Abstract
Insect societies face many social parasites that exploit their altruistic behaviours or their resources. Due to the fitness costs these social parasites incur, hosts have evolved various behavioural, chemical, architectural and morphological defence traits. Similar to bacteria infecting multicellular hosts, social parasites have to successfully go through several steps to exploit their hosts. Here, we review how social insects try to interrupt this sequence of events. They can avoid parasite contact by choosing to nest in parasite-free locales or evade attacks by adapting their colony structure. Once social parasites attack, hosts attempt to detect them, which can be facilitated by adjustments in colony odour. If social parasites enter the nest, hosts can either aggressively defend their colony or take their young and flee. Nest structures are often shaped to prevent social parasite invasion or to safeguard host resources. Finally, if social parasites successfully establish themselves in host nests, hosts can rebel by killing the parasite brood or by reproducing in the parasites' presence. Hosts of social parasites can therefore develop multiple traits, leading to the evolution of complex defence portfolios of co-dependent traits. Social parasites can respond to these multi-level defences with counter-adaptations, potentially leading to geographical mosaics of coevolution.
This article is part of the Theo Murphy meeting issue ‘Evolution of pathogen and parasite avoidance behaviours’.
Keywords: coevolution, resistance, behaviour, cuticular hydrocarbons, defence portfolios
1. Introduction
Social insects such as ants, termites, social wasps and bees represent an enormously successful way of life that plays a dominant role in most terrestrial ecosystems [1]. Their social lifestyle based on altruistic behaviours, however, makes them prone to exploitation [2]. On the other hand, cooperative and altruistic defence strategies have evolved to deal with parasites [3]. For instance, social insects have developed effective cooperative defence behaviours against micro-parasites, termed social immunity [4], which include grooming and other hygienic behaviours [5–8] or the adjustment of social networks following infection [9–11]. Here, we focus on defences against parasites that directly take advantage of the sociality of their hosts, i.e. social parasites. Many social parasites are social themselves, such as the slavemaking ants [12], cleptoparasitic ants and bees [13–15] or ants, wasps and bees that sneak into other nests to reproduce [16–18], while others have social ancestors, such as many workerless ant inquilines [19]. We will restrict our discussion to these cases. There is a variety of other animal taxa that invade insect societies and exploit social behaviour, such as butterfly caterpillars, beetles, spiders and even snails [20–23].
Social parasites are, at first glance, less common in the termites (but see e.g. [24,25]). Young termites often require little brood care beyond the exchange of the gut microflora and already perform worker tasks [26]. Thus, it is not surprising that exploitation of their social lifestyle evolved less frequently. Social hymenoptera show extensive brood care as their larvae are completely dependent on adult workers for food and defence, and many species store food in their nests. Social parasites exploit these behaviours and resources [12,13,27], either by redirecting altruistic social behaviours towards themselves and their offspring or by stealing from the stores of the society or the nest itself (table 1). Given the potential benefits of exploiting others, it is not surprising that social parasitism is an evolutionarily ancient phenomenon [17,61,62] and evolved multiple times in the eusocial Hymenoptera and a number of other arthropod lineages [12,23,63].
Table 1.
Description of the different types of social parasitism.
