Abstract
Background: Clinical and preclinical studies have shown that dietary supplementation with dried plum improves bone health. These osteoprotective effects are a result, in part, of the antiresorptive properties of the fruit, which appear to be mediated by its polyphenolic compounds.
Objective: This study was designed to determine if certain fractions of the polyphenolic compounds in dried plums are responsible for the antiresorptive effects and whether they alter mitogen-activated protein kinase (MAPK) and calcium signaling, which are essential to osteoclast differentiation and activity, under normal and inflammatory conditions.
Methods: Six polyphenolic fractions were derived from the total polyphenolic extract of dried plum based on solubility. Initial screening, with the use of the Raw 264.7 monocyte and macrophage cell line, showed that 3 fractions had the most marked capacity to downregulate osteoclast differentiation. This response was confirmed in 2 of the fractions by using primary bone marrow–derived cultures and in all subsequent experiments to determine how osteoclast differentiation and function were altered with a focus on these 2 fractions in primary cultures. Data were analyzed by using ANOVA followed by post hoc analyses.
Results: Both of the polyphenol fractions decreased osteoclast differentiation and activity coincident with downregulating nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 (Nfatc1), which is required for osteoclast differentiation. Calcium signaling, essential for the auto-amplification of Nfatc1, was suppressed by the polyphenolic fractions under normal conditions as indicated by suppressed mRNA expression of costimulatory receptors osteoclast-associated receptor (Oscar), signaling regulatory protein β1 (Sirpb1), and triggering receptor expressed on myeloid cells 2 (Trem2). In contrast, in the presence of tumor necrosis factor α (TNF-α), only Sirpb1 was downregulated. In addition to calcium signaling, phosphorylation of extracellular signal–regulated kinase (Erk) and p38 MAPK, involved in the expression and activation of Nfatc1, was also suppressed by the polyphenolic fractions.
Conclusion: These results show that certain types of polyphenolic compounds from dried plum downregulate calcium and MAPK signaling, resulting in suppression of Nfatc1 expression, which ultimately decreases osteoclast formation and activity.
Keywords: polyphenols, osteoclasts, osteoporosis, dried plum, calcium signaling
Introduction
Osteoporosis is a major public health threat in the United States, and an estimated 60% of Americans over the age of 50 y either have osteoporosis or are at risk of osteoporosis due to low bone density (i.e., osteopenia) (1, 2). Of the estimated $19 billion in annual health care costs associated with preventing or treating osteoporotic fracture, 76% of those costs are incurred by postmenopausal women (2). Estrogen deficiency results in an uncoupling of the activity of bone-resorbing osteoclasts and bone-forming osteoblasts, with a net effect of a loss of mineralized tissue. The observed estrogen deficiency–induced increase in osteoclast differentiation and activity is due in part to the increase in T cell activation, including proliferation of osteoclast precursors, and a systemic increase in the proinflammatory cytokines TNF-α, IL-1, IL-6, and IL-17 (4–8). TNF-α, for example, increases the sensitivity of osteoclast precursors to receptor activator of NF-κB ligand (RANKL)–stimulated differentiation by upregulating RANK and activating NF-κB (5, 9–12). Disruption of the RANKL receptor binding is the target of the FDA-approved osteoporosis treatment denosumab, a RANKL monoclonal antibody (10, 11, 13).
Stimulation of osteoclast precursor cells by RANKL activates signaling cascades essential to osteoclast differentiation, including TNF receptor–associated factor 6 (TRAF6)–mediated and calcium-regulated signaling pathways (14, 15). Activation of TRAF6 initiates signaling cascades, such as MAPK and NF-κB, that are essential to the initial upregulation of the transcription factor nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 (Nfatc1) (14). Blockade of these signaling pathways by the polyphenolic compound caffeic acid, found in a number of different fruits (e.g., plums and berries), or the phytochemical phenethyl isothiocyanate, found in cruciferous vegetables, has been shown to reduce osteoclast differentiation (16, 17). Although TRAF6-mediated signaling is important for initial upregulation of Nfatc1, the auto-amplification of Nfatc1 necessary for late-stage osteoclast differentiation is modulated by calcium-regulated signaling pathways (15). Costimulatory receptors, such as osteoclast-associated receptor (OSCAR), triggering receptor expressed on myeloid cells 2 (TREM2), and signaling regulatory protein β1 (SIRPB1), regulate calcium oscillations in the differentiating osteoclast (18, 19). Interruption of the influx of calcium into osteoclast precursors and subsequent calcium signaling has been reported with several different types of natural products including some polyphenols (i.e., caffeic acid), coumarins (i.e., praeruptorin A), and lignans (20, 21). Thus, it stands to reason that alterations in calcium signaling as well as TRAF6 represent potential mechanisms through which the bioactive compounds in a number of plant-based products mediate their antiresorptive effects.
Reducing bone resorption is essential to attenuating the loss of bone, especially in postmenopausal osteoporosis. Clinical trials, as well as preclinical studies in animal models, have shown the antiresorptive effects of supplementing the diet with dried plum to prevent or reverse bone loss due to estrogen deficiency (22, 23). Postmenopausal women who consume dried plum (50 g/d) experienced increased whole-body bone mineral density, which was attributed to a decline in serum tartrate-resistant acid phosphatase (TRAP) 5b, which is secreted by osteoclasts during the resorption phase of bone remodeling (22). In preclinical trials that used ovariectomized rat and mouse models, dietary supplementation with dried plum prevented bone loss and decreased bone resorption (23–25). This response has been attributed in part to a decrease in T cell activation, a contributing factor to estrogen deficiency–induced bone loss (23). However, the bioactive components in dried plum that are responsible for inhibiting bone resorption remain in question.
Our laboratory has investigated the effects of polyphenols in dried plums that are known for their potent antioxidant and anti-inflammatory properties (26). The antiresorptive activity of these compounds has been shown in vitro and in vivo. A crude extract of dried plum polyphenols (i.e., total polyphenolic compounds) downregulated Nfatc1 gene expression and attenuated osteoclast differentiation and activity under inflammatory conditions in murine Raw 264.7 cells (27). Preliminary in vivo studies from our laboratory with this crude polyphenol extract indicate that the reduced bone mineral density and trabecular bone loss are reversed in aged osteopenic, estrogen-deficient Sprague-Dawley rats and serum bone resorption biomarkers were decreased. Although the ability of this polyphenolic extract to restore bone to a similar extent as with the whole fruit is a promising advancement in determining the bioactive components, it remains unclear if there are certain types of polyphenolic compounds that are responsible for the decrease in osteoclast activity. Moreover, elucidating the extent to which these polyphenolic compounds alter MAPK and calcium signaling is necessary to fully understand how these effects are mediated.
The purpose of this study was to determine the types of polyphenols within the ethanol polyphenolic extract that suppress osteoclast activity in primary cell culture systems. Furthermore, due to the role of immune cell activation in the bone loss that occurs in postmenopausal women, the mechanisms through which these compounds downregulate osteoclast activity were examined under normal and inflammatory conditions.
Methods
Reagents
Dried plums were supplied by the California Dried Plum Board, the same source of dried plums used in previous in vivo experiments (23–25, 27–30). DMEM; minimum essential medium, α modification (α-MEM); penicillin/streptomycin (P/S); L-glutamine; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT); RANKL; macrophage colony–stimulating factor (M-CSF); LPS; TNF-α and the standards for LC/MS identification of phytochemicals in the fractions (i.e., chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, caffeic acid, quinic acid, o-coumaric acid, m-coumaric acid, ferulic acid, cyanidin 3-rutinoside, cyanidin 3-glucoside, quercetin, rutin, sorbic acid, and 5-hydroxymethyl-2-furaldehyde) were purchased from Sigma-Aldrich. FBS was purchased from Gibco, and collagenase was purchased from Worthington.
Isolation of polyphenolic fractions from dried plum
Chromatography with the use of HP-20 resin (Sigma-Aldrich) was utilized to extract semipurified polyphenolic fractions from dried plum powder. First, a crude polyphenol extract was derived from sonicating 500 g dried plum in 80% methanol under pulsated nitrogen gas 2 times. The liquid phase was subjected to column chromatography by using 300 g HP-20 resin. The column was then rinsed 5 times with 200 mL deionized water to eliminate a water-soluble carbohydrate-rich extract. After the rinses, the column was washed with 100% methanol to yield a water-insoluble total polyphenolic–rich extract. This extract was then subjected to additional column chromatography by using 200 g HP-20 resin. Six semipurified polyphenol fractions of similar solubility properties were eluted from the column with increasing concentrations of methanol (i.e., 0%, 20%, 40%, 60%, 80%, and 100% methanol). These fractions were lyophilized and the weights of each fraction, derived from 500 g dried plum powder, were as follows: dried plum fraction (DP-Fr) A, 17.85 g; DP-FrB, 4.42 g; DP-FrC, 3.27 g; DP-FrD, 4.14 g; DP-FrE, 2.16 g; and DP-FrF, 1.39 g.
