Abstract
Patients with severely decreased glomerular filtration rate (GFR) (i.e., chronic kidney disease [CKD] G4+) are at increased risk for kidney failure, cardiovascular disease (CVD) events (including heart failure), and death. However, little is known about the variability of outcomes and optimal therapeutic strategies, including initiation of kidney replacement therapy (KRT). Kidney Disease: Improving Global Outcomes (KDIGO) organized a Controversies Conference with an international expert group in December 2016 to address this gap in knowledge. In collaboration with the CKD Prognosis Consortium (CKD-PC) a global meta-analysis of cohort studies (n = 264,515 individuals with CKD G4+) was conducted to better understand the timing of clinical outcomes in patients with CKD G4+ and risk factors for different outcomes. The results confirmed the prognostic value of traditional CVD risk factors in individuals with severely decreased GFR, although the risk estimates vary for kidney and CVD outcomes. A 2- and 4-year model of the probability and timing of kidney failure requiring KRT was also developed. The implications of these findings for patient management were discussed in the context of published evidence under 4 key themes: management of CKD G4+, diagnostic and therapeutic challenges of heart failure, shared decision-making, and optimization of clinical trials in CKD G4+ patients. Participants concluded that variable prognosis of patients with advanced CKD mandates individualized, risk-based management, factoring in competing risks and patient preferences.
Keywords: chronic kidney disease, kidney failure, prediction, prognosis, progression, supportive care
Chronic kidney disease (CKD), defined by persistent reduction in glomerular filtration rate (GFR) and/or the presence of other signs of kidney damage, is classified based on GFR and albuminuria categories.1 The risk for adverse outcomes, including mortality and kidney failure, increases with decreasing GFR and increasing albuminuria.2 Individuals with a GFR below 30 ml/min per 1.73 m2 (i.e., CKD G4 or G5) are at particularly high risk across all albuminuria categories (Figure 1). In addition, CKD-specific complications increase markedly at low levels of GFR, with cardiovascular disease (CVD) being a leading cause of morbidity and mortality. Of particular relevance is heart failure (HF), one of the most common CVD conditions for patients with CKD G4 or higher.
Figure 1. Schematic presentation of chronic kidney disease (CKD) categories and conference focus.
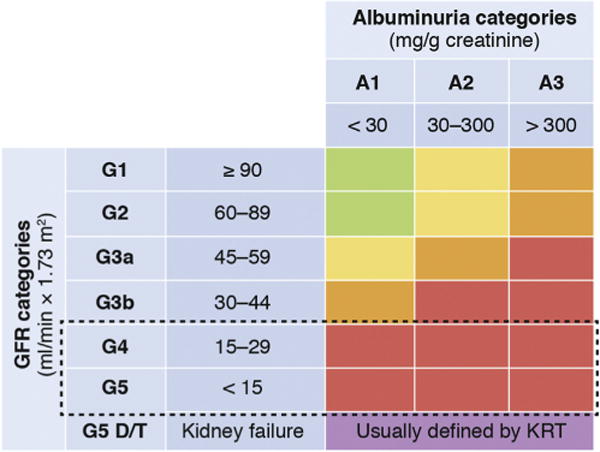
Per definition, CKD G5 includes patients with kidney failure with and without kidney replacement therapy (KRT). The conference focus (dashed line) was on patients within glomerular filtration rate (GFR) categories G4 and G5, excluding individuals already on KRT, but including KRT as an important end point. D = patients on dialysis therapy, T = patients with a kidney transplant. Colors reflect different risk categories for adverse outcomes as compared with individuals without CKD: green = no increase in risk; yellow = slightly elevated risk; orange = moderately elevated risk; and red = severely elevated risk. Adapted with permission from Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150.1
Kidney replacement therapy (KRT; i.e., dialysis or transplantation) can mitigate the consequences of kidney failure and improve prognosis. However, there are large variations in incidence rates of KRT3, and globally only approximately half of those with kidney failure receive KRT.4 Inequalities in access to KRT play an important role, but differences in practice patterns also exist. There is agreement that level of GFR alone should not be used as a trigger for KRT initiation, and that signs and symptoms associated with kidney failure should be considered. Nevertheless, defining the optimal time for KRT initiation remains a challenge.1 Importantly the first few months on dialysis have been identified as a very high-risk period, though it remains unknown to what extent adverse events are triggered by dialysis initiation.5,6 Referral to nephrology services shortly before dialysis initiation has been associated with an increased risk of adverse outcomes as compared with earlier referral.7,8
Thus, low GFR (<30 ml/min per 1.73 m2) corresponding to CKD G4 or G5 (excluding patients on KRT and referred to subsequently as “CKD G4+”) reflects a critical state for patients. A better understanding of prognosis of patients with CKD G4+ may inform treatment strategies, including decision-making for initiation of KRT. Therefore, Kidney Disease: Improving Global Outcomes (KDIGO) collaborated with the CKD Prognosis Consortium (CKD-PC) to initiate a global meta-analysis of cohort studies (population-based cohorts, referred CKD cohorts, and research cohorts). The primary aim was to determine the prognosis of patients with advanced CKD with respect to initiation of KRT, CVD events, mortality, and relative timing of these events,9 with a second aim to determine variability of patient prognosis according to cohort, demographic, or health characteristics.10
The results from the global meta-analysis were presented to an international expert group at a KDIGO Controversies Conference in December 2016, and implications for patient management were discussed. Breakout groups focused on: (i) management of CKD G4+, (ii) diagnostic and therapeutic challenges of HF in CKD G4+, (iii) shared decision-making for KRT initiation, and (iv) optimization of clinical trials in CKD G4+ patients. To curtail the scope of the conference, specific aspects of children and patients with a failing transplant were not addressed.
We present here a summary of the discussion and main conference conclusions with respect to management and future research in patients with CKD G4+. Detailed presentations of the meta-analysis are published in the companion papers.9,10
Prognosis of patients with CKD G4+: novel insights from a global meta-analysis of cohort studies
In preparation for the conference, we conducted a global meta-analysis with the goal of examining absolute and relative risks of outcomes in a large, diverse population of patients with CKD stage G4+. The meta-analysis of risk factors for KRT, CVD events, and death included 28 cohorts (n = 185,024) using standard survival analysis and Cox regression.10 The risk prediction meta-analysis included 29 cohorts (n = 264,296).9
The main findings included that established risk factors for CVD were highly relevant in CKD G4+ patients, but their relative importance differed by outcome (Figure 2). Age and history of CVD were negatively related to risk of KRT but positively related to CVD and death risk. Current smoking was most strongly associated with death. Blood pressure was positively associated with KRT risk but showed a U-shaped association with CVD and mortality. Diabetes and male sex were risk factors for all outcomes but strongest for CVD and KRT, respectively. Black race was only positively related to KRT. Lower estimated GFR (eGFR) and higher albumin-to-creatinine ratio (ACR) were more strongly associated with KRT than other outcomes. Finally, time-varying CVD events and initiation of KRT were strongly associated with subsequent occurrence of death. The second meta-analysis focused on the development of a new risk calculator for CVD events, KRT and death, as diagramed in Supplementary Figure S1.9
Figure 2. Hazard ratios for KRT, CVD events, and death associated with different variables.
