Abstract
Background: Beta blockers are standard therapy for myocardial infarction (MI). Preclinical studies have shown efficacy and safety of thyroid hormone (TH) treatment of cardiovascular disorders. Since THs interact with the sympathoadrenergic system, this study aimed to compare triiodothyronine (T3) and metoprolol (Met) in the treatment of rats with MI on pathophysiology and TH-adrenergic signaling.
Methods: Female Sprague–Dawley rats aged 12 weeks underwent left anterior descending coronary artery ligation (MI) or sham surgeries. T3 (5 μg/kg/day) or Met (100 mg/kg/day) was given in drinking water immediately after surgery for eight weeks. At the terminal of the experiments, the rats were subjected to morphological, functional, and molecular examination.
Results: T3 and Met significantly enhanced left ventricular contractility (left ventricular fractional shortening 21.37 ± 2.58% and 21.14 ± 3.71%, respectively) compared to untreated MI (17.88 ± 1.23%), and decreased the incidence of inducible atrial tachyarrhythmia by 87.5% and 62.5%, respectively. Although both treatments showed efficacy, T3 but not Met showed statistically significant improvements compared to MI in arrhythmia duration, left atrial diameter (T3 vs. MI 4.33 ± 0.63 vs. 5.65 ± 1.32 mm; p < 0.05), fibrosis (6.1 ± 0.6%, 6.6 ± 0.6% vs. 8.2 ± 0.7%, T3, Met vs. MI, respectively), and aortic vasorelaxation responsiveness to acetylcholine (pD2 6.97 ± 0.22, 6.83 ± 0.21 vs. 6.66 ± 0.22, T3, Met vs. MI, respectively). Quantitative polymerase chain reaction showed that T3 and Met attenuated expression of genes associated with inflammation and oxidative stress and restored expression of ion channels and contractile proteins.
Conclusion: These results support comparable efficacy of T3 and Met treatments, suggesting that T3 may provide a therapeutic alternative to standard β-receptor blockade, especially for patients intolerant to treatment with β-blockers after MI.
Keywords: : triiodothyronine, metoprolol, myocardial infarction, heart function
Introduction
Cardiovascular diseases are a leading cause of morbidity and mortality worldwide (1). Thyroid hormones (THs) are essential to maintain homeostasis of the heart and vascular system (2). Even subtle changes in TH levels may adversely impact the cardiovascular system, as evidenced by clinical studies showing that subclinical hypothyroidism and hyperthyroidism and low triiodothyronine (T3) syndrome are associated with poor outcomes in patients with heart diseases (3–7). Low TH function is frequently observed in clinical and experimental studies and is increasingly recognized as a strong, independent risk factor of poor prognosis (8,9), although it may be difficult to determine if the altered TH condition is a cause or consequence of the underlying cardiovascular disease. In addition, independent of serum TH concentrations, reduced cardiac TH receptors (TR alpha and beta) expression and low cardiac T3 levels are frequently observed in severe heart diseases, including acute myocardial infarction (MI) and heart failure (HF), indicating impairment in TH signaling in failing hearts (9–11). Moreover, evidence is accumulating that TH administration post MI and HF improves cardiac contractility and myocardial remodeling, and improves cardiovascular risk factors, especially lipid profiles (12,13). It was recently demonstrated that T3 safely improved heart function and remodeling in experimental models of MI and ischemia–reperfusion injury (14,15). However, it has not been shown whether such a T3 treatment-monitoring protocol can serve as an efficient therapeutic strategy in comparison with existing standard therapies.
The myocardial β-adrenergic signaling pathway plays a key role in the pathogenesis and progression of HF. In early-stage HF, compensatory changes, including activation of the sympathetic nervous system, occur in the cardiovascular system to preserve cardiac output (16,17). Under conditions of increased catecholamine stimulation, β-adrenergic receptors (βARs) are desensitized to prevent overstimulation, which ultimately contributes to attenuated βAR function and decreased cardiac contractility. Cardiac βAR downregulation and desensitization are largely responsible for chronic post-MI decompensation and remodeling. Accordingly, restoration of adrenergic signaling pathway with β-blockers or alternatives preventing further βAR downregulation remains crucial to improving survival and remodeling. It is well established that chronic treatment with β-blockers improves LV contractility and cardiac remodeling and reverses receptor downregulation in failing hearts (18,19). Furthermore, reversal of ventricular remodeling is also associated with favorable changes in expression of key myocardial genes, such as β-myosin heavy chain (MYH7), α-myosin heavy chain (MYH6), and sarco/endoplasmic reticulum calcium-ATPase 2 (SERCA2), all TH-responsive genes (20). The favorable alterations by β-blockers in fetal gene reprogramming of failing hearts resemble the pattern produced by TH administration. A recent study also suggested that gene expression of the stimulatory alpha subunit G-protein, which is activated by the β1AR, may be upregulated toward normal values by low-dose oral T3 therapy following MI in the heart (14). Other previous studies mainly focused on synergistic interactions of both systems on thermogenesis and metabolism, while relationships between β-adrenergic and TH pathways in MI and HF remain poorly understood (21). It was hypothesized that there may be crosstalk between β-adrenergic and thyroid signaling pathways on preserving heart function in a rat model of MI.
This study focused on a direct comparison between therapeutic physiologic doses of T3 and metoprolol (Met), a standard β1-blocker, on pathophysiology and TH-adrenergic signaling in a rat model of MI. This is the first report to show that T3 is comparable to Met in improving left ventricular (LV) function and attenuating atrial tachyarrhythmia (ATA) susceptibility, which was accompanied by alterations in expression of genes involved in TH and adrenergic signaling, inflammation, oxidative stress, ion channels, and contractile proteins.
Materials and Methods
Animals and study design
Adult female Sprague–Dawley rats aged 12 weeks (200–224 g; Envigo, Indianapolis, IN) were subjected to left anterior descending (LAD) coronary artery ligation, as previously published (14). The sham surgery group (n = 8) was similarly treated, except the LAD artery was not occluded. Immediately following MI surgery, survivors were randomly assigned to the following groups: MI + vehicle (Veh; n = 11), MI + T3 (n = 11), and MI + Met (n = 11). T3 was dissolved in ethanol/glycerol and provided in drinking water at a dose of 5 μg/kg/day for eight weeks based on a method previously described (14). Met was provided in drinking water at a dose of 100 mg/kg/day based on previously published studies (18). The efficacy of the selected dose and delivery method was confirmed by preliminary experiments in our laboratory. Sham and MI + Veh rats received ethanol/glycerol formulation in drinking water equivalent to that in the treatment groups. The rats were housed up to three per cage in constant humidity and temperature, and kept on a 12 h light/dark cycle with food and water available ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee at New York Institute of Technology College of Osteopathic Medicine and were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Echocardiographic measurements
At the end of the eight-week treatment period, echocardiography was performed using a GE Vivid 7 Dimension System (GE Vingmed Ultrasound, Horten, Norway) coupled with a M12 L linear (Matrix) array ultrasound transducer probe (5–13 MHz). The rats were anesthetized with isoflurane (1.5%), and a parasternal short-axis view was obtained in B-mode and recorded in M-mode, as previously described (22). Myocardial wall movement was traced over three to five cardiac cycles in the M-mode echocardiogram to determine LV wall thickness and dimensions in systole and diastole. The following parameters were measured: LV fractional shortening (LVFS), anterior and posterior wall thickness in end-diastole and -systole, and LV diastolic and systolic internal diameters. Left atrial diameter was determined at the aortic valve level in long axis view.