Screening of the polyphenolic fractions
Raw 264.7 cells, immortalized monocyte and macrophages that can be differentiated into osteoclasts with RANKL treatment, were used to screen the capacity of the fractions to reduce osteoclast differentiation. Cells were maintained in DMEM supplemented with 10% FBS and 1% P/S. The effects of the polyphenolic fraction on cell growth were assessed in doses ranging from 0.005 to 20 μg/mL by using the MTT assay.
On the basis of the results of the MTT assay, the capacity of the fractions to reduce osteoclastogenesis at doses of 0.1, 1, and 10 μg/mL was examined. Cells were plated at a density of 2 × 103 cells/well in a 96-well plate and treated with RANKL (30 ng/mL) to induce osteoclast differentiation. The cells were treated with polyphenolic fractions (0, 0.1, 1, or 10 μg/mL) beginning 24 h after RANKL treatment. After 5 d of RANKL treatment, the cells were washed with PBS and fixed in a 1:1 mixture of ethanol and acetone for 5 min. After 5 min of fixation, the cells were rinsed with deionized water and the osteoclasts were stained for TRAP (Sigma-Aldrich) expression. Large, multinucleated, TRAP-positive (TRAP+) cells were quantified by counting the number of cells per well with the use of an inverted light microscope (Nikon Instruments, Inc.).
To confirm that the fractions and doses of polyphenols that reduced osteoclast differentiation under normal conditions would have similar effects under inflammatory conditions, a second set of experiments were performed. Raw 264.7 cells were again cultured as described above and then treated with 0 or 1 ng LPS/mL on day 4 of RANKL treatment. The cells were then fixed and TRAP stained and the number of osteoclasts were quantified per well.
All of the screening experiments with Raw 264.7 cells were repeated a minimum of 2–3 times. The mean number of osteoclasts per treatment was expressed relative to the mean of the control group in each experiment (i.e., no polyphenol or LPS). The fractions with the greatest bioactivity in reducing osteoclast differentiation in Raw 264.7 cells were then assessed in primary culture systems.
Animal procedures for primary-derived cells for monocultures and co-cultures
All of the procedures involving the use of animals to derive primary bone marrow cells or neonatal calvarial cells were approved by the Oklahoma State University Institutional Animal Care and Use Committee.
Polyphenolic fractions and primary osteoclast differentiation
Fractions with the greatest bioactivity in suppressing osteoclast differentiation were further examined in primary bone marrow–derived osteoclast cultures. To obtain primary osteoclasts, bone marrow was flushed from long bones of 4- to 6-wk-old C57BL/6 mice (Charles River). Cells were cultured in α-MEM supplemented with 10% FBS, 2 mM L-glutamine, and 1% P/S for 2 d before collecting the nonadherent, hematopoietic bone marrow cells for experiments.
To examine the ability of the polyphenolic fractions to reduce osteoclast differentiation and activity, bone marrow mononuclear cells (BMMCs) were plated (1 × 105 cells/well) in α-MEM supplemented with 10% FBS, 2 mM L-glutamine, 1% P/S, and 30 ng/mL M-CSF in 96-well plates. After 3 d of M-CSF treatment, the media were supplemented with 50 ng RANKL/mL. On the fourth day of RANKL treatment, the cells were treated with 0-, 1-, or 10-μg/mL polyphenolic fractions with or without TNF-α (1 ng/mL) to simulate the inflammatory environment that results from estrogen deficiency. The following day, cells were fixed with a combination of citrate, acetone, and 37% formaldehyde and then stained for TRAP. The number of TRAP+ multinucleated cells (i.e., osteoclasts) were quantified per well.
Polyphenolic fractions alter osteoclast activity
The effect of the polyphenolic fractions on osteoclast activity was assessed by using a resorption pit assay. Dentin discs were prepared following the manufacturer's protocol and BMMCs were then plated in α-MEM supplemented with 10% FBS, 2 mM L-glutamine, 1% P/S, and M-CSF (30 ng/mL) at a density of 2.5 × 105cells/well. After 3 d, the media was replaced and RANKL (50 ng/mL) was added. On day 4 of RANKL treatment, the cells were treated with DP-FrE and DP-FrF (0, 1, or 10 μg/mL), which had the greatest effect on osteoclastogenesis with or without TNF-α (1 ng/mL). After 7 d of treatment, the cells were removed from the dentin discs by incubating in 10% bleach, and the discs were then washed 3 times with deionized water and stained with 1% toluidine blue in 0.5% sodium tetraborate. The discs were rinsed in deionized water until no excess stain remained. Resorption pit area was determined by evaluating light microscopy images with the use of ImageJ software (NIH), and the data were expressed as a percentage of the total area of the dentin disc.
Intracellular calcium in primary bone marrow–derived osteoclasts
For intracellular calcium measurement, BMMCs were plated in 96-well plates and osteoclasts were generated as described above. On day 4 of RANKL treatment, the cells were incubated in Fluo-4 dye (ThermoFisher) for 1 h before treatment with DP-FrE or DP-FrF (0 or 10 μg/mL) and TNF-α (0 or 1 ng/mL). To assess changes over time, intracellular calcium was determined at 1-min intervals for 120 min by measuring fluorescence at an excitation wavelength of 494 nm and an emission at 516 nm on a plate reader (Biotek) with incubation capabilities. Data were expressed as Δ/min and the AUC was calculated by using the trapezoidal approach.
Analysis of gene expression related to osteoclast differentiation and calcium signaling
For gene expression analyses, BMMCs were plated in 24-well plates at a density of 6 × 105 cells/well and osteoclasts were generated. On day 4 of RANKL treatment, the cells were treated with DP-FrE or DP-FrF (0 or 10 μg/mL) and TNF-α (0 or 1 ng/mL). Total RNA was extracted from cells 1 h later by using Trizol reagent (Life Technologies) according to the manufacturer's protocol. The concentration and quality of the RNA were assessed via OD determination at 260 and 280 nm, as well as agarose gel electrophoresis. The relative abundance of mRNA for the following genes related to osteoclast differentiation and calcium signaling was assessed by using SYBR-Green technologies (Life Technologies) and real-time qRT-PCR: Nfatc1 forward, 5′ GCG AAG CCC AAG TCT CTT TCC 3′, and reverse, 5′ GTA TGG ACC AGA ATG TGA 3′ cFos forward, 5′ GGA CAG CCT TTC CTA CTA CCA TTC C 3′, and reverse, 5′ AAA GTT GGC ACT AGA GAC GGA CAG A 3′ Traf6 forward, 5′ CAG CAG TGT AAC GGG ATC TAC 3′, and reverse, 5′ CTG TGT AGA ATC CAG GGC TAT G 3′ Rankl forward, 5′ TCT GCA GCA TCG CTC TGT TC 3′, and reverse, 5′ AGC AGT GAG TGC TGT CTT CTG ATA TT 3′ Opg (osteoprotegerin) forward, 5′ TCC CGA GGA CCA CAA TGA AC 3′, and reverse, 5′ TGG GTT GTC CAT TCA ATG ATG T 3′ Oscar forward, 5′ CGT GCT GAC TTC ACA CCA ACA 3′, and reverse, 5′ CAC AGC GCA GGC TTA CGT T 3′ Sirpb1 forward, 5′ GTC ACT CCT GCT GAT TCG G 3′, and reverse, 5′ GTC ACT GTC TGC TGA GGG AC 3′ and Trem2 forward, 5′ TCC CAA GCC CTC AAC ACC A 3′, and reverse, 5′ TTC CAG CAA GGG TGT CAT CTG CGA 3′. All mRNA expression levels were evaluated via the comparative cycle threshold (CT) method (User Bulletin 2; Applied Biosystems) with the use of Gapdh as an invariant control.
