Abstract
Corticotropin releasing factor receptors (CRFR1) contribute to stress-induced adaptations in hippocampal structure and function that can affect learning and memory processes. Our prior studies showed that female rats with elevated estrogens compared to males have more plasmalemmal CRFR1 in CA1 pyramidal cells, suggesting a greater sensitivity to stress. Here, we examined the distribution of hippocampal CRFR1 following chronic immobilization stress (CIS) in female and male rats using immuno-electron microscopy. Without stress, total CRFR1 dendritic levels were higher in females in CA1 and in males in the hilus; moreover, plasmalemmal CRFR1 was elevated in pyramidal cell dendrites in CA1 in females and in CA3 in males. Following CIS, near-plasmalemmal CRFR1 increased in CA1 pyramidal cell dendrites in males but not to levels of control or CIS females. In CA3 and the hilus, CIS decreased cytoplasmic and total CRFR1 in dendrites in males only. These results suggest that in naive rats, CRF could induce a greater activation of CA1 pyramidal cells in females than males. Moreover, after CIS, which leads to even greater sex differences in CRFR1 by trafficking it to different subcellular compartments, CRF could enhance activation of CA1 pyramidal cells in males but to a lesser extent than either unstressed or CIS females. Additionally, CA3 pyramidal cells and inhibitory interneurons in males have heightened sensitivity to CRF, regardless of stress state. These sex differences in CRFR1 distribution and trafficking in the hippocampus may contribute to reported sex differences in hippocampal-dependent learning processes in baseline conditions and following chronic stress.
Keywords: Electron microscopy, CA1/CA3 pyramidal cells, dentate gyrus, GABAergic interneurons, Learning and memory, addiction
Graphical abstract
INTRODUCTION
Drug addiction is a learning process that reinforces associations of drug use with reward and environmental cues with drug access (O’Brien et al., 1998; Crombag et al., 2008). Clinical studies in humans as well as in animal models found that females express higher sensitivity to craving and rates of relapse than males (Elman et al., 2001). Women are more susceptible to several aspects of addiction than men, including relapse due to stressful events or depression (Becker et al., 2017). The hippocampus is critically involved in ‘cue’ and ‘context’ learning important for drug craving and relapse (Hyman and Malenka, 2001; Nestler, 2002; Volkow et al., 2006). Following chronic stress, male rodents have impaired spatial learning and memory (McEwen, 1999; Sousa et al., 2000; Luine et al., 2007; McEwen and Milner, 2007) and undergo morphological changes and dendritic retraction in CA3 pyramidal cell dendrites (McEwen et al., 2016), suggesting that adaptive mechanisms of the hippocampus to chronic stress differ in females and males.
Evidence from other brain regions suggests that activation of corticotropin releasing factor (CRF) can enhance the acute effects of drugs of abuse and potentiate neuroplasticity induced following drug withdrawal (Haass-Koffler and Bartlett, 2012). Additionally, CRF has been extensively implicated in drug relapse following extended periods of abstinence (Brown et al., 2009; Shalev et al., 2010; Logrip et al., 2011). In the adult rodent hippocampus, endogenous sources of CRF originate from local GABAergic interneurons, especially those containing parvalbumin and somatostatin (SOM) (Yan et al., 1998; Williams and Milner, 2011). Moreover, CRF receptor type 1 (CRFR1) is prominently located on pyramidal neurons and in GABAergic interneurons (Williams et al., 2011a; Chen et al., 2012; Tan et al., 2017). In response to short-term (minutes) stimulation, CRF released in the hippocampus excites synapses and enhances synaptic efficacy (Wang et al., 1998; Wang et al., 2000; Chen et al., 2012).
Stress initiates a cascade of events in the hypothalamic-pituitary-adrenal axis (HPA) that leads to the release of glucocorticoids from the adrenal cortex (Smith and Vale, 2006). Depending on duration, stress can have different effects on CRF-mediated hippocampal neuroplasticity processes important for learning and memory (Smith and Vale, 2006; Regev and Baram, 2014). In response to high-frequency stimulation (e.g., stress), CRF contained in dense-core vesicles (Williams and Milner, 2011) is released from axon terminals (Chen et al., 2012) and, like other peptides, can act in a paracrine manner on CRFRs on neighboring cells (Thureson-Klein and Klein, 1990; Herkenham, 1991). Following 1 hour of acute immobilization stress (AIS), CRF released from male mouse hippocampal nerve terminals can mediate the persistence of long-term potentiation (LTP) population spikes which are essential for enhanced context-dependent fear learning (Blank et al., 2002; Blank et al., 2003). In contrast, early life stress or chronic social stress can result in hippocampal-dependent memory deficits that are ameliorated in CRFR1 forebrain knock-out mice (Wang et al., 2011a; Wang et al., 2011b).
Our previous studies demonstrate that CRF and opioid systems in the hippocampus are closely linked and sex, as well as the hormonal milieu, can alter this relationship. In particular, CRF and delta opioid receptor (DOR) immunoreactivities colocalize in interneurons throughout the rat hippocampus, and proestrus/estrus (high estrogen) females have fewer CRF/DOR interneurons in the hilus of the dentate gyrus (DG) compared to males (Williams and Milner, 2011). However, proestrus female rats compared to males have greater numbers of terminals containing CRF alone in the DG (Williams and Milner, 2011). Moreover, our previous electron microscopic (EM) studies in rats demonstrated that although proestrus females and males had comparable levels of CRFR1 in DOR-containing CA1 pyramidal cell dendrites, proestrus females had increased density of CRFR1 on the plasma membrane (Williams et al., 2011a). Together, these results suggest that females could have elevated sensitivity to CRF at baseline states. However, a systematic evaluation of the subcellular distribution of CRFR1 in the hippocampus of females and males comparing different subregions is lacking.
Additionally, our recent EM studies found that chronic immobilization stress (CIS) affects the subcellular distribution of DORs within CA3 pyramidal cells and DG hilar interneurons in a way that could promote excitation and learning processes in females but not males (Mazid et al., 2016). However, whether CIS alters the subcellular distribution of hippocampal CRFR1 in female and male rats is unknown. Thus, this study used immunoEM to examine sex differences in the subcellular distribution of CRFR1 in CA1 and CA3 pyramidal cells as well as in DG interneurons at baseline states and following CIS.
EXPERIMENTAL PROCEDURES
Animals
Male and female Sprague Dawley rats (N = 24) from Charles River Laboratories (Wilmington, MA; https://www.criver.com/products-services/find-model/sas-sd?region=3611) were 2 to 3 months old upon arrival (males weighed 275 – 325 g and females weighed 225 – 250 g). Animals were pair-housed in cages with a 12-hour light/dark cycle (lights on 0600 – 1800) and ad libitum access to water and food. The rats used in this study were the same as those used in our previous studies (Milner et al., 2013; Pierce et al., 2014; Mazid et al., 2016). All procedures were approved by the Rockefeller University and Weill Cornell Medicine Institutional Animal Care and Use Committees and were in accordance with the 2011 Eighth edition of the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Estrous cycle determination
The study included only female rats that had 2 consecutive, regular, 4–5 day estrous cycles. One week after the rats arrived and were acclimated, estrous cycle stage was determined using vaginal smear cytology (Turner and Bagnara, 1971). Mock estrus cycling on male rats was performed at the same time to control for handling differences. Estrous cycle stage was verified by uterine weight and radioimmunoassay of plasma serum estradiol levels from blood samples from the heart directly before the perfusion procedure. The females used in this study were all diestrus II, the stage in which estrogens and progestins are lowest. This stage was chosen so that we could make direct comparisons with our previous studies examining the effect of CIS on DOR trafficking in the hippocampus (Mazid et al., 2016).
Chronic immobilization stress
Rats were randomly assigned to the unstressed control or CIS experimental groups. To minimize unwanted stress on the control rats, they were housed in a neighboring room to the CIS rats. All CIS procedures were performed between 9:00 a.m. and 1:00 p.m. daily. Rats were subjected to CIS for 10 consecutive days (Lucas et al., 2007; Shansky et al., 2010). For this, rats were placed in plastic cone shaped polyethylene bags with a small apical hole and a Kotex mini-pad underneath them for urine collection. The rats were placed with their nose at the hole of the bag, sealed in with tape and left for 30 min undisturbed. The rats were anesthetized and perfused 1 day after the final CIS session. Control rats were left in their home-room and anesthetized prior to relocation to the procedure room for perfusion.
Immunocytochemistry procedures
Section preparation
Rats were deeply anesthetized with sodium pentobarbital (150 mg/kg, I.P.) in the morning (between 9:30 and 11:30 am) and perfusion fixed through the ascending aorta with: 1) 10-15 ml 0.9% saline containing 2% heparin; 2) 50 ml of 3.75% acrolein and 2% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4); and 3) 200 ml of 2% paraformaldehyde in PB (Milner et al., 2011). The brains then were separated from the skull, cut into 5 mm coronal blocks using a brain mold (Activational Systems, Inc.) and post-fixed in 2% paraformaldehyde in PB for 30 min. The brains were cut into coronal sections (40 μm thick) on a vibrating microtome (Leica Microsystems, Buffalo Grove, IL) into PB and stored in cryoprotectant solution (30% sucrose and 30% ethylene glycol in PB) at −20°C until immunocytochemical processing. Coronal sections containing the dorsal hippocampus [between bregma −3.5 and −4.1; (Swanson, 1992)] of all groups, male and female, control and CIS, (6 rats/group; N=24 rats) were rinsed in PB, coded using hole punches then pooled into single crucibles. The pooled sections were processed together throughout the immunocytochemical procedures to ensure identical labeling conditions (Pierce et al., 1999). Sections were incubated in 1% sodium borohydride in PB for 30 min to neutralize reactive aldehydes (Milner et al., 2011) and rinsed in PB.
Antibody characterization
A goat polyclonal antibody raised against the 425–444 amino acid sequence of the carboxy terminus of the CRF receptor precursor of human origin, which is identical to the corresponding sequence in the rat [C-20 Cat# sc-1757, RRID:AB_673600; Santa Cruz Biochemical, CA (discontinued)], was used in the EM study. On Western blots of mouse hypothalamus, this antibody recognizes 1 band at ~80kD; this band is absent on Western blots from CRFR1 knockout mice (Chen et al., 2000). Moreover, the specificity of this antibody has been shown by absence of labeling in acrolein/paraformaldehyde fixed sections from the dorsal raphe of CRFR1 knockout mice (Waselus et al., 2009). Furthermore, preadsorption with the antigenic peptide sequence produced no labeling in immunoblots (Chen et al., 2000; Bangasser et al., 2010) and tissue sections (Sauvage and Steckler, 2001; Reyes et al., 2006). This antibody was used in our previous EM studies (Williams et al., 2011a).
