Current Anti-caries Approaches
Dental caries is one of the most prevalent bacteria-related infectious diseases worldwide (1,2). Though not life-threatening, it remains a “silent epidemic” and results in a financial burden that leaves many cases untreated in under-privileged socioeconomic regions and countries, eventually resulting in tooth extraction as the last remedy (3,4). Therefore, several measures have been developed for preventing caries, and one of the most effective is the use of sodium fluoride toothpaste and/or rinse. This approach has a well-documented clinical efficacy as it inhibits the activity of cariogenic bacteria, besides the remineralizing capacity and its recovery of demineralized enamel (5,6,7). Previous studies also show that silver diamine fluoride (SDF) treatment is highly efficient in both preventing and arresting dentine caries (8). The treatment procedure is simple, cheap (i.e. it requires no expensive equipment), and non-invasive and thus the risk of spreading infection is low. However, SDF is not a complete solution to caries risk as single application has been reported to be insufficient for sustained benefit (9). Its downsides include a reportedly unpleasant metallic taste, potential to irritate gingival and mucosal surfaces, and the characteristic black staining of the tooth surfaces to which it is applied (8,9).
Cariogenic bacteria have the capacity to consume carbohydrates at a rapid rate, resulting in the accumulation of organic acid in a short period of time (10). This process leads to a dramatic drop in plaque pH, causing the inactivation of both health-associated community members and enamel remineralization processes (11,12). As the pH drops below the demineralization threshold (5.5–5.7) cariogenic bacteria thrive, and the solubilization process of the tooth mineral becomes rapid and irreversible. Despite fluoride being able to prevent plaque formation, it has limited impact on directly killing and extinguishing cariogenic bacteria residing in dental plaque. This is a major explanation to why caries persists in many communities and remains a global health problem. The improved understanding of oral microbial ecology, especially the importance of the balance between cariogenic and commensal residents, has highlighted the fundamental need to develop novel measures to selectively inhibit cariogenic species and modulate the microbial composition of dental plaque for caries control.
Other approaches to reduce caries include the neutralization of plaque pH with sodium bicarbonate (13), abstinence from dietary sugars or substitution with sugar analogues (14), and self-performed mechanical removal of the dental plaque using both tooth brush and interdental floss. The effect of these approaches, however, is unsustainable and requires repeated application or change of dietary habit for sustained effects. Aseptic mouth rinses and indiscriminant topical antibiotics (6,7,15) are also adopted to reduce the total bacterial load in the oral cavity. Though temporarily effective to various degrees in reducing caries incidence, the nonselective interventions often lead to severe antibiotic-associated infections due to the vacated niche available for cariogenic species re-infection (16).
Cariogenic Traits of Streptococcus mutans and its Prevention
According to the “Ecological Plaque Hypothesis” stated by Marsh (17), the microbial homeostasis within dental plaque is suggested to shift when the oral environment changes, such as the uptake of fermentable sugars. Continued acid production from dietary sugars by the acidogenic species eventually reduces the pH below the critical threshold of 5.5, triggering a shift in the enamel demineralization/remineralization equilibrium towards demineralization (18). As the principal causative organism of dental caries (5,19,20), S. mutans possesses many physiological traits relevant to cariogenesis (20). By rapid fermentation of carbohydrates, it can generate acidic end-products (acidogenicity), which is not only the direct cariogenic factor for demineralization of tooth surfaces, but also an environmental determinant that may impact the caries-related microbial flora during cariogenesis (21). Meanwhile, S. mutans has also developed an adaptive acid tolerance response (ATR) to combat the destructive nature of the acidic environment it produces (aciduricity) (22). The ability to produce the insoluble extracellular polysaccharide glucan is another critical virulence trait contributing to S. mutans’ cariogenicity (23). This not only promotes attachment and biofilm formation, but also provides binding sites that fuel accumulation of a variety of microorganisms on the tooth surface. In addition, the produced glucans can also retain protons from the acidic environment to pre-condition the bacterium for acid-stress (24).
