ABSTRACT
RhoB is a Rho family GTPase that is highly similar to RhoA and RhoC, yet has distinct functions in cells. Its unique C-terminal region is subject to specific post-translational modifications that confer different localization and functions to RhoB. Apart from the common role with RhoA and RhoC in actin organization and cell migration, RhoB is also implicated in a variety of other cellular processes including membrane trafficking, cell proliferation, DNA-repair and apoptosis. RhoB is not an essential gene in mice, but it is implicated in several physiological and pathological processes. Its multiple roles will be discussed in this review.
KEYWORDS: cancer progression, development, membrane trafficking, RhoB, Rho GTPases
Introduction
The small Rho GTPase family of signaling molecules are important regulators of cell and tissue morphology and function, acting mainly through the cellular cytoskeleton.1,2 They are key mediators during diverse cellular and physiological processes like cell division, cell migration, wound healing or immune surveillance. The family consists of 20 members in humans and the dysregulation of their function have been linked to different human pathologies.
RhoB, together with RhoA and RhoC, forms the Rho subfamily within the Rho GTPase family. These three proteins have a high degree of similarity (they share around 87% amino acid sequence identity) although RhoB is the most divergent member of the subfamily. In contrast to RhoA and RhoC, RhoB is encoded by a single exon and it is believed to have arisen from a RhoA reverse copy integration during vertebrate evolution. While RhoA and RhoC genes are present in all vertebrates analyzed to date, the RhoB gene is found in many but not all vertebrates, although it is present in some amphibians, reptiles and birds.3,4
RhoB regulation and signaling
Most of the amino acid differences between RhoB and RhoA/RhoC are near the C-terminus, in the region known as the hypervariable region (Fig. 1). The hypervariable region of RhoB contains mostly polar residues compared to the basic residues found in RhoA and RhoC. This affects the effector and regulatory proteins it binds to.5 RhoB also differs from RhoA and RhoC in the C-terminal CAAX box (C =cysteine, A = alphatic amino acid, X = any amino acid), in which the Cys is modified by isoprenoid lipids. RhoB can be modified by both geranyl-geranyl and farnesyl isoprenoids, whereas RhoA and RhoC are only geranylgeranylated. RhoB can also be palmitoylated at Cys189 and 192. This variety of lipid modifications on RhoB affects its localization and indeed RhoB localizes at the plasma membrane, as well as on endosomes and multivesicular bodies (MVB)6,7 whereas RhoA and RhoC are localized mainly on the plasma membrane or in the cytosol. RhoB has also been reported to localize in the nucleus.8
Figure 1.

Schematic of RhoB protein structure highlighting the different protein domains and known phosphorylation sites. A comparison between the hypervariable region sequence of RhoA, RhoC and RhoB is shown in the box. Red amino acid residues in RhoA and RhoC indicate divergence from the RhoB sequence. CAAX box is also highlighted. CK1: Casein kinase 1; GG: geranylgeranylation; P: palmitoylation; F: farnesylation.
Like most Rho GTPases, RhoB activity is regulated by GTP/GDP loading. It cycles between a GTP-bound active state and a GDP-bound inactive state. GTP-bound Rho proteins interact with their downstream effectors to induce cellular responses. GTP/GDP cycling is mainly regulated by guanine nucleotide exchange factors (GEFs), which exchange GDP for GTP, and GTPase-activating proteins (GAPs) that promote rapid GTP hydrolysis. GEF and GAP proteins specifically regulating RhoB and not RhoA or RhoC have not so far been identified, even though the different localization and lipid modifications of RhoB compared to RhoA and RhoC might be expected to expose it to different GEFs and GAPs. Most GEFs and GAPs tested act on RhoA, RhoB and RhoC, at least in vitro. The RhoGEF XPLN/ARHGEF3 binds to RhoA and RhoB but not RhoC.9 The chaperone protein SmgGDS binds to polybasic C-terminal regions in several GTPases and has GEF activity for RhoA and RhoC but does not bind RhoB.10
Of the 3 Rho guanine nucleotide dissociation inhibitors (RhoGDIs), which are negative regulators of some Rho GTPases,11 only RhoGDI-3 has been described to bind RhoB, whereas RhoGDI1 binds RhoA and RhoC but not RhoB.12
RhoB levels are acutely regulated in response to a variety of stimuli. RhoB is target for ubiquitin-mediated proteasomal degradation, with Smurf1 and the Cullin2-RBX1 complex being its best known ubiquitin ligases.13,14 RhoA and RhoC are also ubiquitylated, however, RhoB protein is much more rapidly degraded by the proteasome than RhoA or RhoC, and has a short half-life of about 30 minutes.15 RhoB protein is normally at low steady-state levels in cells, but can be rapidly and transiently upregulated by several stimuli, including UV irradiation, growth factors, cytokines and during the cell cycle (see below). RhoB mRNA can be regulated by several miRNAs in cancer cell lines and endothelial cells. For example miRNA21 regulates proliferation, migration and invasion of colorectal cancer cells and miR19a promotes pancreatic cancer in vitro and in vivo, in both cases by targeting RhoB.16,17 Interestingly, several studies have shown that RhoB expression is increased after the downregulation of RhoA or RhoC,18-21 although it is not clear if this is via miRNAs or changes in protein stability. RhoB may also be regulated by phosphorylation. RhoB, but not RhoA or RhoC, is phosphorylated and inhibited by casein kinase 1 (CK1) on Ser185 (Fig. 1).22 RhoB has been reported to be phosphorylated on tyrosine residues but the functional relevance is not clear (Fig. 1).23
Most effectors tested in vitro bind equally well to RhoA, RhoB or RhoC. ROCK for example is activated by all 3 proteins, although a direct binding might not be required.24 By contrast, the PRK family of protein kinases has a higher affinity for RhoB than RhoA or RhoC, and for PRK3 this involves interaction with the C-terminal region of RhoB.25 Interestingly, PRK1 is localized to endosomes by RhoB in cells,26 indicating that PRKs are likely to preferentially interact with RhoB in vivo as well as in vitro.
