Abstract
Effective screening instruments are necessary for evaluating the outcomes of early interventions for the prevention or delay of disability in older persons. This study examined how transitions in frailty items over 1 year and the baseline components of a comprehensive geriatric assessment were associated with improvements in frailty at a 2-year follow-up in a sample of older patients.
This was a single-center prospective observational study of older patients aged 65 years and over with chronic diseases (n = 103), who were followed through a hospital-based program over 2 years. Frailty was evaluated via the modified Fried Frailty Index and a comprehensive geriatric assessment.
We noted significant improvements in weight loss (P = .016) and self-reported exhaustion (P = .006), and a less decrease in grip strength (P = .009) at the 1-year follow-up. Furthermore, baseline cerebral vascular accident diagnosis (P = .022), high polypharmacy (P = .037), a higher Geriatric Depression Scale score (P = .033), and a lower Mini Nutritional Assessment score (P = .039) were significantly associated with improved frailty at the 2-year follow-up. Furthermore, improvement in self-reported exhaustion (odds ratio [OR]: 4.7, 1.4–16.1, P = .014) and physical activity (OR: 3.8, 1.0–13.7, P = .046), and a less decrease in grip strength (OR: 4.0, 1.3–12.5, P = .017) at the 1-year follow-up were significantly associated with improved frailty at the 2-year follow-up.
Self-reported exhaustion, physical activity, and grip strength are easy, quick, and feasible screening tests for improvements in frailty in clinical practice.
Keywords: elders, exhaustion, frailty transition, grip strength, physical activity
1. Introduction
Frailty is a geriatric syndrome associated with decreased physiologic reserve, functional decline, and increased vulnerability to stressors.[1] Numerous studies have demonstrated that frailty is a major contributor to adverse health outcomes, dependency, institutionalization, and mortality in older people.[2,3] Randomized controlled trials of exercise, comprehensive geriatric assessments (CGA), and interventions targeting multiple risk factors have shown promising, albeit preliminary, results.[4–6] Frail individuals who are not yet disabled and those in the early stages of disability with a high risk of progression are the most likely to benefit from these interventions.[7–10] In order to determine whether these interventions are useful for preventing or delaying disability in older persons at an early stage, clinical staff must employ effective screening instruments for the relevant intervention outcomes.
A systematic review conducted in 2011 found that the Frailty Index (FI) is potentially the most suitable instrument for evaluating the outcomes of frailty interventions in this research field.[11] However, few studies have examined the predictors of improvements in frailty status over time. Identifying these frailty-reversing factors is exceedingly important for frailty prevention and management. Therefore, in the present study, we determined the factors associated with improvements in frailty over a 2-year period, with a focus on changes in frailty items over 1 year and the components of a CGA.
2. Methods
2.1. Study design and participants
This was a prospective observational study conducted in a single medical center from January 2007 to June 2009. Participants were older patients aged 65 years and over who had a diagnosed chronic disease that was being followed by their family physician or geriatrician. Before recruitment, patients’ physicians had experienced study nurses assess patients’ eligibility for participation via a structured CGA. The CGA is a multidimensional and multidisciplinary diagnostic process that aims to determine the clinical profile, pathological risk, residual skills, short- and long-term prognosis, and personalized therapeutic and care plan of individuals who are functionally compromised or frail.[12] The study inclusion criteria were as follows[13]: functional decline (as measured by new disabilities in activities of daily living [ADL] or instrumental ADL [IADL]); a clinical diagnosis of depression or dementia; mobility impairment; ≥1 fall in the past year; weight loss of >5% per year; multiple comorbidities (≥5 diseases); polypharmacy (≥8 classes of drugs per day); multiple specialty physician visits in the past 6 months (≥3 different specialties with ≥2 visits for each specialty); hospitalization in the past year (≥1); frequent emergency room visits in the past year (≥2); and age above 80 years. Patients who were bedridden, in a nursing home, had an expected life expectancy of <6 months, or had a severe hearing or communication disorder were excluded.
