SUMMARY
Most animal embryos display a delay in the activation of zygotic transcription during early embryogenesis [1]. This process is thought to help coordinate rapid increases in cell number during early development [2]. The timing of zygotic genome activation (ZGA) during the maternal to zygotic transition (MZT) remains uncertain, despite extensive efforts. We explore ZGA in the simple protovertebrate, Ciona intestinalis. Single-cell RNA-seq assays identified Cyclin B3 (Ccnb3) as a putative mediator of ZGA. Maternal Ccnb3 transcripts rapidly diminish in abundance during the onset of zygotic transcription at the 8-cell and 16-cell stages. Disruption of Ccnb3 activity results in precocious activation of zygotic transcription, while overexpression abolishes activation. These observations suggest that the depletion of maternal Cyclin B3 products is a critical component of the MZT and ZGA. We discuss evidence that this mechanism might play a conserved role in the MZT of other metazoans, including mice and humans.
eTOC blurb
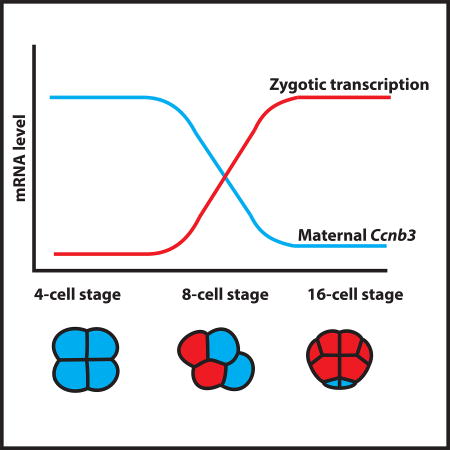
Using a single-cell RNA-seq approach, Treen et al., investigate zygotic genome activation in Ciona and demonstrate that a maternal mRNA encoding Cyclin B3 is a key mediator of the process. This study provides new insights into how the timing of zygotic genome activation can be coordinated with the depletion of maternal mRNAs.
RESULTS
Most animal embryos exhibit a delay in the onset of transcription from the zygotic genome. Early stages of embryogenesis are fueled by maternal products deposited into the unfertilized egg [3]. The onset of zygotic transcription has been shown to coincide with the depletion of maternal mRNAs eventually reaching a transition point when embryogenesis largely depends on the expression of the zygotic genome [1]. This phenomenon is known as the maternal to zygotic transition (MZT). The earliest stages of metazoan development typically involve a number of rapid mitoses that can proceed without the need for newly transcribed mRNAs. The MZT is thought to coordinate rapid increases in cell number [2], resulting in a multicellular embryo defined by differential patterns of zygotic gene activity.
Previous efforts have led to the identification of maternal transcription factors that activate gene expression during zygotic genome activation (ZGA), including Zelda in Drosophila [4] and Nanog/Pou5f1/SoxB1 in zebrafish [5]. In contrast, increasing levels of core histones can have an inhibitory influence on ZGA [6, 7], and there is evidence that the timing of ZGA depends on the depletion of specific maternal mRNAs. For example, in Drosophila, the maternal RNA binding protein Smaug is required for depletion of a variety of maternal mRNAs [8]. Smaug mutants retain many of these RNAs, resulting in the disruption of ZGA and a failure of embryogenesis [9]. A plausible explanation for the sudden onset of ZGA in early development is the occurrence of maternally encoded repressors, which must be depleted in order to enable ZGA. However, their identification has been complicated by the large numbers of maternal mRNAs that display reductions at the onset of ZGA [1].
In the present study, we employ the simple protovertebrate, Ciona intestinalis, to identify maternal repressors that might delay the onset of ZGA. Zygotic transcription is first detected at the 8-cell stage of embryogenesis, followed by a significant increase at the 16-cell stage [10, 11]. During these early stages, Ciona embryos can be easily dissociated into individual blastomeres, thereby permitting single-cell RNA-seq analysis. As we discuss below, these studies identified Cyclin B3 as a putative repressor of ZGA.
Single-cell RNA-seq assays
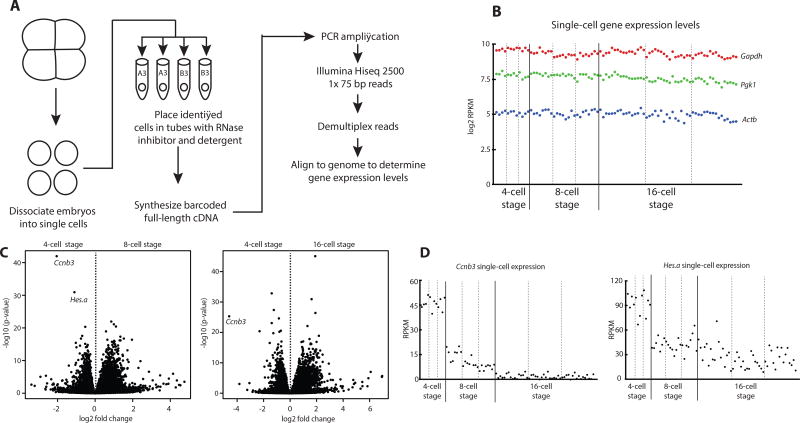
A major strength of the Ciona system is the ease of isolating defined, individual blastomeres from staged embryos. Blastomeres were dissociated by treating embryos with calcium-magnesium free artificial seawater. Every blastomere from 4-cell, 8-cell and 16-cell embryos was isolated in triplicate, spanning the onset of ZGA (Figures 1A, S1). cDNA libraries were prepared from each blastomere and sequenced. These experiments reveal constant expression of constitutive “housekeeping” genes in all blastomeres across the observed time points (Figure 1B).
Figure 1. Single-cell resolution of Ciona early gene expression levels reveal Ccnb3 and Hes.a degradation coincides with the onset of zygotic transcription.
(A) Overview of the procedure used to isolate single-cell transcriptomes of Ciona embryos.
(B) Single-cell expression levels of Gapdh, Pgk1, and Actb. Each dot represents the gene expression levels of a single cell.