RhoB activity in cells can be analyzed using the Rho-binding domain (RBD) of the Rho effector Rhotekin, which interacts with RhoA, RhoB and RhoC, followed by western blotting with RhoB-specific antibodies. Specific molecular FRET biosensors for the study of RhoA and RhoC activity have been described.27 Intermolecular FRET between RhoB and its target mDia2 has been used in the past for the visualization of RhoB activity on endosomes.28 Most recently, a specific molecular FRET biosensor for the detection of RhoB activity in intact cells has been reported and used, together with RhoA/C probes, to analyze the spatiotemporal activity regulation of these molecules in endothelial cells.29
One of the specific cellular functions of RhoB, related to its localization to intracellular membrane vesicles, is the regulation of endosomal dynamics30 (Fig. 2). By controlling intracellular transport, it regulates signaling by the receptors EGFR and CXCR2, and the intracellular kinases Src and Akt,31-34 thus affecting a variety of physiological processes as described below (Fig. 2).
Figure 2.
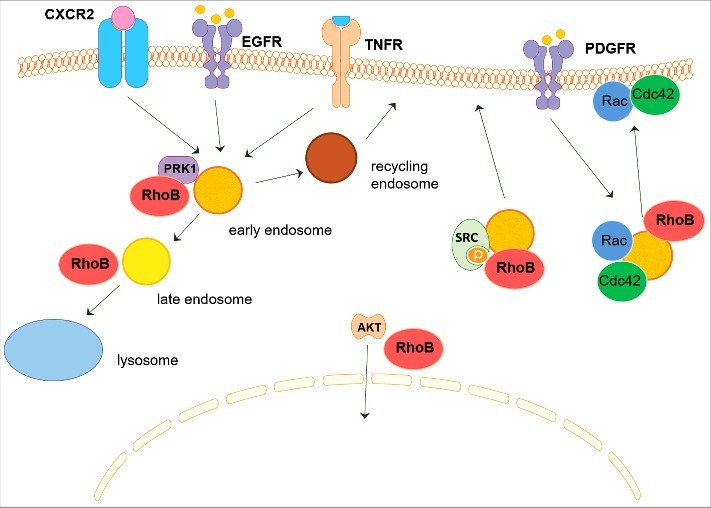
Roles of RhoB in intracellular transport. Endosome-associated RhoB regulates the trafficking and recycling of receptor tyrosine kinases such as EGFR and other receptors like CXCR2 or TNFR, affecting their activity. It also regulates Src activation and transport to membranes and Akt activation and transport to the nucleus. RhoB mediates the PDGF or inflammatory responses by targeting other Rho GTPases like Rac or Cdc42 to the plasma membrane. See text for references.
RhoB function during development
RhoB is not essential for development in mice: RhoB knockout mice are viable and fertile.35 It is possible that signaling by other Rho GTPases is altered in RhoB-null mice, thereby compensating for loss of RhoB. Closer analysis of RhoB-null mice however reveals defects in specific systems and a role for RhoB in several developmental processes. For example, RhoB knockout mice show a reduced thymus weight and cellularity and an increase in TGFβ signaling in the thymic medullary epithelium, where RhoB is normally expressed, implying that RhoB contributes to thymus development and maintenance.36 In addition, RhoB-null mice have retarded vascular development and impaired vessel sprouting in the retina, an effect attributed to a role for RhoB in endothelial cell survival via Akt stabilization in the nucleus.31
RhoB is expressed during mouse development in both neural crest and neural tissues including motor neurons and the floor plate of the neural tube. It is also expressed in the developing endocardial cushions of the atrioventricular and outflow regions of the developing heart and its expression increases as the epithelial-mesenchymal transition (EMT) necessary for generating the valves and septa of the heart progresses.37 This suggests a possible role for RhoB in the formation of the heart membrane valves, although this has not been tested. RhoB is also expressed in glial Müller cells to maintain cellular morphology, in both adult mouse and chick retina.38
In addition to mice, studies in other organisms have demonstrated a role for RhoB in normal development. For example, RhoB activity is necessary for the actin reorganization required for feather bud formation and patterning downstream of Ephrin signaling in chick embryos.39
Where a role for RhoB has been most clearly delineated is in the development of the chick neural crest. RhoB is implicated in the delamination of the neural crest downstream of the transcription factor Slug.35,40 RhoB has also been described to be induced independently of Slug by the transcription factor Sox5 in the pre-migratory chick cephalic neural crest.41 The expression of RhoB is also detected in migrating neural crest cells in Xenopus42 RhoB is proposed to induce neural crest delamination by stimulating cytoskeletal remodeling and the formation of focal adhesions that allow the acquisition of a migratory phenotype in pre-migratory and migratory neural crest cells43 although these early studies relied on the Clostridium botulinum exoenzyme C3 transferase, which inhibits RhoA, RhoB and RhoC. RhoB is also involved in neural crest migration itself, although its overexpression alone is not sufficient to elicit a migratory phenotype.37,42,44 In conclusion, RhoB expression in the neural crest suggests a prominent role in neural crest formation and migration to target tissues, although the mechanism and exact role have not been fully elucidated yet.
