Abstract
Background: Lower-brain glucose uptake is commonly present before the onset of cognitive deterioration associated with aging and may increase the risk of Alzheimer disease. Ketones are the brain's main alternative energy substrate to glucose. Medium-chain triglycerides (MCTs) are rapidly β-oxidized and are ketogenic but also have gastrointestinal side effects. We assessed whether MCT emulsification into a lactose-free skim-milk matrix [emulsified MCTs (MCT-Es)] would improve ketogenesis, reduce side effects, or both compared with the same oral dose of MCTs consumed without emulsification [nonemulsified MCTs (MCT-NEs)].
Objectives: Our aims were to show that, in healthy adults, MCT-Es will induce higher ketonemia and have fewer side effects than MCT-NEs and the effects of MCT-NEs and MCT-Es on ketogenesis and plasma medium-chain fatty acids (MCFAs) will be dose-dependent.
Methods: Using a metabolic study day protocol, 10 healthy adults were each given 3 separate doses (10, 20, or 30 g) of MCT-NEs or MCT-Es with a standard breakfast or no treatment [control (CTL)]. Blood samples were taken every 30 min for 4 h to measure plasma ketones (β-hydroxybutyrate and acetoacetate), octanoate, decanoate, and other metabolites. Participants completed a side-effects questionnaire at the end of each study day.
Results: Compared with CTL, MCT-NEs increased ketogenesis by 2-fold with no significant differences between doses. MCT-Es increased total plasma ketones by 2- to 4-fold in a dose-dependent manner. Compared with MCT-NEs, MCT-Es increased plasma MCFA bioavailability (F) by 2- to 3-fold and decreased the number of side effects by ∼50%.
Conclusions: Emulsification increased the ketogenic effect and decreased side effects in a dose-dependent manner for single doses of MCTs ≤30 g under matching conditions. Further investigation is needed to establish whether emulsification could sustain ketogenesis and minimize side effects and therefore be used as a treatment to change brain ketone availability over a prolonged period of time. This trial was registered at clinicaltrials.gov as NCT02409927.
Keywords: lipid metabolism, medium-chain triglycerides, emulsification, ketogenesis, bioavailability
Introduction
The ketones β-hydroxybutyrate and acetoacetate are the main alternative fuel to glucose for brain energy metabolism and can meet two-thirds or more of the brain's total energy requirements during a prolonged fast or while consuming a very-low-carbohydrate ketogenic diet (1–3). Although brain glucose uptake is not directly related to plasma glucose concentration (4), there is a direct relation between plasma ketones and brain ketone uptake over a wide range of plasma ketone concentrations (5–7).
Medium-chain TGs (MCTs; 6–12 carbons) are well known to be ketogenic in humans (8–10). Their physicochemical properties enable their rapid absorption from the gut through the portal vein to the liver and rapid diffusion into hepatocytes (11). MCTs are more rapidly β-oxidized than long-chain FAs (≥14 carbons) (12), which increases acetyl-CoA in the liver and leads to ketogenesis and ketone release into the circulation (13). The inclusion of MCTs as part of a ketogenic diet to treat intractable epilepsy in children shows their superior ketogenic efficacy over long-chain FAs in humans (14, 15) and rats (16). However, MCTs can be associated with gastrointestinal side effects (9), which can decrease tolerability and the sustainability of their ketogenic effect.
In animal studies, emulsification improves enteral absorption and the bioavailability of MCTs (12, 17, 18), presumably because gastric and pancreatic lipases liberate the medium-chain FAs (MCFAs) more easily from smaller lipid droplets, thereby enhancing their absorption. In humans, emulsification increases absorption and metabolism of long-chain FAs such as DHA (19). Nevertheless, little is known about how emulsification of MCTs affects ketogenesis or their side effects in humans.
The primary objective of the present study was therefore to conduct a dose-response analysis to determine whether emulsification of a single dose of MCTs would improve the acute ketogenic effect in healthy adults compared with the same dose of nonemulsified MCT (MCT-NE) oil. The 2 secondary objectives were to assess the following: 1) the dose-response relation between the change in plasma ketones and change in plasma MCFAs after an oral dose of emulsified MCTs (MCT-Es) or MCT-NEs and 2) whether MCT emulsification would be associated with fewer gastrointestinal side effects. A lactose-free skim-milk matrix was used to emulsify the MCTs to avoid lactose intolerance in susceptible individuals. The metabolic tests were conducted over 4 h, which is sufficient to observe significant increases in plasma ketones postdose (7). A 4-h period is also sufficient to compare the plasma ketone AUCs of the various treatments (9) while limiting potential side effects to the shortest period possible.
Methods
Ethical approval for this study was obtained from the Research Ethics Committee of the Centre Intégré Universitaire de Santé et de Services Sociaux de l'Estrie–Centre Hospitalier Universitaire de Sherbrooke, which oversees all human research conducted at the Research Center on Aging (Sherbrooke, Quebec, Canada). All of the participants provided informed consent before inclusion in the study. This study is registered at clinicaltrials.gov with the identification number NCT02409927.
Participants
There were 10 participants with a mean ± SEM age of 31 ± 3 y (6 men and 4 women), all of whom were judged to be in good health after a review of their medical histories and screening of blood samples obtained after a 12-h overnight fast (Table 1). All of the participants were nonsmokers, nondiabetic (fasting glucose <6.1 mM and glycated hemoglobin <6.0%), and had normal renal function, serum electrolytes, liver function (normal aspartate aminotransferase and alanine aminotransferase), thyroid-stimulating hormone, HDL and LDL cholesterol, TGs, and albumin and no overt nutritional problems.
TABLE 1.
