Abstract
The adeno-associated virus (AAV) vector has been used in preclinical and clinical trials of gene therapy for central nervous system (CNS) diseases. One of the biggest challenges of effectively delivering AAV to the brain is to surmount the blood-brain barrier (BBB). Herein, we identified several potential BBB shuttle peptides that significantly enhanced AAV8 transduction in the brain after a systemic administration, the best of which was the THR peptide. The enhancement of AAV8 brain transduction by THR is dose-dependent, and neurons are the primary THR targets. Mechanism studies revealed that THR directly binds to the AAV8 virion, increasing its ability to cross the endothelial cell barrier. Further experiments showed that binding of THR to the AAV virion did not interfere with AAV8 infection biology, and that THR competitively blocked transferrin from binding to AAV8. Taken together, our results demonstrate, for the first time, that BBB shuttle peptides are able to directly interact with AAV and increase the ability of the AAV vectors to cross the BBB for transduction enhancement in the brain. These results will shed important light on the potential applications of peptides for enhancing brain transduction with systemic administration of AAV vectors.
Keywords: adeno-associated virus, gene therapy, blood-brain barrier shuttle peptide, THR, brain transduction, systemic administration
Graphical Abstract
1. Introduction
Recombinant adeno-associated virus (rAAV) vectors are one of the most promising vehicles for therapeutic gene delivery [1, 2]. Following from extensive preclinical studies, there are numerous ongoing clinical trials using rAAV vectors and great success has been achieved for patients with blindness and hemophilia [3–7]. rAAV vectors have also shown promise as a platform for the treatment of neurological disorders [8, 9]. Upon intraparenchymal injection in mice, some serotypes have demonstrated the ability to efficiently transduce neurons. Of the 13 identified AAV serotypes, serotypes 7, 8, and 9 were shown to effectively transduce neurons [10]. It is worth noting that intraparenchymal injection, adopted by the majority of clinical trials, can successfully deliver the AAV vectors to a local area of the brain. However, most neurodegenerative disorders, such as amyotrophic lateral sclerosis, frontotemporal dementia, Rett syndrome, and Huntington's disease, involve cell damage in multiple areas. Due to the limitations of a local transduction and the side effects of intraparenchymal injection, systemic administration of AAV vectors has been explored for whole brain transduction. AAV9 possesses the remarkable ability to transduce parenchymal brain cells and portions of the BBB endothelium after intravenous injection. Systemic administration of AAV9 results in a widespread neuron gene transfer in the neonatal brain, but has less neuron transduction efficacy in adults [11].
Genetically modifying the viral capsid is commonly used to improve AAV transduction or achieve an anticipated tissue tropism [12–15]. Several groups have developed rAAV vectors with enhanced gene transfer to the CNS after intravenous delivery [16–19]. One study found that one AAV8 mutant (AAV-B1), with 19 amino acid changes and derived from in vivo direct evolution of the AAV shuffling library, resulted in a widespread brain transduction after its systemic administration [16]. A recent study using a novel capsid selection method, named Cre recombinase-based AAV targeted evolution (CREATE), identified an AAV9 variant (AAV.PHP.B), which could transduce a majority of astrocytes and neurons across the CNS when compared to AAV9 after its systemic administration [18]. Another study found that AAV9 vectors with tyrosine capsid mutations significantly enhanced gene delivery to the CNS after systemic injections in neonatal mice [19]. While successful brain neuron transduction in a mouse model has been achieved with these mutants, effective preclinical animal studies with engineered rAAV vectors are not always predictive of a desirable human outcome [20–23], Additionally, it is currently impossible to test these mutants in the human brain, leading to efficacy concerns about the methods [5, 24–26]. In addition to the genetic modification of the AAV capsid, other attempts to augment brain delivery have involved the utilization of mannitol and focused ultrasound in combination with microbubbles [27]. However, these methodologies have the risk of adverse side effects, such as edema, seizures, and the development of neurological disorders. Therefore, it is imperative to explore novel strategies that could effectively increase the ability of rAAV vector to overcome the BBB and safely enhance the brain neuron transduction after a systemic administration.
Among the non-invasive approaches, BBB shuttle peptides have proved their potential in preclinical research and clinical trials, in part because of their ease of affordable access, lower immunogenicity, and higher chemical versatility [28]. BBB shuttle peptides are stably capable of transporting a variety of cargo, such as proteins, antibodies, or nanoparticles, into the brain parenchyma without disrupting the BBB integrity [29, 30]. Of note, several targeted peptides with the ability to cross the BBB for brain delivery have been identified [28]. For example, mediated by a low-density lipoprotein (LDL) receptor-related protein-1, the Angiopep-2 peptide, was shown to effectively transport a wide variety of particles across the BBB [31]. The THR peptide was identified to specifically bind to the transferrin receptor 1 (TfR1) by biopanning a phage display peptide library on a stable TfR1-expressed chicken embryo fibroblasts cell line [32]. It has been demonstrated that the THR peptide is able to be conjugated with gold nanoparticles to increase the permeability of the conjugate in the brain [33]. Previously, we showed that some human serum proteins, such as human serum albumin (HSA), transferrin, and LDL, are able to directly bind to AAV8 and enhance its transduction in the mouse liver [34, 35]. Other research has shown that some cell-penetrating peptides (CPPs) can bind to AAV virions and increase transduction [36]. Based on these observations, we hypothesized that specific BBB shuttle peptides are able to bind to AAV8 virions and increase their ability to cross the BBB for enhanced brain transduction. In this study, we tested several potential BBB permeable peptides and found that the BBB shuttle peptide THR could significantly enhance the brain transduction of AAV8 by crossing the endothelial cell barrier and then transducing in neuronal cells after systemic administration.
2. Materials and methods
2.1. Cell lines and Peptides
HEK293 and human Huh7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10 % (v/v) of heat inactivated fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL). Variant Chinese hamster ovary (CHO)-TRVB cells, devoid of the endogenous TfR1, were kindly provided by Dr. T. E. McGraw (Weill Cornell Medical College, NY) and were grown in F12 mix medium (Lonza, MD, USA) supplemented with 5 % FBS. Human brain endothelial capillary hCMEC/D3 cells (Millipore, USA) were plated in collagen I (Thermo Fisher Scientific, USA)-coated dishes and grown in EBM-2 medium (Lonza, USA) supplemented with 0.025 % vascular endothelial growth factor, 0.025 % LONG® R3 IGF-I, 0.025 % hEGF, 0.0025 % hBFGF, 0.01 % hydrocortisone, penicillin (100 U/mL), streptomycin (100 μg/mL), and 2.5 % FBS. All cells were maintained at 37 °C in an atmosphere of 5 % CO2. All peptides with > 95 % purity (Table 1) were synthesized by GenScript (NJ, USA) and KareBay Biochem, Inc. (NJ, USA). Peptides (stock solution, 10 mM) were dissolved in Dulbecco's phosphate-buffered saline (DPBS) with 10 % DMSO. The 5’-FAM labeled THR peptide was synthesized by High-Throughput Peptide Synthesis and Array Core Facility at the UNC at Chapel Hill.
Table 1.
Sequences and polarity of peptides used in the study
| Peptides | Sequences | Polarity | Proposed transporter |
|---|---|---|---|
| LAH4 | KKALLALALHHLAHLALALKKAC | Basic | N/A |
| Angiopep-2 GSH | TFFYGGSRGKRNNFKTEEY-OH r-g-L-glutamyl-CG-OH | Basic | LRP1 GSH transporter |
| HIV-1 TAT(48 – 60) | GRKKRRQRRRPPQ | Basic | AMT |
| ApoE (159–167)2 | (LRKLRKRLL)2 | Basic | LRP1, LRP2, LDLR |
| Leptin 30 (61–90) | YQQILTSMPSRNVIQISNDLENLRDLLHVL | Neutral | Leptin receptor |
| THR | THRPPMWSPVWP-NH2 | Basic | TfR1 |
| PEPXT-1 | FILMVWAPFI | Neutral | N/A |
| PEPXT-2 | STNQSTNQST | Neutral | N/A |
| PEPXT-3 | DEDEDEDEDE | Acid | N/A |
| PEPXT-4 | RKHRKHRKHR | Basic | N/A |
2.2. Plasmid construction
Plasmids mCherry-TFR-20 (Addgene #55144), expressing human TfR1, and pAcGP67A-murine TfR (Addgene #12392), expressing mouse TfR1, were purchased from Addgene. The mCherry-mTfR-20 plasmid was constructed by PCR with Phusion High-Fidelity DNA polymerase (NEB, USA). Briefly, the mouse TfR1 gene was amplified from the pAcGP67A-murine TfR plasmid using the mTfR1-fwd primer and mTfR1-rev primer (Table S1). The resulting amplicon was digested with restriction enzymes XhoI I and EcoRI I, and then used to replace the human TfR1 gene in the mCherry-TFR-20 plasmid.
