increase PD-L1 expression on tumor cells.11 Interestingly, three of the four long-term survivors on this trial received subsequent anti-PD1 therapy during the course of their therapeutic management.
The results from this study raise some interesting hypotheses with regard to the molecular effects of GMCI on tumor biology. For example, the observation of prolonged survival following checkpoint inhibitors raises the possibility that GMCI could potentially make immunologically “cold” MPE tumors “hot” and thereby increase the response rate to downstream immune checkpoint inhibition.35 Study of patients with earlier stage lung cancer, in which tumors could be directly injected with GMCI followed by on-treatment biopsies, could validate this mechanism. A trial to evaluate the above questions, with a focus on incorporation of tumor biopsies before and after AdV-tk endobronchial injections, has been initiated.
It is important to recognize the limitations of this study. It was relatively small, nonrandomized, conducted at a single center, and included several different histologies. Patients could enter the trial at any point in their therapeutic trajectory; there was no limit on the number of prior treatments. Post-AdV-tk, chemotherapy regimens were at the discretion of the treating oncologist, and as a result, patients received a variety of different regimens. RECIST measurements were performed in a retrospective fashion. Nonetheless, this study showed that intrapleural administration of high-dose AdV-tk plus celecoxib followed by systemic chemotherapy was safe, feasible, and well tolerated in patients with malignant pleural effusion. We noted long-term survival in a subgroup of patients, which may be an indication of successful induction of immune responses with this approach.
Materials and Methods
Study Design
This was an open-label, phase I, dose-escalation trial to evaluate safety of IP administration of AdV-tk followed by valacyclovir and standard of care chemotherapy. The institutional review board at the University of Pennsylvania approved the protocol. Specific written informed consent was obtained from each patient before enrollment. The study was listed on clinicaltrials.gov (NCT01997190).
Patients older than 18 years of age with malignant pleural effusion (defined as positive pleural fluid cytology) and clinical indication for placement of pleural catheter were enrolled. Eligible patients included those with tumors derived from NSCLC, small-cell lung cancer, malignant mesothelioma, breast cancer, and ovarian cancer. For patients with malignant mesothelioma, histologic proof was required, but positive pleural fluid cytology was not required for enrollment. Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and forced expiratory volume (FEV1) at of at least 1 L or 40% of predicted. Laboratory inclusion criteria included bilirubin ≤ 1.5× upper limit of normal (ULN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase ≤2× ULN, hemoglobin ≥9 g/dL, platelets >100,000/mm3, absolute neutrophil count (ANC) >1,000/mm3, serum albumin level ≥2.5 g/dL, serum creatinine <2 mg/dL, and calculated creatinine clearance >30 mL/min.
The AdV-tk vector has been previously described.30 The study had three cohorts: cohort 1, AdV-tk dosed at 1 × 1012 vector particles; cohort 2, AdV-tk at 1 × 1013 vector particles; and cohort 3, AdV-tk at 1 × 1013 vector particles plus celecoxib at a dose of 400 mg orally twice per day starting 3 days before AdV-tk administration and continuing for 2 days after AdV-tk administration. The AdV-tk dose was delivered in 20 mL infused slowly through a pleural catheter after the pleural cavity was maximally drained of pleural fluid. The pleural catheter was then flushed with 30 mL of saline. Patients were monitored in an inpatient setting for 24 hr after IP administration of the vector. Valacyclovir administration began the day after AdV-tk infusion (2 g orally three times a day [TID] for 14 days). Chemotherapy could begin after completion of valacyclovir, at the discretion of the treating medical oncologist. After three patients were evaluated in the third cohort, a planned expansion phase of 10 additional patients was conducted.
Assessments
Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4. DLTs for AdV-tk administration were defined as grade 3 or greater allergic reaction, grade 4 hematologic toxicity persisting for >7 days except lymphopenia, grade 3 hypoxia lasting for 48 hr, grade 4 hypoxia, and any grade 3 or 4 non-hematologic toxicity (except ALT, AST, alkaline phosphatase, bilirubin, or creatinine) lasting for >7 days. Restaging studies were performed at 6–8 weeks post-AdV-tk instillation and then every 8–12 weeks at the discretion of the treating medical oncologist. CT images were reviewed by an attending radiologist specializing in thoracic imaging. Measurements were made using RECIST or modified RECIST for patients with malignant mesothelioma described by Byrne et al.31 The patients were seen every 3 months during the first year and then every 6 months during the second year. After 2 years, clinical assessment of late toxicity and disease status was conducted yearly.