2.2. Measurements
All measures were performed every year at 3 time points over 2 years. Experienced study nurses collected the CGA data using a structured questionnaire containing items on demographic characteristics, diagnosed diseases, smoking and drinking habits, current medications, blood pressure, body mass index (BMI), and geriatric syndromes. Geriatric syndromes are highly prevalent, multifactorial conditions such as delirium, falls, incontinence, and frailty, that are associated with substantial morbidity and poor outcomes.[14] The FI was determined using a modification of Fried criteria.[2] We defined transitions in frailty status according to all items in the modified Fried Frailty Index (FFI) as follows.[2,15]
“Weight loss” was defined as self-reported unintentional weight loss of >3 kg (adjusted from 5 kg to account for Chinese body build)[15,16] or of >5% of the total body weight in the past year.[17] At the 1-year follow-up, we defined transitions in “weight loss” as follows: “improved weight loss” included patients who exhibited weight loss at baseline but not at the 1-year follow-up, and “stable or new onset weight loss” included patients who did not have weight loss at either baseline or the 1-year follow-up assessments or weight loss at the 1-year follow-up (regardless of weight loss at the first assessment).
“Exhaustion” was defined as a positive answer to either of the following statements from the Center for Epidemiologic Studies Depression Scale (CES-D)[18]: “I felt that everything I did was an effort” or “I could not get going.” Patients with positive responses were also asked, “How often in the last week did you feel this way?”, with responses of 0 = rarely or none of the time (<1 day), 1 = some or a little of the time (1–2 days), 2 = a moderate amount of the time (3–4 days), or 3 = most of the time. Participants that answered with “2” or “3” to either of these questions were categorized as “frail” according to this exhaustion criterion. We defined the severity of exhaustion as the sum of these item scores. At the 1-year follow-up, transitions in “exhaustion” were defined as follows: “improved self-reported exhaustion” included patients who showed a change in the total score of <0 at the 1-year follow-up compared with baseline, “stable or worsened self-reported exhaustion” included patients who showed no substantial change in the total score or who showed a change in the total score of more than 0 at 2 assessment points.
“Low physical activity” was defined as low weekly sex-specific energy expenditures according to the Taiwan International Physical Activity Questionnaire–Short Form (IPAQ-SF),[19] instead of the Minnesota Leisure Time Physical Activity Questionnaire.[20] The optimal cutoff point for the transition in physical activity was determined via examination of the receiver operating characteristic (ROC) curve. “Improved physical activity” was defined as an increase of >40% in total Kcals spent per week at the 1-year follow-up compared with baseline. In contrast, “stable or worsening physical activity” was defined as an increase of <40% or a decrease in total Kcals spent per week between these assessment points.
“Slow walking speed” was defined as slow 5-m walking time according to the criteria of the Cardiovascular Health Study Group.[2] According to a study published in 2007,[21] an improvement in gait speed of 0.1 m/s over 1 year predicts better survival in older adults. We, therefore, used this same definition to define transitions in walking speed: the “improved walking speed” group demonstrated such an improvement, while the “stable or worsening walking speed” group showed an improvement of <0.1 m/s or a decrease over 1 year.
“Weak grip strength” was defined as a mean grip strength on the dominant hand 3 times below the criterion-specific thresholds for their sex and BMI; grip strength was determined via a Jamar hand-held dynamometer (Asimow Engineering Co., Los Angeles, California).[2] We examined the optimal cut-off for transition by examining the ROC curve. According to this analysis, “less decreased grip strength” was defined as a decrease of <20% in total kg of grip strength at the 1-year follow-up compared with baseline, while “stable or worsening grip strength” was defined as a decrease of more than this.
We evaluated the modified FFI at baseline and 2-year follow-up. The severity of frailty was defined as the total number of frailty items for which participants met the stated criteria. Specifically, participants were classified into “robust,” “pre-frail,” and “frail” groups if they had 0, 1 to 2, and 3 to 5 items, respectively.[2] Transition in “frailty” over the 2-year period was defined as follows: “improved frailty” included patients showed a decrease of more than 1 point for their modified FFI score at the 2-year follow-up compared with baseline, and “stable or worsening frailty” included patients whose score remained the same or increased by >1 point over this same period. When both the baseline and 2-year follow-up modified FFI scores were in the robust category, the improvement was limited, indicating that this group may have had better baseline data than the other groups. Therefore, this group was excluded to decrease potential bias from the data.
