Abstract
Background
Injection drug use (IDU) in nonurban areas of the United States is a growing public health concern, but there has been no comprehensive assessment of existing research on injection-related HIV and hepatitis C (HCV) in nonurban communities. We conducted a systematic review to assess the current literature and identify knowledge gaps.
Methods
We systematically searched six databases for relevant articles published between January 1990 and June 2016 and screened, extracted, and analyzed the resulting data. Studies were included if they reported original findings from the nonurban U.S. related to 1) IDU and its role in HIV/HCV transmission, and/or 2) HIV/HCV services for people who inject drugs (PWID).
Results
Of 2,330 studies, 34 from 24 unique research projects in 17 states met inclusion criteria. Despite increasing HCV and high vulnerability to injection-related HIV outbreaks in nonurban areas, only three studies since 2010 recruited and tested PWID for HIV/HCV. Twelve reported on sharing injection equipment but used varying definitions of sharing, and only eight examined correlates of injection risk. Nine studies on syringe access suggest limited access through syringe exchange programs and pharmacies. Only two studies addressed HCV testing, none addressed HIV testing, and three examined behavioral or other interventions.
Conclusions
Despite growing concern regarding nonurban IDU there are few studies of HIV/HCV and related services for PWID, and the existing literature covers a very limited geographical area. Current research provides minimal insights into any unique factors that influence injection risk and HIV/HCV service provision and utilization among nonurban PWID.
Keywords: Nonurban, Rural, HIV, HCV, IDU, PWID
1. Introduction
Injection drug use (IDU) is a risk factor for HIV, hepatitis C virus (HCV), and other blood-borne infections. In the United States, six percent of new HIV diagnoses are attributed to IDU and another three percent to men who have sex with men (MSM) and inject drugs (Centers for Disease Control and Prevention, 2015a). While the overall number of newly diagnosed HIV cases among people who inject drugs (PWID) decreased between 2010 and 2014 nationally, cases of acute hepatitis C almost tripled between 2010 and 2015 - an increase largely attributed to increases in IDU in nonurban areas (Centers for Disease Control and Prevention, 2015b) that are generally underserved by syringe access programs (Wejnert et al., 2016). Highly sensitive rapid tests are available to diagnose both HIV and HCV, yet the Centers for Disease Control and Prevention (CDC) estimates that 14% of all HIV-positive individuals and as many as half of those with chronic HCV are unaware of their infection (Hall et al., 2015; National Academies of Sciences, 2016). Early diagnosis of these infections is critical, as there are effective antiviral medications available that manage and cure HIV and HCV, respectively, and prevent onward transmission.
The growth of illicit drug use in U.S. nonurban areas over the past two decades has been covered extensively by the popular press but addressed in only a few peer-reviewed studies. These studies detail increases in methamphetamine use in nonurban areas in the early 2000s (Gruenewald et al., 2010; Gruenewald et al., 2013) and more recent increases in illicit prescription opioid and heroin use (Cicero et al., 2014; Meiman et al., 2015; Paulozzi and Xi, 2008; Rossen et al., 2013), but do not focus on IDU specifically. An HIV outbreak in Indiana received national attention as the first outbreak of its kind in a rural community, underscoring the importance of addressing IDU in nonurban areas. Between November 2014 and November 2015, more than 180 individuals in Scott County, Indiana (population ~23,000), tested positive for HIV, with most (88%) reporting injection of oxymorphone, a prescription opioid, within the past 12 months (Peters et al., 2016). CDC subsequently conducted a nationwide vulnerability assessment to identify counties at high risk of rapid HIV spread and new or continuing high rates of HCV infection among PWID (Van Handel et al., 2016); the 220 counties identified as most vulnerable were “overwhelmingly rural” (pg. 328). CDC also documented an almost four-fold increase in acute HCV infections among persons aged ≤30 years in Kentucky, Tennessee, Virginia, and West Virginia from 2006–2012, noting that IDU was the most commonly reported risk factor and increases were substantially higher in nonurban than urban areas (Zibbell et al., 2015). All of these findings point to a pressing need for research on nonurban IDU, its health impacts, and effective public health responses.
Although there is a wealth of peer-reviewed literature on IDU and HIV/HCV, the vast majority of studies have been conducted in urban areas, and it is unlikely that their findings are broadly generalizable to nonurban areas. Drug-related harms like HIV and HCV are the product of “risk environments” shaped by systemic and social contexts that vary across communities (Rhodes, 2002). For example, drug type and availability differ across geographic regions, resulting in different injection practices and HIV/HCV risk profiles (e.g., powder heroin vs. black tar heroin) (Ciccarone, 2009). The geospatial availability of harm reduction and other health services and access to transportation to reach these services are very different in urban and nonurban areas (Des Jarlais et al., 2015). Social norms and values differ across communities, contributing to variations in the stigma and discrimination that PWID encounter and its impacts on their decisions to seek care as well as their overall mental and physical health (Ahern et al., 2007; Young et al., 2005). Differences in social norms and values can also result in variation in the implementation of laws and policies that govern syringe possession, syringe access, and health services access (Burris et al., 2004; Chiarello, 2016; Pollini, 2017; Strathdee et al., 2015; Taussig et al., 2002). The result is that efforts to understand and address HIV/HCV among PWID should be “locally produced,” taking into consideration the specific factors that influence transmission in different communities (Rhodes, 2002).
To date, there has been no coordinated effort to summarize or synthesize the existing research on IDU and HIV/HCV in nonurban areas. Communities across the country are in need of research-based guidance on both the extent of the problem and effective strategies for HIV/HCV prevention, testing, and care in nonurban settings. In addition, research entities would benefit from insights regarding gaps in knowledge that remain to be filled by methodologically sound epidemiological and health services research studies. To address these needs, we undertook a systematic review of research on IDU and HIV/HCV in nonurban areas of the U.S., guided by three specific aims:
Describe the existing literature on IDU and its role in HIV/HCV transmission in U.S. nonurban areas
Characterize what is known about HIV/HCV-related services for PWID in U.S. nonurban areas
Identify priority areas for research to fill current knowledge gaps regarding IDU, HIV/HCV transmission, and HIV/HCV-related services in U.S. nonurban areas
2. Material and Methods
Our systematic review was guided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). The PRISMA checklist is included in the supplementary material for this article.1
2.1. Search Strategy
We developed a detailed protocol and search-strategy in collaboration with two librarians, including one who specializes in systematic reviews, and registered our protocol with the International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/prospero; CRD42016035780). We ran searches in six research databases (PubMed, CINAHL, PsycINFO, SCOPUS, CENTRAL, and CDSR) for articles published between January 1, 1990, and the search date of June 13, 2016. Searches used database-specific medical subject headings (e.g., MeSH), as well as Boolean operators, truncation, and wildcards in addition to keywords, when possible (Table 1). We imported database search results into EndNote, recorded results on an Excel spreadsheet, and deleted duplicates.
Table 1.
Search terms and subject headings used
| Word group 1 | Word group 2 | Word group 3 |
|---|---|---|
| Search terms | ||
| suburb*, rural, exurb*, non-urban, nonurban, urban fringe, peri-urban, periurban, region* | syringe*, injecti* drug use, needle exchange, syringe exchange, needle sharing, syringe sharing, intravenous injecti*, intravenous drug us*, intravenous drug abus*, IDU, IVDA, PWID | HIV, HCV, hep* C, human immunodeficiency virus, AIDS, acquired immunodeficiency syndrome, acquired immune deficiency syndrome |
| MeSH Headings | ||
| Rural Health Services, Suburban Health Services, Rural Nursing, Suburban Population, Rural Population | Needle-Exchange Programs, Needle Sharing, Injections, Needles, Syringes, (Substance Abuse, Intravenous) | HIV Infections, Hepatitis C |
| PsycINFO Headings | ||
| Rural Environments, Suburban Environments | Needle Sharing, Needle Exchange Programs, Intravenous Injections, Injections, Intravenous Drug Usage | HIV, HIV Testing, AIDS Prevention, AIDS, Hepatitis |
| CINAHL Headings | ||
| Rural Population, Rural Health Services, Rural Health Nursing, Rural Areas, Rural Health, Suburban Areas, Suburban Population, Suburban Health | Needles, Needle Sharing, Needle Exchange Programs, Syringes, (Injections, Intravenous), Injections, (Substance Abuse, Intravenous), Intravenous Drug Users, (Administration, Intravenous) | HIV-Infected Patients, HIV Infections, HIV Education, Hepatitis C, (Hepatitis C, Chronic) |
Note: Search terms within groups combined with OR. Word groups combined with AND. Date restrictions were set for all searches from 01/01/1990 to 12/31/2016. All searches were conducted on 06/13/2016; date restrictions were set to capture pre-publication results available in online databases.