RhoB in inflammation and vasculogenesis
Several studies have implicated RhoB in inflammatory responses, particularly in macrophages and endothelial cells. RhoB but not RhoA is reported to be involved in mannose receptor-mediated phagocytosis in human alveolar macrophages.45 RhoB also regulates the secretion of TNFα and nitric oxide by macrophages in a model of LPS-induced inflammation in mice, possibly through a pathway involving NFκB.46
RhoB affects cell adhesion and migration of macrophages by reducing cell surface expression of β2 and β3 integrins but it is not required for the assembly of podosomes, based on studies with primary macrophages from RhoB-null mice.47 Macrophages lacking both RhoB and RhoA (RhoC is not expressed in macrophages) have impaired lamellipodial retraction and altered cell shape. Similar to RhoB-depleted macrophages47 they migrate faster in vitro, which correlates with increased recruitment in response to peritoneal inflammation in vivo.48
RhoB expression is upregulated in mouse macrophages during the inflammatory response to hypoxia in a mechanism involving JNK, ERK and the hypoxia-inducible transcription regulator HIF1α. RhoB depletion in these cells impairs production of inflammatory cytokines both in normoxia and in response to hypoxia.49 RhoB is also rapidly activated by hypoxia and is required for HIF1α stabilization in endothelial cells and other cell types.50,51 Activation of RhoB by hypoxia increases pulmonary endothelial cell contractility, induces endothelial permeability and promotes cell growth in vitro, mediating adaptive changes to chronic hypoxia in the pulmonary vasculature in vivo.51
The farnesylated but not the geranylgeranylated form of RhoB can activate NFκB in several cell types via its downstream target ROCK-I,52 although whether it contributes to inflammatory responses mediated by NFκB is not known. RhoB is also needed for efficient human cytomegalovirus production and infection of fibroblasts, where it contributes to the actin assembly needed for virus spread.53
RhoB also regulates endothelial cell responses to inflammatory signals. RhoB is strongly upregulated in primary human endothelial cells by the pro-inflammatory stimuli TNFα, IL1β and LPS. It specifically regulates some responses to the TNF receptor by controlling its intracellular traffic, for example TNFα-mediated activation of p38MAP kinase and JNK, the latter presumably in a coordinated manner with RhoA.54 Significant RhoB activation in endothelial cells after TNF stimulation has also been detected using a specific RhoB biosensor.29 The inflammatory response in endothelial cells depleted of RhoB is impaired, as measured by expression of the leukocyte receptor ICAM-1 or production of the cytokines IL6 and IL8. During inflammation, endosomal RhoB in the endothelium is upregulated in response to inflammatory cytokines, and RhoB activity regulates Rac1 trafficking to the plasma membrane to control endothelial barrier integrity.21
RhoB is not only implicated in endothelial cell inflammatory responses but also in the regulation of vascular function and angiogenesis. Using siRNAs, RhoB has been shown to be required for endothelial cell migration, sprouting and capillary morphogenesis.55 The microRNA miRNA-21 targets RhoB in endothelial cells to inhibit migration and tubulogenesis.56 How RhoB exert these functions in endothelial cells is not completely clear. One possibility is that it acts through its effects on the actin cytoskeleton, since RhoB is the main regulator of stress fiber formation in endothelial cells under some conditions.56,57 It is also possible that RhoB regulates the activity of other Rho GTPases, like RhoA, or that it affects endothelial cell morphogenesis by regulating growth factor receptor trafficking and signaling, as VEGF induces RhoB expression.55
Loss of RhoB also decreases pathological angiogenesis in ischemic retina and reduces angiogenesis in response of wounding. Conversely, loss of RhoB increased lymphangiogenesis after wounding or inflammation, indicating that RhoB has different effects in blood vessel versus lymphatic endothelial cells.8 Both effects were linked to RhoB-mediated regulation of gene expression through the transcription factor VEZF1.
RhoB in cancer
Rho GTPases, including RhoB, have been extensively studied for their contribution to cancer progression, given their major roles in regulating cell migration and proliferation.58-61
RhoB was first described to contribute to fibroblast transformation downstream of the Ras onco-protein,62 but has subsequently been described to act predominantly as a tumor suppressor. Indeed, RhoB levels decrease with tumor progression in various solid human tumor types,58 and RhoB knock-out mice are more prone to carcinogen-induced skin cancer.35 This could reflect the role of RhoB in regulating cell cycle progression and apoptosis. For example, RhoB is required for the apoptotic response of transformed fibroblasts to DNA damage or taxol,63 and treatment of anaplastic thyroid carcinoma cells with an agonist of PPARγ (Peroxisome-proliferator-activated-receptor-γ) induces cell cycle arrest by RhoB-mediated activation of the cell cycle inhibitor p21.64 RhoB also inhibits migration, invasion, metastasis and tumor growth in some models.65,66 By contrast, RhoB contributes to tumorigenesis in certain tumor models. For example, RhoB knockdown has been described to induce an apoptotic response in renal cells67 and a recent report shows that, in gliomas, RhoB depletion leads to cell cycle arrest, apoptosis and reduced tumorigenic potential in vivo, possibly through p53 activation.68 However, overexpression of RhoB did not induce cell growth in glioma cells, arguing against a tumor-initiating function for RhoB.
As mentioned above, RhoB expression is rapidly upregulated in cancer cells by multiple stimuli including UV irradiation, cytokines, growth factors or toxin treatment (reviewed in ref. 58). These changes in RhoB levels are mediated by gene transcription (as after toxin A or steroid hormones treatment and after tyrosine kinase stimulation), mRNA stabilization (like after TGFβ stimulation or during cell cycle progression) or both (for example after UV light induction). mRNA stability is controlled by the RNA-binding protein HuR, a substrate of the DNA damage-activated checkpoint kinase Chk2.69,70 Epigenetic changes have also been proposed to regulate the RhoB promoter: histone deacetylase-1 (HDAC1) represses RhoB expression and HDAC inhibitors, known to kill tumor cells, actually induce RhoB expression.71,72 In addition, combined inhibition of HDACs and phosphoinositide 3-kinases (PI3Ks) in Burkitt lymphoma cells increases RhoB expression, correlating with reduced cell proliferation and migration.73 Interestingly, RhoB contributes to either cell cycle arrest or apoptosis depending on which HDAC isoform is inhibited,74 suggesting that RhoB may act together with other HDAC isoform-selective targets to affect cellular responses. On the other hand RhoB expression is reduced by several oncogenes including Ras, EGFR or Akt.66,75 Similarly, thyroid hormone receptor re-expression in thyroid cancer cells activates RhoB transcription inducing cell cycle arrest and reducing invasion.76
In addition to increasing RhoB expression, genotoxic stress rapidly activates RhoB. This appears to be mediated by the nuclear GEFs Ect2 and Net1, as downregulation of these proteins, and not 2 cytoplasmic RhoB GEFs, abrogates DNA damage-mediated RhoB activation.77
Distinct lipid modifications on RhoB may play a role in its tumor suppressive role. Farnesyltranferase inhibitors (FTIs) induce accumulation of geranylgeranylated RhoB, which has been proposed to mediate FTI-induced inhibition of proliferation78. RhoB is certainly required for the apoptotic response to FTIs, which is in part mediated by suppression of Cyclin B1 activity.63,79,80
For some time, it has been known that RhoB deletion confers resistance to DNA damaging agents in vitro.63 New evidence reveals that RhoB affects DNA damage repair and how this could at least in part explain its tumor suppressive role (Fig. 3). A direct link between double strand breaks (DSBs) and RhoB expression has been described, involving RhoB mRNA stabilization by Chk2 via its substrate HuR.70 RhoB-deficient cells fail to repair DNA DSBs effectively by homologous recombination, and this is attributed to reduced de-phosphorylation of the histone γH2AX by the phosphatase PP2A. RhoB can also be induced downstream of the DNA-damage activated kinases ATR and Chk1, which suppress RhoB degradation by the ubiquitin ligase Smurf1, thereby promoting RhoB stabilization and apoptosis.13 These mechanisms together offer an explanation for the association between RhoB depletion, genomic instability and tumor progression.