The associations between the transition in each item of the modified FFI at the 1-year follow-up, the baseline components of a CGA, and the transition in frailty at the 2-year follow-up were examined. The instruments used to evaluate patient functioning were as follows: the Barthel Index of Activities of Daily Living (ADL)[22]; the Lawton and Brody Instrumental Activities of Daily Living (IADL) scale[23]; a dementia screener, the Mini-Mental State Examination (MMSE)[24]; a depression screener, the Geriatric Depression Scale-15 (GDS-15)[25,26]; and a nutrition screener, the Mini Nutritional Assessment (MNA).[27]
2.3. Statistical analysis
Descriptive statistics were summarized as frequencies (percentages) for categorical variables and means with standard deviations for continuous variables. Chi-square tests were conducted to examine the univariate associations. To examine factors associated with frailty transitions, we performed a logistic regression analysis for the factors found to be significant in the chi-square test, while controlling for all other factors. We also used logistic regression to examine how the transition in each item of the modified FFI at the 1-year follow-up was associated with transition in frailty at the 2-year follow-up. A probability of <5% (P < .05) was considered significant. All data were analyzed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL).
2.4. Ethical considerations
The research plan of the study was approved by the institutional review boards of the National Health Research Institutes and National Taiwan University Hospital. Participants that met the inclusion criteria were asked to give their written permission to participate and received evaluation after their enrollment.
3. Results
In total, 189 patients were enrolled in the study. Of these, only 124 (65.6%) completed the study, with 65 (34.4%) dropping out by the 2-year follow-up. Twenty-four (12.7%) of these 65 voluntarily withdrew from the study, 23 (17.5%) were lost to follow-up, 2 (1.1%) moved to a nursing home, 12 (6.3%) died, and 4 (2.1%) could not complete the modified FFI. Only 103 patients were ultimately analyzed because 21 (11.1%) exhibited a robust status at both baseline and the 2-year follow-up, and were thus excluded. Of the 103 pre-frail or frail patients, 26 (25.2%) were in the “improved frailty” group and 77 (74.8%) in the “stable or worsening frailty” group.
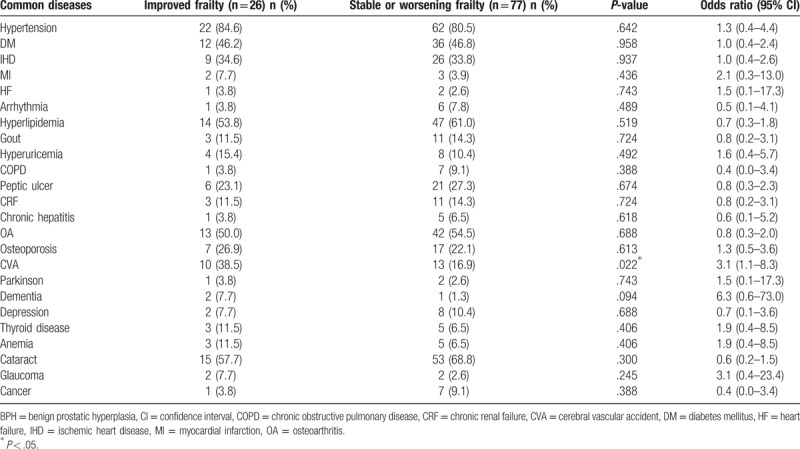
Regarding the baseline demographics and geriatric syndromes, these 2 groups did not significantly differ in age, sex, education, body weight (BW), BMI, number of chronic diseases, Barthel index, IADL score, or MMSE score (Table 1). Compared with the stable or worsening frailty group, a significantly greater proportion of the improved frailty group had polypharmacy (≥8 medicines), and they tended to have a higher GDS score (≥5)[28] and a lower MNA score (≤23.5).[29,30] A significantly greater proportion of the improved frailty group had cerebrovascular accidents (CVA) at baseline compared with the stable or worsening frailty group. There were no other differences in common diseases at baseline between the groups (Table 2).
Table 1.
The demographic characteristics and geriatric syndromes of the study population according to frailty transitions over 2 years.
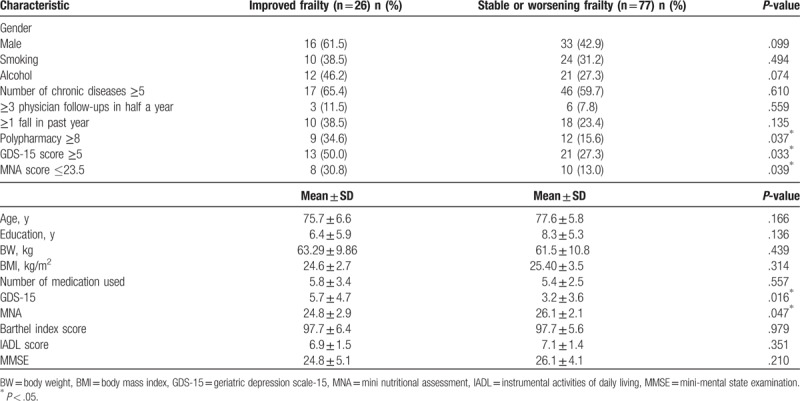
Table 2.