Protein expression analyses
For protein expression analyses, BMMC experiments were plated in 24-well plates and osteoclasts were generated as described above in the gene expression analyses experiments. After 30 min or 1 h of treatment, total protein was harvested by removing the media and washing the cells with PBS and then lysing the cells in radioimmuno-precipitation assay (Cell Signaling) buffer. The lysate was sonicated 6 × 15 s and centrifuged at 16,000 × g and 4°C to remove cellular debris. Total protein (40 μg) was denatured in Laemmli sample buffer at 95°C for 5 min. Protein was separated on a 4–20% gradient polyacrylamide gel by SDS-PAGE and then transferred onto a polyvinylidine difluoride membrane. Ponçeau S staining confirmed equal transfer of all samples. The polyvinylidine difluoride membranes were then blocked in 5% nonfat milk or BSA in Tris-buffered saline and 0.1% Tween-20. Next, the membranes were incubated with phosphorylated (p-) p44/42 [phosphorylated extracellular signal–regulated kinase (pERK)], p44/42 (ERK), p-p38, or p38 antibodies (Cell Signaling Technology), with gentle shaking overnight at 4°C. Actin (Santa Cruz Biotech) was used as a loading control. After overnight incubation, the membranes were washed and incubated with secondary antibody for 1 h before signal detection by using SuperSignal West (ThermoFisher) chemiluminescent substrate. The blots were exposed by using the ProteinSimple Fluorchem R, and the density of the bands was assessed by using UN-SCAN-IT gel analysis software (Silk Scientific, Inc.).
Polyphenolic fractions on osteoclast differentiation in a co-culture system
To determine the most bioactive extract(s) in reducing osteoclast differentiation in an environment that more closely mimics the coupled activity of osteoblasts and osteoclasts in vivo, murine primary co-cultures were used. Osteoblasts were isolated from the calvaria of 3- to 5-d-old C57BL/6 neonate mice by using sequential collagenase digestion. Briefly, the calvaria were rinsed in 0.05% EDTA (pH 7.4) for 20–30 min followed by six 12-min incubations in 0.6 mg collagenase/mL in α-MEM at 37°C on a shaker. The cells liberated during the second to sixth rounds of collagenase digestion were collected and centrifuged at 2000 × g for 6 min at 25°C. The cells were cultured in α-MEM supplemented with 10% FBS, 1% P/S, and 2 mM L-glutamine until used for co-culture experiments. BMMCs were collected as described above for the generation of osteoclasts.
The ability of DP-FrE and DP-FrF to reduce osteoclast differentiation in murine primary co-cultures was examined by quantifying the number of osteoclasts per well. Calvarium-derived osteoblasts were plated in α-MEM supplemented with 10% FBS, 1% P/S, 2 mM L-glutamine, and 10 nM 1,25-dihydroxycholecalciferol at a density of 5 × 104 cells on 6.5-mm polycarbonate Transwell inserts (1 × 108 0.4-μm pores; Corning), and BMMCs were plated at a density of 1.0 × 106 cells/well in a 24-well plate. Osteoblasts were treated with 0 or 10 μg/mL of polyphenolic fractions DP-FrE and DP-FrF with or without TNF-α (1 ng/mL) for 10 d; then, as described previously, TRAP staining of the bone marrow cells was performed. The number of TRAP+, multinucleated, large osteoclasts was quantified per well.
To assess gene expression in primary co-culture experiments, calvarial osteoblasts and nonadherent bone marrow cells were cultured together at a density of 5.0 × 105 cells and 1.5 × 106 cells, respectively, in a 24-well plate in α-MEM supplemented with 10% FBS, 1% P/S, 2 mM L-glutamine, and 10 nM 1,25-dihydroxycholecalciferol. After 6 d of treatment, 0 or 10 μg DP-FrE or DP-FrF/mL under normal or inflammatory (1 ng TNF-α/mL) conditions, RNA was extracted, and qRT-PCR was completed as described above.
Identifying phytochemicals present in DP-FrE and DP-FrF
To characterize DP-FrE and DP-FrF, the fractions were evaluated for the presence of 14 phytochemicals known to be present in dried plum, including chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, caffeic acid, quinic acid, o-coumaric acid, m-coumaric acid, ferulic acid, cyanidin 3-rutinoside, cyanidin 3-glucoside, quercetin, rutin, sorbic acid, and 5-hydroxymethyl-2-furaldehyde (31). LC and MS were used to determine the presence of the phytochemicals by using methods previously described (32). Briefly, known standards of the phytochemicals were used to identify the presence of any of the compounds in the fractions. Quantification of the detected compounds was accomplished by using a standard curve derived from the pure standard of the compound.
Statistical analyses
Statistical analyses were performed by using SAS version 9.3 (SAS Institute). A univariate analysis was completed to test for normal distribution. If the data were not normally distributed, log transformation was completed before statistical analysis. For the screening assays, doses within a given fraction were compared by using ANOVA with Bonferroni adjustment, due to the large number of comparisons, when the overall ANOVA was P < 0.05. For all other assays, the effect of polyphenolic fraction treatment at a given dose was analyzed by using ANOVA and Fisher's least significant difference post hoc analyses. Each experiment (6–8 wells/treatment) was repeated a minimum of 2–3 times. Values are expressed as means ± SEs unless otherwise indicated.
Results
Screening of the polyphenolic fractions in Raw 264.7 cells
To determine the most bioactive fraction(s) capable of reducing osteoclast differentiation, a dose-response study was performed with the use of Raw 264.7 cells treated with RANKL. The polyphenolic fractions that reduced osteoclast differentiation compared with control under normal conditions were DP-FrA, DP-FrE, and DP-FrF. DP-FrA reduced osteoclast number at the 10-μg/mL dose, whereas DP-FrE and DP-FrF reduced osteoclast numbers at 0.1, 1, and 10 μg/mL (Table 1). To assess whether these most bioactive fractions could also suppress osteoclast differentiation under inflammatory conditions, the cells were treated with LPS 24 h before TRAP staining. Under inflammatory conditions, each of the 3 fractions significantly reduced osteoclast differentiation at treatment doses of 1 and 10 μg/mL, whereas DP-FrA and DP-FrF also reduced osteoclast differentiation at a dose of 0.1 μg/mL compared with that of the LPS-treated control (Table 1). From these assays, it was determined that DP-FrA, DP-FrE, and DP-FrF were able to reduce osteoclast differentiation, which occurred under normal as well as inflammatory conditions with the use of a cell line. Therefore, experiments with these 3 fractions were performed to assess their effectiveness in primary cell culture experiments.
TABLE 1.
Screening of the polyphenolic fractions on osteoclast differentiation by using Raw 264.7 cells under normal and inflammatory conditions1
| Normal conditions (0 ng LPS/mL) | Inflammatory conditions (1 ng LPS/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraction | CON (0 μg/mL) | 0.1 μg/mL | 1 μg/mL | 10 μg/mL | P | CON (0 μg/mL) | 0 μg/mL | 0.1 μg/mL | 1 μg/mL | 10 μg/mL | P |
| DP-FrA | 100 ± 7a | 97 ± 11a | 82 ± 5b | 72 ± 6b | 0.049 | 100 ± 4b | 135 ± 7a | 106 ± 9b | 97 ± 3b | 93 ± 3b | 0.001 |
| DP-FrB | 100 ± 7 | 92 ± 7 | 82 ± 5 | 76 ± 6 | 0.099 | — | — | — | — | — | — |
| DP-FrC | 100 ± 7 | 80 ± 3 | 90 ± 6 | 82 ± 8 | 0.161 | — | — | — | — | — | — |
| DP-FrD | 100 ± 7 | 89 ± 6 | 86 ± 5 | 86 ± 5 | 0.051 | — | — | — | — | — | — |
| DP-FrE | 100 ± 7a | 79 ± 4b | 78 ± 6b | 74 ± 6b | 0.034 | 100 ± 4b | 135 ± 7a | 127 ± 2a | 103 ± 5b | 99 ± 3b | <0.001 |
| DP-FrF | 100 ± 7a | 79 ± 2b | 74 ± 2b,c | 66 ± 2c | 0.001 | 100 ± 4b | 135 ± 7a | 102 ± 5b | 100 ± 6b | 88 ± 4b | 0.001 |
Values are mean percentages of control ± SEs. Dried plum fractions (DP-FrA–DP-FrF) were screened for their ability to alter osteoclast differentiation under normal (0 ng LPS/mL) conditions. Fractions with significant effects under normal conditions (DP-FrA, DP-FrE, and DP-FrF) were tested under inflammatory conditions (1 ng LPS/mL). Data presented show osteoclast numbers from 2 to 3 experiments (n = 6 replicates/experiment) expressed relative to controls (0-µg/mL fractions and 0 ng LPS/mL). Within a row, means without a common superscript letter are significantly different from each other, P < 0.05. CON, control; DP-Fr, dried plum fraction.