Cardiac hemodynamic measurements
After echocardiography measurements, right carotid artery catheterization was performed using a Scisense pressure-volume catheter (Transonic Scisense, London, Canada) on rats under isoflurane anesthesia, and data were recorded over 15–20 minutes with maintenance of 1.5% isoflurane in oxygen. The following data were recorded: heart rate (HR), LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), positive change in LV pressure over time (LV +dp/dt), negative change in LV pressure over time (LV −dp/dt), and Tau.
Electrophysiology study and ATA inducibility test
After hemodynamic measurements were recorded, electrophysiology tests were performed, as previously described (22). Briefly, a 1.6F octopolar Millar electrophysiology catheter (EPR-802; Millar Instruments, Inc., Houston, TX) was inserted through the right jugular vein and advanced into the right atrium. The catheter carries eight poles with three pairs of electrodes to record atrial electrocardiograms (ECG) and one pair for pacing. Standard surface ECG lead II and three right atrial ECG were recorded using a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO). Regular pacing and standard S1S2 pacing protocols were consistently used to determine the sinus node recovery time and atrial effective refractory period. Burst pacing containing 200 impulses at 50 Hz was used to induce ATA. Each rat received burst pacing 10 times, and the duration of subsequent spontaneous ATA was recorded. ATA was defined as irregular rapid atrial arrhythmia with varying electrographic morphology lasting ≥0.5 seconds, as previously reported (22). The arrhythmia duration in each animal was based on average of 10 burst pacings.
Serum TH measurements
Following functional measurements on the closed chest animals, a left thoracotomy exposed the heart and blood was collected from the right ventricle and left to clot at room temperature for 30 minutes and then centrifuged at 600 g for 15 minutes at 4°C. The serum was immediately aliquoted and stored at −80°C until assayed for free T3 (fT3), total T3 (TT3), free thyroxine (fT4), total T4 (TT4), and thyrotropin (TSH) using enzyme-linked immunosorbent assay (ELISA) kits (TSH: ALPCO, Salem, NH; fT3, TT3, fT4, TT4: Monobind, Inc., Lake Forest, CA) according to the manufacturers' instructions.
Tissue collection and Masson's trichrome staining
After the heart was isolated, the aorta was clamped, and 0.2 M of KCl was injected slowly into the LV through the apex. Hearts were then immersed in ice-cold phosphate-buffered saline, trimmed of vessels and adipose tissue, and the weights of the heart and LV were recorded. Basal and apical sections were stored at −80°C and used for real time quantitative polymerase chain reaction (qPCR) analysis. A transverse mid-section of LV was preserved in 10% formalin for subsequent sectioning and staining with Masson's Trichrome stain. Images were acquired using an Olympus DP72 microscope. Infarct area and infarct length were determined histologically from a transverse slice at approximately the midpoint of the base-apex axis (e.g., middle of the infarct). Infarct area is the traced area of the infarct from this slice. This provided a consistent estimation of infarct size while also providing tissues for biochemical analyses from adjacent apical and basal transverse slices. Transmural infarct tissue area and fibrosis content were quantified using ImageJ and Image-Pro plus software (Media Cyberetics, Bethesda, MD).
Vascular reactivity
Thoracic aortas were isolated and cleaned of perivascular adipose and connective tissue in oxygenated Krebs solution (130 mM of NaCl, 14.9 mM of NaHCO3, 4.7 mM of KCl, 1.18 mM of KH2PO4, 1.17 mM of MgSO4-7H2O, 1.56 mM of CaCl2-2H2O, 0.026 mM of EDTA, 5.5 mM of glucose; pH 7.4), and cut into segments 2 mm in length. The aortic rings were mounted on a wire myograph (Model 620M; DMT, Copenhagen, Denmark) and placed in a myograph chamber filled with 5 mL of Krebs buffer, maintained at 37°C, and continuously aerated with 95% O2-5% CO2. Changes in force generation were documented using a PowerLab 8/35 data acquisition system (ADInstruments, Colorado Springs, CO). In all experiments, integrity of aortas was assessed by stimulation with 120 mM of KCl. To test for the presence of endothelium, segments were contracted with 1 μM of phenylephrine (PE) from Fluka® (Sigma–Aldrich, St. Louis, MO). Once the vessels reached a stable maximum tension, the vessels were stimulated with 10 μM of ACh from Sigma–Aldrich, and relaxation was confirmed (>80%). After a 20-minute washout period, the aortic rings were precontracted with 1 μM of PE until a stable contraction was reached, followed by a cumulative concentration–response curve of ACh from 1 nM to 10 μM.
Real-time qPCR for gene expression
Total RNA was isolated from left ventricles using TRIzol reagent followed by a RNA purification kit (cat. no. 12183018A; Thermo Fisher Scientific, Pittsburgh, PA) and DNase kit (Qiagen, Valencia, CA). RNA concentration was measured using Nanodrop 1000 (Thermo Fisher Scientific). RNA (1 μg) was reverse transcribed to cDNA using a RT2 First Strand Kit (Qiagen), and then amplified by PCR using a 96-well custom-designed primer plate for more than 90 genes using a SYBR Green Kit (Qiagen) and ABI StepOnePlus. mRNAs were normalized to the expression of housekeeping genes, Ppia (cyclophilin A) and Rplp1 (ribosomal protein, large, P1). Data analysis used the ΔΔCt method according to the SABioscience expression analysis online software (Qiagen). Results for each gene are expressed as 2–ΔΔCt values relative to the mean value of the sham group. Analysis used RNA isolated from six animals per group.
Statistical analysis
Continuous data are expressed as means ± standard deviation and were compared using one-way analysis of variance with Bonferroni correction for multiple comparisons. The incidence of ATA was compared using Fisher's exact test. The ATA duration data are expressed as medians (Q1, Q3) and were compared using a nonparametric Kruskal–Wallis test followed by Dunn's multiple comparison test. The myograph aortic vessel relaxation responses are expressed as a percentage relative to maximal contractile response to 1 μM of PE. The maximal effect elicited by ACh (Emax) and the sensitivity to ACh (pD2) were measured to assess aortic relaxation responsiveness to ACh. GraphPad Prism v7.0 statistical software (GraphPad Software, Inc., San Diego, CA) was used to analyze the data. Significance was accepted at p < 0.05.