Figure 3.

RhoB in DNA-damage responses. Both after ionizing radiation or UV-induced DNA-damage, RhoB expression is increased due to increased mRNA stability, direct promoter activation and reduced degradation. Active RhoB in turn participates in DNA repair and induces cell cycle arrest or apoptosis in damaged cells. UV: ultraviolet radiation; IR: ionizing radiation. See text for references.
In addition to regulating the DNA damage response, RhoB can affect cell proliferation and tumor growth by regulating intracellular trafficking (see Fig. 2). For example, RhoB is responsible for EGFR sorting to lysosomes and recycling, thus modulating the responses mediated by EGF.7 It also influences trafficking of other signaling molecules including the kinases Akt and Src31,33 The effectors that mediate these functions downstream of RhoB are still not known, although PRKs could be involved since RhoB recruits PRK1 to endosomes.26
RhoB can also influence tumor progression by regulating cancer cell migration, invasion and adhesion (Fig. 4). The effect of RhoB on cell migration is cell type and context-dependent, probably because it affects both intracellular protein trafficking and actin organization. Both RhoB deletion and overexpression appears to be able to reduce migration in vitro, highlighting the importance of a tight control on RhoB expression and function.35,66 However, RhoB depletion can also increase migration speed of prostate cancer cells.81 RhoB affects β-integrin expression and localization, thereby modulating cancer cell adhesion and migration.81,82 RhoB regulates signaling mediated by the Urokinase-type plasminogen activator (uPA), affecting uPA-induced cell adhesion, migration and invasion of prostate cancer cells.82 RhoB can also regulate the function of other Rho GTPases affecting cell migration. For example, RhoB controls the trafficking of Cdc42 and Rac to the cell membrane in response to PDGF, mediating cell movement.83
Figure 4.
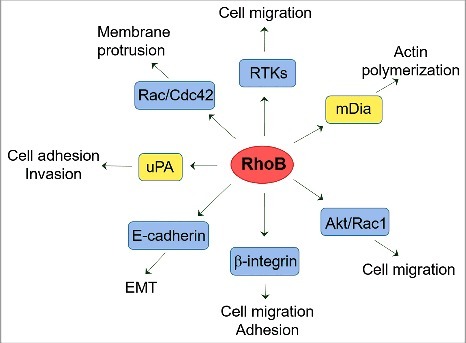
Roles of RhoB in cell migration and adhesion. RhoB acts on several targets (in yellow boxes) and affects the function of adhesion molecules, receptors and/or GTPases to regulate cell migration, invasion and adhesion processes.
Given its role in neural crest development and delamination,35,40 the possible involvement of RhoB in epithelial-mesenchymal transition (EMT) mechanisms during cancer progression has been investigated. RhoB depletion in renal cells did not affect EMT, whereas RhoA and RhoC did contribute to EMT in the same model.67 However, RhoB depletion disrupted cell-cell interaction in prostate cancer cells by altering E-cadherin distribution and levels.84 RhoB depletion promoted Rac1-dependent mesenchymal cell invasion of lung carcinoma cells by mediating induction of the EMT transcription factor Slug and E-cadherin repression. Downregulation of RhoB also induced Akt activation which in turns activates Rac1 via the GEF Trio. The phosphatase PP2A was identified here as a RhoB effector leading to Akt dephosphorylation.85
In summary, RhoB contributes to cancer progression in multiple ways, by regulating DNA damage responses, apoptosis, cell cycle progression, migration and invasion.
Conclusions and perspectives
Despite the sequence similarity with the closely related Rho GTPases RhoA or RhoC, RhoB has proven to have specific and pleiotropic functions in organisms from mammalian development to DNA damage survival responses. Although some of these roles of RhoB can be attributed to regulation of intracellular trafficking of signaling and adhesion molecules, the function of RhoB extends beyond this. More information on how RhoB activity is regulated and identification of RhoB-specific effectors will provide new insight into RhoB function at the cellular level. At the physiological level, it will be important to determine whether RhoB downregulation drives tumor progression and if its signaling could be exploited therapeutically. Given the roles of RhoB in vascular biology and inflammation, its direct involvement in other human pathologies should also be explored.
Funding Statement
This work was supported by Cancer Research UK under Grant C6620/A15961 to Anne J Ridley; Andalucía Talent Hub Program grant launched by the Andalusian Knowledge Agency, co-funded by the European Union's Seventh Framework Program, Marie Skłodowska-Curie actions (COFUND – Grant Agreement no 291780) and the Ministry of Economy, Innovation, Science and Employment of the Junta de Andalucía to Francisco M Vega.