Correlative Studies
Antiviral immune responses in the form of neutralizing adenovirus antibody titers (Nabs) were assessed as previously described.16 To detect induced humoral responses against tumor antigens, we performed immunoblotting against purified extracts from allogeneic mesothelioma cell lines and lung cancer cell lines, as appropriate. Cell lines were derived from patient pleural fluid samples from previous clinical trials and were grown in culture as previously described.19 Extracts from cells or purified proteins were prepared and immunoblotted with patient serum (diluted at 1:1,500) from time points at baseline and 6 weeks after treatment. Multiple exposures were obtained, and comparisons were made only on the exposures in which the major bands detected on pre-treatment blots were of equal intensity in post-treatment blots. Two independent, blinded observers visually scanned each blot to detect new bands or bands that appeared markedly increased in the post-treatment serum and came to a consensus score. The blots were semiquantitatively scored as follows: −1, loss of existing bands; 0, no change in any bands; 1, minimal changes (i.e., increased intensity in one or two bands); and 2, clear increases in >2 bands or appearance of new bands.
Statistics and Observations
The primary endpoint of this dose escalation trial was safety. Adverse events were recorded and summarized according to CTCAE version 4.0. Secondary endpoints included response rate per RECIST and mRECIST, progression free survival (PFS), overall survival (OS), and immune response. PFS and OS were calculated from time of instillation of AdV-tk to progression, death, or date of last follow-up censored by the end of the subject’s observation. Any subject who received AdV-tk was included in the analyses. No comparative statistical analyses were performed.
Author Contributions
Conception and design: C.A, A.R.H., L.K.A., E.A.-C., C.J.L., S.M.A., and D.H.S. Provision of study materials or patients: C.A., A.R.H., E.W.A., T.L.E., J.M.B., R.B.C., C.J.L., and D.H.S. Collection and assembly of data: C.A., S.M., L.K.A., E.A.-C., A.G.M., G.G.-H., S.I.K., S.M.A., and D.H.S. Data analysis and interpretation: C.A., L.K.A., E.A.-C., S.M.A., and D.H.S. Manuscript writing: all authors. Final approval of manuscript: all authors.
Acknowledgments
This work was supported by the National Cancer Institute (NCI) P01 CA66726.
Footnotes
Supplemental Information includes one figure and two tables and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.02.015.
Supplemental Information
References
- 1.Pilling J.E., Dusmet M.E., Ladas G., Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J. Thorac. Oncol. 2010;5:1544–1550. doi: 10.1097/JTO.0b013e3181e95cb8. [DOI] [PubMed] [Google Scholar]
- 2.Pollak J.S., Burdge C.M., Rosenblatt M., Houston J.P., Hwu W.J., Murren J. Treatment of malignant pleural effusions with tunneled long-term drainage catheters. J. Vasc. Interv. Radiol. 2001;12:201–208. doi: 10.1016/s1051-0443(07)61826-0. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar L.K., Guzik B.W., Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J. Cell. Biochem. 2011;112:1969–1977. doi: 10.1002/jcb.23126. [DOI] [PubMed] [Google Scholar]
- 4.Fyfe J.A., Keller P.M., Furman P.A., Miller R.L., Elion G.B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J. Biol. Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 5.Eastham J.A., Chen S.H., Sehgal I., Yang G., Timme T.L., Hall S.J., Woo S.L., Thompson T.C. Prostate cancer gene therapy: herpes simplex virus thymidine kinase gene transduction followed by ganciclovir in mouse and human prostate cancer models. Hum. Gene Ther. 1996;7:515–523. doi: 10.1089/hum.1996.7.4-515. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyama S., Kikukawa M., Masui K., Okuda H., Nakatani T., Akahene T., Mitoro A., Tominaga K., Tsujinoue H., Yoshiji H. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int. J. Cancer. 1999;83:374–380. doi: 10.1002/(sici)1097-0215(19991029)83:3<374::aid-ijc13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Vile R.G., Nelson J.A., Castleden S., Chong H., Hart I.R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 8.Gagandeep S., Brew R., Green B., Christmas S.E., Klatzmann D., Poston G.J., Kinsella A.R. Prodrug-activated gene therapy: involvement of an immunological component in the “bystander effect”. Cancer Gene Ther. 1996;3:83–88. [PubMed] [Google Scholar]