| types of social parasitism | description | examples | references |
|---|---|---|---|
| brood parasitism: inquilinism | Workers or queens enter con- or heterospecific nests, reproduce and exploit the hosts' workforce for brood rearing. This form of parasitism can be temporary, when social parasites depend on hosts only for colony foundation, or permanent, when parasites only produce sexuals, but no or few workers. Thus, they continuously depend on host workers to perform all necessary tasks. In bumblebees, reproductive parasitism is done by both workers (also called ‘egg dumping’), e.g. in Bombus terrestris, and queens, e.g. in B. norvegicus. Brood parasitism can be facultative, e.g. in Melipona stingless bees, Apis mellifera and Vespula vulgaris workers, or obligate, e.g. in cuckoo bumblebees. | ants, bumblebees, stingless bees, honeybees, wasps | [13,16–18,28–38] |
| brood parasitism: dulosis or ‘slave-making’ | Queens establish a new colony by invading a host nest and killing or expelling adults. The host brood, once developed, raises the parasite's own offspring. Slaves are replenished by workers stealing brood from nearby host nests. | ants | [39–50] |
| cleptoparasitism: raiding, agro-predation and xenobiosis | The stealing of food, building material or other valuable resources from a colony. One particular case is ‘agro-predation’, where fungal crops or symbiotic livestock are stolen. Stealing can happen by force, e.g. in Lestrimelitta robber bees or by stealth, e.g. in Ectatomma ants. Another example is xenobiosis, where parasite queen and workers live in the same or an adjacent nest and consume the food of their host. The brood of host and parasite are kept apart. We consider cleptoparasitism a form of social parasitism if parasites take advantage of the social lifestyle of their hosts, e.g. by raiding a colony's resources. | ants, honeybees, stingless bees, wasps | [14,15,51–57] |
| usurpation | Colonies are evicted from their nest by another colony, either from the same species, as in honeybees, or by another species, as in stingless bees and ants. | ants, honeybees, stingless bees, thrips | [13,15,58–60] |
Social parasites are often closely related to their hosts (referred to as ‘Emery's rule’) [64,65], which distinguishes these host–parasite interactions from those of microbial or viral parasites and their multicellular hosts. In cases of intraspecific parasitism, colonies are parasitized by workers or queens of the same species [14,16,18,28,51,52,66,67] or sub-species [17,29] that attempt to raid resources or reproduce. Even in interspecific relationships, such as in the workerless ant inquilines (table 1), they are often their hosts' sister species [19,30]. However, whether host and parasite are closely related also depends on the type of social parasitism: Emery's rule [64] mainly applies to permanent and temporary inquilinism, but less so to dulotic and xenobiotic relationships (table 1) [65]. Host–parasite relatedness also predicts host specificity as more specific parasites are also often more closely related to their hosts.
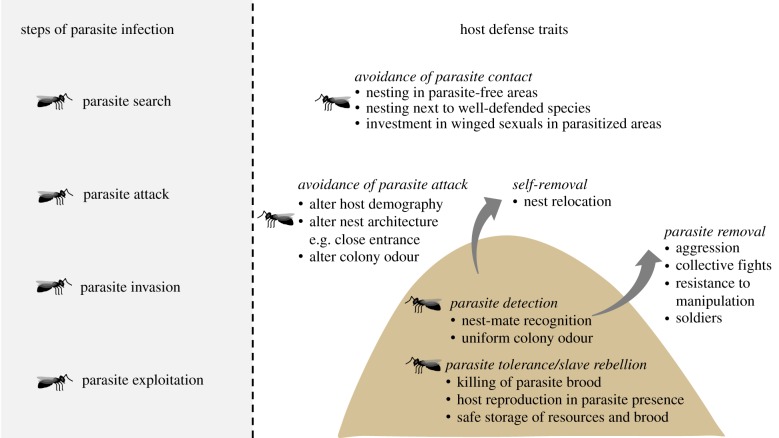
Social parasites use mainly behavioural [31,68,69] and chemical strategies [32] to invade the often well-defended host fortresses. In response, hosts have evolved various behavioural, chemical, architectural or morphological traits, instead of immunological responses, which are so common and effective against microbial or viral parasites. Nonetheless, the different phases of host–parasite interactions resemble those of aforementioned parasites (figure 1) [70]: social parasites also have to find their hosts and detect entrance points into the nest [71]. Once they locate the entrance, they have to either dodge detection [39,72] or overwhelm host defenders [15,73]. We structure our review to follow broadly these stages of parasitism—host detection, infiltration, establishment and exploitation. We focus our attention on the traits and strategies hymenopteran hosts of social parasites use to counteract and interrupt this sequence of events.
Figure 1.
Different host defence traits evolved to interrupt the different steps of successful host exploitation by social parasites. (Online version in colour.)
2. Avoidance of parasite contact or detection
(a). Choosing the right location
It would appear that a very effective anti-parasite defence is the avoidance of contact or detection by parasites altogether [4,11]. However, little is known about how hosts of social parasites can achieve this. Most insect societies typically occupy nests and cannot easily relocate if they detect parasites. Therefore, queens or swarms should start a colony in an area where social parasites are absent or rare. Indeed, social parasites are often patchily distributed [27,74–77], but it remains unclear whether there is reliable information for hosts on how common social parasites are in the vicinity of a potential nesting site.