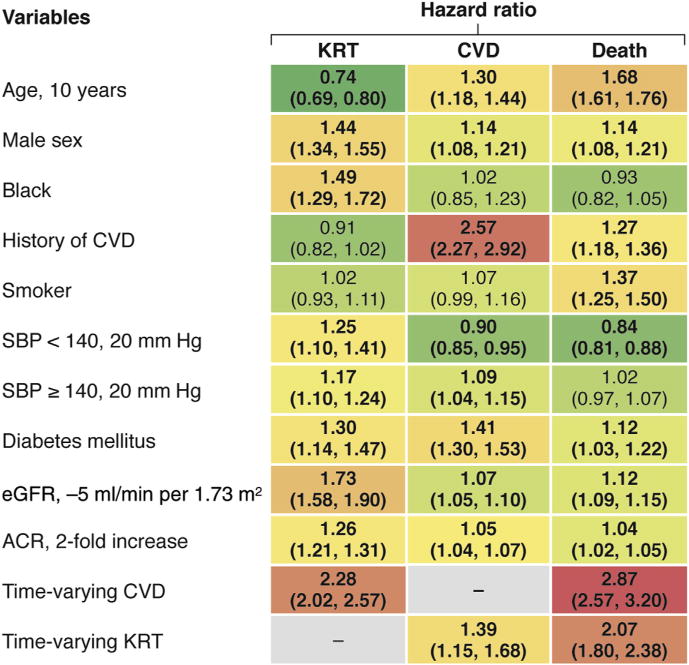
Colors indicate the strength of association, from protective in green to strongly positive in red. Based on 19 cohorts with KRT, CVD, and death outcomes. Bold denotes statistically significant values. ACR, albumin-to-creatinine ratio; CI, confidence interval; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; SBP, systolic blood pressure. Adapted with permission from Evans M, Grams ME, Sang, Y, et al. Risk factors for prognosis in patients with severely decreased GFR. Kidney Int Rep. https://doi.org/10.1016/j.ekir.2018.01.002.10
The CKD G4+ risk calculator uses a pie chart format to show the probability of all outcomes in a given follow-up period (2 or 4 years) (online calculator: http://www.kdigo.org/equation/). For example, as illustrated in Figure 3, at an eGFR of 25 ml/min per 1.73 m2 and the covariates noted in the figure legend, the proportion of participants predicted to receive KRT within 4 years increases from 13% to 32% with increasing albuminuria, while the risk of death increases from 22% to 30%. In the overall population examined who had a median eGFR of 24 ml/min per 1.73 m2 and ACR of 168 mg/g, over 50% of participants were predicted to be event free at 4 years.
Figure 3. Example from the chronic kidney disease (CKD) G4+ risk calculator.
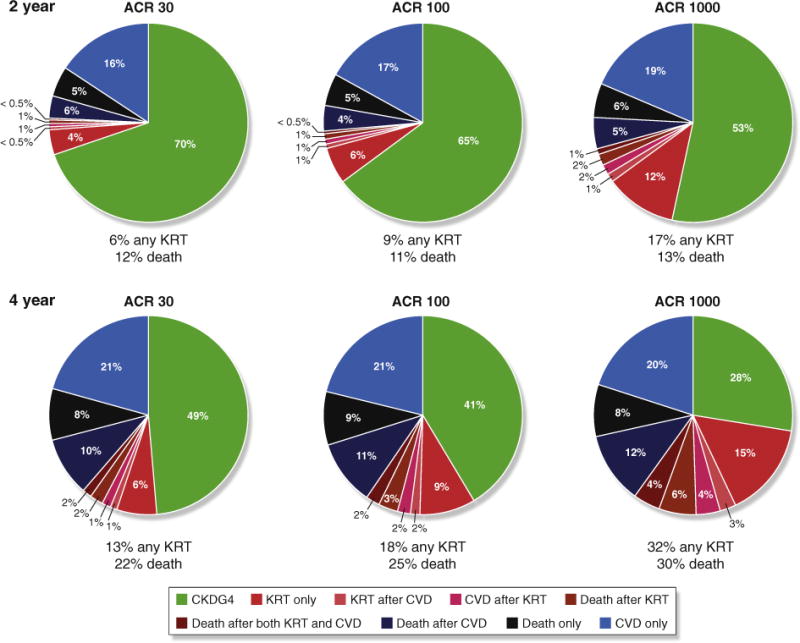
The probability and timing of adverse events at (upper panel) 2 years and (lower panel) 4 years with increasing level of albuminuria. In these models, the scenario was set at age 60 years, male, white, with a history of cardiovascular disease, not a current smoker, systolic blood pressure of 140 mm Hg, with diabetes, and an estimated glomerular filtration rate of 25 ml/min per 1.73 m2. ACR, albumin-to-creatinine ratio; CVD, cardiovascular disease; KRT, kidney replacement therapy. For comparison, risk predictions for individuals with the same patient characteristics but no diabetes are presented in Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int. https://doi.org/10.1016/j.kint.2018.01.009.9
The predicted risk of remaining event free for 2 years varies from less than 20% to greater than 80%, illustrating the predictive power of measured patient characteristics.9
This CKD-PC global meta-analysis9 extends the established kidney failure risk equation (KFRE) for prediction of KRT11,12 as well as confirms its value in CKD G4+. Together with the KFRE, the new CKD G4+ risk calculator provides easily accessible tools to physicians, patients, and policy makers that translate patient characteristics into powerful risk discrimination. It is still important to keep in mind that additional characteristics, often unmeasured, influence risk further, and hence part of individualization should include recognition of the limitations of quantitative risk estimates.
Management of patients with CKD G4+
Risk-based assessment and management
People with CKD G4+ are at risk for kidney failure, hospitalizations, CVD events, death, and often under-recognized outcomes such as disability, cognitive impairment, falls, and infection. The KFRE equation11 and the new CKD G4+ risk calculator provide useful tools in risk prediction, and their consistency is reassuring. Other similar models have also been published.13,14 Further refinement including prediction of additional patient-relevant outcomes remains an important task for future research. Nevertheless, the available models appear sufficient to advocate for their implementation.
Incorporating patient preferences and values
There is growing recognition that patients want to be involved as equal partners in their care.15 Shared decision-making can lead to productive interactions between patients, family, carers, and health care providers, thus actively involving all partners in treatment decisions, providing sufficient education about treatment options and their attributes, utilizing strategies to elicit patients’ values, identifying patient preferences, and achieving agreement about the course of treatment.16,17 Further work to develop and evaluate strategies and resources for shared decision-making within CKD care is required to achieve these goals.
Models of care
So far, data on the systematic implementation of models of care for people with CKD G4+ are relatively sparse, and the relationships between specific elements of CKD care and patient outcomes remain to be determined. Nevertheless a number of overarching considerations appear valid (Table 1), and specific stakeholder issues should be considered (Supplementary Table S1). Moreover, key competencies can be outlined that appear critical for successful implementation of models of care (Supplementary Table S2).
Table 1.
Ten points to consider within models of care for CKD G4+
| 1. Patient-orientated care should be a key priority |
| 2. Categorize CKD according to cause of kidney disease, level of GFR, and degree of albuminuria |
| 3. Estimate risk and prognosis and tailor management accordingly |
| 4. Prevent CKD progression and avoid acute kidney injury where possible |
| 5. Evaluate and manage comorbid conditions, paying particular attention to ischemic heart disease, heart failure, and stroke prevention |
| 6. Identify, prevent, and manage CKD-specific complications (e.g., malnutrition, anemia, bone disease, and acidosis) |
| 7. Plan and prepare for treatment of kidney failure (e.g., choice of modality, access-placement and care, pre-emptive transplantation) and provide conservative care and palliative care options where required |
| 8. Ensure that psychosocial support is provided |
| 9. Maintain continuity across transitions of care |
| 10. Ensure that all communication channels are open: CKD care system to patient and/or carer; between CKD team members; and between CKD team and other health professionals |
CKD, chronic kidney disease; GFR, glomerular filtration rate.