- 9.Chiocca E.A., Aguilar L.K., Bell S.D., Kaur B., Hardcastle J., Cavaliere R., McGregor J., Lo S., Ray-Chaudhuri A., Chakravarti A. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J. Clin. Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles B.J., Shalev M., Aguilar-Cordova E., Timme T.L., Lee H.M., Yang G., Adler H.L., Kernen K., Pramudji C.K., Satoh T. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum. Gene Ther. 2001;12:1955–1967. doi: 10.1089/104303401753204535. [DOI] [PubMed] [Google Scholar]
- 11.Aguilar L.K., Shirley L.A., Chung V.M., Marsh C.L., Walker J., Coyle W., Marx H., Bekaii-Saab T., Lesinski G.B., Swanson B. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol. Immunother. 2015;64:727–736. doi: 10.1007/s00262-015-1679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucharczuk J.C., Raper S., Elshami A.A., Amin K.M., Sterman D.H., Wheeldon E.B., Wilson J.M., Litzky L.A., Kaiser L.R., Albelda S.M. Safety of intrapleurally administered recombinant adenovirus carrying herpes simplex thymidine kinase DNA followed by ganciclovir therapy in nonhuman primates. Hum. Gene Ther. 1996;7:2225–2233. doi: 10.1089/hum.1996.7.18-2225. [DOI] [PubMed] [Google Scholar]
- 13.Sterman D.H. Gene therapy for malignant pleural mesothelioma. Hematol. Oncol. Clin. North Am. 2005;19:1147–1173. doi: 10.1016/j.hoc.2005.09.004. viii. [DOI] [PubMed] [Google Scholar]
- 14.Sterman D.H., Alley E., Stevenson J.P., Friedberg J., Metzger S., Recio A., Moon E.K., Haas E.R., Vachani A., Katz S.I. Pilot and feasibility trial evaluating immuno-gene therapy of malignant mesothelioma using intrapleural delivery of adenovirus-IFNα combined with chemotherapy. Clin. Cancer Res. 2016;22:3791–3800. doi: 10.1158/1078-0432.CCR-15-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterman D.H., Gillespie C.T., Carroll R.G., Coughlin C.M., Lord E.M., Sun J., Haas A., Recio A., Kaiser L.R., Coukos G. Interferon beta adenoviral gene therapy in a patient with ovarian cancer. Nat. Clin. Pract. Oncol. 2006;3:633–639. doi: 10.1038/ncponc0658. [DOI] [PubMed] [Google Scholar]
- 16.Sterman D.H., Haas A., Moon E., Recio A., Schwed D., Vachani A., Katz S.I., Gillespie C.T., Cheng G., Sun J. A trial of intrapleural adenoviral-mediated interferon-α2b gene transfer for malignant pleural mesothelioma. Am. J. Respir. Crit. Care Med. 2011;184:1395–1399. doi: 10.1164/rccm.201103-0554CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterman D.H., Kaiser L.R., Albelda S.M. Gene therapy for malignant pleural mesothelioma. Hematol. Oncol. Clin. North Am. 1998;12:553–568. doi: 10.1016/s0889-8588(05)70008-3. [DOI] [PubMed] [Google Scholar]
- 18.Sterman D.H., Recio A., Carroll R.G., Gillespie C.T., Haas A., Vachani A., Kapoor V., Sun J., Hodinka R., Brown J.L. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin. Cancer Res. 2007;13:4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 19.Sterman D.H., Recio A., Haas A.R., Vachani A., Katz S.I., Gillespie C.T., Cheng G., Sun J., Moon E., Pereira L. A phase I trial of repeated intrapleural adenoviral-mediated interferon-beta gene transfer for mesothelioma and metastatic pleural effusions. Mol. Ther. 2010;18:852–860. doi: 10.1038/mt.2009.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterman D.H., Recio A., Vachani A., Sun J., Cheung L., DeLong P., Amin K.M., Litzky L.A., Wilson J.M., Kaiser L.R. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin. Cancer Res. 2005;11:7444–7453. doi: 10.1158/1078-0432.CCR-05-0405. [DOI] [PubMed] [Google Scholar]