Another way to avoid attacks is to nest near a parasite deterrent. For example, the stingless bee Nannotrigona testaceicornis does not have an effective defence against cleptoparasitic robber bees of the genus Lestrimelitta, but frequently nests near the highly defensive bee Tetragonisca angustula [15,78]. The latter species often comes to the aid of attacked N. testaceicornis colonies [79] (C.G. 2013, personal observation), making it likely that robber bee attacks are occasionally prevented by aggressive T. angustula guards that intercept robber bee scouts. It remains to be tested, however, if less defensive species strategically choose to nest near highly defensive species to avoid being attacked.
(b). Polydomy, polygyny and reproductive investment
If it is not possible to avoid parasite contact, hosts could adapt their colony structure so that they are better prepared in case of an attack. For example, they could decide to either remain in a single nest or split up into several smaller polydomous subnests, thereby spreading the risk of an attack. Some slavemaking ants, such as Temnothorax americanus [40] (figure 2) preferentially attack larger host colonies, so that an increase in polydomy could be regarded as an adaptive response to the presence of slavemakers in the local community. Indeed, in highly parasitized areas, unparasitized host colonies of Temnothorax longispinosus are smaller and contain fewer queens [81]. However, it is unclear whether this is an adaptive defence strategy of the host or the negative outcome of frequent slave raids, as the experimental release of slavemakers caused a local reduction in host colony size [82]. Another potential response of hosts to the presence of slavemakers in a population is refraining from adopting additional queens. Monogynous colonies have a less variable colony odour, making parasite detection more likely [83,84]. Accordingly, queen number is lower in highly parasitized areas, both within populations and across geographically distant sites [75,81] and strictly monogynous species are thought to be less likely to host social parasites [85]. However, here also the question arises whether queen number is the cause or the result of parasite prevalence. Changes in investment strategies could be another potential response to social parasitism. If social parasites are locally common and fitness costs of attacks are high, hosts might do better to invest more into sexuals than into workers [82]. Sexuals in social Hymenoptera are generally winged and, therefore, are more able to escape from highly parasitized locales.
Figure 2.
Defence strategies against ant social parasites. (a) Parasite detection: Temnothorax ambiguus host worker (right) inspects an intruding T. pilagens slavemaking worker. These slavemakers try to undermine the recognition system of their hosts [39]: during some raids slavemakers stay undetected, in others hosts recognize the slavemaker as a parasite and respond by stinging. (b,c) During a slave raid an intruding T. americanus slavemaker is attacked by T. curvispinosus (b) and T. longispinosus (c) host workers, which have to coordinate their attacks to subdue the physically stronger slavemaker [44]. (d) Harpagoxenus sublaevis slavemakers use the secretion of the Dufour gland to elicit fights among host defenders [45,54]. Hosts of slavemakers vary in their resistance to this chemical manipulation [46]. (e) A last line of defence is slave rebellion [47]. Here, enslaved T. longispinosus host workers attack and kill slavemaker brood, a behaviour that can increase their indirect fitness [48,49]. (f) The small T. minutissimus inquiline queens coexist with the host T. curvispinosus host queen. Hosts could either try to expel inquiline queens or become immune to the suppression of their reproduction [80].
(c). Architectural features to prevent invasion
Even if parasites are able to locate a host nest, hosts can use architectural features to prevent parasites from gaining access to the brood or food stores. For instance, many stingless bees close their nest entrance at night when foraging is not possible [86,87]. Partamona bee colonies build intriguing structures that have been interpreted as decoy or false nests [86,88]. These chambers are close to the entrance and often contain empty cells, food pots and wax sheets. Observations suggest that Lestrimelitta robber bees entering Partamona nests may end up in the false nest, thereby giving the host time to seal-off brood and food chambers with building material [88]. Melipona bees make obstacles that block the entrance tube to prevent robbers from entering the inner parts of the nest. While Melipona seminigra makes small balls of mud, which workers use to block the entrance [89], Melipona flavolineata make bee-sized balls of batumen (a mix of mainly wax, resin and earth) to block the entrance tube during Lestrimelitta attacks (figure 3) [90]. In species with small entrance holes, guards in both ants and bees can block the entrance with their heads [15,91].