Electron microscopic immunocytochemistry
Free-floating sections were labeled for CRFR1 using immunogold through previously described methods (Milner et al., 2011). These methods preserve cellular morphology while enabling visualization of an antigen of interest and localization of that antigen to discrete subcellular regions for analysis (Leranth, 1989). To enhance antibody penetration, sections were immersed in a cryoprotectant solution (25% sucrose and 3.5% glycerol in 0.05 M PB) for 15 min then freeze-thawed by placement in a −80°C freezer for 20 min. Sections were then incubated in goat polyclonal CRFR1 (1:100) in 0.1% bovine serum albumin (BSA) in tris-buffered saline (TS) for 72 hours at 4°C. Sections were rinsed in TS followed by washing buffer [0.1 M phosphate-buffered saline (PBS) with 2% gelatin and 0.1% BSA], and incubated overnight at 4°C in a 1:50 dilution of donkey anti-goat IgG conjugated to 1 nm gold particles (Electron Microscopy Sciences (EMS) Cat# 25800, RRID: AB_2631210) in 0.01% gelatin and 0.08% BSA. Sections were rinsed in PBS, post-fixed in 2% glutaraldehyde in PBS for 10 min, rinsed in PBS then in 0.2 M sodium citrate buffer (pH 7.4). The conjugated gold particles were enhanced using silver solution [IntenSE; Amersham Biosciences, Waltham, MA; Cat# RPN491 (discontinued)] for 6 min.
Sections were fixed in 2% osmium tetroxide for 1 hour, dehydrated in increasing ethanol concentrations to propylene oxide and embedded in EMBed 812 (EMS) between two sheets of Aclar plastic (Honeywell, Pottsville, PA). Three rats from each group (control and CIS females, and control and CIS males) were randomly chosen for EM analysis (N = 12). Ultrathin sections (70 nm thick) from the CA1, CA3 and DG from each section were cut on a Leica UCT ultratome. Ultrathin sections were collected on 400 mesh thin-bar copper grids (T400-Cu, EMS) and the grids were counterstained with uranyl acetate (EMS 22400) and Reynold’s lead citrate (lead nitrate EMS, 17900-25).
Electron microscopic localization of CRFR1
All people who performed all data collection and analyses were blinded to experimental conditions. Data were unblinded after the final graphs were generated. Sections were analyzed on a Phillips CM10 transmission electron microscope (FEI, Hillsboro, OR) equipped with an Advanced Microscopy Techniques digital camera (Danvers, MA). Ultra-thin sections were collected from the tissue-plastic interface where immunoreagent access is maximal (Milner et al., 2011) and analyzed. Images were collected at a magnification of 13,500 (DG) or 10,500 (CA1 and CA3). Profiles were identified and categorized by standard morphological criteria as neuronal (soma, dendrites, axons, terminals) or glial (Peters et al., 1991). Dendritic profiles contained regular microtubular arrays and were usually postsynaptic to axon terminal profiles. Dendritic profiles were distinguished by size as large (average diameter > 1.0 μm) and small (average diameter < 1.0 μm), which correspond to proximal and distal, respectively, to the cell body (Peters et al., 1991). Mossy fiber terminals were identified by their large size (~1–1.5 μm in diameter), irregular contour, and the presence of numerous small synaptic vesicles (Amaral and Dent, 1981).
Silver intensified immunogold (SIG) labeling for CRFR1 appeared as black electron-dense particles that varied in size. Criteria for field selection included good morphological preservation, the presence of immunolabeling in the field, and proximity to the tissue-plastic interface (i.e., the surface of the tissue) to avoid problems due to differences in antibody penetration (Milner et al., 2011).
Analysis 1: CRFR1-SIG labeling in pyramidal cell dendrites in CA1 and CA3 and in interneuronal dendrites in DG
Micrographs were taken of CRFR1-SIG labeled dendrites in the stratum radiatum of CA1 and CA3 as well as in the hilus of the DG (Fig. 1A). Photographs were taken of 50 random dendritic profiles in each of the 3 regions for each animal. One thin section per block generally produced 50 dendrites, but in rare cases 2 sections were examined for 1 block and the dendrites were taken from non-overlapping regions of the block. Microcomputer Imaging Device software (MCID Analysis, RRIS:SCR_014278) was used to determine perimeter (i.e., plasma membrane), area, average diameter, and major and minor axis lengths for all labeled dendrites. Dendrites with abnormal shape (form factor value < 0.5) were excluded from the data set. Average diameter measures were used to classify dendrites as large and small. Calculations using CRFR1-SIG particle distribution and morphometry data were used in statistical comparisons.
Fig. 1. Regions of the rat hippocampus sampled for electron microscopy.
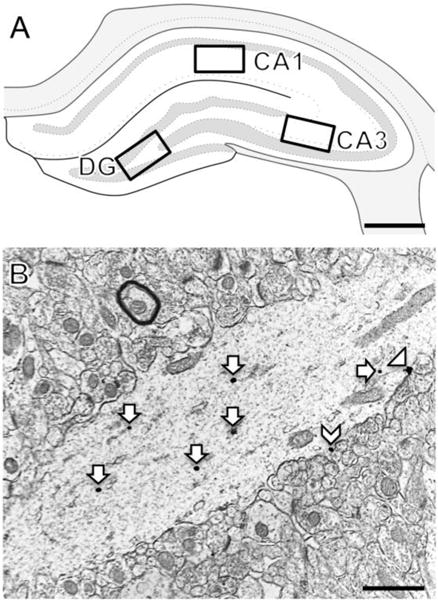
A. Schematic diagram of the rostral rat hippocampus showing the regions of CA1, CA3 and dentate gyrus (DG) sampled for microscopy [modified from diagram 31 (−3.70 from bregma) in Swanson, 1992]. B. Representative electron micrograph shows CRFR1-SIG labeling in a CA1 pyramidal cell dendrite in stratum radiatum. CRFR1-SIG particles were located on the plasma membrane (arrowhead), near the plasma membrane (chevron) and in the cytoplasm (arrow). Scale bar A = 0.5 mm; B = 500 nm.
The density of CRFR1-SIG particles was analyzed in plasma membrane and cytoplasmic dendritic compartments: 1) the number of plasma membrane CRFR1-SIG particles on the dendrite perimeter (On PM:μm); 2) the number of near plasma membrane CRFR1-SIG particles per perimeter (Near:μm); 3) the number of cytoplasmic CRFR1-SIG particles per cross-sectional area (Cyto:μm2); and 4) the total number of CRFR1-SIG particles (sum of on PM, near PM and cytoplasmic) in a dendritic profile/unit area (Total:μm2). Partitioning ratio, which is the proportion of CRFR1-SIG particles in a particular subcellular compartment (e.g., plasma membrane or cytoplasm) divided by the total number of SIG particles, also was determined. In addition to comparisons of CRFR1-SIG particle densities and partitioning ratios within individual subregions (CA1, CA3, and DG), comparisons of CRFR1-SIG particle densities and partitioning ratios between subregions were performed for control groups.
Receptors on the plasma membrane labeled by SIGs identify receptor-binding sites (Boudin et al., 1998). Near plasma membrane receptors constitute a pool from which receptors can be added or removed from the plasma membrane. Receptors in the cytoplasm are either stored during transfer to or from the soma or another cellular compartment, or the receptors are being degraded or recycled (Pierce et al., 2009; Fernandez-Monreal et al., 2012). When stimulated by an agonist, the ratio of receptors on the plasma membrane to those in the cytoplasm declines, as demonstrated by the number of SIG labeled receptors in each compartment (Haberstock-Debic et al., 2003).
Analysis 2: CRFR1-SIG labeling in spines in CA1
From the micrographs in stratum radiatum of CA1 (same micrographs used in analysis 1), 100 spine profiles per rat were randomly identified. Spines were included if contacted by a terminal forming an asymmetric synapse and categorized as labeled (with at least one CRFR1-SIG particle) or unlabeled. CRFR1-SIG particles in labeled spines were classified as in the synapse, on the plasma membrane, or in the cytoplasm.
Analysis 3: CRFR1-SIG labeling in spines contacting mossy fibers in CA3
Fifty mossy fiber profiles per rat were randomly photographed from the tissue-plastic interface of the stratum lucidum of CA3. Mossy fibers were included if contacted by a spine, and spines were categorized as labeled (with at least one CRFR1-SIG particle) or unlabeled.
Figure preparation
Adjustments to brightness, contrast and sharpness were made in Adobe Photoshop 9.0 (Adobe Photoshop, RRID:SCR_014199) on an iMac prior to importing into PowerPoint 2011, where final adjustments to brightness, contrast and size were made. The original content of the images was preserved with any changes made. Illustrations were created in PowerPoint 2011. Graphs were generated in Prism 7 software (Graphpad Prism, RRID:SCR_002798).
Statistical analysis
The independent variables in this study were sex (female vs. male) and treatment (unstressed control vs. CIS). Quantitative dual labeling EM methods are designed to determine relative changes in the subcellular distribution of proteins in dendrites of different sizes following experimental manipulations. For this, protein distribution for CRFR1 in dendritic profiles of different sizes, rather than number of cells or dendrites per animal, is analyzed. Implicit in our analysis are corrections for errors related to spatial location as we only analyze a single plane within each section. In these studies, we measured dendritic profile perimeter, cross-sectional area, and average diameter and used these to determine SIG particle density for each dendritic compartment (e.g., ON/μm) to correct for any size-related differences. To analyze the redistribution of SIG particles within a dendrite, the number of SIG particles in each compartment was divided by the total number of SIG particles in the dendrite (e.g., On/total).
Our previous study (Znamensky et al., 2003) randomly sampled dendritic profiles in 9632 μm2 of tissue and determined that 50 dendritic profiles per block were sufficient to make quantitative comparisons on the subcellular distribution of proteins between groups. In this study (Znamensky et al., 2003), increasing the number of dendrites to 75 or greater per animal did not change the significance of the results.
