Summary
Chimeric mice have been generated by injecting pluripotent stem cells into morula-to-blastocyst stage mouse embryo or by introducing more mature cells into later stage embryos that correspond to the differentiation stage of the donor cells. It has not been rigorously tested, however, whether successful chimera formation requires the developmental stage of host embryo and donor cell to be matched. Here, we compared the success of chimera formation following injection of primary neural crest cells (NCCs) into blastocysts or of embryonic stem cells (ESCs) into E8.5 embryos (heterochronic injection) with that of injecting ESCs cells into the blastocyst or NCCs into the E8.5 embryos (isochronic injection). Chimera formation was efficient when donor and host were matched, but no functional chimeric contribution was found in heterochronic injections. This suggests that matching the developmental stage of donor cells with the host embryo is crucial for functional engraftment of donor cells into the developing embryo.
Keywords: chimera, pluripotent, stem cells, development, neural crest, implantation, isochronicity, contribution, blastocyst
Graphical Abstract
Highlights
-
•
Developmental matching of donor cells and host is crucial for chimera formation
-
•
Heterochronic injection of ESCs to E8.5 mouse embryos failed to yield chimeras
-
•
NCCs injected to blastocyst failed to form chimeras, even when apoptosis is impaired
Cohen at al. compares the efficiency of chimera formation in heterochronic and isochronic injections of ESCs and NCCs. Using two distinct and well-characterized pre- and post-implantation chimeric platforms, they show that matching of developmental age of donor cells and the host is essential for chimera formation.
Introduction
Chimeric animals are typically generated from cells of more than one individual and can be produced by mixing of early embryos (Gardner, 1968, Mintz, 1962, Tarkowski, 1961) or by engrafting cells or tissues into embryos at different stages of development (Le Douarin and Teillet, 1973). In addition to being useful tools for the study of development, the generation of interspecies human-animal chimeras has been proposed as a source of human cells and organs for regenerative medicine (Masaki and Nakauchi, 2017, Wu et al., 2017). To define the parameters that promote functional engraftment of donor cells into embryos and chimera formation is important for the generation of interspecies chimera models.
The most common approach to generate chimeras involves the injection of mouse embryonic stem cells (mESCs) into morula-to-blastocyst stages (E2.5–3.5) of the pre-implantation mouse embryo, producing postnatal chimeric mice with donor cell contribution to all tissues (Tam and Rossant, 2003). This method is routinely used to produce gene-edited mice (Capecchi, 1989). The generation of chimeras at later stages of mouse development is challenging, as the embryo is less accessible to manipulation after implantation into the uterus (E4.5–5). An alternative approach is the introduction of test cells into ex vivo cultured embryos, which enables the study of chimera formation in vitro, but this approach does not allow observations beyond 2 days, the maximal time of embryo cultivation (Huang et al., 2012).
In conventional pre-implantation chimera generation, the donor ESCs are developmentally matched (isochronic) with the host blastocyst. However, it is not clear whether isochronic transplantation of donor cells is necessary for successful engraftment. While a recent study reported that somatic cells, such as primordial germ cells, failed to contribute to chimera formation when heterochronically introduced into blastocysts (Leitch et al., 2014), several groups claimed that transplantation of various somatic stem cell types such as neural precursors, mesenchymal stem cells, or blood stem cells into the blastocyst in a heterochronic injection, induced ‘transdifferentiaton’ and gave rise to chimeras (Clarke et al., 2000, Geiger et al., 1998, Jiang et al., 2002). However, the latter results have not been reproduced under stringent criteria and remain controversial.
We have shown that committed somatic stem cells, when introduced isochronically into the host embryo, generate chimeras efficiently: neural crest cells (NCCs) introduced into the gastrulating embryo at E8.5 contribute to neural-crest-derived lineages of the host such as pigment cells and peripheral nervous system derivatives (Cohen et al., 2016, Huszar et al., 1991, Jaenisch, 1985, and M.A.C., unpublished data). In these experiments, neural crest chimera formation was successful only when the donor cells were microinjected in utero during a narrow time window between E8.5 and E9.25, a time when the endogenous NCCs leave the neural tube and migrate through the embryo. This is consistent with the notion that the developmental stage of the somatic donor cells needs to be matched to that of the host embryo.
The goal of this study was, using ESCs and NCCs, to test previous conclusions and to evaluate whether matching of the developmental stage of donor cells and host embryo is an important parameter for functional integration of the cells and for chimera formation. For this, we used host embryos at two distinct and well-characterized pre- and post-implantation developmental stages, E3.5 and E8.5, respectively, and compared the efficiency of chimera formation by heterochronic and isochronic injection of ESCs and NCCs. Our results argue that matching of developmental age of donor cells and host is critical for chimera formation.
Results and Discussion
Isochronic and Heterochronic Injection of ESCs and NCCs into Embryos
We used two developmentally distinct cell types as donor cells: pluripotent mESCs, which were commonly used for generating chimeras by combining with pre-implantation embryos (E2.5–3.5), and NCCs, which are developmentally restricted and were shown previously to functionally integrate into E8.5 host embryos. NCCs were isolated from C57BL/6;R26(tdTomato) donor mice with about 45% of the cells being positive for HNK-1 and TFAP2a, two typical NCC markers (Figure S1A). ESCs were isolated from the same mouse strain. We injected tdTomato-labeled mESCs or NCCs into blastocysts (E3.5) or E8.5 embryos to compare embryo engraftment of developmentally matched (isochronic) with that of non-matched (heterochronic) donor cells.