The potential benefits of a multidisciplinary team approach have been described over 20 years ago.18 A recent systematic review (18 studies; 8853 patients) found that multidisciplinary care of patients with CKD was associated with lower risks of all-cause mortality, dialysis, and central venous catheter use for dialysis access.19 Although initially associations with improved outcomes have been limited to observational studies and had not been borne out in randomized trials,20 2 recent trials have reported encouraging results. The ESCORT (Effectiveness of Integrated Care on Delaying Progression of Stage 3-4 Chronic Kidney Disease in Rural Communities of Thailand) study, a community-based, cluster randomized controlled trial, reported significant delay in progression of CKD associated with improvements in blood pressure and diabetes control and serum bicarbonate with the introduction of an integrated CKD care program.21 In addition to routine care, comprehensive medical care and education including advice on diet, exercise, and medication was provided as part of the intervention. In another cluster randomized controlled trial, Lalonde and colleagues introduced a training and communication network program for pharmacists as part of the multidisciplinary care of people with CKD in Quebec, Canada.22 In a cohort of patients already benefiting from multidisciplinary care, the introduction of the program improved the quality of medication use and reduced the number of drug-related problems by 15%. It seems intuitive that access to multidisciplinary care providers may be attractive to many patients; however, the impact on patient experience and the optimal design and involvement of team members still remains unclear.23 We suggest that models of care should place patients at the center of a transparent and open structure that ensures optimal communication and use of available resources (Supplementary Figure S2).
Additional research is needed to explore models that address context-specific care in low- and middle-income countries, multi-morbidity and integration of multidisciplinary care providers and other specialists around the patient; new technologies that can enhance communication between stakeholders; and strategies to bridge transitions of care between hospital and community, as well as between phases of CKD, dialysis, and transplant care.
Uncertainties about targets and therapies
There are important uncertainties in CKD G4+ management (Table 2). In general these include interventions that are either targeting the underlying kidney disease or aim at primary and secondary prevention of CVD complications. Frequently, evidence has been extrapolated from studies in other stages of CKD or younger age groups, or is based on observational studies. For example, although some trials of reninangiotensin-aldosterone system (RAAS) blockage have included patients with CKD G4+,24 uncertainty remains about generalizing its efficacy to CKD G4+ patients.25 Results of a trial designed to test the role of stopping treatment with RAAS blockade to stabilize kidney function in patients with progressive or late CKD G4+ are awaited.26 Current evidence and guideline recommendations for several important therapeutic areas are shown in Table S3. Given the high burden and altered metabolism of drugs in CKD G4+, exploration of drug interactions and adverse medication safety events should also be a priority.
Table 2.
Therapeutic uncertainties in CKD G4+ management
| 1. How should we weigh the risks-benefits of common medical and surgical interventions in people with CKD G4+, including blood pressure and glycemic control, major surgery, and other medical procedures or exposures? Do these vary by degree of albuminuria? |
| 2. Should treatment goals, including blood pressure, be modified dependent upon age and/or comorbidity? |
| 3. Can we extrapolate recommended HbA1c targets to people with CKD G4+? |
| 4. Should metformin treatment be discontinued in people with diabetes and CKD G4+? |
| 5. Should we advise a restricted salt intake in people with CKD G4+, and if so what level of intake should we advise? |
| 6. Should we advise modification of dietary protein intake in people with CKD G4+? For example, should we advise more plant-based protein intake? |
| 7. Should we treat acidosis in people with CKD G4+, and if so, at what level of serum bicarbonate should treatment be initiated? |
| 8. Should asymptomatic hyperuricemia be treated in people with CKD G4+, and if so, at what level of serum uric acid should treatment be initiated? |
| 9. Should aspirin for prevention of cardiovascular disease be continued in people with CKD G4+, or does the risk of bleeding outweigh potential benefits? |
| 10. Do other cardiovascular disease prevention strategies convey the same benefits in people with CKD G4+ as compared with people with less advanced CKD? |
| 11. How can the risk of acute kidney injury in people with CKD G4+ be mitigated? Should we advise tablet holidays during intercurrent illness, and if so, what tablets should be temporarily discontinued and for how long? |
| 12. Are there subclinical events such as tubulointerstitial injury, inflammation and fibrosis, and unrecognized episodes of acute kidney injury associated with CKD progression, and are these linked to short-lived prescription of medicines or to nonprescription medication exposures? |
CKD, chronic kidney disease.
Research recommendations for management of patients with CKD G4+ are summarized in Table 3.
Table 3.
Research recommendations for general management of patients with CKD G4+
| 1. Risk-based assessment and management |
| Implementation and evaluation of prognostic models in clinical care to guide risk-based management approaches. Researchers should consider clinical impact analyses of tools that link prognostic information on kidney failure to specific guidance for common CKD management decisions and evaluate the impact on measures of appropriateness, timeliness, patient-centeredness, and efficiency of CKD care. |
| Derivation, validation, and impact analyses of prognostic models for other outcomes in addition to kidney failure. In addition to CVD events, kidney failure, and death, models should consider patient-centered outcomes including quality of life, functional status, cognitive impairment and hospitalization. Prognostic models that help patients and providers weigh the relative benefits and risk of common medical therapies, radiologic procedures (e.g., angiography), and surgery for patients with CKD should also be investigated. |
| 2. Incorporating patient preferences and values |
| Development and evaluation of tools and resources to elicit patient values and preferences for management options throughout CKD G4+. Such studies should evaluate the impact of tools to facilitate shared decision-making on patient-reported outcomes and experience measures, including measures of knowledge transfer and the quality of decision-making processes in CKD management. |
| 3. Models of care |
| Evaluation of the impact of clinic structures and processes on patient experience, outcome measures, and costs of providing care. Specific structural interventions that require further evaluation include financial incentives to support longitudinal patient care rather than episodic health care contacts; novel strategies to address multi-morbidity; technology-based strategies to enhance communication; and transition of care interventions addressing gaps between hospital and community, as well as between phases of pre-dialysis, dialysis, and transplant care. |
| 4. Uncertainties about targets and therapies |
| Evaluation of novel, emerging, and existing pharmacotherapeutic strategies in randomized controlled trials specifically in populations with CKD G4+. Promising therapies include bicarbonate therapy and treatment of asymptomatic hyperuricemia to slow progression in the later stages of CKD, as well as aspirin for primary prevention of cardiovascular events. Inclusion of patients with CKD G4+ should also be a priority for future trials of blood pressure control, glycemic targets, and comparative effectiveness studies of medication safety. |
CKD, chronic kidney disease; CVD, cardiovascular disease.
Cardiovascular complications during CKD G4+: heart failure
A focus of management during CKD G4+ is on preventing CVD, which remains one of the leading causes of morbidity and mortality. HF is of special relevance for CKD G4+ patients as it is one of the most common cardiovascular conditions, and yet there remain many diagnostic and therapeutic uncertainties in the management of HF during CKD G4+, particularly in patients approaching the transition to KRT.
Definition, risk factors, and diagnosis of heart failure in CKD G4+
Patients with CKD have an elevated risk of HF27,28 that increases with severity of CKD.29 Among patients receiving KRT, 40% have HF,30 with higher prevalence rates among patients on hemodialysis compared with peritoneal dialysis and kidney transplant recipients.31 The cumulative incidence of HF is also high among patients with CKD and those receiving KRT (Supplementary Figure S3).32
HF is defined as a syndrome of inadequate filling and/or pumping to meet systemic demands. There are 2 types of HF, with preserved ejection fraction (HFpEF) and reduced ejection fraction (HFrEF), and defining HF subtypes has been an area of ongoing work by the ACCF/AHA33 and ESC34 (Supplementary Table S4). HFpEF is more common in patients with CKD.35
Diagnosis of HF remains challenging in patients with CKD, particularly in CKD G4+, given the difficulty in distinguishing causes of volume overload. HF should be defined as the presence of HF symptoms and structural and/or functional abnormalities on cardiac imaging (Supplementary Table S4).33 In observational studies, imaging data are frequently not available. In fact, in the CKD-PC analysis, HF definitions were not sufficiently harmonized across cohorts to allow a valid analysis of incidence rates and risk prediction. Moreover, despite the recognized importance of HF, it remains unknown whether screening for HF, either with imaging or cardiac biomarkers,36 leads to improved outcomes in patients with CKD G4+.