2.2. Inclusion and Exclusion Criteria
We included articles reporting original findings related to 1) IDU and its role in HIV and/or HCV transmission in nonurban areas of the U.S., and/or 2) availability of, access to, and/or effectiveness of HIV- and/or HCV-related services for PWID in nonurban areas of the U.S. To be eligible, articles had to report results specific to nonurban areas or populations. Nonurban eligibility was determined by study authors’ definition, when available; specifically, articles were eligible if the authors described their sample as “rural” or “nonurban.” For articles with unclear descriptors (e.g., “suburban,” “semi-urban,” etc.), multiple descriptors (e.g., “urban” and “rural”), or no explicit indication of rural/urban context, we used the Census Bureau’s urban-rural classification for cities and towns (e.g., if listed as an urban area or urban cluster, the city or town was considered urban) (United States Census Bureau, 2010) or the USDA Economic Research Service rural-urban continuum codes for counties (i.e., counties with a rural-urban continuum code of 4 to 9 were considered nonurban) (United States Department of Agriculture Economic Research Service, 2013). Studies that included both urban and nonurban areas were included if they reported relevant findings specific to nonurban areas.
Only original peer-reviewed manuscripts were included; we excluded letters, editorials, and news articles. Additional exclusion criteria were dissertations, non-English language publications, non-human study subjects, research conducted outside of the U.S. (all 50 states and the District of Columbia were eligible for inclusion), research conducted solely in urban areas or with urban populations, research conducted in prisons (due to challenges of establishing rural/urban status of prison inmates), and articles published before 1990 (to exclude less relevant research from the early years of the HIV/AIDS epidemic).
2.3. Study Selection
Two reviewers independently applied inclusion and exclusion criteria to each article. Incongruent results were discussed by the two reviewers until they reached consensus, with the option of having any unresolved discrepancies decided by a third reviewer. We calculated inter-rater reliability using kappa statistics, as per recommendations made in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2011). After study selection, we validated our search results by conducting cited and citing reference searches.
2.4. Data Extraction and Analysis
After identifying the final set of eligible manuscripts, we recorded additional information about each study. This included basic study characteristics (publication date, year(s) of data collection, specific location/geographical area studied, and population studied); study aims and methods (research aims, recruitment methods, and primary outcomes and measures); and the study’s primary results, conclusions, and implications in relation to our research questions (including which of our research aims were addressed by the study). Two independent reviewers also made notes about the methodological strengths and weaknesses of each study. We then grouped all results by study aim and examined and analyzed the main findings, identifying primary themes, implications, and gaps in the research.
3. Results
3.1. Search Results
Our initial searches retrieved 3,563 references. We organized and de-duplicated these references, resulting in 2,330 unique references that we considered for inclusion. Based on abstract review we identified 361 articles that were eligible for full-text review. Of these, 230 articles were ineligible based on our exclusion criteria, and 97 did not meet our indicators of relevance. The remaining 34 studies were eligible for inclusion in our review.
3.2. Description of Included Studies
Of the 34 studies included, 26 reported results related to the role of IDU in HIV/HCV transmission in nonurban areas and 13 reported results related to HIV/HCV-related services for PWID in nonurban areas. Publication dates ranged from 1990 to 2016; more than half (56%) were published after 2010, 26 percent were published between 2000 and 2010, and 18 percent were published prior to 2000.
The 34 included studies drew from 24 unique research projects. Three used national samples and the remaining 31 reported data from 17 states. The studies tended to concentrate in a few geographical areas, with half (17 of 34) collecting data in one of only three states: Kentucky, Connecticut, and Florida. Fourteen studies were conducted in Kentucky; of these, 10 (29% of all included studies) were conducted solely in Kentucky, while an additional four used multistate (but not national) samples including Kentucky.
3.3. Role of IDU in HIV/HCV Transmission in Nonurban Areas
A majority of studies (74%, n=25) reported results addressing the role of IDU in HIV/HCV transmission in nonurban areas. These articles typically addressed HIV/HCV prevalence and/or correlates of infection among PWID.
3.3.1. HIV/HCV prevalence
Sixteen studies reported on HIV/HCV prevalence among PWID in nonurban areas. Three used secondary data to examine national or regional trends; five involved HIV/HCV testing with PWID as part of a larger group, providing IDU-specific estimates; five recruited PWID specifically and tested them for HIV and/or HCV; and four collected data on HIV and/or HCV prevalence via self-report methods.
3.3.1.1. Secondary data used to examine national or regional trends
These three studies indicate that HIV diagnoses among PWID have decreased in both urban and nonurban areas in recent years, while HCV diagnoses have been increasing – a change that has been most dramatic in nonurban areas. Using data from the CDC’s National Notifiable Disease Surveillance System, one study found that HIV diagnoses decreased significantly between 2008–2014 in both urban and nonurban areas, though these decreases were smaller for nonurban whites than for other groups (Wejnert et al., 2016). Two others also used CDC data to examine trends in acute HCV infections. Zibbell et al. (2015) found that between 2006 and 2012, 74.8% of HCV cases among persons aged ≤30 years in nonurban areas of Kentucky, Tennessee, Virginia, and West Virginia were related to IDU, and HCV incidence was four times greater in nonurban areas than in urban areas during this period. Van Handel et al. (2016) examined nationwide data at the county level to identify counties at highest risk of an HIV outbreak, using acute HCV infections as a proxy for needle sharing. Although they did not report detailed results related to urbanicity, the authors concluded that the counties most at risk of an HIV outbreak were “overwhelmingly rural” (pg. 328).
3.3.1.2. Estimating IDU-specific burden
Five studies examined HIV and/or HCV burden attributed to IDU within a larger group of study subjects. Two of these recruited individuals living with HIV from clinical settings. Cohn et al. (1994) surveyed 325 HIV-positive patients seen at UNC Hospitals in North Carolina; 10% (n=34) reported a history of IDU. Grace et al. (2000) recruited 119 patients who were treated at HIV specialty clinics in rural Vermont. They reported that IDU and IDU/MSM transmission risk accounted for 23% of HIV cases, noting that a disproportionate number of women were infected via IDU.
The remaining three studies recruited or used data from state or local health departments. A study from rural Appalachian Kentucky recruited individuals who received HCV/hepatitis B virus (HBV) testing at four health departments (Christian et al., 2010). Eight of the 12 individuals who had a positive HCV antibody test had a history of IDU (a 57% HCV-positivity rate among PWID). Conrad et al. (2015) examined data from the Indiana State Department of Health after the 2015 HIV outbreak in rural Indiana; 80% of those infected were PWID. Sheehan et al. (2015) used HIV surveillance data from the Florida Department of Health to examine trends among HIV-positive Latinos between 2000 and 2008. Ten percent of their sample (n=1126) were reported as having a history of IDU, and those with a history of IDU were more likely to live in a rural area.
3.3.1.3. PWID recruited and tested
Of the five studies in this category, two were conducted in the 1990s with small sample sizes. Wykoff et al. (1991) recruited and tested injection partners of people who tested positive for HIV in a rural South Carolina district, documenting 26.7% prevalence. McCoy et al. (1996) recruited 10 PWID in Belle Glade, Florida, and found that four tested positive for HIV. More recent studies had much larger sample sizes. Havens et al. (2013) used respondent-driven sampling (RDS) to recruit and test 392 PWID between 2008 and 2010 in Appalachian Kentucky, finding an HCV prevalence of 54.8% and no positive HIV results. Akselrod et al. (2014) also used RDS to recruit and test 446 PWID in suburban Connecticut between 2008 and 2011, finding an HCV prevalence of 40.1% and HIV prevalence of 3.1%. Zibbell et al. (2014) used modified snowball sampling to recruit and test 123 PWID in rural Courtland County, New York in 2012 and found an HCV prevalence of 34%.