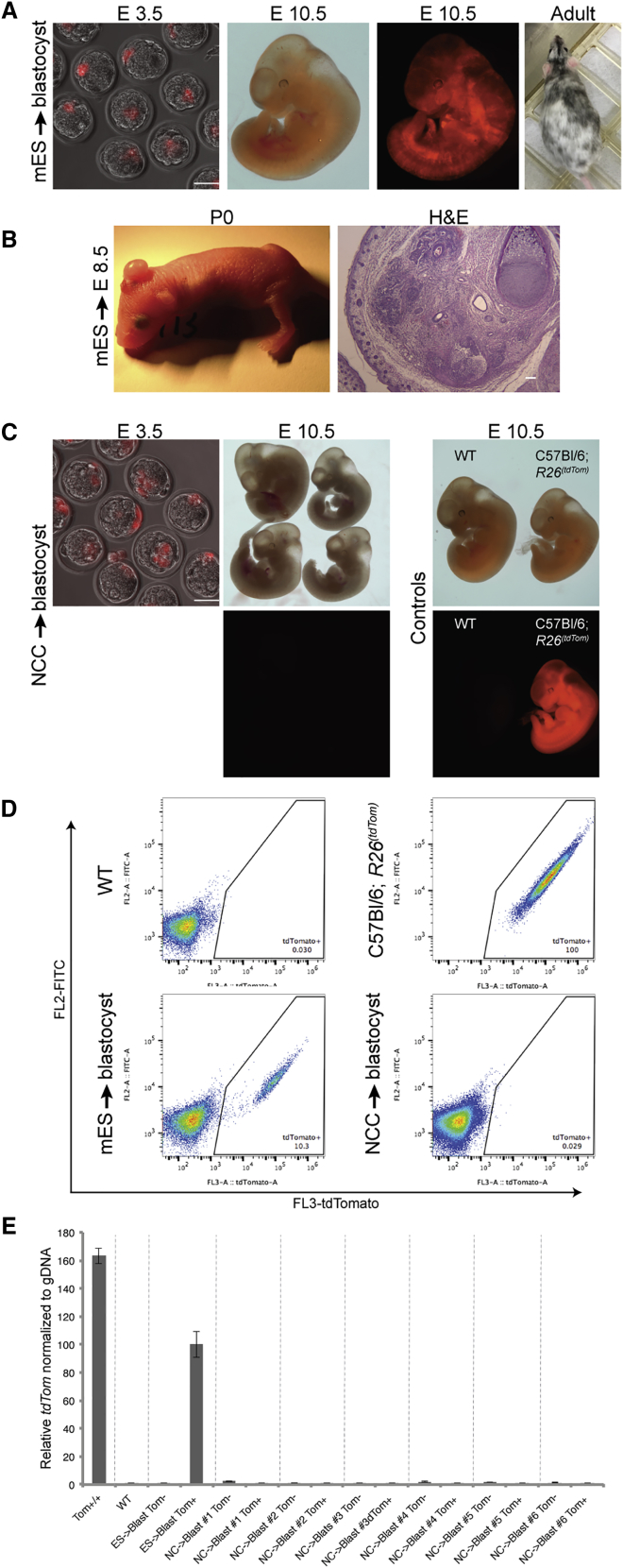
While both cell types integrated into the inner cell mass (ICM; E4.5) after injection into blastocysts, as expected, only mESCs cells formed robust chimeras at E10.5 and postnatal coat chimeras (Figures 1A and 1C; Table 1, top). Similarly, when NCCs were injected into the gastrulating embryo at E8.5 (isochronic injection), robust coat color contribution was found (Figures S1B and S1C; Table 1, bottom). As shown previously, donor NCCs contributed to pigmentation of postnatal mice (Cohen et al., 2016, Huszar et al., 1991, Jaenisch, 1985) with coat color contribution being significantly enhanced when the E8.5 host embryos were mutant for the c-Kit gene (Wsh/Wsh), a mutation, which causes death of melanoblasts thus providing a selective advantage (an “empty niche”) for the donor NCCs.
Figure 1.
Isochronic and Heterochronic Injection of mESCs or Primary NCCs into the Mouse Embryo
(A) TdTomato-labeled mESCs were introduced into mouse blastocysts. Embryos were tested for chimeric contribution using fluorescence at E10.5 and by coat color in adult mice.
(B) mESCs injected into E8.5 host mouse embryos formed massive teratoma outgrowths in postnatal mice (left). Histology of the teratoma stained with H&E is presented (right).
(C) Mouse blastocysts injected with tdTomato-labeled primary NCCs. Embryos were tested at E10.5 for chimeric contribution using fluorescence. Negative and positive controls are presented (right panel; wild-type [WT] and C57BL/6;R26(tdTomato) embryos, respectively).
(D) FACS analysis of representative E10.5 embryos injected with tdTomato-labeled mESCs and NCCs to blastocysts, along with control embryos, as indicated.
(E) Tomato positive and negative cells were sorted and analyzed for the TdTomato gene by qPCR to determine chimeric contribution, along with appropriate negative and positive controls, as indicated. Overall, no cell contribution was found in embryos injected with NCCs. For full statistical analysis of injected embryos, see Table 1. Data are represented as means ± SD.
