Abstract
The cornea accumulates high proportions (can be up to 50%) of taxon-specific, water-soluble, cytoplasmic proteins (often enzymes) that have been considered analogous to the multifunctional lens crystallins. We have shown that gelsolin (an actin-severing protein) is the major water-soluble corneal protein of the zebrafish (Danio rerio) and the ‘four-eyed’ fish (Anableps anableps). Each Anableps eye contains one lens, an aquatic ventral cornea with an epithelium comprising 5–7 cell layers, and an air-exposed flatter dorsal cornea with an epithelium comprising >20 cell layers and appreciably enriched with glycogen. Gelsolin accounts for 38 and 21% of the dorsal and ventral cornea, respectively, suggesting that the abundance of gelsolin in the cornea is not incompatible with its function in air. The thicker, glycogen-enriched, air-exposed dorsal cornea may protect against UV irradiation and desiccation. Gelsolin comprises ~50% of the 5 cell-layer thick aquatic corneal epithelium of zebrafish. Reported zebrafish ESTs have indicated the presence of a second gelsolin gene in this species. We show by RT-PCR that the abundant corneal gelsolin (also expressed weakly in lens) (C/L-gelsolin) is also expressed in early development and differs from a ubiquitously expressed gelsolin (U-gelsolin) that is not specialized for cornea. Microinjection tests showed that overexpression of C/L-gelsolin dorsalizes the embryo and can lead to axis duplication, while interruption of C/L-gelsolin expression with a specific morpholino oligonucleotide ventralizes the embryo and interferes with brain and eye development. The evidence that C/L-gelsolin participates in the bone morphogenetic protein (BMP)/Smad dorsal-ventral signaling pathway is reviewed. Finally, we speculate that soluble C/L-gelsolin:actin complexes in the cornea may be analogous to soluble αA:αB-crystallin complexes in the lens. Together, our data are consistent with an analogy between the abundance of gelsolin in fish corneas and taxon-specific multifunctional crystallins in lenses.
Keywords: crystallin, cornea, gelsolin, zebrafish
1. Introduction
The transparent lens and cornea of the vertebrate eye are responsible for transmitting incident light into the eye and casting an image onto the photoreceptors of the retina (Land and Fernald, 1992). In terrestrial vertebrates, the cornea accounts for about 80% of the refraction of incident light due to the difference in the refractive indices of air (1.00) and cornea (~ 1.37–1.4) (Patel et al., 1995). However, the burden of refraction falls almost entirely on the lens in aquatic animals owing to the similarity of the refractive indices of the cornea and the surrounding water (~1.33) (Sivak et al., 1989; Leonard and Meek, 1997). Crystallins, which represent 80–90% of the water-soluble proteins of the lens, contribute greatly to its optical properties and, in vertebrates, generate a concentration gradient of refractive index responsible for producing a focused image on the retina (Benedek, 1971; Bettelheim and Siew, 1983; Delaye and Tardieu, 1983). Although crystallins play a specialized optical role in the lens, they are often, if not always, related or identical to physiological stress proteins (e.g. the small heat shock protein/α-crystallins) or common metabolic enzymes (e.g. lactate dehydrogenase B4, α-enolase and argininosuccinate lyase, among others) that have non-refractive roles outside of the lens (Ingolia and Craig, 1982; Wistow et al., 1987; Wistow and Piatigorsky, 1987, 1988; de Jong et al., 1989). Another unexpected fact is that crystallins are taxon-specific, i.e. different crystallins or combinations of crystallins are used in lenses of different species (Piatigorsky and Wistow, 1989; Tomarev and Piatigorsky, 1996). The high expression of a ubiquitously expressed enzyme or small heat shock protein in the lens serving predominantly a crystallin role and the low expression of the identical protein and gene in other tissues when serving a metabolic role, such as occurs for duck lactate dehydrogenase B4/ε-crystallin (Hendriks et al., 1988) and argininosuccinate lyase/δ2-crystallin (Piatigorsky et al., 1988), is called ‘gene sharing’ (Piatigorsky and Wistow, 1989).
Similar to the lens, the cornea accumulates high proportions of a few intracellular, water-soluble proteins (Piatigorsky, 1998). BCP 54, subsequently identified as active aldehyde dehydrogenase 3 (ALDH3), accounts for 20–40% of the water-soluble protein of the bovine cornea (Evces and Lindahl, 1989; Abedinia et al., 1990; Cooper et al., 1991; Verhagen et al., 1991). ALDH3 accumulates in the corneas of most mammals but not in other classes, indicating that it is taxon-specific, like the enzyme-crystallins of the lens (Alexander et al., 1981; Jester et al., 1999). Indeed, there are marked species-specific differences in the major enzymes of the cornea. Examples include isocitrate dehydrogenase in the bovine cornea (Sun et al., 1999), ALDH1 in rabbit cornea (Jester et al., 1999), ALDH3 (Nees et al., 2002) and transketolase in most mammalian corneas (Sax et al., 1996) but not in bird, reptile or fish corneas (Sax et al., 2000). A significant feature of the abundant water-soluble, cytoplasmic proteins of the cornea that resembles the lens crystallins is that they are not always enzymes. Gelsolin, an actin-modulating protein is the predominant water-soluble, intracellular protein in the corneas of the zebrafish, rosey barb and tricolor shark (Xu et al., 2000) and of Anableps anableps, the ‘four-eyed’ surface fish (Swamynathan et al., 2003). Actin is the second most abundant water-soluble protein (~14%) in the zebrafish and Anableps cornea. It remains to be shown how widespread the abundant corneal expression of gelsolin is among different species of fish.
