Abstract
Background
Chronic pain and depression often co-occur, and pain may exacerbate depression in people with dementia.
Objective
The objective of this study was to assess the efficacy and safety of analgesic treatment for depression in nursing home patients with advanced dementia and clinically significant depressive symptoms.
Methods
We conducted a multicentre, parallel-group, double-blind, placebo-controlled trial in 47 nursing homes, including 162 nursing home patients aged ≥ 60 years with dementia (Mini-Mental State Examination ≤ 20) and depression (Cornell Scale for Depression in Dementia ≥ 8). Patients were randomised to receive active analgesic treatment (paracetamol or buprenorphine transdermal system) or identical placebo for 13 weeks. The main outcome measure was the change in depression (Cornell Scale for Depression in Dementia) from baseline to 13 weeks, assessed using linear mixed models with fixed effects for time, intervention and their interaction in the models. Secondary outcomes were to assess whether any change in depression was secondary to change in pain (Mobilisation-Observation-Behaviour-Intensity-Dementia-2 Pain Scale) and adverse events.
Results
The mean depression change was − 0.66 (95% confidence interval − 2.27 to 0.94) in the active group (n = 80) and − 3.30 (− 4.68 to −1.92) in the placebo group (n = 82). The estimated treatment effect was 2.64 (0.55–4.72, p = 0.013), indicating that analgesic treatment had no effect on depressive symptoms from baseline to 13 weeks while placebo appeared to ameliorate depressive symptoms. There was no significant reduction in pain in the active treatment group (paracetamol and buprenorphine combined) vs. placebo; however, a subgroup analysis demonstrated a significant reduction in pain for paracetamol vs. placebo [by − 1.11 (− 2.16 to − 0.06, p = 0.037)] from week 6 to 13 without a change in depression. Buprenorphine did not have significant effects on depression [3.04 (− 0.11 to 6.19), p = 0.059] or pain [0.47 (− 0.77 to 1.71), p = 0.456] from 0 to 13 weeks. Thirty-five patients were withdrawn from the study because of adverse reactions, deterioration or death: 25 (31.3%) during active treatment [23 (52.3%) who received buprenorphine], and ten (12.2%) in the placebo group. The most frequently occurring adverse events were psychiatric (17 adverse reactions) and neurological (14 adverse reactions).
Conclusion
Analgesic treatment did not reduce depression while placebo appeared to improve depressive symptoms significantly by comparison, possibly owing to the adverse effects of active buprenorphine. The risk of adverse events warrants caution when prescribing buprenorphine for people with advanced dementia.
Trial registration
ClinicalTrials.gov NCT02267057 (registered 7 July, 2014) and Norwegian Medicines Agency EudraCT 2013-002226-23.
Electronic supplementary material
The online version of this article (10.1007/s40266-018-0546-2) contains supplementary material, which is available to authorized users.
Key Points
| Contrary to our hypothesis, patients who received active analgesic treatment had more persistent depressive symptoms |
| The buprenorphine transdermal system may exacerbate neuropsychiatric symptoms in dementia and should be used with caution in this group |
Introduction
Approximately 40% of nursing home patients receive antidepressants [1], and over 80% have dementia [2]. Although some studies suggest that antidepressants may be beneficial for depression in people with dementia [3, 4], several later studies have found negative results [5, 6]. The most commonly prescribed antidepressants are selective serotonin reuptake inhibitors such as sertraline, and noradrenergic and specific serotonergic antidepressants such as mirtazapine [7]. Lyketsos et al. found that sertraline reduced depression in Alzheimer’s disease compared with placebo (n = 44) [4], this result was followed by a larger study from the same group which found no benefit of sertraline compared with placebo (n = 131) [6]. Banerjee et al. found that sertraline or mirtazapine did not reduce depression in dementia, and that participants who received active treatment had significantly higher rates of adverse events such as nausea and sedation compared with placebo (n = 326) [5]. Updated systematic reviews and meta-analyses conclude that the current evidence base for antidepressants in dementia is equivocal [8, 9].
More than 60% of nursing home patients experience pain, often of moderate-to-severe intensity [10, 11]. Failure to systematically assess and treat pain leads to the risk of chronic pain, particularly in people with dementia who gradually lose their ability to reliably describe symptom severity [12]. Pain has been identified as a possible contributing factor to depression in nursing homes, even in patients with advanced dementia [13, 14]. Pain and depression share a complex relationship, known as the pain-depression dyad, implying that the conditions commonly coexist, exacerbate each other, share common signal pathways and neurotransmitters, and respond to similar treatments [15]. A previous cluster randomised trial suggests that a 12-week stepwise protocol for treating pain with paracetamol, buprenorphine transdermal system (TDS), morphine or pregabalin may reduce depressive symptoms in people with advanced dementia and agitation [16]. However, depression was not an inclusion criterion in this study, and the pain intervention was not placebo controlled.
Buprenorphine is currently recommended for opioid analgesia in the elderly [17]. As a partial agonist/antagonist, it provides effective analgesia with a low potential for serious adverse effects including respiratory depression [17]. Because it undergoes hepatic metabolism and excretion, it does not require dose adjustment in renal insufficiency [17]. Some evidence suggests that buprenorphine may also have a potential for mood-elevating effects in depression [18]. Paracetamol is the most widely used non-opioid analgesic in the elderly, and may also exert an effect in the central processing and response to emotional stimuli [19].
Therefore, we wished to examine whether a stepwise protocol for treating pain using paracetamol or buprenorphine ameliorated depressive symptoms in nursing home patients with moderate-to-severe dementia and clinically significant depressive symptoms, controlling for the choice of analgesic, the presence of moderate-to-severe pain and dementia severity. To assess whether any change in depressive symptoms was secondary to an analgesic effect, we also examined whether the intervention effectively reduced pain compared with placebo.
Materials and Methods
Study Design
This was a 13-week, multicentre, parallel-group, double-blind, randomised placebo-controlled trial conducted in long-term and dementia wards in 47 nursing homes from 12 municipalities in Norway (Bergen, Baerum, Fjell, Kvam, Meland, Os, Oslo, Sandnes, Stavanger, Sula, Sund and Aalesund). Depending on ongoing medical treatment and clinical investigation, participants were prescribed either paracetamol tablets (maximum 3 g/day) or buprenorphine TDS (maximum 10 µg/hour), and were randomised to receive either active treatment or placebo.