All scale bars represent 100 μm.
Table 1.
Chimeric Contribution of Donor Cells after Injection into Pre- and Post-implantation Mouse Embryos
| Cells | Host Strain | No. of Blastocysts Injected + Transferred | Total No. of Embryos Dissected | No. of Chimeric Embryos | % Chimeric Embryos | Total No. of Mice Born | No. of Chimeric Mice | % Chimeric Mice | |
|---|---|---|---|---|---|---|---|---|---|
| Injections into Blastocysts | |||||||||
| mESCs | C57BL/6;R26(tdTomato/M2rtTA) | A | 96 | 36 | 20 | 55.5 | NA | NA | NA |
| In vitro differentiated NCCs | C57BL/6;R26(tdTomato/M2rtTA) + Teto::Bcl2No Dox | A | 70 | 22 | 0 | 0.0 | 22 | 0 | 0.0% |
| C57BL/6;R26(tdTomato/M2rtTA) + Teto::Bcl2 +Dox | A | 158 | 47 | 6 | 12.7 | 41 | 4 | 9.7% | |
| Primary NCCs | C57BL/6;R26(tdTomato) | A | 223 | 97 | 0 | 0.0% | NA | NA | NA |
| C57BL/6;R26(tdTomato) | W | 53 | NA | NA | NA | 4 | 0 | 0.0% | |
| C57BL/6;R26(tdTomato/M2rtTA) + Teto::Bcl2+ Dox | A | 185 | 15 | 0 | 0.0% | 39 | 0 | 0.0% | |
| Cells | Host Strain | No. of Embryos Injected (E8.5) | Total No. of Mice Born | No. of Mice with Teratoma | % Mice with Teratoma | No. of Chimeric Mice | % Chimeric Mice | |
|---|---|---|---|---|---|---|---|---|
| Injections into E8.5 Embryos | ||||||||
| mESCs | C57BL/6;R26(tdTomato/M2rtTA) or C57BL/6;Col1a1(GFP) | W | 72 | 29 | 12 | 41.3 | 0 | 0.0 |
| Primary NCCs | C57BL/6;Col1a1(GFP) | W | 33 | 27 | 0 | 0.0 | 9 | 33.3a |
Top: Mouse blastocysts, injected with mESCs, in vitro differentiated NCCs, or primary NCCs, as indicated, were isolated at embryonic stages (E10.5–16.5) and fluorescence was used to measure chimeric contribution. Alternatively, injected embryos were allowed to develop to term and coat color was used to assess chimeric contribution. The total number of injected blastocysts and the number of chimeric embryos/mice are presented. Chimeric contribution was found when mESCs were injected isochronically into blastocysts. When mESCs were differentiated in vitro to NCCs and injected into mouse blastocysts, only cells that overexpressed Bcl2 contributed to chimeras. However, chimeric contribution was found to be due to contaminating mESCs. No chimeric contribution was found when primary NCCs were used as donor cells. Furthermore, even when primary NCCs overexpressing Bcl2 were used as donor cells, no chimeric contribution was detected. Bottom: Mice injected at E8.5 with either mESCs or NCCs were examined postnatally for coat color contribution. The total numbers of injected embryos, the numbers of chimeric mice as well as the number of mice with teratoma outgrowth are presented. Teratoma formation rather than chimeric contribution was observed when mESCs were injected into E8.5 embryos.
A, albino CD1 mice; W, Wsh/Wsh white spotted mice.
Data summarized from Cohen et al. (2016).
In contrast, heterochronic injection of NCCs into blastocysts, although integrated efficiently into the ICM (Figure 1C), failed to yield chimeras: none or only 0.03% tdTomato positive cells were detected in E10.5 embryos from NCC injected blastocysts (Figures 1C and 1D). Similarly, when mESCs were introduced into E8.5 embryos (heterochronic injection), the donor cells did not functionally engraft to form chimeras but rather developed massive teratoma outgrowths mainly in the backside of the head (Figure 1B; Table 1, bottom), which is the site where donor cells typically enter the embryo after E8.5 injection (Cohen et al., 2016, Huszar et al., 1991, Jaenisch, 1985).
We assessed whether the few tdTomato+ cells seen in embryos from NCCs injected into blastocysts (Figure 1D) were derived from the donor cells, rather than from auto-fluorescent dead cells. Genomic DNA was extracted from sorted populations and qPCR was used to detect the presence of tdTomato DNA (Figure 1E). Control C57BL/6;R26(tdTomato) embryos, which are homozygous for the reporter gene, demonstrated a high level of tdTomato DNA. TdTomato DNA positive cells were found in embryos derived from blastocysts injected with C57BL/6;R26(tdTomato/M2rtTA) mESCs (heterozygous for the tdTomato) at a lower level consistent with chimeric embryos consisting of heterozygous tdTomato cells. Importantly, the rare (0.03%) tdTomato+ sorted cells of embryos derived from blastocysts injected with NCCs were similar to negative controls and showed no tdTomato amplification signal (Figure 1E). These qPCR results confirm that NCCs injected at the blastocyst stage cannot functionally engraft into post-implantation embryos and that the rare tdTomato positive cells are due to auto-fluorescence. Table 1 summarizes our experiments of injecting mESCs or NCCs into blastocysts (top) and the injections of mESCs or primary NCCs into E8.5 embryos (bottom). The results indicate that matching the developmental stage of donor and host, i.e., the injection of ESCs into blastocysts and NCCs into E8.5 embryos, leads to functional engraftment of mESCs and NCCs and chimera formation. In contrast, when the developmental stage of donor and host are not matched (heterochronic injection), no functional engraftment of the donor cells is observed.