(C) Differential expression of all genes comparing cells at the 4-cell stage with the 8-cell stage and the 4-cell stage with the 16-cell stage.
(D) Single-cell expression levels of Ccnb3 and Hes.a. Cells from individual embryos are separated by dotted lines. See also Figure S1.
Known marker genes were identified in appropriate blastomeres. Among these are Pem and Foxa.a (Figure S1C). Pem maternal mRNAs are localized to the future germline [12]. Pem is thought to repress transcription in the germline lineage by direct inhibition of RNA polymerase II elongation in a manner that appears analogous to the germline repressor PIE-1 in C. elegans [13–15]. Foxa.a is a zygotically expressed transcription factor that is required in the early differentiation of several cell lineages [16]. Using these single-cell RNA-seq datasets, we attempted to identify global repressors of zygotic transcription in somatic cell lineages.
Among thousands of different maternal transcripts (Figure S1), Cyclin B3 (Ccnb3) mRNAs were found to exhibit the most dramatic decline between the 4-cell and 16-cell stages of development (Figure 1C). This drop is seen for every blastomere at the 8-cell and 16-cell stages (Figure 1D), and was confirmed by qPCR assays (Figure S1D). Maternal transcripts encoded by Hes.a also display diminishing levels, albeit less dramatic than that seen for Ccnb3 (Figure 1 C–D). Ccnb3 encodes a cyclin that is associated with meiosis in mammals [17]. In early Drosophila embryos, the homologue of Ccnb3 has been shown to be dispensable for mitosis [18] but its degradation is required to proceed to later stages of development [19]. Hes genes encode sequence-specific helix-loop-helix transcriptional repressors that have been implicated in a variety of developmental processes including neurogenesis and somitogenesis [20].
Ccnb3 knockdown can initiate precocious ZGA
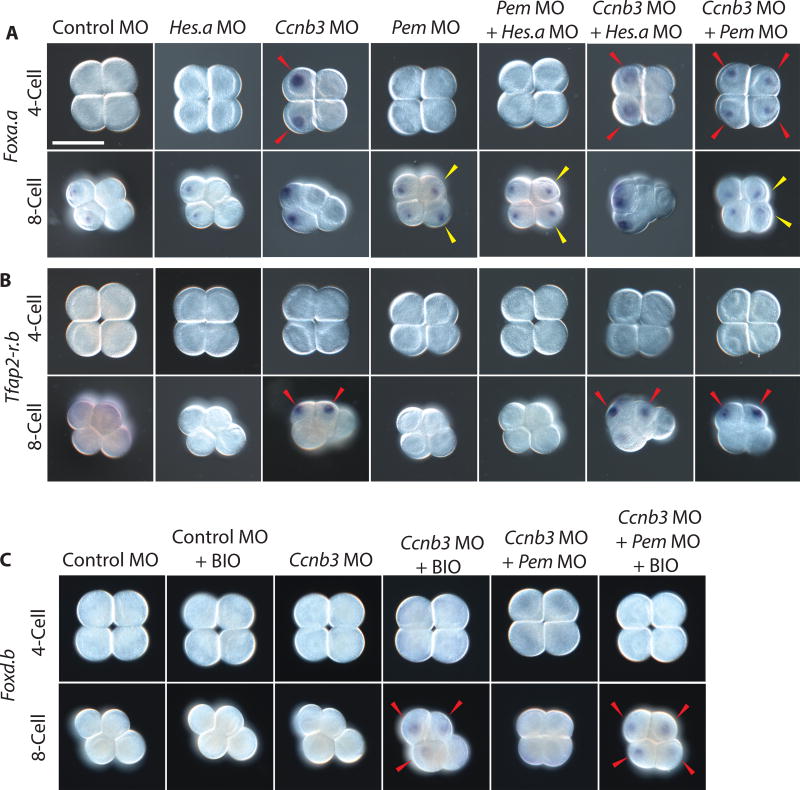
To determine whether Ccnb3 or Hes.a plays a role in the MZT, we inhibited synthesis of the encoded proteins using sequence-specific morpholinos (MOs) spanning the translation initiating region of the coding sequence. Foxa.a and Tfap2-r.b are among the first zygotic genes to be activated during Ciona embryogenesis at the 8-cell and 16-cell stages, respectively [10, 21]. Inhibition of Ccnb3 resulted in precocious activation of Foxa.a and Tfap2-r.b transcription, whereas inhibition of Hes.a had no discernible effect on their expression profiles (Figures 2A–B).
Figure 2. Ccnb3 knockdown results in precocious gene expression.
(A) In situ hybridizations of Foxa.a in early Ciona embryos injected with MOs targeting Hes.a, Ccnb3, and Pem individually or in combination.
(B) In situ hybridizations of Tfap2-r.b under the same experimental conditions as A.
(C) In situ hybridizations of Foxd.b with BIO treatments and Ccnb3 or Ccnb3 + Pem targeting MO injections. Expression in cells matching controls are indicated with a white arrowhead. The expansion of expression into additional cells is indicated with a yellow arrowhead. Precocious expression is indicated with a red arrowhead. Embryos are orientated anterior left. Scale bar = 50 µm. See also Figures S2–S3.
Foxa.a and Tfap2-r.b are activated at the 4-cell and 8-cell stages, respectively, in Ccnb3 morphants (Figures 2 A–B). In both cases, precocious expression is detected in the mother cells of the normal lineages of expression. Foxa.a expression is restricted to A3 blastomeres but absent in B3 blastomeres, probably due to localized Pem repressors in the B3 lineage [12]. MO-targeted inhibition of Pem did not cause precocious expression of Foxa.a or Tfap-r.b, but instead resulted in the previously described expansion of Foxa.a expression in the b/B-lineages of 8-cell embryos [13] (Figure 2A). These results suggest that two independent mechanisms are functioning to inhibit transcription in early Ciona embryos. Pem functions as a localized germline repressor, whereas Ccnb3 may be a general inhibitor of transcription in somatic blastomeres.