Common diseases among the study population according to frailty transitions over 2 years.
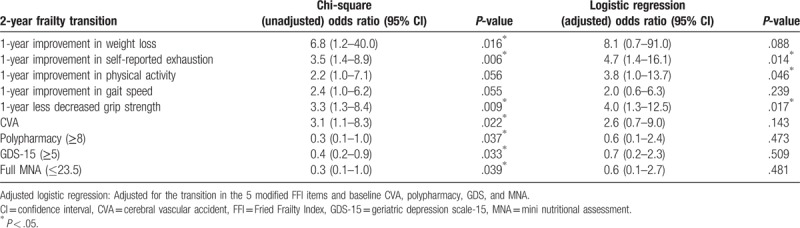
A chi-square test was used to examine the associations of the transition in frailty at the 2-year follow-up with the transitions in the 5 modified FFI items at the 1-year follow-up, as well as baseline CVA diagnosis, polypharmacy, GDS-15 score ≥5, and MNA score ≤23.5. We found that improvement in weight loss (P = .016) and self-reported exhaustion (P = .006), and a less decreased grip strength (P = .009) at the 1-year follow-up were associated with improved frailty at the 2-year follow-up, as were baseline CVA diagnosis (P = .022), polypharmacy (P = .037), higher GDS-15 score (≥5) (P = .033), and lower MNA scores (≤23.5) (P = .039) (Table 3). The multivariate logistic regression analysis, after adjusting for these predictors, showed that only improvement in self-reported exhaustion (odds ratio: 4.7, 1.4–16.1, P = .014), physical activity (odds ratio: 3.8, 1.0–13.7, P = .046), and less decreased grip strength (odds ratio: 4.0, 1.3–12.5, P = .017) at the 1-year follow-up had significant associations with improved frailty at the 2-year follow-up (Table 3).
Table 3.
Two-year frailty transition and its associations with 1-year improvement in the modified Fried Frailty Index (FFI) items, specific diseases, and geriatric syndromes.
4. Discussion
Transitions in frailty have been discussed relatively infrequently in previous studies.[31–34] This study is believed to be the first to examine whether geriatric syndromes and transitions in the modified FFI items are predictors of changes in frailty. The results showed that only improved self-reported exhaustion, increased physical activity, and less decreased grip strength over 1 year were significantly associated with improvements in frailty at the 2-year follow-up.
These results differed from those of a previous study, wherein transitions in self-reported questionnaires including the Medical Outcomes Study 36-item Short Form Health Survey, EuroQol scale, and the basic and instrumental ADL scales from the National Health Interview Survey over 1 year showed no significant associations with mortality at an 8-year follow-up.[21] “Self-reported exhaustion” in this study was defined using 2 items from the CES-D scale[2,18] relating to the somatic domain.[35] Furthermore, items on the GDS focus less on the somatic symptoms of depression and more on the mood symptoms.[36] Feeling exhausted or declining physical function might be the first signs of a poor prognosis, rather than depressed mood or functional disability, in the robust and pre-frail groups in our study. However, depression is highly prevalent among Chinese frail elderly.[37] A previous longitudinal population-based study in 2017 hypothesized that depressive symptoms serve as moderators of the relationship of dementia and all-cause mortality with frailty.[38] In our study, a higher GDS-15 score (≥5) at baseline was significantly associated with improvements in frailty in the chi-square test, but were no longer significant after adjusting for other variables in the multivariate logistic regression. Therefore, early interventions for depression provided by geriatric assessment teams might help reduce frailty transition; however, further studies are still needed to clarify the relationship between depression and frailty transition.