Dried plum fractions on primary osteoclast differentiation and activity under normal conditions
Next, the capacity of DP-FrA, DP-FrE, and DP-FrF to alter osteoclast differentiation in primary bone marrow–derived osteoclasts was assessed. Both the 1-μg/mL (P = 0.003) and 10-μg/mL (P = 0.001) doses of DP-FrE and DP-FrF reduced the number of multinucleated TRAP+ osteoclasts (Figure 1A, B). DP-FrA was not able to significantly decrease osteoclastogenesis in the primary cultures and, as a result of this observation, all subsequent experiments focused on the DP-FrE and DP-FrF fractions.
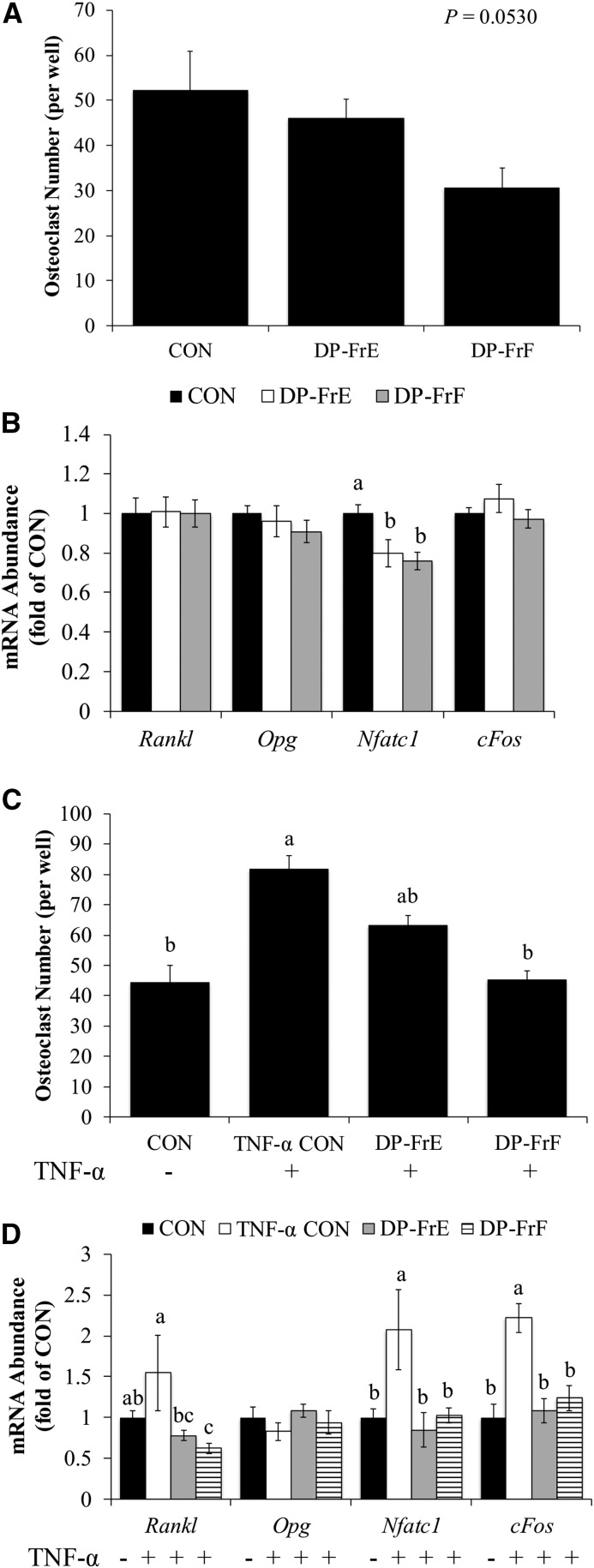
FIGURE 1.
Dried plum polyphenolic fractions (1 and 10 µg/mL) alter osteoclast differentiation and activity in primary bone marrow–derived cultures. Multinucleated TRAP-positive osteoclasts are indicated in the representative micrographs with arrows (A) and were quantified per well (B). Resorption pit assays were used to assess osteoclast activity. The resorbed area was stained (see arrows) (C) and was then quantified (D). The relative mRNA abundance of Nfatc1, Traf6, and cFos (E) during osteoclast differentiation was evaluated after 1 h of treatment with DP-FrE and DP-FrF (10 μg/mL). The relative abundance of p-p38 and pErk1/2 to total p38 and Erk was evaluated via immunoblotting after 1 h of treatment with dried plum polyphenolic fractions (10 μg/mL) and expressed relative to the control. Representative immunoblots are shown (F), and immunoblots (n = 3 replicates/experiment × 3 experiments) were quantified (G). Values in panels B, D, E, and G are means ± SEs. Data were analyzed by using ANOVA followed by Fisher's least significant difference test. Bars without a common letter are significantly different from each other, P < 0.05. CON, control; DP-Fr, dried plum fraction; Erk, extracellular signal–regulated kinase; Nfatc1, nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1; p-, phosphorylated; RANKL, receptor activator of NF-κB ligand; Traf6, TNF receptor–associated factor 6; TRAP, tartrate-resistant acid phosphatase.
To determine if the decrease in osteoclast number observed with polyphenolic fraction treatment resulted in a decrease in the activity of osteoclasts, primary BMMCs were cultured on dentin discs to assess resorption pit area. DP-FrE and DP-FrF at both doses (i.e., 1 and 10 μg/mL) significantly reduced resorption pit area compared with the control (Figure 1C, D).
Dried plum fractions alter gene and protein expression related to osteoclast differentiation under normal conditions
To examine alterations in regulators of osteoclast differentiation, mRNA expression of genes essential to the differentiation of osteoclasts was assessed. The relative abundance of Nfatc1, a key transcription factor that regulates osteoclastogenesis, was also suppressed by DP-FrE and DP-FrF (Figure 1E). Upstream of Nfatc1, the expression of Traf6, which upon RANKL binding to RANK initiates signaling cascades (e.g., MAPK and NF-κB) essential for osteoclast differentiation, was downregulated only by DP-FrF (Figure 1F). However, cFos mRNA, the protein of which heterodimerizes with cJun to form the transcription factor activator protein 1 (AP-1), which induces Nfatc1 expression, was downregulated by both DP-FrE and DP-FrF (Figure 1E).
Protein analyses were performed to determine if RANKL-stimulated MAPK activation was suppressed with dried plum fraction treatment, and representative blots are shown in Figure 1F. After 1 h of treatment, DP-FrF suppressed p38 phosphorylation (Figure 1G). Phosphorylation of Erk1/2 was decreased by DP-FrE, and to a lesser extent by DP-FrF, compared with control (Figure 1G). These data indicate that DP-FrE and DP-FrF downregulated Nfatc1, which is in part due to the suppression of cFos upstream of Nfatc1, as well as reduced activation of p38 and Erk1/2.
Effects of dried plum fractions on osteoclast differentiation and activity under inflammatory conditions
The ability of DP-FrE and DP-FrF to reduce osteoclast differentiation and activity in an inflammatory environment was also assessed. Osteoclast differentiation was significantly upregulated in TNF-α–stimulated cultures, and both DP-FrE and DP-FrF at 1 and 10 μg/mL attenuated this response (Figure 2A, B). In fact, osteoclast differentiation was suppressed by 36% by the higher dose of DP-FrE and by 43% by DP-FrF in TNF-α–treated cultures. These responses resulted in the number of osteoclasts being reduced to the level of the control cells cultured under normal conditions.