Traditional as well as novel risk factors, including metabolic abnormalities, uremic toxins, and sympathetic over-activity, accelerate the development of HF in patients with CKD G4+ (Supplementary Table S5).37,38
Outcomes associated with heart failure in CKD G4+
HF is associated with poor outcomes in patients with CKD, including greater risk of death, particularly in patients who are older, have HFrEF (Supplementary Table S6),39,40 or have severely decreased GFR (Figure 4). HF contributes significantly to morbidity among CKD patients, leading to frequent hospitalizations and re-hospitalizations.41–44 HF is also associated with episodes of acute kidney injury45 and progression of CKD.46,47 Among incident dialysis patients, volume overload compared with other dialysis indications is associated with the greatest risk of post-KRT mortality.48
Figure 4. Unadjusted survival in patients with heart failure, by chronic kidney disease (CKD) status, 2010 to 2011.
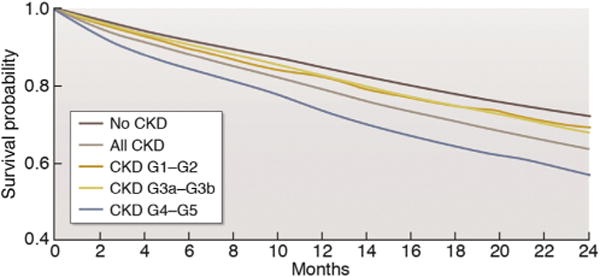
Reproduced from United States Renal Data System. 2015 USRDS annual data report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2015.Available at: www.usrds.org/2013/pdf/v1_ch4_13.pdf.40 Accessed February 28, 2017.
Progression of clinical and subclinical heart failure after initiation of dialysis
Only a few studies have evaluated longitudinal changes in subclinical HF, as assessed by echocardiograms, in patients with CKD G4+, and the results are not consistent. In the CRIC study, mean left ventricular mass index did not change after the initiation of KRT. However, there was a modest but statistically significant decline in left ventricular ejection fraction.49 The CASCADE study examined echocardiograms in patients with CKD G3+50 and reported that left ventricular mass index and left atrial volume both increased within a year; however, the change in left ventricular ejection fraction was not statistically significant. In the IDEAL trial, serial echocardiograms performed 12 months apart showed no change in left ventricular mass index, left atrial diameter, diastolic dysfunction, or left ventricular ejection fraction after dialysis initiation.51 It should be noted, however, that in this study, over 40% initiated peritoneal dialysis (PD) versus hemodialysis (HD). In another study of 41 HF patients, left ventricular mass index decreased after initiation of HD.52
Vascular access and heart failure
Vascular access preparation is a key component of management in CKD G4+ patients. There are numerous postulated changes in the cardiovascular system after creation of an arteriovenous fistula53 (Supplementary Table S7). Small studies or case reports have suggested that arteriovenous fistula may lead to development of high-output HF.54–58 Arteriovenous fistula creation has also been reported in small studies to worsen right ventricular hypertrophy and pulmonary hypertension54,59 and found to be associated with significant right ventricular dilatation and remodeling and an increased risk for development of incident heart failure.60 On the other hand, other studies found a stabilization of kidney function after access creation.61 Larger prospective studies are needed to understand optimal access management in patients with CKD G4+ and HF.
Management of heart failure in CKD G4+
Management of HF in patients with CKD, particularly CKD G4+, is complicated (Table 4). Almost all HF trials have excluded patients with advanced CKD, and few have been successful in improving outcomes in patients with HFpEF. Post hoc analyses have included some patients with moderate CKD, but suggest an attenuated effect of therapies such as β-blockers and implantable cardioverter defibrillators.62–64 In addition, the presumed risk of hyperkalemia limits the use of RAAS inhibitors and mineralocorticoid receptor antagonists in CKD G4+.44,65 Among patients receiving KRT with known HF, the proportion of patients with prescribed therapies such as RAAS inhibitors and β-blockers remains low.31 Further studies of HF therapies and cardiac devices specifically in CKD G4+ are needed, particularly for HFpEF, which remains the leading type of HF in patients with CKD G4+ (Supplementary Table S4 and Table 5). Although the rates of incident (i.e., de novo) and recurrent heart failure are reported to be lower in PD versus HD patients,66–68 in patients with established HF the mortality rate may be higher in PD versus HD patients.69 This likely reflects confounding by indication because frail HF patients are preferentially offered PD as it causes less acute hemodynamic stress. Prospective clinical trials comparing PD with HD are warranted.
Table 4.
Management of HF in CKD G4+
| Approach | Are there data in CKD G4+? | Limitations for use in CKD G4+? |
|---|---|---|
| β-Blockers | Yes: observational data and small trials | May have more adverse effects |
| RAAS inhibitors | No | Hyperkalemia |
| Risk of progressive loss of eGFR | ||
| MRAs | Ongoing trials | Hyperkalemia |
| Risk of progressive loss of eGFR | ||
| Neprilysin inhibitors | No | May have higher risk of hyperkalemia |
| Unknown dose | ||
| Treatment of anemia | Yes | May be linked with worse outcomes |
| Treat mineral metabolism abnormalities | Yes | Not a clear benefit |
| Frequent dialysis | Yes | Many patients cannot do home therapies, and frequent in-center dialysis is not always available |
| Ultrafiltration | Small observational studies and trials | May cause intradialytic hypotension and/or myocardial stunning |
| Cardiac resynchronization therapy | Small observational studies | May have more adverse effects, such as infections |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HF, heart failure; MRA, mineralocorticoid receptor antagonist; RAAS, renin-angiotensin-aldosterone system.
Table 5.
Research recommendations for HF diagnosis and management in CKD G4+
| Areas for future research | Research recommendations |
|---|---|
| Screening for HF in CKD G4+ | • Does screening lead to better outcomes? |
| • Is screening cost effective? | |
| • What is the best way to screen for HF (e.g., imaging biomarkers)? | |
| Definition and classification of HF in CKD G4+ | • What is the incidence of HFpEF versus HFrEF? |
| • Conduct study of the burden and outcomes associated with right ventricular dysfunction | |
| HF risk factors | • What is the relationship between residual kidney function in HD and risk of progression of subclinical and clinical HF? |
| • Assess contribution of ischemic heart disease to development of HF | |
| Outcomes related to HF in CKD G4+ | • Pay specific focus on progression of CKD and initiation of dialysis |
| • Examine risk of other CVD subtypes and death related to HF specifically in CKD G4 + | |
| • Conduct quality of life and other patient-reported outcome studies after dialysis initiation | |
| Management of HF in CKD G4+ | • What is the strategy for use of RAAS inhibitors, beta-blockers, MRAs, and neprilysin? |
| • What are point-of-care tests for kidney function, electrolytes, and surrogate markers of heart failure (e.g., BNP)? | |
| • What are test interventions for novel risk factors? | |
| • Determine optimal targets for volume management | |
| • What is the efficacy of invasive and noninvasive devices for volume assessment? | |
| • What is the efficacy of mechanical circulatory support? | |
| • What are the desirable outcomes for heart-kidney transplants? | |
| • What is the optimal vascular access strategy? | |
| • What is the role of urgent start PD for acute HF and CKD/AKI? | |
| • What is the role of conservative care in this population? |
AKI, acute kidney injury; BNP, B-type natriuretic peptide; CKD, chronic kidney disease; CVD, cardiovascular disease; HD, hemodialysis; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist; PD, peritoneal dialysis; RAAS, renin-angiotensin-aldosterone system.