Baseline participant demographic and plasma variables1
| Value | |
|---|---|
| Age, y | 31 ± 3 |
| Sex (M/F), n/n | 6/4 |
| Plasma measurements | |
| Acetoacetate, µM | 61 ± 11 |
| β-Hydroxybutyrate, µM | 114 ± 18 |
| Glucose, mM | 5.1 ± 0.5 |
| Insulin, IU/L | 3.4 ± 0.9 |
| Octanoate, µg/mL | 1.1 ± 0.6 |
| Decanoate, µg/mL | 0.7 ± 0.3 |
Values are means ± SEMs unless otherwise indicated; n = 10.
MCT emulsification
The composition of the MCTs was 60% octanoate (8:0) and 40% decanoate (10:0), and they contained no protein or carbohydrates (Alpha Health Products). The 3 separate doses of MCT-NEs were manually stirred into lactose-free skim milk to a final volume of 300 mL. The same MCT oil was used for the MCT-Es and at the same doses as for the MCT-NEs but was emulsified into lactose-free skim milk (lactose-free; 0 g lipids, 9 g carbohydrates, and 12 g proteins/250 mL; Natrel brand) to a concentration of 10% by using a high-pressure homogenizer at 2000 pounds/square inch (Dairy Products Pilot Plant, Institute of Nutrition and Functional Foods, Université Laval). The final mean MCT particle diameter was ∼0.7 µm, and the emulsion was shown to be stable at room temperature for ≥28 d.
Metabolic test protocol
There were 7 test conditions: a no-treatment control (CTL) and 3 matching doses each of MCT-Es and MCT-NEs (10, 20, and 30 g). Participants received the 3 doses of MCT-NEs or MCT-Es in random order; each test was performed in the morning, with test days separated by a minimum of 3 d. Participants were blinded to the form and dose of MCT they would receive. Before each metabolic test, participants underwent a 12-h overnight fast. At 0800 on the morning of the metabolic test, a venous forearm catheter was installed for blood sampling, and a baseline sample was taken to evaluate fasting ketones, glucose, insulin, cholesterol, TGs, lactate, and FFAs. After the placement of the catheter and collection of the baseline blood sample, a standardized breakfast consisting of 2 pieces of toast with jelly and 300 mL of the test supplement was served and consumed within 15 min. For the MCT-Es, the volume of pre-emulsified product was proportional to the MCT dose (10 g = 100 mL, 20 g = 200 mL, 30 g = 300 mL), with the final 300-mL volume of the 10- and 20-g doses being made up with lactose-free skim milk. After consuming the drink and breakfast, blood samples were collected every 30 min for the next 4 h. Participants were asked to stay as relaxed as possible, to not engage in any physical exertion (because it might stimulate ketogenesis), and to consume only water. At the end of each metabolic test, participants completed a questionnaire on the side effects they experienced.
MCFA analysis
Plasma samples were prepared for MCFA [C8:0 (octanoic or caprylic acid) and C10:0 (decanoic or capric acid)] analysis with modification of a previously reported method (20). Plasma samples (25 µL) were enriched with isotopic standards (10 µL each of 1,2,3,4-13C4 octanoic acid and methyl-D3 decanoic acid). After mixing 5 µL of 9 M KOH with a 25-µL sample of plasma, the tubes were placed in a water bath at 60°C for 0.5 h. After adjusting the pH by adding 20 µL of 2.25 M HCl, 450 µL acetonitrile was added and the samples centrifuged at 16,400 × g for 0.5 h at at 20°C. Finally, 80 µL of the supernatant was added to 120 µL of 5 mM ammonium bicarbonate and stored at 4°C until analysis. MCFA analysis was performed by ultra-HPLC (Nexera X2; Shimadzu) coupled to tandem MS (MS/MS; API-3000; AB Sciex).
Chromatography was carried out by using an Acquity UPLC HSS T3 1.8-µm column fitted with a BEH C18 1.7-µm VanGuard precolumn, both of which were maintained in a heating compartment at 30°C (Waters). The MCFAs were eluted by a binary gradient starting at 75% solvent A and 25% solvent B and increasing linearly to 100% solvent B in 5 min, at a flow rate of 0.5 mL/min. Solvent A was an aqueous solution of ammonium bicarbonate (5 mM) adjusted to pH 6 by the addition of formic acid, and solvent B was 90% acetonitrile in water. The gradient was held at 100% solvent B for 4 min before equilibrating for another 4 min at initial conditions. A 10-µL injection volume was used. Under these conditions, C8:0 and C10:0 MCFAs eluted at ∼2.5 and 3.3 min, respectively. The natural and isotopic derivatives of MCFAs were detected by MS/MS in negative mode by using multiple reaction monitoring with both quadruples (Q1 and Q3) set to the corresponding molecular ions: natural octanoic acid (m/z 143), isotopic octanoic acid (m/z 147), natural decanoic acid (m/z 171), and isotopic decanoic acid (m/z 174). The source temperature was 500°C and the collision energy was set to −15 eV on the instrument. The concentration of MCFAs in the plasma samples was determined from the ratio of natural compound to isotopic standard added before sample preparation. An isotopically labeled internal standard was added before sample preparation to take into account any changes in extraction efficiency and sensitivity of detection by LC-MS/MS. Calibrations were performed for each assay to ensure the precision of the method and that the LC-MS/MS response was linear over the entire range of concentrations of MCFAs measured in plasma samples. The linearity was verified after every batch of 50–100 samples, and the slope remained fairly constant, with a batch-to-batch variation of ∼20%.