Hand grip strength or muscle strength is a significant predictor of physical disability, functional impairment, and mortality.[39–42] However, a previous systematic review showed that, of all physical frailty indicators, handgrip strength was a less powerful predictor of ADL disability than was slow gait speed and low physical activity in community-dwelling elderly.[43] Another study also revealed that improvements in usual gait speed (specifically, an increase of 0.1 m/s over 1 year) predicts a substantial reduction in mortality[21] at an 8-year follow-up. In the present study, a less decreased grip strength and increased physical activity at 1 year, but not improved gait speed, had significant associations with improvement in frailty at the 2-year follow-up. This suggests that changes in handgrip strength and increases in physical activity are more sensitive, or at least earlier, predictors of frailty when compared with gait speed. Additionally, handgrip strength is an easier, quicker, and more feasible screening test for use in clinical practice, as it can even be performed by patients with osteoarthritis, diabetes, and cognitive function impairment.[39,42] Therefore, handgrip strength should be checked regularly at clinics as part of the early screening of patients at high risk of frailty.
A 2001 review paper revealed that minimal adherence to current physical activity guidelines — that is, an energy expenditure of approximately 1000 kcal per week (4200 kJ per week) was associated with a significant reduction of about 20% to 30% in risk of all-cause mortality.[44] In our study, physical activity was defined as energy expenditure (kcal) resulting from physical activity per week, rather than as engagement in specific activities. We found that an increase in energy expenditure (compared with baseline) of >40% of the total kcal per week at the 1-year follow-up was significantly associated with improvements in frailty at the 2-year follow-up. This finding suggests that frailty can improve so long as elders increase their total energy expenditure per week, regardless of whether the intensity of the physical activity is light (i.e., doing laundry), moderate (i.e., walking for exercise), or vigorous (i.e., dancing). Therefore, a simple lifestyle modification goal can be provided for the elderly and their families. However, further studies are needed to clarify the relationships of the intensity, duration, and frequency of physical activity with frailty.
Regarding the weight loss item, individuals with “stable or new onset weight loss” were included in the same (a heterogeneous) group because we wanted to assess the contribution of improvement in weight loss to changes in frailty. However, improvements in weight loss did not appear to benefit transition in frailty status, possibly because these improvements were reduced by patients’ previous frailty status.
Previous stroke and lower cognitive function have shown associations with worsening or lower improvement in frailty among community-dwelling older adults.[45] In our study, the baseline medical history of CVA differed significantly between improved frailty and stable or worsening frailty groups (Table 2). However, after adjusting for the covariates in the multivariate logistic regression, this factor was no longer significant. The 2 groups also did not significantly differ in terms of the Barthel index, IADL score, or MMSE (Table 1). This finding might indicate that baseline cognitive (MMSE) and functional status (IADL) had stronger contributions to improving frailty than did the patient's CVA medical history. Among the frailty phenotypes, cognitive frailty has been proposed as a clinical entity characterized by cognitive impairments with physical causes, and that is potentially reversible; this has made it an important target of secondary interventions for individuals in the early or asymptomatic stages of dementia. “Cognitive frailty” can be more specifically defined as the presence of both physical frailty and cognitive impairment in the absence of dementia (i.e., a Clinical Dementia Rating (CDR) scale score of 0.5).[46] The relationship between the CDR scale and frailty transitions would need further study to clarify.
Numerous studies have determined an association between malnutrition in the elderly and poor disease prognosis, and the MNA is a well validated and useful tool for measuring nutritional status in elderly persons in clinical practice.[27,29,30] Furthermore, high-risk prescribing behavior, defined in terms of polypharmacy (≥5 medicines), hyperpolypharmacy (≥10 medicines), and the Drug Burden Index (DBI), appears to be more common in frail individuals compared with robust individuals, and has been directly implicated as a potential contributor to frailty in community-dwelling older men after a 2-year follow-up.[47] However, in our study, lower MNA scores (≤23.5) and polypharmacy (≥8 medicines) showed significant associations with improved frailty in the chi-square test (although these were no longer significant after adjustments in the multivariate logistic regression). Potentially, early interventions for malnutrition and polypharmacy provided by geriatric assessment teams might benefit frailty transitions.