Participants
We screened 2323 nursing home patients for inclusion from 18 August, 2014 to 13 September, 2016. Data collection was completed by 20 December, 2016. Eligible participants were elderly (≥ 60 years) long-term patients (i.e. residents with permanent placement) who had been living in the participating ward for at least 4 weeks prior to screening, with dementia according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for major neurocognitive disorders, Mini-Mental State Examination (MMSE) score ≤ 20 [20] and clinically significant depression [Cornell Scale for Depression in Dementia (CSDD) score ≥ 8 at screening] of at least 4 weeks’ duration [21]. Exclusion criteria were: cognitive impairment related to other diagnoses than Alzheimer’s disease; frontotemporal dementia; vascular dementia; dementia with Lewy bodies or mixed dementia (e.g. traumatic head injury, chronic alcohol abuse or Huntington’s disease; assessed by a review of medical records); life expectancy < 6 months; severe pain [Mobilisation-Observation-Behaviour-Intensity-Dementia-2 (MOBID-2) Pain Scale score ≥ 8] [22]; severe aggression (with Neuropsychiatric Inventory—Nursing Home version aggression item ≥ 8) [23]; suicide risk; severe hepatic or renal insufficiency; anaemia (haemoglobin < 8.5 mmol/L in men, < 7.5 mmol/L in women); severe disease or injury that could interfere with study participation; comatose state; participation in another experimental trial; having no carer who was familiar with the patient; diagnosis of psychosis or other severe mental disorder prior to dementia diagnosis (e.g. schizophrenia, schizoaffective disorder and bipolar disorder); severe psychiatric or neurological disorder; uncontrolled epilepsy; the clinician responsible for care or study clinician considered that the patient had any physical condition that would make participation in the trial distressing or likely to increase patient discomfort; contraindication, known allergy, adverse reaction or clinically significant drug interaction to the assigned study treatment; and scheduled prescriptions for any opioid analgesic other than or exceeding buprenorphine 5 µg/hour. When a patient at any point fulfilled any one exclusion criterion, we conducted no further assessments and the reason for exclusion was recorded.
At baseline, after a minimum of 4 weeks, we re-assessed the eligible patients for depression. To avoid false negatives at this point, we excluded patients who scored below the cut-off for greatest sensitivity on the CSDD scale (CSDD score ≥ 6). The screening cut-off value of ≥ 8 and the re-assessment threshold for persistent depression of ≥ 6 on the CSDD scale correspond to the optimal cut-off points for specificity and sensitivity, respectively, as summarised in a recent meta-analysis [24].
Randomisation and Masking
The trial was double blinded, and participants were randomly allocated to each arm in a 1:1 ratio according to computer-generated random numbers in blocks of ten (paracetamol) and 12 (buprenorphine) with no stratification factors. Statisticians generated and sent the randomisation lists directly to the production and packing facilities without researcher involvement. Paracetamol and identical inert placebo tablets were purchased from Kragero Tablettproduksjon A/S, Norway. Mundipharma Research Limited, UK provided buprenorphine TDS and identical inert placebo. The patients, carers, clinicians, pharmacy, researchers and study statistician were masked to group identity until completion of the protocol.
Intervention
As shown in Table 1, participants without current scheduled analgesics or who received ≤ 1 g/day of paracetamol were allocated to step 1, oral paracetamol (increased to a maximum of 3 g/day, active or placebo). Participants who were already prescribed regular doses of > 1 g/day of paracetamol, buprenorphine 5 µg/hour or non-steroidal anti-inflammatory drugs (except low-dose acetylsalicylic acid) were allocated to step 2, buprenorphine TDS (maximum dose of 10 µg/hour, active or placebo). Patients with dysphagia, to whom it was not deemed feasible to administer oral tablets, were allocated to step 2 regardless of whether they were already using paracetamol.
Table 1.
Study treatment steps 1–2
| Step | Regular analgesic treatment | Study treatment | Dose |
|---|---|---|---|
| 1 | No analgesics (1a) or paracetamol ≤ 1 g/day (1b) | Paracetamol tablets | Maximum 3 g/day |
| Placebo tablets | Inert placebo | ||
| 2 | Non-opioid analgesics (paracetamol > 1 g/day, and/or NSAID), or no analgesics, but with difficulty swallowing tablets (2a), or buprenorphine 5 µg/h (2b) | Buprenorphine TDS | 5 µg/h (maximum 10 µg/h in 2b) |
| Placebo TDS | Inert placebo |
NSAID non-steroidal anti-inflammatory drug, except low-dose acetylsalicylic acid, TDS transdermal system
We used a fixed-dose regimen throughout the 13-week treatment period: paracetamol 1 g tablet/placebo was administered at breakfast, lunch and dinner (approximately 8:00 a.m., noon, 6:00 p.m.) for a total daily dose of 3 g in the active group (corresponding to step 1; see Table 1). If the patient was using paracetamol ≤ 1 g/day prior to study inclusion, the study treatment was prescribed in addition to the basis dose, giving a maximum total dose of 1 g three times daily (supplement active or placebo) [step 1b; Table 1]. Buprenorphine/placebo TDS was changed weekly for a total dose of 5 µg/hour in the active group (step 2a; Table 1). However, if the patient was using buprenorphine TDS 5 µg/hour prior to study inclusion, the study treatment was administered as an additional 5 µg/hour TDS (active or placebo) to yield a total dose of 10 µg/hour in the active group (step 2b; Table 1). Patients who were unable to tolerate study treatment were withdrawn from the study and treated as clinically appropriate.
Concomitant Drugs
All participants continued their usual medical treatment after inclusion in the study (including any regular or ‘as needed’ analgesic). The use of ‘as needed’ analgesics was allowed and monitored during the study, ensuring that all patients received adequate pain treatment irrespective of group allocation. Ongoing treatment with antidepressants, other psychotropic drugs and regular analgesics was allowed if the dose had remained stable for 4 weeks prior to study inclusion. Clinicians were advised to keep doses of psychotropic and analgesic drugs unchanged during the study period if possible. If lasting changes were made to regular analgesic treatment or antidepressants, the patient was withdrawn from the study. Lists of regular and ‘as needed’ prescriptions and documentation of administered doses were extracted from medical records at each visit.
Primary and Secondary Outcome Measures
Depressive symptoms were assessed using the CSDD scale, which has been validated and used in clinical studies including people with and without dementia [24]. Each of the 19 items is rated from zero (no symptoms) to two (severe symptoms), and yields a sum score of between zero (no depression) and 38 (most severe depression). While the CSDD scale alone cannot be used to accurately diagnose depression in dementia, it is useful as a screening tool and sufficiently precise to assess change in depressive symptom burden over time. Pain was assessed using the MOBID-2 Pain Scale, a two-part staff-administered behavioural instrument to assess pain in older persons with advanced dementia (see the Electronic Supplementary Material 1) [22]. The evaluation of inferred pain intensity is based on the patient’s pain behaviours during standardised guided movements of different body parts (Part 1), and pain behaviours that might be related to internal organs, head and skin are recorded on an anatomical figure along with inferred pain intensity for each region to allow monitoring over time (Part 2). Excellent interrater and test-retest reliability, internal consistency and validity have been reported [22]. The tool has also demonstrated responsiveness to treatment, as it is able to detect change in the total score (range 0–10) after pain treatment has been initiated [22].
For subgroup analyses, mild/no pain was defined as MOBID-2 < 3 and moderate/severe pain as MOBID-2 ≥ 3. To assess cognitive function at inclusion, we used the MMSE as a screening tool, with MMSE scores of 0–10 defined as severe and MMSE scores of 11–20 defined as moderate dementia [25]. Although the MMSE scale poorly distinguishes between patients with no/questionable dementia, it has shown high agreement with the Clinical Dementia Rating scale for the staging of moderate and severe dementia using these cut-off scores [25]. Assessments of depression (CSDD) and pain (MOBID-2) were made at baseline and 6 and 13 weeks. Adverse events and tolerability were monitored and recorded at each visit. The primary outcome was the effect of analgesic treatment on change in depressive symptoms (CSDD) from baseline to 13 weeks. Secondary outcomes were the effect of analgesic treatment (paracetamol or buprenorphine) on change in pain (MOBID-2) from baseline to 13 weeks, and adverse events and dropout from treatment.