2.3. Virus production
Recombinant AAV full particles expressing luciferase or eGFP, driven by the CBA promoter or the CBh promoter, respectively, were produced using a triple transfection in HEK293 cells as previously described [13]. In brief, the AAV transgene plasmid pTR/CBA-luc, AAV helper plasmid containing AAV Rep and Cap genes, and Ad helper plasmid pXX6-80 were co-transfected into HEK293 cells. HEK293 cells were collected and were lysed 48 h post-transfection. The supernatant was then subjected to a CsCl gradient ultra-centrifugation. Fractions containing AAV were collected and viral titer was determined by real-time quantitative PCR (qPCR) using the Light Cycler 480 instrument with SYBR green (Roche, USA) and a pair of primers (Table S1) that were designed to bind to a homologous sequence on the inverted terminal repeats (ITR) region.
2.4. MTT assay
The 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay was performed to measure the cytotoxicity of peptides using the Vybrant® MTT cell proliferation assay kit (Thermo Fisher Scientific, USA) according to the manufacture’s instruction. In brief, cells were grown in a 48-well plate and transduced with the AAV8/luc and peptides complex. After 48 h, the medium was removed and replaced with fresh culture medium. MTT (10 μl) was added to the cells and incubated for 4 h at 37 °C, then 100 μl of the SDS-HCl solution was added into the wells and further incubated for 4 h at 37 °C. Absorption was measured at 595 nm using the Victor instrument (Perkin-Elmer, MA).
2.5. In vitro transduction assay
At least 4–5 h before AAV transduction, the cells were seeded in 48-well plates with a density of 1×105 cells per well. THR at different concentrations (0.001 mM, and 0.01 mM) were incubated with AAV8/luc at 4 °C for 2 h. Then the cells were infected with 1×104 vector genomes (vg) per cell with AAV8/luc virus or the incubated complex of AAV8-THR and harvested after 48 h. Luciferase activity was measured using the Promega Luciferase assay system based on the manufacturer’s instructions (Promega, Madison, WI).
2.6. AAV8 binding assay
The complex of AAV8/luc and THR or human apo-transferrin protein (Sigma, 616395, USA) was incubated at 4 °C for 2 h. Either 10,000 vg of the AAV8/luc virus alone or the complex was added into Huh7 cells at a cell density of 4×105 per well for 1 h at 4 °C. The cells were then washed three times with DPBS. Genome DNA (gDNA) was extracted from the cells with the DNeasy blood & tissue kit (Qiagen, USA) and was quantified by qPCR with luciferase primers and reference GAPDH primers (Table S1).
2.7. Transcytosis assay on the hCMEC/D3 cell line
The hCMEC/D3 cells were seeded into Transwell®-COL collagen-coated membrane inserts (24 Well Permeable Support with 0.4 μm Pore Polycarbonate Membrane and 6.5 mm Inserts, Sigma, USA) at a density of 5×104 cells per well in EBM-2 cultured medium. The medium was changed every 2–3 days. After about 2 weeks, the cells were washed with DPBS and cultured in serum-free EBM-2 medium. Three cohorts were designed: AAV8 incubated with DPBS at 4 °C for 2 h (AAV8), a brief mixture of AAV8 with 0.1 mM THR prior to infecting cells (AAV8/0.1 mM THR), and AAV8 incubated with 0.1 mM THR at 4 °C for 2 h (AAV8-0.1 mM THR). The hCMEC/D3 cells on the trans-membrane were treated with the AAV8 vector, the medium in the basal chamber was collected at the different time points of 0.25-, 0.5-, 2-, 4-, 6-, and 24- h. Viral titers were calculated by qPCR according to established procedures using primers that were designed against the ITR region (Table S1).
2.8. AAV8 competitive binding assay
For THR competitive binding analysis, 10 μl of Protein G resin (Thermo Fisher Scientific, USA) was incubated with 1 μg of either a human transferrin (hTf) antibody or a goat IgG antibody control at 4 °C overnight. The next day, 1×1010 vg of AAV8/luc was incubated with HSA of physiological concentration, THR or the PEPXT-1 control peptide at different dilutions, or DPBS on ice for 2 h. HTf was added into the complex at a 1:100 dilution of the physiological concentration and incubated on ice for another 2 h. After that, the mixture was added to the complex of protein G resin and transferrin antibody and then incubated at 4 °C overnight. Finally, the complex was stringently washed three times with cold DPBS. DNA from the complex was extracted and subjected to qPCR to determine AAV genome copy number per cell using luc specific primers (Table S1).
2.9. Animal study
C57BL/6 female mice, at 5–6 weeks of age, were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were randomly divided into groups of 5 animals each and maintained in a specific pathogen-free facility at the UNC-Chapel Hill. All procedures were approved by the UNC Institutional Animal Care and Use Committee. Mice were administered either AAV8/Luc or scAAV8-CBh-eGFP incubated with peptides via the retro-orbital injection. Following the intraperitoneal injection of a D-luciferin substrate (Nanolight Pinetop, AZ), luciferase expression was imaged at the indicated time points using a Xenogen IVIS Lumina (Caliper Lifesciences, Waltham, MA). Bioluminescent images were analyzed using Living Image (PerkinElmer, Waltham, MA). At the time points of 5 min, 2-, 24-, and 48- h after injection, blood was collected from the retro-orbital plexus, and the viral titers were determined by qPCR.
2.10. Quantitation of luciferase expression in tissues
Animals utilized for imaging studies were sacrificed either three or one week after imaging work. The tissues of the heart, liver, spleen, skeletal muscle, and brain were collected, minced, and homogenized in passive lysis buffer. The lysates were centrifuged at 12,000 g for 5 min to remove cellular debris. The supernatant was transferred to 96-well plates for luciferase activity analysis as described above. Total protein concentration in tissue lysates was measured using the Bradford assay (Bio-Rad, Philadelphia, PA).
2.11. Measurement of AAV genome copy number in the tissues
The different minced tissues and the blood collected at different time points were treated with Protease K, and the total gDNA was isolated using the DNeasy blood & tissue kit (Qiagen, USA). The luciferase gene was measured by qPCR assay. The mouse lamin gene served as an internal control.
2.12. Histological processing and immunohistochemistry
Mice were anesthetized 4 weeks post-injection and transcardially perfused with 253ml of DPBS followed by 153ml of ice-cold 4 % paraformaldehyde (PFA) in DPBS. The brains were extracted and post-fixed in 4 % PFA overnight at 4 °C. Brains were then dehydrated in 30 % sucrose in DPBS overnight at 4 °C. Serial 40 μm free-floating sections of the entire brain were cut with a Cryostat (Leica Biosystems, USA). Immunohistochemistry (IHC) was performed on floating sections with primary and secondary antibodies. In brief, free-floating brain sections were permeated in Tris-buffered saline (TBS) containing 0.5 % Triton X-100 (Sigma, St. Louis, MO) (TBST) for 30 min at RT, and then blocked in 0.05 % TBST containing 3 % donkey serum (Sigma, D9663, St. Louis, MO) for 1 h at RT. Then sections were incubated at 4 °C overnight with primary antibodies diluted in blocking buffer. The following day, tissue sections were washed in 0.05 % TBST and incubated with the appropriate secondary antibodies in blocking buffer for 2 h at RT. Finally, the sections were washed and were mounted onto slides with DAPI (Sigma, St. Louis, MO). The whole brain was examined under an Olympus epifluorescence microscope for general GFP expression. Z-stack confocal imaging was acquired for the region with the most abundant GFP expression. All images were captured on a Zeiss LSM 710 Spectral Confocal Laser Scanning Microscope using a 20- objective. The primary and second antibodies used in this study were as follows: rabbit anti-GFP (Abcam, Cambridge, MA, ab6556), goat anti-CD31 (Abcam), mouse anti-NeuN (Millipore, MAB377, Billerica, MA), rat anti-GFAP (Thermo Fisher Scientific, 13-0300, USA). Alexa Fluor 488-conjugated donkey anti-rabbit (Invitrogen A21206), Alexa Fluor 594-conjugated donkey anti-goat (Invitrogen A11058), Alexa Fluor 594-conjugated donkey anti-mouse (Invitrogen A21203), and Alexa Fluor 594-conjugated donkey anti-rat (Invitrogen A21209).
2.13. Statistical analysis
All of the quantitative data is presented as mean3±3standard deviation (SD) using GraphPad Prism 6.0 (GraphPad, La Jolla, CA). All of the statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test. Only the p values of < 0.05 were considered statistically significant. Graphs are representative of the data sets from at least three independent assays.