In recent years, the zebrafish has emerged as a popular model organism for studying different aspects of vertebrate eye development (Glass and Dahm, 2004). Here we review our findings that gelsolin accumulates in the corneal epithelium of the zebrafish (Xu et al., 2000) and Anableps (Swamynathan et al., 2003), and that the abundant zebrafish corneal gelsolin is also expressed in the early embryo, where it has a dorsal/ventral signaling role (Kanungo et al., 2003). In addition, we provide evidence by reverse transcriptase-polymerase chain reaction (RT-PCR) that there are at least two, differentially expressed gelsolin genes in the zebrafish. The ubiquitous gelsolin (U-gelsolin) gene is expressed in all the tissues examined, in contrast to the cornea-preferred gelsolin (C/L-gelsolin) gene described earlier. Finally, we propose a possible analogy between the ability of zebrafish C/L gelsolin to maintain actin in the soluble form in the cornea and the ability of αA-crystallin to maintain αB-crystallin in soluble form in the murine lens (Brady et al., 1997). Although the corneal function of the abundant gelsolin in fish is not known yet, our data support the idea that the taxon-specific abundant cytoplasmic proteins in the cornea evolved, like the multifunctional lens crystallins, by a gene sharing strategy (Piatigorsky, 1998, 2001).
2. Materials and methods
2.1. RNA isolation and northern blot analysis
Total RNA was isolated from the brain, cornea, lens, heart and skin of adult zebrafish as described earlier (Xu et al., 2000). A 5 μg aliquot of total RNA (2 μg total RNA from cornea) was separated on formaldehyde-containing 1.2% agarose gel and transferred to a Nylon membrane (NEN Life Science Products, Boston, MA, USA). Northern blot analysis was performed essentially as described earlier (Xu et al., 2000).
2.2. Reverse transcriptase-polymerase chain reaction (RT-PCR) and oligonucleotide primers
To study the expression of C/L- and U- gelsolins in various tissues, RT-PCR was performed with the following pairs of oligonucleotide primer sequences: 5′-1274 GTGCTGGACTACCAGCCACACC 1295 -3′ and 5′-1671 TTCTGTAGGGCAGCAGTCTAG 1692 -3′ for C/L-gelsolin, and 5′-TGCCCTGTCCACGCGAAAC-3′ and 5′-CTCATCTTGCGTGCAGTAGTC-3′ for U-gelsolin (derived from EST Sequence: gi 5588679). About 1 μg total RNA from brain, cornea, heart and lens of adult zebrafish was employed in making first strand cDNA with avian myeloblastosis virus-reverse transcriptase (Roche Diagnostics Corp., Indianapolis, IN, USA); 1/10th of the resulting cDNA was used for PCR reactions.
2.3. Immunoblot analysis and immunocytochemistry
Procedures were essentially as reported earlier (Xu et al., 2000; Swamynathan et al., 2003).
2.4. Zebrafish and anableps
WT zebrafish were maintained as described by Westerfield (1995) and according to the regulations of the Animal Use and Care Committee of the NEI. Embryos were obtained by natural matings. Live Anableps were procured from a commercial vendor (Nova Tropicals, Alexandria, VA, USA) and maintained and dissected as described (Swamynathan et al., 2003). Water-soluble proteins were extracted from the excised corneal epithelia and separated on SDS-PAGE gels (Xu et al., 2000).
2.5. Antisense morpholino oligonucleotide (MO)
Sequences of control and gelsolin MOs and microinjection procedures were as described earlier (Nasevicius and Ekker, 2000; Kanungo et al., 2003).
2.6. In situ hybridization of zebrafish embryos and histological analysis
Procedures for these analyses have already been described in our previous reports (Xu et al., 2000; Swamynathan et al., 2003).
3. Results
Anableps anableps, the ‘four-eyed fish’ lives at the water surface, with the dorsal half of its eyes exposed to air and the ventral half exposed to water (Fig. 1). Medial extensions of the iris divide the pupil into a dorsal, aerial pupil and a ventral aquatic pupil. Their unique habitat presents us with an opportunity to study visual adaptations for amphibious vision.
Fig. 1.