- 21.Sterman D.H., Treat J., Litzky L.A., Amin K.M., Coonrod L., Molnar-Kimber K., Recio A., Knox L., Wilson J.M., Albelda S.M., Kaiser L.R. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum. Gene Ther. 1998;9:1083–1092. doi: 10.1089/hum.1998.9.7-1083. [DOI] [PubMed] [Google Scholar]
- 22.Hasenburg A., Tong X.W., Fischer D.C., Rojas-Martinez A., Nyberg-Hoffman C., Kaplan A.L., Kaufman R.H., Ramzy I., Aguilar-Cordova E., Kieback D.G. Adenovirus-mediated thymidine kinase gene therapy in combination with topotecan for patients with recurrent ovarian cancer: 2.5-year follow-up. Gynecol. Oncol. 2001;83:549–554. doi: 10.1006/gyno.2001.6442. [DOI] [PubMed] [Google Scholar]
- 23.Teh B.S., Ayala G., Aguilar L., Mai W.Y., Timme T.L., Vlachaki M.T., Miles B., Kadmon D., Wheeler T., Caillouet J. Phase I-II trial evaluating combined intensity-modulated radiotherapy and in situ gene therapy with or without hormonal therapy in treatment of prostate cancer-interim report on PSA response and biopsy data. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:1520–1529. doi: 10.1016/j.ijrobp.2003.09.083. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler L.A., Manzanera A.G., Bell S.D., Cavaliere R., McGregor J.M., Grecula J.C., Newton H.B., Lo S.S., Badie B., Portnow J. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro-oncol. 2016;18:1137–1145. doi: 10.1093/neuonc/now002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smythe W.R., Hwang H.C., Amin K.M., Eck S.L., Davidson B.L., Wilson J.M., Kaiser L.R., Albelda S.M. Use of recombinant adenovirus to transfer the herpes simplex virus thymidine kinase (HSVtk) gene to thoracic neoplasms: an effective in vitro drug sensitization system. Cancer Res. 1994;54:2055–2059. [PubMed] [Google Scholar]
- 26.Smythe W.R., Hwang H.C., Elshami A.A., Amin K.M., Albelda S.M., Kaiser L.R. Differential sensitivity of thoracic malignant tumors to adenovirus-mediated drug sensitization gene therapy. J. Thorac. Cardiovasc. Surg. 1995;109:626–630. doi: 10.1016/S0022-5223(95)70342-X. [DOI] [PubMed] [Google Scholar]
- 27.Tong X., Shine D.H., Agoulnik I., Freund C.T., Hasenburg A., Aguilar-Cordova E., Woo S.L., Kieback D.G. Adenovirus mediated thymidine kinase gene therapy may enhance sensitivity of ovarian cancer cells to chemotherapeutic agents. Anticancer Res. 1998;18(5A):3421–3426. [PubMed] [Google Scholar]
- 28.Vlachaki M.T., Chhikara M., Aguilar L., Zhu X., Chiu K.J., Woo S., Teh B.S., Thompson T.C., Butler E.B., Aguilar-Cordova E. Enhanced therapeutic effect of multiple injections of HSV-TK + GCV gene therapy in combination with ionizing radiation in a mouse mammary tumor model. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:1008–1017. doi: 10.1016/s0360-3016(01)01698-4. [DOI] [PubMed] [Google Scholar]
- 29.Fridlender Z.G., Sun J., Singhal S., Kapoor V., Cheng G., Suzuki E., Albelda S.M. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol. Ther. 2010;18:1947–1959. doi: 10.1038/mt.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S.H., Shine H.D., Goodman J.C., Grossman R.G., Woo S.L. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc. Natl. Acad. Sci. USA. 1994;91:3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrne M.J., Nowak A.K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann. Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 32.DeLong P., Tanaka T., Kruklitis R., Henry A.C., Kapoor V., Kaiser L.R., Sterman D.H., Albelda S.M. Use of cyclooxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res. 2003;63:7845–7852. [PubMed] [Google Scholar]
- 33.Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O., Patt D., Chen T.T., Berman D.M., Wolchok J.D. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlom J., Arlen P.M., Gulley J.L. Cancer vaccines: moving beyond current paradigms. Clin. Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowenstein P.R., Castro M.G. Evolutionary basis of a new gene- and immune-therapeutic approach for the treatment of malignant brain tumors: from mice to clinical trials for glioma patients. Clin. Immunol. 2017 doi: 10.1016/j.clim.2017.07.006. Published online July 15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.