Results
Morphometric changes
As presented in Table 1, body weights were similar among the four groups. In MI + Veh, MI + T3, and MI + Met groups, heart weight, LV weight, and their ratios with respect to body weight were significantly increased compared to the sham group, indicating hypertrophy after MI.
Table 1.
Morphometric Data
| Sham | MI + Veh | MI + T3 | MI + Met | |
|---|---|---|---|---|
| BW (g) | 266 ± 22 | 288 ± 20 | 285 ± 20 | 270 ± 13 |
| HW (mg) | 881 ± 93 | 1191 ± 201* | 1345 ± 173* | 1228 ± 165* |
| LVW (mg) | 609 ± 67 | 771 ± 79* | 848 ± 117* | 755 ± 80* |
| HW/BW (mg/g) | 3.3 ± 0.1 | 4.1 ± 0.5* | 4.7 ± 0.4* | 4.6 ± 0.7* |
| LVW/BW (mg/g) | 2.3 ± 0.1 | 2.7 ± 0.2* | 3.0 ± 0.3* | 2.7 ± 0.3* |
Values are expressed as means ± standard deviation.
p < 0.05 vs. sham; n = 8 for sham and n = 11 for the other three groups.
MI, myocardial infarction; Veh, vehicle; T3, triiodothyronine; Met, metoprolol; BW, body weight; HW, heart weight; LVW, left ventricle weight.
Serum TH levels
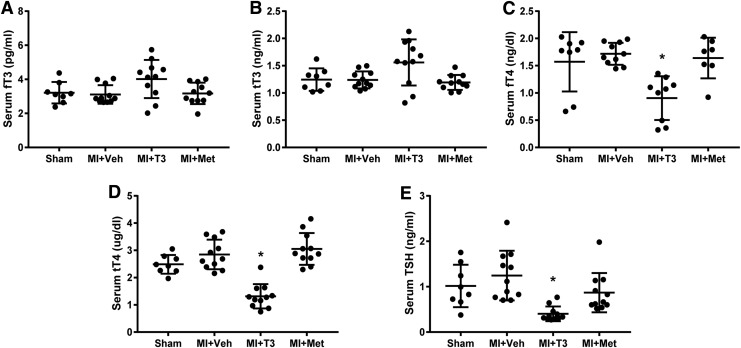
Serum THs are shown in Figure 1. Following T3 treatment, serum fT3 and TT3 levels (Fig. 1A and B) were not significantly increased above normal. It has been shown in multiple animal models of HF that the administered dose of T3 preserves cardiac tissue T3 levels while maintaining serum THs within the reference range (9,23,24). As expected, fT4, TT4, and TSH levels (Fig. 1C–E) were slightly yet significantly decreased in MI + T3 rats, indicating a mild feedback inhibition response to the hypothalamic–pituitary–thyroid axis. These data confirmed a physiological effect of the treatment with T3.
FIG. 1.
Serum thyroid hormone levels. (A) free triiodothyronine (fT3); (B) total triiodothyronine (TT3); (C) free thyroxine (fT4); (D) total thyroxine (TT4); (E) thyrotropin (TSH). *p < 0.05 vs. sham, MI + Veh and MI + Met; n = 8 for sham and n = 11 for the other three groups. MI, myocardial infarction; Veh, vehicle; Met, metoprolol.
T3 and Met improved LV morphological and functional changes
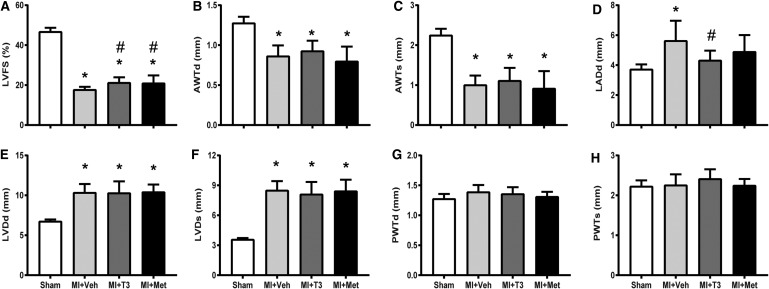
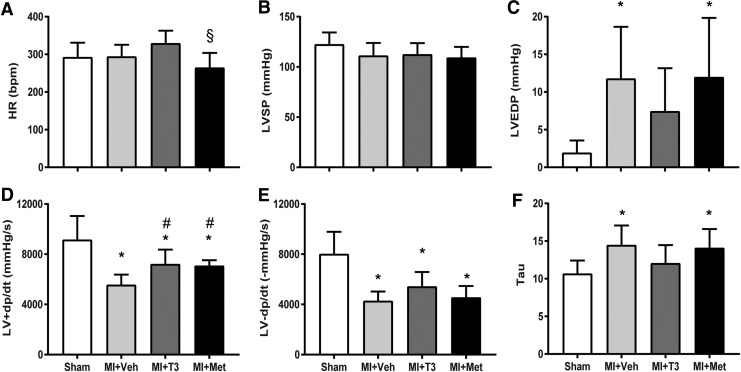
There was a significant decrease in LVFS and anterior wall thickness and an increase in LV dimension after MI. T3 and Met treatments both markedly increased LVFS, without affecting LV dimension and wall thickness (Fig. 2A–H). Following MI, LA diameter was significantly enlarged in MI and was prevented by T3, although it was not significantly different between MI + Veh and MI + Met groups (Fig. 2D). HR was not significantly increased in T3-treated rats compared to shams. However, a slight increase in the T3-treated group coupled with a slight reduction in Met resulted in a significant difference between the two treatment groups (Fig. 3A). MI resulted in a significant reduction in LV +dp/dt and −dp/dt, and an increase in LVEDP and Tau, without affecting LV systolic pressure (Fig. 3B–F). T3 and Met treatments significantly improved LV +dp/dt and tended to increase LV −dp/dt compared to the MI + Veh group. LVEDP and Tau were decreased by T3 but unaffected by Met treatment.
FIG. 2.
Echocardiographic parameters. Values are expressed as means ± standard deviation (SD). (A) Left ventricular fractional shortening (LVFS); (B) left ventricular anterior wall thickness in diastole (AWTd); (C) left ventricular anterior wall thickness in systole (AWTs); (D) left atrial diameter in diastole (LADd); (E) left ventricular diameter in diastole (LVDd); (F) left ventricular diameter in systole (LVDs); (G) left ventricular posterior wall thickness in diastole (PWTd); (H) left ventricular posterior wall thickness in systole (PWTs). *p < 0.05 vs. sham; #p < 0.05 vs. MI + Veh; n = 8 for sham and n = 11 for the other three groups.