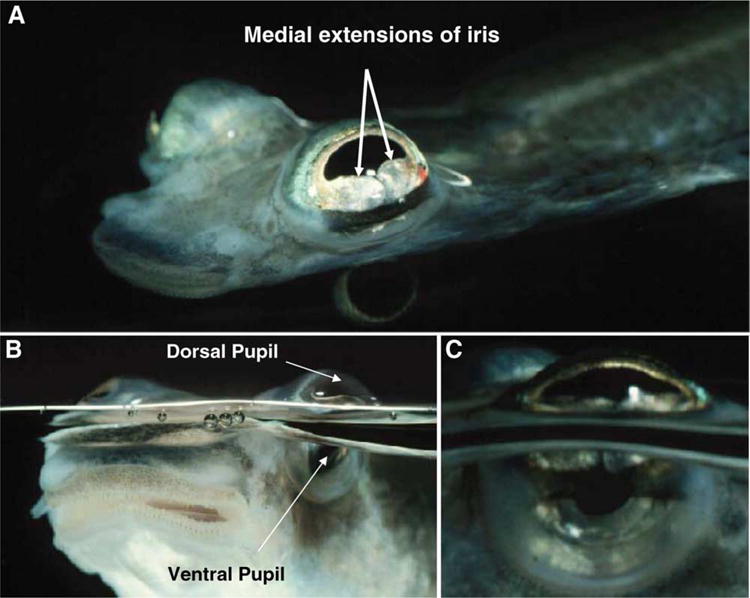
Anableps anableps, the ‘four-eyed fish’. Anableps lives at the water surface, with its eyes capturing aerial and aquatic images simultaneously. (A) Anableps viewed from the top, showing the medial flaps of iris. (B) Anableps viewed from the surface, showing the dorsal pupil and ventral pupil. (C) Close up of the Anableps eye. Photographs by David Denning qBioMEDIA ASSOCIATES. (Reproduced with permission.)
The structure of the Anableps eye is presented in Fig. 2(A) (see Swamynathan et al., 2003). The dorsal corneal epithelium composed of >20 cell layers, is thicker and flatter than the ventral corneal epithelium of 5–7 cell layers. The stroma is thinner than the overlying epithelium in the most dorsal part of the dorsal cornea. It shows a gradual increase in thickness in the ventral direction, forms an inward bulge at the junction of dorsal and ventral corneas, and abruptly becomes thin in the ventral cornea. A strip of pigmented cells is present in the anterior part of the bulged stroma at the junction of dorsal and ventral corneas. Two horizontal flaps of the iris divide the pupil into a larger aerial pupil (dorsal) and a smaller aquatic pupil (ventral). The pyriform lens is positioned such that the longer axis serves the aquatic vision and the shorter axis serves the aerial vision. Such an arrangement of the pyriform lens helps Anableps make up for the loss of refractive power of the ventral cornea in water compared to that of the dorsal cornea in air.
Fig. 2.
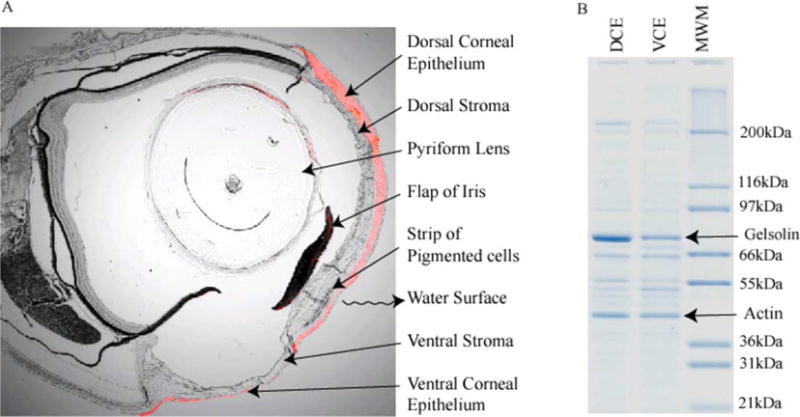
Adaptations in the structure and water-soluble protein compositions of Anableps corneas. (A) Superimposition of bright field photomicrograph with fluorescence image of immunohistochemistry with anti-gelsolin antibodies (red color). Magnification: 25 ×. (B) Profile of water-soluble proteins from the dorsal corneal epithelial cells (DCE) and the ventral corneal epithelial cells (VCE) of Anableps. Gelsolin and actin are indicated.
Densitometric scan of the SDS-PAGE gel of the water-soluble proteins from the dorsal and ventral corneas of Anableps (Fig. 2(B)) indicated that an ~80 kDa protein identified as gelsolin by the Edman degradation N-terminal sequencing constitutes 38 and 21% of the water-soluble protein in the dorsal and ventral corneal epithelial cells, respectively. Actin, the second most abundant band on the gel, corresponds to about 14% of the water-soluble protein in both the dorsal and ventral corneal epithelial cells. Superimposition of the fluorescence image of immunohistochemistry using anti-gelsolin antibodies with the corresponding bright field image shows that gelsolin expression is more abundant in the dorsal than the ventral epithelium of the Anableps corneas (red staining in Fig. 2(A)). In addition, we have shown that the Anableps dorsal corneal epithelium has ~15 times more glycogen than the ventral corneal epithelium. Abundant glycogen, through its water retention capacity, probably helps the dorsal corneal epithelial cells exposed to air resist desiccation (Swamynathan et al., 2003).