3.3.1.4. Self-report
Four studies used self-report methods to quantify HIV or HCV prevalence. Two of these were conducted by the same research team in rural Appalachian Kentucky in the early 2000s. In one, the researchers recruited 800 felony probationers between 2001 and 2004; of the 179 individuals with a lifetime history of IDU, 12.8% reported HCV-positive status and none reported HIV infection (Havens et al., 2011). In the other, Havens et al. (2007) recruited 184 opioid users, including 19 who were currently injecting drugs, from 2004 to 2005. They found that 14.8% of current PWID self-reported HCV-positive status. In their study of PWID in New York, Zibbell et al. (2014) found no self-reported cases of HIV. In rural Kentucky, Staton-Tindall et al. (2015b) facilitated focus groups with 22 women from three rural Kentucky jails who reported drug use; the authors reported that most women perceived IDU was extremely common in their communities and that HCV was widespread.
3.3.2. HIV/HCV risk behaviors
Fourteen studies reported on injection practices that facilitate HIV/HCV transmission. These include sharing syringes, other injection equipment (e.g., cotton, cookers), and water used for dissolving drugs or rinsing injection equipment. Studies varied in the measures used to assess injection-related behaviors; for example, some used measures of “syringe sharing” while others considered “receptive” and “distributive” sharing separately. Similarly, some reported broadly on injection “works” or “other injection paraphernalia” while others reported separately estimates for sharing cotton, cookers, and/or water. Timeframes for assessing injection risk behaviors also varied, encompassing the periods past 30 days, past 6 months, past year, and lifetime. Three studies went beyond measuring the prevalence of these behaviors to investigate factors associated with engaging in them, while an additional four undertook biological testing for HIV/HCV and identified statistically significant correlates of infection. Ten studies used community-based samples and four derived samples from criminal justice populations.
3.3.2.1. Syringe sharing
Twelve studies reported measures of syringe sharing. Three from a single cohort of PWID in southwestern Connecticut reported syringe-mediated sharing (not defined) at 13% (Akselrod et al., 2014), receptive sharing at 21.9% (Grau et al., 2016), and “syringe sharing” at 20.5% (Heimer et al., 2014). A community-based sample in Appalachian Kentucky documented receptive and distributive syringe sharing at 10.5% and 26.3%, respectively (Havens et al., 2007), while a later study documented prevalence of 16.7% for receptive and distributive sharing combined (Young et al., 2013b). A third study from this region estimated receptive syringe sharing at 30.2% and 15.2% for HCV-positive and HCV-negative participants, respectively (Havens et al., 2013). The highest community-based estimate of syringe sharing came from central New York State, where Zibbell et al. (2014) found that 44.4% of young adult PWID reported sharing needles in the past 12 months. A final community-based, multi-site study reported that 12.9% of participants in “low population density communities” reported using “unclean works” in the past 30 days, which we interpreted as including syringes (Leukefeld et al., 2001).
The remaining four studies, conducted among criminal justice populations, documented higher syringe sharing prevalence than the aforementioned community-based samples. These include two studies of probationers in Kentucky; the first documented lifetime receptive and distributive syringe sharing at 34.5% and 97.1%, respectively (Havens et al., 2011). The second found that distributive syringe sharing ranged from 32.6% to 54% depending on recipient type (e.g., other users, sexual partners, or friends) and that 75.3% “often” sold or gave away their needles or works without cleaning (Oser et al., 2006). The third study, among women currently in jail in Kentucky, reported that 70.3% shared needles in the past year (Staton-Tindall et al., 2015a). The fourth, from Indiana, reported that 6% of jail inmates and 3% of jail staff cited needle use as a mode of HIV transmission in jails (Kane and Dotson, 1997).
3.3.2.2. Sharing other injection equipment
Eight studies addressed injection equipment other than syringes. Two, from the same Connecticut-based study, reported slightly differing prevalence for sharing cookers (18.8% and 17.8%), rinse water (31.2%, 30.0%), and drug mixing water (33.8%, 34.8%) in the past 30 days (Grau et al., 2016; Heimer et al., 2014). Other studies grouped non-syringe injection equipment together; these included two community-based Kentucky studies, with one reporting that 42.1% of PWID shared cotton/cookers/rinse water (Havens et al., 2007) and the other that 41.9% of HCV-positive and 26.0% of HCV-negative PWID, respectively, shared cookers/cotton/rinse water in the past 6 months (Havens et al., 2013). In New York, Zibbell et al. (2014) reported that 68.3% of young adult PWID shared cotton/cookers/water in the past year. Among female jail inmates in Kentucky, 96.6% reported sharing cookers/cotton/rinse water in past year (Staton-Tindall et al., 2015a). In a sample of Kentucky probationers, 44% had shared injection equipment like cotton and cookers in their lifetime (Havens et al., 2011); a second study of Kentucky probationers by Oser et al. (2006) combined reporting of sharing “works” with sharing needles, as noted above.
3.3.2.3. Correlates of risky injection and HIV/HCV infection
Five studies tested for HIV and/or HCV and examined correlates of infection. Among young adult PWID in central New York, sharing any injection equipment (excluding syringes) and injecting prescription opiates were independently and positively associated with HCV infection, while female gender was inversely associated (Zibbell et al., 2014). In Kentucky, Havens et al. (2013) identified an independent association between syringe sharing and HCV but not sharing cotton/cookers/water. White race, prescription opioid injection, cocaine injection, testing positive for herpes simplex virus 2, having injected for at least one year, and “eigenvector centrality” (a social network measure) were also independently positively associated with HCV, while having a PTSD diagnosis was inversely associated with infection (Havens et al., 2013). Young et al. (2013a) found that recent IDU was associated with HCV infection. A third study of one urban and two nonurban Florida communities showed a positive association between IDU and HIV seropositivity only in the urban site in univariate analysis (McCoy et al., 1999). In Connecticut, Akselrod et al. (2014) undertook biological testing for HIV/HCV/HBV and conducted multivariate analysis to identify independent associations with testing positive for one or more viruses but did not appear to examine sharing of syringes and/or other injection equipment as a covariate in the analysis.
Three additional studies looked at factors associated with risky injection behaviors rather than HIV/HCV. Two of these studies, from the Connecticut cohort, looked at factors associated with a composite injection-associated risk behavior outcome variable based on participants’ reports of engaging in up to six listed risk behaviors in the last 30 days (i.e., receptive sharing, “syringe mediated sharing,” sharing drug in liquid form, sharing cookers, sharing drug-mixing water, sharing rinse water). Grau et al. (2016) looked at factors associated with reporting at least one of these behaviors and identified White race, having at least a high school degree, and having ≥6 injection partners in past 6 months as positively associated with injection risk behavior(s). Heimer et al. (2014) used this measure as a 6-item continuous outcome variable and found that older age, CES-D depression score normal or mild, injecting in one’s own residence, and higher social support scores were negatively associated with higher risk scores, while larger injection network size and higher hepatitis knowledge score were positively associated with higher scores. The third study, among Kentucky probationers, reported that risky injection practices (defined as engaging in receptive syringe sharing or sharing other injection equipment) were positively associated with being male, lifetime cocaine injection, and lifetime opioid injection, and negatively associated with older age and being African American, after controlling for potential confounders (Havens et al., 2011).
3.4. HIV/HCV-Related Services for PWID in Nonurban Areas
Thirty-eight percent of included studies (n=13) examined HIV/HCV-related services for PWID in nonurban areas. These articles examined syringe access, HIV/HCV testing, and other interventions including a risk reduction intervention and a partner notification program for individuals who tested positive for HIV.
3.4.1. Syringe access
Nine studies examined some aspect of syringe access for PWID in nonurban areas of the United States. These studies demonstrate that nonurban areas lack syringe exchange programs (SEPs) and thus PWID in these areas often rely on nonprescription syringe sales at pharmacies or on secondary sources such as friends, drug dealers, and other PWID. Many states permit nonprescription syringe sales at the pharmacists’ discretion; the included studies also highlight how this provision results in inconsistent access to sterile syringes via pharmacies in nonurban areas.