Shared decision-making for kidney failure therapy
Predicting adverse outcomes after initiation of kidney replacement therapy
Several registries70,71 and cohort studies,6 provide population-based risks of mortality in patients initiating KRT. An important observation is that mortality rates are highest within the first 4 months of starting dialysis and decline in subsequent months.6 In the DOPPS cohort, such high early mortality was observed across countries, and differences between early and later mortality were more pronounced among patients aged ≥65 years compared with younger patients.6 Although dialysis withdrawal accounts for some of the early mortality, it does not provide the only explanation. CVD events are also much higher in the first weeks after KRT initiation.5
Studies have also demonstrated a high residual burden of symptoms and geriatric syndromes, such as dementia and disability, and utilization of health care among patients commencing dialysis, especially in those who are frail, have multiple chronic conditions, and start dialysis in the context of a prolonged hospitalization.72–76 These outcomes are important to patients and sometimes more so than mortality.
High early morbidity and mortality raises questions about the potential causes and risk mitigation strategies; in particular it raises concerns that for some patients, initiation of KRT may not be the optimal choice of therapy. Several prognostic tools have been developed to predict short-term mortality among patients who have initiated dialysis.77–80 Nevertheless, it is difficult to predict with sufficient certainty which patients will do poorly on dialysis.81
By providing quantitative estimates of the risk of KRT initiation, CVD events, and mortality based on individual patient characteristics, the novel CKD-G4+ risk calculator may facilitate decision-making9 (Figure 3). For patients not yet in kidney failure, it is also noteworthy to recognize that a substantial proportion of CKD G4+ patients survive without CVD events and KRT over 2- and 4-year observation periods.
Optimal counseling for treatment modality decisions
Counseling for KRT should be risk-based, iterative, and patient-centered. In addition, it should be tailored to the cultural setting, health literacy, and psychosocial and emotional needs while being mindful of the presence of cognitive impairment. Options available once kidney failure is expected include comprehensive conservative care without dialysis, in-center or home HD, PD, and transplantation. Circumstances may exist in which a specific modality of KRT may be contraindicated, but when options exist, a shared decision-making approach to the choice of KRT optimizes patient engagement and may potentially improve outcomes. Discussions should be revisited at regular intervals to ensure that important health or social circumstances have not changed.
Two scenarios require special considerations: counseling for transplantation in older adults and counseling to forgo dialysis. It is accepted that, among patients placed on the waiting list, kidney transplantation improves life expectancy and quality of life compared with those remaining on dialysis across all age ranges.82 In and of itself, older age is not a contraindication to transplantation.83 Although only a proportion of patients will qualify for transplantation, transplantation should be considered in all patients who do not have obvious contraindications for KRT. Referral and evaluation for transplantation should be based on patient characteristics, preferences, and regional circumstances.84 Unwarranted regional variability in access to transplantation is well-documented, and transplant programs should establish transparent policies for accepting patients on the waiting list.
General counseling about KRT should also include information about the option to forgo dialysis and receive conservative care. The Renal Physicians Association has published guidelines regarding circumstances in which forgoing dialysis may be appropriate (Supplementary Table S8).85 Although the benefit of initiating KRT declines with increasing age,86 older age per se should not be considered as a contraindication for KRT; in fact, conference participants questioned the Renal Physicians Association recommendation to generally forgo dialysis in patients ≥75 years with poor prognosis and favored a more individualized approach, taking into account patient preferences and values along with prognosis.
Uncertainties about initiation of kidney replacement therapies and research priorities
A recent meta-analysis of cohort studies and trials has demonstrated that those who commence dialysis with a higher eGFR have a higher mortality.87 It is likely that this is due to reverse causality, with frailty and accumulated comorbidities, in particular HF, pushing the patient and clinician to initiate dialysis. Global differences exist in how planned KRT is initiated. These include a “PD first” approach, commencement with a functioning arteriovenous fistula and differences in site of fistula placement and “incremental” start to dialysis with either reduced blood flow rates, reduced hours, or limited PD exchanges. To which extent these factors influence outcomes is largely unclear. The indication for initiation of dialysis should be recorded routinely in registry data in addition to reporting elective versus unplanned start to dialysis. In the IDEAL study the majority of patients allocated to late start who started early had the indication for start identified as “uremia.”88 Hence, it would be useful to understand the spectrum of symptoms that prompted initiation of dialysis to provide greater clarity regarding the optimum commencement. Research recommendations are summarized in Table 6.
Table 6.
Research recommendations for shared decision-making for KRT
| • Assess optimal ways to deliver information to people and families with CKD |
| • Does provision of prognostic data alter decision-making? |
| • What are the reasons for variation in acceptance onto dialysis or transplantation programs? |
| •Why is morbidity and mortality high in the first 3 months of commencing hemodialysis, and can it be modified? |
| • Is there an optimal approach to the commencement of dialysis to reduce morbidity and mortality? |
| • Can comorbidity be factored into the reporting of kidney outcomes? |
| • Place greater emphasis on collection of patient-centered KRT outcomes, including quality of life, symptom burden, physical and cognitive function, and financial and caregiver burdens |
| • Can the reasons for commencing dialysis be uniformly collected to improve the understanding of variability in the timing of initiation of dialysis? |
CKD, chronic kidney disease; KRT, kidney replacement therapy.
Needs, opportunities, and challenges for clinical trials in patients with CKD G4+
Barriers for clinical trials in patients with CKD G4+ and strategies to overcome them
There is an urgent need to increase the number and quality of completed clinical trials in patients with CKD, including people with CKD G4+ (Supplementary Table S9).89,90 A summary of selected barriers and proposed solutions is shown in Table 7 and Supplementary Table S10. For industry, the potential economic benefits of a successful clinical trial must be offset against the potential financial and nonfinancial risks. Slow recruitment, higher-than-average adverse event rates, a relatively small total patient population (i.e., prospective users), and several recent null clinical trials in CKD populations may all increase perceived risk. However, trials in populations with CKD G4+ also have key advantages, including the large potential economic benefits of preventing kidney failure and CVD events, a relatively captive and highly motivated patient population, and high event rates (reducing the required sample size or duration of follow-up).
Table 7.
Research recommendations for enhancing clinical trials in CKD G4+
| 1. Develop systems to systematically monitor the quantity and quality of clinical trials in nephrology. Output of these monitoring services could be linked to ongoing updates of KDIGO guidelines. |
| 2. Develop new or refine existing clinical trial designs to optimize success rates for clinical trials including enrichment trials, pragmatic trials, or platform trials. |
| 3. Continue to conduct trials on interventions to slow the progressive loss of kidney function and to safely defer the initiation of dialysis. |
| 4. Develop other trial end points that are transferable globally and more aligned to patient research priorities, such as preserving functional status and cognition and reducing infections. |
| 5. Define which end points are relevant for different patient groups; for example, mortality, access to kidney transplantation, and availability to continue to work for younger patients, and delaying initiation of dialysis, avoiding hospitalizations, and living independently for older patients. |
| 6. Conduct clinical trials in which patients are followed beyond dialysis initiation and/or transplantation. |
CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes.
Investigator-initiated trials have different challenges: the nephrology community understands the clinical need and opportunities in the CKD G4+ population but often struggles to complete adequately powered studies.91 Environmental scans of ongoing trials suggest that certain topics (e.g., sodium bicarbonate treatment in CKD G4+) are being independently studied by multiple groups in different countries. One potential solution could be to link multiple existing national trial networks to increase statistical power, either by use of common protocols or by pooling results from similar but not identical protocols using meta-analysis.92
Choosing patient-relevant interventions and outcomes
As for most medical disciplines, available trial evidence in CKD G4+ populations reflects the interests and priorities of clinicians and researchers rather than patients and families. Correcting this misalignment is critical for improving the utility of trial findings and potentially for facilitating trial conduct by increasing participant recruitment and retention. Existing processes for identifying patient-centered research priorities and patient-relevant outcomes (e.g., those identified using the James Lind Alliance methodology) will be helpful for achieving this aim. A list of such priorities for CKD G3a to G5 patients is already available93,94 and could be used as the basis for people with CKD G4+ specifically. Initiation of KRT is clinically meaningful and, therefore, the obvious choice as an outcome in trials of therapies aimed at slowing the loss of kidney function. However, KRT initiation is determined by many factors such as local habits and guidelines, physicians’ and patients’ preferences, and patients’ well-being and comorbidities. Functional and symptomatic outcomes that are patient-oriented, both before and after the initiation of KRT, are important to address for safety as well as efficacy, and are relevant for the patient. A toolbox that includes validated symptomatic assessment will support trials with functional outcomes.