To distinguish the activities of Ccnb3 and Pem as somatic and germline repressors, respectively, we performed double knockdown assays by co-injecting both Ccnb3 and Pem MOs. The double morphants exhibit precocious expression of Foxa.a in all of the blastomeres of 4-cell and 8-cell embryos (Figure 2A), including the B lineage where it is normally silenced. Triple knockdowns of Ccnb3, Hes.a and Pem resulted in the same precocious expression of Foxa.a and Tfap2-r.b as that seen in Ccnb3 + Pem double morphants (Figure S2).
Ccnb3 knockdown did not cause precocious expression of Foxd.b, another early zygotic marker gene. However this might be expected as Foxd.b expression is dependent upon nuclear accumulation of beta-catenin, which does not normally influence gene expression until the 16-cell stage [22]. Precocious expression of Foxd.b was observed at the 8-cell stage with the combination of Ccnb3 MO knockdown and treatment with the GSK3-beta inhibitor BIO [20], which accelerates nuclear accumulation of beta-catenin (Figure 2C).
Ccnb3 overexpression inhibits ZGA
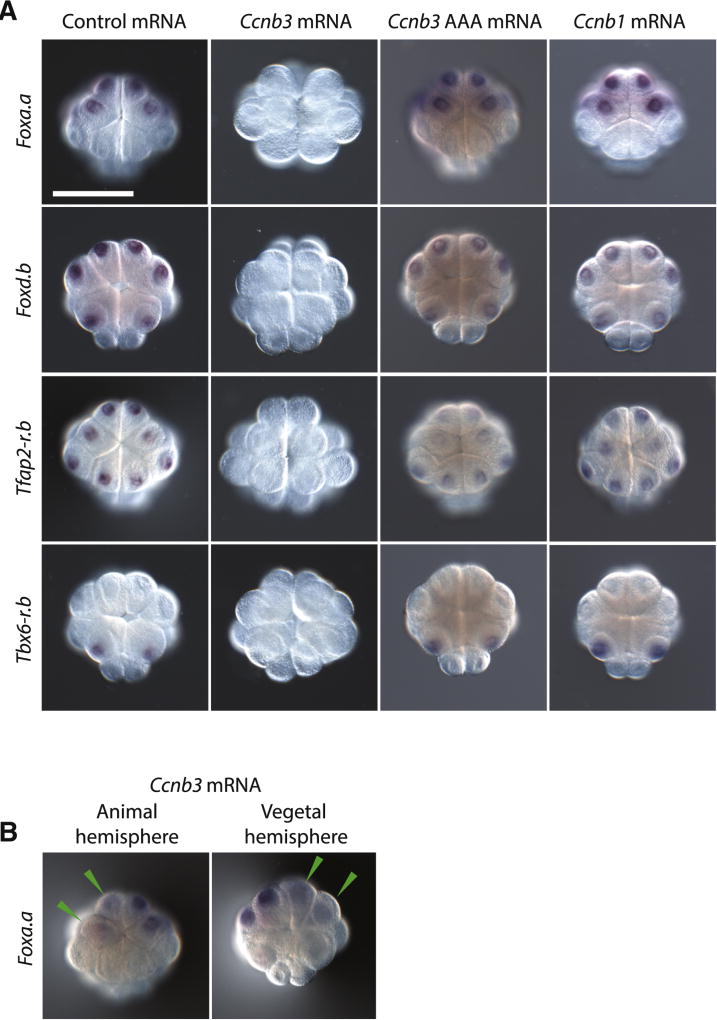
Further evidence that Ccnb3 functions as a somatic repressor of zygotic transcription was obtained by overexpression assays. 5’-capped, polyadenylated Ccnb3 mRNAs were synthesized in vitro using T3 polymerase and injected into fertilized eggs. The injected embryos lack Foxa.a, Foxd.b and Tfap2-r.b transcripts at the 16-cell stage (Figure 3A). They also lack expression of Tbx6-r.b, which is normally restricted to the B5.1 blastomeres [23]. Expression was not lost in embryos injected with a mutant form of the Ccnb3 mRNA harboring inactivating amino acid substitutions in the cdk binding site (Ccnb3 AAA) [24], or in embryos injected with Ccnb1 mRNA. Moreover, mosaic inhibition of Foxa.a expression was obtained by injecting wild-type Ccnb3 mRNAs into a single blastomere at the 2-cell stage (Figure 3B).
Figure 3. Ccnb3 overexpression inhibits gene expression.
(A) In situ hybridizations of early zygotic genes in 16-cell Ciona embryos injected with either in vitro transcribed GFP:H2B control mRNA, Ccnb3 mRNA, Ccnb3 mRNA with mutations in the cdk binding domain (Ccnb3 AAA mRNA) or Ccnb1 mRNA. Ccnb3 overexpression resulted in the failure to detect any expression of early zygotic genes and a loss of cell adhesion.
(B) In situ hybridizations of 16 cell embryos for Foxa.a where one blastomere at the 2-cell stage was injected with Ccnb3 mRNA. Cells that have reduced staining are indicated with a green arrowhead. Embryos are mounted anterior up. For Foxa.a and Tfap2-r.b stainings, the embryo is oriented to show the animal hemisphere and for Foxd.b and Tbx6-r.b stainings, the embryo is oriented to show the vegetal hemisphere. Scale bar = 50 µm. See also Figure S3.
Ccnb3 mRNA overexpression had no effect on the timing of cell divisions from the 1- to 16-cell stage, although there is a minor reduction in timing at the transition from the 16-cell to 32-cell stages (Figure S3A). Ccnb3 morphants exhibit an increase in the duration of the cell cycle during the transition from fertilization to the 1-cell stage, but did not alter the timing of cell cycles at the 2- and 4-cell stages. There is also an increase in the timing during the transition from the 8- to 16-cell stages, from 24 minutes to 42 minutes. Interestingly, this is comparable to the normal timing of the 16- to 32-cell transition, suggesting that precocious ZGA in Ccnb3 morphants might cause a lengthening in the cell cycle (Figure S3A). Mutant embryos arising from overexpression of Ccnb3 mRNAs fail to gastrulate and display severe developmental defects. Ccnb3 morphants gastrulate but fail to neurulate, and therefore display a variety of defects during tail elongation (Figure S3B).