Sample Size
As a preliminary sample size estimate, we used results from Banerjee et al., who found in their updated power analyses that approximately 260 participants would be required to provide 90% power to detect a 2-point difference in the CSDD scale (standard deviation 5; standardised effect size 0.4) between two groups (active and placebo treatment), allowing for 15% dropouts [5]. This estimate was used as a preliminary goal, when inclusion and dropout rates were unknown, and was reviewed when the first 113 patients had completed our 13-week trial protocol (or dropped out). We calculated our revised sample size using a sample size formula for longitudinal data because we have data with repeated measurements. We used a sample size formula for a longitudinal continuous response, where the correlation between repeated measurements (intra-cluster correlation) is taken into account, with the purpose to estimate the intervention effect on average over the total follow-up period [26]. This formula applies for group comparisons with longitudinal data, such as randomised controlled trials. The same parameters (standard deviation 5, standardised effect size 0.4, 90% power, p < 0.05) were used in the revised calculation, but based on available data from the first 113 patients, we were able to estimate the correlation coefficient of repeated measurements within individuals (intra-cluster correlation) with greater precision in the revised sample size calculation (intra-cluster correlation 0.25). The final estimate required 66 patients in each group to obtain 90% power to detect a 2-point CSDD difference. Adjusting for 20% dropouts, our final aim was to include 165 participants in total.
Statistical Analysis
Baseline characteristics were described as mean (standard deviation) for continuous variables, and with the number of patients and percentages of the sample size for categorical variables. Differences in adverse outcomes (deaths) between active treatment and placebo were assessed using the Pearson χ2 test for categorical variables. Treatment effects on both the primary outcome (depression assessed by the CSDD) and the secondary outcome (pain assessed by the MOBID-2 Pain Scale) were assessed separately using linear mixed-effects models, which incorporated all assessments at baseline, 6 and 13 weeks. We treated time as a categorical variable, and included fixed effects for time, intervention and their interaction in the models. To account for clustering, the models were fitted with random intercepts for nursing home units and patients. Treatment effects were calculated for active treatment vs. placebo, these analyses were repeated with the use of other analgesics or antidepressants at baseline as covariates to control for any impact of concomitant drug use. We also conducted pre-planned subgroup analyses for paracetamol tablets compared with placebo tablets, buprenorphine TDS compared with placebo TDS, and to investigate treatment effects stratified for level of cognitive function and for the presence of moderate-to-severe pain. We regarded p < 0.05 as significant. All statistical analyses were conducted with STATA/IC 14 (Stata Corp LP, College Station, TX, USA).
Results
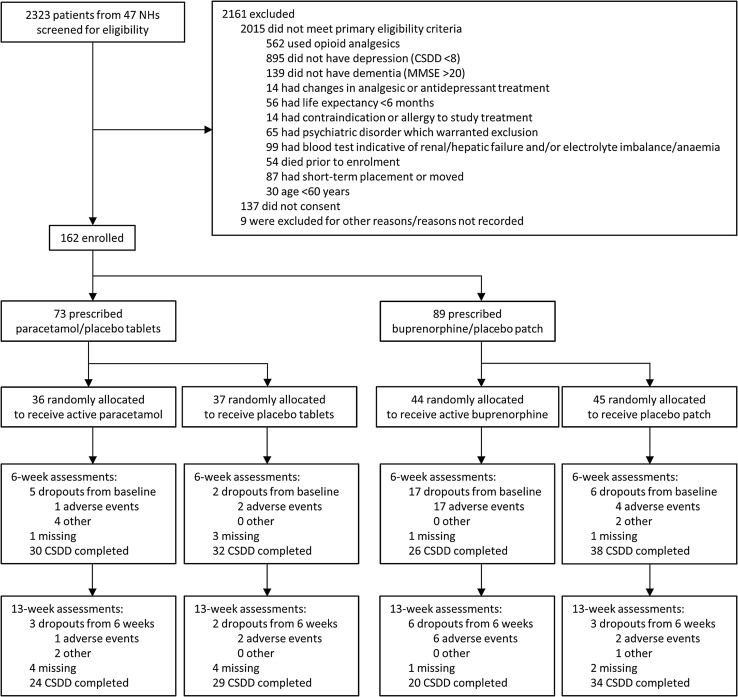
Figure 1 shows the trial profile, wherein each patient is categorised by the first exclusion criterion that was fulfilled, after which no further assessments were made. Table 2 shows group allocation and characteristics of the 162 included patients at baseline. In total, 39 patients (24.1%) reported adverse events (Table 3), most frequently in the active buprenorphine group of whom 23 (52%) withdrew because of adverse events. Thirteen patients discontinued treatment owing to clinical deterioration or death; one in the paracetamol group, two who received placebo tablets, six in the buprenorphine group and four who received placebo TDS. Between-group differences in mortality were not statistically significant (Pearson χ2 test; p = 0.447).
Fig. 1.
Trial profile. Each patient was categorised by the first exclusion criterion that was fulfilled, after which no more assessments were made. CSDD Cornell Scale for Depression in Dementia, MMSE Mini-Mental State Examination, NHs nursing homes
Table 2.
Demographic and clinical characteristics of included patients at baseline
| Total (n = 162) | Placebo (n = 82) | Active (n = 80) | |
|---|---|---|---|
| Age (y) | 85.6 ± 7.4 | 86.2 ± 6.0 | 85.0 ± 8.7 |
| Sex (female) | 122 (75.3) | 63 (76.8) | 59 (73.8) |
| MMSE | 7.8 ± 5.8 | 7.6 ± 5.7 | 8.0 ± 5.9 |
| MOBID-2 | 2.7 ± 2.1 | 3.0 ± 2.3 | 2.4 ± 1.9 |
| CSDD | 11.2 ± 3.7 | 11.7 ± 4.1 | 10.8 ± 3.1 |
| NPI-NH total score | 32.1 ± 19.8 | 31.0 ± 20.1 | 32.8 ± 19.4 |
| NPI-NH depression | 4.4 ± 3.8 | 4.0 ± 3.7 | 5.0 ± 4.0 |
| Analgesic | 81 (50.0) | 41 (50.0) | 40 (50.0) |
| Antidepressant | 81 (50.0) | 50 (61.0) | 31 (38.8) |
| Step 1aa | 68 | 37 | 31 |
| Step 1bb | 5 | 0 | 5 |
| Step 2ac | 74 | 38 | 36 |
| Step 2bd | 15 | 7 | 8 |
Numbers represent mean ± standard deviation or number of patients (%)
CSDD Cornell Scale for Depression in Dementia, MMSE Mini-Mental State Examination, MOBID-2 Mobilisation-Observation-Behaviour-Intensity-Dementia-2 Pain Scale, NPI-NH Neuropsychiatric Inventory-Nursing Home version
aStudy treatment: paracetamol 1 g/placebo tablet three times daily
bStudy treatment: paracetamol 1 g/placebo tablet two times daily + usual treatment: paracetamol ≤ 1 g/day
cStudy treatment: buprenorphine 5 µg/h/placebo transdermal system
dStudy treatment: buprenorphine 5 µg/h/placebo transdermal system + usual treatment: buprenorphine 5 µg/h transdermal system
Table 3.