References
- [1].Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol 2013; 92(10-11):303-15; PMID:24183240; https://doi.org/ 10.1016/j.ejcb.2013.09.002 [DOI] [PubMed] [Google Scholar]
- [2].Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol 2015; 36:103-12; PMID:26363959; https://doi.org/ 10.1016/j.ceb.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kersey PJ, Allen JE, Armean I, Boddu S, Bolt BJ, Carvalho-Silva D, Christensen M, Davis P, Falin LJ, Grabmueller C, et al.. Ensembl Genomes 2016: more genomes, more complexity. Nucleic Acids Res 2016; 44(D1):D574-80; PMID:26578574; https://doi.org/ 10.1093/nar/gkv1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et al.. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics 2016; 54:1. [DOI] [PubMed] [Google Scholar]
- [5].Schaefer A, Reinhard NR, Hordijk PL. Toward understanding RhoGTPase specificity: structure, function and local activation. Small GTPases 2014; 5(2):6; https://doi.org/ 10.4161/21541248.2014.968004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adamson P, Paterson HF, Hall A. Intracellular localization of the P21rho proteins. J Cell Biol 1992; 119(3):617-27; PMID:1383236; https://doi.org/ 10.1083/jcb.119.3.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wherlock M, Gampel A, Futter C, Mellor H. Farnesyltransferase inhibitors disrupt EGF receptor traffic through modulation of the RhoB GTPase. J Cell Sci 2004; 117(Pt 15):3221-31; PMID:15226397; https://doi.org/ 10.1242/jcs.01193 [DOI] [PubMed] [Google Scholar]
- [8].Gerald D, Adini I, Shechter S, Perruzzi C, Varnau J, Hopkins B, Kazerounian S, Kurschat P, Blachon S, Khedkar S, et al.. RhoB controls coordination of adult angiogenesis and lymphangiogenesis following injury by regulating VEZF1-mediated transcription. Nat Commun 2013; 4:2824; PMID:24280686; https://doi.org/ 10.1038/ncomms3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem 2002; 277(45):42964-72; PMID:12221096; https://doi.org/ 10.1074/jbc.M207401200 [DOI] [PubMed] [Google Scholar]
- [10].Hamel B, Monaghan-Benson E, Rojas RJ, Temple BR, Marston DJ, Burridge K, Sondek J. SmgGDS is a guanine nucleotide exchange factor that specifically activates RhoA and RhoC. J Biol Chem 2011; 286(14):12141-8; PMID:21242305; https://doi.org/ 10.1074/jbc.M110.191122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93(1):269-309; PMID:23303910; https://doi.org/ 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- [12].Zalcman G, Closson V, Camonis J, Honore N, Rousseau-Merck MF, Tavitian A, Olofsson B. RhoGDI-3 is a new GDP dissociation inhibitor (GDI). Identification of a non-cytosolic GDI protein interacting with the small GTP-binding proteins RhoB and RhoG. J Biol Chem 1996; 271(48):30366-74; PMID:8939998; https://doi.org/ 10.1074/jbc.271.48.30366 [DOI] [PubMed] [Google Scholar]
- [13].Wang M, Guo L, Wu Q, Zeng T, Lin Q, Qiao Y, Wang Q, Liu M, Zhang X, Ren L, et al.. ATR/Chk1/Smurf1 pathway determines cell fate after DNA damage by controlling RhoB abundance. Nat Commun 2014; 5:4901; PMID:25249323; https://doi.org/ 10.1038/ncomms5901 [DOI] [PubMed] [Google Scholar]
- [14].Xu J, Li L, Yu G, Ying W, Gao Q, Zhang W, Li X, Ding C, Jiang Y, Wei D, et al.. The neddylation-cullin 2-RBX1 E3 ligase axis targets tumor suppressor RhoB for degradation in liver cancer. Mol Cell Proteomics 2015; 14(3):499-509; PMID:25540389; https://doi.org/7539118 10.1074/mcp.M114.045211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zalcman G, Closson V, Linares-Cruz G, Lerebours F, Honore N, Tavitian A, Olofsson B. Regulation of Ras-related RhoB protein expression during the cell cycle. Oncogene 1995; 10(10):1935-45; PMID:7539118 [PubMed] [Google Scholar]
- [16].Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng Y, Bi F. miR-21 targets the tumor suppressor RhoB and regulates proliferation, invasion and apoptosis in colorectal cancer cells. FEBS Lett 2011; 585(19):2998-3005; PMID:21872591; https://doi.org/ 10.1016/j.febslet.2011.08.014 [DOI] [PubMed] [Google Scholar]
- [17].Tan Y, Yin H, Zhang H, Fang J, Zheng W, Li D, Li Y, Cao W, Sun C, Liang Y, et al.. Sp1-driven up-regulation of miR-19a decreases RHOB and promotes pancreatic cancer. Oncotarget 2015; 6(19):17391-403; PMID:26041879; https://doi.org/ 10.18632/oncotarget.3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ho TT, Merajver SD, Lapiere CM, Nusgens BV, Deroanne CF. RhoA-GDP regulates RhoB protein stability. Potential involvement of RhoGDIalpha. J Biol Chem 2008; 283(31):21588-98; PMID:18524772; https://doi.org/ 10.1074/jbc.M710033200 [DOI] [PubMed] [Google Scholar]
- [19].Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol 2011; 193(4):655-65; PMID:21576392; https://doi.org/ 10.1083/jcb.201011038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol 2011; 21(12):718-26; PMID:21924908; https://doi.org/ 10.1016/j.tcb.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marcos-Ramiro B, Garcia-Weber D, Barroso S, Feito J, Ortega MC, Cernuda-Morollon E, Reglero-Real N, Fernandez-Martin L, Duran MC, Alonso MA, et al.. RhoB controls endothelial barrier recovery by inhibiting Rac1 trafficking to the cell border. J Cell Biol 2016; 213(3):385-402; PMID:27138256; https://doi.org/ 10.1083/jcb.201504038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tillement V, Lajoie-Mazenc I, Casanova A, Froment C, Penary M, Tovar D, Marquez R, Monsarrat B, Favre G, Pradines A. Phosphorylation of RhoB by CK1 impedes actin stress fiber organization and epidermal growth factor receptor stabilization. Exp Cell Res 2008; 314(15):2811-21; PMID:18590726; https://doi.org/ 10.1016/j.yexcr.2008.06.011 [DOI] [PubMed] [Google Scholar]