Analyses
Plasma ketone concentrations were evaluated by automated colorimetric assay as previously described (10, 21–23). Calibrations and quality controls were performed for each assay (CV between tests was 5% ± 1% based on n = 360 measurements). Plasma insulin was analyzed by ELISA (Alpco Diagnostics Ltd.) with a microplate reader (Victor Multi-label Plate Reader 2030; Perkin Elmer). Plasma glucose, cholesterol, TGs (Siemens Medical Solutions USA, Inc.), and FFAs (Wako Diagnostics) were measured by using commercially available kits.
MCFA bioavailability
Two variables were determined for all of the test conditions: AUC (0–4 h postdose; Prism 6.0; GraphPad) and relative bioavailability (F; percentage) of the MCFAs, defined as AUC(MCT-E)/AUC(MCT-NE) × 100.
Statistical analysis
All of the statistical analyses were performed by using SPSS 15.0 software (SPSS, Inc.). Due to the small sample size (n <30) and the non-normality of the distribution as calculated by Fisher's test, a nonparametric test (Friedman's test) with an α set at 0.05 was used to determine whether there were significant differences between the response to the test substances. If a difference was observed by using Friedman's test at a given time point, post hoc Wilcoxon's rank-sum test was used to determine the level of significance (α = 0.05). Correlations were assessed by using the Spearman correlation coefficient. The comparisons then underwent a P ≤ 0.05 false discovery rate correction (24).
Results
Metabolic variables
Ten participants completed all 7 tests with no dropouts. Data from men and women were pooled because previous studies showed that there were no sex differences in short-term ketogenesis after a dose of MCTs given to healthy adults (9, 10). All of the participants had plasma metabolite values in the normal range at baseline (Table 1). Changes in plasma cholesterol, TGs, FFAs, glucose, and insulin responses did not differ significantly between any of the 7 metabolic tests (data not shown).
Plasma MCFAs
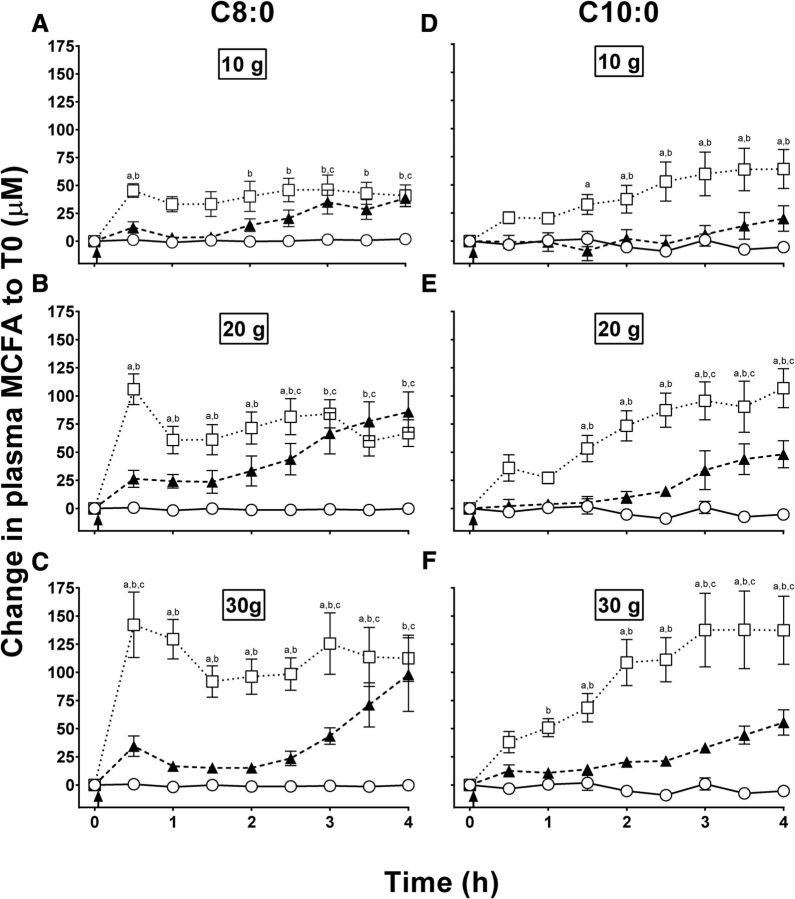
At the pre-MCT baseline (time 0 h), plasma octanoic and decanoic acid were 4.2 ± 0.7 and 9.3 ± 1.7 µM, respectively. Throughout the CTL test, plasma octanoic and decanoic acid averaged 2.8 ± 0.7 and 11.6 ± 1.7 µM, respectively, values that did not differ significantly from baseline. The MCT-NEs induced a gradual increase in plasma C8:0, which started at 3 h with the 10-g dose and at 30 min with both the 20- and 30-g doses (Figure 1A–C). The highest plasma C8:0 concentration attained with all 3 doses of MCT-NEs was at 4 h, with a dose-response effect between the 10- and 20-g doses (45.7 ± 9.0 compared with 92.2 ± 19.4 µM; Figure 1A, B), but no significant difference between the 20- and 30-g doses (92.2 ± 19.4 compared with 99.8 ± 35.6 µM; P = 0.33; Figure 1B, C). All 3 doses of MCT-Es significantly increased plasma C8:0 starting at 30 min postdose. A significant dose-response relation was observed with MCT-Es in which the maximum plasma C8:0 attained increased from 47.8 ± 5.5 to 108.8 ± 13.2 to 144.8 ± 29 µM for the 10-, 20-, and 30-g doses, respectively (P < 0.001; Figure 1A–C).
FIGURE 1.