Inhibition of Apoptosis Does Not Promote Heterochronic Chimera Formation
Recently, Nakauchi and colleagues reported that inhibition of apoptosis by overexpression of Bcl2 can induce Sox17+ endoderm precursors cells to engraft into embryos after heterochronic injection into the blastocyst: the Sox17+ were found to have colonized gut endoderm and yolk sac of E9.5 embryos (Masaki et al., 2016). To test whether anti-apoptotic activity would allow NCCs to functionally contribute to blastocyst chimera formation, we generated mESCs that conditionally overexpressed Bcl2. C57BL/6;R26(tdTomato/M2rtTA) mESCs were infected with FUW-Teto:Bcl2-T2A-PuroR lentivirus and clones carrying the transgene were isolated by drug selection and differentiated into NCCs. When injected into blastocysts, these cells formed chimeras, as indicated by fluorescence in embryos or coat color contribution in adult mice (Table 1 and Figure S2A). These results suggest that NCCs can functionally integrate into the blastocyst. Alternatively, BCL2 expression may enhance survival and delay differentiation of ESCs and enhance the maintenance of undifferentiated cells as has been reported previously (Ardehali et al., 2011, Yamane et al., 2005). Indeed, we found that the in vitro derived NCCs cultures still contained a small fraction (1.7% ± 0.24%) of contaminating pluripotent mESCs as detected by pluripotent colony-forming assay. To test whether chimera formation upon Bcl2 overexpressed was due to contaminating pluripotent mESCs rather than NCCs, we examined the chimeric contribution to a non-NC-derived cell type. Peripheral blood cells (PBCs), isolated from the chimeras and from their littermates were tested for tdTomato fluorescence by fluorescence-activated cell sorting (FACS). A significant fraction of PBCs from all chimeras, but none of their littermates, were found to be tdTomato positive (Figures S2B and S2C), indicating that the donor cells in these chimeras were derived from the pluripotent contaminating mESCs rather than from the NCCs. These results suggest that BCL2 delays differentiation of mESCs, leaving contaminating pluripotent cells in culture, which contribute to chimera formation.
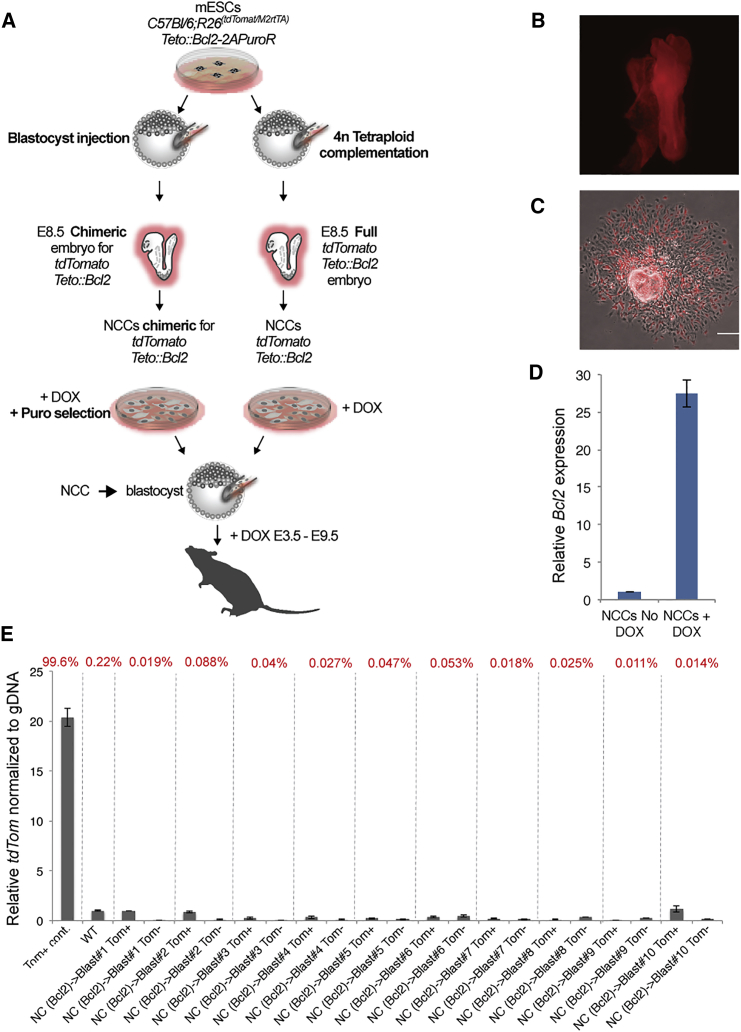
To stringently test the potential of BCL2-expressing NCCs to generate chimeras upon injection into the blastocyst, we prepared primary NCCs overexpressing BCL2 from E8.5 embryos. C57BL/6;R26(tdTomato/M2rtTA);Bcl2-T2A-PuroR mESCs were injected into 4n blastocysts to form all-ESC embryos or into 2n blastocysts to form chimeric embryos. Primary tdTomato+ NCCs that conditionally overexpressed Bcl2 were isolated from tetraploid explanted E8.5 mouse embryos (Figures 2A and 2B) or from chimeric embryos and selected for puromycin resistance (Figure 2C). To induce Bcl2 expression, NCCs were cultured with Dox for 24 hr (Figure 2D). The C57BL/6;R26(tdTomato/M2rtTA);Bcl2-T2A-PuroR primary NCCs were injected into albino mouse blastocysts to assess whether the Bcl2 anti-apoptotic activity supported chimera formation after heterochronic injection into the blastocyst. To maintain Bcl2 expression during the relevant developmental stages, Dox was added to the drinking water of foster mice until mid-gestation. As shown in Table 1 and Figure 2E, we failed to detect donor cell contribution to E10.5 embryos or to coat color in postnatal mice. We conclude that matching the developmental stage of NC donor cells with the host embryo is essential for neural crest chimera formation even when apoptosis is inhibited by BCL2. Our data contrast with Sox17+ endoderm donor cells expressing a Bcl2 transgene generating endoderm chimeras when injected into the blastocyst (Masaki et al., 2016). The different results in our and the previous study may be due to the different experimental designs and may reflect differences in how BCL2 affects cells differentiated in vitro versus cells derived from the embryo. Alternatively, inhibition of apoptosis may allow functional engraftment and chimera formation after heterochronic injection in some but not other lineages.