Data are expressed as means ± SEM. Two-way analysis of variance (ANOVA) followed by Tukey’s HSD post-hoc tests at a 5% confidence interval was used in control rats for: 1) comparisons in total CRFR1-SIG densities in dendrites between and within hippocampal regions; and 2) comparisons in all of the CRFR1-SIG densities and partitioning ratios in the CA3 and DG. One-way ANOVA and post-hoc Welch t-test for samples with unequal variances at a 5% confidence interval was used for: 1) comparisons in the densities of CRFR1-SIGs in individual dendritic subcompartments (due to low numbers in the on and near plasma membrane compartments); and 2) in the density and partitioning ratio of CRFR1-SIGs in all cellular compartments within CA1 dendrites (due to significant differences in controls). All statistical analyses were conducted using JMP 12 Pro software (JMP, RRID:SCR_014242).
RESULTS
Without stress, densities of CRFR1 in CA1, CA3, and DG dendrites differ between males and females
Three regions of the hippocampus were chosen for EM analysis of CRFR1 containing dendrites: stratum radiatum of CA1, stratum radiatum of CA3 and the hilus of the DG (Fig. 1A). A CA1 pyramidal cell dendrite showing an example of CRFR1-SIG particles on the membrane, near the membrane and in the cytoplasm is shown in Figure 1B. In addition to dendrites, CRFR1-labeled terminals, axons and glia (not shown) were observed. The distribution of labeled profiles containing CRFR1 is consistent with previous studies (Chen et al., 2004; Williams et al., 2011a).
Sex differences in the densities and partitioning ratios of CRFR1-SIG particles of unstressed control animals were found in all hippocampal subregions (Fig. 2). Examples of micrographs of dendrites containing CRFR1-SIG particle labeling in CA1, CA3, and DG are shown (Figs. 3A,C; 4A,C; 5A,C, respectively). For total density of dendritic CRFR1-SIG particles, two-way ANOVA revealed a significant main effect of hippocampal region (CA1, CA3, DG; F2,951 = 130; p < 0.0001) and in the interaction between region and sex (F2,951 = 71.7; p < 0.0001). There was no significant main effect of sex (F1,951 = 0.0012; p = 0.9723) on total density of dendritic CRFR1-SIG particles.
Fig. 2. Sex differences in distribution of CRFR1-SIG particles in CA1, CA3, and DG in the unstressed control groups.
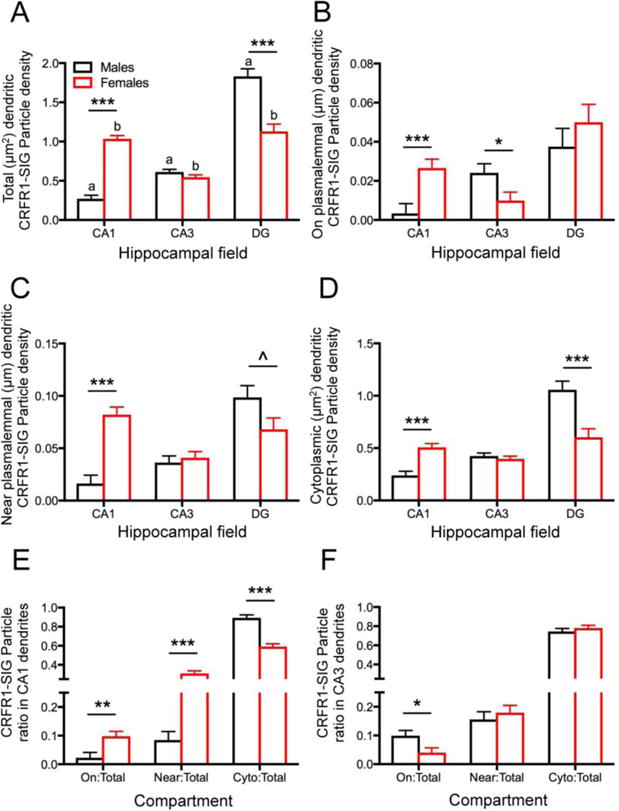
A. In control rats, the total density of CRFR1-SIG particles in dendrites (# SIG/μm2) was significantly lower in CA1 and higher in the DG of males compared to females. Moreover, significantly lower densities of CRFR1-SIG particles in dendrites were seen in CA1 compared to CA3 and DG in males and higher densities of CRFR1 SIG particles in CA1 and DG compared to CA3 in females. B. The density of CRFR1 SIG particles on the plasma membrane (# SIG/μm) in dendrites was significantly lower in CA1 and higher in CA3 of males compared to females. C. The density of CRFR1-SIG particles near the plasma membrane (# SIG/μm) in dendrites was significantly lower in CA1 and higher in DG of males compared to females. D. The density of CRFR1 SIG particles in the cytoplasm (# SIG/μm2) of dendrites was significantly lower in CA1 and higher in DG of males compared to females. E. In CA1, a smaller partitioning ratio of CRFR1-SIG particles was on the plasma membrane (On/Total) and near the plasma membrane (Near/Total), and a greater ratio of cytoplasmic CRFR1-SIG particles (Cyto/Total) in males compared to females. F. In CA3, males compared to females had a greater ratio of CRFR1-SIG particles on the plasma membrane (PM/total). No significant differences in partitioning ratio were observed in hilar interneurons. ***p < 0.001; **p < 0.01; *p < 0.05; ^p = 0.052. a p < 0.001 (CA1 vs. DG; CA3 vs. DG) and p < 0.01 (CA1 vs. CA3); b p < 0.001 (CA1 vs. CA3; CA3 vs. DG). N = 3 rats/group; n = 50 dendrites/rat/area.
Fig 3. Sex differences in the distribution of CRFR1-SIG particles in CA1 pyramidal cell dendrites after CIS.
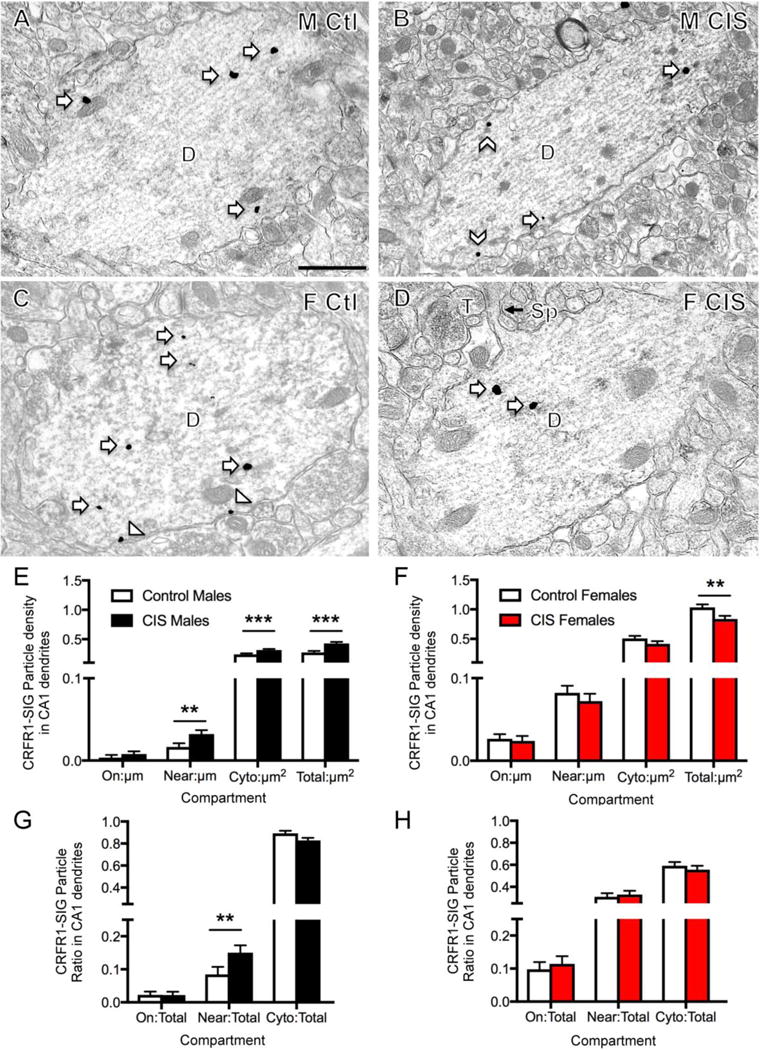
A–D. Representative electron micrographs show the distribution of CRFR1-SIG particles in dendrites from a control male (A), CIS male (B), control diestrus female (C) and CIS diestrus female (D). Examples of CRFR1-SIG particles are shown on the plasma membrane (arrowhead), near the plasma membrane (chevron) and in the cytoplasm (arrow). Scale bar = 500 nm. E. In CIS males, near plasma membrane, cytoplasmic and total CRFR1-SIG particle density significantly increased compared to control males. F. In CIS females compared to control females total CRFR1-SIG particle density was reduced. G, H. In males, but not females, the proportion of CRFR1 SIG particles near the plasma membrane significantly increased (p < 0.05) after CIS (G). ***p < 0.001; **p < 0.01; *p < 0.05. N = 3 rats/group; n = 50 dendrites/rat/area.
Fig. 4. Sex differences in the distribution of CRFR1-SIG particles in CA3 pyramidal cell dendrites after CIS.