Changes in plasma octanoic acid (C8:0) (left panels) and decanoic acid (C10:0) (right panels) normalized to T0 during the metabolic test days with the CTL (○), MCT-NEs (▴), or MCT-Es (□) at the 10-g (A and D), 20-g (B and E), and 30-g (C and F) doses. aMCT-Es compared with MCT-NEs, bMCT-Es compared with CTL, cMCT-NEs compared with CTL (all P < 0.05). CTL, no-treatment control; MCFA, medium-chain FA; MCT-E, emulsified medium-chain TG; MCT-NE, nonemulsified medium-chain TG; T0, time 0; ↑, medium-chain TGs consumed.
The 10-g dose of MCT-NEs did not significantly increase plasma C10:0 (Figure 1D), but the 20- and 30-g doses did increase plasma decanoic acid at 4 h to 59.8 ± 15.7 and 63.3 ± 12.2 µM, respectively (Figure 1E, F). MCT-Es significantly increased plasma decanoic acid starting at 2.5, 2, and 1.5 h for the 10-, 20-, and 30-g doses, respectively. A significant dose-response relation for plasma decanoic acid was observed after the 10-, 20-, and 30-g doses of MCT-Es, with the highest concentrations being 68.0 ± 18.0, 106.8 ± 17.4, and 139.4 ± 34.3 µM, respectively (P = 0.003; Figure 1D–F).
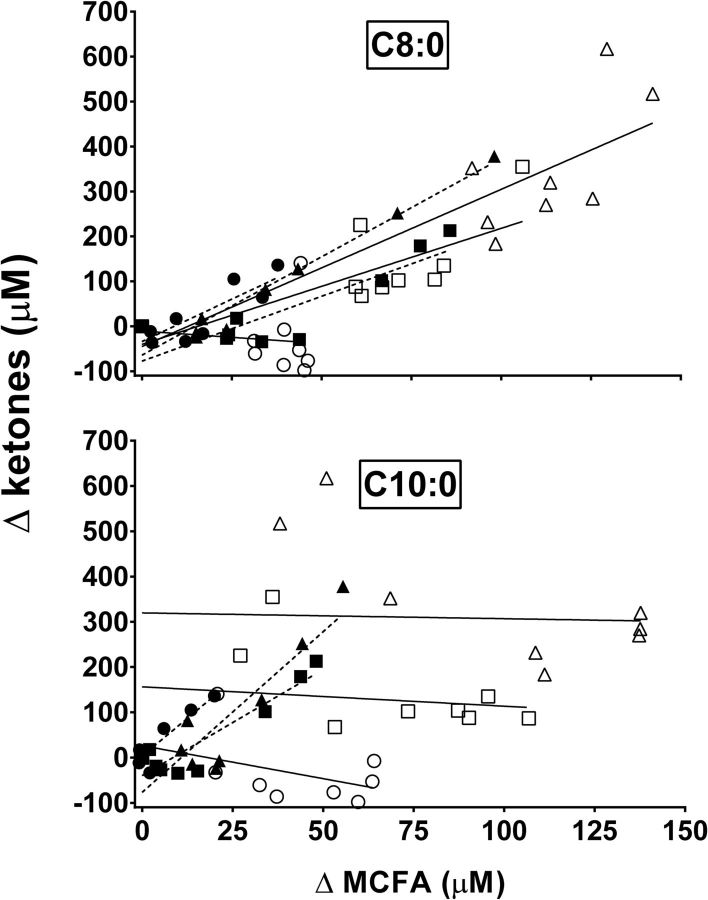
The increase in plasma octanoic acid from baseline was significantly correlated with the increase in plasma ketones with the 10-, 20-, and 30-g doses of MCT-NEs (Figure 2A; r = +0.84, P = 0.005; r = +0.86, P = 0.003; and r = +0.98, P < 0.001, respectively). The increase in plasma octanoic acid from baseline was not significantly correlated with the increase in plasma ketones after the 10-g dose of MCT-Es (Figure 2A; r = −0.11, P = 0.78), but the correlation was significant after the 20- and 30-g doses of MCT-Es (Figure 2A; r = +0.72, P = 0.03, and r = +0.80, P = 0.009, respectively). The increase in plasma decanoic acid from baseline was significantly correlated with the increase in plasma ketones with the 10-, 20-, and 30-g doses of MCT-NEs (Figure 2B; r = +0.93, P < 0.001; r = +0.92, P < 0.001; and r = +0.89, P = 0.001, respectively). The correlation between the increase in plasma C10:0 from baseline and the increase in plasma ketones was not significant for any of the 3 doses of MCT-Es (Figure 2B; r = −0.46, P = 0.22; r = −0.15, P = 0.70; and r = −0.04, P = 0.93, respectively).
FIGURE 2.
Correlations between differences in plasma MCFAs octanoic acid (C8:0) (upper panel) and decanoic acid (C10:0) (lower panel) and differences in plasma ketones after consumption of MCT-NEs (dotted lines) at 10 g (● C8:0: r = 0.84, P = 0.0048; C10:0: r = 0.93, P = 0.0002), 20 g (▪ C8:0: r = 0.86, P = 0.0029; C10:0: r = 0.92, P = 0.0004), and 30 g (▴ C8:0: r = 0.98, P < 0.0001; C10:0: r = 0.89, P = 0.0012) or MCT-Es (solid lines) at 10 g (○ C8:0: r = −0.11, P = 0.7768; C10:0: r = −0.46, P = 0.2174), 20 g (□ C8:0: r = 0.72, P = 0.0291; C10:0: r = −0.15, P = 0.7005), and 30 g (△ C8:0: r = 0.80, P = 0.0091; C10:0: r = −0.04, P = 0.9274). MCFA, medium-chain FA; MCT-E, emulsified medium-chain TG; MCT-NE, nonemulsified medium-chain TG.