Figure 2.
Primary NCCs Overexpressing Bcl2 Fail to Contribute to Blastocyst Chimeras
(A) Schematic of the experiment: to derived NCCs overexpressing Bcl2, C57BL/6;R26(tdTomato/M2rtTA); FUW-Teto:Bcl2-T2A-PuroR mESCs were injected into mouse 4n fused blastocysts (right) or 2n blastocysts (left). NCCs were derived from E8.5 neural tube explants and selected as indicated. To activate Bcl2 expression, NCCs were treated with Dox 24 hr prior to injection. NCCs were then injected into mouse blastocysts, transplanted into foster mothers, and tested for chimeric contribution.
(B) A representative all-ESC E8.5 embryo, derived from prior injection of tdTomato-labeled Teto:Bcl2-mESCs into a 4n-tetraploid blastocyst. The whole embryo is derived from the injected cell, and it is all tdTomato positive. NCCs derived from this embryo (no need for puromycin selection) were then injected into blastocysts for secondary chimera formation.
(C) A neural tube explant derived from a chimeric E8.5 mouse embryo, which was injected at the blastocyst stage with Teto:Bcl2 tdTomato-labeled mESCs. Explanted cells were treated with Dox and puromycin for Bcl2 overexpression and drug selection of the transgenic NCCs. Selected NCCs were then injected into blastocysts. Scale bar represents 100 μm.
(D) qPCR assay demonstrating Bcl2 overexpression upon Dox treatment in NCCs (n = 2).
(E) Blastocysts injected with tdTomato-labeled primary NCCs overexpressing Bcl2 were analyzed at E10.5 for contribution of donor cells by FACS. The percentages of tdTomato positive cells of each embryo are presented in red on the top. Tomato positive and negative cells were analyzed by qPCR to determine chimeric contribution, along with appropriate negative and positive controls, as indicated. Overall, even when BCL2 was overexpressed, no cell contributions were found in embryos injected with NCCs. For full statistical analysis of injected embryos, see Table 1. Data are represented as means ± SD.
Human ESCs Do Not Functionally Integrate into the Gastrulating Mouse Embryo
To test whether heterochronic injection of human ESCs (hESCs) would contribute to chimera formation, we introduced GFP-labeled hESCs into E8.5 mouse embryos. The data summarized in Table S1 indicate that donor hESCs did not functionally engraft into the mouse host but rather developed small clusters of outgrowths mainly in the backside of the head (Figure S3A). Moreover, when tdTomato-labeled hESCs were co-injected along with GFP-labeled human NCCs into E8.5 host mouse embryos, hESCs formed clusters of cells, whereas human NCCs were found migrating and contributing to host development, as previously reported (Cohen et al., 2016; Figure S3A). In some cases, hESCs injected into E8.5 embryo were found to form small teratoma outgrowths in postnatal mice (Figure S3B).
Our results are consistent with previous observations where mouse or human ESCs were injected into in vitro cultured mouse embryos. Thus, epiblast stem cells (EpiSCs), which correspond to the post-implantation stage embryo, differentiated to cells of all germ layers when grafted into cultured post-implantation mouse embryos. In contrast, mESCs, which correspond to the pre-implantation stage embryo, failed to do so (Huang et al., 2012). Similarly, hESCs, which are equivalent to mouse EpiSCs, when grafted into cultured gastrula stage mouse embryos, contributed to multiple tissue layers following 2 days of in vitro culture (Mascetti and Pedersen, 2016, Wu et al., 2015), supporting the notion that developmental matching of donor cells and host is important for interspecies cell engraftment. These observations are consistent with the failure of conventional hESCs to form mouse-human interspecies chimeras when injected into mouse blastocysts (James et al., 2006). In efforts to adjust the developmental stage of hPSCs, different protocols have been used to convert conventional hESCs to a naive state that would correspond to mESCs (Gafni et al., 2013, Takashima et al., 2014, Theunissen et al., 2014, Theunissen et al., 2016), but chimera formation was inefficient. In contrast, a recent study used chemical compounds to generate modified human PSCs; when injected into mouse blastocysts, engraftment of the human donor cells was observed in the mid-gestation host embryos (Yang et al., 2017). Thus, the question whether current naive human PSCs are chimera competent and are developmentally equivalent to the mouse blastocyst remains unresolved. Moreover, other parameters such as the proliferation rate of the donor cells, or the evolutionary distance between the host and injected cells serve as a barrier for interspecies chimera formation (Cohen et al., 2016, Masaki and Nakauchi, 2017, Wu et al., 2016).
