Abstract
The relationship between vitamin D and breast cancer is still controversial. The present meta-analysis examines the effects of the 25(OH)D, 1,25(OH)2D and vitamin D intake on breast cancer risk. For this purpose, a PubMed, Scopus and Web of Science-databases search was conducted including all papers published with the keywords “breast cancer” and “vitamin D” with at least one reported relative risk (RR) or odds ratio (OR). In total sixty eight studies published between 1998 and 2018 were analyzed. Information about type of study, hormonal receptors and menopausal status was retrieved. Pooled OR or RR were estimated by weighting individual OR/RR by the inverse of their variance Our study showed a protective effect between 25 (OH) D and breast cancer in both cohort studies (RR = 0.85, 95%CI:0.74–0.98) and case-control studies (OR = 0.65, 95%CI: 0.56–0.76). However, analyzing by menopausal status, the protective vitamin D – breast cancer association persisted only in the premenopausal group (OR = 0.67, 95%CI: 0.49–0.92) when restricting the analysis to nested case-control studies. No significant association was found for vitamin D intake or 1,25(OH)2D. Conclusion: This systematic review suggests a protective relationship between circulating vitamin D (measured as 25(OH) D) and breast cancer development in premenopausal women.
Introduction
Breast cancer is an important public health problem in developed countries as it is one of the most common cancers, being the most if only the female population is considered1. The incidence is decreasing every year, which is partly due to early detection programs2.
In the last decades, cellular in vitro experiments and in vivo models have evaluated the role of vitamin D in the development of breast cancer, finding a protective anticancer role of 1,25(OH)D33. It has been demonstrated that treating breast cancer cells with 1,25(OH)D3 induces two beneficial effects: an anti-proliferative effect4 and a pro-apoptotic effect5,6. The former is linked to the suppression of growth stimulatory signals and the potentiation of growth inhibitory signals, whilst the second one is explained by the bcl-2 family proteins. The interaction between vitamin D and its receptors induces an increase in the expression of pro-apoptotic family member (bax and bak protein) and simultaneously a decrease of anti-apoptotic (bcl-2/bcl-XL)6. In addition, the breast tissue contains the 1-α-hydroxylase, allowing for the generation of the active vitamin D metabolite (1,25 dihydroxyvitamin D) from the circulating precursor (25 hydroxyvitamin D). As vitamin D receptors are found in the breast6, an autocrine role of vitamin D has been suggested7.
Despite this biological background, literature shows inconsistent results8–16 (Table 1). Several additional observational studies have appeared since the last meta-analysis publication (including articles until 2013). The main purpose of the present meta-analysis is to update the relationship between vitamin D exposure and breast cancer risk by adding the studies published more recently. Thus sixty-eight observational studies: thirty of these were case-control, twenty-one were nested case-control and the remaining were cohort studies.
Table 1.
RR of breast cancer and vitamin D in previous meta-analysis.
| Source | Type of vitamin D | Number of included studies | Type of included studies | RR (95%IC) |
|---|---|---|---|---|
| Bauer SR et al. (2013) | 25(OH)D | 9 | Cohort & nested case-control studies | 0.9 (0.97–1.00) |
| Chen P et al. (2010) | 25(OH)D | 21 | Case control, cohort, & cross-sectional studies | 0.55 (0.38–0.80) |
| Intake of vitamin D | 0.91 (0.85–0.97) | |||
| 1,25(OH)2D | 0.99 (0.68–1.44) | |||
| Chen P et al. (2013) | 25(OH)D | 21 | Nested case-control & retrospective studies | 0.86 (0.75–1.00) |
| Population based case control studies | 0.35 (0.24–0.52) | |||
| Hospital based case-control studies | 0.08 (0.08–0.33) | |||
| Gandini S et al. (2011) | 25(OH)D | 10 | Case-control | 0.83 (0.79–0.87) |
| Nested case-control & cohort studies | 0.97 (0.92–1.03) | |||
| Gissel T et al. (2008) | Intake of vitamin D | 6 | Cross sectional, Case-control, cohort & r&omized-control trials | 0.98 (0.93–1.03) |
| Kim Y and Je Y. (2014) | Intake of vitamin D | 24 | Cohort & nested case-control studies | 0.95 (0.88–1.01) |
| 25(OH)D | 0.92 (0.83–1.02) | |||
| Wang D et al. (2013) | 25(OH)D | 14 | Cohort & nested case-control studies | 0.84 (0.75–0.95) |
| Mohr SB et al. (2011) | 25(OH)D | 11 | All | 0.61 (0.47–0.80) |
| Case-control studies | 0.87 (0.77–0.99) | |||
| Nested case-control studies | 0.41 (0.31–0.56) | |||
| Yin L et al. (2010) | 25(OH)D | 9 | All | 0.73 (0.60–0.88) |
| Nested case-control | 0.92 (0.82–1.04) | |||
| Case- control | 0.59 (0.48–0.73) |
Methods
Search strategy
Firstly, the following inclusion criteria were defined: we looked for cohort or case-control studies performed in humans, which reported, at least, one relative risk (RR) or odds ratio (OR) with confidence interval at 95%. (95% CI)
We began our search in Pub-Med, Scopus and Web of Science database using “breast cancer” and “vitamin D” as keywords, finding 2313 articles. After having read the title and abstract, 2123 articles that did not meet the above criteria were eliminated. Next, we carried out a more exhaustive and complete reading, which allowed us to reject another additional 69 articles (Fig. 1). Finally, sixty eight studies meeting our inclusion criteria were identified: fifty one case-control10,17–65 and seventeen cohort studies65–81. Tables 2 and 3 summarize the main characteristics of the included articles.
Figure 1.
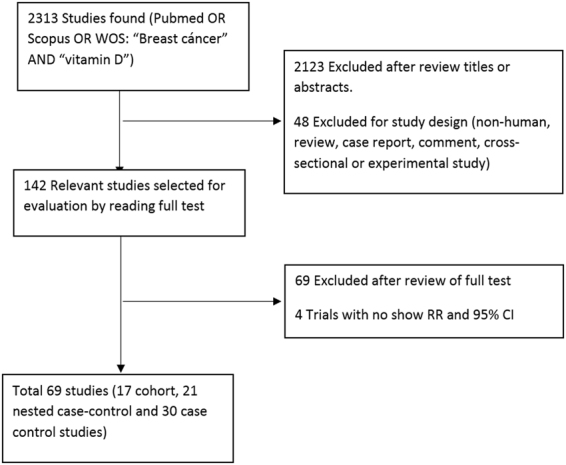
Flowchart which describes the methodology of selection of the articles.
Table 2.
Studies included in our meta-analyses of blood 25-hydroxyvitamin D and breast cancer risk.