We must note 3 potential limitations of this study, largely related to the fact that the study was observational in nature. First, as several previous studies have shown significant associations between frailty and poor health outcomes,[3] we assume that improved frailty status would predict better health outcomes. However, we believe that more time would be needed to demonstrate an association between frailty transition and mobility or mortality in our study group. Second, patients in the robust group at baseline and at the 2-year follow-up were excluded in order to avoid possible data bias, as this group might have had better baseline data compared with the other groups and would therefore show limited improvement. These patients could not be included in the “improved frailty” group because their conditions were already good in the beginning. Finally, we excluded patients who withdrew from the study, were lost to follow-up, moved to a nursing home, died, or did not complete the modified FFI, which means that participants with the poorest outcomes were not analyzed. This might have affected our final results. Potentially, however, these patients’ baseline conditions were too poor to show improvement, even with appropriate assessment and intervention.
5. Conclusions
In conclusion, improved self-reported exhaustion, increased physical activity, and less decreased grip strength over 1 year correlated positively with improvement in frailty at a 2-year follow-up in older outpatients with chronic diseases. These measurements might serve as easy, quick, and feasible screening instruments in clinical practice. Further studies are needed, however, to determine how effectively a better frailty transition predicts outcomes.
Acknowledgment
The authors wish to thank all participants and testers for their collaboration in this study. The authors kindly thank the National Health Research Institutes for financial support (Grant 04A1-PHPP24–014).
Author contributions
Conceptualization: Yu-Ching Pao, Chin-Ying Chen.
Data curation: Yu-Ching Pao, Chin-Ying Chen, Ching-I Chang.
Formal analysis: Yu-Ching Pao, Chin-Ying Chen, Ching-I Chang.
Funding acquisition: Ching-Yu Chen.
Investigation: Chin-Ying Chen, Ching-I Chang.
Methodology: Chin-Ying Chen, Ching-I Chang.
Project administration: Ching-I Chang, Ching-Yu Chen.
Resources: Chin-Ying Chen, Ching-Yu Chen.
Software: Yu-Ching Pao, Chin-Ying Chen, Ching-I Chang.
Supervision: Chin-Ying Chen, Ching-Yu Chen, Jaw-Shiun Tsai.
Validation: Yu-Ching Pao, Ching-I Chang.
Visualization: Yu-Ching Pao, Ching-I Chang.
Writing – original draft: Yu-Ching Pao.
Writing – review & editing: Chin-Ying Chen, Ching-Yu Chen, Jaw-Shiun Tsai.
Footnotes
Abbreviations: ADL = activities of daily living, BMI = body mass index, BW = body weight, CDR = clinical dementia rating, CES-D = Center for Epidemiologic Studies Depression Scale, CGA = comprehensive geriatric assessments, CVA = cerebrovascular accidents, DBI = Drug Burden Index, FFI = Fried Frailty Index, FI = Frailty Index, GDS-15 = Geriatric Depression Scale-15, IADL = instrumental activities of daily living, IPAQ-SF = International Physical Activity Questionnaire–Short Form, MMSE = Mini-Mental State Examination, MNA = Mini Nutritional Assessment, MNA-SF = Mini Nutritional Assessment-Short Form, OR = odds ratio, ROC = receiver operating characteristic.
The authors report no conflicts of interest.
References
- [1].Topinková E. Aging, disability and frailty. Ann Nutr Metab 2008;52(suppl 1):6–11. [DOI] [PubMed] [Google Scholar]
- [2].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- [3].Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–63. [DOI] [PubMed] [Google Scholar]
- [4].Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008;168:382–9. [DOI] [PubMed] [Google Scholar]
- [5].Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009;57:492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: The MOBILIZE Boston Study. J Am Geriatr Soc 2009;57:1532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gill TM, Baker DI, Gottschalk M, et al. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med 2002;347:1068–74. [DOI] [PubMed] [Google Scholar]
- [8].Reuben DB. Organizational interventions to improve health outcomes of older persons. Med Care 2002;40:416–28. [DOI] [PubMed] [Google Scholar]
- [9].Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012. CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004;52:625–34. [DOI] [PubMed] [Google Scholar]
- [11].de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011;10:104–14. [DOI] [PubMed] [Google Scholar]
- [12].Panza F, Solfrizzi V, Lozupone M, et al. An old challenge with new promises: a systematic review on comprehensive geriatric assessment in long-term care facilities. Rejuvenation Res 2018;21:3–14. [DOI] [PubMed] [Google Scholar]