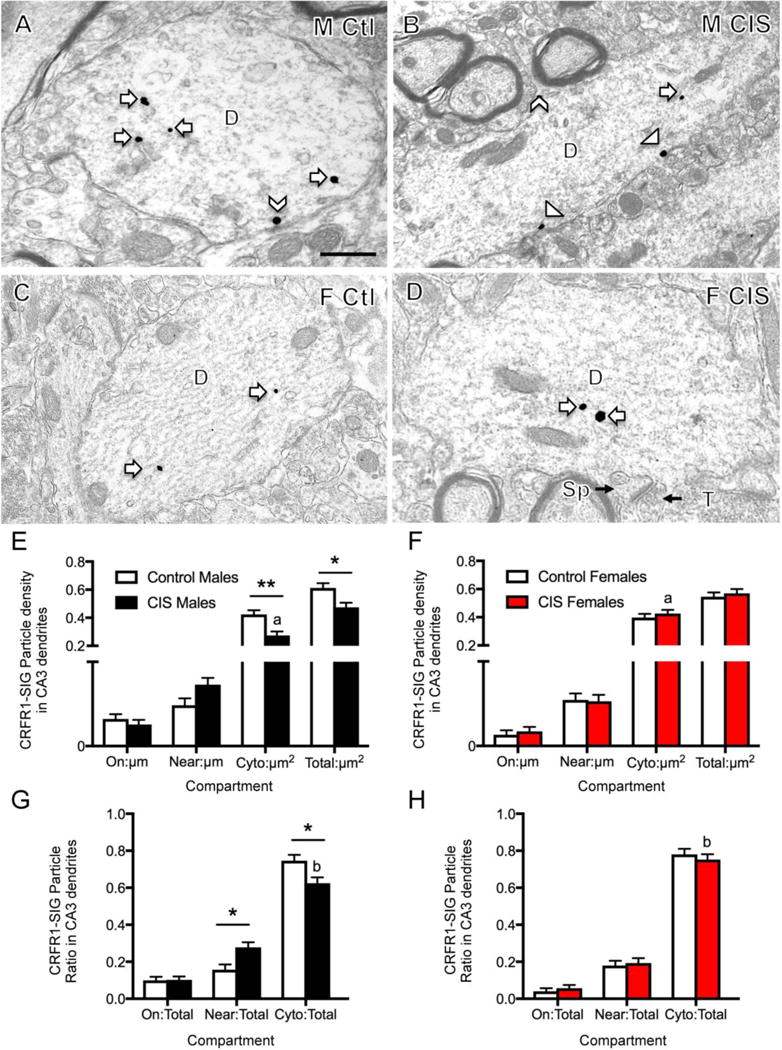
A–D. Representative electron micrographs show the distribution of CRFR1-SIG particles in dendrites from a control male (A), CIS male (B), control diestrus female (C) and CIS diestrus female (D). Examples of CRFR1-SIG particles are shown on the plasma membrane (arrowhead), near the plasma membrane (chevron) and in the cytoplasm (arrow). A spine (sp) and terminal (T) contacting the spine are shown (D). Scale bar = 500 nm. E. In CIS males, CRFR1-SIG particle density in dendrites significantly decreased in the cytoplasm and in total. Moreover, following CIS, males had less cytoplasmic CRFR1-labeling in dendrites than CIS females. F. Females did not show differences in density of CRFR1 in any dendritic compartment after CIS. G, H. In males, but not females, the proportion of CRFR1 near the plasma membrane significantly increased and the proportion of CRFR1 in the cytoplasm significantly decreased in dendrites following CIS. Moreover, the proportion of cytoplasmic to total CRFR1-SIG particles in dendrites from CIS males was significantly less than females. **p < 0.01; *p < 0.05. a p < 0.001; b p < 0.05. N = 3 rats/group; n = 50 dendrites/rat/area.
Fig. 5. Sex differences in the distribution of CRFR1-SIG particles in DG hilar interneuron dendrites after CIS.
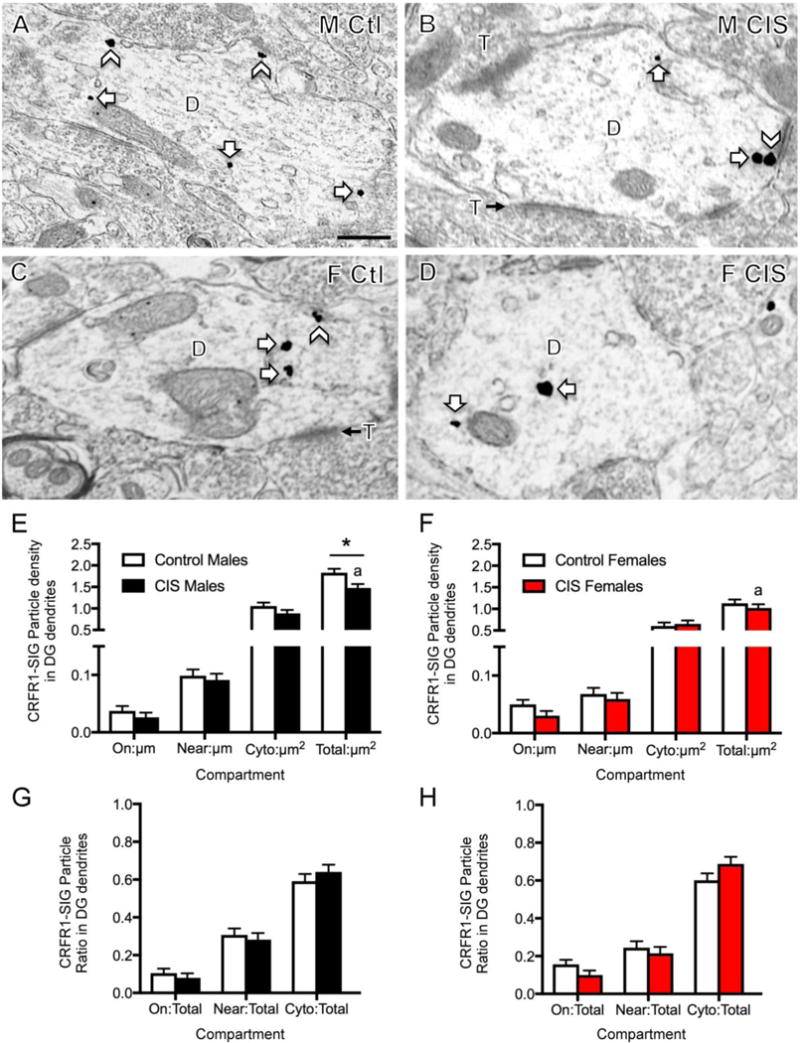
A–D. Representative electron micrographs show the distribution of CRFR1-SIG particles in dendrites from a control male (A), CIS male (B), control diestrus female (C) and CIS diestrus female (D). Examples of CRFR1-SIG particles are shown on near the plasma membrane (chevron) and in the cytoplasm (arrow). Scale bar = 500 nm. E. In males, total CRFR1-SIG particle density in dendrites was significantly reduced after CIS. CIS males had more total CRFR1 than CIS females. F. No significant differences in the density of CRFR1 in any dendritic compartment were found in females. G, H. No significant differences were found in the partitioning ratios of CRFR1 in any dendritic compartment in either males or females following CIS. *p < 0.05. a p < 0.01. N = 3 rats/group; n = 50 dendrites/rat/area.
Post-hoc analysis revealed that the three hippocampal regions had different densities and subcellular distributions of CRFR1-SIG particles in dendrites. Males had more total CRFR1-SIG particle labeling in hilar interneuron dendrites than in CA1 (p < 0.0001) and CA3 (p < 0.0001) dendrites (Fig. 2A). Moreover, males showed more CRFR1-SIG particles in CA3 dendrites than in CA1 dendrites (p = 0.0018; Fig. 2A). Females had more total CRFR1-SIG particles in both DG and CA1 dendrites than they did in CA3 dendrites (p < 0.0001; p < 0.0001, respectively; Fig. 2A). Males displayed more CRFR1-SIG particles in the DG than females (p < 0.0001), however they showed fewer CRFR1-SIG particles in the CA1 than females (p < 0.0001; Fig. 2A). No sex differences were found in total CRFR1-SIG particles in the CA3 dendrites (Fig. 2A).
The subcellular densities of CRFR1-SIG particles in individual dendritic compartments were analyzed further by one-way ANOVA. Like the total densities, males displayed fewer CRFR1-SIG particles on the plasma membrane of CA1 dendrites compared to females (F1,201 = 14.2, p = 0.0002; Fig. 2B). However, unlike total densities, males had more CRFR1-SIG particles on the plasma membrane of CA3 dendrites in comparison to females (F1,245 = 4.70, p = 0.0311; Fig. 2B). There was no sex difference in the density of CRFR1-SIG particles on the plasma membrane in DG dendrites (Fig. 2B).
The subcellular densities of CRFR1-SIG particles in both the near plasma membrane and cytoplasmic dendritic compartments in CA1, CA3, and DG dendrites were similar to those observed for total densities. Specifically, males had fewer near plasma membrane (F1,231 = 44.2, p < 0.0001) and cytoplasmic (F1,215 = 30.6, p < 0.0001) CRFR1-SIG particles in CA1 dendrites compared to females; however, males had more near plasma membrane (F1,276 = 3.81, p = 0.052) and cytoplasmic (F1,212 = 14.7, p = 0.0002) CRFR1-SIG particles in the DG dendrites compared to females (Fig. 2C,D).
Within the CA1 and CA3, partitioning ratios of dendritic CRFR1-SIG particles reflected the differences observed in density. Specifically, males had a lower proportion of CRFR1-SIG particles on (F1,264 = 10.3, p = 0.0015) and near (F1,289 = 35.8, p < 0.0001) the plasma membrane and a higher proportion of CRFR1-SIG particles in the cytoplasm (F1,296 = 54.8, p < 0.0001) in CA1 dendrites compared to females (Fig. 2E). Likewise, males had a greater proportion of CRFR1-SIG particles on the plasma membrane (F1,256 = 5.30, p = 0.0221) of CA3 dendrites compared to females (Fig. 2F). There were no sex differences in the partitioning ratio of CRFR1-SIG particles in any dendritic compartments in the DG (not shown). However, there were additional significant sex differences in the subcellular densities and partitioning ratios between regions (not shown).
CIS has opposite effects on CRFR1 densities in CA1 dendrites of males and females
In agreement with our previous study (Williams et al., 2011a) dendrites containing CRFR1 labeling in stratum radiatum of CA1 had the morphological characteristics of pyramidal cells in all groups (Fig. 3A–D). In particular, many had recognizable spines that were contacted by terminals forming asymmetric synapses (Harris et al., 1992) (Fig. 3D). Due to significant sex differences in the distribution of CRFR1 in control groups, densities and partitioning ratios for CRFR1 in CA1 were analyzed by sex as a factor of treatment in a one-way ANOVA. Males showed considerable shifts in density and partitioning ratio of CRFR1-SIG particles following CIS. Compared to unstressed control males, CIS males had significantly increased CRFR1-SIG particle density near the plasma membrane (F1,228 = 8.45, p = 0.0040), in the cytoplasm (F1,268 = 11.1, p = 0.0010) and in total (F1,199 = 22.3, p < 0.0001) in CA1 dendrites (Fig. 3E). Similarly, CIS males had an increased partitioning ratio of CRFR1-SIG particles near the plasma membrane of CA1 dendrites compared to control males (F1,278 = 5.08, p = 0.025; Fig. 3G). Conversely, CIS females showed a significant decrease in the total density of CRF1-SIG particles in CA1 dendrites compared to control females (F1,346 = 7.48, p = 0.0066; Fig. 3F). In females, no differences were found between unstressed control and CIS groups in the partitioning ratio of CRFR1-SIG particles in any dendritic compartment (Fig. 3H). When dendrites were further subdivided by size (i.e. small vs. large), the distribution of CRFR1-SIG particles was similar to that shown for all dendrites (not shown).