FIG. 3.
Left ventricular hemodynamics. Values are presented as means ± SD. (A) Heart rate (HR); (B) left ventricular systolic pressure (LVSP); (C) left ventricular end-diastolic pressure (LVEDP); (D) positive change in pressure over time (+dP/dt); (E) negative change in pressure over time (–dP/dt); (F) Tau. *p < 0.05 vs. sham; #p < 0.05 vs. MI + Veh; §p < 0.05 vs. MI + T3; n = 8 for sham and n = 11 for the other three groups.
T3 and Met reduced ATA inducibility
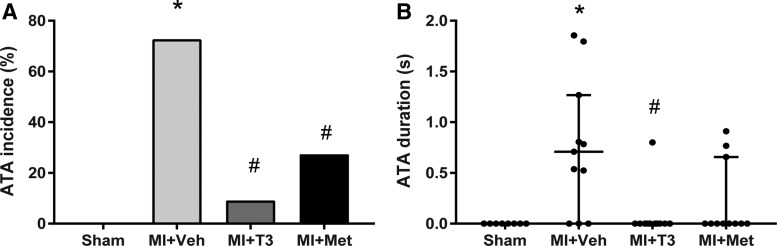
There was no ATA induction in sham rats. ATA inducibility was significantly increased in MI + Veh rats (p < 0.05) and was significantly attenuated by both T3 (p < 0.05) and Met (p < 0.05; Fig. 4A). Following MI, induced ATA duration was also increased. The duration was significantly decreased with T3 treatment (p < 0.05) but not affected by Met treatment (p = 0.1; Fig. 4B).
FIG. 4.
Atrial tachyarrhythmias (ATA) inducibility. ATA incidence (A) and duration (B) in the studied groups are shown. ATA duration data are presented as median (Q1, Q3). *p < 0.05 vs. sham; #p < 0.05 vs. MI + Veh; n = 8 for sham and n = 11 for the other three groups.
Changes in infarct parameters and left atrial fibrosis
Compared to MI + Veh hearts, histological analysis indicated a significant reduction of 19% in the infarct area of T3-treated MI hearts (Table 2). A comparable reduction was noted in the Met treated MI hearts. The MI + T3 group was associated with a significant reduction in infarct length and increased viable tissue area within the infarct segment. As shown in Table 2, T3 treatment significantly decreased left atrial interstitial fibrosis by 25.2% compared with the vehicle-treated MI groups.
Table 2.
Changes in Infarct Parameters and Left Atrial Fibrosis
| Sham | MI + Veh | MI + T3 | MI + Met | |
|---|---|---|---|---|
| Infarct area (mm2) | N/A | 10.33 ± 0.51 | 8.38 ± 0.69# | 8.06 ± 0.85# |
| Infarct length (mm) | N/A | 9.24 ± 0.41 | 7.81 ± 0.46# | 7.58 ± 0.56# |
| Percent viable area within infarct zone (%) | N/A | 21.51 ± 2.69 | 23.90 ± 3.94# | 20.46 ± 1.40 |
| Left atrial fibrosis (%) | 3.35 ± 0.23 | 8.16 ± 0.71 | 6.10 ± 0.55*,# | 6.57 ± 0.64* |
Values are expressed as means ± standard error of the mean.
p < 0.05 vs. sham; #p < 0.05 vs. MI + Veh; n = 8 for sham and n = 11 for the other three groups.
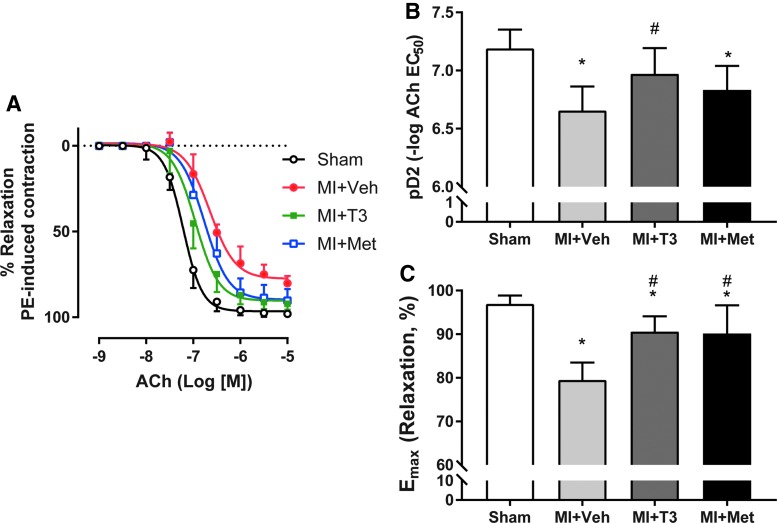
T3 ameliorated MI-induced endothelial dysfunction
Aortas from MI + Veh rats had severe impairment in endothelium-dependent relaxation in response to ACh compared to sham, with a rightward shift of the ACh dose–response curve (Fig. 5A). Aortas from the MI + Veh group exhibited less sensitivity to ACh (pD2: 6.66 ± 0.21) in comparison to aortas from the sham group (pD2: 7.19 ± 0.16; p < 0.05; Fig. 5B). Furthermore, the maximal effect of ACh (Emax) was significantly lower in MI + Veh rats (79.5 ± 4.0%) in comparison to the sham group (96.9 ± 1.9%; p < 0.05; Fig. 5C). This effect was reversed by T3 (90.6 ± 3.5%) and Met treatment (90.1 ± 6.5%) versus vehicle treatment (79.5 ± 4.0%; Fig. 5C). Importantly, ACh-induced endothelium-dependent vasorelaxation was significantly potentiated by T3 (6.97 ± 0.22; p < 0.05) but not by Met (6.83 ± 0.21; p > 0.05) treatment compared to the MI + Veh rats. pD2 was normalized by T3 treatment, with no significant difference between MI + T3 and sham groups.
FIG. 5.
Effect of T3 and Met administration in MI-induced impaired endothelium-dependent relaxation. (A) Cumulative concentration–response curves to ACh for the sham, MI + Veh, MI + T3, and MI + Met groups. Values of pD2 (B) and Emax (C) are shown. *p < 0.05 vs. sham; #p < 0.05 vs. MI + Veh; n = 8 for sham and n = 9 for the other three groups.