The relative bioavailabilities (F) of octanoic acid from the MCT-E and MCT-NE values were 179%, 148%, and 309% for the 10-, 20-, and 30-g doses, respectively. For decanoic acid, F values were 196%, 236%, and 298% for the 10-, 20-, and 30-g doses, respectively, in favor of MCT-Es. For octanoic and decanoic acid combined, F values were 188%, 185%, and 304% for the 10-, 20-, and 30-g doses, respectively, in favor of MCT-Es.
Ketogenic response
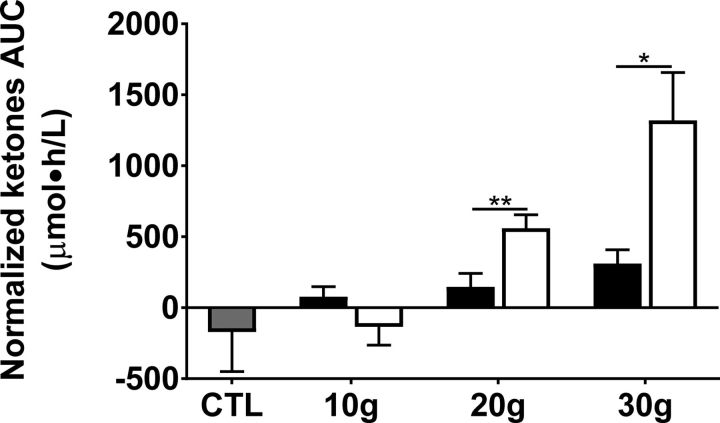
The CTL test did not induce ketogenesis over 4 h; indeed, plasma ketones decreased after the meal and did not return to baseline during the next 4 h (Figure 3). The MCT-NEs induced a ketogenic response starting 2.5 h after the 10-g dose and 3 h after the 20- or 30-g doses (Figure 3). The MCT-Es induced a modest, but rapid transient ketogenic effect at the 10-g dose, which was more pronounced and sustained at the 20- and 30-g doses (Figure 3). At the 20-g dose, there was a higher and more sustained ketogenic effect of MCT-Es that peaked at +355 ± 56 μM after 30 min compared with MCT-NEs, for which ketones reached a maximum concentration of +213 ± 51 μM after 4 h (Figure 1B). The 30-g dose of MCT-Es induced the largest ketogenic response, peaking at 1 h postdose at +617 ± 139 μM (Figure 3C). Ketosis with 30 g MCT-Es was also more sustained and did not go below +190 μM during the 4-h metabolic study period. The ketogenic response to the 30-g dose of MCT-NEs was slower than for MCT-Es and reached the highest concentration at 4 h of +378 ± 73 μM (Figure 3C). There were no significant differences in ketone AUCs at the 10-g dose of either treatment compared with CTL (ketone AUC was −171 ± 88 μM ⋅ h/L for CTL compared with 78 ± 70 μM ⋅ h/L for MCT-NEs and −135 ± 129 μM ⋅ h/L for MCT-Es; Figure 4). An incremental ketogenic dose-effect was seen with MCT-NEs, with the AUCs increasing from 78 ± 70 to 147 ± 94 and 311 ± 97 μM ⋅ h/L for the 10-, 20-, and 30-g doses, respectively (P = 0.01; Figure 4). There was also an incremental dose-effect relation with the 4-h AUCs for the MCT-Es, increasing from −135 ± 129 to 560 ± 95 and 1320 ± 336 μM ⋅ h/L for the 10-, 20-, and 30-g doses, respectively (P < 0.001; Figure 4).
FIGURE 3.
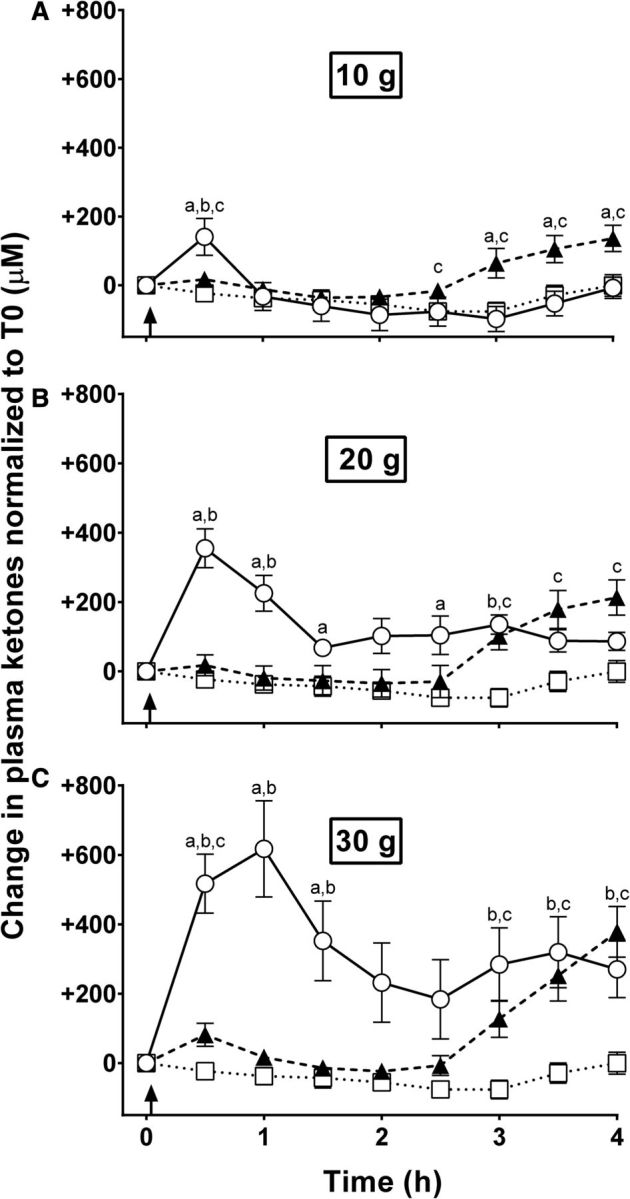
Changes in plasma ketones normalized to T0 during CTL (□ dotted lines) or metabolic tests with 10-g (A), 20-g (B), and 30-g (C) doses of MCT-NEs (▴ dashed lines) or MCT-Es (○ solid lines). aMCT-Es compared with MCT-NEs, bMCT-Es compared with CTL, cMCT-NEs compared with CTL (all P < 0.05). CTL, no-treatment control; MCT-E, emulsified medium-chain TG; MCT-NE, nonemulsified medium-chain TG; T0, time 0; ↑, medium-chain TGs consumed.