CIS alters CRFR1 trafficking in CA3 dendrites of males but not in females
Examples of CRFR1 labeling in CA3 dendrites for all four groups are shown in Figure 4A–D. Similar to CA1 pyramidal cells, CA3 pyramidal cells had spines, many of which were contacted by terminals forming asymmetric synapses (Fig. 4D). In CA3, two-way ANOVA showed significant main effects of sex on CRFR1-SIG density on the plasma membrane (F1,665 = 6.165, p = 0.0133) and in the cytoplasm (F1,665 = 5.061, p = 0.0248) of dendrites. Moreover, a sex by treatment interaction was shown for CRFR1-SIG density in the cytoplasm (F1,665 = 10.30, p = 0.0014) and in total (F1,665 = 6.314, p = 0.0122) of CA3 dendrites. A significant main effect of treatment (unstressed control vs. CIS) was found in the cytoplasm only (F1,665 = 4.798, p = 0.0288).
Post-hoc tests showed that significantly fewer dendritic CRFR1-SIG particles were present in the cytoplasm (p = 0.0014) and in total (p = 0.0188) in CIS males compared to control males (Fig. 4E). Additionally, CIS males had significantly greater CRFR1-SIG particle density in the cytoplasm (p = 0.0006) of CA3 dendrites when compared to CIS females (Fig. 4E,F). Like densities, the ratio of cytoplasmic CRFR1-SIG particles decreased in CIS males (p = 0.0344; Fig. 4G). However, the proportion of CRFR1-SIG particles in CIS males increased near the plasma membrane compared to control males (p = 0.0146; Fig 4G). CIS males also demonstrated a significantly lower CRFR1-SIG particle cytoplasmic partitioning ratio in CA3 dendrites than CIS females (p = 0.0161; Fig. 4G,H). Unlike males, CIS females showed no significant differences from unstressed control females in density or partitioning ratio in any dendritic compartment (Fig. 4F,H).
In DG, CIS alters the dendritic distribution of CRFR1 in males but not in females
Examples of CRFR1 labeling in DG dendrites from all groups are shown in Figure 5A–D. These dendrites are identified as interneurons as they lack spines and receive multiple contacts from terminals, forming asymmetric synapses on their dendritic shafts (Ribak et al., 1990). In the DG, two-way ANOVA showed significant main effects by sex in dendritic CRFR1-SIG particles near the plasma membrane (F1,587 = 8.308, p = 0.0041), in the cytoplasm (F1,587 = 17.89, p < 0.0001), and in total density (F1,587 = 42.63, p < 0.0001). Additionally, total density of CRFR1-SIG particles demonstrated a significant main effect of treatment (unstressed control vs. CIS; F1,587 = 6.800, p = 0.0093).
Post-hoc analyses showed that CIS males had decreased total dendritic CRFR1-SIG particle density (p = 0.0284) compared to control males (Fig. 5E). CIS males showed significantly greater total CRFR1-SIG particle density (p = 0.0017) compared to CIS females (Fig. 5E,F). CIS females showed no significant change in the density of CRFR1-SIG particles in any cellular compartment (Fig. 5F). Partitioning ratios of dendritic CRFR1-SIG particles were not significantly different in the DG of male and female CIS rats (Fig. 5G,H).
In CA1 and CA3, few CRF-labeled dendritic spines were seen in males and females
As sample sizes were too low to perform statistical analyses for CRFR1-labeled dendritic spines in CA1 and CA3, qualitative descriptions are presented for this data. Less than 4% of CRFR1-labeled dendritic spines were seen in CA1 and CA3 in both groups of males and females (Table 1). There was no significant difference in the number of total labeled spines between unstressed or CIS females and males in CA1 stratum radiatum. Of the labeled spines in CA1, CRFR1-SIG particles were primarily found on the plasma membrane or in the cytoplasm (Fig. 6A,B).
Table 1.
Distribution of CRFR1-SIG particles in dendritic spines
| Group | % ± SEM | # | Location* | ||
|---|---|---|---|---|---|
| synapse | membrane | cytoplasm | |||
| CA1 | |||||
| Ctl males | 1 ± 0.6 | 3 | 0 | 0 | 3 |
| CIS males | 3 ± 0.6 | 9 | 1 | 3 | 5 |
| Ctl females | 3.3 ± 1.2 | 10 | 0 | 4 | 6 |
| CIS females | 1.7 ± 0.3 | 5 | 1 | 1 | 3 |
| CA3 | |||||
| Ctl males | 2.5 ± 1.0 | 10 | 1 | 5 | 5 |
| CIS males | 4.8 ± 1.0 | 17 | 0 | 5 | 12 |
| Ctl females | 2.1 ± 0.3 | 9 | 2 | 3 | 4 |
| CIS females | 2.3 ± 0.8 | 9 | 4 | 2 | 3 |
Some spines had more than one CRFR1-SIG particle. CA1: percentage of CRFR1-labeled spines determined from 100 randomly selected spines per rat. CA3: percentage of spines determined from dendritic spines contacted by 50 randomly selected mossy fiber terminals per rat. N = 3 rats per condition.
Fig. 6. Examples of CRFR1-labeled spines in CA1 and CA3.
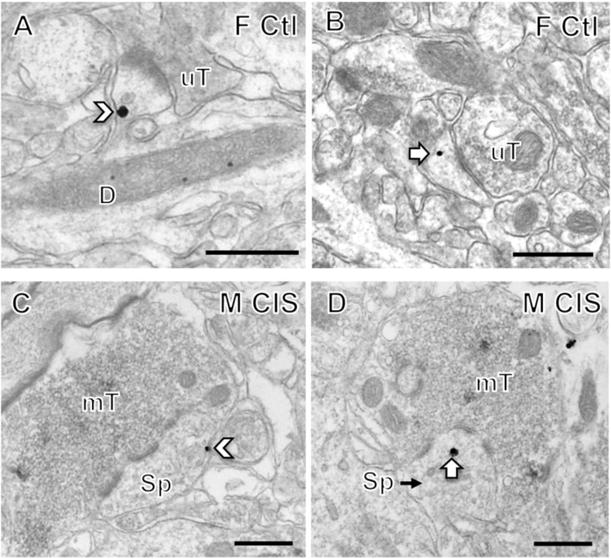
In stratum radiatum of CA1, CRFR1-SIG particles were found on the plasma membrane (chevron, A) or in the cytoplasm (arrow, B) of dendritic spines contacted by unlabeled terminals (uT). In A, the labeled spine emanates from a dendritic shaft (D). In stratum lucidum of CA3, CRFR1-SIG particles were found on the plasma membrane (chevron, C) or in the cytoplasm (arrow, D) of dendritic spines contacted by mossy fiber terminals (mT). Scale bar = 500 nm.
Similarly, the percent of CRFR1-labeled dendritic spines contacted by mossy fibers in stratum lucidum CA3 was not significantly different between the four groups. Of the labeled spines in CA3, CRFR1-SIG particles were primarily found on the plasma membrane or in the cytoplasm (Fig. 6C,D). However, the CIS males had about twice as much CRFR1 labeling in the cytoplasm of CA3 spines than the other 3 groups (Table 1).
DISCUSSION
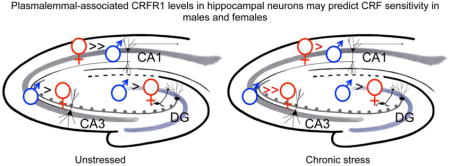
Sex differences in CRFR1 density and trafficking in neurons were found in unstressed controls and CIS animals throughout the hippocampus (Fig. 7). In each hippocampal subregion, unstressed and CIS females and males exhibited distinct patterns of CRFR1 compartmentalization within neurons. In the absence of stress, females displayed higher total CRFR1 dendritic levels in the CA1, whereas unstressed males had higher total levels in the DG hilus. Within neuronal dendrites, unstressed rats also showed sex differences in CRFR1’s location relative to the plasma membrane in CA1, CA3 and DG. Sex differences persisted after CIS; in males, near plasma membrane CRFR1 increased in CA1 pyramidal cell dendrites but not to levels seen in either unstressed or CIS females. In males only, CIS decreased both cytoplasmic and total CRFR1 in dendrites in CA3 and the hilus. These changes in CRFR1 density and distribution may contribute to the reported sex differences in hippocampal-dependent learning and memory processes in basal states as well as those influenced by stress, and have relevance to anxiety and drug addiction and other neurological disorders (Nemeroff and Vale, 2005; Logrip et al., 2011; Dong et al., 2012; Haass-Koffler and Bartlett, 2012; Leuner and Shors, 2013; Bangasser et al., 2016; Becker et al., 2017; McEwen and Milner, 2017).
Fig. 7. Schematic diagram depicting sex differences in the subcellular distribution of CRFR1 in hippocampal dendrites without stress and following CIS.