T3 and Met attenuated inflammation and oxidative stress, regulated cardiac genes, and altered genes in thyroid–adrenergic signaling pathways
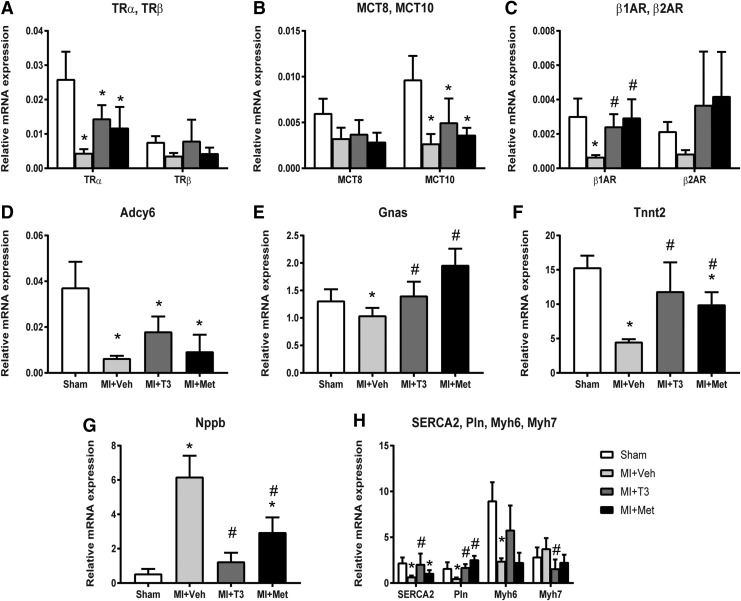
As shown in Figure 6A–H, the expression of thyroid hormone receptor alpha (TRα) was significantly decreased in the MI + Veh group, whereas T3 (p = 0.08) and Met (p = 0.33) treatments had a tendency to increase its expression compared to vehicle-treated rats. This trend in expression was similar for thyroid hormone receptor beta (TRβ), although statistical significance was not obtained. The primary membrane transporter of T3, monocarboxylic acid transporter 10 (MCT10), was decreased in MI and increased 1.85-fold with T3 treatment, although this did not reach significance. β1AR was significantly decreased in failing hearts and increased with T3 and Met treatments. β2AR was downregulated following MI and upregulated by both T3 and Met treatments, although this was not statistically significant. In addition, adenylate cyclase 6 (Adcy6) and stimulatory G-protein α (Gnas) expression were downregulated in MI + Veh rats. T3 and Met treatments increased the expression of Adcy6 2.82-fold and 1.46-fold, respectively, but these changes did not reach statistical significance. T3 and Met treatments significantly increased expression of Gnas to levels comparable with sham rats. Cardiac troponin T (Tnnt2) was significantly decreased in MI + Veh rats compared to sham rats and was normalized by T3 and Met treatments. Natriuretic peptide precursor B (Nppb) was increased in MI + Veh and significantly decreased in MI + T3 and MI + Met rats. SERCA2 and phospholamban (Pln) were downregulated in failing hearts and upregulated to sham values by T3 treatment. Met significantly increased Pln but had no effect on SERCA2. The reduction of Myh6 following MI was prevented by T3 treatment, as it was 2.25-fold higher than MI + Veh rats. The increase (1.33-fold) in Myh7 in MI + Veh rats was reduced 0.38-fold with T3 treatment. Met was without effect on Myh6 or Myh7 expression.
FIG. 6.
Expression of thyroid hormone signaling, adrenergic signaling, and cardiac genes. Gene expression was normalized using cyclophilin A and Rlpl 1. (A) Thyroid hormone receptor α (Trα), thyroid hormone receptor β (TRβ); (B) monocarboxylate transporter (MCT); (C) adrenergic receptors (AR); (D) adenylate cyclase 6 (Adcy6); (E) G-protein stimulatory α subunit (Gnas); (F) troponin T type 2 (Tnnt2); (G) natriuretic peptide precursor B (Nppb); (H) sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 (SERCA2), phospholamban (Pln); myosin heavy chain α isoform (Myh6), myosin heavy chain β isoform (Myh7). *p < 0.05 vs. sham; #p < 0.05 vs. MI + Veh; n = 6 per group.
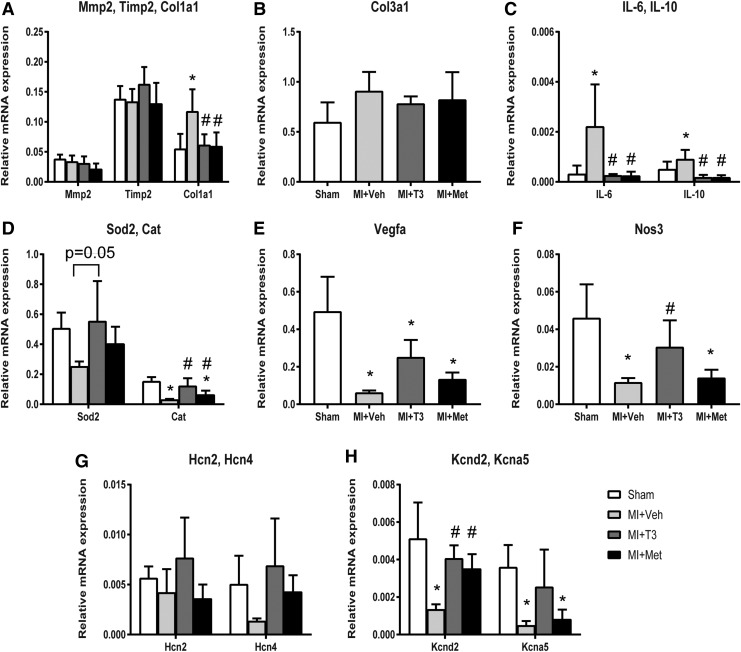
T3 and Met significantly reduced the expression of collagen type I (Col1a1; Fig. 7). Tissue inhibitor of metalloproteinase 2 (Timp2) was upregulated, and Collagen type III alpha 1 (Col3a1) was downregulated, although statistical significance was not reached following T3 and Met treatments. The increases in inflammatory markers, interleukin-6 (IL-6) and IL-10, following MI were completely abrogated and prevented by T3 and Met treatments. Catalase was decreased in MI + Veh and rescued by T3 and Met treatments. The reduction in superoxide dismutase 2 (Sod2) was prevented by T3 treatment (p = 0.05). T3 restored the expression of nitric oxide synthase 3 (Nos3) and increased vascular endothelial growth factor A (Vegfa) levels 3.98-fold (p = 0.08). Both T3 and Met significantly increased voltage-gated potassium channel Kv4.2 (Kcnd2), which was depressed in MI + Veh hearts. While T3 partially returned voltage-gated potassium channel Kv1.5 (Kcna5) expression to almost that of sham levels, Met did not.
FIG. 7.