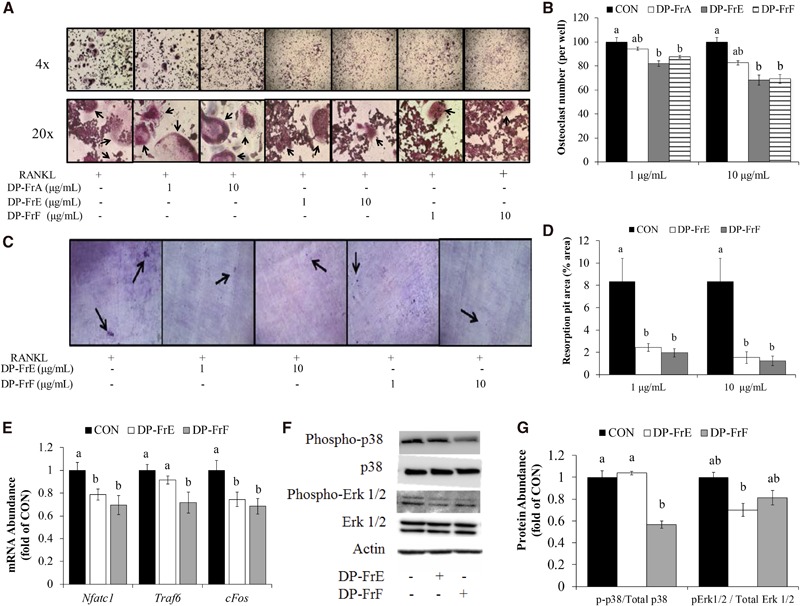
FIGURE 2.
Select polyphenolic fractions of dried plum (1 and 10 µg/mL) reduced osteoclast differentiation and osteoclast activity under inflammatory conditions (1 ng TNF-α/mL) in primary bone marrow–derived cultures. Multinucleated TRAP-positive osteoclasts are shown in the representative micrographs (see arrows) (A) and were quantified (B). Resorption pits were stained (see arrows) (C) and then quantified (D). The relative mRNA abundance of Nfatc1, Traf6, and cFos (E) was assessed after treatment for 1 h with TNF-α (1 ng/mL) and dried plum polyphenolic fractions (10 μg/mL). The relative abundance of p-p38 and pErk1/2 to total p38 and Erk was evaluated after 1 h of treatment with TNF-α (1 ng/mL) and dried plum polyphenolic fractions (10 μg/mL) via immunoblotting. Representative immunoblots are shown (F), and immunoblots n = 3–4 × 3 experiments were quantified (G). Values in panels B, D, E, and G are means ± SEs. Data were analyzed by using ANOVA followed by Fisher's least significant difference test. Bars without a common letter are significantly different from each other, P < 0.05. CON, control; DP-Fr, dried plum fraction; Erk, extracellular signal–regulated kinase; Nfatc1, nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1; p-, phosphorylated; RANKL, receptor activator of NF-κB ligand; Traf6, TNF receptor–associated factor 6; TRAP, tartrate-resistant acid phosphatase.
To determine whether DP-FrE and DP-FrF could also reduce osteoclast activity in an inflammatory environment, primary bone marrow–derived osteoclasts were cultured on dentin discs and treated with the polyphenolic fractions and TNF-α (1 ng/mL). TNF-α increased osteoclast activity as indicated by resorption pit area by ∼4-fold (P = 0.0009) compared with the untreated control cells (Figure 2C, D). Treatment with DP-FrE at the 1 μg/mL attenuated the TNF-α–induced increase (P = 0.0028) in resorption pit area, although not to the same magnitude as that of DP-FrF. Treatment with DP-FrF at 1 μg/mL resulted in a 73% decrease (P = 0.0004) in resorption pit area compared with TNF-α–treated control cells, and this reduction in resorption resulted in a resorbed area similar to that of the control that was not stimulated with TNF-α. At a treatment dose of 10 μg/mL, both DP-FrE and DP-FrF attenuated TNF-α–induced bone resorption to that of the control (0ngTNF-α/mL). Because a dose of 10 μg DP-FrE/mL was more effective than a dose of 1 μg/mL, all subsequent experiments examining mechanisms by which the polyphenolic fractions downregulate osteoclast differentiation were treated with 10 μg DP-FrE or DP-FrF/mL.
Dried plum fractions alter gene and protein expression related to osteoclast differentiation under inflammatory conditions
To determine the effects of DP-FrE and DP-FrF on regulators of osteoclast differentiation in an inflammatory environment, the relative abundance of mRNA for genes encoding for proteins that are essential to the differentiation of osteoclasts was assessed after 1 h of treatment with DP-FrE or DP-FrF, with or without TNF-α. Expression of Nfatc1 was upregulated by TNF-α treatment, and both DP-FrE and DP-FrF were able to attenuate this response, with DP-FrF having a more robust effect than DP-FrE (Figure 2E). Upstream of Nfatc1, the expression of Traf6 was upregulated by TNF-α, and both DP-FrE and DP-FrF were able to suppress this response (Figure 2E). In fact, mRNA expression of Traf6 in DP-FrE– and DP-FrF–treated cultures stimulated with TNF-α was similar to that of the control under normal conditions. In addition, cFos expression, which is induced by TRAF6 and NF-κB signaling and results in the induction of Nfatc1, was not altered by either TNF-α or dried plum phenolic fraction treatment (Figure 2E). In summary, gene expression indicative of osteoclast differentiation was suppressed by treatment with the polyphenolic fractions under inflammatory conditions.
Next, protein analyses were performed to determine if RANKL-stimulated MAPK activation was suppressed with dried plum fraction treatment under inflammatory conditions, and representative blots are shown in Figure 2F. After 1 h of treatment, p38 was not significantly altered by TNF-α or the fractions, although there was a trend (P = 0.051) for the upregulation of p-p38 with TNF-α and suppression of this response with both fractions (Figure 2G). Both DP-FrE and DP-FrF suppressed phosphorylation of Erk1/2 after 1 h of treatment compared with the TNF-treated control (Figure 2G). Likewise, phosphorylation of Erk1/2, but not p38, was significantly upregulated by TNF-α after 30 min of treatment, and both fractions attenuated this response (data not shown). These data indicate that, under inflammatory conditions, DP-FrE and DP-FrF downregulated Nfatc1, which is in part due to suppression of Traf6 and cFos upstream of Nfatc1, as well as reduced activation of p38 and Erk1/2, which is similar to observations under normal conditions.
Effects of dried plum fractions on osteoclast differentiation in primary co-cultures
To assess the bioactivity of the fractions in a system that allows for osteoblast and osteoclast interaction, primary osteoblasts derived from fetal murine calvaria and BMMCs were co-cultured. Under normal conditions, osteoclast differentiation tended to be suppressed (P = 0.0530) with treatment with the dried plum fractions (Figure 3A). Regulators of osteoclast differentiation are produced by the osteoblasts in the co-culture system in response to stimulation by 1,25-dihydroxycholecalciferol and TNF-α. Therefore, the effect of the dried plum fractions on osteoblast expression of signaling molecules that affect osteoclast differentiation was assessed. The relative mRNA abundance of Opg and Rankl was not altered by either DP-FrE or DP-FrF in the co-cultures (Figure 3B). The relative mRNA abundance of regulators of osteoclast differentiation was also assessed. Expression of Nfatc1 was downregulated by both DP-FrE and DP-FrF after 6 d of co-culture and treatment with the polyphenolic fractions (Figure 3B). However, Traf6 (data not shown) and cFos (Figure 3B) were not altered by treatment with dried plum fractions in the osteoblast and osteoclast co-cultures in normal conditions at this time point.
FIGURE 3.
Osteoclast differentiation is suppressed by a polyphenolic fraction of dried plum in TNF-α–stimulated co-cultures. Primary calvarial osteoblasts were treated with 1,25-hydroxyvitamin D3 (10 nM) and dried plum polyphenolic fractions (10 μg/mL) and co-cultured with BMMCs. (A) Multinucleated TRAP-positive osteoclasts were quantified per well. (B) The relative mRNA abundance of Rankl, Opg, Nfatc1, and cFos was assessed with qRT-PCR by using Gapdh as a control. (C) In addition, multinucleated TRAP-positive osteoclasts were quantified after 10 d of co-culture in the presence of TNF-α (1 ng/mL). (D) After 6 d of treatment with dried plum polyphenolic fractions and TNF-α, the relative mRNA abundance of Rankl, Opg, Nfatc1, and cFos was assessed with Gapdh as a control. Values are means ± SEs. Experiments were repeated 2–3 times with 6 replicates/experiment, and data were analyzed by using ANOVA followed by Fisher's least significant difference test. Bars without a common letter are significantly different from each other, P < 0.05. BMMC, bone marrow mononuclear cell; CON, control; DP-Fr, dried plum fraction; Nfatc1, nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1; Opg, osteoprotegerin; Rankl, receptor activator of NF-κB ligand; TRAP, tartrate-resistant acid phosphatase.