Adverse reactions that may be related to study treatment
| Placebo tablets (n = 37) | Paracetamol (n = 36) | Placebo TDS (n = 45) | Buprenorphine TDS (n = 44) | All patients (n = 162) | |
|---|---|---|---|---|---|
| Patients with adverse reactionsa | 4 (10.8%) | 2 (5.6%) | 8 (17.8%) | 25 (56.8%) | 39 (24.1%) |
| Gastrointestinal | 0 | 0 | 0 | 7 | 6 |
| Neurological | 0 | 0 | 2 | 12 | 14 |
| Dermatological | 0 | 0 | 1 | 0 | 1 |
| Psychiatric | 0 | 0 | 0 | 17 | 17 |
| Infection | 1 | 0 | 0 | 1 | 2 |
| Falls/fractures | 1 | 1 | 1 | 4 | 7 |
| Major clinical changes, including hospitalisation/death | 2 | 1 | 4 | 7 | 14 |
TDS transdermal system
aEach patient may have had several reported reactions
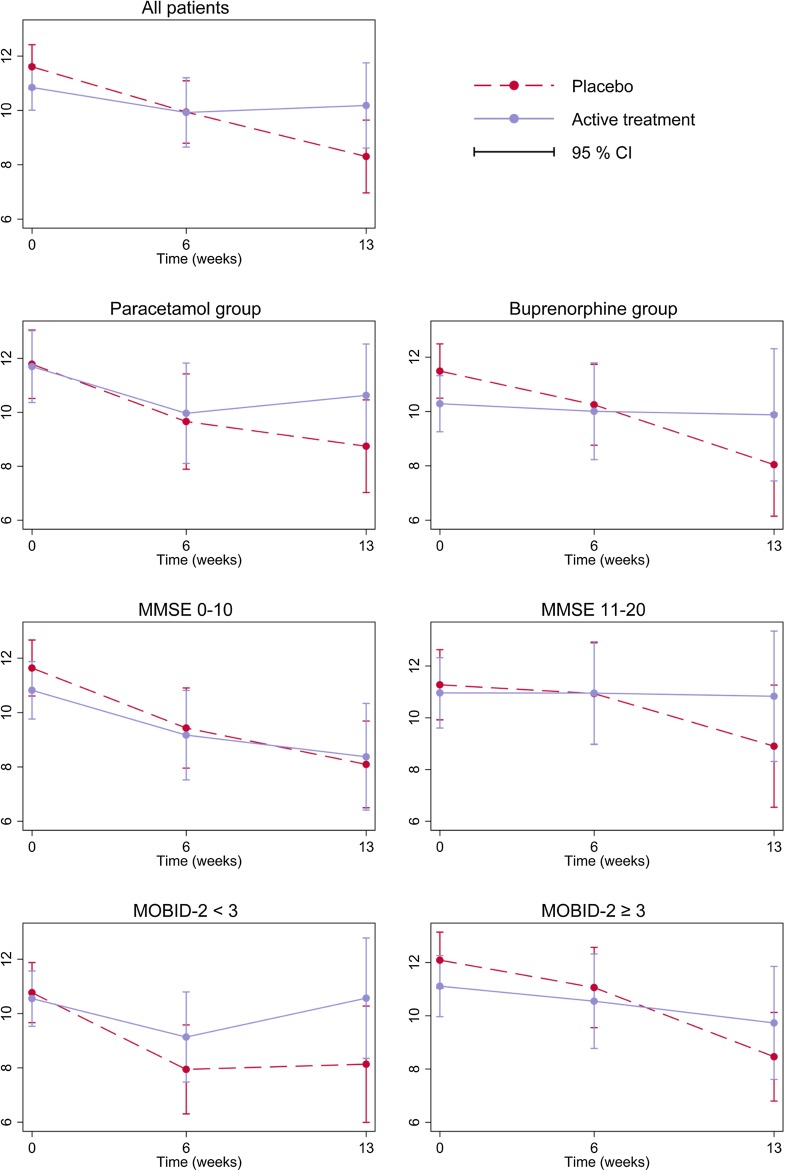
From the linear mixed-model analysis (Table 4, Fig. 2), we found that the placebo group had a significant reduction in depressive symptoms (CSDD score) of −3.30 (95% confidence interval −4.68 to −1.92) from baseline to the 13-week follow-up. The active treatment group did not have a significant CSDD change in the same period [mean change −0.66 (−2.27 to 0.94)]. The estimated treatment effect from baseline to 13 weeks was 2.64 (0.55–4.72, p = 0.013), thus receiving placebo was associated with a significant reduction in depressive symptoms from baseline to 13 weeks compared with those who received active treatment. The observed treatment effects were not affected by concomitant use of antidepressants or analgesics. Analysing patients in the different treatment groups separately, we found that neither active paracetamol nor buprenorphine had significant treatment effects on depressive symptoms from 0 to 13 weeks compared with placebo (Table 4, Fig. 2). The estimated treatment effects were 1.98 (−0.79 to 4.74, p = 0.162) for paracetamol vs. placebo tablets, and 3.04 (−0.11 to 6.19, p = 0.059) for buprenorphine vs. placebo TDS. Grouping patients according to whether they had moderate-to-severe pain at baseline did not yield significant treatment effects on depression compared with placebo; nor did separate analyses for patients with moderate and severe dementia (Table 4, Fig. 2).
Table 4.
Estimated effect of active analgesic treatment on primary outcome (Cornell Scale for Depression in Dementia depressive symptoms) compared with placebo; mixed-model analysis including exploratory subgroup analyses
| N | From baseline to 13 wk | From baseline to 6 wk | From 6 to 13 wk | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | (95% CI) | P value | C | (95% CI) | P value | C | (95% CI) | P value | ||
| Primary analysis | ||||||||||
| All patients | 160 | 2.64 | (0.55–4.72) | 0.013 | 0.74 | (− 1.03 to 2.52) | 0.411 | 1.89 | (− 0.29 to 4.08) | 0.090 |
| Stratified on cognition | ||||||||||
| MMSE 11–20 | 49 | 2.24 | (− 1.24 to 5.72) | 0.207 | 0.33 | (− 2.50 to 3.16) | 0.818 | 1.91 | (− 2.05 to 5.86) | 0.344 |
| MMSE 0–10 | 92 | 1.10 | (− 1.49 to 3.69) | 0.405 | 0.56 | (− 1.75 to 2.86) | 0.635 | 0.54 | (− 2.27 to 3.35) | 0.705 |
| Stratified on drug type | ||||||||||
| Paracetamol/placebo tablets | 73 | 1.98 | (− 0.79 to 4.74) | 0.162 | 0.40 | (− 2.39 to 3.18) | 0.780 | 1.58 | (− 1.49 to 4.64) | 0.313 |
| Buprenorphine/placebo TDS | 89 | 3.04 | (− 0.11 to 6.19) | 0.059 | 0.96 | (− 1.45 to 3.37) | 0.433 | 2.07 | (− 1.06 to 5.20) | 0.194 |
| Stratified on pain level | ||||||||||
| MOBID-2 < 3 | 57 | 2.65 | (− 0.49 to 5.80) | 0.098 | 1.42 | (− 1.00 to 3.83) | 0.251 | 1.24 | (− 1.40 to 3.87) | 0.357 |
| MOBID-2 ≥ 3 | 103 | 2.25 | (− 0.55 to 5.04) | 0.115 | 0.47 | (− 1.98 to 2.91) | 0.709 | 1.78 | (− 1.31 to 4.88) | 0.260 |
| MOBID-2 ≥ 3 and paracetamol | 47 | 1.63 | (− 2.68 to 5.94) | 0.459 | − 0.38 | (− 4.51 to 3.76) | 0.858 | 2.01 | (− 2.52 to 6.53) | 0.385 |
| MOBID-2 ≥ 3 and buprenorphine | 61 | 2.19 | (− 1.35 to 5.73) | 0.226 | 1.32 | (− 1.87 to 4.51) | 0.418 | 0.87 | (− 2.84 to 4.58) | 0.646 |
C coefficient for time × treatment interaction, CI confidence interval, MMSE Mini-Mental State Examination, MOBID-2 Mobilisation-Observation-Behaviour-Intensity-Dementia-2 Pain Scale, N number of patients with at least one valid assessment, TDS transdermal system. See also the Electronic Supplementary Material 2, which reports all corresponding coefficients for change
Fig. 2.