Expression of extracellular matrix pathway, inflammation, oxidative stress, and ion channel genes. Gene expression was normalized using cyclophilin A and Rlpl 1. (A) Matrix metallopeptidase 2 (Mmp2), tissue inhibitor of metalloproteinase 2 (Timp2); (B) collagen type I alpha 1 (Col1a1), collagen type III, alpha 1 (Col3a1); (C) interleukin 6 (IL-6), interleukin 10 (IL-10); (D) superoxide dismutase 2 (Sod2), catalase (Cat); (E) vascular endothelial growth factor A (Vegfa); (F) nitric oxide synthase 3 (Nos3); (G) hyperpolarization activated cyclic nucleotide–gated channel (Hcn); (H) potassium voltage-gated channel, Shal-related subfamily, member 2 (Kcnd2), potassium voltage-gated channel, shaker-related subfamily, member 5 (Kcna5). *p < 0.05 vs. sham; #p < 0.05 vs. MI + Veh; n = 6 per group.
Discussion
Recent studies suggest a growing need to reexamine THs as a potential therapy for cardiovascular disorders (9,13–15,24,25). However, THs have not been experimentally tested in comparison with existing standard therapies. This study directly compared the effects of T3 replacement therapy with Met, a commonly used β-blocker, on myocardial and electrical remodeling, LV function, vascular reactivity, and gene regulation in a rat model of MI. The findings further extended past observations by showing that: (i) treatment with a physiological dose of T3 for eight weeks improved endothelial and heart physiology, with arrhythmia inducibility duration, left atrial diameter/fibrosis, and aortic vasorelaxation responsiveness being statistically different from untreated MI, whereas Met treatment was not; (ii) these treatments were associated with favorable alterations in genes regulating contractile function, thyroid signaling, adrenergic signaling, inflammation, and oxidative stress; and (iii) beneficial crosstalk between cardiac TH and β-adrenergic signaling pathways after MI may be induced by either therapy.
In the present study, a strategy was used to investigate arrhythmias that was identical to that of a previous MI study using a similar physiological T3 dosage regimen (14). The only significant difference is that in this study, the number of burst pacings per animal was doubled for all the groups, which would increase precision and rigor of the experiment. As expected, the current MI + T3 group showed a similar improvement in attenuation of inducible ATA incidence, as observed in the previous study (i.e., 85.2% vs. 87.9%). However, Met offered a 62.5% improvement. Of note, Met did not improve ATA duration significantly, while T3 did in both cases. AF is considered to be the most common cardiac arrhythmia and an independent risk factor for mortality (26). In long-term post-MI follow-up, AF can be observed in as many as 39% of patients (27). However, the incidence of AF in patients with heart disease related to borderline low THs is not clear at present. These results suggest that physiological doses of T3 in low TH conditions do not promote arrhythmias, a finding confirmed recently by the TRUST trial (28). Wandell et al. also showed that women diagnosed with AF who were being treated with T4 had a decreased risk of mortality (29). Previous studies reported that mutations in Kcna5 could enhance AF susceptibility (30). T3 but not Met restored expression of this gene, which may be related to greater reduction in AF inducibility by T3. Taken together, this study expands the possibility of T3 replacement therapy as a potentially valuable approach to prevent or reduce arrhythmias in HF and warrants well-designed clinical trials.
It was previously shown that chronic serum hypothyroidism eventually leads to dilated HF involving myocyte lengthening from series addition of sarcomeres, despite cardiac atrophy from hemodynamic unloading (31). This unique remodeling pattern of increased length:width ratio is specific to dilated HF. Hypothyroidism, like all heart diseases leading to HF, results in re-expression of the fetal gene program of which many genes have been shown to be transcriptionally regulated by T3, thus reflecting cardiac tissue hypothyroidism in HF. Indeed, alterations in serum THs does not necessarily suggest altered thyroid status in the heart (32). In the current study and past related studies on myocardial ischemic injury (14,15,33), T3 (the potent and active form of TH) was not decreased in MI + Veh, although expression of deiodinases, TRs, MCT transporters, midkine, and/or myosin heavy chains were impaired. Experimental evidence revealed that changes in cardiac tissue T3 concentrations in MI and HF were a result of increased deiodinase 3 activity and reduced deiodinase 1 and deiodinase 2 activity (34,35). Additionally, downregulation of TRs also contributes to a lower level of T3 action in cardiac tissue, resulting in tissue hypothyroidism (10,36,37). Furthermore, systemic TH treatment partially or wholly restored these molecular and other physiological defects. These repeated observations strongly indicate the importance of localized TH signaling and availability in the ischemic heart (13,25). A safe and efficacious TH treatment strategy has now been repeatedly demonstrated in this and other rat models of HF, offering optimism for pursuing similar trials in human.
THs regulate β1AR density, with downregulation observed in hypothyroidism and upregulation in hyperthyroidism (38). This mechanism is believed to be largely responsible for changes in HR observed in chronic hypothyroidism and hyperthyroidism. This study confirmed downregulation of β1/β2 receptors in MI and restoration with T3 or Met treatment. Downstream signaling mediators including Adcy6 and Gnas were downregulated in MI, and T3 restored Gnas expression (14). Similar to T3, Met led to partial or complete restoration in TRα, βARs, Gnas, Pln, Nppb, Tnnt 2, Col1a1, IL-6, IL-10, Catalase, and voltage-gated potassium channel Kv4.2 expression. These results suggest extensive crosstalk between cardiac thyroid and cardiac adrenergic signaling systems, which is in accordance with previous reports (39). Physiological improvements significant for some parameters with T3, but not Met, were likely mediated by reversal of fetal genes (SERCA2, Myh6, and Myh7), which were not observed with Met treatment. Human studies showing improvement in TH signaling with β-blocker treatment of HF support the relevancy of the current findings (20). While the present study suggests that low TH function post MI or HF may underlie downregulation of βARs, it is not feasible to investigate this in humans, since β-blockers are standard therapy. However, some patients do not tolerate β-blockers well. The findings suggest that it may be worthwhile to explore the potential therapeutic utility of THs in this patient population in addition to restoration of depressed TH function in HF patients.
Endothelial dysfunction is frequently observed in the evolution and progression of ischemic HF and is associated with poor prognosis (40). This study found that endothelium-dependent vasodilatation is severely impaired in MI + Veh rats and was significantly improved by T3 and Met treatments. Many mechanisms can contribute to the improvement of endothelial function. Restoration of TRα following T3 and Met treatments can increase coronary blood flow and decrease coronary artery resistance (41). Increased activation of the β2AR signaling pathway by T3 was previously shown to modulate vascular reactivity, which may also play a role, since obvious upregulation of β2AR was observed from T3 and Met therapies, though it did not reach significance due to some values with high variation (42). Besides genomic signaling effects, T3 also potentiates vasodilatory effects via the activation of PI3K/Akt signaling and nitric oxide synthase in endothelial and vascular smooth muscle cells (43). Recently, T3-induced rapid vascular relaxation via a PKG/VASP signaling pathway was shown, highlighting non-genomic and direct effect of T3 in the vasculature (44). In the present study, Nos3 and Vegfa (to a lesser extent) were upregulated by T3 treatment. These changes contribute to the amelioration of endothelial dysfunction in MI. Low-grade inflammation post-MI might also contribute to endothelial dysfunction. Inflammation was attenuated by T3 and Met treatments here, as confirmed by a reduction in elevated IL-6 and IL-10 mRNA in MI hearts, thus leading to improved vascular reactivity (45).