In an inflammatory environment, TNF-α upregulated osteoclast differentiation in the co-cultures and treatment with DP-FrF reduced the number of multinucleated TRAP+ cells compared with the TNF-α control cells (P = 0.0001; Figure 3C). In fact, the number of multinucleated TRAP+ osteoclasts in the DP-FrF–treated cultures was similar to that of the control (i.e., no TNF-α). Although mRNA expression of Rankl was not altered under normal conditions, both DP-FrE and DP-FrF downregulated Rankl expression in TNF-α–stimulated co-cultures (Figure 3D). The expression of Opg was not altered by dried plum fractions or TNF-α (Figure 3D). Further explanation for the reduction in osteoclast number was the expression of Nfatc1, which was upregulated by TNF-α, and both DP-FrE and DP-FrF were able to attenuate this response (Figure 3D). Similar to the co-cultures under normal conditions, Traf6 expression was not altered by dried plum fractions or TNF-α (data not shown). However, cFos expression was upregulated by TNF-α, and both DP-FrE and DP-FrF attenuated this response (Figure 3D). In fact, expression of both cFos and Nfatc1 were suppressed by DP-FrE and DP-FrF to the level of the untreated control cells in co-cultures under inflammatory conditions. These findings suggest that the polyphenolic fractions may both directly and indirectly reduce osteoclast differentiation in a co-culture model, in which osteoblast and osteoclast activities are coupled. The suppression of Rankl expression suggests that the polyphenolic fractions can downregulate osteoclast differentiation by reducing the production of stimulatory molecules by osteoblasts, whereas Nfatc1 expression data from the monocultures and these co-cultures suggest that the dried plum fractions can also directly downregulate differentiation pathways in osteoclast precursors.
Calcium signaling in primary bone marrow–derived osteoclasts under normal conditions
The differentiation of osteoclasts requires intracellular calcium oscillations regulated by costimulatory membrane-bound receptors, including OSCAR, SIRPB1, and TREM2. Kinetic studies showed that treatment of primary bone marrow–derived pre-osteoclasts with DP-FrF and DP-FrE under normal conditions failed to alter extracellular calcium uptake by the cells in a significant manner (Figure 4A). DP-FrF treatment resulted in an ∼2-fold downregulation of Oscar (Figure 4B), an osteoclast-specific receptor that is transcriptionally regulated by Nfatc1 and that is important in the auto-amplification of Nfatc1 via its role in activating phospholipase Cγ and calcium signaling. In addition, DP-FrF treatment resulted in decreased mRNA expression of Sirpb1, a costimulatory receptor expressed by myeloid cells that is known to regulate calcium signaling (Figure 4B). Furthermore, treatment with both DP-FrE and DP-FrF resulted in an ∼2-fold downregulation of Trem2, which activates phosphoinositide 3-kinase and subsequently calcium release from the endoplasmic reticulum (Figure 4B). These data suggest that DP-FrE and DP-FrF may reduce osteoclast differentiation, at least in part, by suppressing intracellular calcium signaling in the differentiating osteoclast, but not extracellular uptake.
FIGURE 4.
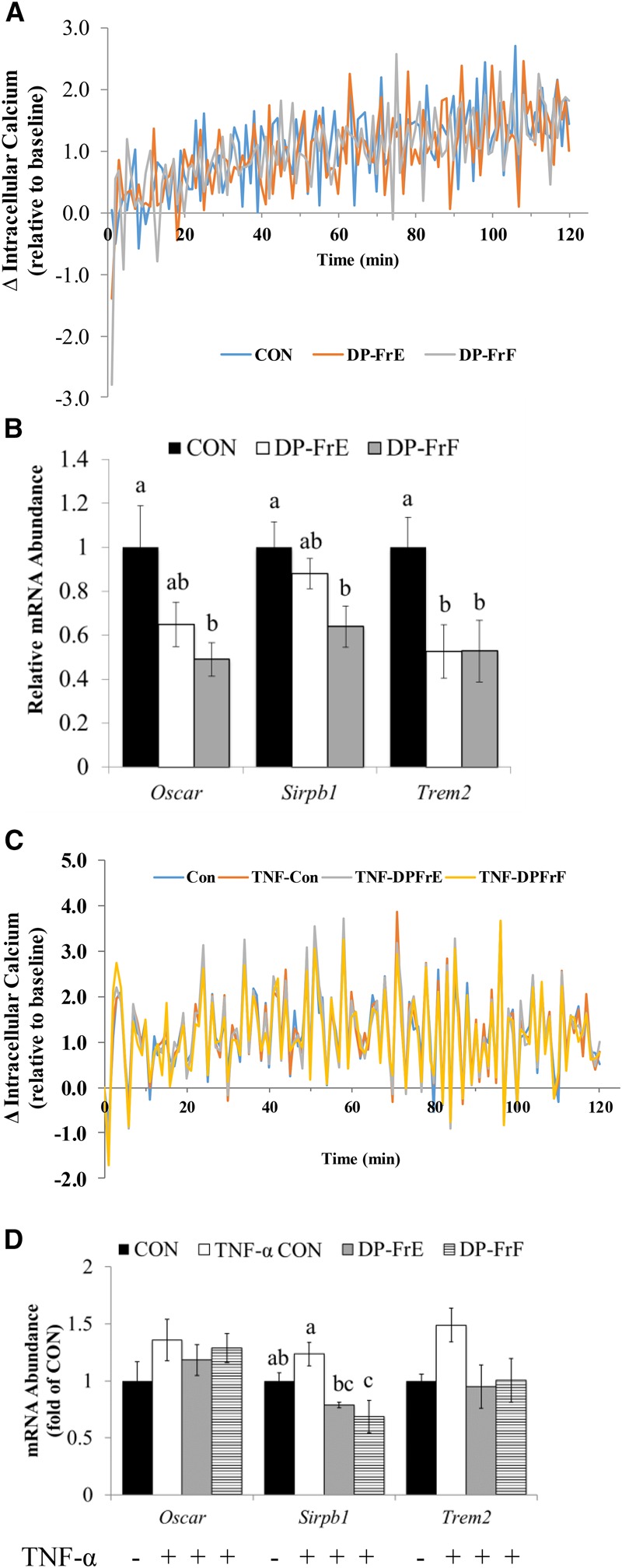
Treatment with DP-FrE or DP-FrF (10 μg/mL) altered calcium transport in primary BMMC cultures. Intracellular calcium was assessed under normal (A) and inflammatory (C) conditions and the relative mRNA abundance of Oscar, Sirbp1, and Trem2 mRNA was assessed after treatment with the dried plum fractions (10 µg/mL) for 1 h under normal (B) and inflammatory (D) conditions. Line graphs (A and C) show the mean change (Δ) in intracellular calcium per minute relative to baseline values. Values in panels B and D are means ± SEs. Data were analyzed by using ANOVA followed by Fisher's least significant difference test. Bars without a common letter are significantly different from each other, P < 0.05. BMMC, bone marrow mononuclear cell; CON, control; DP-Fr, dried plum fraction; Oscar, osteoclast-associated receptor; Sirpb1, signaling regulatory protein β1; Trem2, triggering receptor expressed on myeloid cells 2.
Calcium signaling in primary bone marrow–derived osteoclasts under inflammatory conditions
Similar to the response under normal conditions, calcium uptake was not altered by the 2 fractions in an inflammatory environment. Over the course of 120 min of treatment with TNF-α and the fractions, no significant alterations in intracellular calcium were observed (Figure 4C). Examination of mRNA expression of the costimulatory receptors that initiate the calcium oscillations required for osteoclastogenesis indicated that Sirpb1 was suppressed by both DP-FrE and DP-FrF in TNF-α–stimulated cultures compared with the TNF-α–treated control (Figure 4D). In fact, DP-FrF suppressed Sirpb1 to a level even lower than that of the untreated control. Unlike in the osteoclast cultures under normal conditions (i.e., no TNF-α), treatment with the dried plum phenolic fractions did not alter the expression of Oscar or Trem2 (Figure 4D).
Characterization of polyphenolic compounds in DP-FrE and DP-FrF
Dried plums are rich in polyphenols, especially phenolic acids. The presence of 14 phytochemicals (chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, caffeic acid, quinic acid, o-coumaric acid, m-coumaric acid, ferulic acid, cyanidin 3-rutinoside, cyanidin 3-glucoside, quercetin, rutin, sorbic acid, and 5-hydroxymethyl-2-furaldehyde) known to be in dried plums was assessed in DP-FrE and DP-FrF. Interestingly, of the 14 compounds, only cryptochlorogenic acid, neochlorogenic acid, and rutin were detected in the 2 fractions. Each of the chlorogenic acid isomers was more abundant in the fraction extracted with the highest concentration of methanol, DP-FrF, than in DP-FrE.