SDS-PAGE analysis of water-soluble proteins from the cornea, brain, lens, heart and skin of zebrafish showed that gelsolin (Xu et al., 2000) is abundant in cornea but not in other tissues (Fig. 3(A)). A specific polyclonal antibody developed against the cornea-abundant gelsolin recognized the protein in the cornea and to a much lesser extent in lens but not in the brain, heart and skin (Fig. 3(B)), suggesting preferred if not specific expression of C/L-gelsolin in the adult cornea and lens. In situ hybridization, however, showed all the blastomeres in the gastrula stage embryo expressed C/L-gelsolin mRNA (Fig. 4(A)). Some blastomeres at the anterior (future head region) part of the embryo showed a stronger hybridization signal than those in the other regions (Fig. 4(A)). In the hatching stage embryo (Stage 24) (Westerfield, 1995), C/L-gelsolin mRNA hybridization was abundant at the surface of the eye (Fig. 4(B)). Histological analysis revealed that the abundant gelsolin mRNA resides in the corneal epithelium (Fig. 4(C)).
Fig. 3.
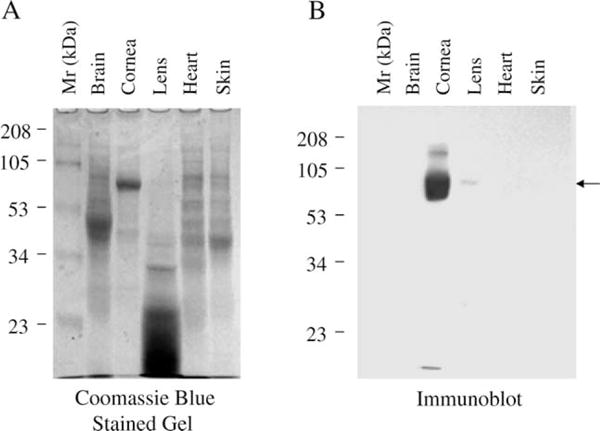
C/L-gelsolin is abundant in the cornea and present in the lens but not in other tissues. Coomassie Blue-stained SDS-polyacrylamide gel (A) and Western immunoblot (B) of approximately 15 μg of water-soluble proteins of the specified zebrafish tissues. The Western blot in (B) was performed from a duplicate of the gel shown in (A) The rabbit antiserum raised against a synthetic peptide comprising amino acids 329–345 of C/L-gelsolin was used to probe the blot.
Fig. 4.
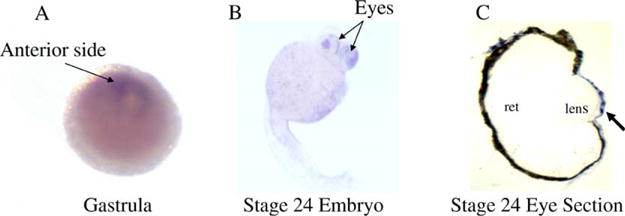
Developmental expression of C/L-gelsolin mRNA by in situ hybridization. C/L-gelsolin mRNA expression is shown in gastrula (A) and hatching stage embryos (B) at 70 hr of development (at 25 × magnification). A histological section of the eye (at 100 × magnification) shows the abundance of gelsolin message in the cornea (C). Locations of retina (ret) and lens are indicated. Arrow indicates the location of the cornea.
Northern blot analyses confirmed that the 2.7 kb C/L-gelsolin mRNA was abundantly expressed in the cornea but not detectable in the brain, heart, lens and skin (Fig. 5(A)). RT-PCR analysis was employed to probe the expression of the C/L-gelsolin and a putative second gelsolin gene, the existence of which was suggested by the EST sequence (gi 5588679) in different tissues of adult zebrafish. This more sensitive assay also confirmed that C/L-gelsolin is expressed abundantly in the cornea and at a very low but detectable level in the lens (Fig. 5(B)). By contrast, the second gelsolin gene was expressed in all the tissues examined and hence labeled ubiquitous gelsolin (U-gelsolin) (Fig. 5(B)).
Fig. 5.
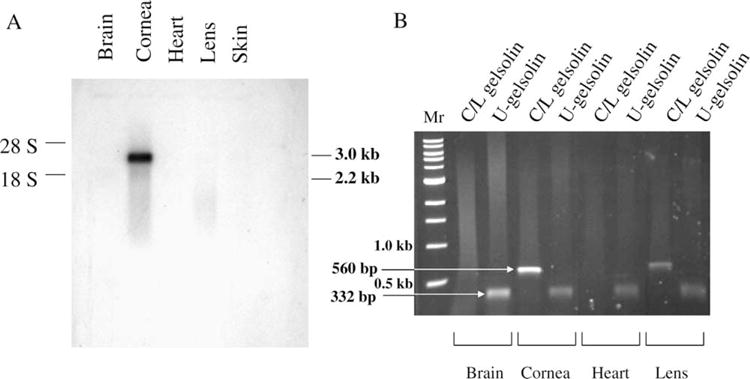
Northern blot and RT-PCR analyses of the tissue-specific expression of the two gelsolin genes in zebrafish. (A) 5 μg (2 μg from cornea) of total RNA from various tissues of zebrafish were analysed for Northern blotting; the blot was probed with a 5′ fragment of the C/L-gelsolin cDNA. (B) RT-PCR for C/L-gelsolin or U-gelsolin mRNA using RNA synthesized from cDNA obtained from the specified tissues of adult zebrafish.