Figure 3.
Defensive strategies in the bee Melipona flavolineata. (a) A first line of defence against robber bees is formed by highly aggressive entrance guards. (b,c) Workers also make bee-sized balls from resin, wax and earth which they use to block the entrance tube during a robber bee raid (from [90]). (Online version in colour.)
3. Parasite detection
If contact with social parasites cannot be avoided, the next step is the detection and rejection of these enemies. In cavity-nesting species, entrance guards are often tasked with preventing unwanted individuals from entering the nest. To do so, ants, bees and wasps rely on chemical cues encoded in their cuticular hydrocarbon profile to discriminate nest-mates from non-nest-mates [92–97]. Nest-mates share a colonial profile, because of a continuous exchange of genetically and environmentally determined recognition cues through grooming, trophallaxis or via nesting material [98–101]. This colonial label is thought to act as a template, enabling individuals to distinguish between nest-mates and aliens [95,102]. In addition, some species use visual cues to recognize parasites [103]. In turn, social parasites have evolved physiological and behavioural adaptations to crack the nest-mate recognition code of their host, such as mimicry or camouflage of their host's recognition profile, or the evolution of chemical insignificance [32,41,72,104–106].
Although the development of a narrower acceptance range in hosts may provide protection against mimetic parasites, it could also lead to rejection errors when individuals falsely reject nest-mates [43]. Selection may thus favour hosts that exhibit flexibility in their acceptance threshold, lowering it only when the likelihood of invasion by social parasites is high. Temnothorax longispinosus colonies only increase their level of aggression towards conspecific workers during the raiding season [107] and, in particular, following an encounter with a T. americanus slavemaker [42]. Host colonies that showed elevated aggression following slavemaker encounter were better able to save their brood [43]. In honeybees, entrance guards adjust their aggression towards incoming bees according to the risk of robbing: when robbing of honey by bees from other colonies is more likely, both nest-mates and non-nest-mate bees are more likely to be aggressed by guards [52,108]. Thus, conditional adjustment of acceptance thresholds provide fitness benefits when parasitism is eminent, without imposing costs when heightened defences are not needed.
Host colonies could also adapt to mimetic parasites by diversifying their chemical profile compared to adjacent conspecific colonies. Larger inter-colonial variation in recognition cues impairs adaptive mimicry by the parasite to its host population as a whole. Rare host profiles may be favoured by selection because parasites that adapt to common host profiles will be more successful. Martin et al. [109] showed that populations of the ant Formica fusca that are targeted by a large variety of temporary social parasites exhibit a high diversity in nest-mate recognition cues, whereas almost unparasitized populations were low in recognition cue diversity. Similarly, nest-mate recognition cue diversity among, but not within, T. longispinosus host colonies was larger in the presence of the slavemaker T. americanus [110]. Thus, social parasites may drive chemical cue diversity as a host defence trait, in much the same way that cuckoos promote egg polymorphism in their avian hosts [111,112].
4. Avoidance of parasite establishment
(a). Coordinated defence
Recognizing a parasite in itself does little to stop the threat. Therefore, accurate detection needs to be paired with strategies that prevent parasite establishment and a number of adaptive host responses have evolved. Hosts could fight collectively against intruders and try to overwhelm them [58]. To mobilize a coordinated response, hosts often use alarm pheromones to alert nest-mates to the presence of a parasite (e.g. [90,113]). Coordinated, cooperative fighting strategies are especially beneficial if invading social parasites are stronger than their hosts, as found in Asian honeybees where hundreds of bees form a ball around raiding giant hornets and increase the temperature inside the ball to levels that are lethal for the hornet [114]. However, if hosts stand little chances to fight off the attack, e.g. because the latter outnumber their hosts or are much stronger, flight might be the better response. Accordingly, hosts of some slavemaking ant species respond with nest evacuation instead of collective fights in highly parasitized locales [44].