Change in depressive symptoms (Cornell Scale for Depression in Dementia) throughout the study period. CI confidence interval, MMSE Mini-Mental State Examination, MOBID-2 Mobilisation-Observation-Behaviour-Intensity-Dementia-2 Pain Scale
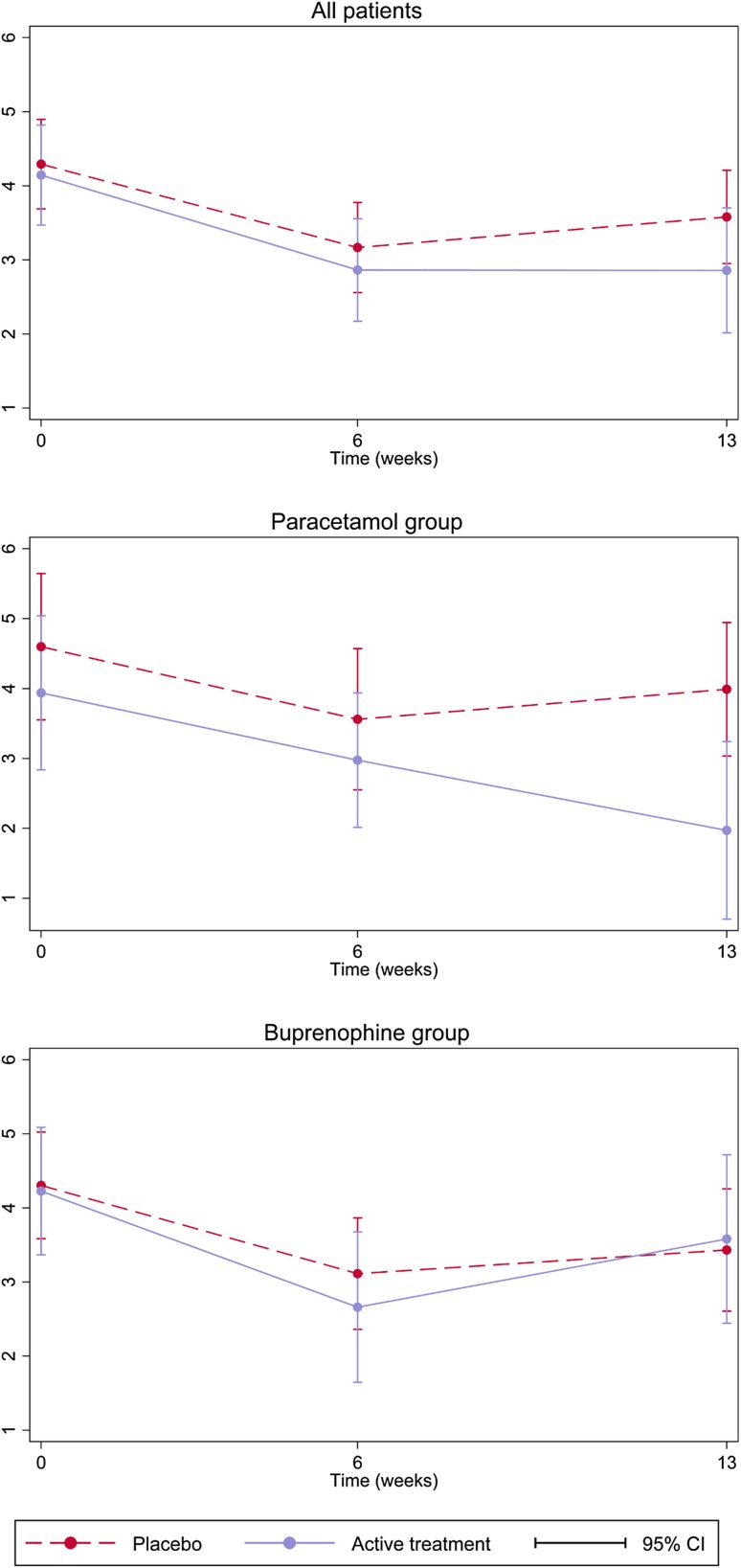
There was no significant reduction in pain in the combined active treatment group (paracetamol and buprenorphine) compared with placebo (Table 5, Fig. 3). Active paracetamol was associated with a significant decrease in pain from 6- to 13-week assessments compared with placebo tablets, with an estimated treatment effect of −1.11 (−2.16 to −0.06, p = 0.037). This effect was not observed for active buprenorphine [coefficient 0.26 (−1.06 to 1.59), p = 0.697].
Table 5.
Estimated effect of active analgesic treatment on secondary outcome [Mobilisation-Observation-Behaviour-Intensity-Dementia-2 Pain Scale (MOBID-2) pain intensity] compared with placebo; mixed-model analysis including exploratory subgroup analyses
| N | From baseline to 13 wk | From baseline to 6 wk | From 6 to 13 wk | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | (95% CI) | P value | C | (95% CI) | P value | C | (95% CI) | P value | ||
| Secondary analysis | ||||||||||
| All patients | 147 | − 0.19 | (− 1.02 to 0.64) | 0.652 | 0.19 | (− 0.59 to 0.97) | 0.634 | − 0.38 | (− 1.25 to 0.49) | 0.389 |
| Stratified on cognition | ||||||||||
| MMSE 11–20 | 44 | − 1.01 | (− 2.44 to 0.41) | 0.162 | 0.39 | (− 0.94 to 1.73) | 0.563 | − 1.41 | (− 2.83 to 0.01) | 0.051 |
| MMSE 0–10 | 87 | 0.12 | (− 1.02 to 1.26) | 0.838 | 0.03 | (− 1.05 to 1.11) | 0.960 | 0.09 | (− 1.13 to 1.32) | 0.884 |
| Stratified on drug type | ||||||||||
| Paracetamol/placebo tablets | 69 | − 0.98 | (− 2.00 to 0.05) | 0.061 | 0.14 | (− 0.83 to 1.10) | 0.779 | − 1.11 | (− 2.16 to −0.06) | 0.037 |
| Buprenorphine/placebo TDS | 78 | 0.47 | (− 0.77 to 1.71) | 0.456 | 0.21 | (− 0.98 to 1.39) | 0.733 | 0.26 | (− 1.06 to 1.59) | 0.697 |
| Stratified on pain level | ||||||||||
| MOBID-2 ≥ 3 | 90 | − 0.57 | (− 1.77 to 0.62) | 0.347 | −0.16 | (− 1.24 to 0.93) | 0.779 | − 0.42 | (− 1.63 to 0.79) | 0.498 |
| MOBID-2 ≥ 3 and paracetamol | 38 | − 1.36 | (− 3.27 to 0.55) | 0.164 | 0.07 | (− 1.67 to 1.82) | 0.933 | − 1.43 | (− 3.25 to 0.39) | 0.123 |
| MOBID-2 ≥ 3 and buprenorphine | 52 | 0.23 | (− 1.32 to 1.77) | 0.775 | −0.38 | (− 1.81 to 1.05) | 0.607 | 0.60 | (− 1.04 to 2.25) | 0.474 |
C coefficient for time × treatment interaction, CI confidence interval, CSDD Cornell Scale for Depression in Dementia, MMSE Mini-Mental State Examination, N number of patients with at least one valid assessment, TDS transdermal system. See also the Electronic Supplementary Material 3, which reports all corresponding coefficients for change
Fig. 3.