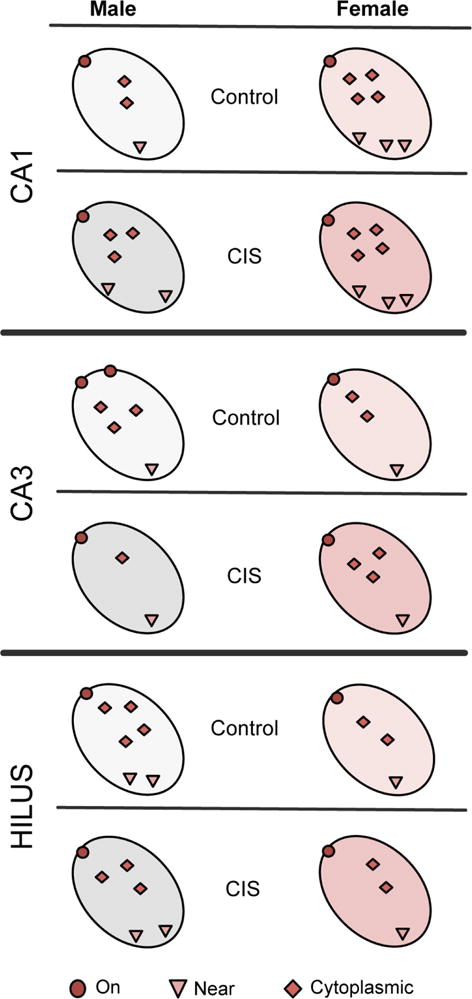
Unstressed (control): In CA1 pyramidal cell dendrites, females compared to males had more CRFR1 in every cellular compartment. In CA3 pyramidal cell dendrites, males had more CRFR1 on the plasma membrane than females. In hilar interneuron dendrites, males compared to females had more cytoplasmic and total CRFR1. CIS: In CA1 pyramidal cell dendrites, CIS males compared to control males had more CRFR1 near the plasma membrane, in the cytoplasm and in total. However, although CIS females compared to control females had less total CRFR1 in CA1 dendrites, they still had more CRFR1 than control and CIS males. In CA3 pyramidal cell dendrites, CIS decreased cytoplasmic and total CRFR1 in males only. Likewise, hilar interneuron dendrites of CIS males had less total CRFR1 than control males.
In unstressed rats, sex differences in CRFR1 distribution varied between hippocampal regions
Our studies show sex differences exist without exposure to CIS in the levels and distribution of CRFR1 in CA1 and CA3 pyramidal cells as well as in DG hilar interneurons. The largest sex differences in the distribution of CRFR1 are observed in CA1 pyramidal cell dendrites: diestrus (low estrogen) females compared to males have greater total CRFR1 density as a result of elevated densities of CRFR1 on the plasma membrane, near the plasma membrane and in the cytoplasm. This agrees with our previous finding that proestrus (high estrogen) females exhibit increased CRFR1 density on the plasma membrane of CA1 pyramidal cell dendrites compared to males; however, total and cytoplasmic CRFR1 densities are not different (Williams et al., 2011a). These findings are supported by the increased binding capacities for CRF detected in CA1 pyramidal neurons in females, regardless of estrogen state, compared to males (Boudin et al., 1998). Moreover, they suggest that at low estrogen states, females, but not males, have elevated reserve pools of CRFR1s in the process of being stored, degraded or recycled that could be inserted into the plasma membrane (Fernandez-Monreal et al., 2012; Kneussel and Hausrat, 2016).
In unstressed females, the CA1 and DG show comparable total densities of dendritic CRFR1, which are greater than the total dendritic CRFR1 density in CA3. In contrast, unstressed males display a greater density of total dendritic CRFR1 in the DG compared to CA1 and CA3, with CA1 levels even lower than CA3. Although unstressed males and females have similar total levels of CRFR1 in CA3 pyramidal cell dendrites, the males have increased CRFR1 on the plasma membrane compared to females. This finding agrees with a previous study in prepubescent rats indicating that CRFR1 binding in the CA3 hippocampal region is increased in males compared to females; however, they do not observe this sex difference in CRFR1 binding in adult rats (Weathington et al., 2014). This is similar to reports in the locus coeruleus where CRFR1 is elevated on the plasma membrane of dendrites in unstressed male rats compared to females (Reyes et al., 2008; Bangasser et al., 2010). In the locus coeruleus, increased localization of CRFR1 to the plasma membrane in males, but not females, is associated with beta arrestin 2, an important protein for the CRFR1 internalization process (Bangasser et al., 2010).
Altogether, these baseline CRFR1 levels in unstressed females and males in all hippocampal regions suggest that CRF differentially affects the balance of excitation and inhibition in a sex-dependent manner. Specifically, unstressed females could have a heightened sensitivity to CRF in excitatory CA1 pyramidal cells (Freund and Buzsáki, 1996), whereas unstressed males could have an elevated sensitivity to CRF in excitatory CA3 pyramidal cells as well as in inhibitory hilar interneurons (Freund and Buzsáki, 1996). These regional differences could impact the processing of information from inputs to the granule cells (e.g., the entorhinal cortex) as well as outputs within and external to the hippocampus.
Alternatively, although lower levels of CRFR1 are detected in the DG and CA3 females relative to males, the females may have an elevated sensitivity to CRF. In support, evidence for the locus coeruleus shows that CRF-mediated beta arrestin signaling is dysfunctional in females rendering CRF-receptive neurons more vulnerable to low levels of CRF (Bangasser et al., 2010; Bangasser et al., 2012).
CIS differentially impacts CRFR1 density in CA1 pyramidal cells in males and females
Following CIS, males compared to females have elevated total levels of CRFR1 in CA1 pyramidal cell dendrites as well as increased densities of CRFR1 near the plasma membrane and in the cytoplasm of these dendrites. In contrast, females have decreased total levels of CRFR1 in CA1 pyramidal cell dendrites. However, CIS females continue to have greater total levels of CRFR1 as well as higher densities of dendritic CRFR1 in every synaptic compartment than unstressed or CIS males. Thus, although CA1 pyramidal cell dendrites in males could have an elevated sensitivity to CRF following CIS, CA1 pyramidal cells in females would still be more responsive to CRF than males. The sexual dimorphism in the redistribution of cytoplasmic CRFR1 in CA1 dendrites following CIS is similar to that seen in the dendrites of locus coeruleus neurons 1 day after 15 min of swim stress (Bangasser et al., 2010).
Our previous EM studies in CA1 (Williams et al., 2011a) demonstrated that there are similar levels of CRFR1 labeled SIG particles in pyramidal cell DOR dendrites in unstressed females and males but dual labeled dendrites are increased proestrus females. We recently have found that after CIS, plasma membrane DORs in CA1 pyramidal cell dendrites decrease in males, but not females (Reich et al., unpublished). Together with the present results, these findings suggest that female and male CA1 pyramidal cells, which demonstrate sex differences in the absence of stress, show even greater differences after CIS by shifting their cellular responses in opposing directions. As DORs are thought to be neuroprotective (Hayashi et al., 2002; Charron et al., 2008; Feng et al., 2009), this redistribution of CRFR1 and DORs could render CA1 pyramidal cells in males more susceptible to neuronal damage from CRF after experiencing CIS (Maecker et al., 1997). Interestingly, as some studies show CRF decreases glutamate excitotoxicity (Hollrigel et al., 1998; Elliott-Hunt et al., 2002), this unopposed increased CRFR1 signaling in CA1 dendrites could also enhance neuroprotection through not yet described mechanisms (Charron et al., 2009). DOR can modulate CRFR1 signaling through cyclic AMP-phosphokinase A pathways, although other G-protein coupled receptors and signaling pathways contribute to the diverse responses to CRF (Markovic et al., 2006; Williams et al., 2011a; Dunn et al., 2013).
In CA3 pyramidal cells, CIS alters the distribution of CRFR1 only in males
CIS results in a redistribution of CRFR1 from the cytoplasm to near the plasma membrane as well as decreased total CRFR1 in CA3 pyramidal cell dendrites in males but not females. Moreover, although few CRFR1s are detected in the CA3 pyramidal cell dendritic spines contacted by mossy fibers, the number of CRFR1-labeled spines appears to increase following CIS in males but not females. In contrast, CIS does not affect the distribution or levels of CRFR1 in any cellular compartment in the CA3 pyramidal cell dendrites of females. These findings support our recent studies demonstrating a down-regulation of Crhr1 rna in the CA3 following CIS in male, but not female, rats (Randesi et al., 2018). These sex differences in the redistribution of CRFR1 in CA3 pyramidal cell dendrites may contribute to the dendritic retraction of CA3 dendrites in males after long-term stress (McEwen et al., 2016).
CA3 pyramidal cells contain both CRFR1s and DORs in males and females (Chen et al., 2000; Mazid et al., 2016). Following CIS, DORs redistribute within CA3 pyramidal cell dendrites: plasma membrane DORs decrease in males while cytoplasmic and total DORs decrease in females (Mazid et al., 2016). Additionally, males have approximately three times less CA3 pyramidal cell DOR-labeled spines contacted by mossy fibers compared to proestrus females (Harte-Hargrove et al., 2015), and the proportion of CA3 DOR-labeled spines is unchanged in females following CIS (Mazid et al., 2016). Similar to the CA1, the response to CIS is not only sex dependent, but both CRFR1 and DORs redistribute in CA3 pyramidal cell dendrites in opposite manners. As a consequence, this redistribution of CRFR1 and DORs in CA3 pyramidal cells could affect mossy fiber-CA3 synaptic function. In particular, the presence of postsynaptic DORs in CA3 dendrites results in proestrus females, but not diestrus females or males, exhibiting a novel form of mossy fiber LTP (Harte-Hargrove et al., 2015). Moreover, the opioid system in the hippocampal CA3 region has been implicated in visual-spatial pattern completion, an important component of context learning (Meilandt et al., 2004; Kesner and Warthen, 2010).
CIS has limited effects on CRFR1 levels in DG interneurons
CIS has few effects on the levels or subcellular distribution of CRFR1 in hilar interneuron dendrites and these occur only in males. Similar to CA3, in DG hilar interneuron dendrites, males exhibit a decrease in total levels of CRFR1 following CIS; however, the total levels of CRFR1 in interneuron dendrites in males are still elevated compared to CIS females. These results are consistent with our previous finding that CIS does not alter the density of DORs in GABAergic hilar dendrites in either males or females (Mazid et al., 2016). However, as the present study examines CRFR1 redistribution following CIS in unidentified interneurons, it is also possible that changes in the redistribution of CRFR1 in hilar interneurons are limited to a specific subtype of interneurons. Moreover, previous studies have shown that parvalbumin-containing hilar interneurons decrease by about 30% in males, but not females, following CIS (Czeh et al., 2005; Hu et al., 2010; Milner et al., 2013). Thus, it is likely that CRFR1 labeling following CIS is measured in a smaller portion of interneurons in the males compared to the females.