Increasing and sustaining patient engagement in trials and other clinical research is critical, but no consensus was reached on how this goal should be achieved or approached. A key starting point could be to describe lessons learned from leading patient-centered initiatives (e.g., SONG-HD95 and NICE) and establish an organization responsible for patient engagement in CKD G4+ research specifically.
Increasing the success of clinical trials in CKD G4+ by optimizing other aspects of trial design
Besides outcomes, other aspects of clinical trial design could be optimized. Thus, the KFRE and the newly developed predictive instrument specific for CKD G4+ could be used in potential trial participants to inform power calculations or enrich recruitment of participants at higher-than-average risk.11 Alternative methods (e.g., pragmatic trials, stepped-wedge designs, or registry-based studies) could also be used to facilitate trial conduct.96 Pragmatic trials are well suited for patients with CKD G4+ because such trials can enroll socially disadvantaged populations (usually excluded from traditional randomized controlled trials), are directly applicable to patient care, allow assessment of a range of interventions, include patient-centered outcomes, and are cheaper than traditional randomized controlled trials. There are also some challenges, such as lack of experience, collection and ascertainment of outcomes, and informed consent procedure, which may pose challenges given the increasing use of electronic health records.
Conclusions and perspectives
Patients with CKD G4+ represent a high-risk population that requires specialized care and expertise that should ideally be coordinated by nephrologists. Despite their severely reduced level of GFR, the prognosis of patients with CKD G4+ is variable, with a substantial proportion of up to more than 50% surviving CVD event- and KRT-free for at least several years. The newly developed risk prediction tool specific for CKD G4+ may help to establish a comprehensive quantitative analysis of possible adverse outcomes, including CVD events, kidney failure and mortality, and thereby guide therapy. Such prognostic information can be factored into decisions for surveillance, CVD risk reduction, and eventual preparation for KRT. Another important finding of the meta-analysis is that traditional CVD risk factors appear to be relevant in CKD G4+, like in earlier stages of CKD and in the absence of CKD. Although such associations do not prove the efficacy of risk factor targeting, it appears rational to apply such strategies as long as no opposing evidence is available. Finally, there was general agreement that the complex comorbidities of people with CKD G4+, particularly those with functional impairment, older age, and limited life expectancy, mandate a patient-centric approach with joint decision-making both in routine practice as well as during the design of trials to optimize management and outcomes.
Supplementary Material
Table S1. Various ways that individualized risk-based information may be used by different stakeholders involved in CKD care.
Table S2. Key competencies required for delivery of CKD G4+ care.
Table S3. Selected therapies for future research in CKD G4+.
Table S4. Definition of HF according to ACCF/AHA and ESC.
Table S5. Risk factors for HF in patients with CKD G4+.
Table S6. Adjusted hazard ratios of all-cause death in patients with heart failure, by CKD status, 2010–2011.
Table S7. Consequences of arteriovenous fistula on the cardiovascular system.
Table S8. Recommendations from the Renal Physicians Association regarding forgoing dialysis.
Table S9. Key goals and activities identified by the Clinical Trials Group at the Vancouver Kidney Health Summit.
Table S10. Challenges, potential solutions, and suggested actions related to increasing the number and quality of clinical trials in CKD G4+ populations.
Figure S1. Markov model: modified graph illustrating the different possible pathways.
Figure S2. CKD chronic care model.
Figure S3. Cumulative probability of heart failure in incident patients.
Acknowledgments
DISCLOSURE
The conference was sponsored by KDIGO and supported in part by unrestricted educational grants from Akebia Therapeutics, AMAG Pharmaceuticals, Amgen, AstraZeneca, FibroGen, Fresenius Medical Care, Keryx Biopharmaceuticals, Relypsa, Roche, and Vifor Fresenius Medical Care Renal Pharma.
CAH declared having received consultancy fees from AbbVie, FibroGen, Relypsa, Sanifit, and ZS Pharma; and research support from Amgen and Zoll. MTJ declared having received research support from Amgen Canada. HJLH declared having received consultancy fees from AbbVie, AstraZeneca, Boehringer Ingelheim, Fresenius, Janssen, and Merck; speaker honoraria from AstraZeneca and Boehringer Ingelheim; and research support from AstraZeneca and Boehringer Ingelheim. PES declared having received speaker honorarium from Janssen-Cilag. MKT declared having received research support from National Institutes of Health, Veterans Administration, and Gordon and Betty Moore Foundation. DCW declared having received consultancy fees from Akebia, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Vifor Fresenius Medical Care Renal Pharma; speaker honoraria from Amgen and Vifor Fresenius Medical Care Renal Pharma; and research support from AstraZeneca. WCW declared having received consultancy fees from Akebia, AMAG Pharmaceuticals, Amgen, AstraZeneca, Bayer, Daiichi Sankyo, Relypsa, and ZS Pharma; speaker honoraria from FibroGen; and research support from National Institutes of Health.
APPENDIX
Other Conference Participants
Ali K. Abu-Alfa, Lebanon; Shuchi Anand, USA; Mustafa Arici, Turkey; Shoshana H. Ballew, USA; Geoffrey A. Block, USA; Rafael Burgos-Calderon, Puerto Rico; David M. Charytan, USA; Zofia Das-Gupta, UK; Jamie P. Dwyer, USA; Danilo Fliser, Germany; Marc Froissart, Switzerland; John S. Gill, Canada; Kathryn E. Griffith, UK; David C. Harris, Australia; Kate Huffman, Canada; Lesley A. Inker, USA; Kitty J. Jager, Netherlands; Min Jun, Australia; Kamyar Kalantar-Zadeh, USA; Bertrand L. Kasiske, USA; Csaba P. Kovesdy, USA; Vera Krane, Germany; Edmund J. Lamb, UK; Edgar V. Lerma, USA; Andrew S. Levey, USA; Adeera Levin, Canada; Juan Carlos Julián Mauro, Spain; Danielle M. Nash, Canada; Sankar D. Navaneethan, USA; Donal O’Donoghue, UK; Gregorio T. Obrador, Mexico; Roberto Pecoits-Filho, Brazil; Bruce M. Robinson, USA; Elke Schäffner, Germany; Dorry L. Segev, USA; Bénédicte Stengel, France; Peter Stenvinkel, Sweden; Navdeep Tangri, Canada; Francesca Tentori, USA; Yusuke Tsukamoto, Japan; Mintu P. Turakhia, USA; Miguel A. Vazquez, USA; Angela Yee-Moon Wang, Hong Kong; Amy W. Williams, USA.
Footnotes
All the other authors declared no competing interests.