3.4.1.1. Limited SEPs and reliance on pharmacies and secondary sources
Only one study examined access to sterile syringes through SEPs in nonurban areas. In a survey of SEPs across the United States, Des Jarlais et al. (2015) found that only 20% of SEPs were located in rural areas, and 9% in suburban areas. The authors noted that in addition to having fewer SEPs, nonurban areas typically had smaller SEPs which served fewer clients and had less funding. The lack of access to free syringes in nonurban areas may result in a reliance on pharmacies and secondary sources for sterile syringes. Akselrod et al. (2014) found that in suburban Connecticut, where at the time of the study there was only one SEP in each county studied, 74.2% of PWID usually purchased syringes from a pharmacy, while only 3.3% obtained syringes from a SEP in the past 30 days. In Kentucky, Havens et al. (2011) found that only a third of felony probationers with a lifetime history of IDU reported obtaining syringes from legal sources. In a later study, Havens et al. (2013) found that among participants with a lifetime history of IDU, fewer than 4% regularly bought syringes from pharmacies, while most got them from family, friends, or dealers. Relatedly, in qualitative interviews with methamphetamine users in Tennessee, MacMaster et al. (2008) reported that their participants identified syringe access (including nonprescription pharmacy syringe sales) as a service that was lacking in their community.
3.4.1.2. Inconsistent pharmacy access
The reliance on pharmacies for sterile syringes can be problematic because state laws generally either allow nonprescription syringe sales at the individual pharmacist’s discretion or more proactively promote nonprescription syringe sales but only with voluntary pharmacy participation. Reich et al. (2002) found that in Missouri, Kentucky, and Colorado pharmacies were the most common source of syringes for PWID, but PWID in urban areas had an easier time accessing syringes at pharmacies than those in rural areas. Compton et al. (2004) found similar results in Kentucky, where rates of successful syringe purchases were higher in urban regions than in rural regions. In that study, however, they found an opposite trend in Colorado, Connecticut, and Missouri (i.e., rural areas had higher rates of successful syringe purchase than urban areas in those states). Stopka et al. (2002) found that in urban and suburban areas of Connecticut, syringe purchase attempts overall were significantly more successful in suburban vs. urban pharmacies but purchase attempts at chain pharmacies were more successful in urban areas. Overall, these studies highlight inconsistent access to syringes at pharmacies: access appears to vary from state to state and across urban vs. nonurban areas within states and may also vary by pharmacy type.
3.4.2. HIV/HCV-related services
Five studies examined services for PWID related to HIV and/or HCV in nonurban areas. These studies highlight a lack of access to HIV and HCV testing in these areas, as well as an overall lack of effective risk reduction interventions.
3.4.2.1. Testing
Two studies examined the availability of HCV testing in nonurban areas, while none reported results related to the availability of HIV testing. Staton-Tindall et al. (2015b) reported that the women they interviewed in rural Kentucky jails believed services and testing for HCV were very limited, and the authors noted that the local Health Department did not offer HCV testing. Barocas et al. (2014) surveyed PWID who utilized an SEP in Southern Wisconsin in 2012. The researchers found that PWID who lived in rural areas were less likely to report recent HCV testing than those who lived in urban and suburban areas, and that participants who lived in urban areas were more likely to have been tested in the past year than those who lived in rural and suburban areas. In that study, lack of transportation was identified as a barrier to testing.
3.4.2.2. Risk reduction
Two studies examined programs or interventions which aimed to reduce HIV/HCV risk among PWID in nonurban areas. Knittel et al. (2010) studied clients who enrolled in an SEP at an HIV/AIDS resource center in Ypsilanti, MI between 2004 and 2006 to examine the impact of SEP participation on risk behaviors. The researchers found that at six months after enrollment, clients were less likely to report giving someone else a used syringe than at enrollment; no other variables related to HIV risk reduction reached significance. Warner and Leukefeld (2001) studied an HIV risk reduction intervention for PWID and people who used crack/cocaine in Kentucky. The researchers found that PWID in their “most” rural site actually increased injection risk behaviors after the intervention.
3.4.2.3. Other interventions
One additional study examined the acceptability of a partner notification intervention for sex and injection partners of individuals who tested positive for HIV in a rural South Carolina district between 1988 and 1989. Jones et al. (1990) reported that most respondents in all groups, including injection partners, were in favor of the partner notification program.
4. Discussion
Our review documented only a small number of studies examining IDU and HIV/HCV in non-urban areas of the U.S. between 1990 and 2016. This finding is at odds with the extensive media and policy focus on nonurban illicit drug use in the past two decades and indicates an urgent need for targeted, high quality public health research on HIV/HCV transmission, testing, and treatment among PWID in these underserved areas.
The national epidemiological assessments in our review indicate that HCV is rising in nonurban areas and that these areas are at disproportionately high risk of an HIV outbreak (Van Handel et al., 2016; Wejnert et al., 2016; Zibbell et al., 2015). We documented only five studies – just three since 2010 – that specifically recruited and tested nonurban PWID for HIV and/or HCV. The three recent studies used community-based recruitment methods to test a total of 961 PWID for HCV; prevalence ranged from 34 percent in New York (Zibbell et al., 2015) to 55 percent in Kentucky (Havens et al., 2013). Only two of the three studies also tested for HIV, with prevalence ranging from zero (Havens et al., 2013) to three percent (Akselrod et al., 2014). This constitutes a substantial gap in knowledge regarding HIV/HCV prevalence among nonurban PWID, providing insufficient data upon which to base broader estimates of HIV/HCV prevalence in U.S. nonurban areas. Well-designed prevalence studies in strategically selected community-based samples across the U.S. are critical to efforts to establish nationwide prevalence estimates and establish a broad strategy for targeting and implementing HIV/HCV prevention and treatment strategies for nonurban PWID.
The limited number of community-based studies also raises concerns regarding methodological limitations of the current literature regarding nonurban IDU and HIV/HCV. Specifically, our review documented an overreliance on convenience samples, primarily recruited from health care settings and often resulting in samples of less than 100 PWID. A growing number of studies document the marginalization of PWID from society in general (e.g., Capitanio and Herek, 1999) and health services in particular (e.g., Ding et al., 2005; Etesam et al., 2014; Luoma et al., 2007), raising concerns about the generalizability of existing findings. Further, some studies used problematic exposure and outcomes measures, with at least two relying on self-report of HIV and/or HCV status. Many of these limitations may have resulted from difficulties in recruiting community-based PWID in nonurban areas, where until recently there were few harm reduction programs in operation that could link researchers with eligible research participants. Limited funding for nonurban research on these issues may also have played a role. As nonurban harm reduction programs expand, there should be additional opportunities for research using recruitment methods like RDS that extend recruitment beyond service PWID who access health services. With regard to funding, we note that the National Institute on Drug Abuse, in collaboration with several other federal agencies, is making targeted funding available to address opioid use in rural U.S. regions (RFA-DA-17-014). Nonetheless, the findings of this review highlight the need for rigorous recruitment and data collection methods in this area, including recruitments of sufficient size and statistical power to draw conclusions about appropriately measured outcomes of interest.
With regard to testing, we identified only two studies on HCV testing in nonurban areas, both conducted with convenience samples, and none addressing HIV testing. Notably, one of the two studies found lower HCV testing among nonurban participants. Biological testing is critical to national efforts to “seek, test, treat, and retain” (NIDA, 2013) those infected with HIV and identify HCV-infected individuals who may benefit from new highly effective antiviral therapies. Research characterizing the availability of HIV/HCV testing and barriers to testing among PWID in nonurban areas could make invaluable contributions to both HIV/HCV surveillance efforts and interventions to improve testing engagement and timely entry into care. Research on barriers to HIV/HCV treatment for those who test positive is especially needed, as our review failed to identify even one study examining HIV/HCV treatment engagement and retention among nonurban PWID.
We identified a comparatively large number of studies examining HIV/HCV risk factors among PWID, albeit from a very small number of states, in both community and institutional samples. Definitions of syringe sharing and the inclusion and/or categorization of other injection equipment varied across studies; promoting standardization in these measures would facilitate comparisons across nonurban PWID studies in geographically diverse areas of the U.S. and help prioritize interventions. Such standardization might include consistently measuring the frequency of both receptive and distributive syringe sharing and individually assessing the sharing of “other” injection equipment like cotton, cookers, and rinse water. That said, recent work by Heimer et al. (2017) indicates that it is the process of preparing drugs with used syringes, rather than the sharing of preparation paraphernalia itself, that contributes to HCV transmission. This finding should be incorporated into nonurban and urban surveys alike by including standardized questions on “booting” (pulling drug back into the syringe after injecting and then reinjecting), and using previously used syringes to add water for dissolving and/or apportioning drugs after mixing.
