Abstract
Background: High-intensity exercise (HIEX) suppresses appetite in adults and is thought to be mediated by appetite-regulating hormones. However, the effects of HIEX-induced inflammatory and stress biomarkers on appetite control and body weight have not been reported in children or adults.
Objective: The objective of this study was to describe the effects of acute HIEX at 70% peak oxygen consumption (VO2peak) on postexercise appetite and selective biomarkers of inflammation, stress, and appetite regulatory hormones in normal-weight (NW) and in overweight/obese boys.
Methods: NW (n = 11) and overweight/obese (n = 11) boys aged 10–18 y were randomly assigned in a crossover design to either rest or HIEX. Visual analog scale appetite ratings and plasma biomarkers of appetite, inflammation, stress, and glucose control were measured after HIEX or rest.
Results: Appetite increased from baseline to 110 min (P < 0.001), but was lower after HIEX (P = 0.04), with no difference between body weight groups. HIEX also resulted in lower active ghrelin (P < 0.001) and increased interleukin-6 (IL-6; P < 0.001), tumor necrosis factor-α (P < 0.001), and cortisol (P < 0.001) concentrations, independent of body weight. It increased blood glucose (P = 0.002) and insulin (P = 0.028) concentrations in NW but not overweight and obese boys. Leptin, glucagon-like peptide 1, peptide tyrosine tyrosine, C-reactive protein, and cortisol were not affected by HIEX. An inverse correlation was found between IL-6 and appetite (r = −0.379; P = 0.012), but not any other biomarkers.
Conclusions: HIEX resulted in reduced appetite that correlated with an increase in IL-6 in both NW and overweight/obese boys. However, although a role for IL-6 in the response can be suggested, the suppression of appetite was potentially mediated by the decrease in active ghrelin and/or increase in cortisol. This trial was registered at clinicaltrials.gov as NCT02619461.
Keywords: appetite regulation, biomarkers, childhood obesity, clinical trials, eating behavior, energy metabolism, exercise, hormonal regulation, inflammation, obesity
Introduction
The short-term effect of exercise on energy intake (EI) regulation is dependent on intensity, modality (1) and duration (2). Acute low intensity produces no or only moderate effects on appetite (3, 4), whereas exercise at a high intensity above 70% peak oxygen consumption (VO2peak) suppresses appetite (5, 6) and EI (3, 7) in healthy adults and obese men (8). High-intensity exercise (HIEX), defined by the American College of Sports Medicine as exercise intensity ≥70% VO2peak (9), is not to be confused with high-intensity interval or intermittent training.
The role of appetite hormones in reduced appetite after HIEX is uncertain. Appetite hormones are well known to play a critical role in nutrient signaling and subsequently in controlling appetite (10). The ingestion of food stimulates the release of appetite hormones such as ghrelin, peptide tyrosine tyrosine (PYY), and glucagon-like peptide 1 (GLP-1). In one study, an acute bout of HIEX lowered active ghrelin and increased PYY, as well as GLP-1 (7, 11). In contrast, HIEX has been reported to increase active ghrelin (12), decrease GLP-1 (13), and have no effect on PYY (12). Thus, as concluded in a meta-analysis of current studies, acute bouts of exercise may have only slight to moderate effects on appetite-regulating hormones (14).
Acute HIEX elicits many physiologic responses other than those on appetite hormones, such as those on the immune and stress systems (15, 16), which may account for postexercise appetite suppression. Similar to appetite hormones such as active ghrelin, GLP-1, PYY, and leptin, inflammatory cytokines such as IL-6 and TNF-α cross the blood-brain barrier and directly interact with the luminal surface of brain endothelial cells to release substances that can affect appetite (17). For example, IL-6 and TNF-α stimulate expression of pro-opiomelanocortin (18), a neuropeptide that suppresses appetite in the hypothalamus. Hypothalamic IL-6 receptors are also present in the arcuate, dorsomedial, ventromedial, lateral, paraventricular, and supraoptic nucleus, exerting their effect on appetite (19–22).
An increase in the inflammatory biomarkers IL-6, TNF-α, and C-reactive protein (CRP) has been associated with a suppression of appetite (23, 24). In rodents, elevated inflammation is associated with suppression of appetite when induced by LPS injection (25) or by direct exogenous administration of inflammatory cytokines such as IL-6 and TNF-α (26). Furthermore, IL-6–deficient mice develop mature onset obesity (27) and display greater EI than do wild-type mice (28). In humans, an inverse association between inflammation and suppression of appetite and EI is also found during cachexia (29, 30), a condition characterized by a rapid loss of appetite and body weight (30) in which IL-6 and TNF-α have been hypothesized to be major driver in the loss of appetite (24). After HIEX in overweight/obese adults, a more pronounced increase in the inflammatory biomarkers IL-6 and TNF-α is found than with normal-weight (NW) individuals (31). Obese adolescents also display reduced EI after intensive exercise compared with NW individuals (32).
Because IL-6 is derived from muscle and increases in blood with HIEX, it is a possible cause of the suppression of appetite. IL-6 is a mediator of muscle protein biosynthesis (33), thermoregulation (34), energy expenditure (35), and the regulation of body weight (27); in contrast to with cachexia, TNF-α is not increased by exercise (36). However, to our knowledge, only one study has reported that an increase in IL-6 with HIEX is associated with suppression of appetite and EI (37). In this study of twins, one twin was exposed to 45 min of submaximal exercise intensity near the anaerobic threshold and 7 min of 90% of VO2peak. Plasma IL-6 concentrations increased and postexercise EI decreased. However, to our knowledge, there has been no report in either adults or children of HIEX-induced suppression of appetite with its concurrent effects on appetite hormones, stress, and inflammatory responses.
Therefore, we hypothesized that acute suppression of appetite after HIEX (at 70% VO2peak) was associated with IL-6 but not appetite hormone response in both overweight/obese and NW children and adolescents. The objective of this study (NCT02619461) was to describe the effects of acute HIEX at 70% VO2peak on postexercise appetite and selective biomarkers of inflammation, stress, and appetite regulatory hormones in NW and overweight/obese boys.
Methods
Participants
Eleven NW (BMI for age percentile: 15th–85th) and 11 overweight/obese (BMI for age percentile: >90th) boys aged 10–18 y (38) were recruited through local advertisements. The participant characteristics are shown in Table 1. Sample size was determined from previous studies in our laboratory. Eligibility for the study was determined via a telephone screening questionnaire. Boys who answered “yes” to one of the questions on the physical activity readiness questionnaire, displayed a form of hematophobia, were dieters, had been diagnosed with diabetes or other metabolic diseases, or scored ≥11 on an eating habit questionnaire were excluded from the study. All experimental procedures were approved by the University of Toronto Health Sciences Research Ethics Board, and informed consent was obtained from all adult participants, parents of the children, and the children themselves. Four participants did not complete the study because of difficulties accessing the veins or a mild form of vasovagal syncope, likely because of the intravenous catheter insertion.