As emphasized, serum TH levels may not reflect cardiac tissue T3 content. Normalization of myocardial TH content with TH replacement therapy in different cardiac disease models has been clearly demonstrated (9,23,24). However, it remains unknown whether Met can also affect cardiac tissue T3 levels. Identification of a serum biomarker tracking with cardiac tissue T3 levels would be a major diagnostic advance in the field. Some variation was observed in serum T3 levels with the method of administration of T3 in the drinking water. However, a similar variation is typically observed with other modes of treatment (46,47). The reasons the method of administration of T3 in the drinking water was chose are that the effective performance of T3 is repeatedly seen without producing untoward side effects, the procedure is minimally invasive, similar to human treatment, and can be readily adjusted by changing the T3 concentration in water. Finally, since the focus of the study was to assess the effect of T3 treatment on MI, and not investigation of hyperthyroidism, a T3 + sham group was not included.
Conclusions
Short-term, therapeutic treatment of T3 in HF patients has been well-tolerated and efficacious (48,49). However, long-term studies are lacking. The findings from this animal study support growing evidence that long-term T3 therapy in HF patients may confer comparable benefits with those of current standard therapy. These data suggest that improvements in β-adrenergic signaling offered by β-blockade may also result from TH treatment.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Bethesda, MD) under Award Number R01HL103671 (AG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledged the National Natural Science Foundation of China (No. 81470485), Capital Clinical Featured Application Research Project (No. z151100004015175), and CAMS Innovation Fund for Medical Sciences (CIFMS 2016-I2M-1-009).
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. 2017. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein I, Ojamaa K. 2001. Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 [DOI] [PubMed] [Google Scholar]
- 3.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L'Abbate A, Donato L. 2003. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation 107:708–713 [DOI] [PubMed] [Google Scholar]
- 4.Biondi B, Cooper DS. 2008. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- 5.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. 2006. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. 2005. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 165:2467–2472 [DOI] [PubMed] [Google Scholar]
- 7.Iervasi G, Molinaro S, Landi P, Taddei MC, Galli E, Mariani F, L'Abbate A, Pingitore A. 2007. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med 167:1526–1532 [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Guan H, Gerdes AM, Iervasi G, Yang Y, Tang YD. 2015. Thyroid status, cardiac function, and mortality in patients with idiopathic dilated cardiomyopathy. J Clin Endocrinol Metab 100:3210–3218 [DOI] [PubMed] [Google Scholar]
- 9.Weltman NY, Ojamaa K, Schlenker EH, Chen YF, Zucchi R, Saba A, Colligiani D, Rajagopalan V, Pol CJ, Gerdes AM. 2014. Low-dose T(3) replacement restores depressed cardiac T(3) levels, preserves coronary microvasculature and attenuates cardiac dysfunction in experimental diabetes mellitus. Mol Med 20:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantos C, Mourouzis I, Galanopoulos G, Gavra M, Perimenis P, Spanou D, Cokkinos DV. 2010. Thyroid hormone receptor alpha1 downregulation in postischemic heart failure progression: the potential role of tissue hypothyroidism. Horm Metab Res 42:718–724 [DOI] [PubMed] [Google Scholar]
- 11.Kinugawa K, Minobe WA, Wood WM, Ridgway EC, Baxter JD, Ribeiro RC, Tawadrous MF, Lowes BA, Long CS, Bristow MR. 2001. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation 103:1089–1094 [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, Liu L, Wang F, Yuan Z, Zhang X, Xu C, Song Y, Guan Q, Gao L, Shan Z, Zhang H, Zhao J. 2016. A worthy finding: decrease in total cholesterol and low-density lipoprotein cholesterol in treated mild subclinical hypothyroidism. Thyroid 26:1019–1029 [DOI] [PubMed] [Google Scholar]
- 13.Gerdes AM, Ojamaa K. 2016. Thyroid hormone and cardioprotection. Compr Physiol 6:1199–1219 [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan V, Zhang Y, Ojamaa K, Chen YF, Pingitore A, Pol CJ, Saunders D, Balasubramanian K, Towner RA, Gerdes AM. 2016. Safe oral triiodo-L-thyronine therapy protects from post-infarct cardiac dysfunction and arrhythmias without cardiovascular adverse effects. PLoS One 11:e0151413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopalan V, Zhang Y, Pol C, Costello C, Seitter S, Lehto A, Savinova OV, Chen YF, Gerdes AM. 2017. Modified low-dose triiodo-L-thyronine therapy safely improves function following myocardial ischemia–reperfusion injury. Front Physiol 8:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. 1986. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73:913–919 [DOI] [PubMed] [Google Scholar]
- 17.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. 2009. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762 [DOI] [PubMed] [Google Scholar]
- 18.Rinaldi B, Donniacuo M, Sodano L, Gritti G, Martuscelli E, Orlandi A, Rafaniello C, Rossi F, Calzetta L, Capuano A, Matera MG. 2015. Effects of chronic treatment with the new ultra-long-acting beta2-adrenoceptor agonist indacaterol alone or in combination with the beta1-adrenoceptor blocker metoprolol on cardiac remodelling. Br J Pharmacol 172:3627–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bristow MR. 2011. Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res 109:1176–1194 [DOI] [PubMed] [Google Scholar]
- 20.Kao DP, Lowes BD, Gilbert EM, Minobe W, Epperson LE, Meyer LK, Ferguson DA, Volkman AK, Zolty R, Borg CD, Quaife RA, Bristow MR. 2015. Therapeutic molecular phenotype of beta-blocker-associated reverse-remodeling in nonischemic dilated cardiomyopathy. Circ Cardiovasc Genet 8:270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva JE, Bianco SD. 2008. Thyroid–adrenergic interactions: physiological and clinical implications. Thyroid 18:157–165 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Dedkov EI, Lee B, 3rd, Li Y, Pun K, Gerdes AM. 2014. Thyroid hormone replacement therapy attenuates atrial remodeling and reduces atrial fibrillation inducibility in a rat myocardial infarction-heart failure model. J Card Fail 20:1012–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weltman NY, Ojamaa K, Savinova OV, Chen YF, Schlenker EH, Zucchi R, Saba A, Colligiani D, Pol CJ, Gerdes AM. 2013. Restoration of cardiac tissue thyroid hormone status in experimental hypothyroidism: a dose–response study in female rats. Endocrinology 154:2542–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weltman NY, Pol CJ, Zhang Y, Wang Y, Koder A, Raza S, Zucchi R, Saba A, Colligiani D, Gerdes AM. 2015. Long-term physiological T3 supplementation in hypertensive heart disease in rats. Am J Physiol Heart Circ Physiol 309:H1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. 2017. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 14:39–55 [DOI] [PubMed] [Google Scholar]