Numerous efforts have been attempted to prevent S. mutans from acid production via replacement therapy, which includes applying a genetically engineered S. mutans strain to outcompete indigenous acidogenic bacteria (25). Other methods include colonization control via anticaries vaccines, e.g. immunization against either cell surface adhesins (26) or a glucosyltransferase enzyme that is responsible for glucan production (27). However, no favorable results have yet been reported from these treatment approaches (28,29). The current treatments frequently rely on general biocide mouthwashes and broad-spectrum antibiotics administered to the oral cavity. Treatment with broad-spectrum antibacterial agents is well known to cause destruction of the entire oral bacterial flora, thus allowing for equal competition between S. mutans and commensal organisms to re-colonize the tooth surface. If an individual has poor oral hygiene and a high uptake of dietary sugars, S. mutans will re-infect the oral cavity without difficulty (30,31), and the re-established oral biofilm will retain a persistent cariogenic condition. Conversely, individuals with low levels of S. mutans are resistant to exogenous colonization from cariogenic species and have shown long-term protection from dental caries (6,15,32). Therefore, there is a need to develop an antimicrobial agent with the specific ability to kill S. mutans that can eradicate the primary pathogen of dental caries from the oral microbial community while leaving the remaining commensal organisms intact. If this can be achieved, the major initiator of caries, S. mutans, can be eliminated and a healthy oral biofilm established, which might provide long-term caries protection (6).
Application of Oral Antimicrobial Peptides
As part of the innate immunity, antimicrobial peptides (AMPs) have been shown to play important roles in controlling viability of a vast range of pathogens (33,34). Many AMPs have been identified in the oral cavity and represent promising candidates for the development of new oral antimicrobial therapeutics (35,36). The known AMPs belong to six functional families, including cationic peptides, bacterial agglutination and adhesion, metal ion chelators, peroxidases, protease inhibitors, and AMPs with activity against bacterial cell walls (39). The physical traits of these peptides include amphipathic mixtures of α-helical and β-sheet structures and an overall cationic charge (40). Their mode of action often involves binding to the bacterial membrane and then disrupts the phospholipid bilayer (41). Due to their attraction to negatively charged structural molecules on the bacterial membrane, development of resistance to these peptides is rare (42), making them potentially useful as antibiotics. However, the broad-spectrum antimicrobial characteristics of AMPs alter the ecological balance of the oral microbial community and eliminate the entire oral flora, along with any protective benefits provided (43), which has prompted interest in the design of target-specific AMPs.
Specifically-Targeted Anti Microbial Peptides (STAMPs)
Our research group has initiated a targeted approach to control oral microbial pathogenesis via a new class of antimicrobials, called specifically-targeted AMPs (STAMPs) (44). The STAMP requires two functionally independent peptide domains, a killing moiety comprised of a non-specific AMP that can rapidly kill bacterial cells, and a targeting moiety consisting of a species-specific, high-affinity binding peptide (44,45). The two moieties are then integrated through a small linker, generating a fusion AMP without detrimental changes in the independent functions of the two domains. The major strength of such an AMP is that the targeting moiety can guide the conjoined peptide to selectively recognize the target organism, allowing peptide-guided killing. Furthermore, the fusion peptide, which is constructed from two short moieties can be chemically synthesized with high yields.
By using the structure of STAMP as a template, a number of novel STAMPs with S. mutans-selective activity were generated (46). These potential STAMPs were investigated for their killing potency and selectivity against S. mutans. Among them, C16G2 was selected due to its improved minimum inhibitory concentration (MIC), greatly enhanced killing kinetics, and selectivity against S. mutans (44) (Figure 1). The STAMP C16G2 was designed by utilizing an S. mutans produced pheromone, i.e. a competence stimulating peptide (CSP) as the STAMP targeting domain for effective delivery of the STAMP antimicrobial domain to the cell surface of S. mutans (44). The 16 amino acids (TFFRLFNRSFTQALGK) in the C-terminal of the CSP sequence (SGSLSTFFRLFNRSFTQALGK), called CSPC16, which was shown to maintain pheromone activity (47), could be used as a substitute for CSP. Further studies demonstrated that an eight-amino-acid region (TFFRLFNR) within CSPC16, called CSP M8, was sufficient for targeted delivery of the antimicrobial peptide domain to S. mutans. The STAMP killing domain, AMP G2 (48), was designed as a truncated version (16 amino acids) of the broad-spectrum killing peptide novispirin G10. The final molecule, C16G2, consisted of (from the N to C terminus) CSPC16, a flexible tri-glycine peptide linker (GGG), and AMP G2 at either the C terminus or the N terminus (48). In another study (49), CSP was fused to a killing domain consisting of an N-terminal portion of the marine-derived, broad-spectrum AMP, NRC-4, to generate another target-specific AMP, named IMB-2, which can also kill S. mutans specifically, suggesting that the targeted peptide CSP predominantly bound to S. mutans to mediate selective killing.
FIGURE 1.

Electron microscopy images of S. mutans bacteria, before (left panel) and after treatment (right panel) with C16G2. Courtesy C3J Therapeutics, Inc.