The delayed entry to the 2-cell stage seen for Ccnb3 morphants raises the possibility that precocious transcription is due to an absolute timing mechanism (Figure S3A). Foxa.a transcripts are normally detected at the onset of the 8-cell stage (~104 min following fertilization) in wild-type embryos, and are first seen at ~96 min (4-cell stage) of Ccnb3 morphants. To distinguish the contributions of mitotic cycle number and absolute time in the onset of ZGA, we experimentally extended the duration of the 4-cell stage by treatment with the DNA polymerase inhibitor aphidicolin [25], which inhibits entry into S-phase without interfering with transcription [26]. After permitting development to the equivalent of the 16-cell stage (~140 min), the treated embryos failed to show detectable Foxa.a transcripts (Figure S3C). This observation suggests that absolute timing is not sufficient for the onset of ZGA, although it is conceivable that the degradation of maternal Ccnb3 mRNAs depends on a combination of cell number and absolute timing.
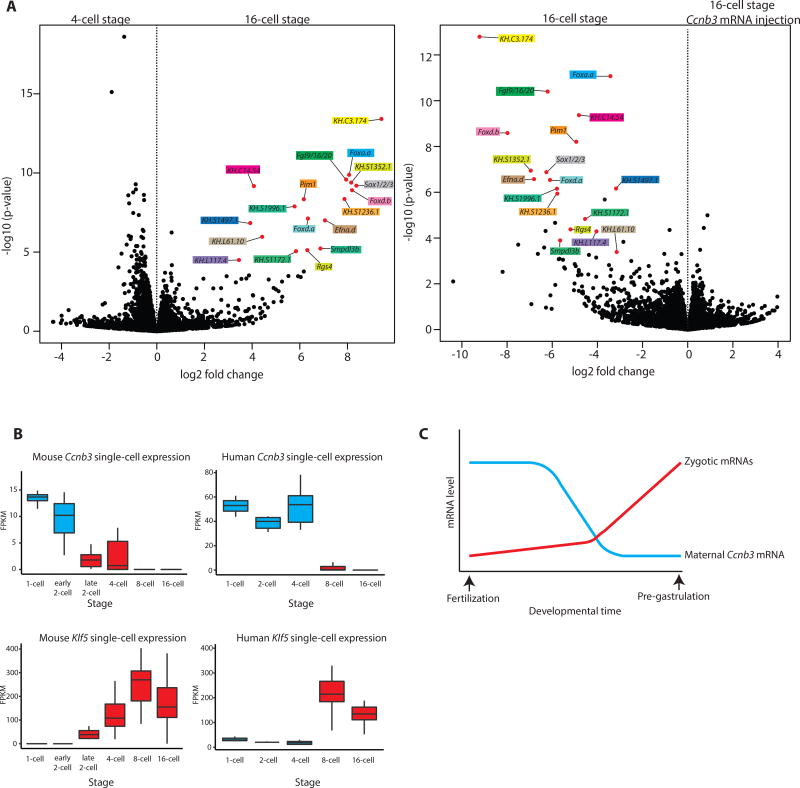
To determine the full impact of Ccnb3 on ZGA, we performed RNA-seq assays on pooled total RNA obtained from embryos injected with Ccnb3 MOs or Ccnb3 mRNA. Housekeeping genes display constant expression in all control and mutant embryos (Figure S4A). In contrast, there is a dramatic drop in expression of all known early zygotic genes upon overexpression of Ccnb3 (Figures 4A, S4B–C), and many of these genes display enhanced expression in Ccnb3 morphants. Several new early zygotic genes were identified in these assays, including the serine/threonine kinase Pim1 and KH.C3.174, a putative vanadium binding protein. These findings suggest that Ccnb3 functions as a key mediator of ZGA by inhibiting transcription during the earliest stages of embryogenesis.
Figure 4. Ccnb3 depletion enables ZGA.
(A) Volcano plots depictions differential analysis of gene expression levels from RNA-seq of pooled Ciona embryo total RNA for the 4- vs 16-cell stage and for the 16-cell stage uninjected vs 16- cell stage Ccnb3 mRNA injection. Each stage and experimental condition was performed in biological triplicate (n=3) with embryos from different parent animals. Genes with an adjusted p-value of <0.1 for the 4 – vs 16- cell stage comparison are indicated by a red dot, named and color coded in both plots.
(B) Box plots of single-cell expression levels of Ccnb3 and Klf5 in early mouse and human embryos. Boxes indicates the lower (25%) and upper (75%) quantile and the solid line indicates the median. Whiskers extend to the 10th and 90th percentile of each distribution. Stages before the major onset of zygotic transcription are colored in blue, and stages after the major onset of zygotic transcription are colored in red.
(C) Overview of the relative levels of Cyclin B3 mRNA correlating with the onset of zygotic transcription. See also Figure S4 and Table S1.
DISCUSSION
The mechanism by which Ccnb3 inhibits transcription is uncertain, although disrupting its cdk binding domain abrogates repression by an otherwise normal Ccnb3 mRNA (Figure 3A). The cdk partners of Ccnb3 might include Cdk1 and/or Cdk2 [17, 18], which can influence the activities of RNA polymerase II [27]. Related cyclins and cdks have also been implicated in the regulation of sequence-specific transcription factors [28] and linker histone H1 [29]. All of these functions would be mediated by protein products encoded by Ccnb3 maternal mRNAs. Because cyclin degradation is coordinated with the cell cycle [30] we expect a close correspondence between the loss of Ccnb3 mRNAs and encoded proteins at the onset of ZGA.