Notably absent from these studies was consideration of structural factors and their potential impact on PWID decisionmaking; more specifically, what does the “risk environment” look like in nonurban communities and how does it influence injection risk behaviors? The Des Jarlais et al. study (2015) documented a comparatively small number of SEPs in nonurban compared to urban areas and others documented participant reports of limited sterile syringe access (Havens et al., 2013; Havens et al., 2011; MacMaster et al., 2008; Staton-Tindall et al., 2015b). Understanding how limited syringe access and other structural factors (e.g., policing practices, stigma, transportation issues) influence injection-related HIV/HCV risk in nonurban areas, so that appropriate interventions can be implemented, should be a priority for research. Studies comparing the impact of structural- and individual-level factors that influence HIV/HCV risk behaviors and care seeking among PWID in nonurban and urban areas might also provide insights into whether and how research conducted in urban communities can be applied to nonurban communities and vice versa.
In addition to the small number of studies documented in our review, the lack of geographic breadth in these studies is an issue of significant concern. Only two states west of the Mississippi River were included; large portions of the southern U.S. and most of the Northeast were also excluded, as were states like Ohio and West Virginia that have been hard-hit by the current opioid epidemic. Our study should be viewed as a wake-up call regarding the lack of peer-reviewed research on nonurban drug-related HIV/HCV across the U.S.; such research is integral to inform efforts designed to prevent and address injection-related HIV outbreaks and the continued spread of HCV in these underserved communities.
Our systematic review has limitations. To be included in our review, studies had to include a nonurban identifier (e.g., “rural,” “nonurban,” or “suburban”) or a variant of the word “region” somewhere in the searchable reference text, including title, abstract, and keywords. Studies that do not include such identifiers would have been missed by our searches, and thus our review likely did not identify all relevant studies. This limitation also has important public health implications, as relevant studies that were not found by our review will also not be easily identified by academic and community stakeholders seeking to learn from existing nonurban studies for research, practice, or policy-related purposes. Including a descriptor of the study setting in searchable reference text for would facilitate the dissemination and application of new information on IDU and HIV/HCV in nonurban areas.
5. Conclusions
Our systematic review documented only 34 U.S.-based studies of IDU and HIV/HCV in nonurban areas between 1990 and 2016, covering an extremely limited geographic area. These studies document increasing HCV and high HIV vulnerability but offer limited information on the factors that contribute to HIV/HCV risk among nonurban PWID. There is also extremely limited information about barriers to HIV/HCV testing and treatment among nonurban PWID. Future research should address these issues with rigorous methodological approaches and broader geographic reach, and clearly, indicate that findings refer to nonurban communities.
Supplementary Material
Figure 1.
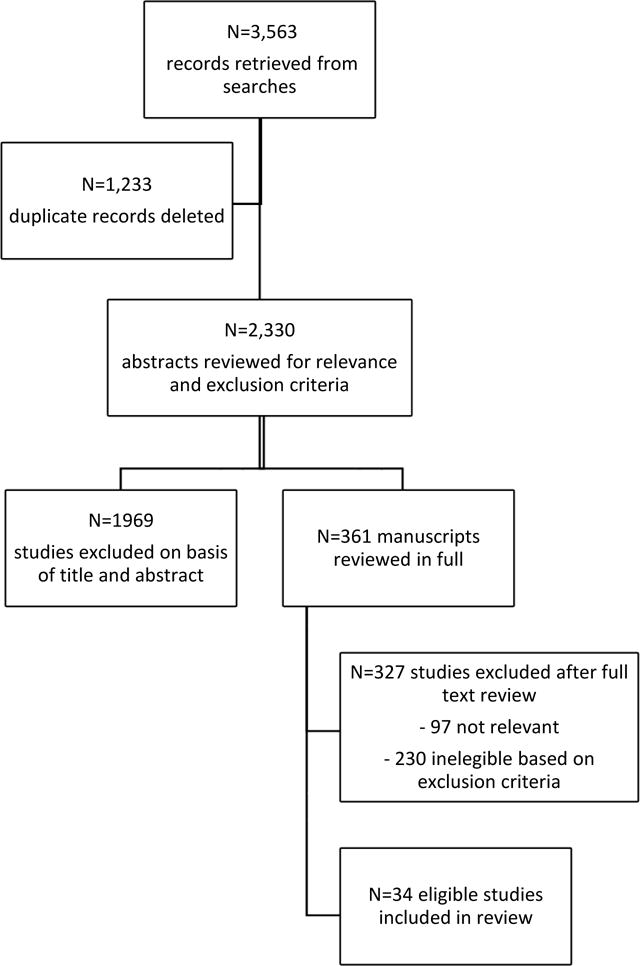
Manuscript Selection Process
Figure 2.
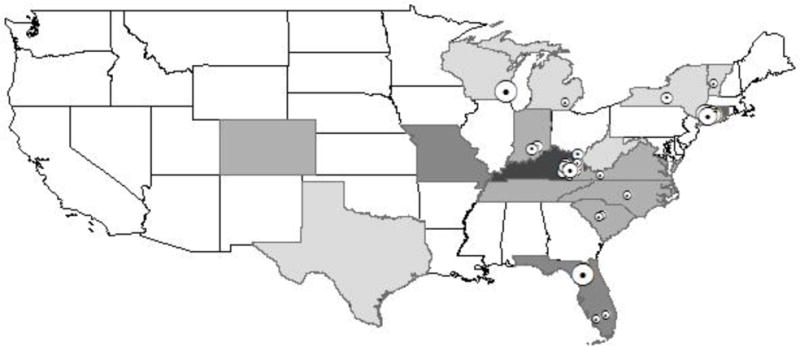
Map of Studies included in systematic review Note: Excludes studies with national samples. Number of PWID in each study is geocoded in the main location of each study, including for studies with multistate samples.
Table 2.