Previous data suggest that stem cells transplanted into more advanced embryos can functionally engraft. For example, primordial germ cells, when introduced into seminiferous tubules of postnatal mice differentiated to functional spermatozoa (Chuma et al., 2005, Ohinata et al., 2009). Similarly, injection of human embryonic glial cells into the P0 mouse brain generated mature human astrocytes in the adult mouse brain (Windrem et al., 2014). Our findings suggest that this might be restricted to some stem cell populations but not to PSCs. While others reported that injection of hESCs cells into the E14 mouse brain generated mature human neurons in the adult mouse brain (Muotri et al., 2005), we show that mouse and human PSCs develop teratomas rather than functionally contributing to chimeras when introduced into E8.5 mouse embryos.
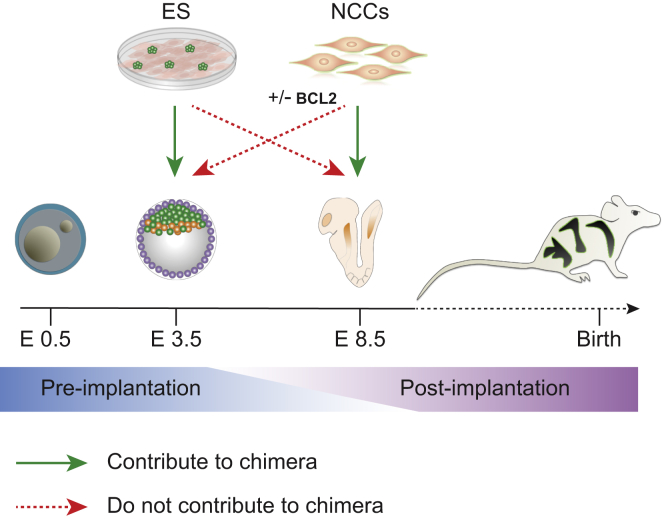
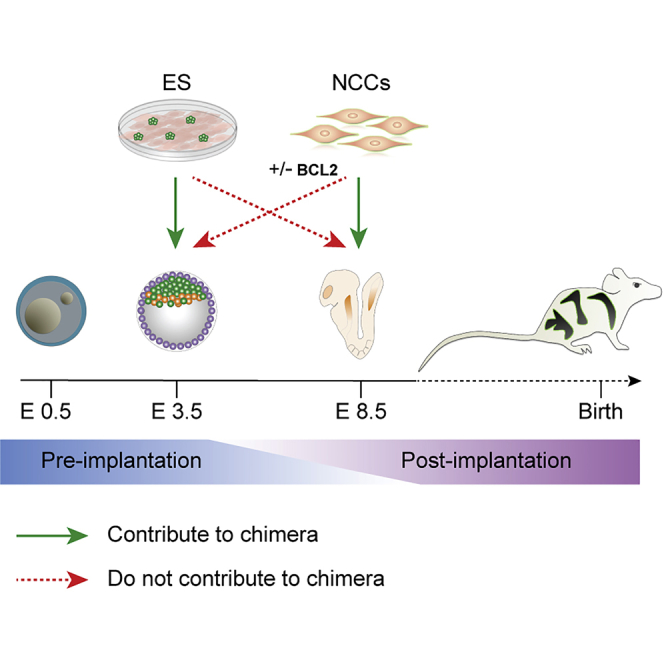
The data presented in this study are consistent with the notion that matching the developmental stage of both the donor and host embryo (isochronic injection) is an important parameter for successful engraftment of ESCs and NCCs into chimeric embryos (Figure 3). Donor cells that were at a more mature or immature stage than the host embryo were not able to contribute to chimera formation. It remains to be seen whether manipulation of the donor cells such as interfering with apoptosis as shown in endodermal donor cells (Masaki et al., 2016) could overcome the inability of some developmentally mismatched donor cell types to engraft.
Figure 3.
Developmental Matching of Donor Cells with the Host Embryo Is Essential for Proper Chimera Formation
The figure summarizes our results. Chimera formation was efficient when the developmental stage of donor and host were matched. In contrast, no chimeric contributions to the host were seen when primary NCCs were injected into blastocysts, even when expression of BCL2 was induced in the NCCs. Similarly, injection of PSCs into gastrulating embryos did not generate chimeras but rather the outgrowth of teratomas. Our data suggest that matching the developmental stage of donor cells with the host embryo is an important parameter for functional engraftment of the donor cells for generating chimeras.
Experimental Procedures
Mouse Lines and Husbandry
Mice were obtained from the Jackson Laboratory and maintained in the Whitehead Institute animal facility. All experiments were approved by the Committee on Animal Care at MIT, and animal procedures were performed following the NIH guidelines.