- [13].Reuben DB, Principles of geriatric assessment. In: Hazzard WR, Blass JP, Ettinger WH, Halter JB, Ouslader JG, eds. Principles of Geriatric Medicine and Gerontology. 4th ed. New York: McGraw-Hill; 1999:467–481. [Google Scholar]
- [14].Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007;55:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsai JS, Wu CH, Chen SC, et al. Plasma adiponectin levels correlate positively with an increasing number of components of frailty in male elders. PLoS One 2013;8:e56250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Walpole SC, Prieto-Merino D, Edwards P, et al. The weight of nations: an estimation of adult human biomass. BMC Public Health 2012;12:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician 2014;89:718–22. [PubMed] [Google Scholar]
- [18].Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- [19].Liu YM. Validation of the Taiwan International Physical Activity Questionnaire - Short Form (Doctoral Dissertation in Chinese). Institute of Nursing, Taipei: National Taiwan University; 2004. [Google Scholar]
- [20].Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–55. [DOI] [PubMed] [Google Scholar]
- [21].Hardy SE, Perera S, Roumani YF, et al. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc 2007;55:1727–34. [DOI] [PubMed] [Google Scholar]
- [22].Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- [23].Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrument activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- [24].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method of grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [25].Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982–1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- [26].Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing 2012;41:148–54. [DOI] [PubMed] [Google Scholar]
- [27].Vellas B, Lauque S, Andrieu S, et al. Nutrition assessment in the elderly. Curr Opin Clin Nutr Metab Care 2001;4:5–8. [DOI] [PubMed] [Google Scholar]
- [28].de Craen AJ, Heeren TJ, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry 2003;18:63–6. [DOI] [PubMed] [Google Scholar]
- [29].Vellas B, Villars H, Abellan G, et al. Overview of the MNA--its history and challenges. J Nutr Health Aging 2006;10:456–63. [PubMed] [Google Scholar]
- [30].Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med 2002;18:737–57. [DOI] [PubMed] [Google Scholar]
- [31].Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc 2012;60:652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gill TM, Gahbauer EA, Han L, et al. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci 2011;66:1238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fallah N, Mitnitski A, Searle SD, et al. Transitions in frailty status in older adults in relation to mobility: a multistate modeling approach employing a deficit count. J Am Geriatr Soc 2011;59:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418–23. [DOI] [PubMed] [Google Scholar]
- [35].Poresky RH, Clark K, Daniels AM. Longitudinal characteristics of the Center for Epidemiologic Studies--Depression Scale. Psychol Rep 2000;86(3 pt 1):819–26. [DOI] [PubMed] [Google Scholar]
- [36].Salamero M, Marcos T. Factor study of the Geriatric Depression Scale. Acta Psychiatr Scand 1992;86:283–6. [DOI] [PubMed] [Google Scholar]
- [37].Chen CY, Wu SC, Chen LJ, et al. The prevalence of subjective frailty and factors associated with frailty in Taiwan. Arch Gerontol Geriatr 2010;50(suppl 1):S43–7. [DOI] [PubMed] [Google Scholar]
- [38].Solfrizzi V, Scafato E, Seripa D, et al. Reversible cognitive frailty, dementia, and all-cause mortality. The Italian Longitudinal Study on Aging. J Am Med Dir Assoc 2017;18:89.e1–8. [DOI] [PubMed] [Google Scholar]
- [39].Giampaoli S, Ferrucci L, Cecchi F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing 1999;28:283–8. [DOI] [PubMed] [Google Scholar]
- [40].Norman K, Stobäus N, Gonzalez MC, et al. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr 2011;30:135–42. [DOI] [PubMed] [Google Scholar]
- [41].Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266–73. [DOI] [PubMed] [Google Scholar]
- [42].Hamasaki H, Kawashima Y, Katsuyama H, et al. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci Rep 2017;7:7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vermeulen J, Neyens JC, van Rossum E, et al. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr 2011;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 2001;33(suppl):S459–71. [DOI] [PubMed] [Google Scholar]
- [45].Lee JS, Auyeung TW, Leung J, et al. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc 2014;15:281–6. [DOI] [PubMed] [Google Scholar]
- [46].Panza F, Lozupone M, Solfrizzi V, et al. Cognitive frailty: a potential target for secondary prevention of dementia. Expert Opin Drug Metab Toxicol 2017;13:1023–7. [DOI] [PubMed] [Google Scholar]
- [47].Gnjidic D, Hilmer SN, Blyth FM, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther 2012;91:521–8. [DOI] [PubMed] [Google Scholar]