FIGURE 4.
Normalized AUCs for plasma ketones (acetoacetate + β-hydroxybutyrate) during the 4-h metabolic test for the CTL (gray bar), MCT-NEs (black bars), or MCT-Es (white bars). Values are means ± SEMs; n = 10/group. *P < 0.05, **P < 0.01. CTL, no-treatment control; MCT-E, emulsified medium-chain TG; MCT-NE, nonemulsified medium-chain TG.
Side effects
With the MCT-NEs, the most common side effects reported were abdominal discomfort and diarrhea, both of which represented 42% of the total number of reported side effects and were reported on all test days except for the CTL. Normally, the abdominal discomfort disappeared 30−60 min postdose. The number and severity of reported side effects were dose-dependent with the MCT-NEs; 2 side effects were noted with the 10-g dose, whereas 11 were reported with the 30-g dose. With the MCT-Es, the most common side effect reported was abdominal discomfort (53% of all side effects; reported on all 6 tests; Table 2). As with MCT-NEs, abdominal discomfort decreased after 30–60 min after taking the MCT-Es. There was no difference in the total number of side effects reported for the 10- and 20-g doses of MCT-Es. With the 30-g dose, almost twice as many side effects were reported for MCT-NEs as for MCT-Es (11 compared with 6), with the main difference being the absence of diarrhea with MCT-Es.
TABLE 2.
Self-reported side effects over 4 h after a single dose of MCT-NEs or MCT-Es1
| MCT-NEs (n = 10) | MCT-Es (n = 10) | |||||
|---|---|---|---|---|---|---|
| 10 g | 20 g | 30 g | 10 g | 20 g | 30 g | |
| Abdominal discomfort | 1 (M) | 3 (S) | 4 (M) | 2 (M) | 2 (L) | 4 (S) |
| Decreased appetite | — | — | 1 (L) | 1 (L) | 1 (L) | 1 (M) |
| Gastric reflux | — | — | 1 (L) | — | 1 (L) | — |
| Nausea | — | — | — | — | 2 (L) | 1 (L) |
| Diarrhea | 1 (M) | 2 (M) | 5 (S) | — | — | — |
| Headache | — | 1 (M) | — | — | — | — |
| Participants reporting side effects, n | 1 | 4 | 5 | 2 | 4 | 5 |
| Total number of side effects reported | 2 | 6 | 11 | 3 | 6 | 6 |
Severity scale (0–10): L, 0–3; M, 4–7; S, 8–10. The severity level was based on the highest score reported. L, light; M, moderate; MCT-E, emulsified medium-chain TG; MCT-NE, nonemulsified medium-chain TG; S, severe.
Discussion
Our main observation was that a stable emulsion of MCTs in lactose-free skim milk improved the ketogenic effect while reducing diarrhea, the side effect most commonly associated with MCTs. Previous studies from our group have shown a potent and rapid effect of MCT-NEs on ketogenesis in humans (9), but this is the first time we report the dose-response effect of emulsification on ketogenesis as well as the side effects after a single dose of MCTs.
Emulsification was previously shown to improve long-chain FA absorption and metabolism (18, 19), but we are not aware of any other studies that reported whether it affects the ketogenicity or side effects of MCTs (11). The significantly higher increase in plasma ketones for the 3 doses of MCT-Es implies faster absorption of MCTs after emulsification. It may be that preformed MCT-rich micelles are more easily hydrolyzed by gastric lipases, or pancreatic lipases before absorption via the portal vein (17, 18), thereby increasing the ketogenic effect and reducing the amount of unabsorbed MCTs reaching the large gut after the consumption of MCT-Es compared with MCT-NEs. Our present results are in line with those previously reported in piglets in which a 2-fold increase in plasma octanoic acid was noted after emulsification of a TG of octanoic acid and where the peak octanoic acid concentration was achieved at 1 h (18). However, the effect of nonemulsified octanoic acid in piglets differed from our results in which the plasma ketone concentration did not start to increase before 3 h postdose. Previous studies have shown that substrate availability is probably a limiting factor in stimulating ketogenesis in adults (25), suggesting that the efficacy of absorption and bioavailability of MCTs is probably directly linked to their subsequent ketogenic effect.