We examined the expression profiles of Ccnb3 in a variety of metazoans to explore the possibility that it might function as a conserved repressor of ZGA. Indeed, maternal expression of different Cyclin B isoforms is seen for every published transcriptome dataset of early embryogenesis. Moreover, each species possesses a maternal Cyclin B that displays diminishing levels of expression, roughly correlating with the onset of zygotic transcription (Table S1). This is best seen for mice and humans, where complete single-cell transcriptomes have been obtained during ZGA [31–33]. These datasets show the elimination of hundreds of maternal mRNAs roughly correlating with the onset of zygotic transcription. Of these eliminated mRNAs, there is a sharp reduction in the levels of Ccnb3 at the onset of zygotic transcription during the early-late 2 cell (mouse) and 4–8 cell (human) stages of development (Figure 4B). These reductions show an inverse correlation with the activation of early zygotic genes such as Klf5, a transcription factor that is involved in the earliest lineage specifications in the mouse embryo [34]. Altogether, these observations suggest that Ccnb3 might function as a key agent of ZGA in Ciona, and possibly other animal embryos.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael Levine (msl2@princeton.edu).
EXEPERIMENTAL MODEL AND SUBJECT DETAILS
Ciona intestinalis (Pacific species also designated Ciona robusta)
Wild-type adult Ciona intestinalis individuals collected from the Pacific Ocean in San Diego County were purchased from M-Rep (San Marcos, CA, USA) and kept in a recirculating aquarium until use. All procedures involving live animals (with the exception of single-cell isolation experiments described below) were performed at 18°C in filtered artificial sea water (Instant Ocean (Blacksburg, VA, USA.) dissolved to 32.5 parts per thousand). Eggs and sperm were extracted by surgical dissection and embryos were cultured in gelatin coated plastic petri dishes.
METHOD DETAILS
Single-cell isolation
Dechorionated Ciona embryos were developed until the appropriate stage. Embryos were transferred to calcium/magnesium free artificial sea water (0.5 M NaCl, 0.01 M KCl, 1.5 mM NaHCO3, 0.02M EGTA, pH 8.0) containing 0.01% sodium thioglucolate, 0.001% pronase, 6 mM NaOH for 1 minute and then transferred to ice-cooled calcium/magnesium free artificial sea water without EGTA. Individual cells were hand-dissected using an eyelash hair held in a hand-pulled glass needle. The procedure was observed using a Leica M165SC dissecting microscope equipped with a Plan APO 1.0× M series objective with fluorescence (Leica, Wetzlar, Germany). Cell identities were determined by embryo geometry and auto fluorescence of vegetal cells. 0.5 µL of calcium/magnesium free artificial sea water without EGTA containing the cell of interest was transferred to a 200 µL tube containing 2.5 µL of RNase free water with 0.075% Triton x100 and 1.2 units of recombinant RNase inhibitor (Takara Bio USA, Mountain View, Ca, USA). The 3 µL single-cell solution was stored at −80°C until processing. All cells used were developed from eggs obtained from the same individual adult Ciona. Embryos that were not used were allowed to develop to larvae and showed normal development.
Single-cell RNA-seq
cDNA was synthesized and amplified from the poly-A containing RNA transcripts in the single-cell lysate using the Smart-Seq2 method [35]. cDNA from cells at 4-cell stage was amplified by 15 cycles of PCR, while 16 cycles of PCR was applied on the cDNA from cells of 8-cell and 16-cell stage embryos. The amplified cDNA samples were cleaned up with Ampure XP beads (Beckman Coulter, CA, USA), quantified by Qubit fluorometer (Invitrogen, CA, USA), and examined on Bioanalyzer with High Sensitivity DNA chips (Agilent, CA, USA) for size distribution. Nextera DNA library prep kit (Illumina, CA, USA) was used to convert the cDNA samples to Illumina sequencing libraries. The libraries were pooled at equal molar amount and sequenced on Illumina HiSeq 2500 Rapid flowcells as single-end 75 nt reads with dual index reads following the standard Illumina protocol. Raw sequencing reads were filtered by Illumina HiSeq Control Software and only Pass-Filter (PF) reads were used for further analysis.
Transcriptome analysis
Sequencing reads were separated according to their barcodes. At least 1.5 million reads were obtained per sample. Reads were aligned to the Ciona genome using the STAR alignment software [36]. Gene expression values were determined by RPKM using the KH2012 gene models [37]. Differential expression analysis was performed using the R package DEseq2 [38]. Following DEseq2, genes were classified as zygotically expressed or Ccnb3 targets if they were found to be upregulated in the 4- vs 16-cell stage comparison or downregulated in the 16-cell vs 16 cell Ccnb3 mRNA overexpression comparison with an adjusted p-value of <0.05. The gene KH.C5.205 met these criteria but was omitted due to high levels of variation between biological replicates.
Quantitative polymerase chain reaction (qPCR)
Dechorionated Ciona embryos were developed until the appropriate stage. For each sample several hundred embryos were lysed in 330 µL RNeasy RLT lysis buffer (Qiagen, Hilden, Germany). Total RNA was isolated from the lysate using RNeasy mini columns according to the manufacturer’s instructions for animal tissue and eluted in 50 µL RNase free water. cDNA synthesis was performed on 2.5 µg total RNA per sample in a 20 µL reaction using the SuperScript IV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and 2.5 µM random hexamers. The reaction conditions were: 23°C for 10 minutes, 50°C for 20 minutes, 80°C for 10 minutes. cDNA samples were then stored at −20°C until processing. qPCR was performed in 25 µl reactions containing 0.5 µl cDNA, 5 pmol of each oligonucleotide primer and 12.5 µL of Power SYBR Green PCR Master Mix (Life Technologies, Warrington, UK). qPCR reactions were performed in a ViiA 7 qPCR instrument (Applied Biosystems, Foster City, CA, USA) in a 96-well plate. The reaction conditions were: 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds, 60°C for 1 minute. Expression levels were determined by comparing the cT of each sample with the cT of the corresponding sample at the 1-cell stage normalized to the cT of Gapdh. All primers sets were validated by the presence of a single peak when a melt-curve analysis was performed.