3. Results
3.1. BBB shuttle peptides enhance AAV8 transduction in the brain
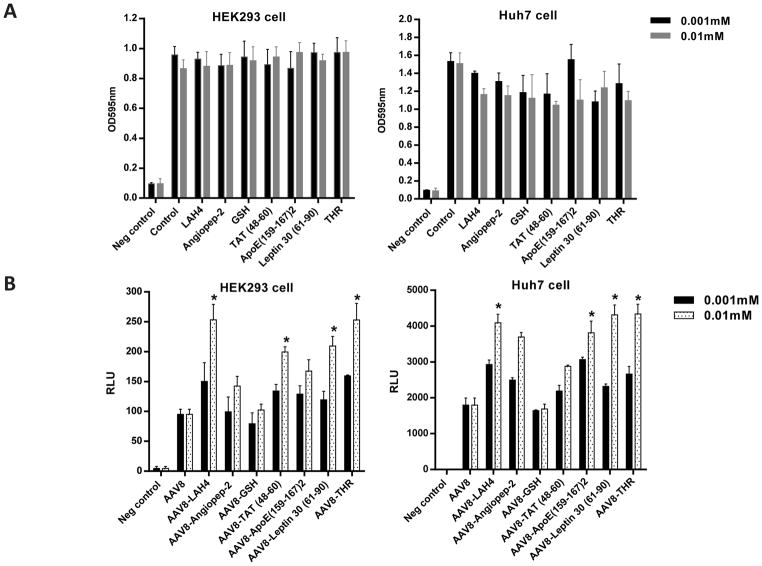
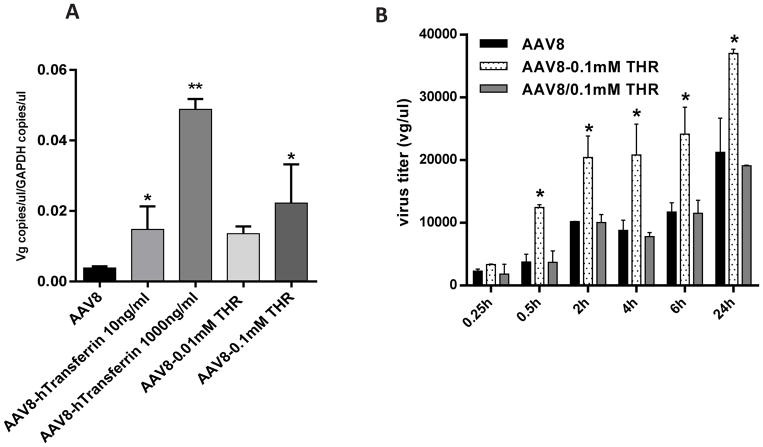
To identify the potential peptides that enhanced AAV8 brain transduction after a systemic administration, we screened several reported peptides based on their predicted actions on the BBB (Table 1). First, we tested the cytotoxicity of each peptide at different concentrations on either HEK293 or Huh7 cells in culture using the MTT assay. No cytotoxicity was observed in the cells treated with the peptides (Fig. 1A). Next, we studied the effect of the peptides on rAAV transduction in vitro. After incubating 10,000 vg of AAV8/luc with either 0.001 mM or 0.01 mM of the peptides for 2 h at 4 °C, the mixtures were applied to either HEK293 or Huh7 cells. The cell lysate was collected after 48 h for luciferase analysis. Several peptides, including LAH4, HIV-1 TAT (48–60), leptin 30 (61–90), and THR, significantly enhanced AAV8 transduction in both cell lines (Fig. 1B). Of these, THR increased AAV8 transduction the most.
Fig. 1. Screening of the peptides to enhance AAV8 transduction without cytotoxicity in vitro.
(A) Different concentrations of the peptides (0.001 mM and 0.01 mM) were first incubated with 1×108 vg of AAV8/luc at 4 °C for 2 h, and then added into 1×105 cells for 48 h. After that, the cytotoxicity of the peptides was measured using the MTT assay. (B) The transduction efficiency of the different AAV8 (1×104 vg per cell) and peptide complexes were tested in the HEK293 and Huh7 cell lines. *p < 0.05 compared to the AAV8 transduction alone group. All error bars represent the SD of the mean from at least a triplicate of experiments.
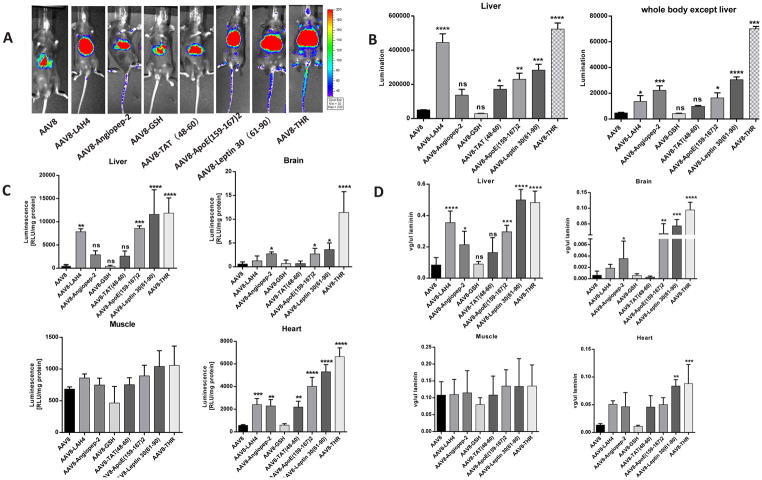
Previous research has shown that the THR peptide can interact with the human TfR1 [32]. Here, the immunofluorescence assay results showed that THR could also interact with the mouse TfR1 in CHO-TRVB cells (Fig. S1). Next, we assessed the effect of the peptides on AAV transduction in vivo. The AAV8/luc vector (5×1010 vg) was incubated with 0.1 mM of peptides for 2 h at 4 °C and injected into C57BL/6 mice via the retro-orbital vein. After 7 days, the images were taken. The transgene expression in the liver was higher in the AAV8 vectors treated with peptides LAH4, Angiopep-2, TAT, ApoE (159–167)2, leptin 30 (61–90), and THR (Fig. 2A). Consistent with the result observed in cell lines, the THR peptide induced the greatest level of transduction throughout the whole body, including in the brain (Fig. 2B). We also evaluated luciferase gene expression and viral genome number by qPCR in different tissues. Consistent with the imaging results, the incubation of peptides Angiopep-2, ApoE (159–167)2, leptin 30 (61–90), and THR increased the rAAV8 genome and mRNA expression in the brain (Fig. 2C, D). The bio-distribution analysis showed that a much higher AAV genome copy number was detected in the brains of the mice receiving AAV8 vectors treated with peptides, including Angiopep-2, ApoE (159–167)2, leptin 30 (61–90), and THR. A higher AAV genome copy number was not detected in the brains of the mice receiving AAV8 vectors treated with LAH4 and HIV-1 TAT (48–60), although an increased liver transduction was achieved by these peptides (Fig. 2C, D). These results indicate that BBB shuttle peptides can increase the ability of the AAV8 vector to cross the BBB and enhance brain transduction.
Fig. 2. THR peptide significantly enhances AAV8 transduction in vivo, especially in the brain.
5×1010 vg of AAV8/luc alone or the complex of pre-incubated AAV8 and 0.1 mM of peptide at 4 °C for 2 h were administered via an intravenous injection. At one-week post- injection, in vivo luminescence imaging (A) was performed and the photon signal (B) was measured and calculated. At three-week post-injection, the mice were euthanized and their tissues were harvested for DNA extraction. Relative luciferase expression levels (C) and vector copy numbers (D) of different tissue lysates were determined separately. The data of each group represent the average and SD from five mice. *** p < 0.001, ** p < 0.01, and *p < 0.05 compared to the control mice with an AAV8 treatment only. The “ns” indicates no significant difference (p> 0.05).
It is well known that polarity plays an important role in peptide function. To rule out the possibility of a non-specific peptide interaction with AAV8 virions increasing the ability of AAV to cross the BBB and enhance brain transduction, we designed four control peptides with different polarities (Table 1). The images were taken seven days after the systemic injection of AAV8/luc and peptide complexes into the C57BL/6 mice. There was no observed enhancement of transgene expression in the liver or the brain following the treatment with these control peptides (Fig. S2). These results, in combination with those obtained using peptides LAH4 and HIV-1 TAT (48–60), indicate that the BBB shuttle peptides specifically facilitate AAV8 virions to increase the ability of AAV8 to cross the BBB and enhance brain transduction.