Change in pain intensity [Mobilisation-Observation-Behaviour-Intensity-Dementia-2 Pain Scale (MOBID-2)] throughout the study period in patients with moderate-to-severe pain at baseline (MOBID-2 ≥ 3). CI confidence interval
Discussion
This is the first placebo-controlled study investigating the efficacy of analgesic treatment for depressive symptoms in people with moderate-to-severe cognitive impairment and dementia. We have found that a stepwise increase of analgesic treatment, using either paracetamol tablets or buprenorphine TDS, was not effective as a means of reducing depressive symptoms in these patients. Contrary to our initial hypothesis, we found that the placebo group had a significant decrease in depressive symptoms from baseline to the 13-week follow-up compared with the active treatment group. We did not find an overall benefit of active treatment on pain compared with placebo, but paracetamol reduced pain significantly from 6 to 13 weeks compared with placebo tablets (Table 5). Despite this, depressive symptoms did not decrease in the same group (Table 4).
While our results appear to indicate the reverse effect: a significant decrease in depressive symptoms in the placebo group compared with the active treatment group, this result must be interpreted with caution for several reasons. This study includes people with severe dementia, in whom symptoms of both pain and depression are difficult to assess. We excluded patients in whom severe pain (MOBID-2 ≥ 8) was identified because it would be unethical to risk prolonged untreated pain by randomising these patients to receive active treatment or placebo, and recommended instead that the responsible physician should initiate appropriate analgesic treatment.
Therefore, our results may not be generalisable to nursing home patients with dementia and severe pain. Most of the included patients were unable to self-report pain reliably because of advanced cognitive impairment. Although proxy-rated pain is the best available pain assessment method in this group, we have no method to ascertain the patients’ subjective pain experience. In patients with very limited verbal and non-verbal expression, pain intensity may be underestimated by proxy rating. Our initial hypothesis was therefore that undiagnosed and therefore untreated painful symptoms may cause exacerbated depressive symptoms in people with advanced dementia.
The CSDD scale has been developed for use in people with dementia, and has shown good sensitivity and specificity. However, as noted in a recent systematic review and meta-analysis, most studies that have tested the scale have excluded people with severe dementia or communication deficits, thus limiting the majority of the evidence to people with mild-to-moderate dementia [24]. In cognitively intact populations, the efficacy of pharmaceutical therapies for both depression and pain is difficult to isolate from expectation effects, including both placebo and nocebo effects [27]. Although people with advanced dementia may have a diminished or absent placebo response [28], the proxy raters are prone to observer bias such as the Hawthorne effect, which could potentially skew the observed difference between the treatment groups. Furthermore, we did not assess raters’ expectation of group allocation, a factor that has been shown to interfere strongly with observed effects in placebo-controlled trials [27]. As shown in the first graph of Fig. 2, all patients had a trend towards decreasing severity of depressive symptoms from baseline to the 6-week follow-up. Similarly, Fig. 3 shows that pain tended to decrease from baseline to the 6-week follow-up, regardless of group allocation. This initial improvement across all groups exaggerates the apparent benefit of placebo on depressive symptoms, and may be caused by observer bias. Similar trends have been shown in other studies [5, 16].
The high dropout rate observed in the group receiving active buprenorphine may reduce comparability between active treatment and placebo conditions, but represents an important finding as it suggests lower than expected tolerability in this population, which warrants further investigation. In active treatment, only 44 of the 66 planned for in the final power analysis completed 13-week assessments. This may further limit our ability to detect a positive effect of treatment compared with placebo. However, our data are significantly in favour of the placebo condition (p = 0.013), probably because the obtained mean CSDD difference of 2.64 at 13 weeks was larger than the threshold for a clinically relevant difference of 2.0 (standardized effect size 0.4) used in the power analysis. This means that the sample size was sufficient to explore our primary aim, and may indicate that adverse effects of active treatment led to apparent worsening of depressive symptoms. Known adverse effects of buprenorphine include symptoms such as sedation, reduced appetite and anxiety, which may overlap with items assessed by the CSDD scale and possibly be interpreted as increased depression. Secondary analyses, in which patients were grouped based on the presence of moderate-to-severe pain, cognitive status and choice of analgesic treatment all show a similar trend in favour of the placebo condition, although these associations did not reach significance, probably because the sample size did not provide sufficient power for subgroup analyses.
An important limitation to the interpretation of our results is therefore that we do not have a sufficient sample size to determine whether there was a significant differential effect between paracetamol and buprenorphine on depressive symptoms. Furthermore, the extensive list of exclusion criteria was necessary to include this frail population in the current trial, but also limits the generalisability of our results to a more heterogeneous group of nursing home patients. A recent study found that patients with depression were more likely to be prescribed analgesic treatments [29]. This means that an unknown proportion of patients who theoretically may have benefited from the intervention were excluded from our study: 562 patients (38% of the 2323 patients screened) were excluded because of opioid analgesic use, without any further assessments of eligibility. This choice was made intentionally to assess treatment effects in patients who were not already using high doses of the study drugs, and in whom untreated pain was not identified as a primary clinical issue.
Several previous studies have suggested that depression in nursing home patients with cognitive impairment may be related to untreated pain. The association between pain and depression, also known as the pain-depression dyad, has been observed in nursing home patients at all stages of cognitive impairment [13, 14]. Secondary analyses from a previous cluster-randomised study, which assessed the efficacy of a stepwise increase in analgesic treatment for depressive symptoms in 175 nursing home patients with dementia and agitation, found a significant but small benefit on the mood syndrome cluster assessed with the Neuropsychiatric Inventory – Nursing Home version [16]. They included patients with agitation, whereas in our study depression was an inclusion criterion. Furthermore, a higher proportion of patients were allocated to receive paracetamol relative to our study [120 (69%) and 36 (45%), respectively]. They had an open-label design with the control group receiving usual care, consequently their results may have been biased owing to a Hawthorne effect. These methodological differences may in part explain our apparently opposing result.
Nonetheless, our rigorous placebo-controlled design justifies our conclusion that analgesic treatment alone is not sufficient to improve depressive symptoms in nursing home patients with dementia and depression in the absence of severe pain. By excluding patients with severe pain from the trial, we may have limited the potential to find beneficial effects of analgesic treatment for depression. However, subgroup analyses stratified on pain level did not indicate that patients with moderate-to-severe pain had a more beneficial effect of active treatment on depressive symptoms. Although the group that received active paracetamol had a significant decrease in pain compared with those who received placebo tablets, there was a trend towards more persistent depressive symptoms in this group during the same period. While the latter result was not statistically significant, it indicates that the negative result on the main outcome of the current trial cannot be explained by the absence of pain at baseline.