The functional relevance of the C/L-gelsolin was tested by knock-down experiments. An antisense morpholino oligonucleotide against the C/L-gelsolin cDNA (gelsolin MO) was designed (Kanungo et al., 2003). Microinjection of the MO that was capable of specifically inhibiting the translation of the C/L-gelsolin resulted in 30-hr-old zebrafish embryos with underdeveloped heads and eyes. In the present histological analyses, we show in a 52-hr embryo, the malformation of the eye and brain (Fig. 6). In comparison to the wild type embryo (Fig. 6(A)), eosin– hematoxylin stained cross sections of the MO-injected embryo (Fig. 6(B)) showed a developmentally-lagged lens placode resulting in a strikingly underdeveloped eye. In short, the MO injected embryos displayed a ventralized phenotype.
Fig. 6.
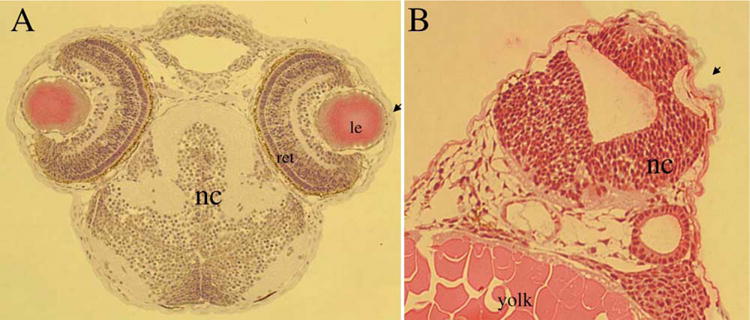
MO knockdown of C/L-gelsolin results in abnormal brain and eye development in zebrafish. Histological analyses using hematoxylin/eosin staining compare the anterior cephalic structures in control (A) and gelsolin MO-injected (B) embryos at 30 hr of development. Locations of notochord (nc), lens (le), retina (ret) and yolk are indicated. Magnification: 50 ×.
Microinjection of in vitro synthesized C/L-gelsolin mRNA into the one- to two-cell embryos resulted in dorsalized embryos (Fig. 7(A)). Dorsalization consisted of an excess of dorsal mesodermal tissue mass or, in advanced cases, partial body axis duplication. Additionally, microinjection of purified human plasma gelsolin protein into the embryos resulted in axis duplication. Axially duplicated embryos developed two heads, each with eyes (Fig. 7(B)). Thus, the experimental results obtained by increased expression of C/L gelsolin complemented those of the ventralization obtained by decreased expression of C/L-gelsolin in MO-injected embryos.
Fig. 7.
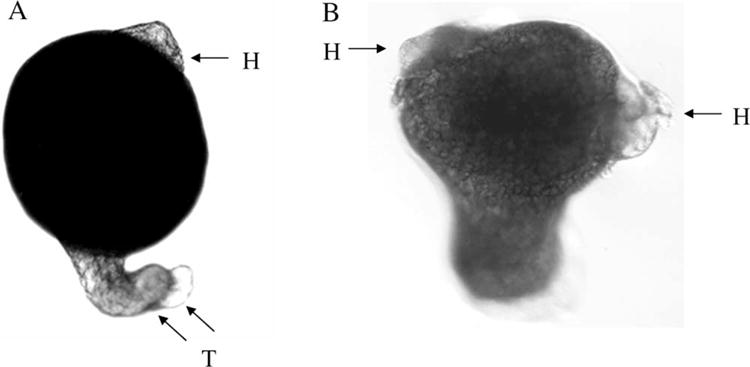
Gelsolin overexpression results in dorsalized and axis-duplicated embryos. Whole mounts of 30 hr-old zebrafish embryos that were microinjected with C/L-gelsolin mRNA (50 pg/embryo) (A), or with human plasma gelsolin protein (4 ng/embryo) (B), at the 1–2 cell stage. Arrows indicate the location of head (H) or tail (T). Magnification: 50 ×.