TABLE 1.
Participant characteristics1
| Measurement | NW | OW/OB |
|---|---|---|
| Age, y | 15.4 ± 0.8 | 14.5 ± 0.9 |
| Weight, kg | 60.6 ± 4.8 | 80.9 ± 5.5* |
| Height, cm | 166.5 ± 3.8 | 165.6 ± 3.4 |
| BMI, kg/m2 | 21.5 ± 1.2 | 29.2 ± 1.3*** |
| BMI percentile | 59.8 ± 8.2 | 96.4 ± 0.7*** |
| VO2peak, mL ⋅ kg−1 ⋅ min−1 | 43.5 ± 2.3 | 30.9 ± 1.7*** |
| VO2peak, %VET | 63.2 ± 3.3 | 57.8 ± 2.7 |
| HRmax, bpm | 187 ± 3.0 | 187 ± 2.4 |
| HREX, bpm | 151 ± 5.3 | 152 ± 5.6 |
| Body fat, % | 20.1 ± 1.7 | 39.3 ± 1.1*** |
| Tanner stage | 3.5 ± 0.4 | 2.9 ± 0.4 |
1Values are means ± SEMs, n = 22 (n = 11 NW and n = 11 OW/OB subjects). *,***Significantly different from NW by unpaired t test, *P < 0.05, ***P < 0.001. bpm, beats per minute; HREX, average heart rate achieved during high-energy exercise sessions from 10 to 43 min; HRmax, maximum average heart rate achieved at the screening; NW, normal weight; OW/OB, overweight/obese; VET, ventilation threshold; VO2peak, maximum oxygen consumption.
Subject assessment
For the screening session, parents and their children attended the University of Toronto Goldring Center for High Performance Sport during the week or on weekends. The screening session was conducted to determine physical fitness, anthropometric measurements, habitual physical activity levels, and pubertal status with the use of Tanner stages (39). Physical fitness was determined via the measurement of VO2peak and the ventilation threshold with the use of a continuous incremental cycling protocol on a Kettler RE7 recumbent bicycle. Participants cycled for 3 min at 25 W, after which the intensity was increased every minute by 15 W for boys who weighed <60 kg and 20 W for boys who weighed >60 kg. The ventilation threshold was assessed with the use of the ventilation equivalent method (40), and VO2peak was determined with the use of the highest 6 consecutive breaths (41). BMI was used to identify participants with healthy body weight (15th–85th BMI for age percentile) and those who were overweight/obese (90th–100th BMI for age percentile) according to CDC (2000) growth charts (38). Bioelectrical impedance analysis was used to estimate body fat mass and fat-free mass (101Q; RJL Systems) based on the Horlick equation (42).
Study design and experimental protocol
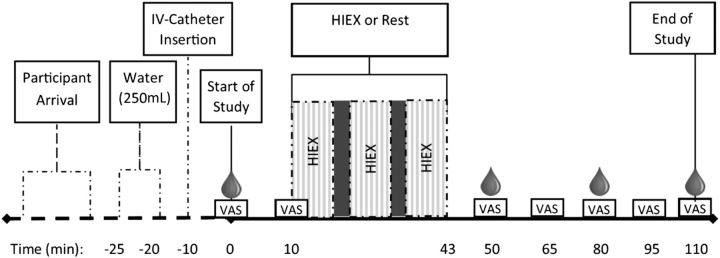
The study used a 3-level mixed factorial (activity × weight status × time) repeated-measures randomized design. All participants completed 2 test sessions: 1) exercise, with 30 min of HIEX at 70% VO2peak, or 2) no exercise, with 30 min of rest. The randomization schedule was generated with the use of the SAS PROC PLAN procedure (SAS version 9.3). Experimental sessions were conducted at the University of Toronto Athletic center on weekends between 0900 and 1000 after a 12-h overnight fast. Children were asked to refrain from exercise ≥24 h before the experimental session, and parents were asked to encourage their children to drink water ≤1 h before the scheduled session, refrain from physical activity, and maintain the same dietary patterns the evening before each test. The experimental exercise protocol (Figure 1) was adopted with slight modifications from a previously published paper by Thivel et al. (43). Before the test, subjects were fitted with a heart rate (HR) monitor (Polar). Participants were asked to complete the baseline questionnaire and then drink 250 mL water (within 5 min) followed by the insertion of an intravenous catheter. For the HIEX session, at 9 min, participants were seated on the recumbent bicycle and asked to start peddling [at 25 W and 60–70 revolutions per minute (rpm)] for 1 min before the first HIEX bout started. The HIEX protocol consisted of three 10-min bouts of HIEX at 70% VO2peak at 60–70 rpm with 1.5 min active rest interposed at 25 W with 60–70 rpm. For the resting session, participants spent the entire time resting, reading a book, or doing homework. The blood samples were drawn at baseline (0 min), 50 min, 80 min, and at the end of the study at 110 min. To ensure consistency, each child attended experimental sessions after a 12-h fast, at the same time and day of the week, 1 wk apart.
FIGURE 1.
Study protocol flow diagram. The light gray area = 10 min HIEX bout, the dark gray area = 1.5 min rest, and the blood drop = time point for each blood draw. HIEX, high-intensity exercise; IV, intravenous; VAS, visual analog scale.
Visual analog scales
A visual analog scale (VAS) was used to assess subjective appetite based on the following questionnaires: determination to eat (DTE), hunger, fullness, and prospective food consumption (PFC). Appetite scores were calculated as a function with the use of the following formula: average appetite score (millimeters) = [DTE + hunger + (100 − fullness) + PFC]/4 (44). Physical comfort, nausea, and thirst were also measured to determine possible confounders for the appetite scores as previously described (2, 41, 45). Furthermore, nausea has been shown to be associated with exercise-induced anorexia (46). Participants were instructed to read each question and place an “X” along a 100-mm line, depending on how they felt at the current moment. VAS questionnaires were administered at baseline (0 min) and 10, 50, 65, 80, 95, and 110 min after the start of each session.
Blood collection and plasma sample processing
Blood samples were collected into prechilled 10-mL BD Vacutainer blood collection tubes (BD Diagnostics) that contained spray-dried K2EDTA anticoagulant and a protease inhibitor cocktail composed of a proprietary mix of Sitagliptin, Aprotinin and 4-(2-Aminomethyl)benzenesulfonyl fluoride hydrochloride to prevent the proteolytic breakdown of hormones. Immediately after collection, plasma was separated by centrifugation for 15 min at 2000 × g at 4°C, then portioned in 2-mL aliquots into Eppendorf tubes and stored at −80°C for later analyses. In addition, to enhance the active ghrelin stability in blood samples, 200 µL 1 N HCl was added to every 1 mL plasma collected for ghrelin analysis.