Discussion
This study is the first to our knowledge to determine the fractions of a polyphenolic extract from dried plums that are responsible for reducing osteoclast differentiation and activity. The fractions extracted in the highest concentration of methanol (i.e., DP-FrE and DP-FrF) were the most effective at downregulating osteoclast differentiation and activity in primary bone marrow–derived osteoclasts, which was mediated through the suppression of Nfatc1, the master regulator of osteoclast differentiation. The ability of the DP-FrE and Dp-FrF to suppress Nfatc1 expression in primary cell cultures is consistent with our previous findings with an extract of dried plum total polyphenols that utilized a cell line as well as in the bone tissue of ovariectomized mice fed a dried plum–supplemented diet (24, 27). The expression of Nfatc1 is regulated in part by TRAF6-mediated and calcium-dependent signaling pathways. TRAF6-mediated NF-κB activation results in the induction of cFos expression, a major component of the transcription factor AP-1, which regulates Nfatc1 expression (14). In the current study, the fractions suppressed the expression of cFos under normal conditions and both cFos and Traf6 expression in the presence of TNF-α.
The induction of Nfatc1 is also regulated by TRAF6-mediated MAPK signaling, including the activation of p38 and Erk (14). The activation of AP-1 by Erk-mediated phosphorylation induces Nfatc1 mRNA expression (33). Furthermore, the presence of p38 at the promoter region of Nfatc1 is required for its transcription (34). In the current study, treatment with DP-FrE and, to a lesser degree, DP-FrF attenuated the RANKL-stimulated phosphorylation of Erk and tended to reduce the phosphorylation of p38 under normal and inflammatory conditions. The ability of plant-derived polyphenolic compounds to inhibit MAPK signaling and subsequent osteoclast differentiation has been shown previously in vitro (16, 17). For example, the polyphenols phenethyl isothiocyanate, found in cruciferous vegetables, and caffeic acid 3,4-dihydroxy-phenethyl ester, found in various medicinal plants, have been shown to downregulate Erk and p38 activation and result in a decrease in osteoclast differentiation in Raw 264.7 cells and primary BMMCs (16, 17). The inhibition of MAPK activation and cFos expression provides a plausible explanation for the suppression of Nfatc1 mRNA and osteoclast numbers observed with DP-FrE and DP-FrF.
It is well known that osteoclast differentiation and activity are enhanced in a proinflammatory environment (5, 9–12). In our studies, treatment with TNF-α increased osteoclast differentiation and activity. This upregulated differentiation is due in part to an increased expression of RANK by osteoclast precursors, as well as an enhanced sensitivity to RANKL stimulation due to increased activation of NF-κB and AP-1 (5, 12). The TNF-α–induced increase in osteoclast number was suppressed by both DP-FrE and DP-FrF. The expression of Traf6 was also significantly downregulated by both DP-FrE and DP-FrF, suggesting that the treatments may attenuate the TNF-α–enhanced sensitivity of osteoclast precursors to RANKL stimulation. Surprisingly, cFos expression was not significantly altered by either TNF-α or treatment with the dried plum fractions. Despite a decrease in osteoclast number with treatment of either fraction in the presence of TNF-α, Nfatc1 expression was significantly suppressed only with treatment of DP-FrF, suggesting that the involvement of other signaling mechanisms in the downregulation of osteoclast differentiation or other factors (e.g., increased apoptosis) could be responsible for the decrease in osteoclast number and activity observed with certain dried plum polyphenolic fractions.
Although the assessment of how the fractions alter signaling pathways in bone marrow–derived osteoclast monocultures provides valuable insight into the mechanisms involved in the antiresorptive capacity of dried plums, supraphysiologic doses of RANKL are traditionally used to stimulate osteoclast differentiation (18). Furthermore, the monoculture system does not allow for the study of coupled osteoblast and osteoclast activity that occurs in vivo. Therefore, the ability of the polyphenolic fractions to reduce osteoclast differentiation in osteoblast and osteoclast co-cultures was assessed in the present study. Osteoclast differentiation tended to be suppressed with the polyphenolic fractions under normal conditions, which can be attributed, at least in part, to a significant downregulation of Nfatc1 by both DP-FrE and DP-FrF. Under inflammatory conditions, the magnitude of response to DP-FrF was greater than that to DP-FrE in downregulating osteoclast differentiation. In this co-culture system, it is likely that osteoclast differentiation was upregulated by TNF-α by both direct and indirect effects, including induced stromal cell and osteoblast production of RANKL, as well as increased sensitivity of osteoclast precursors to RANKL stimulation (7, 9–11). Both DP-FrE and DP-FrF reduced Rankl expression in the co-culture system. Furthermore, both fractions downregulated cFos and, subsequently, Nfatc1 expression. Therefore, it is not clear why only DP-FrF significantly reduced osteoclast numbers, which indicates that other signaling mechanisms are likely involved. However, the downregulation of Rankl and Nfatc1 has also been observed in vivo with dried plum supplementation in gonadal hormone deficiency models of osteoporosis (24, 35).
The initial expression of Nfatc1 requires TRAF6-mediated signaling cascades, but subsequent calcium-dependent auto-amplification of Nfatc1 requires sustained intracellular calcium oscillations, mediated by costimulatory receptors expressed by osteoclast precursors including OSCAR, TREM2, and SIRPB1 (15, 18, 19). In our studies, DP-FrF significantly reduced costimulatory receptors under normal conditions but not intracellular calcium concentration in differentiating osteoclasts. DP-FrE had a similar, but less robust, effect on calcium signaling as DP-FrF. Previous in vivo and in vitro studies showed that osteoclast differentiation is impaired if these membrane-bound costimulatory receptors or adaptor proteins involved in their signaling cascades are inhibited (19, 36–39). Therefore, the inhibition of calcium signaling may provide a further explanation for the suppression of Nfatc1 expression and reduction in osteoclast number. However, the lack of an effect of treatment on calcium uptake by differentiating osteoclasts with the Fluo-4 dye assay raises the question of whether or not cellular calcium uptake is altered by the fractions. Follow-up studies are needed that use more sensitive techniques (fluorescence microscopy) to confirm these findings.
To assess the role of inflammation in the suppression of Nfatc1 expression, intracellular calcium and the expression of costimulatory receptors were examined in the presence of TNF-α. The fractions failed to alter calcium uptake and the alteration in costimulatory receptors by DP-FrE and DP-FrF was not of the magnitude observed under normal conditions. In fact, only Sirpb1 was significantly downregulated by the polyphenolic fractions in the TNF-α–treated cultures. Both SIRPβ1 and TREM2 are DNAX-activating protein of 12 kDa (DAP12)-associated receptors, whereas OSCAR recruits Fc receptor γ (FcRγ) adaptor proteins (19). There is evidence to suggest that DAP12 is a predominant regulator of calcium signaling over FcRγ in osteoclasts, suggesting a major role for DAP12-associated receptors in calcium signaling in the differentiating osteoclast (40).
In summary, this study is the first to our knowledge to examine fractions of a polyphenolic extract from dried plum that downregulate osteoclast differentiation and activity in murine primary monocultures and co-cultures. Although both fractions were able to suppress osteoclast differentiation and activity, DP-FrF appeared to have a more robust effect, especially under inflammatory conditions. Of the 14 polyphenols assessed in the fractions, both DP-FrE and DP-FrF contained only neochlorogenic acid, cryptochlorogenic acid, and rutin. However, because of the differences in ability to alter signaling in differentiating osteoclasts observed, further characterization of DP-FrF, the most bioactive fraction, is warranted. We have also shown that both fractions downregulate Nfatc1 expression, at least in part, by suppressing MAPK and calcium-signaling pathways, providing insight into the mechanism through which polyphenols from dried plum decrease osteoclast differentiation and activity in vivo. Therefore, the development of a therapeutic treatment option from dried plum will require further investigation of whether these most bioactive fractions of the polyphenolic extract of dried plum suppress bone resorption in vivo.