(b). Larger fighters
Not only the number of defenders is important, but also their ‘muscle power’. In Polistes wasps, for instance, the relative physical size and strength of the P. sulcifer parasite and its P. dominulus host is the determining factor for successful nest usurpation [96]. Larger hosts are better able to defend their colony, and females from highly parasitized populations are larger than those from unparasitized populations [33]. These patterns suggest host body size is an adaptive defence trait in Polistes wasps. Similarly, some Neotropical stingless bee species have evolved a guard caste of increased body size (i.e. soldiers), most likely as a response to cleptoparasitic bees [115,116]. Increased body size seems to benefit hosts in at least two ways. First, as is the case with Polistes wasps, body size affects the fighting ability of guards when facing robber bees [115]. Second, body size positively correlated with the ability of entrance guards to recognize intruders based on chemical cues [117]. A possible explanation is that larger bees have more sensory sensilla on their antennae, which is an important determinant of chemosensory sensitivity in ants and bees [117–119]. In Sericomyrmex fungus growing ants, the task of defending the colony against a fungus raiding ‘agro-predator’ is performed by another ant species, a parasitic guest ant of the genus Gnamptogenys [53]. Here, one parasite defends the host against another parasite.
Behavioural specialization during social parasite attack is also found in the absence of specialized morphological worker castes. For instance, some T. longispinosus workers fight against their slavemaker attackers, while others start to evacuate the nest and flee with the brood [120]. However, division of labour can also be maladaptive when facing slavemakers [121]: manipulation of the level of division of labour in T. longispinosus hosts showed that colonies composed of generalist workers saved more brood and inflicted more casualties among their slavemaker attackers than colonies composed of specialist workers. Moreover, comparing the natural level of division of labour in host colonies across populations confirmed that hosts were less specialized when threatened by slavemakers.
(c). Unresponsiveness to parasite manipulation
Social parasites frequently use appeasement, repellent and propaganda substances, which pacify the host, cause panic and confusion in host workers, or even lead to fights among host nest-mates (ants: [32]; wasps: [122]; bees: [79,123]). These substances are often produced in the Dufour's gland, and its usage in both ant, bee and wasp social parasites suggest convergent evolution of host manipulation (e.g. [34,124–129]). In Lestrimelitta robber bees, the repellent chemicals used during raids appear to originate from the labial and the mandibular glands [123].
Despite the success of chemical manipulation by parasites, host colonies are not entirely defenceless. Indeed, several studies have shown that host species and populations differ markedly in their susceptibility to manipulation by parasites. For instance, the slavemaker Harpagoxenus sublaevis applies its Dufour's gland substances to Leptothorax workers during slave raids, which elicits deadly fights among hosts. Leptothorax acervorum workers from unparasitized British populations are more aggressive towards the secretion than workers from parasitized populations, suggesting that host populations that occur in sympatry with the slavemaker developed some resistance to the parasite's chemical weaponry [45]. Similar patterns of variation in host susceptibility were found across host species and populations in Europe, where some hosts responded with flight rather than intracolonial fights. As flight resulted in fewer host fatalities, it could be an adaptive host response to manipulation by the slavemaker [130]. A similar pattern is observed in stingless bees, where some species do not show aggression towards robbers, but hide in their nest during a raid. To reduce the losses caused by raids, workers from these ‘pacifist’ species consume as much food as they can when attacks start and regurgitate this food after the raid has ended, i.e. they use the communal crop as a temporary safe for valuable resources [15].
Lack of host susceptibility to manipulation by social parasites can have far reaching consequences for the eco-evolutionary dynamics between antagonists, as has been recently demonstrated for the North-American slavemaker ant T. americanus [46]. Like H. sublaevis, T. americanus uses its Dufour's gland secretions to manipulate its hosts into attacking nest-mates [129], which may deter defenders away from the parasitic invader during invasion. Temnothorax host species and populations not only varied substantially in their responsiveness to the slavemaker's Dufour's gland secretions, but hosts that were less susceptible to manipulation were also more successful against an intruding slavemaker and, subsequently, suffered far lower slavemaker pressure. Thus, successful host counter-adaptations can have important implications for the prevalence and host preference of social parasites.