In vitro mRNA synthesis
The Ccnb3 open reading frame was amplified from Ciona cDNA using the primers agtcgaattcATGCCTCGTACTTCAATTTTCG and gctggaattcTTAAAGTTGAGTGAAATGTTGG and the Ccnb1 open reading frame was amplified using the primers agtcgaattcATGGCAACTACTCTTGCAAAC and gctggaattcTCATAACTTGTGACTTGCTGC (restriction sites in lower case) by PCR using a proof reading polymerase (PrimeSTAR, Takara Bio USA). The PCR product was digested with EcoRI (New England Biolabs, Ipswich, MA, USA) and ligated into a pHTB vector containing the Halocynthia beta actin untranslated regions [39]. Point mutations to inactivate the Ccnb3 cdk binding site were introduced by amplifying the pHTB Ccnb3 plasmid with the primers GGCGATTGATCTGGCCTCAGGTGTTATCTCGGTTTGC and GTTGACGCCATGGTTGAGGTGCAAGAGAAC. The PCR product was phosphorylated and self-ligated. 1 µg of the ligated vector was linearized with KpnI (New England Biolabs) and purified by agarose gel extraction. In vitro transcription was performed using a Megascript T3 kit (Ambion, Foster City, CA, USA) according to the manufacturer’s instructions for a 20 µL reaction with the amendment that 2 µL of GTP was replaced with 0.4 µL of GTP and 1.6 µL of m7G(5')ppp(5')G RNA Cap Structure Analog (New England Biolabs). The reaction was performed for 6 hours. After digestion with DNase I a Poly(A) tailing reaction was performed using a Poly(A) tailing Kit (Invitrogen) according to the manufacturer’s instructions for a 107 µL reaction. mRNA was precipitated using the Megascript T3 kit’s LiCl precipitation solution and the pellet was washed 3 times in 75% ethanol. The control mRNA was synthesized following the same procedure but with the open reading frame of GFP:H2B.
Embryo microinjections
Dechorionated Ciona eggs were microinjected as previously described [40] using custom pulled needles. The microinjection media contained Fast Green and rhodamine dextran to screen successfully microinjected embryos. mRNA injections contained 300 ng/µL mRNA except for injections at the 2-cell stage that contained 150 ng/µL mRNA. Less severe mRNA overexpression phenotypes could be observed at ng/µL. Control MO injections used the human beta-globin MO (CCTCTTACCTCAGTTACAATTTATA, Gene Tools, Philomath, OR, USA) at a concentration of 2mM. Individual gene specific MOs were injected at concentration of 0.65 mM. The Ccnb3 MO could produce the reported phenotypes in all embryos when injected at a concentration of 0.65 mM–2 mM, in approximately 50% of embryos when injected at 0.325 mM and was ineffective at 0.216 mM. Based on previous estimates [41] approximately 30 pL of solution was injected into each egg.
Drug treatments
Ciona embryos were treated with 2.5 µL of 10 mM BIO ((2’Z,3’E)-6-Bromoindirubin-3′-oxime, Millipore Sigma, MO, USA) dissolved in DMSO, to a final concentration of 2.5 µM in 10 mL of artificial sea water. Control embryos were treated with 2.5 µL of DMSO only in 10 mL of artificial sea water. Treatments were performed 15 minutes after fertilization. Cytochalasin B and aphidicolin (both Millipore Sigma, MO, USA) treatments were performed as previously described [25]. Control embryos were treated with 5 µL of DMSO and experimental embryos were treated with either 2.5 µL of 10 mg/mL cytochalasin B dissolved in DMSO, to a final concentration of 2.5 µg/mL in 10 mL of artificial sea water by itself, or in combination with 2.5 µL of 10 mg/mL aphidicolin dissolved in DMSO, to a final concentration of 2.5 µg/mL in 10 mL of artificial sea water.
RNA probe synthesis
cDNA templates for probes were amplified from the appropriate plasmid from a cDNA library by PCR (Table S2) [42]. PCR products were verified by agarose gel electrophoresis and purified by gel extraction. Digoxigenin labeled antisense RNA probes were synthesized in a 20 µL reaction containing T7 RNA polymerase, digoxigenin labelling mix (Roche, Basel, Switzerland) and 500 ng of DNA template at 37°C for 2 hours. Reactions were cleaned up using RNeasy mini columns (Qiagen) according to the manufacturer’s instructions eluting in 50 µL of RNase free water and diluting 1:1 in formamide.
Whole mount in situ hybridization
The procedure performed closely resembled a previously published protocol [43]. Embryos were fixed in a fixation buffer (4% paraformaldehyde, 0.1 M MOPS pH 7.4, 0.5 M NaCl, 1 mM EDTA, 1mM MgSO4, 0.1% Tween 20) at 4°C overnight, washed in PBST and stored in 80% ethanol at −20°C until processing. Embryos were then rehydrated in PBST and treated with PBST containing 2 µg/mL proteinase K at 37°C for 20 minutes, washed twice in PBST and fixed in the fixation buffer for 1 hour at room temperature. Embryos were then washed 4 times in PBST, pre-hybridized for 1 hour at 50°C in 6× SSC, 50% formamide, 5× Denhardt’s solution, 100 µg/mL yeast tRNA, 0.1% tween 20. Hybridizations were performed in 200 µL of pre-hybridization buffer containing approximately 100ng of digoxigenin labeled antisense RNA probes for the gene of interest at 50°C for 16–20 hours. Embryos were then washed twice in 4× SSC, 50% formamide, 0.1% tween 20 for 15 minutes at 50°C, twice in 2× SSC, 50% formamide, 0.1% tween 20 for 15 minutes at 50°C, twice in 2× SSC, 0.1% tween 20 at room temperature. Embryos were then treated with 20 µg/mL RNase A at 37 °C for 30 minutes, washed once in 2× SSC, 0.1% tween 20 at room temperature and twice in 0.5× SSC, 50% formamide, 0.1% tween 20 for 15 minutes at 50 °C. Embryos were then washed 4 times in PBST and blocked for 1 hour at room temperature in PBST containing 0.5× Western blocking reagent (Roche). Embryos were then incubated overnight in PBST containing 0.5× Western blocking reagent with 1/2000 diluted anti-dig AP antibody (Roche, product SKU: 11093274910) at 4°C. Embryos were then washed 6 times in PBST over 1.5 hours and washed 3 times in TMN (100mM NaCl, 50mM MgCl2, 100 mM Tris-Cl pH 8.0). Embryos were then stained in TMN containing NBT/BCIP in the dark for 6 hours. Embryos were then washed in PBST, fixed in the fixation solution overnight at 4°C. Embryos were then washed 4 times in PBST and dehydrated to 100% ethanol to remove background staining, rehydrated to PBST and mounted on a depression glass slide in a drop of 100% glycerol. Images were taken with a Zeiss Axio Imager.A2 microscope with a Zeiss Plan-Apochomat 10×/0.43 objective (Zeiss, Oberkochen, Germany).