| Nested Case-Control | Country | Exposition | Group | OR 95% CI | No. of participants | Age at baselinea | Follow-up period | Upper vs lower cut off levels | Adjusted by Time of blood draw |
|---|---|---|---|---|---|---|---|---|---|
| Almquist M et al.(2010)£,¥,§,φ | Sweden | 25(OH)D3 | All | 0.99 (0.72–1.36) | 1524 | 57 years | 1991–2006 | ≥106 vs ≤70 ng/mL | Yes |
| 25(OH)D3 + D2 | All | 1.01 (0.73–1.40) | ≥107 vs ≤71 ng/mL | ||||||
| 25(OH)D3 | PRE | 1.58 (0.77–3.25) | ≥106 vs ≤70 ng/mL | ||||||
| POST | 0.88 (0.60–1.28) | ≥107 vs ≤71 ng/mL | |||||||
| 25(OH)D3 + D2 | PRE | 1.74 (0.84–3.60) | ≥106 vs ≤70 ng/mL | ||||||
| POST | 0.88 (0.60–1.29) | ≥107 vs ≤71 ng/mL | |||||||
| Amir E et al. (2012)£ | Canada | 25(OH)D | All | 0.86 (0.62–1.21) | 1087 | 53.6 years | 1992–1997 | ≥34.4 vs <12 ng/mL | No |
| Bertone-Johnson ER et al. (2005)£,¥,§ | USA | 25(OH)D | All | 0.73 (0.49–1.07) | 1425 | 52.7 cases 57.1 controls | 1989–1996 | ≥48 vs <20 ng/mL | No |
| 1,25(OH)D | All | 0.76 [0.52–1.11] | ≥38.2 vs <28.5 ng/mL | ||||||
| Chlebowski RT et al. (2008)€,£,§,ǂ,$ | USA | 25(OH)D | POST | 0.82 (0.60–1.12) | 2134 | 50–79 years | 1995–2002 | ≥27.04 vs <12.96 ng/mL | Yes |
| Deschasaux M et al. (2016)£, ¥,ǂ,φ | France | 25(OH)D | All | 0.98 (0.60–1.61) | 699 | 49.3 cases 49.1 controls | 1994–2007 | ≥23.5 vs <11.4 ng/mL | Yes |
| Eliassen AH et al. (2011)£,¥ | USA | 25(OH)D | All | 1.20 (0.88–1.63) | 1827 | 45 cases 44.9 controls | 1996–2007 | ≥30.6vs <18.4 ng/mL | No |
| ER+ | 1.21 (0.84–1.75) | ||||||||
| ER− | 1.31 (0.63–2.74) | ||||||||
| Eliassen AH et al.(2016)£,¥ | USA | 25(OH)D | All | 0.84 (0.58–1.21) | 3012 | 56.7 cases 56.8 controls | 1989–2010 | ≥32.7 ng/ml vs <17.5 | No |
| ER+ | 0.89 (0.74–1.08) | ≥30 ng/ml vs <30 | |||||||
| ER− | 0.87 (0.63–1.20) | ||||||||
| Engel P et al. (2010)€,£, ¥, ǂ | France | 25(OH)D | All | 0.73 (0.55–0.96) | 1908 | 56.9 years | 1995–2005 | >27 vs <19.8 ng/ml | Yes |
| PRE | 0.37 (0.12–1.15) | ||||||||
| POST | 0.80 (0.60–1.07) | ||||||||
| Freedman M et al. (2008)€,£,¥¥,§ | USA | 25(OH)D | POST | 1.04 (0.72–1.51) | 2010 | 55–74 years | 1993–2005 | 33.7 vs 18.3 ng/mL | Yes |
| Hiatt RA et al. (1998)¥,φ | USA | 1,25(OH)2D | All | 1.00 (0.20–3.40) | 192 | >55 years | 1980–1991 | ≥51 vs <32 pg/ml | No |
| Kim Y et al. (2014)£,¥,$ | USA | 25(OH)D | White | 0.13 (0.03–0.71) | 1414 | 68.5 cases 68.4 controls | 2001–2006 | >0 vs 0 ng/mL | Yes |
| African-american | 1.35 (0.65–2.78) | ||||||||
| Hawaian | 1.35 (0.23–7.69) | ||||||||
| Japanese | 1.04 (0.51–2.13) | ||||||||
| Latino | 1.11 (0.51–2.44) | ||||||||
| Kühn T et al. (2013)£,¥,ǂ,φ | Europe | 25(OH)D | All | 1.07 (0.85–1.36) | 2782 | 50.7 years | 1992–2006 | >63 vs ≤39.3nmol/L | No |
| ER+ | 0.97 (0.67–1.38) | ||||||||
| ER− | 0.97 (0.66–1.42) | ||||||||
| McCullough ML et al.(2009)£,¥,$ | USA | 25(OH)D | All | 1.09 (0.70–1.68) | 1032 | 69.5 cases 69.4 controls | 1998–2005 | >76.2vs <36.7 nmol/ml | Yes |
| ER+ | 1.15 (0.80–1.65) | >64.2 vs <45.9 nmol/ml | |||||||
| ER− | 0.95 (0.43–2.06) | ||||||||
| Mohr SB et al. (2013)$ | USA | 25(OH)D | All | 0.84(0.56–1.25) | 1200 | 39.6 years | 1994–2009 | ≥35.2 vs ≤14.9 ng/mL | No |
| Neuhouser ML et al. (2012)£,ǂ | USA | 25(OH)D | POST | 0.94 (0.70–1.28) | 2160 | 50–79 years | 1994–2005 | ≥25.96vs ≤14.68 ng/mL | No |
| Rejnmark L et al. (2009)# | Denmark | 25(OH)D | All | 0.52 (0.32–0.85) | 562 | 58 years | 2003–2007 | >33.6 vs <24 ng/mL | No |
| PRE | 0.38 (0.15–0.97) | ||||||||
| POST | 0.71 (0.38–1.30) | ||||||||
| Scarmo S et al. (2013)£,¥,§ | USA&Sweden | 25(OH)D | All | 0.94 (0.76–1.16) | 4525 | 34–69 years | 1985–2007 1995–2010 | N.A. (Quintiles) | No |
| PRE | 0.67 (0.48–0.92) | ||||||||
| POST | 1.21 (0.92–1.58) | ||||||||
| Shirazi L et al. (2016)€,£, ¥,§ | Sweden | 25(OH)D3 | All | 0.97 (0.75–1.25) | 1520 | 46–73 years | 1991–1996/2006 | ≥98nmol/L vs ≤76nmol/L | Yes |
| Wang J et al. (2014)£,¥ | USA | 25(OH)D | All | 0.95 (0.67–1.36) | 1168 | 45 years | >= 5.59 vs <3.76nmol/L | No | |
| Case-Control | Country | Exposition | Group | OR 95% CI | No. of participants | Age at baseline | Follow-up period | Upper cut off levels | |
| Abbas S et al. (2009)£,¥,φ | Germany | 25(OH)D | PRE | 0.45 (0.29–0.70) | 884 | 42.1 cases 41.6 controls | 1992–1995 | ≥60 vs <30nmol/L | Yes |
| ER+ | 0.56 (0.31–1.00) | ||||||||
| ER− | 0.40 (0.20–0,81) | ||||||||
| Abbas S et al. (2008)£,¥,§ | Germany | 25 (OH)D | POST | 0.31 (0.24–0.42) | 2759 | 63.6 cases 63.5 controls |
2001–2005 | > = 75 vs <30nmol/L | Yes |
| Alipour S et al. (2014)€, ¥ | Iran | 25 (OH)D | All | 0.33 (0.12–0.91) | 500 | 44.2 cases 43.2 controls |
N.A. | >35 ng/ml vs <12.5 ng/ml | No |
| Bilinski K et al. (2012) €,φ | Australia | 25(OH)D | All | 0.43 (0.23–0.77) | 1066 | 55.4 cases 55.5 controls |
2008–2011 | ≥75nmol/L vs <25nmol/mL | Yes |
| <50years | 0.29 [0.08–1] | ||||||||
| ≥50 years | 0.45 [0.23–0.71] | ||||||||
| Chen P et al. (2013)€, ¥,§ | China | 25(OH)D | All | 0.11 (0.07–1.17) | 1173 | 53.0 cases 55.3 controls |
2005–2008 | >17.9 ng/ml vs <10.4 ng/ml | Yes |
| Colagar AH et al. (2015)# | Iran | 25(OH)D | All | 0.26 (0.13–0.50) | 261 | 48.7 cases 47.0 controls |
2009–2013 | ≥16 vs <9 ng/mL | No |
| Crew KD et al. (2009) €,£,¥,§,ǂ,$ | USA | 25(OH)D | All | 0.56 (0.41–0.78) | 2101 | 58.6 cases 56.1 controls |
1996–1997 | ≥40 vs <20 ng/mL | Yes |
| PRE | 0.83 [0.36–1.30] | ||||||||
| POST | 0.46 [0.09–0.83] | ||||||||
| Fedirko V et al. (2012)£,¥¥,§,ǂ,φ | Mexico | 25(OH)D3 | All | 0.53 (0.36–0.78) | 2074 | 53.1 cases 51.3 controls |
2004–2007 | >25 vs ≤20 ng/mL | Yes |
| PRE | 0.40 (0.30–0.81) | ||||||||
| POST | 0.55 (0.33–0.90) | ||||||||
| Jamshidinaein Y et al. (2016)£,§,φ,$ | Iran | 25(OH)D | All | 0.26 (0.12–0.59) | 270 | 50.4 cases 50.0 controls |
2013–2014 | ≥29.5 vs <10.30 ng/ml | Yes |
| PRE | 0.25 (0.09–0.69) | ||||||||
| POST | 0.42(0.15–1.17) | ||||||||
| Janowsky EC et al. (1999)€ | USA | 1,25(OH)2D | All | 0.31 (0.17–0.59) | 331 | NA | 1990–1991 | ≤34.6 vs>63.6pmol/ml | Yes |
| Lowe LC et al.(2005)€ | UK | 25(OH)D | All | 0.17 (0.07–0.43) | 358 | 58.0 cases 58.0 controls |
1998–2003 | ≥150 vs ≤50 nM | Yes |
| Oliveira-Sediyama CM et al.(2016)ǂ | Brazil | 25(OH)D | All | 0.34 (0.16–0.71) | 378 | 54.0 cases 47.5 controls |
NA | ≥20vs <20 ng/mL | No |
| Park S et al. (2015)€,£, ¥,§ | Korea | 25(OH)D | All | 0.82 (0.75–0.90) | 20767 | 50.7 cases 49.7 controls |
2006–2012 | ≥20 vs <20 ng/mL | Yes |
| PRE | 0.84 (0.74–0.96) | ||||||||
| POST | 0.82 (0.73–0.93 | ||||||||
| Sofi NY et al. (2016)# | India | 25(OH)D | All | 0.40 (0.14–1.11) | 200 | 45.0 cases 46.0 controls |
2014–2015 | ≥20 ng/mL vs <20 ng/mL | No |
| Sofi NY et al. (2018)# | India | 25(OH)D | All | 0.42 (0.20–0.83) | 400 | 45.0 cases 47.0 controls |
2015–2017 | ≥20 ng/mL vs <20 ng/mL | No |
| Yao S et al. (2011)€,£,¥ | USA | 25(OH)D | All | 0.37 (0.27–0.51) | 1153 | NA | 2003–2008 | ≥30 vs <20 ng/mL | Yes |
| PRE | 0.57 (0.34–0.93) | ||||||||
| POST | 0.29 (0.19–0.45) | ||||||||
| Yousef FM et al. (2013)€,£,φ | Saudi Arabia | 25(OH)D | All | 0.16 (0.07–0.42) | 240 | 18–75 years | 2009 | ≥20 vs <10 ng/mL | No |
| Ordoñez-Mena JM et al. (2016)€,£,ǂ,φ | Europe | 25(OH)D | POST | 0.73 (0.22–2.43) | 252 | > = 60 years | 1992–2000 | >50 vs <30 nmol/L | Yes |
| Cohort | Country | Exposition | Group | RR 95% CI | Cases (No. of participants) | Age at baseline | Follow-up period | Upper cut off levels | |
| Skaaby T et al. (2014)£,ǂ,φ | Denmark | 25(OH)D | All | 1.1 (0.7–1.71) | 159 (5606) | 18–71 years | 1993–2008 | N.A. (Quartiles) | Yes |
| O´Brien KM (2017) et al€,£, ¥,§,ǂ,φ,$ | USA | 25(OH)D | All | 0.79 (0.63–0.98) | 1600 (3422) | 35–74 years | 2003–2009 | >38 vs <24.6 ng/mL | Yes |
| Ordonez-Mena JM et al. (2013)€,£,ǂ,φ | Germany | 25(OH)D | All | 1.08 (0.72–1.6) | 137 (5261) | 50–74 years | 2000–2002 | <30 vs >55 nmol/L* | No |
| Palmer JR et al. (2016)€,£, ¥,§ | USA (African American Women) | 25(OH)D | All | 0.81 (0.68–0.96) | 1454 (2856) | 21–69 years | 2012–2017 | ≥49 vs <21 ng | No |
| Ordonez-Mena JM et al. (2016)€,£, ǂ,φ | Germany | 25(OH)D | POST | 1.35 (0.38–2.27) | 63 (4990) | 63 years | 2000–2002 | >50 vs <30nmol/L | Yes |
| Ordonez-Mena JM et al. (2016)€,£,ǂ,φ | Norway | 25(OH)D | POST | 2.63 (0.82–8.33) | 89 (2471) | 62 years | 1994–1995 | >50 vs <30nmol/L | Yes |
aMean or range of age.