Biochemical plasma measurements
Plasma concentrations of glucose, insulin, CRP, and cortisol were analyzed at Mount Sinai Hospital (Toronto, Canada). All other analyses were performed in the Department of Nutritional Sciences at the University of Toronto. Leptin, total PYY [i.e., PYY (1–36 amide) and PYY (3–36 amide)], and the biologically active forms of ghrelin and GLP-1 [i.e., GLP-1 (7–36 amide)] were analyzed by using commercial ELISA kits purchased from Millipore as follows: leptin–catalog no. EZHL-80SK, sensitivity: 0.5 ng/mL, range: 0.5–100 ng/mL; active ghrelin–catalog no. EZGRA-88K, sensitivity: 8 pg/mL, range: 25–2000 pg/mL; GLP-1–catalog no. EGLP-35K, sensitivity: 2 pM, range: 2–100 pM; and PYY–catalog no. EZHPYYT66K, sensitivity: 6.5 pg/mL, range: 14–1800 pg/mL. IL-6 and TNF-α were analyzed with the use of sandwich ELISA kits from R&D systems as follows: IL-6–catalog no. HS600B, sensitivity: 0.11 pg/mL, range: 0.156–10 pg/mL; and TNF-α–catalog no. HSTA00D, sensitivity: 0.191 pg/mL, range: 0.5–32 pg/mL. For all assays, intra-CV was <4% and inter-CV was <8%.
Statistical analysis
All statistical analyses were performed with the use of SAS version 9.3. The data were tested for normality with the use of the SAS PROC UNIVARIATE procedure. Student's t test was used to compare subject characteristics, baseline VAS, and baseline biomarker measurements for NW compared with overweight/obese subjects. Baseline measurements are presented as absolute means ± SEMs. VAS and blood biomarker changes over time were analyzed with the use of a 3-factor ANOVA by the SAS PROC MIXED procedure followed by a Tukey-Kramer post hoc test, with activity (HIEX compared with rest), weight status (NW compared with overweight/obese), and time as independent variables. VAS and blood biomarkers over time are presented as means ± SEMs and percentage mean ± SEM change from baseline to allow a direct comparison between NW and overweight/obese participants. For clarity, combined data for NW and overweight/obese subjects are presented in results if no main effect of weight status or interaction with weight status was detected. Pearson correlation analysis was conducted between mean percentage changes from baseline of each biomarker (leptin, active ghrelin, PYY, GLP-1, blood glucose, insulin, CRP, IL-6, TNF-α, and cortisol) and each of the mean changes in VAS scores (appetite, DTE, fullness, hunger, and PFC). Statistical significance was declared at P < 0.05.
Results
Twenty-two participants, 11 NW and 11 overweight/obese boys, completed the study. Overweight and obese participants had higher body weight (P = 0.012), BMI (P < 0.001), BMI percentile (P < 0.001), and percentage body fat (P < 0.001), but lower physical fitness (VO2peak) (P < 0.001) than did NW subjects. The participants' ages (P = 0.464), heights (P = 0.862), Tanner stages (P = 0.302), heart rates (HRs) during the HIEX bout (P = 0.898), and maximum HRs during the screening session (P = 0.99) were not significantly different (Table 1).
HR
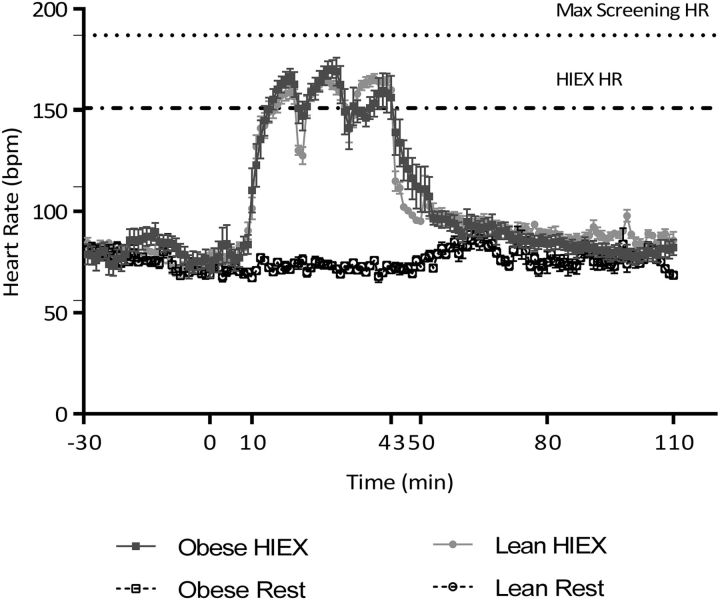
HR increased with HIEX (P < 0.001), weight status (P < 0.001) and time (P < 0.001). HR during HIEX (10–43 min) did not differ between NW and overweight/obese boys (P = 0.898) (Figure 2).
FIGURE 2.
Heart rate during HIEX and rest. Values are means ± SEMs, n = 22 (11 normal-weight and 11 overweight/obese subjects). bpm, beats per minute; HIEX, high-intensity exercise; HR, heart rate.
Baseline appetite, physical comfort scores, and blood markers.
At baseline, appetite (P = 0.026) and PFC (P = 0.009) scores were lower and fullness ratings were higher (P < 0.001) in overweight/obese boys than they were in NW boys (Table 2). The VAS scores for hunger, DTE, physical comfort, nausea, and thirst were not significantly different.
TABLE 2.
Baseline measurements in NW and OW/OB subjects1
| Measurement | NW | OW/OB |
|---|---|---|
| Visual analog scales, mm | ||
| Appetite | 68.8 ± 4.7 | 53.3 ± 2.7* |
| DTE | 55.6 ± 6.7 | 48.7 ± 4.3 |
| Hunger | 56.0 ± 6.0 | 49.5 ± 3.7 |
| PFC | 71.6 ± 5.9 | 53.3 ± 3.3** |
| Fullness | 19.9 ± 3.8 | 38.1 ± 3.1* |
| Plasma biomarkers of appetite | ||
| Leptin ng/mL | 3.8 ± 0.7 | 11.3 ± 1.5*** |
| Ghrelin active, pg/mL | 7.3 ± 1.3 | 8.3 ± 1.1 |
| PYY, pg/mL | 3.7 ± 0.3 | 3.1 ± 0.3 |
| GLP-1, pM | 5.4 ± 0.9 | 4.1 ± 0.4 |
| Glucose, mmol/L | 5.0 ± 0.1 | 5.3 ± 0.1** |
| Insulin, pmol/L | 71.3 ± 7.2 | 124.2 ± 17.6** |
| HOMA-IR | 2.3 ± 0.2 | 4.4 ± 0.7** |
| Plasma biomarkers of stress and inflammation | ||
| Cortisol, nmol/L | 326.4 ± 29.2 | 296.3 ± 44.8 |
| CRP, mg/L | 0.9 ± 0.4 | 0.6 ± 0.3 |
| IL-6, pg/mL | 1.3 ± 0.4 | 0.9 ± 0.2 |
| TNF-α, pg/mL | 1.6 ± 0.2 | 1.4 ± 0.2 |
1Values are means ± SEMs, n = 22 (n = 11 NW and n = 11 OW/OB subjects). *,**,***Significantly different from NW by unpaired t test, *P < 0.05, **P < 0.01, ***P < 0.001. CRP, C-reactive protein; DTE, determination to eat; GLP-1, glucagon-like peptide 1; NW, normal weight; OW/OB, overweight/obese; PFC, prospective food consumption; PYY, peptide tyrosine tyrosine.