Pooled embryo RNA-seq
Injected embryos were developed until the appropriated stage. For each condition 8 embryos were taken in triplicate and total RNA was isolated for each sample using 1 mL of Trizol (Invitrogen) according to the manufacturer’s instructions. The total RNA was dissolved in 10 µL of RNase free water and stored at −80 °C until processing. cDNA synthesis and sequencing was performed as described for single-cells with the exception that 12 cycles of PCR were performed.
QUANTIFICATION AND STATISTICAL SIGNIFICANCE
All experiments where statistical significance is reported were performed in triplicate (three whole individual embryos for each developmental stage for the single-cell analysis as well as three biological replicates from different parent animals for the pooled RNA analysis). Gene expression levels were normalized for visualization by RPKM. Statistical significance for changes in gene expression levels were determined by DEseq2 based on raw read counts. Genes selected for further analysis were selected based on their exceptional levels of fold change expression levels and p-values determined by DEseq2.
Supplementary Material
Highlights.
A single-cell transcriptomic study of Ciona ZGA.
Ccnb3 and Hesa.a are the only mRNAs to be depleted during ZGA.
Ccnb3 knockdown or overexpression disrupts ZGA.
Acknowledgments
We thank Shelby Blythe, Amanda Amodeo, Yosuke Ogura and Yasunori Sasakura for feedback on the manuscript. We thank the Lewis-Sigler Institute Genomics Core Facility for preparing cDNA libraries and sequencing services, and members of the Levine laboratory for helpful discussions. This study was supported by an NIH grant to M.L. (NS076542). T.H. is supported by funding from the NHGRI (T32HG003284).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information includes four figures and two tables.
AUTHOR CONTRIBUTIONS
N.T. and M.L. designed the experiments. N.T. performed the experiments. W.W designed and supervised sequencing assays. N.T. and T.H. performed bioinformatic analyses. N.T, T.H., and M.L. wrote the manuscript.
DECLERATION OF INTERESTS
The authors declare no competing interests.
References
- 1.Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development. 2009;136:3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- 2.O’Farrell PH, Stumpff J, Su TT. Embryonic cleavage cycles: how is a mouse like a fly? Curr. Biol. 2004;14:R35–45. doi: 10.1016/j.cub.2003.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859–870. doi: 10.1242/dev.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang HL, Nien CY, Liu HY, Metzstein MM, Kirov N, Rushlow C. The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature. 2008;456:400–403. doi: 10.1038/nature07388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Flemming ES, Giraldez AJ. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature. 2013;503:360–364. doi: 10.1038/nature12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amodeo AA, Jukam D, Straight AF, Skotheim JM. Histone titration against the genome sets the DNA-to-cytoplasm threshold for the Xenopus midblastula transition. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E1086–E1095. doi: 10.1073/pnas.1413990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph SR, Palfy M, Hilbert L, Kumar M, Karschau J, Zarburaev V, Shevchenko A, Vastenhouw NL. Competition between histone and transcription factor binding regulates the onset of transcription in zebrafish embryos. Elife. 2017:e23326. doi: 10.7554/eLife.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tadros W, Goldman AL, Babak T, Menzies F, Vardy L, Orr-Weaver T, Hughes TR, Westwood JT, Smibert CA. SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev. Cell. 2007;12:143–155. doi: 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Benoit B, He CH, Zhang F, Votruba SM, Tadros W, Westwood JT, Simbert CA, Lipshitz HD, Theurkauf WE. An essential role for the RNA-binding protein Smaug during the Drosophila maternal-to-zygotic transition. Development. 2009;136:923–932. doi: 10.1242/dev.031815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamy C, Rothbacher U, Caillol D, Lemaire P. Ci-FoxA-a is the earliest zygotic determinant of the ascidian anterior ectoderm and directly activates Ci-sFRP1/5. Development. 2006;133:2835–2844. doi: 10.1242/dev.02448. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire P. Unfolding a chordate developmental program, one cell at a time: invariant cell lineages, short-range inductions and evolutionary plasticity in ascidians. Dev. Biol. 2009;332:48–60. doi: 10.1016/j.ydbio.2009.05.540. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S, Marikawa N, Satoh N. Posterior end mark, a novel maternal gene encoding a localized factor in the ascidian embryo. Development. 1996;122:2005–2012. doi: 10.1242/dev.122.7.2005. [DOI] [PubMed] [Google Scholar]
- 13.Shirae-Kurabayshi M, Matsuda K, Nakamura A. Ci-Pem-1 localizes to the nucleus and represses somatic transcription in the germline of Ciona intestinalis embryos. Development. 2011;138:2871–2881. doi: 10.1242/dev.058131. [DOI] [PubMed] [Google Scholar]
- 14.Kumano G, Takatori N, Negishi T, Takada T, Nishida H. A maternal factor unique to ascidians silences the germline via binding to P-TEFb and RNAP II regulation. Curr. Biol. 2011;21:1308–1313. doi: 10.1016/j.cub.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 15.Seydoux G, Mello CM, Pettitt J, Wood WB, Priess JR, Fire A. Repression of gene expression in the embryonic germ lineage of C. elegans. Nature. 1996;382:713–716. doi: 10.1038/382713a0. [DOI] [PubMed] [Google Scholar]