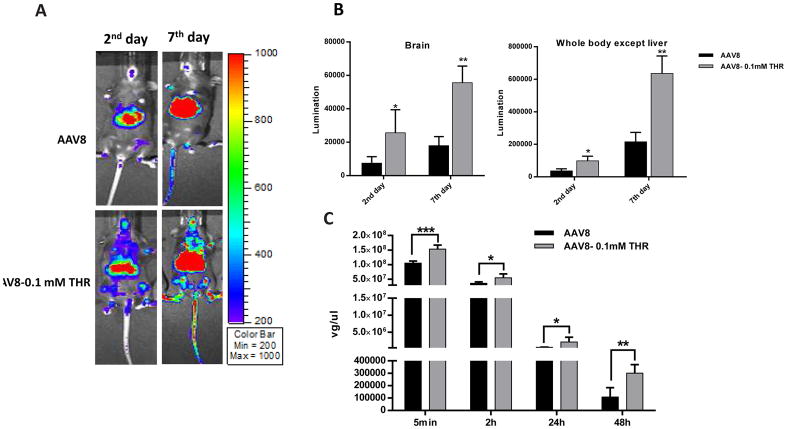
3.2. The effect of THR on enhanced AAV8 transduction is dose-dependent
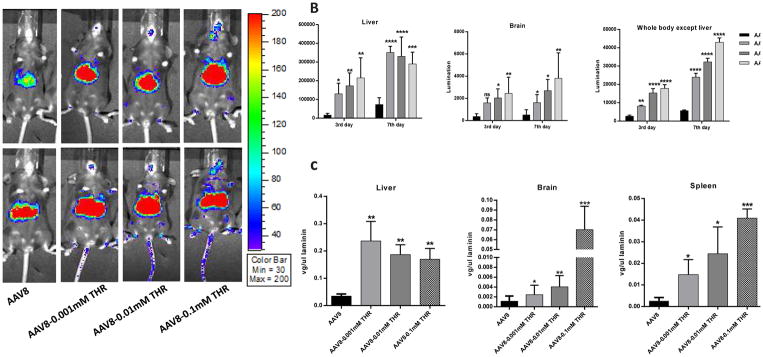
Given that the THR peptide was the most effective at enhancing the transduction of AAV8, it was chosen for the following experiments. To verify the effect of the THR peptide further, six-week old female C57BL/6 mice received 5×1010 particles of AAV8/luc pre-incubated with different concentrations of the THR peptide (0.001 mM, 0.01 mM, and 0.1 mM) for 2 h at 4 °C via the retro-orbital injection. Luciferase expression showed that a higher brain transduction was achieved at days 3 and 7 with the higher concentration of THR (Fig. 3A, B). We also measured the viral genome number in the various tissues. As expected, it was achieved that increased genomic copy number in the THR treated groups compared to the AAV8 alone in the liver, brain, and spleen (Fig. 3C). Surprisingly, slightly decreased transduction and genomic copy number in the liver of the group with higher concentration of THR at day 7 were observed, even though no significant difference among them. One possible explanation is that higher concentration of THR accelerates distribution of AAV8 in the whole body.
Fig. 3. THR enhances AAV8 transduction in vivo in a dose-dependent manner, especially in the brain.
(A) On days 3 and 7 after the administration of AAV8 or different doses of complexes, the images were taken for luminescence analysis. (B) The average luciferase signal for the liver and whole body (excluding the liver) in the mice treated with different doses of THR was calculated. (C) The gene copy numbers in liver, brain, and spleen were determined separately. The data of each group represents the average and SD from five mice. *** p < 0.001, ** p < 0.01, and *p < 0.05 compared to control mice with AAV8 treatment only. The “ns” indicates no significant difference (p> 0.05).
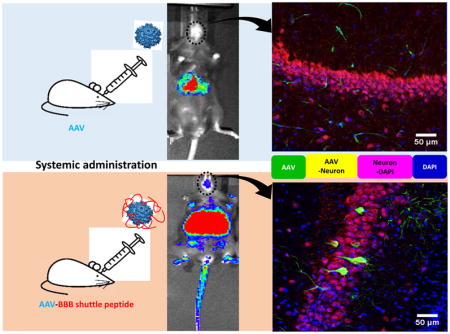
3.3. THR promotes AAV8 crossing the BBB and targeting neuron cells in the brain
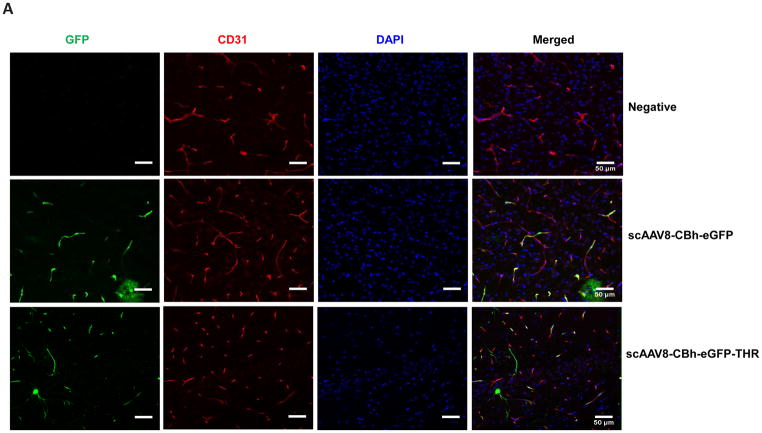
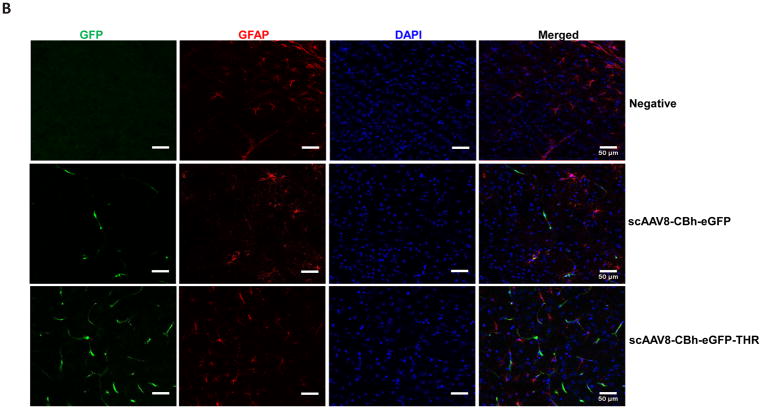
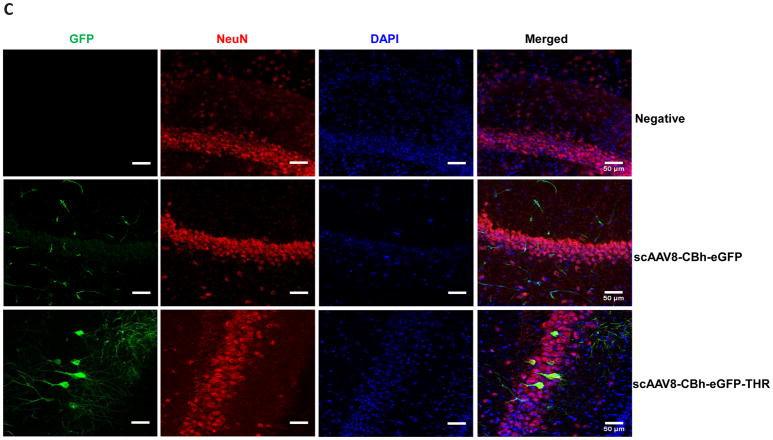
Based on the transgene expression and AAV genome copy number, systemic administration of AAV8/luc vectors pre-incubated with THR induced high transduction in the brain. The luciferase analysis results were unable to differentiate whether the high transduction levels were from either the endothelial or brain parenchymal cells. Therefore, we assessed the transgene expression in different cells of the brain 4 weeks after the intravenous injection of 2×1011 vg of scAAV8-CBh-eGFP pre-incubated with 0.4 mM of THR (scAAV8-eGFP-THR) for 2 h at 4 °C. An efficient transduction of brain endothelial cells was observed in the mice treated with either the scAAV8-CBh-eGFP viruses or scAAV8-CBh-eGFP-THR (Fig. 4A). A higher neuron transduction was observed in the mice receiving scAAV8-eGFP-THR than was observed in the mice treated with scAAV8-CBh-eGFP (Fig. 4C). No astrocyte transduction was observed in the mice treated with either scAAV8-CBh-eGFP or scAAV8-eGFP-THR (Fig. 4B). These results confirm the notion that THR has the potential to increases the ability of AAV8 to cross the BBB and enhance brain transduction, especially in the neuronal cells.
Fig. 4. Intravenous injection of the THR and scAAV8-CBh-eGFP complex leads to neuron cell transduction in the brain.
2×1011 vg of either scAAV8-CBh-eGFP or scAAV8-eGFP-0.4 mM THR was retro-orbitally injected into the mice (n = 3). Representative images of the EGFP expression (green) of AAV8 was colocalized with CD31 (red, endothelial marker) in the cortex (A), GFAP (red, astrocyte marker) in the cortex (B), and NeuN (red, neuronal marker) in the hippocampal CA1 region (C) in the mice brain, and were merged with DAPI (blue), were captured and assessed after 4 weeks. Scale bars, 50 μm (Objective 20× optical axis).