Microinjection into Pre-implantation Embryos
Diploid embryos were obtained following standard superovulation methods using pregnant mare serum and human chorionic gonadotropin (hCG). To obtain tetraploid (4n) blastocysts, electrofusion was performed at 44–47 hr post hCG using a BEX LF-101 cell fusion apparatus (Protech International). To generate chimeric embryos, 5–6 cells were injected into E3.5 blastocysts and about 6–20 injected blastocysts were surgically transferred into 2.5 days postcoitum pseudo-pregnant CD1 female mice following standard procedures.
Microinjection into Mid-gestation Embryos
Microinjections were performed as previously described (Cohen et al., 2016, Jaenisch, 1985). Laparotomy of E8.5 pregnant females was performed by a long, vertical incision, and the uterus was exposed. Cells were drawn into a glass micropipette and injected into the distal third of the decidual swelling. Roughly, 2–5 × 103 cells (suspended in 0.25–0.75 μL of cell culture medium) were injected per embryo.
Derivation of Primary Mouse NCCs
Female mice were timed pregnant, and primary NCCs were isolated from E8.5 embryos as previously described (Cohen et al., 2016). Briefly, neural tubes from E8.5 embryos were cultured in tissue culture dishes pre-coated with collagen (Thermo Fisher) in DMEM/F12 medium (Thermo Fisher) containing 5% fetal bovine serum (FBS; HyClone), 5% horse serum (ATCC), 1% penicillin/streptomycin (Thermo Fisher), 1 mM L-glutamine (Thermo Fisher), and 1% nonessential amino acids (Thermo Fisher). For gene activation or cell selection, cells were cultured with Dox (2 mg/mL) and puromycin (2 μg/mL; Sigma). The neural tubes were removed after 2 days of culture, and at day 3, the migrating NCCs were dissociated and harvested using Accutase (Thermo Fisher) prior to microinjection.
Immunostaining
Cells were fixed in 4% paraformaldehyde in PBS and immunostained according to standard protocols. Cells were mounted with Fluoro-mount G (Electron Microscopy Sciences) and imaged using a Zeiss LSM 710 laser scanning confocal microscope. Mouse tissues were dissected and fixed in 10% formalin overnight. Tissues were embedded in paraffin, sectioned, and stained for H&E.
Assessment of Cell Contribution to Chimeras
Embryos were harvested between E10.5 to E16.5 of gestation, and the cell contribution to the embryos were determined by the presence of a fluorescent protein signal. Embryos were imaged using a Nikon SMZ18 stereomicroscope. For further analysis, whole embryos were dissociated using Papain (Worthington Biochem) and sorted using Sony SH800S Cell Sorter.
qPCR
For the cell contribution in chimeric embryos, genomic DNA was extracted from sorted cells. All DNA samples were run in technical triplicates using Fast SYBR Green Master Mix (Thermo Fisher) in the QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher). Relative quantification was determined by the changes in steady-state donor cells (tdTomato) across multiple samples and its relative level to DNA internal control (UCNE TFAP2A#463). For Bcl2 expression, total RNA was isolated (RNeasy Kit, QIAGEN) and reverse transcribed (Superscript III First Strand Synthesis kit, Invitrogen). qRT-PCR analysis was performed in triplicate. Gene expression was normalized to GAPDH expression. Error bars represent the SD of triplicate reactions.
Author Contributions
M.A.C. and R.J. conceived of the study. M.A.C. designed experiments, cultured cells, performed post-implantation injections, and analyzed chimeras. S.M. performed pre-implantation injections. M.A.C. and R.J. wrote the manuscript.
Acknowledgments
We thank Y. Stelzer, F. Soldner, T. Theunissen, and P. Nicholls for advice and W. Salmon from the W.M. Keck biological-imaging facility, P. Wisniewski and P. Autissier from the Whitehead FACS facility, R. Alagappan, D. Fu, J. Drotar, R. Flannery, and D. Rooney for experimental assistance. This work was supported by the Emerald Foundation and by R37HD045022, R01-NS088538, and R01-MH104610 NIH grants. R.J. is a co-founder of Fate Therapeutics, Fulcrum Therapeutics, and Omega Therapeutics.
Published: March 29, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one table and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.03.004.