Overweight and obese boys had higher concentrations of circulating baseline leptin (P < 0.001), blood glucose (P < 0.001), insulin (P = 0.003), and HOMA-IR (P = 0.005) than did NW boys (Table 2). No differences in baseline concentrations were found for active ghrelin, GLP-1, PYY, cortisol, CRP, IL-6, and TNF-α.
VAS appetite response to HIEX
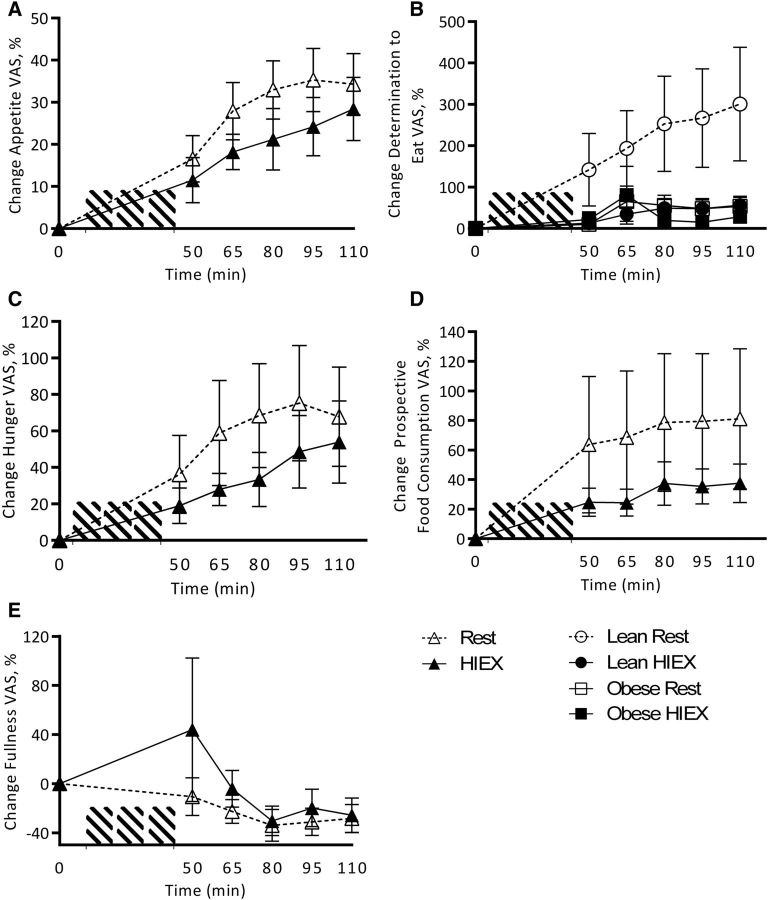
HIEX decreased appetite (P = 0.04) and DTE (P = 0.019) compared with rest, with no effect by weight status (P = 0.299). Total appetite increased over time (P < 0.001) (Figure 3A, B). There was an activity × weight status interaction for DTE (P = 0.043) resulting from the fact that overweight/obese subjects were less hungry than NW subjects after HIEX (Figure 3C). PFC was not affected by activity (P = 0.346) or weight status (P = 0.327), but was increased over time (P < 0.001) (Figure 3D). Fullness was not affected by activity (P = 0.29), weight status (P = 0.85), or time (P = 0.072) (Figure 3E). Physical comfort was not affected by activity (P = 0.081) or weight status (P = 0.51), but decreased over time (P = 0.017). Thirst and nausea were not affected by activity, weight status, or time. No other interactions were found.
FIGURE 3.
Change from baseline in appetite (A), determination to eat (B), hunger (C), prospective food consumption (D), and fullness (E) VAS response to HIEX at 0, 50, 65, 80, 95, and 110 min. Values are means ± SEMs, n = 22 (11 normal-weight and 11 overweight/obese subjects). For clarity, combined data for NW and overweight/obese subjects are presented in results if no main effect of weight status or interaction with weight status was detected. HIEX, high-intensity exercise; VAS, visual analog scale.
Plasma appetite hormone response to HIEX
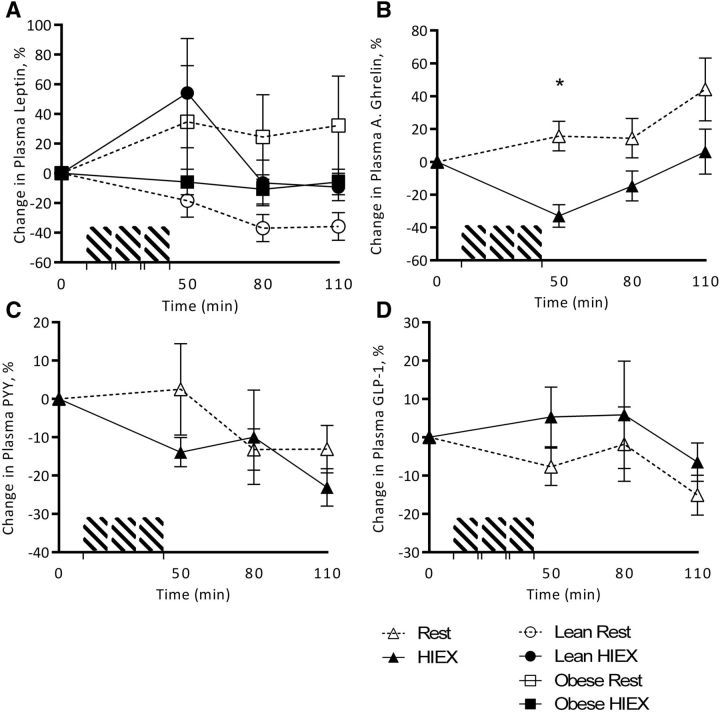
Plasma leptin concentrations, shown as a percentage change from baseline, were not affected by activity (P = 0.835), weight status (P = 0.389), or time (P = 0.008). An interaction was found for activity × weight status (P < 0.001), showing that leptin response to HIEX was increased and more consistent compared with rest in NW subjects than in overweight/obese children (Figure 4A). Plasma active ghrelin concentrations were decreased after HIEX (P < 0.001) independent of weight status (P = 0.808) and affected by time (P = 0.013). There was an interaction of activity × time (P = 0.03), with plasma ghrelin concentrations decreasing in response to HIEX (0–50 min) and returning to baseline concentrations at 110 min (Figure 4B). Plasma PYY concentrations were not affected by time (P = 0.26), activity (P = 0.391), or weight status (P = 0.392). No other interactions were found (Figure 4C). GLP-1 was not affected by activity (P = 0.527), time (P = 0.143), weight status (P = 0.408), or their interactions (Figure 4D).