STAMPs – Selectivity and Killing Ability
C16G2 has been shown to specifically eliminate S. mutans, without affecting closely related non-cariogenic oral streptococci, in both planktonic and saliva-derived biofilm systems (44,50). Our group further investigated the antimicrobial specificity of C16G2 by expanding the panel of streptococci species closely related to S. mutans. This study showed that C16G2 treatment did not significantly affect the diversity of total Streptococcus spp. A panel of 20 different bacterial species, including both oral and non-oral Gram-positive and Gram-negative bacteria in monoculture was also tested. The results revealed an overall low capacity of C16G2 against Gram-negative species, and among the oral Gram-positive bacteria tested, C16G2 was most potent in killing S. mutans (51).
C16G2 has a rapid mechanism of action, affecting bacteria in less than one minute of exposure, a duration short enough for the application of most oral care products. It is also soluble in aqueous solutions, indicating that the STAMP is readily amendable for delivery to the oral cavity in a mouth rinse vehicle (44,46,52,53). In another study (50), a 40 s rinse with a mouth rinse formulation containing 0.04% C16G2 was administered only once at the start of a four-day test phase (no fluoride toothpaste was used during this time period). We observed that C16G2 was highly effective in decreasing levels of both plaque and salivary S. mutans. The fact that the placebo group showed a significant increase in the relative amounts of S. mutans confirms that growth conditions were favorable. The study also supported that at day four, the concentration of S. mutans was significantly lower in the C16G2-treated group, which suggests that the antimicrobial activity of C16G2 is S. mutans selective. In addition, further evidence for S. mutans selectivity was shown, as the overall bacterial community composition at day four was highly similar for the C16G2 treated and placebo groups. This study also strongly suggested C16G2 had high efficacy at preventing S. mutans from regrowing, despite frequent exposure to sugar during the four-day period (50). Although C16G2 show strong inhibitory effects, reinfection is highly likely due to shared lifestyles and environments among family members who may be S. mutans carriers. Therefore, it is likely that the C16G2 treatment will have to be repeated.
Modes of Action
The STAMP-targeting region drives the enhancement of antimicrobial activity due to increased binding to the surface of a targeted pathogen, utilizing specific determinants such as overall membrane hydrophobicity, charge, and/or pheromone receptors, which in turn leads to increased selective accumulation of the killing moiety (44,48). The exact mechanism through which AMPs kill targeted bacteria is not well understood and likely varies peptide by peptide, but membrane disruption and subsequent interference with intracellular targets are thought to be the main processes responsible (54,55,56,57). Sequence analysis of C16G2 suggests that it is an amphipathic and cationic α-helical peptide, similar to traditional AMPs (54). The hydrophobic moment of C16G2 is considerably greater than that of its individual moieties due to the stacking of hydrophobic residues in the STAMP. Our group’s data suggest that CSPC16-S. mutans binding is species-specific but is independent of the ComD surface receptor (44), which can sense pheromone CSP and triggers the signaling cascade for bacteriocin production and other cell density-dependent activities (58). A natural S. mutans-specific targeting sequence in this pheromone might bind to an alternative receptor (e.g. lipids, exopolysaccharides, or teichoic acids) on the bacterial surface prior to interaction with ComD. An explanation of the selective killing activity against S. mutans by CSPC16 might be the absent avidity or hydrophobic interactions of CSP16 with the membrane of untargeted oral organisms, resulting in poor binding and/or retention, as well as a lack of α-helical adoption, resulting in decreased hydrophobic moment and membrane activity. The proper folding of CSPC16 on the surface of S. mutans may retain a role in sequestering and retaining STAMP. Although the exact mechanism of selective membrane disruption by C16G2 remains unclear, it may involve early membrane binding or partition steps governed by the targeting moiety of C16G2 (53). Recent studies have indicated that C16G2 kills S. mutans through membrane disruption, with small molecules subsequently leaking out of the cell, which is followed by a loss of membrane potential and cell death (53). It seems likely that the amphipathic characteristic shared between C16G2 and AMPs results in the STAMP functioning as a membrane disrupting peptide, but with greater specificity for its target (53).
Both our study and the study by Eckert et al. (44) showed significantly enhanced killing of S. mutans cells, but no activation of the signal transduction pathway or its regulated genes (59,50). This may be because all fusion peptides lack a C-terminal structural motif of CSP, which is known to activate the signal transduction pathway (59).