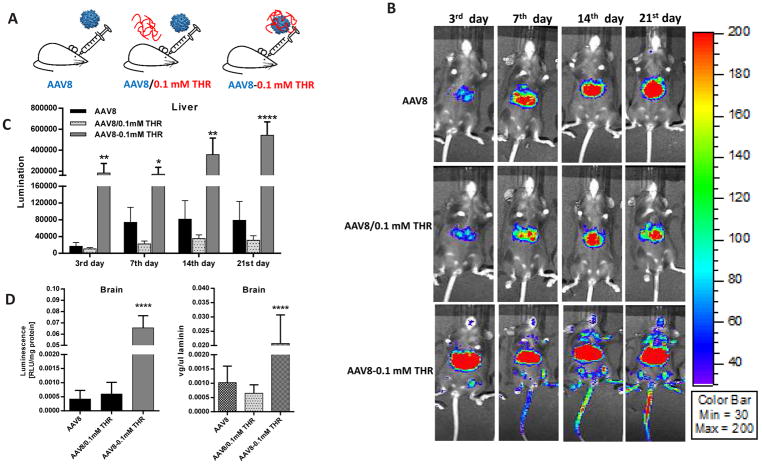
3.4. Direct interaction between THR and AAV8 is required for enhanced transduction
Incubation of THR with AAV8 enhances its brain transduction, as described above. However, it is unclear whether the effect of THR on the ability of AAV8 to cross the BBB and enhance brain transduction requires a direct interaction between THR and the AAV8 virions. To answer this question, AAV8/luc vectors were incubated with 0.1 mM of the THR peptide at 4 °C for 2 h (AAV8-0.1 mM THR cohort) and administered via the retro-orbital vein injection. The other two groups (the AAV8 vector pre-incubated with the PBS-AAV8 cohort and a brief mixture of AAV8 with 0.1 mM of THR prior to administration-AAV8/0.1 mM THR cohort) were used as the controls (Fig. 5A). The effect of systemic administration of the AAV8 and the THR peptide mixture into mice was analyzed at different time points. Surprisingly, although not significant, a slightly lower transduction was observed in the liver of the mice receiving AAV8/0.1 mM THR when compared to the control AAV8 cohort (Fig. 5B, C. One possible explanation is that the free THR in the AAV8/0.1 mM THR cohort preferably binds to the TfR1 on liver cells, and then blocks the interaction of AAV8 with the liver cells, resulting in a lower liver transduction. As expected, the AAV8-0.1 mM THR group showed significantly enhanced transduction in the brain (Fig. 5B, D). This result implies that the increased ability of AAV8 to cross the BBB requires a direct interaction between the virus and the THR peptide.
Fig. 5. THR binding to AAV8 is necessary for THR-mediated enhanced transduction.
(A) A schematic administration of the AAV8 vectors. Three groups were assigned: AAV8 only, AAV8/0.1 mM THR and AAV8-0.1 mM THR (B) Images were taken for luminescence expression on days 3, 7, 14, and 21 following the AAV8 only, AAV8/0.1 mM THR, and AAV8-0.1 mM THR after a systemic administration. (C) The average liver luciferase signal was calculated for the different groups. (D) Relative luciferase expression level and vector copy numbers in the brain of each group were determined separately. The data of each group represents the average and SD from five mice. ****p < 0.001 compared to the control mice with the AAV8 treatment only.
3.5. THR enhanced the AAV8 binding to cells and endothelial cell permeability
The THR peptide can increase vascular permeability by binding to the transferrin receptor on endothelial cells [33]. To study whether THR affects the ability of AAV8 to cross the endothelial cell layer, we first performed an AAV8 virion-binding assay in Huh7 cells. The incubation of THR with AAV8 dramatically increased the binding of the AAV8 virions to the surface of Huh7 cells, similar to that observed for hTf (Fig. 6A). We then performed an in vitro endothelial cell permeability analysis in a well-defined system using BBB hCMEC/D3 endothelial cells. We designed three groups: an AAV8 vector incubated with DPBS (AAV8/luc cohort); the addition of THR to culture medium just before the application of an AAV8 vector incubated with DPBS (AAV8/0.1 mM THR cohort); and an AAV8 vector incubated with THR (AAV8-0.1 mM THR cohort). The integrity of the BBB membrane was confirmed by an FITC-dextran fluorescence intensity analysis (Fig. S3A), and by the similar expression levels of the zona occludens-1 (ZO- 1) major junction protein of BBB (Fig. S3B, C). A significant increase in the endothelial cell permeability was observed when the AAV8 vector was pre-incubated with the THR peptide (AAV8-0.1 mM THR cohort) at every time point assessed, except for 15 min after the application of the AAV8 vector (Fig. 6B). However, no marked difference was observed between the AAV8/0.1 mM THR and AAV8 cohorts. These observations indicate that a direct interaction of AAV8 with the THR peptide increased the binding of the AAV virions to the cell surface, resulting in an enhanced ability of AAV8 to cross the BBB.
Fig. 6. THR dose dependently enhances the binding ability of AAV8 and transcytosis in hCMEC/D3 cells following AAV8 and THR complex exposure.
(A) The ability of the Huh7 cells to bind to either AAV8 alone or the AAV8 complexed with either transferrin or THR was assessed. The transduced cells were incubated at 4 °C for 1 h, and the AAV8 genome copy number was measured and normalized to GAPDH. (B) hCMEC/D3 cells were cultured in a monolayer and incubated with AAV8 only, a brief mixture of AAV8 with 0.1 mM THR prior to infecting cells (AAV8/0.1 mM THR), or the incubated AAV8-0.1 mM THR complex. The media in the basal chamber was collected at different time points and the viral titer was analyzed by qPCR. All treatments were performed in triplicate. *p < 0.05, **p < 0.01 compared to cells with the AAV8 treatment only.
3.6. THR decreases AAV8 clearance in blood
Slow AAV vector clearance may increase its global transduction [37]. Next, we examined whether the interaction of THR with the AAV8 virions affected its clearance in the blood after a systemic administration. C57BL/6 mice were intravenously administered a high dose (1×1011 vg per mouse) of AAV8/luc pre-incubated with THR (AAV8-0.1 mM THR). The blood was collected at the indicated time points, and the AAV genome copy number in the plasma was detected via quantitative PCR. Consistent with previous findings, the THR peptide enhanced the AAV8 transduction in the brain (Fig. 7). The mice injected with the AAV8-0.1 mM THR complex consistently exhibited a delayed blood clearance at both the early and late time points. This observation may suggest that a slow clearance of the AAV vector increases the brain transduction after a systemic administration [37].
Fig. 7. THR enhanced AAV8 transduction by decreasing AAV8 clearance in the blood.
(A, B) The mice were immunized with the incubated complex of 2×1011 vg of AAV8/luc and 0.1 mM THR peptide via a retro-orbital injection. The luciferase expression was measured on days 2 and 7. (C) The blood was also collected from the retro-orbital plexus at various time points as shown after injection, and the viral titers were tested by qPCR. The data represents the average and SD from five mice. Asterisks indicate the statistical significance when compared to the AAV8-CBA-luc alone treatment group (*p < 0.05; **p < 0.01, ***p < 0.001).
3.7. THR does not affect AAV8 infection biology
To determine whether THR could interfere with the infection biology of AAV8, we performed in vitro neutralization assays. Complexes of AAV8/luc and various concentrations of THR were incubated at 4 °C for 2 h, and then different dilutions of mouse AAV8 sera were incubated with either the AAV8/luc virus or the AAV8/luc and THR complex for 1 h at 37 °C. The various combinations of AAV8, mouse sera, and THR were then applied to HEK293 or Huh7 cells and the luciferase expression was detected after 48 h. The presence of THR, regardless of the concentration, did not change the sera neutralizing antibody (Nab) titer against AAV8 (Fig. S4). This result suggests that the interaction between THR and the AAV8 virions does not influence AAV8 transduction biology.