Adjusted by: €age; £BMI; ¥reproductive factors (menopausal status, age at menopause, age at menarche, parity, etc); §hormone therapy; ǂphysical activity; φeducative or socioeconomic variables; $race or sun exposure.
#Unadjusted.
Abbreviations: CI = confidence interval; POST = postmenopausal; PRE = premenopausal; OR = odds ratio; NA: Not available.
Table 3.
Studies included in our meta-analyses of dietary or supplements vitamin D and breast cancer risk.
| Case-Control | Country | Exposition | Group | OR (95% CI) | No. of participants | Age at baseline | Follow-up period | Upper vs lower cut off levels |
|---|---|---|---|---|---|---|---|---|
| Abbas S et al. (2007)€,£,¥ | Germany | Dietary Vitamin D | PRE | 0.50 (0.26–0.96) | 944 | 41.7 cases 41.6 controls |
1992–1995 | ≥200 vs <80 IU/day |
| Anderson LN et al. (2010)€,¥,ǂ,φ | Canada | Total vitamin D intake | All | 0.99 (0.78–1.26) | 6572 | 56 years | 2002–2003 | ≥15 vs <2.5 mg/day |
| Dietary Vitamin D | 1.13 (0.88–1.45) | ≥10 vs <2.5 mg/day | ||||||
| Vitamin D supplement | 0.76 (0.59–0.98) | ≥10 vs 0 mg/day | ||||||
| Anderson LN et al. (2011)€ | Canada | Vitamin D supplement | All | 0.80 (0.60–1.08) | 3616 | 56 years | 2002–2003 | >400 vs 0 IU/day |
| Total Vitamin D intake | 0.87 (0.71–1.06) | ≥600 vs <200 IU/day | ||||||
| Bidgoli SA et al. (2014)# | Iran | Vitamin D supplement | PRE | 0.89 (0.84–0.95) | 176 | 36.5 cases 34.2 controls | 2010–2012 | Yes vs No |
| Jamshidinaein Y et al. (2016)€,£,¥,§,φ | Iran | Dietary vitamin D | All | 0.38 (0.18–0.83) | 270 | 50.4 cases 50 controls | 2013–2014 | NA (Quartile) |
| Dietary vitamin D | PRE | 0.39 (0.15–1.00) | ||||||
| Dietary vitamin D | POST | 0.40 (0.15–1.12) | ||||||
| Total vitamin D intake | All | 0.52 (0.25–1.14) | ||||||
| Total vitamin D intake | PRE | 0.36 (0.13–1.06) | ||||||
| Total vitamin D intake | POST | 0.70 (0.27–1.82) | ||||||
| Kawase T et al. (2010)£,¥,§,ǂ | Japan | Dietary Vitamin D | All | 0.76 (0.63–0.90) | 5409 | 20–79 | 2001–2005 | >6.66 vs <2 mg/day |
| PRE | 0.65 (0.50–0.86) | |||||||
| POST | 0.83 (0.64–1.07) | |||||||
| Lee MS et al. (2011)€,£,¥,φ | Taiwan | Dietary Vitamin D | All | 0.57 (0.28–1.19) | 400 | 52.5 cases 48.9 controls | 2004–2005 | >5 vs <2 mg/day |
| Dietary Vitamin D | PRE | 0.38 (0.14–0.98) | ||||||
| Dietary Vitamin D | POST | 0.60 (0.20–1.69) | ||||||
| Total vitamin D intake | All | 0.52 (0.25–1.07) | NA (Quartile) | |||||
| Total vitamin D intake | PRE | 0.47 (0.18–1.23) | ||||||
| Total vitamin D intake | POST | 0.68 (0.23–1.27) | ||||||
| Levi F et al.(2001)€,£,¥,φ | Switzerland | Vitamin D supplement | All | 1.43 (0.90–2.26) | 731 | 23–74 | 1993–1999 | ≥2.7 vs <1.4 mg/day |
| Leung et al.(2016)€ | China | Vitamin D supplement | All | 0.78 (0.63–0.98) | 323612 | >18 | 2000–2011 | ≤15 DDD |
| Potischman N et al. (1999)€,¥,§,φ | USA | Dietary Vitamin D | All | 0.98 (0.80–1.20) | 2019 | 20–44 | 1990–1992 | ≥400 vs <0 IU |
| Rollison DE et al. (2012)€,£,¥,§,ǂ | USA | Dietary Vitamin D | All | 1.35 (1.15–1.60) | 4839 | 24–79 | 1999–2004 | 7.71 vs <3.06 mg/day |
| Vitamin D supplement | All | 0.79 (0.65–0.96) | 24–79 years | 1999–2004 | 0 vs>10 mg/day | |||
| Rossi M et al. (2009)€,£,¥,§,φ | Italy | Dietary Vitamin D | All | 0.76 (0.58–1.00) | 5157 | 55 years cases 56 controls | 1991–1994 | >3.57 vs ≤3.57 mg |
| PRE | 0.80 (0.64–0.99) | |||||||
| POST | 0.78 (0.66–0.92) | |||||||
| Salarabadi A et al. (2015)# | Iran | Vitamin D supplement | PRE | 0.53 (0.14–1.96) | 152 | NA | 2012–2014 | Yes vs No |
| Cohort | Country | Exposition | Group | RR (95% CI) | Cases/Total | Age at baseline | Follow-up period | Upper cut off levels |
| John EM et al. (1999)€,£,¥,ǂ,φ | USA | Dietary vitamin D | All | 0.85 (0.59–1.24) | 190/5009 | 25–74 | 1971–1992 | ≥200 vs <100 IU/day |
| Vitamin D supplement | All | 0.89 (0.60–1.32) | 25–74 | 1971–1993 | Daily vs never | |||
| Total vitamin D intake | All | 0.86 (0.61–1.2) | 25–74 | 1971–1994 | ≥200 or daily suppl vs <100 IU/day without daily suppl | |||
| Shin MH et al. (2002)€,£,¥,ǂ | USA | Total vitamin D intake | PRE | 0.89 (0.68–1.15) | 3482/88 691 | 46.7 | 1980–1996 | >500 vs ≤150 IU/day |
| POST | 0.93 (0.8–1.08) | |||||||
| Dietary Vitamin D | PRE | 0.84 (0.59–1.18) | ||||||
| POST | 0.86 (0.7–1.05) | |||||||
| Lin J et al. (2007)€,£,¥,§,ǂ | USA | Total vitamin D intake | PRE | 0.65 (0.42–1) | 1019/31487 | 55 (≥45) | 1993–2003 | ≥548 vs <162 IU/d |
| POST | 1.30 (0.97–1.73) | |||||||
| Dietary vitamin D | PRE | 1.02 (0.69–1.53) | ≥319 vs <142 IU/d | |||||
| POST | 1.22 (0.95–1.55) | |||||||
| Vitamin D supplement | PRE | 0.76 (0.5–1.17) | ≥400 vs 0 IU/d | |||||
| POST | 0.87 (0.68–1.12) | |||||||
| Robien K et al. (2007)€,£,¥,§,φ | EEUU | Vitamin D supplement | POST | 0.89 (0.74–1.08) | 2440/34321 | 61 (55–69) | 1986–2004 | ≥800 IU/d vs No |
| Dietary Vitamin D | POST | 0.55 (0.24–1.22) | ≥800 vs <400 IU/d | |||||
| Total vitamin D intake | POST | 0.89 (0.77–1.03) | ≥800 vs <400 IU/d | |||||
| Kuper H et al. (2009)€,£,¥,§,ǂ | Sweden | Dietary vitamin D | All | 0.90 (0.80–1.1) | 848/41889 | 30–49 | 1991–2003 | N.A. (Quartile) |
| Cadeau C et al. (2015) €,£,¥,§,ǂ | France | Vitamin D supplement | All | 1.10 (0.92–1.31) | 2482/57403 | 40–65 | 1995–2008 | Current vs never |
| ER+ | 1.23 (1–1.51) | 40–65 | 1995–2008 | Current vs never | ||||
| ER− | 0.93 (0.55–1.55) | 40–65 | 1995–2008 | Current vs never | ||||
| Abbas S et al. (2013)€,¥,§,ǂ,φ | Europe | Dietary vitamin D | All | 1.04 (0.94–1.14) | 7760/319985 | 50.2 | 1992–2005 | ≥5.46 vs <1.85 mg/day |
| PRE | 1.07 (0.87–1.32) | ≥5.46 vs <1.85 mg/day | ||||||
| POST | 1.02 (0.9–1.16) | ≥5.46 vs <1.85 mg/day | ||||||