To the best of our knowledge, since the study by Freund and Weinsier (8), this is the first study to report the dose-response relation of an oral dose of MCTs on plasma ketones and MCFAs and the first study to look at the effect of MCT formulation on ketogenesis in humans. The results show a direct relation between the oral dose of MCTs given (≤30 g) and the bioavailability of MCFAs in the plasma of adults, a relation that also correlates directly with the change in plasma ketones. A previous review that explored this dose-response relation came to the same conclusion (7). The strong positive correlation between plasma ketones and plasma octanoic acid after MCT-E consumption was not observed with decanoic acid, implying that the increase in plasma ketones was more a function of octanoic than decanoic acid. The 4-h metabolic tests did not, however, permit us to follow plasma decanoic acid to its peak, so it was not possible to assess the full extent of the impact of octanoic and decanoic acid on plasma ketones beyond 4-h postdose. Recent work from our group showed that octanoic acid increases ketones significantly more in humans than does decanoic acid over the course of an 8-h study day (26). The same results were observed in neuronal cell culture in which octanoic but not decanoic acid stimulated the production of ketones from astrocytes (27).
There was no significant difference in the total number of reported side effects between MCT-Es and MCT-NEs. However, more diarrhea was reported with the highest dose of MCT-NEs than with MCT-Es (P < 0.001). In contrast, nausea of short duration (≤30 min) was more common with MCT-Es than with the MCT-NEs. In our 1- to 6-mo feeding studies with the same MCT emulsion, we observed better tolerability when the participants started at a lower dose (typically 5 g in the morning and again in the evening) and gradually titrated toward 15 g/dose twice a day over ≥1 wk.
The present study has some limitations. In particular, the single-dose design does not provide information about the effect of MCTs on ketogenesis and side effects over the long term. Nevertheless, there is presently no evidence that the ketogenic response to MCTs differs after a long-term intervention than after a single dose (9, 28). Our 4-h metabolic study day model was therefore appropriate to evaluate the acute dose-response effects of MCT-Es or MCT-NEs without side effects from repeated doses, which potentially cause lower compliance. The differences in AUCs reported from this 4-h metabolic study day may, however, not reflect differences in the AUCs that would be observed during a longer metabolic study day or prolonged MCT consumption.
Several studies have shown a 10–15% lower brain glucose uptake in the elderly, a deficit that increases to 20–25% in Alzheimer disease (29–32). Because this deterioration of brain glucose uptake is commonly present before the clinical (cognitive) onset of Alzheimer disease, it was postulated that deteriorating brain energy metabolism could be an important step in the evolution of the disease (7, 33, 34). However, brain ketone uptake is not affected in Alzheimer disease (35, 36). When given as a single dose (28, 37) or as a daily supplement for 3 mo (28), MCTs have been shown to improve cognition in mild to moderate Alzheimer disease, so it seems plausible that the beneficial cognitive effect of MCTs occurs through the stimulation of ketogenesis (28, 37). Hence, optimizing the ketogenic effect and tolerability of MCTs could potentially be beneficial in Alzheimer disease (37, 38).
In conclusion, emulsification of MCTs into a lactose-free skim-milk matrix improved their ketogenic effect by ≤4-fold over 4 h and also decreased diarrhea, a key side effect observed particularly at the 30-g dose of MCTs. The effect on tolerability and compliance of emulsifying MCTs therefore needs testing in longer-term studies.
Acknowledgments
We thank our research nurse Christine Brodeur-Dubreuil for her invaluable help with participant screening, blood sampling, and care of the participants. The authors' responsibilities were as follows—AC-L, MF, C-AC, JRW, and SCC: designed the research; AC-L, C-ML, VS-P, and CV: conducted the research; AC-L and C-ML: analyzed the data; AC-L, C-ML, VS-P, JRW, and SCC: wrote the manuscript; and all authors: read and approved the final manuscript.
Abbreviations
- CTL
control
- MCFA
medium-chain FA
- MCT
medium-chain TG
- MCT-E
emulsified medium-chain TG
- MCT-NE
nonemulsified medium-chain TG
- MS/MS
tandem MS
Footnotes
Supported by the Natural Sciences and Engineering Research Counsel of Canada, the Fonds de Recherche du Québec–Santé, and the University of Sherbrooke.
References
- 1. Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr. Brain metabolism during fasting. J Clin Invest 1967;46:1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drenick EJ, Alvarez LC, Tamasi GC, Brickman AS. Resistance to symptomatic insulin reactions after fasting. J Clin Invest 1972;51:2757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 1980;60:143–87. [DOI] [PubMed] [Google Scholar]
- 4. Robinson PJ, Rapoport SI. Glucose transport and metabolism in the brain. Am J Physiol 1986;250:R127–36. [DOI] [PubMed] [Google Scholar]
- 5. Blomqvist G, Thorell JO, Ingvar M, Grill V, Widen L, Stone-Elander S. Use of R-beta-[1-11C]hydroxybutyrate in PET studies of regional cerebral uptake of ketone bodies in humans. Am J Physiol 1995;269:E948–59. [DOI] [PubMed] [Google Scholar]
- 6. Courchesne-Loyer A, Croteau E, Castellano CA, St-Pierre V, Hennebelle M, Cunnane SC. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab 2017;37:2485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, Croteau E, Bocti C, Fulop T, Castellano CA. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci 2016;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freund G, Weinsier RL. Standardized ketosis in man following medium chain triglyceride ingestion. Metabolism 1966;15:980–91. [DOI] [PubMed] [Google Scholar]