- 26.Andersson T, Magnuson A, Bryngelsson IL, Frobert O, Henriksson KM, Edvardsson N, Poci D. 2013. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case-control study. Eur Heart J 34:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jons C, Jacobsen UG, Joergensen RM, Olsen NT, Dixen U, Johannessen A, Huikuri H, Messier M, McNitt S, Thomsen PE. 2011. The incidence and prognostic significance of new-onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: a CARISMA substudy. Heart Rhythm 8:342–348 [DOI] [PubMed] [Google Scholar]
- 28.Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, Sattar N, Aubert CE, Aujesky D, Bauer DC, Baumgartner C, Blum MR, Browne JP, Byrne S, Collet TH, Dekkers OM, den Elzen WPJ, Du Puy RS, Ellis G, Feller M, Floriani C, Hendry K, Hurley C, Jukema JW, Kean S, Kelly M, Krebs D, Langhorne P, McCarthy G, McCarthy V, McConnachie A, McDade M, Messow M, O'Flynn A, O'Riordan D, Poortvliet RKE, Quinn TJ, Russell A, Sinnott C, Smit JWA, Van Dorland HA, Walsh KA, Walsh EK, Watt T, Wilson R, Gussekloo J. 2017. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 376:2534–2544 [DOI] [PubMed] [Google Scholar]
- 29.Wandell P, Carlsson AC, Holzmann MJ, Arnlov J, Sundquist J, Sundquist K. 2017. Comparison of mortality and nonfatal cardiovascular events in adults with atrial fibrillation with versus without levothyroxine treatment. Am J Cardiol 120:1974–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christophersen IE, Olesen MS, Liang B, Andersen MN, Larsen AP, Nielsen JB, Haunso S, Olesen SP, Tveit A, Svendsen JH, Schmitt N. 2013. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J 34:1517–1525 [DOI] [PubMed] [Google Scholar]
- 31.Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. 2005. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation 112:3122–3130 [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Redetzke RA, Said S, Pottala JV, de Escobar GM, Gerdes AM. 2008. Serum thyroid hormone levels may not accurately reflect thyroid tissue levels and cardiac function in mild hypothyroidism. Am J Physiol Heart Circ Physiol 294:H2137–2143 [DOI] [PubMed] [Google Scholar]
- 33.Chen YF, Pottala JV, Weltman NY, Ge X, Savinova OV, Gerdes AM. 2012. Regulation of gene expression with thyroid hormone in rats with myocardial infarction. PLoS One 7:e40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wassner AJ, Jugo RH, Dorfman DM, Padera RF, Maynard MA, Zavacki AM, Jay PY, Huang SA. 2017. Myocardial induction of type 3 deiodinase in dilated cardiomyopathy. Thyroid 27:732–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Berghe G. 2014. Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid 24:1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mourouzis I, Kostakou E, Galanopoulos G, Mantzouratou P, Pantos C. 2013. Inhibition of thyroid hormone receptor alpha1 impairs post-ischemic cardiac performance after myocardial infarction in mice. Mol Cell Biochem 379:97–105 [DOI] [PubMed] [Google Scholar]
- 37.Adamopoulos S, Gouziouta A, Mantzouratou P, Laoutaris ID, Dritsas A, Cokkinos DV, Mourouzis I, Sfyrakis P, Iervasi G, Pantos C. 2013. Thyroid hormone signalling is altered in response to physical training in patients with end-stage heart failure and mechanical assist devices: potential physiological consequences? Interact Cardiovasc Thorac Surg 17:664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilezikian JP, Loeb JN. 1983. The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev 4:378–388 [DOI] [PubMed] [Google Scholar]
- 39.Pantos C, Xinaris C, Mourouzis I, Perimenis P, Politi E, Spanou D, Cokkinos DV. 2008. Thyroid hormone receptor alpha 1: a switch to cardiac cell “metamorphosis”? J Physiol Pharmacol 59:253–269 [PubMed] [Google Scholar]
- 40.Shantsila E, Wrigley BJ, Blann AD, Gill PS, Lip GY. 2012. A contemporary view on endothelial function in heart failure. Eur J Heart Fail 14:873–881 [DOI] [PubMed] [Google Scholar]
- 41.Suarez J, Wang H, Scott BT, Ling H, Makino A, Swanson E, Brown JH, Suarez JA, Feinstein S, Diaz-Juarez J, Dillmann WH. 2014. In vivo selective expression of thyroid hormone receptor alpha1 in endothelial cells attenuates myocardial injury in experimental myocardial infarction in mice. Am J Physiol Regul Integr Comp Physiol 307:R340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pappas M, Mourouzis K, Karageorgiou H, Tesseromatis C, Mourouzis I, Kostopanagiotou G, Pantos C, Cokkinos DV. 2009. Thyroid hormone modulates the responsiveness of rat aorta to alpha1-adrenergic stimulation: an effect due to increased activation of beta2-adrenergic signaling. Int Angiol 28:474–478 [PubMed] [Google Scholar]
- 43.Carrillo-Sepulveda MA, Ceravolo GS, Fortes ZB, Carvalho MH, Tostes RC, Laurindo FR, Webb RC, Barreto-Chaves ML. 2010. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc Res 85:560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel S, Zhang K, Tang YD, Gerdes AM, Carrillo-Sepulveda MA. 2017. Triiodothyronine potentiates vasorelaxation via PKG/VASP signaling in vascular smooth muscle cells. Cell Physiol Biochem 41:1894–1904 [DOI] [PubMed] [Google Scholar]
- 45.Turemen EE, Cetinarslan B, Sahin T, Canturk Z, Tarkun I. 2011. Endothelial dysfunction and low grade chronic inflammation in subclinical hypothyroidism due to autoimmune thyroiditis. Endocr J 58:349–354 [DOI] [PubMed] [Google Scholar]
- 46.Chen YF, Weltman NY, Li X, Youmans S, Krause D, Gerdes AM. 2013. Improvement of left ventricular remodeling after myocardial infarction with eight weeks L-thyroxine treatment in rats. J Transl Med 11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henderson KK, Danzi S, Paul JT, Leya G, Klein I, Samarel AM. 2009. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction-induced congestive heart failure. Circ Heart Fail 2:243–252 [DOI] [PubMed] [Google Scholar]
- 48.Pingitore A, Galli E, Barison A, Iervasi A, Scarlattini M, Nucci D, L'Abbate A, Mariotti R, Iervasi G. 2008. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab 93:1351–1358 [DOI] [PubMed] [Google Scholar]
- 49.Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, Chopra IJ, Moriguchi JD, Hage A. 1998. Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. Am J Cardiol 81:443–447 [DOI] [PubMed] [Google Scholar]