- [23].Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res 2008; 7(1):311-8; PMID:18034455; https://doi.org/ 10.1021/pr0701254 [DOI] [PubMed] [Google Scholar]
- [24].Truebestein L, Elsner DJ, Fuchs E, Leonard TA. A molecular ruler regulates cytoskeletal remodelling by the Rho kinases. Nat Commun 2015; 6:10029; PMID:26620183; https://doi.org/ 10.1038/ncomms10029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hutchinson CL, Lowe PN, McLaughlin SH, Mott HR, Owen D. Differential binding of RhoA, RhoB, and RhoC to protein kinase C-related kinase (PRK) isoforms PRK1, PRK2, and PRK3: PRKs have the highest affinity for RhoB. Biochemistry 2013; 52(45):7999-8011; PMID:24128008; https://doi.org/ 10.1021/bi401216w [DOI] [PubMed] [Google Scholar]
- [26].Mellor H, Flynn P, Nobes CD, Hall A, Parker PJ. PRK1 is targeted to endosomes by the small GTPase, RhoB. J Biol Chem 1998; 273(9):4811-4; PMID:9478917; https://doi.org/ 10.1074/jbc.273.9.4811 [DOI] [PubMed] [Google Scholar]
- [27].Donnelly SK, Bravo-Cordero JJ, Hodgson L. Rho GTPase isoforms in cell motility: Don't fret, we have FRET. Cell Adh Migr 2014; 8(6):526-34; PMID:25482645; https://doi.org/ 10.4161/cam.29712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wallar BJ, Deward AD, Resau JH, Alberts AS. RhoB and the mammalian Diaphanous-related formin mDia2 in endosome trafficking. Exp Cell Res 2007; 313(3):560-71; PMID:17198702; https://doi.org/ 10.1016/j.yexcr.2006.10.033 [DOI] [PubMed] [Google Scholar]
- [29].Reinhard NR, van Helden SF, Anthony EC, Yin T, Wu YI, Goedhart J, Gadella TW, Hordijk PL. Spatiotemporal analysis of RhoA/B/C activation in primary human endothelial cells. Sci Rep 2016; 6:25502; PMID:27147504; https://doi.org/ 10.1038/srep25502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci 2005; 118(Pt 12):2661-70; PMID:15944396; https://doi.org/ 10.1242/jcs.02384 [DOI] [PubMed] [Google Scholar]
- [31].Adini I, Rabinovitz I, Sun JF, Prendergast GC, Benjamin LE. RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev 2003; 17(21):2721-32; PMID:14597666; https://doi.org/ 10.1101/gad.1134603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gampel A, Parker PJ, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase rhoB. Curr Biol 1999; 9(17):955-8; PMID:10508588; https://doi.org/ 10.1016/S0960-9822(99)80422-9 [DOI] [PubMed] [Google Scholar]
- [33].Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell 2004; 7(6):855-69; PMID:15572128; https://doi.org/ 10.1016/j.devcel.2004.09.019 [DOI] [PubMed] [Google Scholar]
- [34].Neel NF, Lapierre LA, Goldenring JR, Richmond A. RhoB plays an essential role in CXCR2 sorting decisions. J Cell Sci 2007; 120(Pt 9):1559-71; PMID:17405813; https://doi.org/ 10.1242/jcs.03437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol 2001; 21(20):6906-12; PMID:11564874; https://doi.org/ 10.1128/MCB.21.20.6906-6912.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bravo-Nuevo A, O'Donnell R, Rosendahl A, Chung JH, Benjamin LE, Odaka C. RhoB deficiency in thymic medullary epithelium leads to early thymic atrophy. Int Immunol 2011; 23(10):593-600; PMID:21865151; https://doi.org/ 10.1093/intimm/dxr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Henderson DJ, Ybot-Gonzalez P, Copp AJ. RhoB is expressed in migrating neural crest and endocardial cushions of the developing mouse embryo. Mech Dev 2000; 95(1-2):211-4; PMID:10906464; https://doi.org/ 10.1016/S0925-4773(00)00333-6 [DOI] [PubMed] [Google Scholar]
- [38].Santos-Bredariol AS, Belmonte MA, Kihara AH, Santos MF, Hamassaki DE. Small GTP-binding protein RhoB is expressed in glial Muller cells in the vertebrate retina. J Comp Neurol 2006; 494(6):976-85; PMID:16385489; https://doi.org/ 10.1002/cne.20861 [DOI] [PubMed] [Google Scholar]
- [39].McKinnell IW, Makarenkova H, de Curtis I, Turmaine M, Patel K. EphA4, RhoB and the molecular development of feather buds are maintained by the integrity of the actin cytoskeleton. Dev Biol 2004; 270(1):94-105; PMID:15136143; https://doi.org/ 10.1016/j.ydbio.2004.02.007 [DOI] [PubMed] [Google Scholar]
- [40].del Barrio MG, Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 2002; 129(7):1583-93; PMID:11923196 [DOI] [PubMed] [Google Scholar]
- [41].Perez-Alcala S, Nieto MA, Barbas JA. LSox5 regulates RhoB expression in the neural tube and promotes generation of the neural crest. Development 2004; 131(18):4455-65; PMID:15306568; https://doi.org/ 10.1242/dev.01329 [DOI] [PubMed] [Google Scholar]
- [42].Vignal E, de Santa Barbara P, Guemar L, Donnay JM, Fort P, Faure S. Expression of RhoB in the developing Xenopus laevis embryo. Gene Expr Patterns 2007; 7(3):282-8; PMID:17049930; https://doi.org/ 10.1016/j.modgep.2006.09.002 [DOI] [PubMed] [Google Scholar]
- [43].Liu JP, Jessell TM. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 1998; 125(24):5055-67; PMID:9811589 [DOI] [PubMed] [Google Scholar]
- [44].Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell 2005; 8(2):179-92; PMID:15691760; https://doi.org/ 10.1016/j.devcel.2004.12.010 [DOI] [PubMed] [Google Scholar]