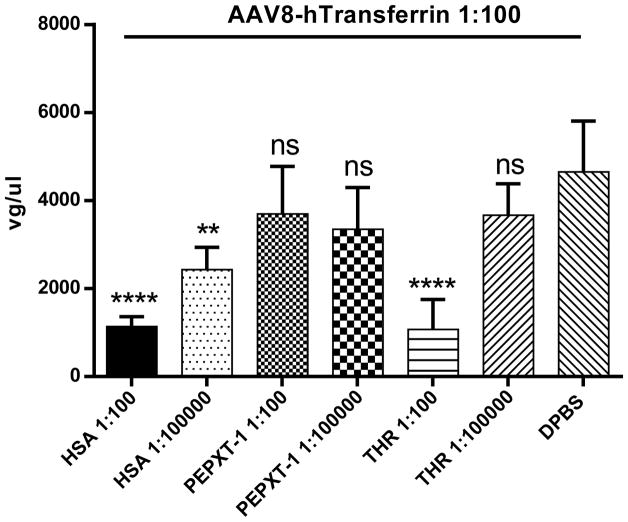
3.8. THR competes with the human transferrin for AAV8 virion binding
Previously, we showed that several serum proteins (transferrin, albumin, and ApoB) are able to directly interact with AAV8 and enhance liver transduction in vitro and in vivo. Further studies showed that these proteins competitively bind at the same location on the AAV8 virions [34, 35]. To investigate whether THR also binds at this AAV8 virion location, we performed a competitive binding assay. AAV8 was incubated with HSA, control peptide PEPXT-1, or different amounts of THR for 2 h at 4 °C. After this, the human transferrin was added to the mixture for another 1 h. The transferrin antibody was used to pull down the transferrin bound AAV8 virions and the gene copy number was measured by qPCR. Consistent with our previous report, HSA blocked the transferrin binding to AAV8, while the control peptide PEPXT-1 had no effect on the transferrin binding to AAV8. However, at high concentrations, the THR peptide exhibited a blocking effect on the transferrin binding to the AAV8 virions, similar to that observed for HSA (Fig. 8). This result implies that the THR increases the ability of AAV8 to cross the BBB by binding to the same location on the AAV virions as do the human serum proteins.
Fig. 8. The THR peptide competes with the human transferrin binding to AAV8.
Diluted HSA (1:100 and 1:100,000 of physiological concentration), peptide control (1:100 and 1:100,000 dilution), THR (1:100 and 1:100,000 dilution), and DPBS control were used to block the AAV8 capsid on Huh7 cells in vitro. Then, the human transferrin (1:100 of physiological concentration) was added to the mixture separately, the transferrin antibody was used for pull down, and the AAV8 gene copy number was measured by qPCR. All of the data were shown as the means of the triplicate experiments and SD. Asterisks indicate the statistical significance when compared to the DPBS group (**p < 0.01, ****p < 0.001). The “ns” indicates no significant difference (p> 0.05).
4. Discussion
To date, increasing number of therapeutic peptides are used in a wide range of medical applications [38]. Our objective was to explore the potential of using BBB shuttle peptides to enhance the ability of AAV vectors to cross the BBB for efficient brain transduction. We found that several peptides increased AAV8 brain transduction after a systemic administration, with the THR peptide having the greatest effect. Furthermore, we showed that the direct interaction between THR and the AAV8 virions was required for the THR-mediated increase in the ability of AAV8 to cross the BBB and transduce brain cells, especially the neuronal cells.
Tremendous efforts have been devoted to surmount the BBB for cerebral disease treatments. However, for the rAAV vectors to effectively cross the BBB and transduce the brain after its peripheral application remains challenging. While a substantial amount of research into engineered AAVs has displayed promising results in rodents, additional studies suggest that the possibility of cross-reactivity in large animals and humans that may not be accurately predicted by studies in rodents remains a large concern [20, 21, 23, 39]. For example, the systemic injection of AAV.PHP.B significantly enhanced the ability of crossing the BBB in C57BL/J mice [18], but did not correspondingly increase the CNS transduction in BALB/cJ mice and marmosets [23, 40]. Therefore, it is important to explore a generic strategy that which is able to overcome the differences across species for brain transduction after systemic administration of the AAV vector. The BBB is a complex regulatory interface that possesses barrier, secretory, and transporter activities [41]. In general, substances permeate the BBB by a variety of either active or passive mechanisms [28, 41]. Compared to the transcellular passive diffusion and carrier-mediated transport of the passive pathway, the adsorptive- or receptor-mediated endocytosis and transcytosis pathways could transport a wider variety of cargo with a high molecular weight, including proteins, viruses, peptides, and nanocarriers for their potential targeting capacity [28]. The BBB shuttle peptides are able to provide broadly applicable, selective, and safer delivery systems. Until now, several BBB shuttle peptides have reached most the advanced stages in the route towards clinical application, including Angiopep-2 and GSH [42, 43], but little research has reported the application of these peptides for rAAV-based gene therapy. Interestingly, among the peptides tested in this study, the enhancement effect on the brain transduction of AAV8 was inconsistent. The GSH peptide did not show any enhanced effect on brain transduction, and the peptide THR induced the best transduction in the brain. There are several possibilities for these discrepancies. Firstly, this could be accounted for by the ability of specific peptides to bind to the AAV8 virions, with some peptides able to interact with the AAV virions, such as THR, while others are not, for example the control peptides. Secondly, it is possible that the structure of the peptide changes to prevent its binding to corresponding receptors on the cell surface after interacting with the AAV capsids. Lastly, the differences in the brain transduction enhancement observed between the peptide-AAV virions complexes may be affected by the stability of the complexes in the endosomes of endothelial cells.
There are several lines of evidence that support a higher chance of success for future clinical trials to apply the BBB shuttle peptides to enhance AAV vector brain transduction after a systemic administration. 1) BBB shuttle peptides use normal physiological mechanism to enhance the BBB permeability, but do not disrupt the physical integrity of the BBB. 2) Unlike AAV mutants which have unknown tissue tropism, the application of the BBB shuttle peptides does not change the structure or affect the receptor binding and intracellular trafficking pathway of AAV, therefore there is no change of the AAV tropism in the brain. 3) Since there is a similar mechanism used by receptor mediated BBB permeability between different species, the results obtained from the mouse model would predict the AAV transduction efficiency in humans.
In this study, less glial cells and more neurons were transduced after a systemic administration of the AAV8-THR complex. Until now, little research has been done concerning the AAV8 tropism in the brain after a systemic injection. There are several reports about the local brain injection of AAV8 and tests of the tropism [44, 45]. After direct injection into the brain, AAV8 is able to preferably transduce the brain neurons, along with glial cells. The mechanism for the low glial cells transduction is unknown after a systemic administration of AAV8. It is possible that the interaction of the AAV8 virions with either serum proteins or BBB shuttle peptides may affect the AAV8 tropism in the brain.
A major concern in this study is whether the binding of the peptides to AAV8 would interfere with the AAV8 infection mechanism. Recently, it was reported that an artificial peptide could mimic the activity of the Nab to bind with the influenza virus and block infection [46]. Here, the Nab analysis showed that THR did not influence the AAV8 Nab titer irrespective of the THR concentration (Fig. S4.B). TfR1 is highly expressed in brain endothelial cells [47, 48] and together with either the drug or antibody conjugates has been actively explored as a means to deliver protein therapeutics to the brain [49, 50]. Our study demonstrates that THR can compete with the transferrin binding to AAV8. Collectively, these results indicate that the THR peptide binds to the non-functional sites of AAV8 and serves as a secondary ligand for endothelial cell binding to enhance the AAV BBB permeability.
The other concern raised from this study is the high level of liver transduction. Indeed, more AAV virions were detected in the liver following a systemic administration of the complex AAV8/peptide. It has been suggested that in clinical trials for patients with hemophilia, the capsid specific cytotoxic T lymphocytes (CTLs) are able to eliminate the AAV transduced hepatocytes, leading to therapeutic failure [6]. The capsid antigen presentation in AAV transduced cells is dose-dependent [51]. To avoid a high liver tropism and maintain the increased BBB permeability, it is imperative to identify novel BBB peptides with a liver de-targeting capacity and the ability to bind to AAV virions in future studies.
The third concern is whether there is an increased susceptibility for other pathogenic infections/crossing BBB when the THR shuttle peptide is used to increase the AAV brain transduction after a systemic administration. THR is able to interact with AAV8 and specifically bind to the TfR1 without physically changing the BBB integrity. Therefore, an increased susceptibility for other pathogenic infections is not expected.
A delayed blood clearance plays an important role in the robust cardiac transduction of AAV9 following a systemic administration [37]. Here, we investigated the THR peptide’s role in a possible delayed blood clearance. As expected, our analysis revealed that, when compared with that of the control, THR delayed the blood clearance (Fig. 7C). Whether the THR peptide-mediated slow blood clearance of the AAV vector after a systemic administration directly contributes to the increased ability of the AAV vector to cross the vascular vessels (including the BBB) for a higher global transduction warrants further investigation.