FIGURE 4.
Change from baseline in plasma concentrations of leptin (A), active ghrelin (B), PYY (C), and GLP-1 (D) in response to HIEX at 0, 50, 80, and 110 min. *Significantly different (HIEX compared with rest) at each time point (3-factor ANOVA, Tukey-Kramer post hoc test, P < 0.05). For clarity, combined data for normal-weight and overweight/obese subjects are presented in results if no main effect of weight status or interaction with weight status was detected. A., active; GLP-1, glucagon-like peptide 1; HIEX, high-intensity exercise; PYY, peptide tyrosine tyrosine.
Plasma glucose and insulin response to HIEX
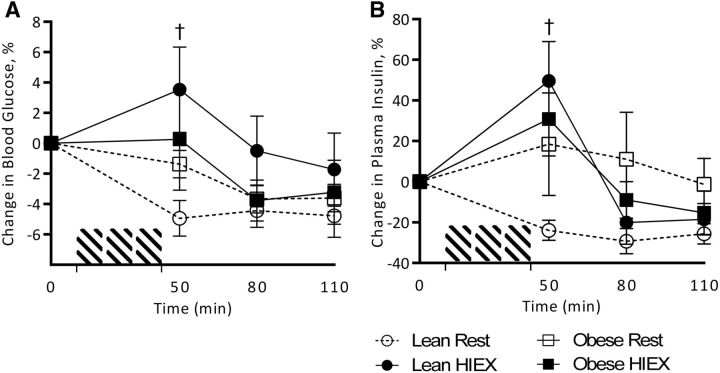
Blood glucose concentrations (percentage change from baseline) were increased by HIEX (P = 0.002) and decreased over time (P < 0.001), with an interaction between activity and weight status (P = 0.032). The interaction was explained by an increase in glucose after HIEX and a decrease at rest in NW children, with little effect in overweight/obese subjects (Figure 5A). Insulin was also increased by HIEX (P = 0.028) and decreased by time (P < 0.001), with an interaction between activity and time (P = 0.001). Again, the interaction was explained by the increase after HIEX and decrease at rest in NW but not overweight/obese children (Figure 5B).
FIGURE 5.
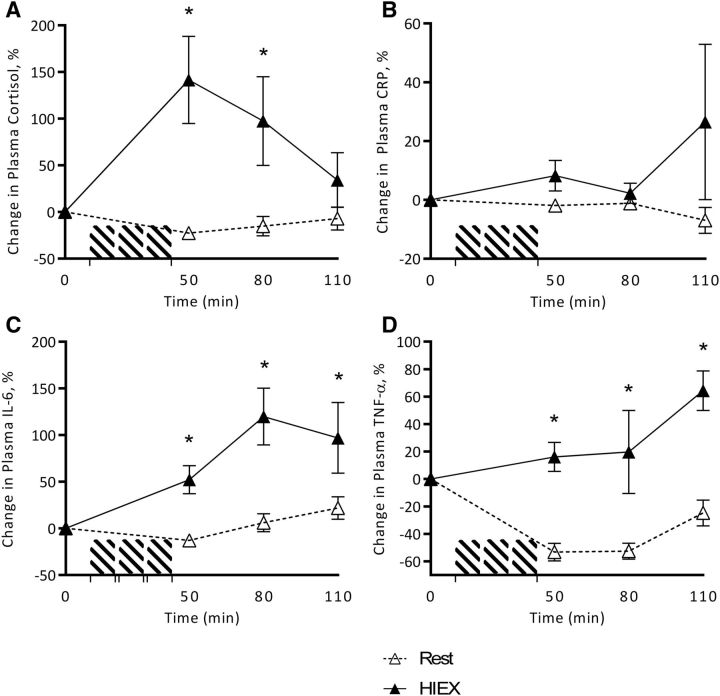
Change from baseline plasma concentrations of cortisol (A), CRP (B), IL-6 (C), and TNF-α (D) in response to HIEX at 0, 50, 80 and 110 min. *Significantly different (HIEX compared with rest) at each time point (3-factor ANOVA, Tukey-Kramer post hoc test, P < 0.05). Values are means ± SEMs, n = 22 (11 NW and 11 overweight/obese). CRP, C-reactive protein; HIEX, high-intensity exercise; NW, normal weight.
Plasma inflammatory and stress responses to HIEX
Cortisol was increased with HIEX (P < 0.001) and over time (P = 0.018), but was not affected by weight status (P = 0.408). There was a time × activity interaction (P < 0.001) because of a 142% increase from baseline during HIEX (0–50 min), followed by a decrease from 50 to 110 min to baseline concentrations (Figure 6A). CRP tended to increase with HIEX (P = 0.072), but was not affected by weight status (P = 0.703) or time (P = 0.707) (Figure 6B). IL-6 was increased by HIEX (P < 0.001) and over time (P = 0.026), but was not affected by weight status (P = 0.713). There was an interaction of activity and time (P = 0.014) because of a 120% increase in IL-6 from 0 to 80 min compared with rest (Figure 6C). TNF-α also increased with HIEX (P < 0.001) and over time (P = 0.003), but was not affected by weight status (P = 0.097). (Figure 6D). No other interactions were found.
FIGURE 6.
Change from baseline plasma concentrations of blood glucose (A) and insulin (B) in response to HIEX at 0, 50, 80, and 110 min. †Statistical significance (HIEX compared with rest) is denoted in NW at each time point (3-factor ANOVA, Tukey-Kramer post hoc test, P < 0.05). Values are means ± SEMs, n = 22 (11 NW and 11 overweight/obese). HIEX, high-intensity exercise; NW, normal weight.