- 9. Courchesne-Loyer A, Fortier M, Tremblay-Mercier J, Chouinard-Watkins R, Roy M, Nugent S, Castellano CA, Cunnane SC. Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition 2013;29:635–40. [DOI] [PubMed] [Google Scholar]
- 10. Freemantle E, Vandal M, Tremblay Mercier J, Plourde M, Poirier J, Cunnane SC. Metabolic response to a ketogenic breakfast in the healthy elderly. J Nutr Health Aging 2009;13:293–8. [DOI] [PubMed] [Google Scholar]
- 11. Ramírez M, Amate L, Gil A. Absorption and distribution of dietary fatty acids from different sources. Early Hum Dev 2001;65(Suppl):S95–101. [DOI] [PubMed] [Google Scholar]
- 12. Johnson RC, Young SK, Cotter R, Lin L, Rowe WB. Medium-chain-triglyceride lipid emulsion: metabolism and tissue distribution. Am J Clin Nutr 1990;52:502–8. [DOI] [PubMed] [Google Scholar]
- 13. Guillot E, Vaugelade P, Lemarchal P, Rerat A. Intestinal absorption and liver uptake of medium-chain fatty acids in non-anaesthetized pigs. Br J Nutr 1993;69:431–42. [DOI] [PubMed] [Google Scholar]
- 14. Wibisono C, Rowe N, Beavis E, Kepreotes H, Mackie FE, Lawson JA, Cardamone M. Ten-year single-center experience of the ketogenic diet: factors influencing efficacy, tolerability, and compliance. J Pediatr 2015;166:1030–6, e1. [DOI] [PubMed] [Google Scholar]
- 15. Liu YM, Wang HS. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed J 2013;36:9–15. [DOI] [PubMed] [Google Scholar]
- 16. Likhodii SS, Musa K, Mendonca A, Dell C, Burnham WM, Cunnane SC. Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia 2000;41:1400–10. [DOI] [PubMed] [Google Scholar]
- 17. Wieland TM, Lin X, Odle J. Emulsification and fatty-acid chain length affect the utilization of medium-chain triglycerides by neonatal pigs. J Anim Sci 1993;71:1869–74. [DOI] [PubMed] [Google Scholar]
- 18. Odle J, Lin X, Wieland TM, van Kempen TA. Emulsification and fatty acid chain length affect the kinetics of [14C]-medium-chain triacylglycerol utilization by neonatal piglets. J Nutr 1994;124:84–93. [DOI] [PubMed] [Google Scholar]
- 19. Garaiova I, Guschina IA, Plummer SF, Tang J, Wang D, Plummer NT. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr J 2007;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gagné S, Crane S, Huang Z, Li CS, Bateman KP, Lévesque JF. Rapid measurement of deuterium-labeled long-chain fatty acids in plasma by HPLC-ESI-MS. J Lipid Res 2007;48:252–9. [DOI] [PubMed] [Google Scholar]
- 21. Li PK, Lee JT, MacGillivray MH, Schaefer PA, Siegel JH. Direct, fixed-time kinetic assays for beta-hydroxybutyrate and acetoacetate with a centrifugal analyzer or a computer-backed spectrophotometer. Clin Chem 1980;26:1713–7. [PubMed] [Google Scholar]
- 22. Harano Y, Ohtsuki M, Ida M, Kojima H, Harada M, Okanishi T, Kashiwagi A, Ochi Y, Uno S, Shigeta Y. Direct automated assay method for serum or urine levels of ketone bodies. Clin Chim Acta 1985;151:177–83. [DOI] [PubMed] [Google Scholar]
- 23. Tremblay-Mercier J, Tessier D, Plourde M, Fortier M, Lorrain D, Cunnane SC. Bezafibrate mildly stimulates ketogenesis and fatty acid metabolism in hypertriglyceridemic subjects. J Pharmacol Exp Ther 2010;334:341–6. [DOI] [PubMed] [Google Scholar]
- 24. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–84. [DOI] [PubMed] [Google Scholar]
- 25. Courchesne-Loyer A, St-Pierre V, Hennebelle M, Castellano CA, Fortier M, Tessier D, Cunnane SC. Ketogenic response to cotreatment with bezafibrate and medium chain triacylglycerols in healthy humans. Nutrition 2015;31:1255–9. [DOI] [PubMed] [Google Scholar]
- 26. Vandenberghe C, St-Pierre V, Pierotti T, Fortier M, Castellano CA, Cunnane SC. Tricaprylin alone increases plasma ketone response more than coconut oil or other medium-chain triglycerides: An acute crossover study in healthy adults. Curr Dev Nutr 2017;1:257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thevenet J, De Marchi U, Domingo JS, Christinat N, Bultot L, Lefebvre G, Sakamoto K, Descombes P, Masoodi M, Wiederkehr A. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J 2016;30:1913–26. [DOI] [PubMed] [Google Scholar]
- 28. Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 2011;27:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet 2006;368:387–403. [DOI] [PubMed] [Google Scholar]
- 31. Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, Li Y, Boppana M, de Leon MJ. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology 2005;64:1860–7. [DOI] [PubMed] [Google Scholar]
- 32. Ogawa M, Fukuyama H, Ouchi Y, Yamauchi H, Kimura J. Altered energy metabolism in Alzheimer's disease. J Neurol Sci 1996;139:78–82. [PubMed] [Google Scholar]
- 33. Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA 2004;101:284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mosconi L. Glucose metabolism in normal aging and Alzheimer's disease: methodological and physiological considerations for PET studies. Clin Transl Imaging 2013;1:217–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, Imbeault H, Turcotte E, Fulop T, Cunnane SC. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer's disease dementia. J Alzheimers Dis 2015;43:1343–53. [DOI] [PubMed] [Google Scholar]
- 36. Castellano CA, Paquet N, Dionne IJ, Imbeault H, Langlois F, Croteau E, Tremblay S, Fortier M, Matte JJ, Lacombe G, et al. 3-Month aerobic training program improves brain energy metabolism in mild Alzheimer's disease: preliminary results from a neuroimaging study. J Alzheimers Dis 2017;56:1459–68. [DOI] [PubMed] [Google Scholar]
- 37. Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging 2004;25:311–4. [DOI] [PubMed] [Google Scholar]
- 38. Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001;51:241–7. [DOI] [PubMed] [Google Scholar]