5. Tolerance towards the parasite
Even if social parasites manage to establish themselves within host colonies, second lines of defence can limit the negative effects of parasites and allow parasitized hosts to partially rescue their fitness [131]. During ant slave rebellions [47,48], host workers rise up against their oppressors, mostly by attacking and killing the parasite's offspring. As a consequence, these colonies grow more slowly and attack fewer neighbouring host colonies in order to replenish their work force. If those spared adjacent colonies are related to the enslaved workers [49], the slaves are indirectly helping their free-living relatives, which are less likely to end up being attacked.
Detection does not necessarily have to occur towards adult social parasites. The social wasp Polistes biglumis is able to discriminate between alien eggs and their own, and founding females selectively remove alien eggs that are destined to become reproductives [132]. Although direct evidence that brood discrimination in this system evolved as a defence against social parasites is lacking, it is the most likely driver because P. biglumis lacks reproductive competition within the colony. Some hosts of slavemaking ants also evolved recognition of nest-mate larvae [133], which allows them to discriminate against slavemaker brood [47].
Another form of tolerance against parasite manipulation was detected in workers of the paper wasp P. dominulus [134]: while parasites often suppress host reproduction, host workers parasitized by the socially parasitic P. sulcifer were found to develop their ovaries more. Opportunistic egg laying by host workers from parasitized colonies suggests that this host has evolved successful counter-defences against the behavioural suppression of worker reproduction by P. sulcifer.
6. Multi-level defences or the evolution of defence portfolios
Most host species do not develop a single defence trait, but use multiple defences against parasites. These sets of defence traits have been described as defence portfolios [135,136]. Some of these defences are rather indirect and unspecific, such as being very aggressive or closing the nest entrance at night. Other traits are highly specific and directly target a particular parasite species, such as hosts that evolved unresponsiveness towards a specific manipulative secretion of a social parasite thereby becoming ‘immune' to this parasites' chemical tricks [129,46]. This raises the question of whether and how these different defence traits interact. How are these defence portfolios structured? If a frontline defence is highly effective, for example, a host colony invariably detects a social parasite and can deny access to its nest, secondary defences, such as slave-rebellion, might not evolve or be lost secondarily. Likewise, if colonies evolve a flight strategy, they might lose counter-attack responses. Accordingly, in some highly parasitized populations, ant hosts concentrate on flight and do not attempt to fight off intruding social parasites [46].
There are cases where two different defence traits clearly support each other. We already mentioned the example where entrance guards that are better at detection are also better at fighting, both due to their increased body size [115,117]. In T. longispinosus, aggressive colonies are able to save more brood if they encounter a slavemaker scout before the slave raid has begun [43]. Thus, only colonies that detected the enemy (parasite detection) and show a certain trait expression (high aggression) managed to defend their nest effectively.
7. Geographical mosaic of coevolution
The geographical mosaic theory of coevolution posits that variation in selection, genetic drift or gene flow across coevolutionary hot and cold spots can lead to spatial differences in the strength of biotic interactions [137]. Social parasites are often patchily distributed, leading to selection mosaics, which can result in the evolution of host defences only in heavily parasitized locations. Indeed, defence trait expression covaries with parasite pressure from the slavemaking ant T. americanus over the geographical ranges of two Temnothorax host species [44,46,138]. A similar association between parasite presence and defence trait expression was found in Polistes wasps, where foundresses in parasitized locales have enlarged body size and are more often present on the nest during mid-day, which is when parasitic wasps are most likely to attack [33,139]. On the other hand, spatial association between parasite occurrence and defence trait expression can also be the result of induced defences following parasite contact. In the eusocial bee T. angustula, the investment in soldiers covaries with the presence of robber bees. Experimental introduction of chemical robber bee cues revealed an induced long-term upregulation of the number of soldiers over several worker generations [140].
Social insect populations can be attacked by multiple social parasites at the same time. Studies of such a tripartite coevolutionary arms race in Temnothorax ants revealed that intraguild interactions among the two slavemaking ants strongly affected selection pressures on hosts, potentially contributing to the geographical mosaic of coevolution [141].