Supplementary References
Supplementary material is linked to the online version of the paper at www.kidney-international.org.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 3.Robinson BM, Akizawa T, Jager KJ, et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/S0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 5.Eckardt KU, Gillespie IA, Kronenberg F, et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015;88:1117–1125. doi: 10.1038/ki.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85:158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med. 2011;124:1073–1080. doi: 10.1016/j.amjmed.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Innes A, Rowe PA, Burden RP, et al. Early deaths on renal replacement therapy: the need for early nephrological referral. Nephrol Dial Transplant. 1992;7:467–471. [PubMed] [Google Scholar]
- 9.Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int. 2018;93:1442–1451. doi: 10.1016/j.kint.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans M, Grams ME, Sang Y, et al. Risk factors for prognosis in patients with severely decreased GFR. Kidney Int Rep. 2018;3:625–637. doi: 10.1016/j.ekir.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 13.Maziarz M, Black RA, Fong CT, et al. Evaluating risk of ESRD in the urban poor. J Am Soc Nephrol. 2015;26:1434–1442. doi: 10.1681/ASN.2014060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder EB, Yang X, Thorp ML, et al. Predicting 5-year risk of RRT in stage 3 or 4 CKD: development and external validation. Clin J Am Soc Nephrol. 2017;12:87–94. doi: 10.2215/CJN.01290216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulter A. Do patients want a choice and does it work? BMJ. 2010;341:c4989. doi: 10.1136/bmj.c4989. [DOI] [PubMed] [Google Scholar]
- 16.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions! Cochrane Database Syst Rev. 2014:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 17.Becker H, Winterbottom A, Mooney A. Patient information and decision-making processes. Br J Renal Med. 2009;14:12–14. [Google Scholar]
- 18.Hakim RM, Lazarus JM. Initiation of dialysis. J Am Soc Nephrol. 1995;6:1319–1328. doi: 10.1681/ASN.V651319. [DOI] [PubMed] [Google Scholar]
- 19.Wang SM, Hsiao LC, Ting IW, et al. Multidisciplinary care in patients with chronic kidney disease: A systematic review and meta-analysis. Eur J Intern Med. 2015;26:640–645. doi: 10.1016/j.ejim.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Barrett BJ, Garg AX, Goeree R, et al. A nurse-coordinated model of care versus usual care for stage 3/4 chronic kidney disease in the community: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1241–1247. doi: 10.2215/CJN.07160810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiamjariyapon T, Ingsathit A, Pongpirul K, et al. Effectiveness of integrated care on delaying progression of stage 3-4 chronic kidney disease in rural communities of Thailand (ESCORT study): a cluster randomized controlled trial. BMC Nephrol. 2017;18:83. doi: 10.1186/s12882-016-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalonde L, Quintana-Barcena P, Lord A, et al. Community pharmacist training-and-communication network and drug-related problems in patients with CKD: a multicenter, cluster-randomized, controlled trial. Am J Kidney Dis. 2017;70:386–396. doi: 10.1053/j.ajkd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Levin A, Steven S, Selina A, et al. Canadian chronic kidney disease clinics: a national survey of structure, function and models of care. Can J Kidney Health Dis. 2014;1:29. doi: 10.1186/s40697-014-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A, Jorna T, Bhandari S. Should we STOP angiotensin converting enzyme inhibitors/angiotensin receptor blockers in advanced kidney disease? Nephron. 2016;133:147–158. doi: 10.1159/000447068. [DOI] [PubMed] [Google Scholar]
- 26.Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant. 2016;31:255–261. doi: 10.1093/ndt/gfv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 28.Bansal N, Katz R, Robinson-Cohen C, et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2017;2:314–318. doi: 10.1001/jamacardio.2016.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 30.USRDS. Chapter 9: Cardiovascular Disease in Patients With ESRD. 2015 USRDS Annual Data Report. Available at: https://www.usrds.org/2015/download/vol2_09_CVD_15.pdf. Accessed March 21, 2018.
- 31.USRDS. Atlas of CKD & ESRD. 2013 Available at: https://www.usrds.org/atlas13.aspx. Accessed February 28, 2017.
- 32.USRDS. Annual Data Report. 2007 Available at: https://www.usrds.org/atlas07.aspx. Accessed February 28, 2017.
- 33.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 35.Smith DH, Thorp ML, Gurwitz JH, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6:333–342. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal N, Hyre Anderson A, Yang W, et al. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26:946–956. doi: 10.1681/ASN.2014010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bansal N, McCulloch CE, Lin F, et al. Different components of blood pressure are associated with increased risk of atherosclerotic cardiovascular disease versus heart failure in advanced chronic kidney disease. Kidney Int. 2016;90:1348–1356. doi: 10.1016/j.kint.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubin RF, Deo R, Bansal N, et al. Associations of conventional echocardiographic measures with incident heart failure and mortality: the chronic renal insufficiency cohort. Clin J Am Soc Nephrol. 2017;12:60–68. doi: 10.2215/CJN.02700316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United States Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. Available at: https://www.usrds.org/2013/pdf/v1_ch4_13.pdf. Accessed February 28, 2017. [Google Scholar]
- 41.Harel Z, Wald R, McArthur E, et al. Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2015;26:3141–3150. doi: 10.1681/ASN.2014060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erickson KF, Winkelmayer WC, Chertow GM, et al. Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25:2079–2087. doi: 10.1681/ASN.2013080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall RK, Toles M, Massing M, et al. Utilization of acute care among patients with ESRD discharged home from skilled nursing facilities. Clin J Am Soc Nephrol. 2015;10:428–434. doi: 10.2215/CJN.03510414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronksley PE, Hemmelgarn BR, Manns BJ, et al. Potentially preventable hospitalization among patients with CKD and high inpatient use. Clin J Am Soc Nephrol. 2016;11:2022–2031. doi: 10.2215/CJN.04690416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C, Yang X, Lei Y, et al. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol. 2016;11:1536–1544. doi: 10.2215/CJN.00910116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sud M, Tangri N, Pintilie M, et al. Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation. 2014;130:458–465. doi: 10.1161/CIRCULATIONAHA.113.007106. [DOI] [PubMed] [Google Scholar]
- 47.Hsu RK, Chai B, Roy JA, et al. Abrupt decline in kidney function before initiating hemodialysis and all-cause mortality: the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2016;68:193–202. doi: 10.1053/j.ajkd.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivara MB, Chen CH, Nair A, et al. Indication for dialysis initiation and mortality in patients with chronic kidney failure: a retrospective cohort study. Am J Kidney Dis. 2017;69:41–50. doi: 10.1053/j.ajkd.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bansal N, Keane M, Delafontaine P, et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol. 2013;8:355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai QZ, Lu XZ, Lu Y, et al. Longitudinal changes of cardiac structure and function in CKD (CASCADE study) J Am Soc Nephrol. 2014;25:1599–1608. doi: 10.1681/ASN.2013080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whalley GA, Marwick TH, Doughty RN, et al. Effect of early initiation of dialysis on cardiac structure and function: results from the echo substudy of the IDEAL trial. Am J Kidney Dis. 2013;61:262–270. doi: 10.1053/j.ajkd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Ganda A, Weiner SD, Chudasama NL, et al. Echocardiographic changes following hemodialysis initiation in patients with advanced chronic kidney disease and symptomatic heart failure with reduced ejection fraction. Clin Nephrol. 2012;77:366–375. doi: 10.5414/cn107169. [DOI] [PubMed] [Google Scholar]
- 53.Korsheed S, Eldehni MT, John SG, et al. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant. 2011;26:3296–3302. doi: 10.1093/ndt/gfq851. [DOI] [PubMed] [Google Scholar]