- 16.Hudson C, Sirour C, Yasuo H. Co-expression of Foxa.a, Foxd and Fgf9/16/20 defines a transient mesendoderm regulatory state in ascidian embryos. Elife. 2016:e14692. doi: 10.7554/eLife.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen T, Manova K, Capodieci P, Lindon C, Bottega S, Wang XY, Refik-Rogers J, Pines J, Wolgemuth DJ, Koff A. Characterization and expression of mammalian cyclin b3, a prepachytene meiotic cyclin. J. Biol. Chem. 2002;277:41960–41969. doi: 10.1074/jbc.M203951200. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs HW, Knoblich JA, Lehner CF. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 1998;23:3741–3751. doi: 10.1101/gad.12.23.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan K, O’Farrell PH. Cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition. Curr. Biol. 2015;25:811–816. doi: 10.1016/j.cub.2015.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 21.Oda-Ishii I, Kubo A, Kari W, Suzuki N, Rothbacher U, Satou Y. A maternal system initiating the zygotic developmental program through combinatorial repression in the ascidian embryo. PLoS Genet. 2016;12:e1006045. doi: 10.1371/journal.pgen.1006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai KS, Satoh N, Satou Y. An essential role of a FoxD gene in notochord induction in Ciona embryos. Development. 2002;129:3441–3453. doi: 10.1242/dev.129.14.3441. [DOI] [PubMed] [Google Scholar]
- 23.Takatori N, Hotta K, Mochizuki Y, Satoh G, Mitani Y, Satoh N, Satou Y, Takahashi H. T-box genes in the ascidian Ciona intestinalis: characterization of cDNAs and spatial expression. Dev. Dyn. 2004;230:743–753. doi: 10.1002/dvdy.20082. [DOI] [PubMed] [Google Scholar]
- 24.Bendris N, Lemmers B, Blanchad JM, Arsic N. Cyclin A2 mutagenesis analysis: a new insight into CDK activation and cellular localization requirements. PLoS One. 2011:e22879. doi: 10.1371/journal.pone.0022879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh N. DNA replication is required for tissue-specific enzyme development in ascidian embryos. Differentiation. 1982;21:37–40. doi: 10.1111/j.1432-0436.1982.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 26.Hassan AB, Errington RJ, White NS, Jackson DA, Cook PR. Replication and transcription sites are colocalized in human cells. J. Cell. Sci. 1994;107:425–434. doi: 10.1242/jcs.107.2.425. [DOI] [PubMed] [Google Scholar]
- 27.Lolli G, Johnson LN. Recognition of cdk2 by cdk7. Proteins. 2007;67:1048–1059. doi: 10.1002/prot.21370. [DOI] [PubMed] [Google Scholar]
- 28.Wells AD, Morawski PA. New roles for cyclin-dependent kinases in T cell biology: linking cell division and differentiation. Nat. Rev. Immunol. 2014;14:261–270. doi: 10.1038/nri3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu G, Ghadam P, Sirotkin A, Khochbin S, Skoultchi AI, Clarke HJ. Mouse oocytes and early embryos express multiple histone H1 subtypes. Biol. Reprod. 2003;68:1569–1576. doi: 10.1095/biolreprod.102.012336. [DOI] [PubMed] [Google Scholar]
- 30.Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 31.Deng Q, Ramskold D, Reinius B, Sandberg R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science. 2014;343:193–196. doi: 10.1126/science.1245316. [DOI] [PubMed] [Google Scholar]
- 32.Yan L, Yang M, Guo H, Yang L, Wu J, Li R, Liu P, Lian Y, Zheng X, Yan J, et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 2013;20:1131–1139. doi: 10.1038/nsmb.2660. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Lu X, Dean J. The maternal to zygotic transition in mammals. Mol. Aspects Med. 2014;34:919–938. doi: 10.1016/j.mam.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azami T, Waku T, Matsumoto K, Jeon H, Muratani M, Kawashima A, Yanagisawa J, Manabe I, Nagai R, Kunath T, et al. Klf5 maintains the balance of primitive endoderm to epiblast specification during mouse embryonic development by suppression of Fgf4. Development. 2017;144:3706–3718. doi: 10.1242/dev.150755. [DOI] [PubMed] [Google Scholar]
- 35.Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 36.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingaras TR. STAR: ultrafast universal RNA-seq aligner. Bioninformatics. 2013;291:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satou Y, Mineta K, Ogasawara M, Sasakura Y, Shoguchi E, Ueno K, Yamada L, Matsumoto J, Wasserscheid J, Dewar K, et al. Improved genome assembly and evidence-based global gene model set for the chordate Ciona intestinalis: new insight into intron and operon populations. Genome Biol. 2008;14:R152. doi: 10.1186/gb-2008-9-10-r152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Love MI, Huberm W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akanuma T, Hori S, Darras S, Nishida H. Notch signaling is involved in nervous system formation in ascidian embryos. Dev. Genes Evol. 2002;212:459–472. doi: 10.1007/s00427-002-0264-x. [DOI] [PubMed] [Google Scholar]
- 40.Hikosaka A, Kusakabe T, Satoh N, Makabe KW. Introduction and expression of recombinant genes in ascidian embryos. Develop. Growth & Differ. 1992;34:627–634. doi: 10.1111/j.1440-169X.1992.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 41.Satou Y, Imai KS, Satoh N. Action of morpholinos in Ciona embryos. Genesis. 2001;30:103–106. doi: 10.1002/gene.1040. [DOI] [PubMed] [Google Scholar]
- 42.Satou Y, Yamada L, Mochizuki Y, Takatori N, Kawashima T, Sasaki A, Hamaguchi M, Awazu S, Yagi K, Sasakura Y, et al. A cDNA resource from the basal chordate Ciona intestinalis. Genesis. 2002;33:153–154. doi: 10.1002/gene.10119. [DOI] [PubMed] [Google Scholar]
- 43.Satou Y, Kusakabe T, Araki I, Satoh N. Timing of initiation of muscle-specific gene expression in the ascidian embryo precedes that of developmental fate restriction in lineage cells. Develop. Growth & Differ. 1995;37:319–327. doi: 10.1046/j.1440-169X.1995.t01-2-00010.x. [DOI] [PubMed] [Google Scholar]
- 44.Imai KS, Levine M, Satoh N, Satou Y. Regulatory blueprint for a chordate embryo. Science. 2006;312:1183–1187. doi: 10.1126/science.1123404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.