Acknowledgments
The authors' responsibilities were as follows—BJS, JLG, and EAL: designed the research; JLG, BJS, LW, DL, EAL, ER-R, EKC, and PO: conducted the research; BJS, JBK, and RHC: provided essential reagents and materials; JLG, LW, DL, EAL, and BJS: analyzed the data; JLG, EAL, DL, and BJS: wrote the manuscript; BJS: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Abbreviations
- AP-1
activator protein 1
- BMMC
bone marrow mononuclear cell
- DAP12
DNAX-activating protein of 12 kDa
- DP-Fr
dried plum fraction
- Erk
extracellular signal-regulated kinase
- M-CSF
macrophage colony stimulating factor
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Nfatc1
nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1
- OPG
osteoprotegerin
- OSCAR
osteoclast-associated receptor
- p-
phosphorylated
- P/S
penicillin/streptomycin
- RANKL
receptor activator of NF-κB ligand
- SIRPB1
signaling regulatory protein β1
- TRAF6
TNF receptor–associated factor 6
- TRAP
tartrate-resistant acid phosphatase
- TREM2
triggering receptor expressed on myeloid cells 2
- α-MEM
minimal essential medium, α modification
Footnotes
Supported by NIH/National Center for Complimentary and Alternative Medicine R21AT006580. The California Dried Plum Board provided the dried plum powder used for this project.
References
- 1. Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C.. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 2012;23:2239–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A.. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465–75. [DOI] [PubMed] [Google Scholar]
- 3. Parfitt AM.. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 1994;55:273–86. [DOI] [PubMed] [Google Scholar]
- 4. Shevde NK, Bendixen AC, Dienger KM, Pike JW.. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA 2000;97:7829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R.. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 2000;106:1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roggia C, Tamone C, Cenci S, Pacifici R, Isaia GC.. Role of TNF-alpha producing T-cells in bone loss induced by estrogen deficiency. Minerva Med 2004;95:125–32. [PubMed] [Google Scholar]
- 7. Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R.. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA 2003;100:10405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D.. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS One 2012;7:e44552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S.. Interleukin-1B and tumor necrosis factor-a, but not interleukin-6, stimulate osteoprotogerin ligand gene expression in human osteoblastic cells. Bone 1999;25:255–9. [DOI] [PubMed] [Google Scholar]
- 10. Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL.. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000;106:1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sherman ML, Weber BL, Datta R, Kufe DW.. Transcriptional and posttranscriptional regulation of macrophage-specific colony stimulating factor gene expression by tumor necrosis factor. Involvement of arachidonic acid metabolites. J Clin Invest 1990;85:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y.. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem 2001;276:563–8. [DOI] [PubMed] [Google Scholar]
- 13. Dubois EA, Rissmann R, Cohen AF.. Denosumab. Br J Clin Pharmacol 2011;71:804–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boyle WJ, Simonet WS, Lacey DL.. Osteoclast differentiation and activation. Nature 2003;423:337–42. [DOI] [PubMed] [Google Scholar]
- 15. Hwang SY, Putney JW.. Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J 2012;26:1484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murakami A, Song M, Ohigashi H.. Phenethyl isothiocyanate suppresses receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis by blocking activation of ERK1/2 and p38 MAPK in RAW264.7 macrophages. Biofactors 2007;30:1–11. [DOI] [PubMed] [Google Scholar]
- 17. Wu X, Li Z, Yang Z, Zheng C, Jing J, Chen Y, Ye X, Lian X, Qiu W, Yang F, et al. Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-kappaB ligand-induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+-nuclear factor of activated T-cells cytoplasmic 1 signaling pathways. J Bone Miner Res 2012;27:1298–308. [DOI] [PubMed] [Google Scholar]
- 18. Kim N, Takami M, Rho J, Josien R, Choi Y.. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med 2002;195:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 2004;428:758–63. [DOI] [PubMed] [Google Scholar]
- 20. Yeon JT, Choi SW, Ryu BJ, Kim KJ, Lee JY, Byun BJ, Son YJ, Kim SH.. Praeruptorin A inhibits in vitro migration of preosteoclasts and in vivo bone erosion, possibly due to its potential to target calmodulin. J Nat Prod 2015;78:776–82. [DOI] [PubMed] [Google Scholar]
- 21. An J, Hao D, Zhang Q, Chen B, Zhang R, Wang Y, Yang H.. Natural products for treatment of bone erosive diseases: the effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int Immunopharmacol 2016;36:118–31. [DOI] [PubMed] [Google Scholar]
- 22. Hooshmand S, Kern M, Metti D, Shamloufard P, Chai SC, Johnson SA, Payton ME, Arjmandi BH.. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos Int 2016;27:2271–9. [DOI] [PubMed] [Google Scholar]
- 23. Rendina E, Lim YF, Marlow D, Wang Y, Clarke SL, Kuvibidila S, Lucas EA, Smith BJ.. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss in C57BL/6 mice and modulates the immune response. J Nutr Biochem 2012;23:60–8. [DOI] [PubMed] [Google Scholar]
- 24. Rendina E, Hembree KD, Davis MR, Marlow D, Clarke SL, Halloran BP, Lucas EA, Smith BJ.. Dried plum's unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS One 2013;8:e60569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith BJ, Bu SY, Wang Y, Rendina E, Lim YF, Marlow D, Clarke SL, Cullen DM, Lucas EA.. A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone 2014;58:151–9. [DOI] [PubMed] [Google Scholar]
- 26. Fang N, Yu S, Prior RL.. LC/MS/MS characterization of phenolic constituents in dried plums. J Agric Food Chem 2002;50:3579–85. [DOI] [PubMed] [Google Scholar]
- 27. Bu SY, Lerner M, Stoecker BJ, Boldrin E, Brackett DJ, Lucas EA, Smith BJ.. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif Tissue Int 2008;82:475–88. [DOI] [PubMed] [Google Scholar]
- 28. Smith BJ, Graef JL, Wronski TJ, Rendina E, Williams AA, Clark KA, Clarke SL, Lucas EA, Halloran BP.. Effects of dried plum supplementation on bone metabolism in adult C57BL/6 male mice. Calcif Tissue Int 2014;94:442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bu SY, Lucas EA, Franklin M, Marlow D, Brackett DJ, Boldrin EA, Devareddy L, Arjmandi BH, Smith BJ.. Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos Int 2007;18:931–42. [DOI] [PubMed] [Google Scholar]
- 30. Bu SY, Hunt TS, Smith BJ.. Dried plum polyphenols attenuate the detrimental effects of TNF-alpha on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J Nutr Biochem 2009;20:35–44. [DOI] [PubMed] [Google Scholar]
- 31. Prior RL, Wu X, Gu L.. Identification and urinary excretion of metabolites of 5-(hydroxymethyl)-2-furfural in human subjects following consumption of dried plums or dried plum juice. J Agric Food Chem 2006;54:3744–9. [DOI] [PubMed] [Google Scholar]
- 32. Kammalla AK, Ramasamy MK, Chintala J, Dubey GP, Agrawal A, Kaliappan I.. Comparative pharmacokinetic interactions of quercetin and rutin in rats after oral administration of European patented formulation containing Hipphophae rhamnoides and co-administration of quercetin and rutin. Eur J Drug Metab Pharmacokinet 2015;40:277–84. [DOI] [PubMed] [Google Scholar]
- 33. Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem 2007;282:18245–53. [DOI] [PubMed] [Google Scholar]
- 34. Sharma SM, Bronisz A, Hu R, Patel K, Mansky KC, Sif S, Ostrowski MC.. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem 2007;282:15921–9. [DOI] [PubMed] [Google Scholar]
- 35. Franklin M, Bu SY, Lerner MR, Lancaster EA, Bellmer D, Marlow D, Lightfoot SA, Arjmandi BH, Brackett DJ, Lucas EA, et al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone 2006;39:1331–42. [DOI] [PubMed] [Google Scholar]
- 36. Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, Nakamura M, Ivashkiv LB.. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J Immunol 2009;183:2444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mócsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC.. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA 2004;101:6158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ishikawa S, Arase N, Suenaga T, Saita Y, Noda M, Kuriyama T, Arase H, Saito T.. Involvement of FcRgamma in signal transduction of osteoclast-associated receptor (OSCAR). Int Immunol 2004;16:1019–25. [DOI] [PubMed] [Google Scholar]
- 39. Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, Seaman WE, Nakamura MC.. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J Bone Miner Res 2006;21:237–45. [DOI] [PubMed] [Google Scholar]
- 40. Kameda Y, Takahata M, Komatsu M, Mikuni S, Hatakeyama S, Shimizu T, Angata T, Kinjo M, Minami A, Iwasaki N.. Siglec-15 regulates osteoclast differentiation by modulating RANKL-induced phosphatidylinositol 3-kinase/Akt and Erk pathways in association with signaling adaptor DAP12. J Bone Miner Res 2013;28:2463–75. [DOI] [PubMed] [Google Scholar]