- [45].Zhang J, Zhu J, Bu X, Cushion M, Kinane TB, Avraham H, Koziel H. Cdc42 and RhoB activation are required for mannose receptor-mediated phagocytosis by human alveolar macrophages. Mol Biol Cell 2005; 16(2):824-34; PMID:15574879; https://doi.org/ 10.1091/mbc.E04-06-0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang XH, Wang Y, Diao F, Lu J. RhoB is involved in lipopolysaccharide-induced inflammation in mouse in vivo and in vitro. J Physiol Biochem 2013; 69(2):189-97; PMID:22869204; https://doi.org/ 10.1007/s13105-012-0201-z [DOI] [PubMed] [Google Scholar]
- [47].Wheeler AP, Ridley AJ. RhoB affects macrophage adhesion, integrin expression and migration. Exp Cell Res 2007; 313(16):3505-16; PMID:17692842; https://doi.org/ 10.1016/j.yexcr.2007.07.014 [DOI] [PubMed] [Google Scholar]
- [48].Konigs V, Jennings R, Vogl T, Horsthemke M, Bachg AC, Xu Y, Grobe K, Brakebusch C, Schwab A, Bahler M, et al.. Mouse macrophages completely lacking Rho subfamily GTPases (RhoA, RhoB, and RhoC) have severe lamellipodial retraction defects, but robust chemotactic navigation and altered motility. J Biol Chem 2014; 289(44):30772-84; PMID:25213860; https://doi.org/ 10.1074/jbc.M114.563270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huang G, Su J, Zhang M, Jin Y, Wang Y, Zhou P, Lu J. RhoB regulates the function of macrophages in the hypoxia-induced inflammatory response. Cell Mol Immunol 2015; 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Skuli N, Monferran S, Delmas C, Lajoie-Mazenc I, Favre G, Toulas C, Cohen-Jonathan-Moyal E. Activation of RhoB by hypoxia controls hypoxia-inducible factor-1alpha stabilization through glycogen synthase kinase-3 in U87 glioblastoma cells. Cancer Res 2006; 66(1):482-9; PMID:16397264; https://doi.org/ 10.1158/0008-5472.CAN-05-2299 [DOI] [PubMed] [Google Scholar]
- [51].Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, et al.. Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circ Res 2012; 110(11):1423-34; PMID:22539766; https://doi.org/ 10.1161/CIRCRESAHA.112.264473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rodriguez PL, Sahay S, Olabisi OO, Whitehead IP. ROCK I-mediated activation of NF-kappaB by RhoB. Cell Signall 2007; 19(11):2361-9; PMID:17728102; https://doi.org/ 10.1016/j.cellsig.2007.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goulidaki N, Alarifi S, Alkahtani SH, Al-Qahtani A, Spandidos DA, Stournaras C, Sourvinos G. RhoB is a component of the human cytomegalovirus assembly complex and is required for efficient viral production. Cell Cycle 2015; 14(17):2748-63; PMID:26114383; https://doi.org/ 10.1080/15384101.2015.1066535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kroon J, Tol S, van Amstel S, Elias JA, Fernandez-Borja M. The small GTPase RhoB regulates TNFalpha signaling in endothelial cells. PloS One 2013; 8(9):e75031; PMID:24086429; https://doi.org/ 10.1371/journal.pone.0075031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Howe GA, Addison CL. RhoB controls endothelial cell morphogenesis in part via negative regulation of RhoA. Vascular Cell 2012; 4:1; PMID:22316440; https://doi.org/ 10.1186/2045-824X-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, Colige A, Rakic JM, Noel A, Martial JA, et al.. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PloS One 2011; 6(2):e16979; PMID:21347332; https://doi.org/ 10.1371/journal.pone.0016979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gottesbuhren U, Garg R, Riou P, McColl B, Brayson D, Ridley AJ. Rnd3 induces stress fibres in endothelial cells through RhoB. Biol Open 2013; 2(2):210-6; PMID:23430146; https://doi.org/ 10.1242/bio.20123574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Huang M, Prendergast GC. RhoB in cancer suppression. Histol Histopathol 2006; 21(2):213-8; PMID:16329046 [DOI] [PubMed] [Google Scholar]
- [59].Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett 2008; 582(14):2093-101; PMID:18460342; https://doi.org/ 10.1016/j.febslet.2008.04.039 [DOI] [PubMed] [Google Scholar]
- [60].Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta 2009; 1796(2):91-8; PMID:19327386 [DOI] [PubMed] [Google Scholar]
- [61].Ridley AJ. RhoA, RhoB and RhoC have different roles in cancer cell migration. J Microsc 2013; 251(3):242-9; PMID:23488932; https://doi.org/ 10.1111/jmi.12025 [DOI] [PubMed] [Google Scholar]
- [62].Prendergast GC, Khosravi-Far R, Solski PA, Kurzawa H, Lebowitz PF, Der CJ. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene 1995; 10(12):2289-96; PMID:7784077 [PubMed] [Google Scholar]
- [63].Liu A, Cerniglia GJ, Bernhard EJ, Prendergast GC. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc Natl Acad Sci U S A 2001; 98(11):6192-7; PMID:11353846; https://doi.org/ 10.1073/pnas.111137198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Marlow LA, Reynolds LA, Cleland AS, Cooper SJ, Gumz ML, Kurakata S, Fujiwara K, Zhang Y, Sebo T, Grant C, et al.. Reactivation of suppressed RhoB is a critical step for the inhibition of anaplastic thyroid cancer growth. Cancer Res 2009; 69(4):1536-44; PMID:19208833; https://doi.org/ 10.1158/0008-5472.CAN-08-3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chen Z, Sun J, Pradines A, Favre G, Adnane J, Sebti SM. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J Biol Chem 2000; 275(24):17974-8; PMID:10770919; https://doi.org/ 10.1074/jbc.C000145200 [DOI] [PubMed] [Google Scholar]