Characteristics of included studies
| Author(s), year | Location(s) | Study population | Sample size | Recruitment | Reported HIV/HCV prevalence and risk behaviors | Other results |
|---|---|---|---|---|---|---|
| Akselrod et al. (2014) | Nonurban towns in Fairfield and New Haven Counties, CT | Suburban PWID | n=454 | RDS1 (seeds recruited through local service agencies) | 3.1% HIV+, 40.1% HCV+; Syringe-mediated sharing (not defined) 13% | 74.2% of PWID usually purchased syringes from a pharmacy; 3.3% obtained syringes from an SEP in the past 30 days |
| Barocas et al. (2014) | Madison and surrounding rural communities, Milwaukee and suburbs, cities of Kenosha, Waukesha, Janesville, and Beloit, WI | PWID who utilized an SEP2 in Southern Wisconsin | n=520 | Via SEP2 | N/A | Rural PWID less likely to report recent and past year HCV testing than urban PWID |
| Christian et al. (2010) | KY River Area Development District | People receiving HCV/HBV testing at Kentucky River District Health Department | n=119 total, n=14 PWID | During testing at health department | 57% of PWID HCV+ | |
| Cohn et al. (1994) | Chapel Hill, NC | HIV+ patients seen at UNC hospitals | n=325 total, n=34 PWID | Outpatient clinics, inpatient services, clinical research center | 10% (n=34) reported IDU history | |
| Compton et al. (2004) | Rural and urban regions of CO, CT, KY, MO | Pharmacy purchase trial | n=400 pharmacies (200 rural, 200 urban) | N/A | N/A | Rates of successful syringe purchase higher in rural than urban regions |
| Conrad et al. (2015) | Rural county in southeastern IN. | HIV+ patients identified during outbreak | n=135 total, n=108 PWID | Data from Indiana State Department of Health | 80% of HIV cases were PWID; 84% HCV-coinfected. | |
| Des Jarlais et al. (2015) | National | SEPs2 across US | n=153 SEPs2 | SEP2 directors surveyed via mail/phone | N/A | 20% of SEPs located in rural areas, 9% in suburban areas |
| Grace et al. (2000) | Rural VT | Patients at HIV specialty clinics | n=119 total, n=27 PWID | Three rural specialty clinics | 23% IDU or IDU-MSM3 | |
| Grau et al. (2016) | Nonurban towns in Fairfield and New Haven Counties, CT | PWID | n=462 | RDS1 | Receptive syringe sharing 21.9%; sharing cookers 18.8%, rinse water 31.2%, drug mixing water 33.8% (past 30 days) | |
| Havens et al. (2013) | Rural Appalachian KY | Drug users (past 30 days) with lifetime history of IDU | n=392 PWID | RDS1 | 54.8% HCV+; 0 HIV+; Syringe sharing 30.2% for HCV+ and 15.2% and HCV-participants; sharing cookers/cottons/rinse water 41.9% of HCV+ and 26.0% of HCV− participants (past 6 months) | Among participants with lifetime history of IDU, fewer than 4% regularly bought syringes from pharmacies |
| Havens et al. (2011) | Rural Appalachian KY | Felony probationers | n=800 total, n=179 PWID | Probation offices | Of those w/self-reported lifetime IDU history: 12.8% HCV+, 0 HIV+ ; Receptive syringe sharing 34.5%; distributive sharing 97.1%; sharing other injection equipment 44% (lifetime) | One third of those with lifetime history of IDU obtained syringes from legal sources |
| Havens et al. (2007) | Two rural counties in Appalachian KY | Opioid users who used OxyContin® ≥ once in prior 3 years and used any opioid analgesic in prior 30 days (medically or nonmedically) | n=184 total, n=81 PWID | Community outreach then snowball sampling | Self-reported HCV prevalence among PWID 14.8%; receptive syringe sharing 10.5%; distributive sharing 26.3%; sharing cottons/cookers/rinse water 42.1% | |
| Heimer et al. (2014) | Nonurban towns in Fairfield and New Haven Counties, CT | PWID | N=454 | RDS1 | Syringe sharing 20.5%; sharing cookers 17.8%, rinse water 30.0%, and drug mixing water 34.8% (past 30 days) | |
| Jones et al. (1990) | Rural district in SC | Sex and injection partners of people who tested HIV+ | n=9 PWID | Contact tracing and partner notification program | N/A | Most respondents were in favor of the partner notification program. |
| Kane et al. (1997) | South and southcentral IN | Jail inmates and staff | n=137 PWID | Within jails | 6% of inmates and 3% of staff cited needle use as mode of HIV transmission in jails. | |
| Knittel et al. (2010) | Ypsilanti, MI | SEP2 clients | n=88 | SEP2 client data | Clients were less likely to report giving someone else a used syringe after 6 months than at enrollment | |
| Leukefeld et al. (2001) | Areas in KY, MO, NC, TX, DC, VA | PWID (injected past 30 days) and people who use crack/cocaine (past 48 hours) | N=3,130 total, n=1,659 PWID | Five sites participating in a Cooperative Agreement used NIDA-approved sampling plan | 12.9% of participants in “low population density communities” reported using “unclean works” (past 30 days) | |
| MacMaster et al. (2008) | Harlan County, TN | Rural key informants were current or former methamphetamine users | N=97 | Snowball sampling; initial recruitment via flyers | N/A | Participants identified syringe access as lacking in their community |
| McCoy et al. (1999) | Miami, Immokalee, and Belle Glade, FL | People who used drugs in past 30 days | n=3,555 | Snowball sampling; initial recruitment via community outreach | N/A | Positive association between IDU and HIV in urban but not rural sites (univariate analysis) |
| McCoy et al. (1996) | Belle Glade, FL | People who reported any drug use (injected or noninjected) in past six months and their sexual partners | n=269 drug users, n=40 PWID | Snowball sampling; initial recruitment via outreach in high HIV prevalence areas | 4/10 current PWID were HIV+ | |
| Oser et al. (2006) | 30 counties in rural Appalachian KY | Probationers | n=800 total, n=166 PWID | Via probation offices | Distributive syringe sharing ranged from 32.6% to 54% depending on recipient type (e.g., other users, sexual partners, or friends); 75.3% “often” sold or gave away needles or works without cleaning | |
| Reich et al. (2002) | Urban and rural areas in MO, KY, and CO, and CT | PWID | n=79 | From treatment centers and by word of mouth | N/A | Pharmacies were most common source of syringes in MO, KY, and CO, but easier to access in urban vs. rural areas |
| Sheehan et al. (2015) | FL | HIV+ Latinos | n=10,989 total, n=1126 PWID | HIV surveillance records from FL Department of Health | 10% (n=1126) of cases had IDU or IDU-MSM3 listed as a mode of HIV transmission | |
| Staton-Tindall, Harp, et al. (2015) | Three Appalachian KY counties | Female jail inmates who had a NIDA-modified ASSIST (NM-ASSIST) score of 4+ for any drug, and engagement in at least one sex risk behavior in the past 3 months | N=136 total, N=103 PWID | Jail-based | Past year syringe sharing 70.3%; sharing cookers/cottons/rinse water 96.6% | |
| Staton-Tindall, Webster, et al. (2015) | Three Appalachian KY counties | Female jail inmates who had a NIDA-modified ASSIST (NM-ASSIST) score of 4+ for any drug, and engagement in at least one sex risk behavior in the past 3 months | n=22 | Jail-based | Most perceived IDU extremely common in their communities and HCV widespread | Most were not aware of any services for HCV in their area |
| Stopka et al. (2002) | CT | Pharmacy purchase trial | n=100 pharmacies | N/A | N/A | Syringe purchase attempts significantly more successful in suburban vs. urban pharmacies but purchase attempts at chain pharmacies more successful in urban areas |
| Van Handel et al. (2016) | National | Acute HCV other indicators used to assess counties at highest risk of HIV outbreak. | N/A | HCV reported via National Notifiable Disease Surveillance System | Counties at highest risk of HIV outbreak “overwhelmingly rural” | |
| Warner & Leukefeld (2001) | Rural Appalachian Eastern KY | Current PWID and people who use crack/cocaine (verified by urine screen) | n=1,393 total, n=199 PWID | Recruited by outreach workers | N/A | PWID at the “most” rural site reported increased injection risk behaviors after a risk reduction intervention |
| Wejnert et al. (2016) | National | PWID diagnosed with HIV during 2008–2014 | Not reported | Data from CDC’s National HIV Surveillance System | HIV diagnoses decreased significantly between 2008–2014 in urban and nonurban areas; decreases smaller for nonurban whites than other groups | |
| Wykoff et al. (1991) | Six-county rural health district in SC | Sex and injection partners of people testing HIV+ | n=485 total, n=26 PWID | Contact tracing and partner notification program | 26.7% HIV+ among injection partners | |
| Young, Jonas, & Havens (2013) | Rural Appalachian KY | Nonmedical prescription opioid users who used prescription opioids, heroin, crack/cocaine and/or methamphetamine to get high in past 30 days | N=436 total, N=77 PWID | RDS1 | N/A | Recent IDU was associated with HCV infection |
| Young, Jonas, Mullins, et al. (2013) | Rural Appalachian KY | Individuals who used prescription opioids, heroin, crack/cocaine and/or methamphetamine to get high in past 30 days | N=503 total, N=394 PWID | RDS1 | Receptive and distributive sharing combined 16.7% | |
| Zibbell et al. (2015) | KY, TN, VA, WV | Confirmed acute HCV infections reported 2006–2012 | N=1,377 total, N=95 nonurban PWID | HCV reported via National Notifiable Disease Surveillance System | 74.8% of HCV cases in nonurban areas related to IDU. Four-fold greater increase in diagnoses in nonurban vs. urban areas. | |
| Zibbell et al. (2014) | Courtland County, NY | Young adult PWID | n=123 | Modified snowball sampling | 34% tested HCV+; 0 self-reported HIV+ status; past 12 months syringe sharing 44.4%; sharing cottons/cookers/water 68.3% |
Respondent-driven sampling
Syringe exchange program
Men who have sex with men
Highlights.
Injection drug use (IDU) in the nonurban U.S. is a growing public health concern.
Studies document increasing HCV and high HIV vulnerability related to nonurban IDU.