4. Discussion
Our results indicate that the corneas of zebrafish and the ‘four-eyed’ fish, Anableps, accumulate C/L-gelsolin, an actin-severing protein, as its major water-soluble protein. Since the zebrafish cornea and the ventral cornea of the Anableps eye are always submerged in water and used exclusively for aquatic vision, while the dorsal cornea of Anableps is amphibious and often exposed to air, we conclude that gelsolin accumulation is not incompatible with corneal function in air. We also describe structural differences in the dorsal and ventral corneas of Anableps that are of interest in view of the fact that the former is strictly aquatic while the latter is amphibious and must cope with an air environment. We propose that the thicker and flatter epithelium and high glycogen content of the dorsal cornea reflect the need for amphibious function and protection against UV radiation and desiccation (Swamynathan et al., 2003).
The reason for the corneal specialization of gelsolin in zebrafish and Anableps is still enigmatic, as is the reason for the high proportion of different proteins in the corneas of other species (Piatigorsky, 2001). To explore the possible analogy with the taxon-specific multifunctional lens crystallins, we have investigated other cellular functions of gelsolin (Kanungo et al., 2003). The results reviewed here show that, in addition to the accumulation of C/L-gelsolin in the adult cornea of zebrafish, this protein also has a non-refractive role in cell fate determination by modulating dorsal/ventral patterning in the early embryo. This is consistent with our finding that C/L-gelsolin mRNA is expressed already before the onset of post-zygotic transcription (Kanungo et al., 2003). Moreover, dorsalization enhanced expression of chordin and goosecoid mRNAs, while ventralization enhanced expression of Vent mRNA. These molecular markers suggest that gelsolin participates in the BMP/Smad dorsal-ventral signaling cascade during early development. Reduction of C/L-gelsolin by micro-injecting antisense MO into 1 to 2-cell embryos resulted in the loss of proper head and eye development, and over expression of C/L-gelsolin mRNA or human gelsolin protein resulted in dorsalized zebrafish embryos with partial axis duplications. Dorsalized cell fate occurs in vertebrate embryos when the ventralizing properties of the bone morphogenetic proteins (BMPs) are antagonized (Harland and Gerhart, 1997; Schulte-Merker et al., 1997; Dale and Wardle, 1999). Our results suggest that gelsolin antagonizes the BMP pathway (Kanungo et al., 2003). Besides their early developmental roles, BMPs are known to induce bone growth and interestingly, aging gelsolin-null mice show excessive bone growth (Chellaiah et al., 2000) possibly due to excess BMP activity. Gelsolin is known to have still other roles, such as in transcriptional regulation in association with androgen receptors in mammalian cells (Nishimura et al., 2003). Thus, the multifunctionality of C/L-gelsolin is reminiscent of the multifunctional nature of lens crystallins (Wistow and Piatigorsky, 1988; de Jong et al., 1989): both accumulate in transparent, refractive eye tissues and both play non-refractive roles elsewhere in the body, suggesting a gene sharing strategy for their evolution (Piatigorsky, 1998).
It is interesting to note that gelsolin-null mice are developmentally normal, with head and eye formation unaffected (Witke et al.), although cells from these mice show reduced cell motility and wound-healing ability (Cunningham et al., 1991). The fact that mice have one gelsolin gene (Kwiatkowski et al., 1986) whereas zebrafish have at least two gelsolin genes may explain this difference. Gelsolin is not the only gene that has duplicated in zebrafish. Indeed, fish appear to have undergone an ancient genome doubling (Van der Peer et al., 2003). Other important regulatory genes that contain additional copies in zebrafish include the hox clusters (Amores et al., 1998), Pax2 (Krauss et al., 1991; Pfeffer et al., 1998) and Pax6 (Nornes et al., 1998). It has been argued that the gene doublings in zebrafish permit subfunctionalization that reveals hidden functions of the single gene in other species (Vogel, 1998). This may be the situation concerning the two gelsolin genes in zebrafish. Our results suggest that following gene duplication, zebrafish C/L-gelsolin has subspecialized for both high expression in the cornea (and lower expression in the lens) as well as a regulatory role in the early embryo, leaving the second, ubiquitously expressed gelsolin (U-gelsolin) with the more generalized tasks for this protein. It is also possible that the regulatory, dorsal/ventral patterning role of gelsolin in the zebrafish embryo is compensated for in mice by the presence of numerous actin-modulating proteins. In other words, a functionally redundant pathway not present in zebrafish could annul the effect of the loss of gelsolin in mice.
Although the functional significance of the abundant C/L-gelsolin in the zebrafish or Anableps cornea is not known, we end by speculating on a further analogy with lens crystallins. In zebrafish and Anableps, actin is the second most abundant soluble protein in the corneal epithelium (Xu et al., 2000; Swamynathan et al., 2003). Despite the abundance of actin, however, it is almost entirely in the soluble, G-actin state in the cytoplasm, as judged by phalloidin staining (Xu et al., 2000; Swamynathan et al., 2003). The paucity of filamentous F-actin in the zebrafish and Anableps cornea is presumably due to the prevalence of cytoplasmic gelsolin, which is an actin-depolymerizing protein. We thus suggest an analogy between the abundant gelsolin and actin in the cornea and the abundant αA- and αB-crystallins in the lens: in order to remain soluble, αB-crystallin requires the presence of αA-crystallin in the murine lens (Brady et al., 1997), just as the presence of soluble G-actin requires the presence of abundant gelsolin in the zebrafish cornea (Fig. 8). Further studies are necessary to determine the possible relevance of this analogy for the optical or other biological properties of the transparent, refractive lens and cornea.