In this study, we demonstrated that several BBB shuttle peptides were able to enhance the ability of AAV8 to cross the BBB after systemic administration. One of the issues is whether there is a serotype dependency to the BBB shuttle-peptide. To address this, we tested the possible serotype dependency of a BBB shuttle-peptide with AAV9. After a systemic administration of AAV9 pre-incubated with THR, we did not observe a significant increase in the transduction of the brain and liver (Fig. S5). The results suggest that there is a serotype dependency to the BBB shuttle-peptide. A systemic administration of AAV9 has been used in several clinical trials for patients with CNS disorders. Other serotypes, such as AAV7 and AAVrh10, also demonstrate the ability to cross the BBB in animal models. In further studies, we will be focusing on identifying the novel BBB shuttle peptides that are able to bind to AAV9, AAV7, or AAVrh10 for further increased brain transduction after systemic administration.
5. Conclusion
Herein, we demonstrate that the BBB shuttle peptide can directly interact with AAV and facilitate its targeting of brain cells across the BBB. This approach uses a natural mechanism that would potentially apply to any species, including humans, and importantly, without theoretically changing the AAV capsid structure. The results presented here advance and expand our understanding of the roles of BBB shuttle peptides for use in rAAV-based systemic gene delivery. Therefore, these findings are of importance in clinical trials for patients with CNS disorders who require a systemic administration of an AAV vector. Our results explore a novel and effective strategy to enhance the AAV penetration of the BBB and hold a potential for future human clinical trials.
Supplementary Material
Fig. S1. THR interacts with the human and mouse TfR1. CHO-TRVB cells, after a co-incubation with 1 nM of the 5’-FAM labeled THR peptide and transiently expressed the RFP labeled human TfR1 (A) and mouse TfR1 (B) at 37 °C for 1 h were acid-washed with a low pH buffer and fixed on slides. The images for the RFP labeled TfR1 proteins of either the human or mouse were overlaid with those of the 5’-FAM-THR peptide. All of the cells were nuclear counter stained with DAPI. All of the data were shown as the means of triplicate experiments.
Fig. S2. No significant effect of peptide polarity on AAV8 transduction. (A) Seven days after the administration of either AAV8 or the complexes, imaging was taken to assess luminescence expression. (B) The average luciferase signal for the liver and the whole body (excluding liver) in different groups were calculated. At three weeks post-injection, the mice were euthanized and the tissues were harvested for DNA extraction. The relative luciferase expression level (C) and the vector copy numbers (D) in the liver and brain were determined separately. The data represents the average and SD from five mice. *p < 0.05 compared to control mice with AAV8 treatment only.
Fig. S3. hCMEC/D3 cells maintain baseline activation after AAV8 and THR complex exposure. (A) The membrane permeability assay was conducted with FITC-dextran following different treatments. Briefly, the FITC-dextran was added into the apical of cell layer for 30 min. The medium in the basal chamber was analyzed for FITC-dextran fluorescence intensity with the excitation and emission wavelengths of 485 and 5303nm, respectively. No significant difference in permeability was observed. (B) The western blot showed similar expression levels of the major junction protein ZO-1 in hCMEC/D3 cells at the early and late time points in different groups. GAPDH is presented as a loading control for equal protein. All of the data were shown as the means of at least a triplicate of experiments and their SD. The “ns” indicates no significant difference (p> 0.05).
Fig. S4. THR does not interfere with the AAV8 biology. (A) HEK293 and Huh7 cells were transduced with 1×108 vg of either AAV8/luc alone or the complex of AAV8 and THR at different concentrations. The luciferase expression was analyzed 48 h later. (B) The effect of the THR on Nab titer. 1×108 vg of AAV8/luc in 50 μl was incubated with different concentrations of THR at 4 °C for 2 h. Equal volumes of the sera at different dilutions, or with DPBS, were added and the mixture was incubated at 37 °C for 1 h before the mixture of AAV and the peptide and serum were added into cells. Finally, the cells were lysed for luciferase assay at 48 h post-transduction, and the Nab titer was evaluated. The data represents an average of three separate infections, with the SD indicated by an error bar.
Fig. S5. THR does not significantly enhance the AAV9 transduction. (A) 5×1010 vg of either AAV9/luc alone or the complex of the pre-incubated AAV9 and 0.1 mM THR peptide at 4 °C for 2 h were administered via a retro-orbital injection. At the days 3 and 7 post-injection, in vivo luminescence imaging (A) was performed and the photon signal of the liver and the brain (B) was measured and calculated. The data of each group represents the average and SD from five mice. The “ns” indicates no significant difference (p> 0.05).
Primer sequences utilized in this study
Highlight.
BBB shuttle peptides enhance AAV8 brain transduction after systemic administration
BBB shuttle peptide THR directly binds to AAV8 and facilitates its crossing the BBB
THR primarily promotes AAV8 targeting neurons in the brain after systemic delivery
THR competitively binds to AAV8 with serum proteins
THR does not interfere with AAV8 infection biology
Acknowledgments
We thank Dr. T. E. McGraw for generously providing the CHO-TRVB cell line. We thank Violeta Zaric and Kelly Michelle Rigsbee for their great technical assistance, thank Dr. Lauriel Earley and Kelly Michelle Rigsbee for critical reading and improving the manuscript. We acknowledge the UNC Biomedical Research Imaging Center (BRIC) Small Animal Imaging (SAI) facility for assistance with mouse imaging. This work was supported by National Institutes of Health Grants R01AI117408, R01HL125749 (to C.L.), P30-CA016086-35-37, and U54-CA151652-01-04 (to the BRIC SAI facility).
Footnotes
Author contributions: C.L and X.Z designed research. X.Z performed experiments, analyzed the data, prepared figures, and wrote the manuscript. X.Z and T.H executed the brain section preparation and IHC. Z.C contributed to the animal imaging, tissue processing, analysis of results, and discussion. R.J.S discussed the results, provided advice, and understood all the data. C.L supervised the project and revised the manuscript and figures. All authors edited and approved the manuscript.
Conflicts of interest
R. Jude Samulski is the founder, and a shareholder, at Asklepios BioPharmaceutical. He receives research support through the UNC from Asklepios BioPharmaceutical. He holds patents that have been licensed by UNC to Asklepios Biopharmaceutical, for which he receives royalties. He has consulted for Baxter Healthcare and has received payment for speaking.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hastie E, Samulski RJ. Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success--a personal perspective. Human gene therapy. 2015;26:257–265. doi: 10.1089/hum.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, McCague S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Mingozzi F, High KA, Maguire AM. AAV2 gene therapy readministration in three adults with congenital blindness. Science translational medicine. 2012;4:120ra115. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, Hauswirth WW. Improvement and decline in vision with gene therapy in childhood blindness. The New England journal of medicine. 2015;372:1920–1926. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daya S, Berns KI. Gene Therapy Using Adeno-Associated Virus Vectors. Clinical Microbiology Reviews. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature medicine. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 7.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O'Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. The New England journal of medicine. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet (London, England) 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 9.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. The Lancet Neurology. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 10.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature biotechnology. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R, Yadav S, DiPrimio N, Nam HJ, Agbandje-McKenna M, McPhee S, Wolff J, Samulski RJ. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nature biotechnology. 2010;28:79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai Z, Sun JJ, Rigsbee KM, Wang M, Samulski RJ, Li CW. Application of polyploid adeno-associated virus vectors for transduction enhancement and neutralizing antibody evasion. Journal of Controlled Release. 2017;262:348–356. doi: 10.1016/j.jconrel.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller OJ, Kaul F, Weitzman MD, Pasqualini R, Arap W, Kleinschmidt JA, Trepel M. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nature biotechnology. 2003;21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- 15.Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, Flotte T, Muzyczka N. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. Journal of virology. 2000;74:8635–8647. doi: 10.1128/jvi.74.18.8635-8647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhury SR, Fitzpatrick Z, Harris AF, Maitland SA, Ferreira JS, Zhang Y, Ma S, Sharma RB, Gray-Edwards HL, Johnson JA, Johnson AK, Alonso LC, Punzo C, Wagner KR, Maguire CA, Kotin RM, Martin DR, Sena-Esteves M. In Vivo Selection Yields AAV-B1 Capsid for Central Nervous System and Muscle Gene Therapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24:1247–1257. doi: 10.1038/mt.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhury SR, Harris AF, Cabral DJ, Keeler AM, Sapp E, Ferreira JS, Gray-Edwards HL, Johnson JA, Johnson AK, Su Q, Stoica L, DiFiglia M, Aronin N, Martin DR, Gao G, Sena-Esteves M. Widespread Central Nervous System Gene Transfer and Silencing After Systemic Delivery of Novel AAV-AS Vector. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24:726–735. doi: 10.1038/mt.2015.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nature biotechnology. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iida A, Takino N, Miyauchi H, Shimazaki K, Muramatsu S. Systemic delivery of tyrosine-mutant AAV vectors results in robust transduction of neurons in adult mice. BioMed research international. 2013;2013:974819. doi: 10.1155/2013/974819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, Bakar Y, Nathwani AC. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Molecular therapy : the journal of the American Society of Gene Therapy. 2018 doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM, Nygaard S, Grompe M, Alexander IE, Kay MA. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki Y, Konno A, Mochizuki R, Shinohara Y, Nitta K, Okada Y, Hirai H. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neuroscience letters. 2017 doi: 10.1016/j.neulet.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 24.Salmon F, Grosios K, Petry H. Safety profile of recombinant adeno-associated viral vectors: focus on alipogene tiparvovec (Glybera(R)) Expert review of clinical pharmacology. 2014;7:53–65. doi: 10.1586/17512433.2014.852065. [DOI] [PubMed] [Google Scholar]