Associations between appetite and biomarkers of appetite, inflammation, and stress
No significant correlations were found between mean percentage changes in appetite, hunger, fullness, DTE, and PFC and mean percentage changes in leptin, PYY, GLP-1, cortisol, CRP, and TNF-α. However, IL-6 was inversely correlated with appetite (r = −0.379; P = 0.012) and fullness (r = 0.446; P = 0.004). Active ghrelin was inversely correlated with fullness (r = −0.341; P = 0.025), but not appetite. Blood glucose was positively correlated with fullness (r = 0.326; P = 0.035). Correlations were negative between leptin and GLP-1 (r = −0.477; P = 0.001) and active ghrelin and cortisol (r = −0.352; P = 0.021), but positive between TNF-α and IL-6 (r = 0.337; P = 0.027).
Discussion
HIEX suppressed appetite in boys, as previously shown in adults (5, 6), and EI in obese but not lean adolescents (32). In addition, the hypothesis that an acute suppression of appetite after HIEX (at 70% VO2peak) is associated with IL-6 but not appetite hormone responses was partially supported. First, IL-6 increased, whereas appetite VAS scores were suppressed after HIEX, and IL-6 was the only biomarker that correlated with appetite. Second, the appetite hormones leptin, PYY, and GLP-1 were not affected by HIEX and did not correlate with appetite. However, HIEX increased cortisol and decreased active ghrelin, which contribute to food intake regulation, but neither correlated with appetite scores.
Because IL-6, but not TNF-α or CRP, matched the time course of VAS appetite scores and correlated with VAS scores for appetite and fullness, a possible role for IL-6 in the relation between appetite post-HIEX can be suggested. Although both TNF-α and IL-6 concentrations are high during sepsis and cachexia and are derived predominantly by macrophages, exercise induced an increase in IL-6 produced by the muscle (47). Although TNF-α was different from rest during HIEX, the difference arose primarily from a decrease in TNF-α during rest, perhaps mediated by diurnal changes (48). CRP was measured because it is also a sensitive marker of inflammation that is often used to quantify low systemic inflammation in lean and obese individuals (49), and it has been positively associated with the loss of appetite and weight during cancer (50). However, it was not different between the body-weight groups, and its lack of change with HIEX is consistent with the fact that it is known to be a tonic indicator of systemic inflammation and is less variable to acute stimuli such as exercise (34). CRP was also not correlated with appetite.
The results from this experiment support a previous review that concluded that appetite hormones have only negligible or small effects on the suppression of appetite after HIEX (14). Leptin, PYY, and GLP-1 were not affected by HIEX (70% VO2peak), similar to studies in adults (8, 9, 35–38), and did not correlate with appetite. Furthermore, leptin, PYY, and GLP-1 did not match the time course of appetite or correlate with any of the VAS measurements. This is not surprising, because most studies reporting an effect of appetite hormones on EI are based on the effects of peripheral injections resulting in alterations in appetite and EI (51–54).
HIEX-induced changes in ghrelin and cortisol occurred, but their contribution to the decrease in appetite is unclear. HIEX decreased active ghrelin, which steadily increased during rest, as shown by previous studies (55), but this was not correlated with overall average appetite. However, the decrease in active ghrelin correlated with an increase in one component on the appetite scale, fullness, making it a possible candidate for the HIEX-induced suppression of appetite. How the effect of HIEX on active ghrelin secretion associates with fullness is not known. However, HIEX increases sympathetic nervous activity and facilitates the redistribution of splanchnic blood flow to the peripheries (56), leading to gastric mucosal ischemia, possibly resulting in a decreased secretion and lower ghrelin plasma concentrations (57). Ghrelin also previously has been shown to be inversely associated with cortisol (58). In our study, blood cortisol mirrored active ghrelin concentrations, increasing to 150% at 50 min with HIEX, and declined from 50 to 110 min, similar to another study in adults (59). Plasma cortisol concentrations were not correlated with appetite VAS or any other appetite markers. The injection of cortisol has been shown to decrease food intake, and the anorexic effect of cortisol has been hypothesized to act via 2 possible pathways: 1) a decrease in ghrelin receptor expression in the hypothalamus and ghrelin secretion, and 2) a decrease in neuropeptide Y expression (60). However, the exact mechanisms of how cortisol can affect appetite need to be elucidated.
Blood glucose and insulin are well-described biomarkers of appetite (61). During exercise, blood glucose concentrations are usually stable in healthy individuals (41). However, overweight/obese subjects display higher insulin, glucose, and HOMA-IR concentrations at baseline (Table 2), indicating increased insulin resistance and metabolic inflexibility (62). This may lead to increased blood glucose and insulin concentrations in overweight/obese subjects compared with lean subjects during and after HIEX, resulting in increased hyperglycemia in overweight/obese subjects compared with lean participants. However, whereas blood glucose concentrations differed between NW and overweight/obese subjects, appetite response did not, suggesting that the blood glucose response to HIEX was not driving the appetite.
This study had several limitations that future research needs to address. First, appetite was measured, but not EI. Currently, there are only limited data available on the suppression of appetite in children. The time course of appetite and hormonal response to HIEX is largely unknown in NW and overweight/obese children. EI was not measured in this study to investigate the time point of the strongest appetite suppression within this 1-h time period. Furthermore, previous research has shown an uncoupling effect of EI and VAS appetite scores in NW and overweight/obese children and adolescents (63); therefore, future studies need to assess EI. Second, although the results show that IL-6 is correlated with appetite after HIEX in boys, this association does not indicate a functional relation. Studies with varying concentrations of IL-6 or IL-6 antagonists are needed to further understand the role if IL-6 in appetite control. Finally, the effect of sex on the exercise-induced suppression of appetite was not addressed. Women have an attenuated inflammatory response to exercise (64), and may increase their EI in response to HIEX (65).
In conclusion, HIEX resulted in reduced appetite that correlated with an increase in IL-6 in both NW and overweight/obese boys. However, although a role for IL-6 in the response can be suggested, the suppression of appetite was potentially mediated by the decrease in active ghrelin and/or increase in cortisol.