| McCullough ML et al. (2005)€,¥,§,ǂ,φ | USA | Total vitamin D intake | POST | 0.94 (0.8–1.1) | 2855/68567 | 50–74 | 1992–2001 | >700 vs ≤100 IU/day |
| Dietary vitamin D | POST | 0.87 (0.75–1) | >300 vs ≤100 IU/day | |||||
| Edvarsen K et al. (2011) €,£,¥,§ | Norway | Dietary vitamin D | All | 1.07 (0.87–1.32) | 948/41811 | 40–70 | 1997–2007 | 12.31 vs <3.99 mg/day |
| Frazier et al. (2004)€,£,¥,§ | USA | Dietary vitamin D | All | 0.92 (0.66–1.27) | 838/47355 | 34–51 | 1989–1998 | 591 vs 159.6 IU/day |
| Engel P et al. (2011)£,¥,§,ǂ | France | Total vitamin D intake | All | 0.94 (0.86–1.03) | 2871/67721 | 41.8–72 | 1990–2008 | >113 vs <80 IU/day |
| PRE | 1.03 (0.85–1.25) | |||||||
| POST | 0.92 (0.86–1.03) | |||||||
| Nested Case-Control | Country | Exposition | Group | OR (95% CI) | No. of participants | Age at baseline | Follow-up period | Upper vs lower cut off levels |
| Simard A et al. (1991)# | Canada | Dietary Vitamin D | All | 2.79 (0.85–9.15) | 430 | 40–59 | 1981–1983 | >200 vs <50 IU/day |
| Kim Y et al. (2014)£,¥,ǂ | USA | Vitamin D supplement | White | 1.29 (0.75–2.23) | 1414 | 67.8 | 2001–2010 | > = 16 ng/mL vs <16 ng/mL |
| African-american | 0.29 (0.12–0.70) | |||||||
| Hawaian | 0.46 (0.16–1.34) | |||||||
| Japanese | 1.32 (0.90–1.93) | |||||||
| Latino | 0.85(0.46–1.56) | |||||||
| PRE | 1.03 (0.85–1.25) | |||||||
| POST | 0.92 (0.86–1.03) |
aMean or range of age.
Adjusted by: €age; £BMI; ¥reproductive factors (menopausal status, age at menopause, age at menarche, parity, etc); §hormone therapy; ǂphysical activity; φeducative or socioeconomic variables; $race or sun exposure.
#Unadjusted.
Abbreviations: CI = confidence interval; POST = postmenopausal; PRE = premenopausal; OR = odds ratio; NA: Not available.
Data extraction
The following step was to create a database to gather all relevant information extracted from each article: year of publication, author, journal, follow up, country, sample size, exposure levels, units of measure, data for the creation of the contingency table and RR/OR with 95% CI; as well as a section to assess the quality of the study using the STROBE scale82.
Statistical analysis
Statistical analysis was performed separately for cohort and case-control studies. In the case control studies a sensitivity analysis was also carried-out including only nested case-control studies. We performed separate analyses for any type of vitamin D exposure reported in at least three studies: 25(OH)D, dietary intake of vitamin D, 1,25(OH)2D and vitamin D supplements.
The ways that doses or levels of vitamin D were reported in each individual article were not standardized across studies (for instance, some papers reported vitamin D levels in quartiles; others in tertiles, and so on), making it difficult to extract them in an analyzable form. Therefore, in order to provide a consistent criterion of comparability, we selected the OR or RR reported for the highest category compared to the lowest one.
Regarding the type of breast cancer, we analyzed all invasive breast cancers together, and breast cancer stratified according to the cancer estrogen receptor status and woman’s menopausal status. Pooled OR or RR were estimated by weighting individual OR/RR by the inverse of their variance. OR or RR heterogeneity was measured using Q and I2 statistics83. A fixed-effect model was preferred if the Q statistic was higher than 0.1 or I2 lower than 25%, indicating no relevant heterogeneity; a random-effect model was otherwise chosen84. The presence of small-study bias was explored with Rosenthal model and with Egger test85; due to the low sensitivity of Egger test, the cut-off was set at p = 0.1. Funnel plots86 were applied to detect publication bias.
An analysis of influence was performed via the re-estimation of pooled OR/RR by removing one study at a time. Studies that, when removed, strongly changed the OR/RR would be considered as highly influential. Results are displayed as forest plots showing OR/RR and their 95% confidence intervals for each individual study and for the pooled result. Cumulative meta-analyses were carried out to deem the stability of the OR/RR estimates. In order to do that, all studies considered were arranged from oldest to neweest. Then an OR/RR estimate was obtained for the two eldest studies; another for the three eldest, and so on, adding a study each time. Results are reported as forest plots.
All the statistical analyses were carried out with the package Stata 14/SE (Stata Corporation, College Station, TX, US).
Results
Relationship between 25(OH) D and breast cancer
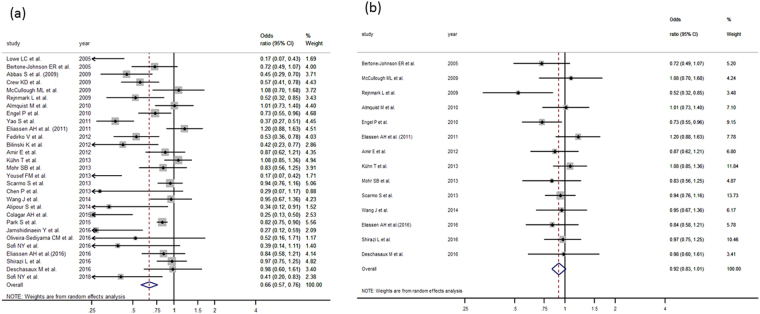
Twenty-nine case control studies were analyzed to study the relationship between 25 (OH) D and breast cancer10,19–22,25,27,29–35,38,42,44–46,48,49,51,55,56,58–63 obtaining a pooled OR of 0.65 (95%CI: 0.56–0.76) (Fig. 2a, Table 4). This value was calculated using the random effects model because of the high heterogeneity (I2 = 77.76%) of the fixed-effect. Although Egger test cannot rule out a small-study effect (p = 0.001), no study shows a relevant influence. The funnel plot shows asymmetry (Supplementary Fig. 1a), indicating either publication bias or heterogeneity that cannot be explained by a random-effect meta-analysis. Rosenthal model shows that 1194 negative studies would be needed to lose statistical significance. In order to further clarify the heterogeneous result, we carried out a sensitivity analysis including only nested case-control studies21,22,25,31–34,42,45,46,51,55,56,59 reaching a pooled OR = 0.92 (95%CI: 0.83–1.01) (Fig. 2b) with I2 = 15.87%, Q-based p value = 0.22 and a very symmetrical-looking funnel plot (Supplementary Fig. 1b).