Impacts on Microbial Community Ecology
Microbial communities usually result from complex intraspecies, interspecies, and microbe–host interactions. Any change in the abundance of a particular species within the community could have drastic effects on its interacting partners, eventually resulting in a change of community profile as well as community level functions. To explore if the application of C16G2 impacts the composition shift of the oral microbial community, a saliva-derived in vitro model system containing over 100 species approaching the diversity and overall metabolic functionality of the human oral microbiome (60) was applied. We treated S. mutans-containing in vitro planktonic oral microbial communities with C16G2 for 30 minutes followed by extensive washing to remove the residual C16G2. The treated communities were then allowed to recover by being cultured in fresh non-selective medium. The microbial composition of the recovered community was determined by 454 pyrosequencing analyses to examine how the removal of S. mutans may impact other species within the same community (51).
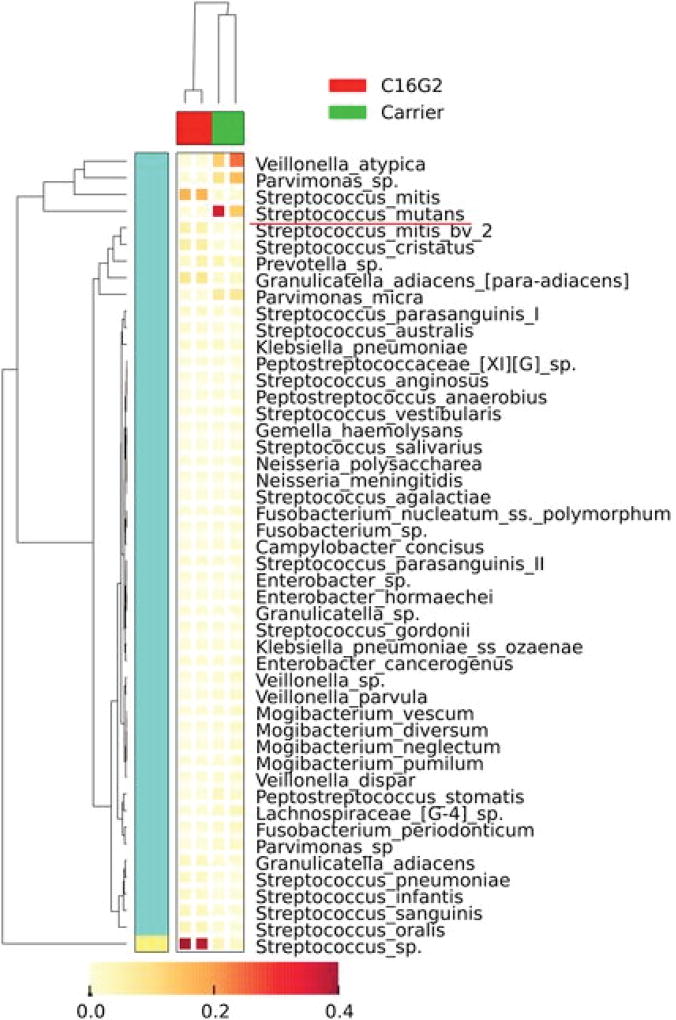
The most intriguing finding was that the targeted removal of S. mutans had a community-level impact on the species composition and abundance within the same community (51). Data showed that 21 bacterial genera could be detected from regrowth of untreated samples, with Streptococcus, Veillonella, Parvimonas, Prevotella, and Peptostreptococcus spp. being the most dominant genera (Figure 2). In contrast, only 16 bacterial genera were detected from the regrowth of the C16G2-treated samples, with Streptococcus, Granulicatella, and Prevotella being the most dominant ones (Figure 2). Interestingly, although the relative abundance of S. mutans reduced drastically, the overall sequence counts of all Streptococcus spp. increased from 30% to 81% in the culture recovered after C16G2 treatment. Meanwhile, many bacterial genera, most of which were Gram-negative bacteria, including Fusobacteria, Campylobacter, Neisseria, and Parvimonas spp., which were present at less than 5%, could no longer be detected at the depth of sequencing obtained from the regrowth of the C16G2-treated samples, whereas genera, such as Veillonella, suffered drastic reductions in relative abundance within the community (from 20% to less than 1%) (51).
FIGURE 2.
Cluster analyses of oral taxa-weighted abundance profiles obtained from regrowth after treatment with Carrier (negative control) and C16G2. Relative proportions of the total taxa abundance are indicated in the heat map, which shows how the dominant taxa varied. The figure is modified from Guo and colleagues (51).