- [66].Jiang K, Sun J, Cheng J, Djeu JY, Wei S, Sebti S. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol Cell Biol 2004; 24(12):5565-76; PMID:15169915; https://doi.org/ 10.1128/MCB.24.12.5565-5576.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hutchison N, Hendry BM, Sharpe CC. Rho isoforms have distinct and specific functions in the process of epithelial to mesenchymal transition in renal proximal tubular cells. Cell Signal 2009; 21(10):1522-31; PMID:19477269; https://doi.org/ 10.1016/j.cellsig.2009.05.012 [DOI] [PubMed] [Google Scholar]
- [68].Ma Y, Gong Y, Cheng Z, Loganathan S, Kao C, Sarkaria JN, Abel TW, Wang J. Critical functions of RhoB in support of glioblastoma tumorigenesis. Neuro Oncol 2015; 17(4):516-25; PMID:25216671; https://doi.org/ 10.1093/neuonc/nou228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Westmark CJ, Bartleson VB, Malter JS. RhoB mRNA is stabilized by HuR after UV light. Oncogene 2005; 24(3):502-11; PMID:15543229; https://doi.org/ 10.1038/sj.onc.1208224 [DOI] [PubMed] [Google Scholar]
- [70].Mamouni K, Cristini A, Guirouilh-Barbat J, Monferran S, Lemarie A, Faye JC, Lopez BS, Favre G, Sordet O. RhoB promotes gammaH2AX dephosphorylation and DNA double-strand break repair. Mol Cell Biol 2014; 34(16):3144-55; PMID:24912678; https://doi.org/ 10.1128/MCB.01525-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mazieres J, Tovar D, He B, Nieto-Acosta J, Marty-Detraves C, Clanet C, Pradines A, Jablons D, Favre G. Epigenetic regulation of RhoB loss of expression in lung cancer. BMC Cancer 2007; 7:220; PMID:18047684; https://doi.org/ 10.1186/1471-2407-7-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang S, Yan-Neale Y, Fischer D, Zeremski M, Cai R, Zhu J, Asselbergs F, Hampton G, Cohen D. Histone deacetylase 1 represses the small GTPase RhoB expression in human nonsmall lung carcinoma cell line. Oncogene 2003; 22(40):6204-13; PMID:13679859; https://doi.org/ 10.1038/sj.onc.1206653 [DOI] [PubMed] [Google Scholar]
- [73].Ferreira AC, de-Freitas-Junior JC, Morgado-Diaz JA, Ridley AJ, Klumb CE. Dual inhibition of histone deacetylases and phosphoinositide 3-kinases: effects on Burkitt lymphoma cell growth and migration. J Leukoc Biol 2016; 99(4):569-578; PMID:26561567; https://doi.org/ 10.1189/jlb.2A0415-162R [DOI] [PubMed] [Google Scholar]
- [74].Marlow LA, Bok I, Smallridge RC, Copland JA. RhoB upregulation leads to either apoptosis or cytostasis through differential target selection. Endocrine Related Cancer 2015; 22(5):777-92; PMID:26206775; https://doi.org/ 10.1530/ERC-14-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jiang K, Delarue FL, Sebti SM. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene 2004; 23(5):1136-45; PMID:14647415; https://doi.org/ 10.1038/sj.onc.1207236 [DOI] [PubMed] [Google Scholar]
- [76].Ichijo S, Furuya F, Shimura H, Hayashi Y, Takahashi K, Ohta K, Kobayashi T, Kitamura K. Activation of the RhoB signaling pathway by thyroid hormone receptor β in thyroid cancer cells. PloS One 2014; 9(12):e116252; PMID:25548921; https://doi.org/ 10.1371/journal.pone.0116252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Srougi MC, Burridge K. The nuclear guanine nucleotide exchange factors Ect2 and Net1 regulate RhoB-mediated cell death after DNA damage. PloS One 2011; 6(2):e17108; PMID:21373644; https://doi.org/ 10.1371/journal.pone.0017108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mazieres J, Tillement V, Allal C, Clanet C, Bobin L, Chen Z, Sebti SM, Favre G, Pradines A. Geranylgeranylated, but not farnesylated, RhoB suppresses Ras transformation of NIH-3T3 cells. Exp Cell Res 2005; 304(2):354-64; PMID:15748883; https://doi.org/ 10.1016/j.yexcr.2004.10.019 [DOI] [PubMed] [Google Scholar]
- [79].Kamasani U, Huang M, Duhadaway JB, Prochownik EV, Donover PS, Prendergast GC. Cyclin B1 is a critical target of RhoB in the cell suicide program triggered by farnesyl transferase inhibition. Cancer Res 2004; 64(22):8389-96; PMID:15548709; https://doi.org/ 10.1158/0008-5472.CAN-04-2437 [DOI] [PubMed] [Google Scholar]
- [80].Kamasani U, Liu AX, Prendergast GC. Genetic response to farnesyltransferase inhibitors: proapoptotic targets of RhoB. Cancer Biol Ther 2003; 2(3):273-80; PMID:12878865; https://doi.org/ 10.4161/cbt.2.3.385 [DOI] [PubMed] [Google Scholar]
- [81].Vega FM, Colomba A, Reymond N, Thomas M, Ridley AJ. RhoB regulates cell migration through altered focal adhesion dynamics. Open Biol 2012; 2(5):120076; PMID:22724071; https://doi.org/ 10.1098/rsob.120076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Alfano D, Ragno P, Stoppelli MP, Ridley AJ. RhoB regulates uPAR signalling. J Cell Sci 2012; 125(Pt 10):2369-80; PMID:22366462; https://doi.org/ 10.1242/jcs.091579 [DOI] [PubMed] [Google Scholar]
- [83].Huang M, Satchell L, Duhadaway JB, Prendergast GC, Laury-Kleintop LD. RhoB links PDGF signaling to cell migration by coordinating activation and localization of Cdc42 and Rac. J Cell Biochem 2011; 112(6):1572-84; PMID:21344485; https://doi.org/ 10.1002/jcb.23069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vega FM, Thomas M, Reymond N, Ridley AJ. The Rho GTPase RhoB regulates cadherin expression and epithelial cell-cell interaction. Cell Commun Signal 2015; 13:6; PMID:25630770; https://doi.org/ 10.1186/s12964-015-0085-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bousquet E, Calvayrac O, Mazieres J, Lajoie-Mazenc I, Boubekeur N, Favre G, Pradines A. RhoB loss induces Rac1-dependent mesenchymal cell invasion in lung cells through PP2A inhibition. Oncogene 2015; 35:1760-9; PMID:26148238 [DOI] [PubMed] [Google Scholar]