There is limited information on factors that contribute to nonurban HIV/HCV risk.
Few studies examine HIV/HCV testing or treatment among nonurban injectors.
There is an urgent need for research to inform IDU interventions in nonurban areas.
Acknowledgments
Author Disclosures
Role of Funding Source
This research was funded by grants from the National Institute on Drug Abuse (1R01DA035098), the National Institute of General Medical Sciences (1U54GM104942), and the National Center for Injury Control and Prevention (R49CE002109). Funders had no role in study design; in the collection, analysis or interpretation of data; in the writing of the manuscript; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: …
Contributors
Dr. Pollini conceived of the study and oversaw all research activities. Ms. Paquette led the development of the systematic review protocol, managed abstract and manuscript review, and developed the final list of articles. Dr. Pollini and Ms. Paquette collaborated closely in writing the manuscript and both approve the final version.
Conflict of Interest
No conflicts declared.
References
- Ahern J, Stuber J, Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88:188–196. doi: 10.1016/j.drugalcdep.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Akselrod H, Grau LE, Barbour R, Heimer R. Seroprevalence of HIV, hepatitis B virus, and HCV among injection drug users in Connecticut: Understanding infection and coinfection risks in a nonurban population. Am J Public Health. 2014;104:1713–1721. doi: 10.2105/AJPH.2013.301357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barocas JA, Brennan MB, Hull SJ, Stokes S, Fangman JJ, Westergaard RP. Barriers and facilitators of hepatitis C screening among people who inject drugs: A multi-city, mixed-methods study. Harm Reduct J. 2014;11:1. doi: 10.1186/1477-7517-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris S, Blankenship KM, Donoghoe M, Sherman S, Vernick JS, Case P, Lazzarini Z, Koester S. Addressing the “risk environment” for injection drug users: the mysterious case of the missing cop. Milbank Q. 2004;82:125–156. doi: 10.1111/j.0887-378X.2004.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Herek GM. AIDS-related stigma and attitudes toward injecting drug users among black and white Americans. Am Behav Sci. 1999;42:1148–1161. [Google Scholar]
- Centers for Disease Control and Prevention. HIV Surveillance Report. 2015a;27 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (Accessed October 22, 2017). [Google Scholar]
- Centers for Disease Control and Prevention. Surveillance for viral hepatitis – United States, 2015. 2015b https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm#Ref17 (Accessed October 22 2017)
- Chiarello E. Nonprescription syringe sales: Resistant pharmacists’ attitudes and practices. Drug Alcohol Depend. 2016;166:45–50. doi: 10.1016/j.drugalcdep.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Christian WJ, Hopenhayn C, Christian A, McIntosh D, Koch A. Viral hepatitis and injection drug use in Appalachian Kentucky: A survey of rural health department clients. Public Health Rep. 2010;125:121–128. doi: 10.1177/003335491012500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D. Heroin in brown, black and white: Structural factors and medical consequences in the US heroin market. Int J Drug Policy. 2009;20:277–282. doi: 10.1016/j.drugpo.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the united states: A retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Cohn SE, Klein JD, Mohr JE, van der Horst CM, Weber DJ. The geography of AIDS: Patterns of urban and rural migration. South Med J. 1994;87:599–606. doi: 10.1097/00007611-199406000-00004. [DOI] [PubMed] [Google Scholar]
- Compton WM, Horton JC, Cottler LB, Booth R, Leukefeld CG, Singer M, Cunningham-Williams R, Reich W, Fortuin Corsi K, Staton M, Fink JL, Stopka TJ, Spitznagel EL. A multistate trial of pharmacy syringe purchase. J Urban Health. 2004;81:661–670. doi: 10.1093/jurban/jth149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, Hillman D, Hon J, Hoover KW, Patel MR, Perez A, Peters PJ, Pontones P, Roseberry JC, Sandoval M, Shields J, Walthall J, Waterhouse D, Weidle PJ, Wu H, Duwve JM. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone–Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:443–444. [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Nugent A, Solberg A, Feelemyer J, Mermin J, Holtzman D. Syringe Service Programs for Persons Who Inject Drugs in Urban, Suburban, and Rural Areas-United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:1337–1341. doi: 10.15585/mmwr.mm6448a3. [DOI] [PubMed] [Google Scholar]
- Ding L, Landon BE, Wilson IB, Wong MD, Shapiro MF, Cleary PD. Predictors and consequences of negative physician attitudes toward HIV-infected injection drug users. Arch Intern Med. 2005;165:618–623. doi: 10.1001/archinte.165.6.618. [DOI] [PubMed] [Google Scholar]
- Etesam F, Assarian F, Hosseini H, Ghoreishi FS. Stigma and its determinants among male drug dependents receiving methadone maintenance treatment. Arch Iran Med. 2014;17:108–114. [PubMed] [Google Scholar]
- Grace C, Richardson-Nassif K, Rolley LA, Kutzko D, Alston K, Ramundo M. Injection drug use (IDU) related human immunodeficiency virus infection in rural Vermont: A comparison with non-IDU related infections. Drugs Soc. 2000;16:223–235. [Google Scholar]
- Grau LE, Zhan W, Heimer R. Prevention knowledge, risk behaviours and seroprevalence among nonurban injectors of southwest Connecticut. Drug Alcohol Rev. 2016;35:628–636. doi: 10.1111/dar.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald PJ, Johnson FW, Ponicki WR, Remer LG, Lascala EA. Assessing correlates of the growth and extent of methamphetamine abuse and dependence in California. Subst Use Misuse. 2010;45:1948–1970. doi: 10.3109/10826081003682867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald PJ, Ponicki WR, Remer LG, Waller LA, Zhu L, Gorman DM. Mapping the spread of methamphetamine abuse in California from 1995 to 2008. Am J. 2013;103:1262–1270. doi: 10.2105/AJPH.2012.300779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HI, An Q, Tang T, Song R, Chen M, Green T, Kang J. Prevalence of Diagnosed and Undiagnosed HIV Infection — United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64:657–662. [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Lofwall MR, Frost SD, Oser CB, Leukefeld CG, Crosby RA. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. Am J Public Health. 2013;103:e44–52. doi: 10.2105/AJPH.2012.300874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Oser CB, Leukefeld CG. Injection risk behaviors among rural drug users: implications for HIV prevention. AIDS Care. 2011;23:638–645. doi: 10.1080/09540121.2010.516346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2007;87:98–102. doi: 10.1016/j.drugalcdep.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Heimer R, Barbour R, Palacios WR, Nichols LG, Grau LE. Associations between injection risk and community disadvantage among suburban injection drug users in southwestern Connecticut, USA. AIDS Behav. 2014;18:452–463. doi: 10.1007/s10461-013-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer R, Binka M, Koester S, Grund JC, Patel A, Paintsil E, Lindenbach BD. Recovery of infectious hepatitis c virus from injection paraphernalia: Implications for prevention programs serving people who inject drugs. J Infect Dis. 2018;217:466–173. doi: 10.1093/infdis/jix427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green SE, editors. Cochrane handbook for systematic reviews of interventions, Version 5.1.0. The Cochrane Collaboration. 2011;2011 http://handbook.cochrane.org. [Google Scholar]
- Jones JL, Wykoff RF, Hollis SL, Longshore ST, Gamble WB, Jr, Gunn RA. Partner acceptance of health department notification of HIV exposure, South Carolina. JAMA. 1990;264:1284–1286. [PubMed] [Google Scholar]
- Kane S, Dotson CJ. HIV risk and injecting drug use: Implications for rural jails. Crime Delinq. 1997;43:169–185. [Google Scholar]
- Knittel AK, Wren PA, Gore L. Lessons learned from a peri-urban needle exchange. Harm Reduct J. 2010;7 doi: 10.1186/1477-7517-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leukefeld CG, Farabee D, McDermeit M, Dennis ML, Wechsberg WM, Inciardi JA, Surratt HL, Compton WM, Cottler LB, Klein H, Hoffman JA, Desmond D, Logan TK. Real and perceived HIV risk by population density: An exploratory examination. J Drug Issues. 2001;31:889–904. [Google Scholar]
- Luoma JB, Twohig MP, Waltz T, Hayes SC, Roget N, Padilla M, Fisher G. An investigation of stigma in individuals receiving treatment for substance abuse. Addict Behav. 2007;32:1331–1346. doi: 10.1016/j.addbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- MacMaster SA, Tripp K, Argo S. Perceptions of HIV risk behaviors and service needs among methamphetamine users in rural Appalachian Tennessee. J Ethn Subst Abuse. 2008;7:115–130. doi: 10.1080/15332640802083329. [DOI] [PubMed] [Google Scholar]
- McCoy CB, Metsch LR, Inciardi JA, Anwyl RS, Wingerd J, Bletzer K. Sex, drugs, and the spread of HIV/AIDS in Belle Glade, Florida. Med Anthropol Q. 1996;10:83–93. doi: 10.1525/maq.1996.10.1.02a00090. [DOI] [PubMed] [Google Scholar]
- McCoy CB, Metsch LR, McCoy HV, Weatherby NL. HIV seroprevalence across the rural/urban continuum. Subst Use Misuse. 1999;34:595–615. doi: 10.3109/10826089909037233. [DOI] [PubMed] [Google Scholar]
- Meiman J, Tomasallo C, Paulozzi L. Trends and characteristics of heroin overdoses in Wisconsin, 2003–2012. Drug Alcohol Depend. 2015;152:177–184. doi: 10.1016/j.drugalcdep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Eliminating the public health problem of hepatitis b and c in the United States: Phase one report. The National Academies Press; Washington, DC: 2016. [PubMed] [Google Scholar]
- NIDA. Seek-Test-Treat-Retain To Stop the Spread of HIV. 2013 https://www.drugabuse.gov/news-events/nida-notes/2013/02/seek-test-treat-retain-to-stop-spread-hiv. (Accessed October 22 2017).