Our study indicated that the reduction in the S. mutans population by C16G2 was accompanied by an increase in the abundance of several streptococci from the mitis group, including S. mitis, S. cristatus, S. oralis, and S. sanguinis, signature bacterial species identified from the oral microbial community of healthy subjects (61,62,63). The antagonism between S. mutans and streptococci of the mitis group, particularly S. sanguins and S. gordonii, at the ecological level has been well-documented (64). Epidemiological studies revealed that high levels of S. mutans are always concurrent with low levels of S. sanguinis (64), whereas high levels of S. sanguinis in the oral cavity correlate with delayed S. mutans colonization (30). Recent work by Kreth et al. (66) showed sophisticated interspecies interactions between these two species that might play an essential role in balancing competition and coexistence within the oral community. The targeted removal of S. mutans could shift the balance and provide a competitive growth advantage to the mitis group.
After overnight regrowth, the C16G2-treated community showed decreased microbial diversity compared with the negative control. Many Gram-negative species, such as Veillonella, experienced drastic reductions in abundance, whereas F. periodonticum, Campylobacter, Gemella, and Neisseria, which are implicated in the pathogenesis of periodontal disease (67), could not be detected by pyrosequencing from communities recovered from the C16G2 treatment, although they were only present at abundances of less than 5%. The results might be caused by the nonspecific killing of the peptide; however, the data showed that some of these species, including F. periodonticum, displayed high levels of resistance against the C16G2 treatment, suggesting that the reduction or elimination of certain species could be directly or indirectly associated to the removal of S. mutans. For example, it has been shown that lactic acid, a metabolic product of S. mutans, is required for the growth of Veillonella spp. (68). The reduction in the S. mutans population as a result of the C16G2 treatment may, therefore, have had a negative effect on the growth of Veillonella spp., such as was seen in our metagenomic data.
The use of STAMP C16G2 to modulate the microbiome structure allows insight into the therapeutic potential of C16G2 to achieve a healthy oral microbiome, since several bacterial species with metabolic dependency or physical interactions with S. mutans suffered drastic reduction in their abundance, whereas S. mutans’ natural competitors, including health-associated oral streptococci, became dominant (51).
STAMP Stability and Safety
The half-life of C16G2 was estimated to be 18.8 minutes in pooled human saliva, suggesting the STAMP is unlikely to be retained at meaningful quantities in the oral cavity after long durations and indicating its favorable safety (50). C16G2 could be formulated in PBS with at least overnight stability at 4°C, without excipients or stabilizers, it remained active and capable of penetrating dental plaque to inhibit S. mutans, and could be freshly prepared up to four hours before treatment if stored at room temperature (50). Also, the therapeutic concentrations of 25–100 µM had no hemolytic activity against human red blood cells, isolated human cells or defined tissue (50), which suggests that C16G2 does not interfere with human host cell integrity and is therefore relatively safe.
STAMP C16G2 protective effects
Compared to AMPs with wide spectra of activity, the STAMP C16G2 has demonstrated specificity for S. mutans in multispecies communities, resulting in the complete killing of S. mutans, while leaving noncariogenic oral streptococci in the environment unaffected (53). Moreover, 0.04% (w/v) C16G2 rinse usage can effectively lessen lactic acid production and protect enamel against demineralization in an intra-oral model during the course of a four-day treatment period even under the conditions of accelerated demineralization induced by frequent exposure to sucrose, which suggests that C16G2 is effective against S. mutans and its cariogenesis in vivo (50).
C16G2 was also shown to significantly elevate the resting pH of dental plaque compared to the placebo rinse (50). The higher resting pH creates conditions that are favorable for growth of healthy bacteria and unfavorable for cariogenic (acidoduric) bacteria. This may be in part responsible for helping keep the S. mutans population from recovering in spite of the frequent exposure to sugar.
According to the report by Sullivan (2011) (50), a single STAMP treatment was able to selectively eliminate S. mutans from plaque and salivary bacterial populations while leaving the remaining flora relatively undisturbed. The effect resulted in an S. mutans-free “healthy plaque” that resisted S. mutans overgrowth despite sucrose challenges of up to four times daily for the entire course of treatment. It is well known that S. mutans is the critical and central facilitator of caries development, at least for caries linked to intake of dietary sugars and not resulting from pre-existing pathologies. Therefore, it may be possible to generate a “healthy” non-cariogenic microbial ecosystem in the oral cavity through STAMP intervention at the clinical level, as has been demonstrated (50). An intact dental biofilm without S. mutans could resist future exogenous S. mutans colonization, or overgrowth due to sucrose consumption, and could delay or postpone cariogenesis. The oral community that recovered from C16G2 treatment exhibited a health condition with an increase in the population of the noncariogenic species, S. mitis and S. sanguinis, and a reduction in many periodontitis-associated Gram-negative species, such as Fusobacteria (51).