Application of CRF to the DG of male rats produces long-lasting synaptic efficacy of neurons in this region (Wang et al., 2000). Although the phenotype of CRFR1-containing neurons in the rat hilus in not known, their topographic distribution by light microscopy (Chen et al., 2000; Tan et al., 2017) indicates that they likely contain SOM and/or neuropeptide Y (NPY) (Freund and Buzsáki, 1996). Our previous studies have shown that SOM/NPY-containing interneurons and, to a lesser extent, parvalbumin-labeled interneurons colocalize CRF as well as DORs in the male and female rat hilus (Williams and Milner, 2011; Williams et al., 2011b). Hilar SOM/NPY-containing neurons project to the outer molecular layer of the DG, where they inhibit the induction and maintenance of lateral perforant pathway LTP (Sperk et al., 2007); the lateral perforant pathway also contains enkephalins (Drake et al., 2007). Thus, together with this study, these findings suggest that activation of CRFR1 in males could have a greater effect on synaptic plasticity and LTP in the DG, particularly in relation to the opioid system, than in females.
Functional considerations
The present study adds to the growing body of literature that stress affects hippocampal protein and gene expression as well as plasticity processes in females and males differently (Marrocco et al., 2017; McEwen and Milner, 2017; Randesi et al., 2018). As depicted in the examples below, sex differences in the densities and subcellular distributions of CRFR1 in hippocampal subregions could contribute to a wide range of outcomes depending on the age of the animal, type and duration of stressor.
Together with our previous studies (Williams et al., 2011a), the present studies indicate that more CRFR1 is found on the plasma membrane of CA1 pyramidal cell dendrites in females, regardless of estrogen state, compared to males. This could differentially impact excitatory glutamatergic transmission (Oberlander and Woolley, 2017) as well as stress-associated changes in the structure and function of this region (McEwen et al., 2015). For example, proestrus females compared to diestrus females and males have greater numbers of dendritic spines on CA1 pyramidal cell neurons (McEwen and Milner, 2017). Thus, elevated CRFR1 in CA1 synapses from females, especially during proestrus, would be expected to promote enhanced levels of synaptic efficacy following a short excitatory stimulus that release CRF compared to males (Chen et al., 2012). However, the presence of higher levels of CRFR1 in CA1 pyramidal cells in females may render females more sensitive to longer periods of CRF release. Specifically, approximately 75 minutes after application of CRF to CA1 pyramidal cells in male rodents, synaptic transmission is depressed and thin dendritic spines are lost, resulting in impaired synaptic plasticity (Chen et al., 2013). Thus, we would predict that the interval in which synaptic efficacy begins to decline following CRF application would be shorter in female rodents. Moreover, as females also have higher levels of CRFR1 in other cellular compartments (i.e., near plasma membrane and cytoplasmic) of CA1 pyramidal neurons compared to males, we would predict that they would be more sensitive to acute stressors, especially when the protective effects of estrogen (Zárate et al., 2017) are low. In support of this idea, when diestrus female rats are subjected to acute (30 sec) stress and analyzed in proestrus, the increase in CA1 dendritic spines is not observed (Shors et al., 2001). In contrast, the same acute stress in male rats increases CA1 dendritic spines (Shors et al., 2001).
In the DG, the higher density of CRFR1 on GABAergic interneurons in males compared to females suggests that inhibitory neurons would be more sensitive to CRF in males. However, the effect of CRF on these interneurons would vary depending on the duration of exposure. As previous studies have shown in male rats (Wang et al., 2000), a brief (seconds to minutes) exposure to CRF activates CRFR1 on inhibitory neurons to promote long lasting potentiation of DG neurons. However, in male but not female rats chronic stress results in a loss of DG parvalbumin interneurons (Hu et al., 2010; Milner et al., 2013), known to contain CRFR1 (Yan et al., 1998; Williams and Milner, 2011) and this upsets inhibitory-excitatory DG networks important for cognitive processes (Hu et al., 2010). Moreover, GABAergic interneurons are important for coordinating the activity of preexisting as well as newly born granule cells (Markwardt et al., 2011). The presence of elevated levels of CRFR1 in GABAergic interneurons in males compared to females could indirectly contribute to reduction of hippocampal adult neurogenesis in male rodents, but not females following chronic stress (Mirescu and Gould, 2006; Surget et al., 2008; Masiulis et al., 2011; Marques et al., 2016). These decreases in adult neurogenesis can increase anxious behavior in males (Revest et al., 2009). Although the developmental time-point at which sex differences in the subcellular distribution and density of CRFR1 within interneurons emerge is unknown, evidence from the hypothalamus suggests that sex hormone influences on CRH gene expression emerge during early prenatal development (Patchev et al., 1999). Thus, it is possible that the lower density of CRFR1 in DG interneurons in females compared to males may contribute to the decreases in granule cell number and DG size seen in females following maternal deprivation stress (Oomen et al., 2011).
The higher number of CRFR1s on the plasma membrane along with colocalization with DORs in CA3 pyramidal cell dendrites in males could influence plasticity and neuroprotective processes in response to chronic stress. CRFR1 and DORs are known to utilize common signaling pathways including protein kinase C and mitogen-activated protein kinase (Battaglia et al., 1987; Quock et al., 1999; Blank et al., 2003; Hillhouse and Grammatopoulos, 2006). Moreover, CRF induced activation of CRFRs leads to production of intracellular cyclic AMP that can be attenuated by DOR agonists (Williams et al., 2011a), suggesting receptor cross-talk between the opioid and CRF receptor systems. In the rat hippocampus, our EM observations would suggest that sex differences in the responses of CA3 neurons to DOR agonists could be attributed to the balance of DORs and CRFR1 within these neurons. Thus, elevated plasma membrane CRFR1 but lower DORs on CA3 dendrites in unstressed males compared to unstressed females (Mazid et al., 2016) may permit greater excitation and plasticity to responses to CRF in favor of DOR-mediated LTP (Harte-Hargrove et al., 2015). However, in males following CIS, the balance of CRFR1 and DORs on the plasma membrane of CA3 neurons shift even more to favor of CRFR1 (Mazid et al., 2016). This elevation in CRFR1s on and near the plasma membrane of CA3 pyramidal cell dendrites in males would make them more vulnerable to CRF-induced cyclic AMP-dependent hyperexcitability (Hollrigel et al., 1998; Elliott-Hunt et al., 2002) and would contribute to processes that promote spine loss and dendritic retraction following CIS (Wang et al., 2013; McEwen et al., 2015). In contrast, the balance of CRFR1 and DORs in CA3 pyramidal neurons is similar in unstressed and CIS female rats (Mazid et al., 2016) and thus would be predicted may allow for the DOR responses of CA3 neurons to still be in place. In particular, opioid effects on CA3 pyramidal cell neurons are important for contextual learning processes (Meilandt et al., 2004; Kesner and Warthen, 2010). We recently found that conditioned place preference to oxycodone, a behavior that involves contextual learning, is present in female rats, but not male rats, following CIS (Milner et al., unpublished).
In conclusion, the demonstrated sex differences in the density and subcellular distribution of CRFR1 in excitatory and inhibitory neurons that could differentially affect the network properties of the hippocampus. In particular, the CA1 may be more sensitive to CRF in females whereas the CA3 and DG may be more sensitive to CRF in males. Thus, as CA3 pyramidal neurons receive afferents from granule cells and project to CA1 as well as back to the DG (Scharfman and MacLusky, 2017), differences in the weight of CRF sensitivity in one subregion would affect the responses of other subregions. Thus, the reported sex differences in hippocampal CRFR1 distributions could affect learning and memory processes in different ways in females and males, including those relevant for addictive processes, and also contribute to sex differences in these processes in response to stress (McEwen et al., 2016).
Highlights.
Female rats have higher CRFR1 levels in CA1 pyramidal cell dendrites even after chronic immobilization stress (CIS).
After CIS, near plasmalemmal CRFR1 increases in CA1 pyramidal cell dendrites in males.
Unstressed male rats have higher CRFR1 in inhibitory interneurons in the dentate gyrus even after CIS.
Males have elevated plasmalemmal CRFR1 on CA3 pyramidal cell neurons, even following CIS.
Sex differences in hippocampal CRFR1 may contribute to effects of stress on learning and memory.
Acknowledgments
This work was supported by the National Institutes of Health [grants DA08259 (TAM, BSM), HL098351 & HL096571 (TAM)] and the Hope for Depression Research Foundation (BSM)
ABBREVIATIONS
- AIS
acute immobilization stress
- BDNF
brain derived neurotrophic factor
- BSA
bovine serum albumin
- CIS
chronic immobilization stress
- CRF
corticotropin releasing factor
- CRFR1
corticotropin releasing factor receptor 1
- DG
dentate gyrus
- DOR
delta opioid receptor
- EM
electron microscopic
- HPA
hypothalamic-pituitary-adrenal
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinase
- NPY
neuropeptide Y
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PKC
protein kinase C
- SIG
silver-intensified immunogold
- SOM
somatostatin
- TS
tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS: B.S.M., E.M.W., and T.A.M. designed research; A.G.D., E.M.W., and T.A.M. performed research; H.R.M., B.R., N.H.C., R.P.K., and T.A.M. analyzed data; H.R.M., B.R., N.H.C., and T.A.M. wrote the paper. All authors have approved the final article.