Figure 2.
(a) Forest plot for the relationship between 25(OH)D and breast cancer in case control studies. (b) Forest plot for the relationship between 25(OH)D and breast cancer in nested case control studies.
Table 4.
Results from the meta-analysis.
| Exposition | Group (Number of studies) | Type of study | OR/RR (95% CI) | I2 |
|---|---|---|---|---|
| 25(OH)D | All (n = 29) | Case-control | 0.65 (0.56–0.76) | 40.87% |
| All (n = 4) | Cohort | 0.85 (0.74–0.98) | 3.56% | |
| ER+ (n = 5) | Case-control | 0.98 (0.85–1.13) | 0% | |
| ER– (n = 5) | Case-control | 0.86 (0.64–1.15) | 15.60% | |
| Postmenopausal (n = 19) | Case-control | 0.74 (0.59–0.93) | 13.16% | |
| Postmenopausal (n = 3) | Cohort | 1.15 (0.59–2.23) | 8% | |
| Premenopausal (n = 9) | Case-control | 0.63 (0.49–0.80) | 8.37% | |
| Dietary vitamin D | All (n = 8) | Case-control | 0.91 (0.72–1.17) | 30.73% |
| All (n = 5) | Cohort | 1.00 (0.93–1.07) | 0% | |
| Postmenopausal (n = 4) | Case-control | 0.78 (0.68–0.90) | 0% | |
| Postmenopausal (n = 5) | Cohort | 0.95 (0.83–1.09) | 19.13% | |
| Premenopausal (n = 5) | Case-Control | 0.65 (0.52–0.82) | 0% | |
| Premenopausal (n = 3) | Cohort | 1.01 (0.86–1.18) | 0% | |
| Vitamin D supplements | All (n = 5) | Case-control | 0.78 (0.63–0.98) | 25.94% |
| All (n = 2) | Cohort | 1.06 (0.90–1.25) | 0% | |
| Total Vitamin D intake (dietary + supplements) | All (n = 4) | Case-control | 0.84 (0.68–1.05) | 18.65% |
| All (n = 2) | Cohort | 0.93 (0.86–1.02) | 0% | |
| Postmenopausal (n = 5) | Cohort | 0.94 (0.87–1.02) | 17.64% | |
| Premenopausal (n = 3) | Cohort | 0.90 (0.72–1.12) | 10.83% |
Four cohort studies75,78–80 provided results on 25(OH)D and breast cancer relationship, from which we obtained a pooled RR of 0.85 (95% CI:0.74–0.98).
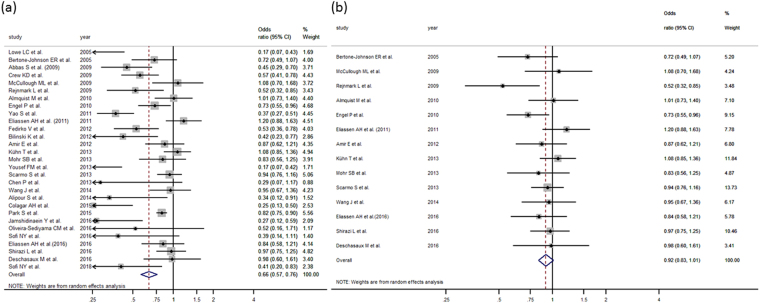
We also analyzed the relationship between 25(OH) D and breast cancer, stratifying results by hormonal receptors (ER+/ER−) and menopausal status (postmenopausal or premenopausal). Regarding hormonal receptors (Table 4), we have found only one cohort study80 and five case-control studies19,32,33,42,45. In both cases (ER+ and ER− tumors) statistical significance was not reached. With respect to menopausal status (Table 4), we obtained a protective effect in both groups: nineteen case-control studies targeted postmenopausal women18,21,28,30,34–36,38,41,47,49,51,55,60,81 with a pooled OR of 0.74 (95%CI: 0.59–0.93), and nine focused on premenopausal21,30,34,35,38,49,51,55,60 obtaining a pooled OR of 0.63 (95%CI: 0.49–0.80) (Fig. 3a). When the sensitivity analysis was carried out including only nested case-control studies, the protective vitamin D – breast cancer association persisted only in the premenopausal group (Fig. 3b, Supplementary Table 1). On the other hand three cohorts studies analyzed separately postmenopausal women79,81 without reaching statistical significance (OR = 1.15 (0.59–2.23)).
Figure 3.
(a) Forest plot for the relationship between 25(OH)D and premenopausal breast cancer in case control studies. (b) Forest plot for the relationship between 25(OH)D and premenopausal breast cancer in nested case control studies.
Relationship between 1,25(OH)2D and breast cancer
Three case-control studies25,37,39 examined the relationship between circulating 1,25(OH)2D and breast cancer; significant association was not found either in the whole analysis (pooled OR = 0.61 (0.33–1.16)) or in postmenopausal women (combined OR = 1.28 IC 95%: 0.98–1.67)36,37.
Relationship between dietary vitamin D and breast cancer
We found eight case-control studies24,38,40,50,52,53,57,64 on the relationship between dietary vitamin D and breast cancer with a pooled OR of 0.91 (95%CI: 0.72–1.17) (Table 4, Supplementary Fig. 2a). In addition, by combining five cohort studies66,68,70–72 we obtained a RR of 1.00 (95% CI 0.93–1.07) (Table 4, Supplementary Fig. 2b).
When stratifying by menopausal status, four case-control38,40,53,64 and five cohort studies66,73,74,76,77 assessed the risk of breast cancer in postmenopausal women. The pooled OR for case-control studies was 0.78 (95%CI: 0.68–0.90) and the pooled RR for cohort studies was 0.95 (95%CI: 0.83–1.09) (Table 4). In both analyses, Egger test rejected the possibility of small study bias (p = 0.536 in case-control studies and p = 0.68 in cohort studies). On the other hand, five case-control studies17,38,40,53,63 and three cohort studies66,73,77 targeted premenopausal women; the pooled OR was 0.65 (95%CI: 0.52–0.82) for case-control studies and the RR for cohort studies was 1.01 (95% CI: 0.86–1.18) (Table 4).
Relationship between supplements of vitamin D and breast cancer
We identified five case-control studies23,24,43,52,65 and two cohort studies67,71 that had evaluated the association between supplements of vitamin D and breast cancer risk. The pooled OR and RR were 0.78 (95% CI: 0.63–0.98) and 1.06(95% IC: 0.90–1.25) respectively (Table 4). Regarding menopausal status, Kim et al.41 published a study on five different populations of postmenopausal women; when combining all five results, we found no significant association (OR: 0.82 95%CI: 0.49–1.35).In addition, we found two case-control studies26,54 focused on premenopausal women obtaining a weak protection (pooled OR 0.89 95%CI (0.84–0.95)).
Relationship between total vitamin D intake (dietary and supplements) and breast cancer
Finally, we found two cohort studies69,71 and four case control studies23,24,38,64 on vitamin D intake (dietary plus supplements) and breast cancer risk, providing no separate results on dietary/supplemented vitamin D origin. We obtained a combined RR = 0.93 (95% CI: 0.86–1.02) for cohort studies, and a combined OR = 0.84 (95% CI: 0.68–1.05) for case-control studies. Five cohort studies69,73,74,76,77 provided results on postmenopausal women (RR = 0.94 95% CI: 0.87–1.00) and three cohort studies69,73,77 on about premenopausal women (RR = 0.90 95% CI: 0.72–1.12) (Table 4). Only two case-control studies provided results according menopausal status38,64 without being significant in both groups.
Discussion
According to our results, 25(OH)D levels were associated with smaller risk of breast cancer in both case-control and cohort studies; these results were consistent on premenopausal women for case-control studies but could not be analyzed for cohort studies. Results for the relationships between breast cancer and dietary vitamin D or between breast cancer and vitamin D supplements, however, showed a protective association only in case-control studies.
In relation to the influence of vitamin D on breast cancer development prospective (cohort and nested case-control) and case control studies tend to show discrepant results: case-control studies usually show a protective effect while prospective studies rarely find it87. This discrepancy might be the result of several factors: Firstly, it is well known that prospective studies are less prone to be affected by both information and reverse-causation bias. Secondly, several authors highlight the season when the vitamin D measurement was made as a potential limitation of case-control studies. Eliassen et al.33 in a nested case-control study found an inverse association between serum 25(OH) D levels and breast cancer limited only to summer measures. It can be assumed that people with low vitamin D levels in summer would also have low levels year-round; therefore, vitamin D levels in summer would be more adequate for analyzing vitamin D – breast cancer relationship than vitamin D levels in any other moment of the year.