Acknowledgments
The authors' responsibilities were as follows—SH: designed and conducted the experiment, analyzed the data, and wrote the research paper; RK and RA: contributed to the analysis of the data and the manuscript editing process; ST and GHA: conceptualized, designed, and supervised the experiment and had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Abbreviations
- CRP
C-reactive protein
- DTE
determination to eat
- EI
energy intake
- GLP-1
glucagon-like peptide 1
- HIEX
high-intensity exercise
- HR
heart rate
- NW
normal weight
- PFC
prospective food consumption
- PYY
peptide tyrosine tyrosine
- rpm
revolutions per minute
- VAS
visual analog scale
- VO2peak
peak oxygen consumption
References
- 1. Panissa VL, Julio UF, Hardt F, Kurashima C, Lira FS, Takito MY, Franchini E.. Effect of exercise intensity and mode on acute appetite control in men and women. Appl Physiol Nutr Metab 2016. Jul 7 (Epub ahead of print; DOI: 10.1139/apnm-2016-0172). [DOI] [PubMed] [Google Scholar]
- 2. Bozinovski NC, Bellissimo N, Thomas SG, Pencharz PB, Goode RC, Anderson GH.. The effect of duration of exercise at the ventilation threshold on subjective appetite and short-term food intake in 9 to 14 year old boys and girls. Int J Behav Nutr Phys Act 2009;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King NA, Burley VJ, Blundell JE.. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur J Clin Nutr 1994;48:715–24. [PubMed] [Google Scholar]
- 4. Thompson DA, Wolfe LA, Eikelboom R.. Acute effects of exercise intensity on appetite in young men. Med Sci Sports Exerc 1988;20:222–7. [DOI] [PubMed] [Google Scholar]
- 5. Blundell JE, King NA.. Exercise, appetite control, and energy balance. Nutrition 2000;16:519–22. [DOI] [PubMed] [Google Scholar]
- 6. Sim AY, Wallman KE, Fairchild TJ, Guelfi KJ.. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int J Obes (Lond) 2014;38:417–22. [DOI] [PubMed] [Google Scholar]
- 7. Ueda SY, Yoshikawa T, Katsura Y, Usui T, Fujimoto S.. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J Endocrinol 2009;203:357–64. [DOI] [PubMed] [Google Scholar]
- 8. Ueda SY, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S.. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J Endocrinol 2009;201:151–9. [DOI] [PubMed] [Google Scholar]
- 9. Pescatello LS, American College of Sports Medicine ACSM's guidelines for exercise testing and prescription. 9th ed.Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. p. 456. [Google Scholar]
- 10. Ahima RS, Antwi DA.. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am 2008;37:811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deighton K, Barry R, Connon CE, Stensel DJ.. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur J Appl Physiol 2013;113:1147–56. [DOI] [PubMed] [Google Scholar]
- 12. Larson-Meyer DE, Palm S, Bansal A, Austin KJ, Hart AM, Alexander BM.. Influence of running and walking on hormonal regulators of appetite in women. J Obes 2012;2012:730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Unick JL, Otto AD, Goodpaster BH, Helsel DL, Pellegrini CA, Jakicic JM.. Acute effect of walking on energy intake in overweight/obese women. Appetite 2010;55:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schubert MM, Sabapathy S, Leveritt M, Desbrow B.. Acute exercise and hormones related to appetite regulation: a meta-analysis. Sports Med 2014;44:387–403. [DOI] [PubMed] [Google Scholar]
- 15. Brown WM, Davison GW, McClean CM, Murphy MH.. A systematic review of the acute effects of exercise on immune and inflammatory indices in untrained adults. Sports Med Open 2015;1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamer M, Taylor A, Steptoe A.. The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biol Psychol 2006;71:183–90. [DOI] [PubMed] [Google Scholar]
- 17. Banks WA.. Anorectic effects of circulating cytokines: role of the vascular blood-brain barrier. Nutrition 2001;17:434–7. [DOI] [PubMed] [Google Scholar]
- 18. Katahira M, Iwasaki Y, Aoki Y, Oiso Y, Saito H.. Cytokine regulation of the rat proopiomelanocortin gene expression in AtT-20 cells. Endocrinology 1998;139:2414–22. [DOI] [PubMed] [Google Scholar]
- 19. Shizuya K, Komori T, Fujiwara R, Miyahara S, Ohmori M, Nomura J.. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sci 1997;61:PL 135–40. [DOI] [PubMed] [Google Scholar]
- 20. Benrick A, Schele E, Pinnock SB, Wernstedt-Asterholm I, Dickson SL, Karlsson-Lindahl L, Jansson JO.. Interleukin-6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol 2009;21:620–8. [DOI] [PubMed] [Google Scholar]
- 21. Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J, De Souza CT, Moraes JC, Prada PO, Guadagnini D, et al. . IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 2010;8:e1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Saper CB, Chou TC, Elmquist JK.. The need to feed: homeostatic and hedonic control of eating. Neuron 2002;36:199–211. [DOI] [PubMed] [Google Scholar]
- 23. Kasapis C, Thompson PD.. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 2005;45:1563–9. [DOI] [PubMed] [Google Scholar]
- 24. Morley JE, Thomas DR, Wilson MM.. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 2006;83:735–43. [DOI] [PubMed] [Google Scholar]
- 25. Boelen A, Platvoet-ter Schiphorst MC, Bakker O, Wiersinga WM.. The role of cytokines in the lipopolysaccharide-induced sick euthyroid syndrome in mice. J Endocrinol 1995;146:475–83. [DOI] [PubMed] [Google Scholar]
- 26. Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO.. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun 2002;293:560–5. [DOI] [PubMed] [Google Scholar]
- 27. Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO.. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 2002;8:75–9. [DOI] [PubMed] [Google Scholar]
- 28. Chida D, Osaka T, Hashimoto O, Iwakura Y.. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes 2006;55:971–7. [DOI] [PubMed] [Google Scholar]
- 29. Solheim TS, Fearon KC, Blum D, Kaasa S.. Non-steroidal anti-inflammatory treatment in cancer cachexia: a systematic literature review. Acta Oncol 2013;52:6–17. [DOI] [PubMed] [Google Scholar]
- 30. Gautron L, Laye S.. Neurobiology of inflammation-associated anorexia. Front Neurosci 2010;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christiansen T, Bruun JM, Paulsen SK, Olholm J, Overgaard K, Pedersen SB, Richelsen B.. Acute exercise increases circulating inflammatory markers in overweight and obese compared with lean subjects. Eur J Appl Physiol 2013;113:1635–42. [DOI] [PubMed] [Google Scholar]