In contrast to current aseptic interventions, the selective hallmark of STAMP C16G2 drives its development into “probiotic” antibiotics, which could selectively eliminate caries-causative species while preserving the protective colonization effects associated with noncariogenic oral flora that overtake S. mutans colonization sites or antagonize the growth of the bacterium directly. The established S. mutans-free biofilms through STAMP treatment can reduce the competitive advantage of S. mutans even in the presence of high sugar content (50), thus preventing the shift in the biofilm composition toward cariogenesis. Furthermore, the prior establishment of an S. mutans-free biofilm provides considerable protection against subsequent reestablishment of this oral pathogen in oral biofilm. In this regard, the STAMP C16G2 may represent a remarkably effective weapon against dental caries that is easy to formulate, easy to administer, complements existing oral hygiene regimens, and can be dosed infrequently compared to other oral care ingredients.
Conclusions and future directions
As an alternative to conventional antibiotics, antibacterial peptides such as C16G2 have been explored for therapeutic uses. C16G2 is a highly attractive solution to caries disease as it has robust and selective activity against cariogenic S. mutans planktonic and biofilm cells in vitro. When available on the market (either as a mouth wash or gel trays) the treatment will likely have multifold benefits, such as an intact oral ecosystem (i.e. no vacated niches open up for pathogens colonization), no threats of drug resistance development. In the future, if C16G2 passes clinical trials, it could be prescribed as a mouth rinse or as gel trays for treating clinically diagnosed caries disease. Monitoring of treatment efficiency would have to be conducted by the treating dentist who also would make decisions on treatment time. Post-treatment with fluoride and follow up visits at the dentist would serve as reinfection prevention. STAMP C16G2 is developed under an Investigational New Drug authorization with the U.S. FDA and is currently in Phase 2 clinical trials. This new technology could have an impact far beyond dentistry and could possibly be used to treat and prevent other microbiome-related diseases.
Acknowledgments
We are thankful to Dr. Xiaoyu Tang for help with editing of the manuscript. We also want to thank Dr. Pierre Kyme and Dr. Brian C. Varnum at C3J Therapeutics, Inc. and Dr. Wenyuan Shi, School of Dentistry, UCLA, for providing images and knowledge on STAMP C16G2 development.
References
- 1.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 2.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 3.Bowen WH. Do we need to be concerned about dental caries in the coming millennium? Crit Rev Oral Biol Med. 2002;13:126–131. doi: 10.1177/154411130201300203. [DOI] [PubMed] [Google Scholar]
- 4.Marsh PD, Bradshaw DJ. Physiological approaches to the control of oral biofilms. Adv Dent Res. 1997;11:176–185. doi: 10.1177/08959374970110010901. [DOI] [PubMed] [Google Scholar]
- 5.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol. 2005;33:248–255. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MH, Shi W. A probiotic approach to caries management. Pediatr Dent. 2006;28:151–153. discussion 192–198. [PubMed] [Google Scholar]
- 7.Tsang PW, Qi F, Huwig AK, et al. A medical approach to the diagnosis and treatment of dental caries. AHIP Cover. 2006;47:38–42. [PubMed] [Google Scholar]
- 8.Chu CH, Lo ECM, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish and arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81:767–770. doi: 10.1177/0810767. [DOI] [PubMed] [Google Scholar]
- 9.Horst JA, Ellenikiotis H, Milgrom PL. UCSF Protocol for caries arrest using silver diamine fluoride: Rationale, indications and consent. J Calif Dent Assoc. 2016;44:16–28. [PMC free article] [PubMed] [Google Scholar]
- 10.Loesche WJ. The identification of bacteria associated with periodontal disease and dental caries by enzymatic methods. Oral Microbiol Immunol. 1986;1:65–72. doi: 10.1111/j.1399-302x.1986.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams MI, Cummins D. The technology behind Colgate Total Advanced Fresh. Compend Contin Educ Dent. 2003;24:4–9. [PubMed] [Google Scholar]
- 12.Tom D. Review: Increasing fluoride concentrations in toothpastes improved prevention of dental caries. Arch Dis Child Educ Pract Ed. 2011;96:159. doi: 10.1136/adc.2010.206615. [DOI] [PubMed] [Google Scholar]