When stratifying by menopausal status, our meta-analysis shows a consistent protective effect of 25(OH) D in both case-control and nested case-control studies, but only in premenopausal women. There are different explanations for the influence of menopausal status in the relationship between vitamin D and breast cancer. One of them may be related to the joint relationship between vitamin D and insulin-like growth factors (IGFs). IGF-I is a mitogenic and antiapoptotic peptide that can stimulate the proliferation of breast epithelial cells, increasing the risk of neoplastic transformation88,89. The active vitamin D metabolite is able to block the mitogenic effects of IGF-I, leading to a decrease in proliferation and an increase in apoptosis90. As there is a physiological decline of the IGF with aging91, the interaction between IGF pathways and vitamin D is likely to be stronger for premenopausal than for postmenopausal women, leading to greater risk reduction in premenopausal breast cancer73,92. Finally, high levels of vitamin D may reduce progesterone and estradiol, providing a potential mechanism for reducing breast cancer risk in young women93.
Previous meta-analyses of prospective studies showed contradictory results. Kim et al.13 (who included 24 studies, 14 of those having measured serum 25(OH)D) found a slightly stronger inverse association among premenopausal than among postmenopausal women but without significant differences, whereas in the meta-analysis of Bauer et al.8 (nine studies included) the inverse association was only observed in postmenopausal women. In our meta-analysis, new prospective studies31,33,41,56,58,59,67,78–81,94 not included in previous reviews, were added and this fact may explain the differences in the results.
Concerning hormonal receptors (ER+/ER−), the relationship with breast cancer remains controversial. On the one hand, a decreased risk in ER+ would be expected, since it seems that sensitivity to 1,25(OH)2D is generally reported as being higher in breast cancer cells that express the estrogen receptor than in those that do not93,95. It has been demonstrated that treating breast cancer cells ER+ with 1,25(OH)D3 induces a cell cycle shutdown in GO/G13,96. On the other hand, two-thirds of triple negative tumors express VDR97 and it has been demonstrated that VDR expression is inversely associated with more aggressive breast cancer98. In consonance with previous epidemiological studies32,33,42,45, our study does not reach significant differences when the analysis was performed separately in ER+ or ER− subgroups. However, other studies found a decreased risk of ER− breast cancer regarding the serum levels of 25 (OH) D18,60.
No relationship is found between the level of circulating 1,25(OH)2D and breast cancer. This result is consistent with previous studies9, while Janowsky et al.39 found an inverse association. Several authors consider that 1,25(OH)2D is not a good indicator of vitamin D status: First, 1,25(OH)2D’s half-life is only 4–6 h, whereas 25(OH)D’s half-life is 3 weeks; second, 1,25(OH)2D is influenced by many factors10, for instance, it can be elevated in patients with vitamin D deficiency as a result of hyperparathyroidism12,99; finally, as 1,25(OH)2D is metabolized by 1-α -hydroxylase in breast tissue, plasma levels may not adequately represent breast tissue levels12,100.
We do not find a relationship between vitamin D intake and breast cancer in the overall analysis. In contrast, when stratifying by menopausal status, a protective effect is observed in case-control studies in both premenopausal and postmenopausal women, whereas this association is not present in cohort studies. On the other hand, when analyzing the influence of vitamin D supplements on breast cancer risk, we find a borderline protective effect.
In the relationship between vitamin D intake (dietary and/or supplements) and breast cancer, most observational studies showed non-significant differences; only two articles17,53 found a protective association. In a previous meta-analysis13, this association was not significant for either vitamin D intake or supplements.
A probable explanation for the lack of association observed in the analysis of dietary intake or supplements compared to the 25(OH)D levels may be that the main source of vitamin D is sunlight rather than food or supplements.
In addition, the French E3N Cohort Study12 reported that high vitamin D intake is associated with lower breast cancer risk in regions with high ultraviolet solar radiance. These results suggested that the total amount of vitamin D needed to reach a protective effect on breast cancer is too high to be achieved in regions with low ultraviolet radiance. Under these circumstances, as the vitamin D intake has to be higher than the usually recommended, it could eventually lead to side effects such as hypercalcemia, constipation or muscle weakness.
Our study has some limitations; firstly each article uses different cutoff points according to serum levels of vitamin D. To analyze it we restricted our analysis to the comparison between the highest vs. lowest category of exposure. This analysis strategy does not allow for a dose-response analysis. Moreover, we carried out a sensitivity analysis excluding one study at a time, showing that no single study substantially affected the pooled RR/OR. Secondly, there is huge variability in the literature on the type of vitamin D studied, which makes it difficult to perform the analysis. In addition, levels of vitamin D depend on the season, so it would be advisable to take all samples at the same time, or at least refer to when they were collected75. Thirdly, case-control studies are more prone to methodological issues, such as recall and selection biases, which limits the strength and quality of evidence. However, about half of the case-control studies included in our meta-analysis are nested in cohort studies, which minimizes the possibility of introducing biases. Finally, breast cancer is a heterogeneous disease and it is possible that vitamin D only affects certain breast cancer subtypes. However, this aspect has been scarcely studied in primary articles, so we have not been able to analyze it in the present meta-analysis.
Despite these limitations, our study also has several strengths; first, we have gathered all the observational studies published in the last twenty years. In addition, we have focused the analysis on different types of vitamin D exposure (diet, supplements and blood-levels of 25(OH) D and 1,25(OH)2D) whereas other meta-analyses are only focused on 25(OH)D levels9,10,16,99 or vitamin D intake12. This strategy allows us to obtain a more detailed analysis of the relationship between vitamin D and breast cancer.
In conclusion, our meta-analysis supports the hypothesis that high serum levels of 25(OH) vitamin D has a protective effect on breast cancer risk in premenopausal women; we cannot draw the same conclusion regarding vitamin D intake or supplements of vitamin D since the number of studies are still limited and publication biases cannot be excluded.
Electronic supplementary material
Author Contributions
N.E., T.D.S. and I.G.A. contributed substantially to the conception, design and acquisition of data. N.E. and T.D.S.: wrote the main manuscript text. N.E. and C.P. prepared figures. T.D.S., I.G.A. and J.L. contributed to the analysis and interpretation of the data. N.E. and T.D.S., I.G.A., C.P. and J.L. contributed to devising the draft of the article and all of the other authors revised it critically. All authors participated in revising the manuscript and in the final approval of the version to be published.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27297-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Habib OS, et al. Epidemiology of Breast Cancer among Females in Basrah. Asian Pac J Cancer Prev. 2016;17:91–5. doi: 10.7314/APJCP.2016.17.S1.91. [DOI] [PubMed] [Google Scholar]
- 3.Colston KW, Hansen M. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer. 2001;9:45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 4.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh JE. 1α,25(OH)2D3 induces morphological and biochemical indices of apoptosis in MCF-7 breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 1996;58:367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 5.Welsh JE. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochemistry and Cell Biology. 1994;72:537–545. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- 6.James SY, Mackay AG, Colston KW. Effects on 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 1996;58:395–401. doi: 10.1016/0960-0760(96)00048-9. [DOI] [PubMed] [Google Scholar]
- 7.Khan QJ, Kimler BF, Fabia CJ. The Relationship Between Vitamin D and Breast Cancer Incidence and Natural History. Curr Oncol Rep. 2010;12:136–142. doi: 10.1007/s11912-010-0081-8. [DOI] [PubMed] [Google Scholar]