Importantly, no clear causal relationship between pain and increased depression, or between depression and increased pain, has been established. Pain and depression are known to mutually exacerbate each other, a relationship that may be most accurately characterised as multifactorial. Although many nursing home patients with depression have comorbid chronic pain, other associated problems such as isolation and lack of social contact or meaningful activity may be equally important [30]. In this perspective, it may not be surprising that an isolated pain intervention is insufficient to improve depressive symptoms. Rather, our results show that careful assessment of painful symptoms, followed by the implementation and continuous re-evaluation of appropriate interventions, is an absolute requirement for adequate care in this population, as both untreated pain and use of unnecessary analgesics may lead to harm. Patients with cognitive impairment are particularly susceptible to the adverse effects of analgesics and antidepressants, and may be unable to communicate verbally the severity of their symptoms. This makes it particularly challenging to ensure that the benefit of pharmacological treatment outweighs any potential harm.
A 2011 study found that physicians in Norwegian nursing homes rarely diagnosed depression before prescribing antidepressants, and that treatment with antidepressants often was continued despite great uncertainty of their effectiveness [31]. Forty percent of nursing home patients in Norway use antidepressants [32]. This is in line with the pooled percentage of antidepressant use in Western European nursing homes [1], and indicates that the need for improved prescribing practice is not exclusive to Norway. Future advances should go towards more comprehensive treatment strategies that include both pharmacological and non-pharmacological interventions, as exemplified by Chen and Lin [33]. Non-pharmacological interventions that have been shown to reduce depressive symptoms in dementia include caregiver education and engagement in physical activity and pleasant events, but more evidence is needed to determine which strategies are most effective [34, 35].
Buprenorphine elicits its pharmacological effects on the opioidergic system, but has previously been suggested as a potential agent for treatment-resistant depression as some patients have had promising results [18]. However, based on the high rate of adverse events and absence of benefit on depressive symptoms, it is unlikely that buprenorphine has any potential as a treatment for depression in nursing home patients with dementia. The efficacy and tolerability of buprenorphine TDS have not previously been investigated in people with dementia in a placebo-controlled study. Buprenorphine has similar pharmacokinetic properties and does not require dose adjustment in the elderly compared with younger patients [36]. In a study comparing healthy elderly people aged ≥ 75 years to those aged 50–60 years, buprenorphine TDS was found to have a slightly lower steady-state concentration with higher variability in the elderly group [37]. The same study found a lower rate of adverse events in the elderly subjects compared with the younger controls [37].
In the current study, the dropout rate owing to adverse events of buprenorphine exceeded that reported in a previous study of buprenorphine in patients with dementia [16], and is more than twice that reported in a study of opioid-naïve, cognitively intact elderly patients (aged ≥ 75 years), which found that 21% dropped out because of adverse events of buprenorphine [38]. This suggests that people with dementia may be more susceptible to adverse events of buprenorphine compared with elderly patients without cognitive impairment.
Because few large-scale safety studies of opioid analgesics in elderly patients exist, and none have included people with advanced dementia, we do not know whether this may represent a class effect of opioid analgesics or whether buprenorphine may be more poorly tolerated in frail elderly people and people with dementia compared with other opioid analgesics. In light of the widespread use of buprenorphine TDS and other opioid analgesics in nursing home patients, particularly in the oldest patients [39, 40], there is an urgent need for high-powered studies investigating the safety and efficacy of buprenorphine and other opioid analgesics for treating pain in people with advanced dementia.
Conclusion
Analgesic treatment did not reduce depression in patients with cognitive impairment and depressive symptoms. Patients who received active treatment had more persistent depressive symptoms than those who received placebo, possibly owing to adverse effects. These results point to the importance of continuous symptom assessment when caring for people with dementia, ensuring that analgesics are given based on the correct indications with a minimal risk of harm, and using both pharmacological and non-pharmacological interventions as appropriate. Active buprenorphine was associated with high rates of adverse events, and should be prescribed cautiously in people with dementia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients, their relatives and the nursing home staff for their willingness and motivation that made this work possible. Bettina S. Husebo would also like to thank the G.C. Rieber Foundation and the Norwegian Directorate of Health for supporting the authors’ work at the Centre for Elderly and Nursing Home Medicine, University of Bergen, Norway. Parts of the work were carried out at the Biostatistics and Data analysis core facility (BIOS), University of Bergen.
Funding
The DEP.PAIN.DEM trial and Ane Erdal and Elisabeth Flo are funded by the Research Council of Norway (sponsor’s protocol code 221951). The DEP.PAIN.DEM trial has received a grant from the University of Bergen. Mundipharma Research Limited, UK provided study treatment.
Author Contributions
All authors contributed significantly to the development of the study design, setting the aims, drafting the manuscript, and finalising this work.
Conflict of interest
Clive Ballard has received consultancy honoraria from Acadia, Lundbeck, Heptares, Roche, Lilly, Otsuka, GSK, Pfizer and Synexus; speaker fees from Lundbeck, Lilly and Otsuka; and grant support from Acadia Pharmaceuticals 2014–2017. Ane Erdal, Elisabeth Flo, Dag Aarsland, Dagrun D. Slettebo and Bettina S. Husebo have no conflicts of interest directly relevant to the content of this article. The sponsors had no influence on the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Prior to enrolment, the trial was registered in ClinicalTrials.gov (NCT02267057), and was approved by the Norwegian Medicines Agency (EudraCT 2013-002226-23) and the Regional Committee for Medical and Health Research Ethics (REC-West 2013/1474).
Consent to participate
Informed consent was obtained from all individual participants included in the study. Verbal and written informed consent was obtained in direct conversation with all patients who were deemed to have medical decision-making capacity. If participants did not have the capacity to give consent, the participant’s next of kin or legal guardian provided informed consent in accordance with ethics committee requirements and Norwegian legislation at the time of the study. We expected that patients with Mini-Mental State Examination scores ≥ 16 would be able to give informed consent [41], but nevertheless we included the closest relatives of all patients in a discussion about consent and provided written information about the trial to ensure full transparency. To empower those patients with a reduced ability to consent, we attempted to adjust the information procedure to enable them to understand the purpose and implications of study participation. We included a verbal and written statement assuring that their decision to give consent would not affect the quality of the medical care provided to the patient. Even though informed consent had been given, all participants were free to decline drug administration and other procedures at any time during the trial, irrespective of cognitive state.