Fig. 8.
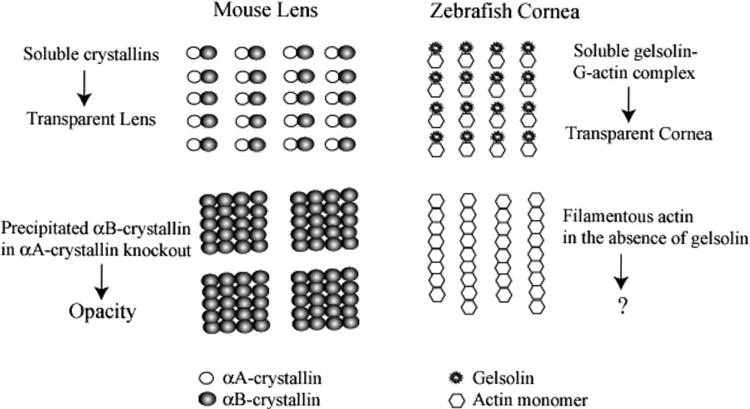
Suggested analogy between mouse lens αA- and αB-crystallins and zebrafish corneal gelsolin and actin. A schematic diagram suggesting an analogy between the mouse lens αA:αB–crystallin complexes and the zebrafish corneal gelsolin:G–actin complexes. The stoichiometry of the complexes is strictly diagrammatic to indicate complex formation. The question mark indicates that the consequence of having large amounts of filamentous actin in the zebrafish cornea is not known.
Acknowledgments
J.P. thanks Dr Abraham Spector, on this occasion of his retirement, for many years of friendship and stimulating scientific interaction.
References
- Abedinia M, Pain T, Algar EM, Holmes RS. Bovine corneal aldehyde dehydrogenase: the major soluble corneal protein with a possible dual protective role for the eye. Exp Eye Res. 1990;51:419–426. doi: 10.1016/0014-4835(90)90154-m. [DOI] [PubMed] [Google Scholar]
- Alexander RJ, Silverman B, Henley WL. Isolation and characterization of BCP 54, the major soluble protein of bovine cornea. Exp Eye Res. 1981;32:205–216. doi: 10.1016/0014-4835(81)90009-9. [DOI] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Benedek G. Theory of the transparency of the eye. Appl Optics. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- Bettelheim FA, Siew EL. Effect of change in concentration upon lens turbidity as predicted by the random fluctuation theory. Biophys J. 1983;41:29–33. doi: 10.1016/S0006-3495(83)84402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Nat Acad Sci USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah M, Kizer N, Silva M, Alvarez U, Kwiatkowski D, Hruska KA. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol. 2000;148:665–678. doi: 10.1083/jcb.148.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DL, Baptist EW, Enghild JJ, Isola NR, Klintworth GK. Bovine corneal protein 54K (BCP 54) is a homologue of the tumor-associated (class 3) rat aldehyde dehydrogenase (RATALD) Gene. 1991;98:201–207. doi: 10.1016/0378-1119(91)90174-a. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Stossel TP, Kwiatkowski DJ. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991;251:1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- Dale L, Wardle FC. A gradient of BMP activity specifies dorsal-ventral fates in early Xenopus embryos. Semin Cell Dev Biol. 1999;10:319–326. doi: 10.1006/scdb.1999.0308. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Hendriks W, Mulders JW, Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989;14:365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–418. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Evces S, Lindahl R. Characterization of rat cornea aldehyde dehydrogenase. Arch Biochem Biophys. 1989;274:518–524. doi: 10.1016/0003-9861(89)90465-7. [DOI] [PubMed] [Google Scholar]
- Glass AS, Dahm R. The zebrafish as a model organism for eye development. Ophthal Res. 2004;36:4–24. doi: 10.1159/000076105. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Ann Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hendriks W, Mulders JWM, Bibby M, et al. Duck lens -E-crystallin and lactate dehydrogenase B4 are identical: a single-copy gene product with two distinct functions. Proc Nat Acad Sci USA. 1988;85:7114–7118. doi: 10.1073/pnas.85.19.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc Nat Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Kanungo J, Kozmik Z, Swamynathan SK, Piatigorsky J. Gelsolin is a dorsalizing factor in zebrafish. Proc Nat Acad Sci USA. 2003;100:3287–3292. doi: 10.1073/pnas.0634473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991;113:1193–1206. doi: 10.1242/dev.113.4.1193. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Stossel TP, Orkin SH, Mole JE, Colten HR, Yin HL. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986;323:455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Land MF, Fernald RD. The evolution of eyes. Ann Rev Neurosci. 1992;15:1–29. doi: 10.1146/annurev.ne.15.030192.000245. [DOI] [PubMed] [Google Scholar]