References
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: Potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BAS, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2012;18:166. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Dong H, Carroll J, Plona Z, Ding H, Rodriguez L, McKennan C, Csernansky JG, Seeholzer SH, Valentino RJ. Corticotropin-releasing factor overexpression gives rise to sex differences in alzheimer’s disease-related signaling. Mol Psychiatry. 2016;22:1126. doi: 10.1038/mp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Webster EL, De Souza EB. Characterization of corticotropin‐releasing factor receptor‐ mediated adenylate cyclase activity in the rat central nervous system. Synapse. 1987;1:572–581. doi: 10.1002/syn.890010610. [DOI] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95:136–147. doi: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: Implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple g-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: Role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Pickel VM, Beaudet A. Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. J Neurosci. 1998;18:8473–8484. doi: 10.1523/JNEUROSCI.18-20-08473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta1-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93:502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Charron C, Schock SC, Proulx G, Thompson CS, Hakim AM, Plamondon H. Protection conferred by corticotropin-releasing hormone in rat primary cortical neurons against chemical ischemia involves opioid receptor activation. Brain Res. 2009;1257:117–127. doi: 10.1016/j.brainres.2008.12.053. [DOI] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (crf(1))-like immunoreactivity in the mouse brain: Light microscopy analysis using an antibody directed against the c-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: Pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Andres A, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: The crh system. Front Cell Neurosci. 2012;6:13. doi: 10.3389/fncel.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kramár E, Chen L, Babayan A, Andres A, Gall C, Lynch G, Baram T. Impairment of synaptic plasticity by the stress mediator crh involves selective destruction of thin dendritic spines via rhoa signaling. Mol Psychiatry. 2013;18:485–496. doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: A review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: Prevention by treatment with a substance p receptor (nk1) antagonist. Neuropsychopharmacology. 2005;30:67–79. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- Dong H, Murphy KM, Meng L, Montalvo-Ortiz J, Zeng Z, Kolber BJ, Zhang S, Muglia LJ, Csernansky JG. Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2012;28:579–592. doi: 10.3233/JAD-2011-111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Dunn HA, Walther C, Godin CM, Hall RA, Ferguson SSG. Role of sap97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling. J Biol Chem. 2013;288:15023–15034. doi: 10.1074/jbc.M113.473660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott-Hunt CR, Kazlauskaite J, Wilde GJC, Grammatopoulos DK, Hillhouse EW. Potential signalling pathways underlying corticotrophin-releasing hormone-mediated neuroprotection from excitotoxicity in rat hippocampus. J Neurochem. 2002;80:416–425. doi: 10.1046/j.0022-3042.2001.00712.x. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chao D, He X, Yang Y, Kang X, L HL, Xia Y. A novel insight into neuroprotection against hypoxic/ischemic stress. Sheng Li Xue Bao. 2009;61:585–592. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Brown TC, Royo M, Esteban JA. The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. J Neurosci. 2012;32:13200–13205. doi: 10.1523/JNEUROSCI.0061-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Bartlett SE. Stress and addiction: Contribution of the corticotropin releasing factor (crf) system in neuroplasticity. Front Mol Neurosci. 2012;5:1–13. doi: 10.3389/fnmol.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (ca1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE. Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat. J Neurosci. 2015;35:1723–1738. doi: 10.1523/JNEUROSCI.0820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Tsao LI, Su TP. Antiapoptotic and cytotoxic properties of delta opioid peptide [d-ala(2),d-leu(5)]enkephalin in pc12 cells. Synapse. 2002;43:86–94. doi: 10.1002/syn.10019. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations: Implications for endocrine functions in brain. In: Fuxe K LFA, editor. Volume transmission in the brain: Novel mechanisms for neural transmission. New York: Raven Press; 1991. pp. 63–87. [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: Implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czeh B, Flugge G, Zhang W. Stress impairs gabaergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK. Implications of ca3 nmda and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–557. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Hausrat TJ. Postsynaptic neurotransmitter receptor reserve pools for synaptic potentiation. Trends Neurosci. 2016;39:170–182. doi: 10.1016/j.tins.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Leranth C. Electron microscopic pre-embedding double immunohistochemical methods. In: Heimer LZL, editor. Neuroanatomical tract tracing methods 2 Recent progress. New York: Plenum; 1989. pp. 129–172. [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction: Potential for pharmacological intervention. CNS Drugs. 2011;25:271–287. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 2007;1155:108–115. doi: 10.1016/j.brainres.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: Accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent crf antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744:166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- Markovic D, Papadopoulou N, Teli T, Randeva H, Levine MA, Hillhouse EW, Grammatopoulos DK. Differential responses of corticotropin-releasing hormone receptor type 1 variants to protein kinase c phosphorylation. J Pharmacol Exp Ther. 2006;319:1032–1042. doi: 10.1124/jpet.106.107441. [DOI] [PubMed] [Google Scholar]
- Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat Neurosci. 2011;14:1407. doi: 10.1038/nn.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AA, Bevilaqua MC, da Fonseca AM, Nardi AE, Thuret S, Dias GP. Gender differences in the neurobiology of anxiety: Focus on adult hippocampal neurogenesis. Neural Plast. 2016;2016:14. doi: 10.1155/2016/5026713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J, Petty GH, Rios MB, Gray JD, Kogan JF, Waters EM, Schmidt EF, Lee FS, McEwen BS. A sexually dimorphic pre-stressed translational signature in ca3 pyramidal neurons of bdnf val66met mice. Nat Commun. 2017;8:808. doi: 10.1038/s41467-017-01014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiulis I, Yun S, Eisch AJ. The interesting interplay between interneurons and adult hippocampal neurogenesis. Mol Neurobiol. 2011;44:287–302. doi: 10.1007/s12035-011-8207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazid S, Hall BS, Odell SC, Stafford K, Dyer AD, Van Kempen TA, Selegean J, McEwen BS, Waters EM, Milner TA. Sex differences in subcellular distribution of delta opioid receptors in the rat hippocampus in response to acute and chronic stress. Neurobiol Stress. 2016;5:37–53. doi: 10.1016/j.ynstr.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95:24–39. doi: 10.1002/jnr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL., Jr Role of hippocampal ca3 mu-opioid receptors in spatial learning and memory. J Neurosci. 2004;24:2953–2962. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, Williams TJ, Schierberl KC, Torres-Reveron A, Gonzales KL, McEwen BS, Waters EM. Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse. 2013;67:757–772. doi: 10.1002/syn.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP. Degenerating processes identified by electron microscopic immunocytochemical methods. In: Manfredi G, Kawamata H, editors. Neurodegeneration, methods and protocols. New York: Springer; 2011. pp. 23–59. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: Inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2017;37:12314–12327. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EMM, Joëls M, Krugers H, Lucassen PJ. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology (Berl) 2011;214:249–260. doi: 10.1007/s00213-010-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hayashi S, Orikasa C, Almeida OFX. Ontogeny of gender-specific responsiveness to stress and glucocorticoids in the rat and its determination by the neonatal gonadal steroid environment. Stress. 1999;3:41–54. doi: 10.3109/10253899909001111. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The fine structure of the nervous system: Neurons and their supporting cells. 3. New York: Oxford University Press; 1991. [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: Dynorphin b-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kievits J, Graustein B, Speth RC, Iadecola C, Milner TA. Sex differences in the subcellular distribution of AT(1) receptors and nadph oxidase subunits in the dendrites of c1 neurons in the rat rostral ventrolateral medulla. Neuroscience. 2009;163:329–338. doi: 10.1016/j.neuroscience.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA. Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J Chem Neuroanat. 2014;55:9–17. doi: 10.1016/j.jchemneu.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quock RM, Burkey TH, Varga E, Hosohata Y, Hosohata K, Cowell SM, Slate CA, Ehlert FJ, Roeske WR, Yamamura HI. The δ-opioid receptor: Molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol Rev. 1999;51:503–532. [PubMed] [Google Scholar]
- Randesi M, Zhou Y, Mazid S, Odell SC, Gray JD, Correa da Rosa J, McEwen BS, Milner TA, Kreek MJ. Sex differences after chronic stress in the expression of opioid-, stress- and neuroplasticity-related genes in the rat hippocampus. Neurobiol Stress. 2018;8:33–41. doi: 10.1016/j.ynstr.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev L, Baram TZ. Corticotropin releasing factor in neuroplasticity. Front Neuroendocrinol. 2014;35:171–179. doi: 10.1016/j.yfrne.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Nitsch R, Seress L. Proportion of parvalbumin-positive basket cells in the gabaergic innervation of pyramidal and granule cells of the rat hippocampal formation. J Comp Neurol. 1990;300:449–461. doi: 10.1002/cne.903000402. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei–potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Sex differences in hippocampal area ca3 pyramidal cells. J Neurosci Res. 2017;95:563–575. doi: 10.1002/jnr.23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of crf and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: Structure of the rat brain. 1. Amsterdam: Elsevier; 1992. [Google Scholar]
- Tan LA, Vaughan JM, Perrin MH, Rivier JE, Sawchenko PE. Distribution of corticotropin-releasing factor (crf) receptor binding in the mouse brain using a new, high-affinity radioligand, [(125) I]-PD-Sauvagine. J Comp Neurol. 2017;525:3840–3864. doi: 10.1002/cne.24307. [DOI] [PubMed] [Google Scholar]
- Thureson-Klein AK, Klein RL. Exocytosis from neuronal large dense-cored vesicles. Int Rev Cytol. 1990;121:67–126. doi: 10.1016/s0074-7696(08)60659-2. [DOI] [PubMed] [Google Scholar]
- Turner CD, Bagnara JT. General endocrinology. Philadelphia: W.B Saunders; 1971. [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Wayner MJ, Chai CY, Lee EH. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur J Neurosci. 1998;10:3428–3437. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Wang HL, Tsai LY, Lee EH. Corticotropin-releasing factor produces a protein synthesis–dependent long-lasting potentiation in dentate gyrus neurons. J Neurophysiol. 2000;83:343–349. doi: 10.1152/jn.2000.83.1.343. [DOI] [PubMed] [Google Scholar]
- Wang X-D, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH, Harbich D, Mayer B, Wurst W, Holsboer F, Deussing JM, Baram TZ, Müller MB, Schmidt MV. Forebrain crhr1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis. 2011a;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-D, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W, Holsboer F, Deussing JM, Baram TZ, Stewart MG, Müller MB, Schmidt MV. Forebrain crf1 modulates early-life stress-programmed cognitive deficits. J Neurosci. 2011b;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-D, Su Y-A, Wagner KV, Avrabos C, Scharf SH, Hartmann J, Wolf M, Liebl C, Kühne C, Wurst W, Holsboer F, Eder M, Deussing JM, Müller MB, Schmidt MV. Nectin-3 links crhr1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci. 2013;16:706. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Hamki A, Cooke BM. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J Comp Neurol. 2014;522:1284–1298. doi: 10.1002/cne.23475. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Akama KT, Knudsen MG, McEwen BS, Milner TA. Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in ca1 pyramidal cell dendrites. Exp Neurol. 2011a;230:186–196. doi: 10.1016/j.expneurol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Milner TA. Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience. 2011;179:9–22. doi: 10.1016/j.neuroscience.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA. Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol Learn Mem. 2011b;95:206–220. doi: 10.1016/j.nlm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (crh)-containing neurons in the immature rat hippocampal formation: Light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zárate S, Stevnsner T, Gredilla R. Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front Aging Neurosci. 2017;9:430. doi: 10.3389/fnagi.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated akt in hippocampal ca1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