- 8.Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma Vitamin D Levels, Menopause, and Risk of Breast Cancer: Dose-Response Meta-Analysis of Prospective Studies. Medicine. 2013;92:123–131. doi: 10.1097/MD.0b013e3182943bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen P, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121:469–77. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, et al. Higher Blood 25(OH)D Level May Reduce the Breast Cancer Risk: Evidence from a Chinese Population Based Case-Control Study and Meta-analysis of the Observational Studies. PLoS One. 2013;8:e49312. doi: 10.1371/journal.pone.0049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandini S, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–24. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 12.Gissel T, Rejnmark L, Mosekilde L, Vestergaard P. Intake of vitamin D and risk of breast cancer–a meta-analysis. J Steroid Biochem Mol Biol. 2008;111:195–9. doi: 10.1016/j.jsbmb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer. 2014;110:2772–84. doi: 10.1038/bjc.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr SB, et al. Serum 25-Hydroxyvitamin D and Prevention of Breast Cancer: Pooled Analysis. Anticancer Res. 2011;31:2939–48. [PubMed] [Google Scholar]
- 15.Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW. Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumor Biology. 2013;34:3509. doi: 10.1007/s13277-013-0929-2. [DOI] [PubMed] [Google Scholar]
- 16.Yin L, et al. Meta-analysis: Serum vitamin D and breast cancer risk. Eur J Cancer. 2010;4:2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Abbas S, Linseisen J, Chang-Claude J. Dietary Vitamin D and Calcium Intake and Premenopausal Breast Cancer Risk in a German Case-Control Study. Nutr Cancer. 2007;59:54–61. doi: 10.1080/01635580701390223. [DOI] [PubMed] [Google Scholar]
- 18.Abbas S, et al. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer—results of a large case–control study. Carcinogenesis. 2008;29:93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 19.Abbas S, Linseisen J, Chang-Claude J. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int J Cancer. 2009;124:250–5. doi: 10.1002/ijc.23904. [DOI] [PubMed] [Google Scholar]
- 20.Alipour S, et al. Levels of Serum 25-Hydroxy-Vitamin D in Benign and Malignant Breast Masse. Asian Pac J Cancer Prev. 2014;15:129–32. doi: 10.7314/APJCP.2014.15.1.129. [DOI] [PubMed] [Google Scholar]
- 21.Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk—a prospective nested case–control study. Int J Cancer. 2010;127:2159–2168. doi: 10.1002/ijc.25215. [DOI] [PubMed] [Google Scholar]
- 22.Amir E, et al. 25-Hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast Cancer Res Treat. 2012;133:1077–1088. doi: 10.1007/s10549-012-2012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson LN, Cotterchio M, Cole DE, Knight JA. Vitamin D-Related Genetic Variants, Interactions with Vitamin D Exposure, and Breast Cancer Risk among Caucasian Women in Ontario. Cancer Epidemiol Biomarkers Prev. 2011;20:1708–17. doi: 10.1158/1055-9965.EPI-11-0300. [DOI] [PubMed] [Google Scholar]
- 24.Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am J Clin Nutr. 2010;91:1699–707. doi: 10.3945/ajcn.2009.28869. [DOI] [PubMed] [Google Scholar]
- 25.Bertone-Johnson ER, et al. Plasma 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D and Risk of Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–7. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 26.Bidgoli SA, Azarshab H. Role of Vitamin D Deficiency and Lack of Sun Exposure in the Incidence of Premenopausal Breast Cancer: a Case Control Study in Sabzevar, Iran. Asian Pac J Cancer Prev. 2014;15:3391–6. doi: 10.7314/APJCP.2014.15.8.3391. [DOI] [PubMed] [Google Scholar]
- 27.Bilinski K, Boyages J. Association between 25-hydroxyvitamin D concentration and breast cancer risk in an Australian population: an observational case–control study. Breast Cancer Res Treat. 2013;137:599–607. doi: 10.1007/s10549-012-2381-1. [DOI] [PubMed] [Google Scholar]
- 28.Chlebowski RT, et al. Calcium Plus Vitamin D Supplementation and the Risk of Breast Cancer. J Natl Cancer Inst. 2008;100:1581–91. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colagar AH, Firouzjah HJ, Halalkho S. Vitamin D Receptor Poly(A) Microsatellite Polymorphism and 25-Hydroxyvitamin D Serum Levels: Association with Susceptibility to Breast Cancer. J Breast Cancer. 2015;18:119–12. doi: 10.4048/jbc.2015.18.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crew KD, et al. Association between Plasma 25-Hydroxyvitamin D and Breast Cancer Risk. Cancer Prev Res. 2009;2:589–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deschasaux M, et al. Weight Status and Alcohol Intake Modify the Association between Vitamin D and Breast Cancer Risk. J Nutr. 2016;143:576–85. doi: 10.3945/jn.115.221481. [DOI] [PubMed] [Google Scholar]
- 32.Eliassen AH, et al. Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses’ Health Study II. Breast Cancer Res. 2011;13:R50. doi: 10.1186/bcr2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eliassen AH, et al. Plasma 25-hydroxyvitamin D and risk of breast cancer in women followed over 20 years. Cancer Res. 2016;76:5423–30. doi: 10.1158/0008-5472.CAN-16-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel P, et al. Serum 25(OH) Vitamin D and Risk of Breast Cancer: A Nested Case-Control Study from the French E3N Cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:2341–50. doi: 10.1158/1055-9965.EPI-10-0264. [DOI] [PubMed] [Google Scholar]
- 35.Fedirko V, et al. Serum 25-hydroxyvitamin D and risk of breast cancer: results of a large population-based case–control study in Mexican wome. Cancer Causes Control. 2012;23:1149–62. doi: 10.1007/s10552-012-9984-z. [DOI] [PubMed] [Google Scholar]
- 36.Freedman M, et al. Serum Levels of Vitamin D Metabolites and Breast Cancer Risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2008;17:889–94. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiatt RA, et al. Prediagnostic Serum Vitamin D and Breast Cancer. J Natl Cancer Inst. 1998;90:461–3. doi: 10.1093/jnci/90.6.461. [DOI] [PubMed] [Google Scholar]
- 38.Jamshidinaeini Y, Akbari ME, Abdollahi M, Ajami M, Davoodi SH. Vitamin D Status and Risk of Breast Cancer in Iranian Women: A Case–Control Stud. J Am Coll Nutr. 2016;35:639–646. doi: 10.1080/07315724.2015.1127786. [DOI] [PubMed] [Google Scholar]
- 39.Janowsky EC, et al. Association between low levels of 1,25-dihydroxyvitamin D and breast cancer risk. Public Health Nutr. 1999;2:283–29. doi: 10.1017/S1368980099000385. [DOI] [PubMed] [Google Scholar]
- 40.Kawase T, et al. Association between vitamin D and calcium intake and breast cancer risk according to menopausal status and receptor status in Japan. Cancer Sci. 2010;101:1234–40. doi: 10.1111/j.1349-7006.2010.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, et al. Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: a nested case-control study in the multiethnic cohort study. BMC Cancer. 2014;17(14):29. doi: 10.1186/1471-2407-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kühn T, et al. Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition: A nested case–control study. Int J Cancer. 2013;133:1689–700. doi: 10.1002/ijc.28172. [DOI] [PubMed] [Google Scholar]
- 43.Levi F, Pasche C, Lucchini F, La Vecchia C. Dietary intake of selected micronutrients and breast-cancer risk. Int J Cancer. 2001;91:260–3. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1041>3.3.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 44.Lowe LC, et al. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–9. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 45.McCullough ML, et al. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009;11:R64. doi: 10.1186/bcr2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohr SB, et al. Serum 25-hydroxyvitamin D and breast cancer in the military: a case–control study utilizing pre-diagnostic serum. Cancer Causes Control. 2013;24:495–504. doi: 10.1007/s10552-012-0140-6. [DOI] [PubMed] [Google Scholar]
- 47.Neuhouser ML, et al. The Influence of Health and Lifestyle Characteristics on the Relation of Serum 25-Hydroxyvitamin D With Risk of Colorectal and Breast Cancer in Postmenopausal Women. Am J Epidemio. 2012;175:673–84. doi: 10.1093/aje/kwr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira-Sediyama CM, et al. Lifestyle and vitamin D dosage in women with breast cancer. Nutr Hosp. 2016;33:1179. doi: 10.20960/nh.584. [DOI] [PubMed] [Google Scholar]
- 49.Park S, et al. Serum 25-hydroxyvitamin D deficiency and increased risk of breast cancer among Korean women: a case–control study. Breast Cancer Res Treat. 2015;152:147–54. doi: 10.1007/s10549-015-3433-0. [DOI] [PubMed] [Google Scholar]
- 50.Potischman N, et al. Intake of food groups and associated micronutrients in relation to risk of early-stage breast cancer. Int J Cancer. 1999;82:315–21. doi: 10.1002/(SICI)1097-0215(19990730)82:3<315::AID-IJC1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 51.Rejnmark L, et al. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2009;18:2655–60. doi: 10.1158/1055-9965.EPI-09-0531. [DOI] [PubMed] [Google Scholar]
- 52.Rollison DE, et al. Vitamin D intake, vitamin D receptor polymorphisms, and breast cancer risk among women living in the southwestern U.S. Breast Cancer Res Treat. 2012;132:683–91. doi: 10.1007/s10549-011-1885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossi M, et al. Vitamin D intake and breast cancer risk: a case-control study in Italy. Ann Oncol. 2009;20:374–8. doi: 10.1093/annonc/mdn550. [DOI] [PubMed] [Google Scholar]
- 54.Salarabadi A, Bidgoli SA, Madani SH. Roles of Kermanshahi Oil, Animal Fat, Dietary and Non- Dietary Vitamin D and other Nutrients in Increased Risk of Premenopausal Breast Cancer: A Case Control Study in Kermanshah, Iran. Asian Pac J Cancer Prev. 2015;16:7473–8. doi: 10.7314/APJCP.2015.16.17.7473. [DOI] [PubMed] [Google Scholar]