- 25.Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Current gene therapy. 2003;3:545–565. doi: 10.2174/1566523034578131. [DOI] [PubMed] [Google Scholar]
- 26.Dismuke DJ, Tenenbaum L, Samulski RJ. Biosafety of recombinant adeno-associated virus vectors. Current gene therapy. 2013;13:434–452. doi: 10.2174/15665232113136660007. [DOI] [PubMed] [Google Scholar]
- 27.Meairs S. Facilitation of Drug Transport across the Blood-Brain Barrier with Ultrasound and Microbubbles. Pharmaceutics. 2015;7:275–293. doi: 10.3390/pharmaceutics7030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oller-Salvia B, Sanchez-Navarro M, Giralt E, Teixido M. Blood-brain barrier shuttle peptides: an emerging paradigm for brain delivery. Chemical Society reviews. 2016;45:4690–4707. doi: 10.1039/c6cs00076b. [DOI] [PubMed] [Google Scholar]
- 29.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharmaceutical research. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malakoutikhah M, Teixido M, Giralt E. Shuttle-mediated drug delivery to the brain. Angewandte Chemie. 2011;50:7998–8014. doi: 10.1002/anie.201006565. [DOI] [PubMed] [Google Scholar]
- 31.Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, Gabathuler R, Castaigne JP, Beliveau R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. Journal of neurochemistry. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Engler JA, Collawn JF, Moore BA. Receptor mediated uptake of peptides that bind the human transferrin receptor. European Journal of Biochemistry. 2001;268:2004–2012. doi: 10.1046/j.1432-1327.2001.02073.x. [DOI] [PubMed] [Google Scholar]
- 33.Prades R, Guerrero S, Araya E, Molina C, Salas E, Zurita E, Selva J, Egea G, López-Iglesias C, Teixidó M, Kogan MJ, Giralt E. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33:7194–7205. doi: 10.1016/j.biomaterials.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 34.Pei X, He T, Hall NE, Gerber D, Samulski RJ, Li C. AAV8 virions hijack serum proteins to increase hepatocyte binding for transduction enhancement. Virology. 2018;518:95–102. doi: 10.1016/j.virol.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Sun J, Crosby A, Woodard K, Hirsch ML, Samulski RJ, Li C. Direct interaction of human serum proteins with AAV virions to enhance AAV transduction: immediate impact on clinical applications. Gene therapy. 2017;24:49–59. doi: 10.1038/gt.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erazo-Oliveras A, Muthukrishnan N, Baker R, Wang T-Y, Pellois J-P. Improving the Endosomal Escape of Cell-Penetrating Peptides and Their Cargos: Strategies and Challenges. Pharmaceuticals (Basel, Switzerland) 2012;5 doi: 10.3390/ph5111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotchey NM, Adachi K, Zahid M, Inagaki K, Charan R, Parker RS, Nakai H. A potential role of distinctively delayed blood clearance of recombinant adeno-associated virus serotype 9 in robust cardiac transduction. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:1079–1089. doi: 10.1038/mt.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug discovery today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Kay MA. Selecting the Best AAV Capsid for Human Studies. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23:1800–1801. doi: 10.1038/mt.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Molecular Therapy. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks WA. Characteristics of compounds that cross the blood-brain barrier. Bmc Neurol. 2009;9 doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaillard PJ, Appeldoorn CC, Rip J, Dorland R, van der Pol SM, Kooij G, de Vries HE, Reijerkerk A. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. Journal of controlled release : official journal of the Controlled Release Society. 2012;164:364–369. doi: 10.1016/j.jconrel.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, Qian Y, Sun X, Jiang X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33:3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 44.Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, Ozawa K, Isa T, Yamamori T. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neuroscience Research. 2015;93:144–157. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Lawlor PA, Bland RJ, Mouravlev A, Young D, During MJ. Efficient Gene Delivery and Selective Transduction of Glial Cells in the Mammalian Brain by AAV Serotypes Isolated From Nonhuman Primates. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2009;17:1692–1702. doi: 10.1038/mt.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadam RU, Juraszek J, Brandenburg B, Buyck C, Schepens WBG, Kesteleyn B, Stoops B, Vreeken R, Vermond J, Goutier W, Tang C, Vogels R, Friesen RHE, Goudsmit J, van Dongen MJP, Wilson IA. Potent peptidic fusion inhibitors of influenza virus. Science. 2017 doi: 10.1126/science.aan0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 48.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological reviews. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 49.Dufes C, Al Robaian M, Somani S. Transferrin and the transferrin receptor for the targeted delivery of therapeutic agents to the brain and cancer cells. Therapeutic delivery. 2013;4:629–640. doi: 10.4155/tde.13.21. [DOI] [PubMed] [Google Scholar]
- 50.Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2013;10:459–472. doi: 10.1007/s13311-013-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Y, Weinberg MS, Hirsch M, Johnson MC, Tisch R, Samulski RJ, Li C. Kinetics of adeno-associated virus serotype 2 (AAV2) and AAV8 capsid antigen presentation in vivo are identical. Human gene therapy. 2013;24:545–553. doi: 10.1089/hum.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. THR interacts with the human and mouse TfR1. CHO-TRVB cells, after a co-incubation with 1 nM of the 5’-FAM labeled THR peptide and transiently expressed the RFP labeled human TfR1 (A) and mouse TfR1 (B) at 37 °C for 1 h were acid-washed with a low pH buffer and fixed on slides. The images for the RFP labeled TfR1 proteins of either the human or mouse were overlaid with those of the 5’-FAM-THR peptide. All of the cells were nuclear counter stained with DAPI. All of the data were shown as the means of triplicate experiments.
Fig. S2. No significant effect of peptide polarity on AAV8 transduction. (A) Seven days after the administration of either AAV8 or the complexes, imaging was taken to assess luminescence expression. (B) The average luciferase signal for the liver and the whole body (excluding liver) in different groups were calculated. At three weeks post-injection, the mice were euthanized and the tissues were harvested for DNA extraction. The relative luciferase expression level (C) and the vector copy numbers (D) in the liver and brain were determined separately. The data represents the average and SD from five mice. *p < 0.05 compared to control mice with AAV8 treatment only.
Fig. S3. hCMEC/D3 cells maintain baseline activation after AAV8 and THR complex exposure. (A) The membrane permeability assay was conducted with FITC-dextran following different treatments. Briefly, the FITC-dextran was added into the apical of cell layer for 30 min. The medium in the basal chamber was analyzed for FITC-dextran fluorescence intensity with the excitation and emission wavelengths of 485 and 5303nm, respectively. No significant difference in permeability was observed. (B) The western blot showed similar expression levels of the major junction protein ZO-1 in hCMEC/D3 cells at the early and late time points in different groups. GAPDH is presented as a loading control for equal protein. All of the data were shown as the means of at least a triplicate of experiments and their SD. The “ns” indicates no significant difference (p> 0.05).
Fig. S4. THR does not interfere with the AAV8 biology. (A) HEK293 and Huh7 cells were transduced with 1×108 vg of either AAV8/luc alone or the complex of AAV8 and THR at different concentrations. The luciferase expression was analyzed 48 h later. (B) The effect of the THR on Nab titer. 1×108 vg of AAV8/luc in 50 μl was incubated with different concentrations of THR at 4 °C for 2 h. Equal volumes of the sera at different dilutions, or with DPBS, were added and the mixture was incubated at 37 °C for 1 h before the mixture of AAV and the peptide and serum were added into cells. Finally, the cells were lysed for luciferase assay at 48 h post-transduction, and the Nab titer was evaluated. The data represents an average of three separate infections, with the SD indicated by an error bar.
Fig. S5. THR does not significantly enhance the AAV9 transduction. (A) 5×1010 vg of either AAV9/luc alone or the complex of the pre-incubated AAV9 and 0.1 mM THR peptide at 4 °C for 2 h were administered via a retro-orbital injection. At the days 3 and 7 post-injection, in vivo luminescence imaging (A) was performed and the photon signal of the liver and the brain (B) was measured and calculated. The data of each group represents the average and SD from five mice. The “ns” indicates no significant difference (p> 0.05).
Primer sequences utilized in this study