Supplemental Information
References
- Ardehali R., Inlay M.A., Ali S.R., Tang C., Drukker M., Weissman I.L. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc. Natl. Acad. Sci. USA. 2011;108:3282–3287. doi: 10.1073/pnas.1019047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M.R. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chuma S., Kanatsu-Shinohara M., Inoue K., Ogonuki N., Miki H., Toyokuni S., Hosokawa M., Nakatsuji N., Ogura A., Shinohara T. Spermatogenesis from epiblast and primordial germ cells following transplantation into postnatal mouse testis. Development. 2005;132:117–122. doi: 10.1242/dev.01555. [DOI] [PubMed] [Google Scholar]
- Clarke D.L., Johansson C.B., Wilbertz J., Veress B., Nilsson E., Karlström H., Lendahl U., Frisén J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Cohen M.A., Wert K.J., Goldmann J., Markoulaki S., Buganim Y., Fu D., Jaenisch R. Human neural crest cells contribute to coat pigmentation in interspecies chimeras after in utero injection into mouse embryos. Proc. Natl. Acad. Sci. USA. 2016;113:1570–1575. doi: 10.1073/pnas.1525518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Gardner R.L. Mouse chimeras obtained by the injection of cells into the blastocyst. Nature. 1968;220:596–597. doi: 10.1038/220596a0. [DOI] [PubMed] [Google Scholar]
- Geiger H., Sick S., Bonifer C., Müller A.M. Globin gene expression is reprogrammed in chimeras generated by injecting adult hematopoietic stem cells into mouse blastocysts. Cell. 1998;93:1055–1065. doi: 10.1016/s0092-8674(00)81210-6. [DOI] [PubMed] [Google Scholar]
- Huang Y., Osorno R., Tsakiridis A., Wilson V. In vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep. 2012;2:1571–1578. doi: 10.1016/j.celrep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Huszar D., Sharpe A., Jaenisch R. Migration and proliferation of cultured neural crest cells in W mutant neural crest chimeras. Development. 1991;112:131–141. doi: 10.1242/dev.112.1.131. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Mammalian neural crest cells participate in normal embryonic development on microinjection into post-implantation mouse embryos. Nature. 1985;318:181–183. doi: 10.1038/318181a0. [DOI] [PubMed] [Google Scholar]
- James D., Noggle S.A., Swigut T., Brivanlou A.H. Contribution of human embryonic stem cells to mouse blastocysts. Dev. Biol. 2006;295:90–102. doi: 10.1016/j.ydbio.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R., Reyes M., Lenvik T., Lund T., Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M., Teillet M.A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Leitch H.G., Okamura D., Durcova-Hills G., Stewart C.L., Gardner R.L., Matsui Y., Papaioannou V.E. On the fate of primordial germ cells injected into early mouse embryos. Dev. Biol. 2014;385:155–159. doi: 10.1016/j.ydbio.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H., Kato-Itoh M., Takahashi Y., Umino A., Sato H., Ito K., Yanagida A., Nishimura T., Yamaguchi T., Hirabayashi M. Inhibition of apoptosis overcomes stage-related compatibility barriers to chimera formation in mouse embryos. Cell Stem Cell. 2016;19:587–592. doi: 10.1016/j.stem.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Masaki H., Nakauchi H. Interspecies chimeras for human stem cell research. Development. 2017;144:2544–2547. doi: 10.1242/dev.151183. [DOI] [PubMed] [Google Scholar]
- Mascetti V.L., Pedersen R.A. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72. doi: 10.1016/j.stem.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Experimental study of the developing mammalian egg: removal of the zona pellucida. Science. 1962;138:594–595. doi: 10.1126/science.138.3540.594. [DOI] [PubMed] [Google Scholar]
- Muotri A.R., Nakashima K., Toni N., Sandler V.M., Gage F.H. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc. Natl. Acad. Sci. USA. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohinata Y., Ohta H., Shigeta M., Yamanaka K., Wakayama T., Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137:571–584. doi: 10.1016/j.cell.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Takashima Y., Guo G., Loos R., Nichols J., Ficz G., Krueger F., Oxley D., Santos F., Clarke J., Mansfield W. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158:1254–1269. doi: 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam P.P., Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155–6163. doi: 10.1242/dev.00893. [DOI] [PubMed] [Google Scholar]
- Tarkowski A.K. Mouse chimaeras developed from fused eggs. Nature. 1961;190:857–860. doi: 10.1038/190857a0. [DOI] [PubMed] [Google Scholar]
- Theunissen T.W., Friedli M., He Y., Planet E., O'Neil R.C., Markoulaki S., Pontis J., Wang H., Iouranova A., Imbeault M. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell. 2016;19:502–515. doi: 10.1016/j.stem.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen T.W., Powell B.E., Wang H., Mitalipova M., Faddah D.A., Reddy J., Fan Z.P., Maetzel D., Ganz K., Shi L. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15:471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem M.S., Schanz S.J., Morrow C., Munir J., Chandler-Militello D., Wang S., Goldman S.A. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J. Neurosci. 2014;34:16153–16161. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Greely H.T., Jaenisch R., Nakauchi H., Rossant J., Belmonte J.C. Stem cells and interspecies chimaeras. Nature. 2016;540:51–59. doi: 10.1038/nature20573. [DOI] [PubMed] [Google Scholar]
- Wu J., Okamura D., Li M., Suzuki K., Luo C., Ma L., He Y., Li Z., Benner C., Tamura I. An alternative pluripotent state confers interspecies chimaeric competency. Nature. 2015;521:316–321. doi: 10.1038/nature14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Platero-Luengo A., Sakurai M., Sugawara A., Gil M.A., Yamauchi T., Suzuki K., Bogliotti Y.S., Cuello C., Morales Valencia M. Interspecies chimerism with mammalian pluripotent stem cells. Cell. 2017;168:473–486 e415. doi: 10.1016/j.cell.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Dylla S.J., Muijtjens M., Weissman I.L. Enforced Bcl-2 expression overrides serum and feeder cell requirements for mouse embryonic stem cell self-renewal. Proc. Natl. Acad. Sci. USA. 2005;102:3312–3317. doi: 10.1073/pnas.0500167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu B., Xu J., Wang J., Wu J., Shi C., Xu Y., Dong J., Wang C., Lai W. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell. 2017;169:243–257 e25. doi: 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.