- 13.Anderson LA, Orchardson R. The effect of chewing bicarbonate-containing gum on salivary flow rate and pH in humans. Arch Oral Biol. 2003;48:201–204. doi: 10.1016/s0003-9969(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 14.Sgan-Cohen HD, Salinger E. Dental caries and sugar intake, during and between meals, in children of an Israeli Kibbutz. Community Dent Oral Epidemiol. 1982;10:52–53. doi: 10.1111/j.1600-0528.1982.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 15.He X, Lux R, Kuramitsu HK, et al. Achieving probiotic effects via modulating oral microbial ecology. Adv Dent Res. 2009;21:53–56. doi: 10.1177/0895937409335626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang MY, Wang JH. Impact of antibiotic use on fungus colonization in patients hospitalized due to fever. J Microbiol Immunol Infect. 2003;36:123–128. [PubMed] [Google Scholar]
- 17.Marsh PD. Are dental diseases examples of ecological catastrophes? Micorbiol. 2003;149(Pt 2):279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 18.Hardie JM. Oral sreptococci. In: Sneath PHAMN, Sharpe ME, Holt JG, editors. Oral sreptococci. Baltimore: William & Wilkins; 1986. pp. 1054–1063. [Google Scholar]
- 19.Corby PM, Lyons-Weiler J, Bretz WA, et al. Microbial risk indicators of early childhood caries. J Clin Microbiol. 2005;43:5753–5759. doi: 10.1128/JCM.43.11.5753-5759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colby SM, Russell RR. Sugar metabolism by mutans streptococci. Soc Appl Bacteriol Symp Ser. 1997;26:80S–88S. [PubMed] [Google Scholar]
- 22.Cotter PD, Hill C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev. 2003;67(3):429–453. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamada S, Koga T, Ooshima T. Virulence factors of Streptococcus mutans and dental caries prevention. J Dent Res. 1984;63:407–411. doi: 10.1177/00220345840630031001. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, McLean JS, Lux R, et al. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep. 2015;5:18015. doi: 10.1038/srep18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillman JD, Brooks TA, Michalek SM, et al. Construction and characterization of an effector strain of Streptococcus mutans for replacement therapy of dental caries. Infect Immun. 2000;68(2):543–549. doi: 10.1128/iai.68.2.543-549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abiko Y. Passive immunization against dental caries and periodontal disease: development of recombinant and human monoclonal antibodies. Crit Rev Oral Biol Med. 2000;11(2):140–158. doi: 10.1177/10454411000110020101. [DOI] [PubMed] [Google Scholar]
- 27.Xu QA, Yu F, Fan MW, et al. Protective efficacy of a targeted anti-caries DNA plasmid against cariogenic bacteria infections. Vaccine. 2007;25(7):1191–1195. doi: 10.1016/j.vaccine.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Carounanidy U, Sathyanarayanan R. Dental caries: A complete changeover, part iii: Changeover in the treatment decisions and treatments. J Conserv Dent. 2010;13:209–217. doi: 10.4103/0972-0707.73383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajishengallis G, Michalek SM. Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol. 1999;14(1):1–20. doi: 10.1034/j.1399-302x.1999.140101.x. [DOI] [PubMed] [Google Scholar]
- 30.Caufield PW, Dasanayake AP, Li Y, et al. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 2000;68:4018–4023. doi: 10.1128/iai.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikx FH, Van Der Hoeven JS, Plasschaert AJ, et al. Effect of Actinomyces viscosus on the establishment and symbiosis of Streptococcus mutans and Streptococcus sanguis in SPF rats on different sucrose diets. Caries Res. 1975;9:1–20. doi: 10.1159/000260138. [DOI] [PubMed] [Google Scholar]
- 32.Marsh PD. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am. 2010;54:441–454. doi: 10.1016/j.cden.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Jenssen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesner J, Vilcinskas A. Antimicrobialpeptides: the ancient arm of the human immune system. Virulence. 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 35.Hancock RE, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mor A. Peptide-based antibiotics: a potential answer to raging antimicrobial resistance. Drug Dev Res. 2000;50:440–447. [Google Scholar]
- 37.Beckloff N, Laube D, Castro T, et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother. 2007;51(11):4125–4132. doi: 10.1128/AAC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porat Y, Marynka K, Tam A, et al. Acyl-substituted dermaseptin S4 derivatives with improved bactericidal properties, including on oral microflora. Antimicrob Agents Chemother. 2006;50(12):4153–60. doi: 10.1128/AAC.00750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorr SU. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–180. doi: 10.1111/j.1600-0757.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 40.Pazgier M, Hoover DM, Yang D, et al. Human beta-defensins. Cell Mol Life Sci. 2006;63:1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vorland LH, Ulvatne H, Rekdal O, et al. Initial binding sites of antimicrobial peptides in Staphylococcus aureus and Escherichia coli. Scand J Infect Dis. 1999;31:467–473. doi: 10.1080/00365549950163987. [DOI] [PubMed] [Google Scholar]