- Leonard DW, Meek KM. Refractive indices of the collagen fibrils and extrafibrillar material of the corneal stroma. Biophys J. 1997;72:1382–1387. doi: 10.1016/S0006-3495(97)78784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ’knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nees DW, Wawrousek EF, Robison WG, Jr, Piatigorsky J. Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice. Mol Cell Biol. 2002;22:849–855. doi: 10.1128/MCB.22.3.849-855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Ting HJ, Harada Y, Tokizane T, Nonomura N, Kang HY, Chang HC, Yeh S, Miyamoto H, Shin M, Aozasa K, Okuyama A, Chang C. Modulation of androgen receptor transactivation by gelsolin: a newly identified androgen receptor coregulator. Cancer Res. 2003;63:4888–4894. [PubMed] [Google Scholar]
- Nornes S, Clarkson M, Mikkola I, Pederson M, Bardsley A, Martinez JP, Krauss S, Johansen T. Zebrafish contains two pax6 genes involved in eye development. Mech Dev. 1998;77:185–196. doi: 10.1016/s0925-4773(98)00156-7. [DOI] [PubMed] [Google Scholar]
- Patel S, Marshall J, Fitzke FW., III Refractive index of the human corneal epithelium and stroma. J Refract Surg. 1995;11:100–105. doi: 10.3928/1081-597X-19950301-09. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Gene sharing in lens and cornea: facts and implications. Prog Ret Eye Res. 1998;17:145–174. doi: 10.1016/s1350-9462(97)00004-9. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the ‘refracton’ hypothesis. Cornea. 2001;20:853–858. doi: 10.1097/00003226-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J, Wistow G. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989;57:197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J, O’Brien WE, Norman BL, Kalumuck K, Wistow GJ, Borras T, Nickerson JM, Wawrousek EF. Gene sharing by δ-crystallin and argininosuccinate lyase. Proc Nat Acad Sci USA. 1988;85:3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax CM, Kays WT, Salamon C, Chervenak MM, Xu YS, Piatigorsky J. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea. 2000;19:833–841. doi: 10.1097/00003226-200011000-00014. [DOI] [PubMed] [Google Scholar]
- Sax CM, Salamon C, Kays WT, Guo J, Yu FX, Cuthbertson RA, Piatigorsky J. Transketolase is a major protein in the mouse cornea. J Biol Chem. 1996;271:33568–33574. doi: 10.1074/jbc.271.52.33568. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Sivak JG, Howland HC, West J, Weerheim J. The eye of the hooded seal, Cystophora cristata, in air and water. J Comp Physiol. 1989;165:771–777. doi: 10.1007/BF00610875. [DOI] [PubMed] [Google Scholar]
- Sun L, Sun TT, Lavker RM. Identification of a cytosolic NADP + -dependent isocitrate dehydrogenase that is preferentially expressed in bovine corneal epithelium. A corneal epithelial crystallin. J Biol Chem. 1999;274:17334–17341. doi: 10.1074/jbc.274.24.17334. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Crawford MA, Robison WG, Jr, Kanungo J, Piatigorsky J. Adaptive differences in the structure and macromolecular compositions of the air and water corneas of the ‘four-eyed’ fish (Anableps anableps) FASEB J. 2003;17:1996–2005. doi: 10.1096/fj.03-0122com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarev SI, Piatigorsky J. Lens crystallins of invertebrates. Diversity and recruitment from detoxification enzymes and novel proteins. Eur J Biochem. 1996;235:449–465. doi: 10.1111/j.1432-1033.1996.00449.x. [DOI] [PubMed] [Google Scholar]
- Van der Peer Y, Taylor JS, Meyer A. Are all fishes ancient polyploids? J. Struct. Funct. Genomics. 2003;3:65–73. [PubMed] [Google Scholar]
- Vogel G. Doubled genes may explain fish diversity. Science. 1998;281:1119–1121. doi: 10.1126/science.281.5380.1119. [DOI] [PubMed] [Google Scholar]
- Verhagen C, Hoekzema R, Verjans GM, Kijlstra A. Identification of bovine corneal protein 54 (BCP 54) as an aldehyde dehydrogenase. Exp Eye Res. 1991;53:283–284. doi: 10.1016/0014-4835(91)90085-s. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene: 1995. [Google Scholar]
- Wistow G, Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987;236:1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Ann Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G, Mulders JWM, de Jong WW. The enzyme lactate dehydrogenase as a structural protein in avian and crocodilian lenses. Nature. 1987;326:622–624. doi: 10.1038/326622a0. [DOI] [PubMed] [Google Scholar]
- Witke W, Sharpe AH, Hartwig JH, Azuma T, Stossel TP, Kwiatkowski DJ. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- Xu YS, Kantorow M, Davis J, Piatigorsky J. Evidence for gelsolin as a corneal crystallin in zebrafish. J Biol Chem. 2000;275:24645–24652. doi: 10.1074/jbc.M001159200. [DOI] [PubMed] [Google Scholar]