References
- 1.Janus SI, van Manen JG. IJzerman MJ, Zuidema SU. Psychotropic drug prescriptions in Western European nursing homes. Int Psychogeriatr. 2016;28(11):1775–1790. doi: 10.1017/S1041610216001150. [DOI] [PubMed] [Google Scholar]
- 2.Helvik AS, Engedal K, Benth JS, Selbaek G. Prevalence and severity of dementia in nursing home residents. Dement Geriatr Cogn Disord. 2015;40(3–4):166–177. doi: 10.1159/000433525. [DOI] [PubMed] [Google Scholar]
- 3.Bergh S, Selbaek G, Engedal K. Discontinuation of antidepressants in people with dementia and neuropsychiatric symptoms (DESEP study): double blind, randomised, parallel group, placebo controlled trial. BMJ. 2012;344:e1566. doi: 10.1136/bmj.e1566. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, Munro C, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60(7):737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):403–411. doi: 10.1016/S0140-6736(11)60830-1. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, Weintraub D, et al. Sertraline for the treatment of depression in Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):136–145. doi: 10.1097/JGP.0b013e3181c796eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabins PV, Blacker D, Rovner BW, Rummans T, Schneider LS, Tariot PN, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry. 2007;164(12 Suppl.):5–56. [PubMed] [Google Scholar]
- 8.Farina N, Morrell L, Banerjee S. What is the therapeutic value of antidepressants in dementia? A narrative review. Int J Geriatr Psychiatry. 2017;32(1):32–49. doi: 10.1002/gps.4566. [DOI] [PubMed] [Google Scholar]
- 9.Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc. 2011;59(4):577–585. doi: 10.1111/j.1532-5415.2011.03355.x. [DOI] [PubMed] [Google Scholar]
- 10.Sandvik RK, Selbaek G, Seifert R, Aarsland D, Ballard C, Corbett A, et al. Impact of a stepwise protocol for treating pain on pain intensity in nursing home patients with dementia: a cluster randomized trial. Eur J Pain. 2014;18(10):1490–1500. doi: 10.1002/ejp.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achterberg WP, Gambassi G, Finne-Soveri H, Liperoti R, Noro A, Frijters DH, et al. Pain in European long-term care facilities: cross-national study in Finland. Italy and The Netherlands. Pain. 2010;148(1):70–74. doi: 10.1016/j.pain.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Hadjistavropoulos T, Herr K, Prkachin KM, Craig KD, Gibson SJ, Lukas A, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216–1227. doi: 10.1016/S1474-4422(14)70103-6. [DOI] [PubMed] [Google Scholar]
- 13.Erdal A, Flo E, Selbaek G, Aarsland D, Bergh S, Slettebo DD, et al. Associations between pain and depression in nursing home patients at different stages of dementia. J Affect Disord. 2017;218:8–14. doi: 10.1016/j.jad.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 14.Leong IY, Nuo TH. Prevalence of pain in nursing home residents with different cognitive and communicative abilities. Clin J Pain. 2007;23(2):119–127. doi: 10.1097/01.ajp.0000210951.01503.3b. [DOI] [PubMed] [Google Scholar]
- 15.Chopra K, Arora V. An intricate relationship between pain and depression: clinical correlates, coactivation factors and therapeutic targets. Expert Opin Ther Targets. 2014;18(2):159–176. doi: 10.1517/14728222.2014.855720. [DOI] [PubMed] [Google Scholar]
- 16.Husebo BS, Ballard C, Fritze F, Sandvik RK, Aarsland D. Efficacy of pain treatment on mood syndrome in patients with dementia: a randomized clinical trial. Int J Geriatr Psychiatry. 2014;29(8):828–836. doi: 10.1002/gps.4063. [DOI] [PubMed] [Google Scholar]
- 17.van Ojik AL, Jansen PA, Brouwers JR, van Roon EN. Treatment of chronic pain in older people: evidence-based choice of strong-acting opioids. Drugs Aging. 2012;29(8):615–625. doi: 10.1007/BF03262278. [DOI] [PubMed] [Google Scholar]
- 18.Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014;75(8):e785–e793. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durso GR, Luttrell A, Way BM. Over-the-counter relief from pains and pleasures alike: acetaminophen blunts evaluation sensitivity to both negative and positive stimuli. Psychol Sci. 2015;26(6):750–758. doi: 10.1177/0956797615570366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 22.Husebo BS, Ostelo R, Strand LI. The MOBID-2 pain scale: reliability and responsiveness to pain in patients with dementia. Eur J Pain. 2014;18(10):1419–1430. doi: 10.1002/ejp.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/WNL.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 24.Goodarzi ZS, Mele BS, Roberts DJ, Holroyd-Leduc J. Depression case finding in individuals with dementia: a systematic review and meta-analysis. J Am Geriatr Soc. 2017;65(5):937–948. doi: 10.1111/jgs.14713. [DOI] [PubMed] [Google Scholar]
- 25.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14(2):139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 26.Twisk JWR. Applied longitudinal data analysis for epidemiology: a practical guide. 2. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 27.Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol. 2016;15(7):736–747. doi: 10.1016/S1474-4422(16)00066-1. [DOI] [PubMed] [Google Scholar]
- 28.Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, et al. Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain. 2006;121(1–2):133–144. doi: 10.1016/j.pain.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Gilmartin JF, Vaatainen S, Tormalehto S, Bell JS, Lonnroos E, Salo L, et al. Depressive symptoms are associated with analgesic use in people with Alzheimer’s disease: Kuopio ALSOVA study. PloS One. 2015;10(2):e0117926. doi: 10.1371/journal.pone.0117926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snowden M, Sato K, Roy-Byrne P. Assessment and treatment of nursing home residents with depression or behavioral symptoms associated with dementia: a review of the literature. J Am Geriatr Soc. 2003;51(9):1305–1317. doi: 10.1046/j.1532-5415.2003.51417.x. [DOI] [PubMed] [Google Scholar]
- 31.Iden KR, Hjorleifsson S, Ruths S. Treatment decisions on antidepressants in nursing homes: a qualitative study. Scand J Prim Health Care. 2011;29(4):252–256. doi: 10.3109/02813432.2011.628240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulla C, Selbaek G, Flo E, Kjome R, Kirkevold O, Husebo BS. Multi-psychotropic drug prescription and the association to neuropsychiatric symptoms in three Norwegian nursing home cohorts between 2004 and 2011. BMC Geriatr. 2016;16(1):115. doi: 10.1186/s12877-016-0287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YH, Lin LC. Ability of the pain recognition and treatment (PRT) protocol to reduce expressions of pain among institutionalized residents with dementia: a cluster randomized controlled trial. Pain Manag Nurs. 2016;17(1):14–24. doi: 10.1016/j.pmn.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kress HG. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain. 2009;13(3):219–230. doi: 10.1016/j.ejpain.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Al-Tawil N, Odar-Cederlof I, Berggren AC, Johnson HE, Persson J. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143–149. doi: 10.1007/s00228-012-1320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karlsson J, Soderstrom A, Augustini BG, Berggren AC. Is buprenorphine transdermal patch equally safe and effective in younger and elderly patients with osteoarthritis-related pain? Results of an age-group controlled study. Curr Med Res Opin. 2014;30(4):575–587. doi: 10.1185/03007995.2013.873714. [DOI] [PubMed] [Google Scholar]
- 39.Sandvik R, Selbaek G, Kirkevold O, Husebo BS, Aarsland D. Analgesic prescribing patterns in Norwegian nursing homes from 2000 to 2011: trend analyses of four data samples. Age Ageing. 2016;45(1):54–60. doi: 10.1093/ageing/afv184. [DOI] [PubMed] [Google Scholar]
- 40.Jensen-Dahm C, Gasse C, Astrup A, Mortensen PB, Waldemar G. Frequent use of opioids in patients with dementia and nursing home residents: a study of the entire elderly population of Denmark. Alzheimers Dement. 2015;11(6):691–699. doi: 10.1016/j.jalz.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Etchells E, Darzins P, Silberfeld M, Singer PA, McKenny J, Naglie G, et al. Assessment of patient capacity to consent to treatment. J Gen Intern Med. 1999;14(1):27–34. doi: 10.1046/j.1525-1497.1999.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.