- 42.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly JP, Bellm LA, Epstein JB, et al. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect Dis. 2003;3:405–412. doi: 10.1016/s1473-3099(03)00668-6. [DOI] [PubMed] [Google Scholar]
- 44.Eckert R, He J, Yarbrough DK, et al. Targeted killing of streptococcus mutans by a pheromone-guided “Smart” antimicrobial peptide. Antimicrob Agents Chemother. 2006;50(11):3651–3657. doi: 10.1128/AAC.00622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu XQ, Wang H, Lu XF, et al. An engineered multidomain bactericidal peptide as a model for targeted antibiotics against specific bacteria. Nat Biotechnol. 2003;21:1480–1485. doi: 10.1038/nbt913. [DOI] [PubMed] [Google Scholar]
- 46.He J, Yarbrough DK, Kreth J, et al. Systematic approach to optimizing specifically targeted antimicrobial peptides against Streptococcus mutans. Antimicrob Agents Chemother. 2010;54(5):2143–51. doi: 10.1128/AAC.01391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi F, Kreth J, Levesque CM, et al. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol Lett. 2005;251(2):321–326. doi: 10.1016/j.femsle.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Eckert R, Qi F, Yarbrough DK, et al. Adding selectivity to antimicrobial peptides: rational design of a multidomain peptide against Pseudomonas spp. Antimicrob Agents Chemother. 2006;50:1480–1488. doi: 10.1128/AAC.50.4.1480-1488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mai J, Tian XL, Gallant JW, et al. A novel target-specific, salt-resistant antimicrobial peptide against the cariogenic pathogen Streptococcus mutans. Antimicrob Agents Chemother. 2011;55(11):5205–5213. doi: 10.1128/AAC.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan R, Santarpia P, Lavender S, et al. Clinical efficacy of specifically targeted antimicrobial peptide mouth rinse: target ed elimination of Streptococcus mutans and prevention of demineralization. Caries Res. 2011;45(5):415–428. doi: 10.1159/000330510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo L, McLean JS, Yang Y, et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci USA. 2015;112(24):7569–7574. doi: 10.1073/pnas.1506207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li LN, Guo LH, Lux R, et al. Targeted antimicrobial therapy against Streptococcus mutans establishes protective non-cariogenic oral biofilms and reduces subsequent infection. Int J Oral Sci. 2010;2:66–73. doi: 10.4248/IJOS10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplan CW, Sim JH, Shah KR, et al. Selective membrane disruption: The mode of action of C16G2, a specifically targeted antimicrobial peptide. Antimicrob Agents Chemother. 2011;55:3446–3452. doi: 10.1128/AAC.00342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hancock REW, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 55.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 56.Shai Y. Molecular recognition between membrane-spanning polypeptides. Trends Biochem Sci. 1995;20:460–464. doi: 10.1016/s0968-0004(00)89101-x. [DOI] [PubMed] [Google Scholar]
- 57.Wu M, Maier E, Benz R, et al. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 58.Senadheera D, Cvitkovitch DG. Quorum sensing and biofilm formation by Streptococcus mutans. Adv Exp Med Biol. 2008;631:178–188. doi: 10.1007/978-0-387-78885-2_12. [DOI] [PubMed] [Google Scholar]
- 59.Syvitski RT, Tian XL, Sampara K, et al. Structure-activity analysis of quorum-sensing signaling peptides from Streptococcus mutans. J Bacteriol. 2007;189:1441–1450. doi: 10.1128/JB.00832-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian XL, Syvitski RT, Liu T, et al. A method for structure-activity analysis of quorumsensing signaling peptides from naturally transformable streptococci. Biol Proced Online. 2009;11:207–226. doi: 10.1007/s12575-009-9009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edlund A, Yang Y, Hall AP, et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome. 2013;1(1):25. doi: 10.1186/2049-2618-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eren AM, Borisy GG, Huse SM, et al. Oligotyping analysis of the human oral microbiome. Proc Natl Acad Sci USA. 2014;111(28):E2875–E2884. doi: 10.1073/pnas.1409644111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kreth J, Zhang Y, Herzberg MC. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 2008;190(13):4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loesche WJ, Rowan J, Straffon LH, et al. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kreth J, Merritt J, Shi W, et al. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187(21):7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Signat B, Roques C, Poulet P, et al. Fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol. 2011;13(2):25–36. [PubMed] [Google Scholar]
- 68.Chalmers NI, Palmer RJ, Jr, Cisar JO, et al. Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque. J Bacteriol. 2008;190(24):8145–8154. doi: 10.1128/JB.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]