- Oser CB, McDonald HMS, Havens JR, Leukefeld CG, Webster JM, Cosentino-Boehm AL. Lack of HIV seropositivity among a Group of rural probationers: Explanatory factors. J Rural Health. 2006;22:273–275. doi: 10.1111/j.1748-0361.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban–rural status and by drug type. Pharmacoepidemiol Drug Saf. 2008;17:997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, Blosser SJ, Spiller MW, Combs B, Switzer WM, Conrad C, Gentry J, Khudyakov Y, Waterhouse D, Owen SM, Chapman E, Roseberry JC, McCants V, Weidle PJ, Broz D, Samandari T, Mermin J, Walthall J, Brooks JT, Duwve JM, et al. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. N Engl J Med. 2016;375:229–239. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- Pollini RA. Self-reported participation in voluntary nonprescription syringe sales in California’s Central Valley. J Am Pharm Assoc. 2017;57:677–685. doi: 10.1016/j.japh.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Compton WM, Horton JC, Cottler LB, Cunningham-Williams RM, Booth R, Singer M, Leukefeld C, Fink J, Stopka TJ, Corsi KF, Tindall MS. Injection drug users report good access to pharmacy sale of syringes. J Am Pharm Assoc (Wash) 2002;42:S68–72. doi: 10.1331/1086-5802.42.0.s68.reich. [DOI] [PubMed] [Google Scholar]
- RFA-DA-17-014. HIV, HCV and Related Comorbidities in Rural Communities Affected by Opioid Injection Drug Epidemics in the United States: Building Systems for Prevention, Treatment and Control (UG3/UH3) National Institutes of Health, Centers for Disease Control and Prevention, Substance Abuse and Mental Health Services Administration, and Appalachian Regional Commission; 2016. [Google Scholar]
- Rhodes T. The ‘risk environment’: A framework for understanding and reducing drug-related harm. Int J Drug Policy. 2002;13:85–94. [Google Scholar]
- Rossen LM, Khan D, Warner M. Trends and geographic patterns in drug-poisoning death rates in the U.S., 1999–2009. Am J Prev Med. 2013;45:e19–e25. doi: 10.1016/j.amepre.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DM, Trepka MJ, Fennie KP, Prado G, Madhivanan P, Dillon FR, Maddox LM. Individual and neighborhood predictors of mortality among HIV-positive Latinos with history of injection drug use, Florida, 2000–2011. Drug Alcohol Depend. 2015;154:243–250. doi: 10.1016/j.drugalcdep.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton-Tindall M, Harp KL, Minieri A, Oser C, Webster JM, Havens J, Leukefeld C. An exploratory study of mental health and HIV risk behavior among drug-using rural women in jail. Psychiatr Rehabil J. 2015a;38:45–54. doi: 10.1037/prj0000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton-Tindall M, Webster JM, Oser CB, Havens JR, Leukefeld CG. Drug use, hepatitis C, and service availability: perspectives of incarcerated rural women. Soc Work Public Health. 2015b;30:385–396. doi: 10.1080/19371918.2015.1021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopka TJ, Singer M, Teng W, Horton J, Compton W. Pharmacy access to over-the-counter syringes in Connecticut: Implications for HIV and hepatitis prevention among injection-drug users. AIDS Public Policy J. 2002;17:115–126. [Google Scholar]
- Strathdee SA, Beletsky L, Kerr T. HIV, drugs and the legal environment. Int J Drug Policy. 2015;26:S27–32. doi: 10.1016/j.drugpo.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig J, Junge B, Burris S, Jones TS, Sterk CE. Individual and structural influences shaping pharmacists’ decisions to sell syringes to injection drug users in Atlanta, Georgia. J Am Pharm Assoc (Wash) 2002;42:S40–45. doi: 10.1331/1086-5802.42.0.s40.taussig. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. Urban and Rural Classification and Urban Area Criteria. 2010 https://www.census.gov/geo/reference/ua/urban-rural-2010.html (Accessed October 22, 2017).
- United States Department of Agriculture Economic Research Service. Rural-Urban Continuum Codes. 2013 https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx (Accessed October 22, 2017)
- Van Handel MM, Rose CE, Hallisey EJ, Kolling JL, Zibbell JE, Lewis B, Bohm MK, Jones CM, Flanagan BE, Siddiqi AE, Iqbal K, Dent AL, Mermin JH, McCray E, Ward JW, Brooks JT. County-Level Vulnerability Assessment for Rapid Dissemination of HIV or HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr. 2016;73:323–331. doi: 10.1097/QAI.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner BD, Leukefeld CG. Assessing the differential impact of an HIV prevention intervention: Who’s putting the message into practice? AIDS Educ Prev. 2001;13:479–494. doi: 10.1521/aeap.13.6.479.21433. [DOI] [PubMed] [Google Scholar]
- Wejnert C, Hess KL, Hall HI, Van Handel M, Hayes D, Fulton P, Jr, An Q, Koenig LJ, Prejean J, Valleroy LA. Vital Signs: Trends in HIV Diagnoses, Risk Behaviors, and Prevention Among Persons Who Inject Drugs - United States. MMWR Morb Mortal Wkly Rep. 2016;65:1336–1342. doi: 10.15585/mmwr.mm6547e1. [DOI] [PubMed] [Google Scholar]
- Wykoff RF, Jones JL, Longshore ST, Hollis SL, Quiller CB, Dowda H, Gamble WB. Notification of the sex and needle-sharing partners of individuals with human immunodeficiency virus in rural South Carolina: 30-month experience. Sex Transm Dis. 1991;18:217–222. doi: 10.1097/00007435-199110000-00004. [DOI] [PubMed] [Google Scholar]
- Young AM, Jonas AB, Havens JR. Social networks and HCV viraemia in anti-HCV-positive rural drug users. Epidemiol Infect. 2013a;141:402–411. doi: 10.1017/S0950268812000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Jonas AB, Mullins UL, Halgin DS, Havens JR. Network structure and the risk for HIV transmission among rural drug users. AIDS Behav. 2013b;17:2341–2351. doi: 10.1007/s10461-012-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M, Stuber J, Ahern J, Galea S. Interpersonal discrimination and the health of illicit drug users. Am J Drug Alcohol Abuse. 2005;31:371–391. doi: 10.1081/ada-200056772. [DOI] [PubMed] [Google Scholar]
- Zibbell JE, Hart-Malloy R, Barry J, Fan L, Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health. 2014;104:2226–2232. doi: 10.2105/AJPH.2014.302142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, Serrecchia J, Blankenship S, Ward JW, Holtzman D. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64:453–458. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