- 32. Thivel D, Isacco L, Montaurier C, Boirie Y, Duche P, Morio B.. The 24-h energy intake of obese adolescents is spontaneously reduced after intensive exercise: a randomized controlled trial in calorimetric chambers. PLoS One 2012;7:e29840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raj DS, Moseley P, Dominic EA, Onime A, Tzamaloukas AH, Boyd A, Shah VO, Glew R, Wolfe R, Ferrando A.. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int 2008;73:1054–61. [DOI] [PubMed] [Google Scholar]
- 34. De Jongh RF, Vissers KC, Booij LH, De Jongh KL, Vincken P, Meert TF.. Interleukin-6 and perioperative thermoregulation and HPA-axis activation. Cytokine 2003;21:248–56. [DOI] [PubMed] [Google Scholar]
- 35. Wernstedt I, Edgley A, Berndtsson A, Faldt J, Bergstrom G, Wallenius V, Jansson JO.. Reduced stress- and cold-induced increase in energy expenditure in interleukin-6-deficient mice. Am J Physiol Regul Integr Comp Physiol 2006;291:R551–7. [DOI] [PubMed] [Google Scholar]
- 36. Croft L, Bartlett JD, MacLaren DP, Reilly T, Evans L, Mattey DL, Nixon NB, Drust B, Morton JP.. High-intensity interval training attenuates the exercise-induced increase in plasma IL-6 in response to acute exercise. Appl Physiol Nutr Metab 2009;34:1098–107. [DOI] [PubMed] [Google Scholar]
- 37. Almada C, Cataldo LR, Smalley SV, Diaz E, Serrano A, Hodgson MI, Santos JL.. Plasma levels of interleukin-6 and interleukin-18 after an acute physical exercise: relation with post-exercise energy intake in twins. J Physiol Biochem 2013;69:85–95. [DOI] [PubMed] [Google Scholar]
- 38. Flegal KM, Wei R, Ogden C.. Weight-for-stature compared with body mass index-for-age growth charts for the United States from the Centers for Disease Control and Prevention. Am J Clin Nutr 2002;75:761–6. [DOI] [PubMed] [Google Scholar]
- 39. Marshall WA, Tanner JM.. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caiozzo VJ, Davis JA, Ellis JF, Azus JL, Vandagriff R, Prietto CA, McMaster WC.. A comparison of gas exchange indices used to detect the anaerobic threshold. J Appl Physiol 1982;53:1184–9. [DOI] [PubMed] [Google Scholar]
- 41. Hunschede S, El Khoury D, Antoine-Jonville S, Smith C, Thomas S, Anderson GH.. Acute changes in substrate oxidation do not affect short-term food intake in healthy boys and men. Appl Physiol Nutr Metab 2015;40:168–77. [DOI] [PubMed] [Google Scholar]
- 42. Horlick M, Arpadi SM, Bethel J, Wang J, Moye J Jr, Cuff P, Pierson RN Jr, Kotler D.. Bioelectrical impedance analysis models for prediction of total body water and fat-free mass in healthy and HIV-infected children and adolescents. Am J Clin Nutr 2002;76:991–9. [DOI] [PubMed] [Google Scholar]
- 43. Thivel D, Metz L, Julien A, Morio B, Duche P.. Obese but not lean adolescents spontaneously decrease energy intake after intensive exercise. Physiol Behav 2014;123:41–6. [DOI] [PubMed] [Google Scholar]
- 44. Anderson GH, Catherine NL, Woodend DM, Wolever TM.. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr 2002;76:1023–30. [DOI] [PubMed] [Google Scholar]
- 45. Tamam S, Bellissimo N, Patel BP, Thomas SG, Anderson GH.. Overweight and obese boys reduce food intake in response to a glucose drink but fail to increase intake in response to exercise of short duration. Appl Physiol Nutr Metab 2012;37:520–9. [DOI] [PubMed] [Google Scholar]
- 46. Kondo T, Nakae Y, Mitsui T, Kagaya M, Matsutani Y, Horibe H, Read NW.. Exercise-induced nausea is exaggerated by eating. Appetite 2001;36:119–25. [DOI] [PubMed] [Google Scholar]
- 47. Pedersen BK, Febbraio MA.. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008;88:1379–406. [DOI] [PubMed] [Google Scholar]
- 48. Muc-Wierzgoń M, Madej K, Baranowski M, Wierzgon J.. Circadian rhythmometry of serum endogenous tumor necrosis factor-alpha in patients with colorectal cancer metastases. Eur Cytokine Netw 1998;9:193–6. [PubMed] [Google Scholar]
- 49. Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, Jilma B, Roggla G, Wolzt M, Widhalm K, et al. . A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol 2006;26:2541–6. [DOI] [PubMed] [Google Scholar]
- 50. Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD.. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 2004;80:299–307. [DOI] [PubMed] [Google Scholar]
- 51. Druce MR, Neary NM, Small CJ, Milton J, Monteiro M, Patterson M, Ghatei MA, Bloom SR.. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int J Obes (Lond) 2006;30:293–6. [DOI] [PubMed] [Google Scholar]
- 52. Friedman JM, Halaas JL.. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- 53. Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR.. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 2003;349:941–8. [DOI] [PubMed] [Google Scholar]
- 54. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. . A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72. [DOI] [PubMed] [Google Scholar]
- 55. Wasse LK, Sunderland C, King JA, Miyashita M, Stensel DJ.. The influence of vigorous running and cycling exercise on hunger perceptions and plasma acylated ghrelin concentrations in lean young men. Appl Physiol Nutr Metab 2013;38:1–6. [DOI] [PubMed] [Google Scholar]
- 56. Otte JA, Oostveen E, Geelkerken RH, Groeneveld AB, Kolkman JJ.. Exercise induces gastric ischemia in healthy volunteers: a tonometry study. J Appl Physiol 2001;91:866–71. [DOI] [PubMed] [Google Scholar]
- 57. Perini R, Veicsteinas A.. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol 2003;90:317–25. [DOI] [PubMed] [Google Scholar]
- 58. Espelund U, Hansen TK, Hojlund K, Beck-Nielsen H, Clausen JT, Hansen BS, Orskov H, Jorgensen JO, Frystyk J.. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J Clin Endocrinol Metab 2005;90:741–6. [DOI] [PubMed] [Google Scholar]
- 59. Duclos M, Gouarne C, Bonnemaison D.. Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J Appl Physiol 2003;94:869–75. [DOI] [PubMed] [Google Scholar]
- 60. Janzen WJ, Duncan CA, Riley LG.. Cortisol treatment reduces ghrelin signaling and food intake in tilapia, Oreochromis mossambicus. Domest Anim Endocrinol 2012;43:251–9. [DOI] [PubMed] [Google Scholar]
- 61. Lemmens SG, Martens EA, Kester AD, Westerterp-Plantenga MS.. Changes in gut hormone and glucose concentrations in relation to hunger and fullness. Am J Clin Nutr 2011;94:717–25. [DOI] [PubMed] [Google Scholar]
- 62. Chiarelli F, Marcovecchio ML.. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159Suppl 1:S67–74. [DOI] [PubMed] [Google Scholar]
- 63. Thivel D, Chaput JP.. Are post-exercise appetite sensations and energy intake coupled in children and adolescents? Sports Med 2014;44:735–41. [DOI] [PubMed] [Google Scholar]
- 64. Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C, Tarnopolsky MA.. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol 2000;89:2325–32. [DOI] [PubMed] [Google Scholar]
- 65. Pomerleau M, Imbeault P, Parker T, Doucet E.. Effects of exercise intensity on food intake and appetite in women. Am J Clin Nutr 2004;80:1230–6. [DOI] [PubMed] [Google Scholar]