- 54.Babadjanov J, Miler R, Niebauer K, et al. Arteriovenous fistula creation for end-stage renal disease may worsen pulmonary hypertension. Ann Vasc Surg. 2016;36:293.e291–293. doi: 10.1016/j.avsg.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Engelberts I, Tordoir JH, Boon ES, et al. High-output cardiac failure due to excessive shunting in a hemodialysis access fistula: an easily overlooked diagnosis. Am J Nephrol. 1995;15:323–326. doi: 10.1159/000168857. [DOI] [PubMed] [Google Scholar]
- 56.Young PR, Jr, Rohr MS, Marterre WF., Jr High-output cardiac failure secondary to a brachiocephalic arteriovenous hemodialysis fistula: two cases. Am Surg. 1998;64:239–241. [PubMed] [Google Scholar]
- 57.Bailey WB, Talley JD. High-output cardiac failure related to hemodialysis arteriovenous fistula. J Ark Med Soc. 2000;96:340–341. [PubMed] [Google Scholar]
- 58.Dikow R, Schwenger V, Zeier M, et al. Do AV fistulas contribute to cardiac mortality in hemodialysis patients? Semin Dial. 2002;15:14–17. doi: 10.1046/j.1525-139x.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- 59.Ragupathi L, Johnson D, Marhefka GD. Right ventricular enlargement within months of arteriovenous fistula creation in 2 hemodialysis patients. Tex Heart Inst J. 2016;43:350–353. doi: 10.14503/THIJ-15-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reddy YNV, Obokata M, Dean PG, et al. Long-term cardiovascular changes following creation of arteriovenous fistula in patients with end stage renal disease. Eur Heart J. 2017;38:1913–1923. doi: 10.1093/eurheartj/ehx045. [DOI] [PubMed] [Google Scholar]
- 61.Sumida K, Molnar MZ, Potukuchi PK, et al. Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease. Nephrol Dial Transplant. 2017;32:1330–1337. doi: 10.1093/ndt/gfw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pun PH, Al-Khatib SM, Han JY, et al. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: a meta-analysis of patient-level data from 3 randomized trials. Am J Kidney Dis. 2014;64:32–39. doi: 10.1053/j.ajkd.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldenberg I, Moss AJ, McNitt S, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Singh SM, Wang X, Austin PC, et al. Prophylactic defibrillators in patients with severe chronic kidney disease. JAMA Intern Med. 2014;174:995–996. doi: 10.1001/jamainternmed.2014.1208. [DOI] [PubMed] [Google Scholar]
- 65.Chang TI, Zheng Y, Montez-Rath ME, et al. Antihypertensive medication use in older patients transitioning from chronic kidney disease to end-stage renal disease on dialysis. Clin J Am Soc Nephrol. 2016;11:1401–1412. doi: 10.2215/CJN.10611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson DW, Dent H, Hawley CM, et al. Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol. 2009;4:1620–1628. doi: 10.2215/CJN.01750309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trespalacios FC, Taylor AJ, Agodoa LY, et al. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis. 2003;41:1267–1277. doi: 10.1016/s0272-6386(03)00359-7. [DOI] [PubMed] [Google Scholar]
- 68.Wang IK, Lu CY, Lin CL, et al. Comparison of the risk of de novo cardiovascular disease between hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Int J Cardiol. 2016;218:219–224. doi: 10.1016/j.ijcard.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 69.Sens F, Schott-Pethelaz AM, Labeeuw M, et al. Survival advantage of hemodialysis relative to peritoneal dialysis in patients with end-stage renal disease and congestive heart failure. Kidney Int. 2011;80:970–977. doi: 10.1038/ki.2011.233. [DOI] [PubMed] [Google Scholar]
- 70.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66:S1–S305. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans R, Caskey F, Fluck R, et al. UK Renal Registry 18th annual report: chapter 12 epidemiology of reported infections amongst patients receiving dialysis for established renal failure in England 2013 to 2014: a joint report from Public Health England and the UK Renal Registry. Nephron. 2016;132(Suppl 1):279–288. doi: 10.1159/000444826. [DOI] [PubMed] [Google Scholar]
- 72.Carson RC, Juszczak M, Davenport A, et al. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4:1611–1619. doi: 10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008;73:1289–1295. doi: 10.1038/ki.2008.62. [DOI] [PubMed] [Google Scholar]
- 74.Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 76.Wong SP, Kreuter W, O’Hare AM. Healthcare intensity at initiation of chronic dialysis among older adults. J Am Soc Nephrol. 2014;25:143–149. doi: 10.1681/ASN.2013050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen LM, Ruthazer R, Moss AH, et al. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol. 2010;5:72–79. doi: 10.2215/CJN.03860609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Couchoud C, Labeeuw M, Moranne O, et al. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24:1553–1561. doi: 10.1093/ndt/gfn698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thamer M, Kaufman JS, Zhang Y, et al. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis. 2015;66:1024–1032. doi: 10.1053/j.ajkd.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wick JP, Turin TC, Faris PD, et al. A clinical risk prediction tool for 6-month mortality after dialysis initiation among older adults. Am J Kidney Dis. 2017;69:568–575. doi: 10.1053/j.ajkd.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 81.Couchoud C, Hemmelgarn B, Kotanko P, et al. Supportive care: time to change our prognostic tools and their use in CKD. Clin J Am Soc Nephrol. 2016;11:1892–1901. doi: 10.2215/CJN.12631115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 83.Rao PS, Merion RM, Ashby VB, et al. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069–1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 84.Jassal SV, Krahn MD, Naglie G, et al. Kidney transplantation in the elderly: a decision analysis. J Am Soc Nephrol. 2003;14:187–196. doi: 10.1097/01.asn.0000042166.70351.57. [DOI] [PubMed] [Google Scholar]
- 85.Moss AH. Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol. 2010;5:2380–2383. doi: 10.2215/CJN.07170810. [DOI] [PubMed] [Google Scholar]
- 86.Kurella M, Covinsky KE, Collins AJ, et al. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146:177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 87.Susantitaphong P, Altamimi S, Ashkar M, et al. GFR at initiation of dialysis and mortality in CKD: a meta-analysis. Am J Kidney Dis. 2012;59:829–840. doi: 10.1053/j.ajkd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 89.Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 90.Baigent C, Herrington WG, Coresh J, et al. Challenges in conducting clinical trials in nephrology: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017;92:297–305. doi: 10.1016/j.kint.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deo A, Schmid CH, Earley A, et al. Loss to analysis in randomized controlled trials in CKD. Am J Kidney Dis. 2011;58:349–355. doi: 10.1053/j.ajkd.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 92.Morrish AT, Hawley CM, Johnson DW, et al. Establishing a clinical trials network in nephrology: experience of the Australasian Kidney Trials Network. Kidney Int. 2014;85:23–30. doi: 10.1038/ki.2013.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemmelgarn BR, Pannu N, Ahmed SB, et al. Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant. 2017;32:847–854. doi: 10.1093/ndt/gfw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813–1821. doi: 10.2215/CJN.01610214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tong A, Manns B, Hemmelgarn B, et al. Standardised outcomes in nephrology–Haemodialysis (SONG-HD): study protocol for establishing a core outcome set in haemodialysis. Trials. 2015;16:364. doi: 10.1186/s13063-015-0895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Boer IH, Kovesdy CP, Navaneethan SD, et al. Pragmatic clinical trials in CKD: opportunities and challenges. J Am Soc Nephrol. 2016;27:2948–2954. doi: 10.1681/ASN.2015111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Various ways that individualized risk-based information may be used by different stakeholders involved in CKD care.
Table S2. Key competencies required for delivery of CKD G4+ care.
Table S3. Selected therapies for future research in CKD G4+.
Table S4. Definition of HF according to ACCF/AHA and ESC.
Table S5. Risk factors for HF in patients with CKD G4+.
Table S6. Adjusted hazard ratios of all-cause death in patients with heart failure, by CKD status, 2010–2011.
Table S7. Consequences of arteriovenous fistula on the cardiovascular system.
Table S8. Recommendations from the Renal Physicians Association regarding forgoing dialysis.
Table S9. Key goals and activities identified by the Clinical Trials Group at the Vancouver Kidney Health Summit.
Table S10. Challenges, potential solutions, and suggested actions related to increasing the number and quality of clinical trials in CKD G4+ populations.
Figure S1. Markov model: modified graph illustrating the different possible pathways.
Figure S2. CKD chronic care model.
Figure S3. Cumulative probability of heart failure in incident patients.