- 55.Scarmo S, et al. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: a nested case-control study. Breast Cancer Res. 2013;133:1689–700. doi: 10.1186/bcr3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shirazi L, Almquist M, Borgquist S, Manjer J. Serum vitamin D (25OHD3) levels and the risk of different subtypes of breast cancer: A nested case-control study. Breast. 2016;28:184–190. doi: 10.1016/j.breast.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Simard A, Vobecky J, Vobecky JS. Vitamin D deficiency and cancer of the breast: an unprovocative ecological hypothesis. Can J Public Health. 1991;82:300–3. [PubMed] [Google Scholar]
- 58.Sofi NY, et al. Nutritional risk factors and status of serum 25(OH)D levels in patients with breast cancer: A case control study in India. J Steroid Biochem Mol Biol. 2016;175:55–59. doi: 10.1016/j.jsbmb.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Eliassen AH, Spiegelman D, Willett WC, Hankinson SE. Plasma free 25-hydroxyvitamin D, vitamin D binding protein, and risk of breast cancer in the Nurses’ Health Study II. Cancer Causes Control. 2014;25:819–27. doi: 10.1007/s10552-014-0383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao S, et al. Pretreatment Serum Concentrations of 25-Hydroxyvitamin D and Breast Cancer Prognostic Characteristics: A Case-Control and a Case-Series Study. PLoS One. 2011;6:e17251. doi: 10.1371/journal.pone.0017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yousef FM, et al. Vitamin D status and breast cancer in Saudi Arabian women: case-control study. Am J Clin Nutr. 2013;98:105–10. doi: 10.3945/ajcn.112.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y, Sarkissyan M, Clayton S, Chlebowski R, Vadgama JV. Association of Vitamin D3 Level with Breast Cancer Risk and Prognosis in African-American and HispanicWomen. Cancers. 2017;9:144. doi: 10.3390/cancers9100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sofi NY, et al. Reproductive factors, nutritional status and serum 25(OH)D levels in women with breast cancer: A case control study. Journal of Steroid Biochemistry and Molecular Biology. 2018;175:200–204. doi: 10.1016/j.jsbmb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Lee MS, et al. Vitamin D Decreases Risk of Breast Cancer in Premenopausal Women of Normal Weight in Subtropical Taiwan. J Epidemiol. 2011;21:87–94. doi: 10.2188/jea.JE20100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leung HWC, Muo CH, Liu CF, Chan ALF. Vitamin D3 Intake Dose and Common Cancer: A Population-Based Case Control Study in a Chinese Population. Journal of Cancer. 2016;7:2028–2034. doi: 10.7150/jca.16505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbas S, et al. Dietary Intake of Vitamin D and Calcium and Breast Cancer Risk in the European Prospective Investigation into Cancer and Nutrition. Nutr Cancer. 2013;65:178–87. doi: 10.1080/01635581.2013.752018. [DOI] [PubMed] [Google Scholar]
- 67.Cadeau C, et al. Interaction between current vitamin D supplementation and menopausal hormone therapy use on breast cancer risk: evidence from the E3N cohort. Am J Clin Nutr. 2015;102:966–73. doi: 10.3945/ajcn.114.104323. [DOI] [PubMed] [Google Scholar]
- 68.Edvardsen K, et al. Vitamin D-effective solar UV radiation, dietary vitamin D and breast cancer risk. Int J Cancer. 2011;128:1425–1433. doi: 10.1002/ijc.25463. [DOI] [PubMed] [Google Scholar]
- 69.Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Joint Effects of Dietary Vitamin D and Sun Exposure on Breast Cancer Risk: Results from the French E3N Cohort. Cancer Epidemiol Biomarkers Prev. 2010;20:187–98. doi: 10.1158/1055-9965.EPI-10-1039. [DOI] [PubMed] [Google Scholar]
- 70.Frazier AL, Li L, Cho E, Willett WC, Colditz GA. Adolescent diet and risk of breast cancer. Cancer Causes Control. 2004;15:73–82. doi: 10.1023/B:CACO.0000016617.57120.df. [DOI] [PubMed] [Google Scholar]
- 71.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and Breast Cancer Risk: The NHANES I Epidemiologic Follow-up Study, 1971–1975 to 1992. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 72.Kuper H, et al. Prospective study of solar exposure, dietary vitamin D intake, and risk of breast cancer among middle-aged women. Cancer Epidemiol Biomarkers Prev. 2009;18:2558–6. doi: 10.1158/1055-9965.EPI-09-0449. [DOI] [PubMed] [Google Scholar]
- 73.Lin J, et al. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–9. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 74.McCullough ML, et al. Dairy, Calcium, and Vitamin D Intake and Postmenopausal Breast Cancer Risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2898–904. doi: 10.1158/1055-9965.EPI-05-0611. [DOI] [PubMed] [Google Scholar]
- 75.Ordóñez-Mena JM, et al. Serum 25-hydroxyvitamin d and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2013;22:905–16. doi: 10.1158/1055-9965.EPI-12-1332. [DOI] [PubMed] [Google Scholar]
- 76.Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women’s Health Study. Cancer causes control. 2007;18:775–782. doi: 10.1007/s10552-007-9020-x. [DOI] [PubMed] [Google Scholar]
- 77.Shin MH, et al. Intake of Dairy Products, Calcium, and Vitamin D and Risk of Breast Cancer. J Natl Cancer Inst. 2002;94:1301–11. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 78.Skaaby T, et al. Prospective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cáncer. Cancer Epidemiol Biomarkers Prev. 2014;23:1220–9. doi: 10.1158/1055-9965.EPI-14-0007. [DOI] [PubMed] [Google Scholar]
- 79.O’Brien KM, Sandler DP, Taylor JA, Weinberg CR. Serum Vitamin D and Risk of Breast Cancer within Five Years. Environ Health Perspect. 2017;25:077004. doi: 10.1289/EHP943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palmer JR, et al. Predicted 25-hydroxyvitamin D in relation to incidence of breast cancer in a large cohort of African American women. Breast Cancer Research. 2016;18:86. doi: 10.1186/s13058-016-0745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ordoñez-Mena JM, et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: an analysis of cohorts participating in the CHANCES consortium. Eur J Epidemiol. 2016;31:311–23. doi: 10.1007/s10654-015-0040-7. [DOI] [PubMed] [Google Scholar]
- 82.Elma EV, et al. Declaración de la Iniciativa STROBE (Strengthening the Reporting of Observational studies inEpidemiology): directrices para la comunicación de estudios observacionales. Revista Española de Salud Pública. 2008;82:144–150. doi: 10.1590/s1135-57272008000300002. [DOI] [PubMed] [Google Scholar]
- 83.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 84.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 85.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Light, R. J. & Pillemer, D. B. Summing up: the science of reviewing research. Cambridge: Harvard University Press. (1984).
- 87.Moukayed M, Grant WB. The roles of UVB and vitamin D in reducing risk of cancer incidence and mortality: A review of the epidemiology, clinical trials, and mechanisms. Rev Endocr Metab Disord. 2017;18:167–182. doi: 10.1007/s11154-017-9415-2. [DOI] [PubMed] [Google Scholar]
- 88.Hankinson SE, et al. Plasma Sex Steroid Hormone Levels and Risk of Breast Cancer in Postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 89.Christopoulos, P. F., Msaouel, P. & Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. 15, 43 (2015). [DOI] [PMC free article] [PubMed]
- 90.Ameri P, et al. Interactions between vitamin D and IGF-I: from physiology to clinical practice. Clin Endocrinol (Oxf). 2013;79:457–63. doi: 10.1111/cen.12268. [DOI] [PubMed] [Google Scholar]
- 91.Gomez M. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 2006;7:125–32. doi: 10.2174/138920106776597621. [DOI] [PubMed] [Google Scholar]
- 92.Chlebowski RT. Vitamin D and breast cancer: interpreting current evidence. Breast Cancer Res. 2011;3:217. doi: 10.1186/bcr2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knight JA, Wong J, Blackmore M, Raboud JM, Vieth R. Vitamin D association with estradiol and progesterone in young women. Cancer Causes Control. 2010;21:479. doi: 10.1007/s10552-009-9466-0. [DOI] [PubMed] [Google Scholar]
- 94.Cadeau C, et al. Postmenopausal breast cancer risk and interactions between body mass index, menopausal hormone therapy use, and vitamin D supplementation: Evidence from the E3N cohort. Int. J. Cancer. 2016;139:2193–2200. doi: 10.1002/ijc.30282. [DOI] [PubMed] [Google Scholar]
- 95.Narvaez CJ, Zinser G, Welsh J. Functions of 1,25-dihydroxyvitamin D3 in mammary gland: from normal development to breast cancer. Steroids. 2001;66:301–8. doi: 10.1016/S0039-128X(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 96.Shao T, Klein P, Grossbard ML. Vitamin D and Breast Cancer. Oncologist. 2012;17:36–45. doi: 10.1634/theoncologist.2011-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thakkar A, et al. Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res Treat. 2016;157(1):77–90. doi: 10.1007/s10549-016-3807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Azhri, et al. Tumor Expression of Vitamin D Receptor and Breast Cancer Histopathological Characteristics and Prognosis. Clin Cancer Res. 2016;23(1):97–103. doi: 10.1158/1078-0432.CCR-16-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garland CF, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 100.Bertone-Johnson ER. Vitamin D and breast cancer. Ann Epidemiol. 2009;19:462–467. doi: 10.1016/j.annepidem.2009.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.