During coevolution, local adaptation is expected to occur in both parasites and hosts of sympatric populations if gene flow between populations is not too high [69]. For example, experimental studies analysing the outcome of interactions between sympatric or allopatric populations showed that the fitness consequences for slavemaker and host depend on a history of co-adaptation [82,142]. Interestingly, a model suggests that local adaptation in parasites is inversely proportional to the fraction of its host's range that they occupy [143]. In other words, parasites with a restricted distribution should be best adapted. A restricted distribution could be the result of local extinction by social parasites, because, for example, hosts developed strong defences. Unfortunately, historic data on social parasite loss are lacking, but there are possible cases, such as the L. acervorum populations of the British Isles, which do not harbour H. sublaevis slavemakers, but are highly effective in defending their nests against their raids [45,142]. However, alternatively, the social parasite H. sublaevis may never have invaded Britain, possibly due to the absence of its more preferred host, L. muscorum [144]. While the coevolution between bacteria and parasitic phages can be directly observed in experimental evolution set-ups, the long generation times of social parasites and their hosts hamper the study of temporal changes in these systems. Instead, geographical comparisons combined with population genetic analyses are required to gain insights into the coevolutionary history of these biotic interactions between parasites and hosts with similar evolutionary potentials [145,146].
8. Future directions
Despite the recent progress in our understanding of how colonies can defend themselves against social parasites, many open questions remain. For instance, can founding queens or swarms actively avoid areas with a higher prevalence of social parasites? How costly are defence strategies against social parasites? The fact that the expression of defence traits often increases with parasite pressure indicates that defence traits are traded-off and are costly in the absence of parasites [44,46,121,140]. Highly specific defence traits might carry low or no costs if the parasite is absent. More general, unspecific defences, such a lower degree of division of labour [121], nest architecture, or guards might lower the productivity of the colony. These costs could be detrimental in a competitive environment. Other questions include whether chemical, morphological or behavioural defence traits are more likely to be specific or costly. Are different types of defence traits more likely to facilitate each other? Are defence portfolios more likely to contain certain types of traits? Social structure and genetic diversity have been shown to be important when dealing with endoparasites (e.g. [147]), but it remains to be explored whether different types of social parasitism are affected by social structure in the same way. One major obstacle to a better understanding is the lack of basic natural history data (e.g. regional variation in social structure, defensive investment or behaviour), which would allow us to evaluate evolutionary patterns in host–parasite interactions. We hope that our review will motivate researchers to fill some of these gaps.
Acknowledgements
The authors would like to thank the reviewers and editors for their helpful comments.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to the literature review and the writing and revision of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
Support for this study came from the Deutsche Forschungsgemeinschaft (Fo 298/9-2, Fo 298/17-1).
References
- 1.Wilson EO. 1990. Success and dominance in ecosystems: the case of the social insects. Oldendorf/Luhe, Germany: Ecology Institute.
- 2.Rubenstein DR, Abbot P.. 2017. The evolution of social evolution. In Comparative social evolution (eds Rubenstein DR, Abbot P), pp. 1–18. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Shorter JR, Rueppell O. 2012. A review on self-destructive defense behaviours in social insects. Insectes Soc. 59, 1–10. ( 10.1007/s00040-011-0210-x) [DOI] [Google Scholar]
- 4.Cremer S, Armitage SAO, Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, R693–R702. ( 10.1016/j.cub.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 5.Tranter C, Hughes WOH. 2015. Acid, silk and grooming: alternative strategies in social immunity in ants? Behav. Ecol. Sociobiol. 69, 1687–1699. ( 10.1007/s00265-015-1980-3) [DOI] [Google Scholar]
- 6.Konrad M, et al. 2012. Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol. 10, e1001300 ( 10.1371/journal.pbio.1001300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigio G, Al Toufailia H, Ratnieks F. 2014. Honey bee hygienic behaviour does not incur a cost via removal of healthy brood. J. Evol. Biol. 27, 226–230. ( 10.1111/jeb.12288) [DOI] [PubMed] [Google Scholar]
- 8.Toufailia H, Alves AD, Bento JMS, Marchini LC, Ratnieks FLW. 2017. Hygienic behaviour in Brazilian stingless bees. Biol. Open 5, 1712–1718. ( 10.1242/bio.018549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ugelvig LV, Cremer S. 2007. Social prophylaxis: group interaction promotes collective immunity in ant colonies. Curr. Biol. 17, 1967–1971. ( 10.1016/j.cub.2007.10.029) [DOI] [PubMed] [Google Scholar]
- 10.Stroeymeyt N, Casillas-Pérez B, Cremer S. 2014. Organisational immunity in social insects. Curr. Opin. Insect Sci. 5, 1–15. ( 10.1016/j.cois.2014.09.001) [DOI] [PubMed] [Google Scholar]
- 11.Meunier J. 2015. Social immunity and the evolution of group living in insects. Phil. Trans. R. Soc. B 370, 20140102 ( 10.1098/rstb.2014.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buschinger A. 2009. Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol. News 12, 219–235. [Google Scholar]
- 13.Breed MD, Cook C, Krasnec MO. 2012. Cleptobiosis in social insects. Psyche 2012, 484765. [Google Scholar]
- 14.McGlynn TP, Graham R, Wilson J, Emerson J, Jandt JM, Jahren AH. 2015. Distinct types of foragers in the ant Ectatomma ruidum: typical foragers and furtive thieves. Anim. Behav. 109, 243–247. ( 10.1016/j.anbehav.2015.08.024) [DOI] [Google Scholar]
- 15.Grüter C, von Zuben L, Segers F, Cunningham J. 2016. Warfare in stingless bees. Insectes Soc. 63, 223–236. ( 10.1007/s00040-016-0468-0) [DOI] [Google Scholar]
- 16.Blacher P, Yagound B, Lecoutey E, Devienne P, Chameron S, Châline N. 2013. Drifting behaviour as an alternative reproductive strategy for social insect workers. Proc. R. Soc. B 280, 20131888 ( 10.1098/rspb.2013.1888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann P, Radloff SE, Moritz RF, Hepburn HR, Reece SL. 2001. Social parasitism by honeybee workers (Apis mellifera capensis Escholtz): host finding and resistance of hybrid host colonies. Behav. Ecol. 12, 419–428. ( 10.1093/beheco/12.4.419) [DOI] [Google Scholar]
- 18.Wenseleers T, Alves DA, Francoy TM, Billen J, Imperatriz-Fonseca VL. 2011. Intraspecific queen parasitism in a highly eusocial bee. Biol. Lett. 7, 173–176. ( 10.1098/rsbl.2010.0819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen G, Savolainen R, Vepsäläinen K. 2010. Phylogeny, divergence-time estimation, biogeography and social parasite-host relationships of the Holarctic ant genus Myrmica (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 56, 294–304. ( 10.1016/j.ympev.2010.01.029) [DOI] [PubMed] [Google Scholar]
- 20.Als TD, Vila R, Kandul NP, Nash DR, Yen SH, Hsu YF, Mignault AA, Boomsma JJ, Pierce NE. 2004. The evolution of alternative parasitic life histories in large blue butterflies. Nature 432, 386–390. ( 10.1038/nature03020) [DOI] [PubMed] [Google Scholar]
- 21.von Beeren C, Maruyama M, Rosli H, Witte V. 2011. Differential host defense of multiple parasites in ants. Evol. Ecol. 25, 259–276. ( 10.1007/s10682-010-9420-3) [DOI] [Google Scholar]
- 22.Witte V, Janssen R, Eppenstein A, Maschwitz U. 2002. Allopeas myrmekophilos (Gastropoda, Pulmonata), the first myrmecophilous mollusc living in colonies of the ponerine army ant Leptogenys distinguenda (Formicidae, Ponerinae). Insectes Soc. 49, 301–305. (doi:0.1007/PL00012646) [Google Scholar]
- 23.Yamamoto S, Maruyama M, Parker J. 2016. Evidence for social parasitism of early insect societies by Cretaceous rove beetles. Nat. Commun. 7, 13658 ( 10.1038/ncomms13658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristaldo P, Rosa C, Florencio D, Marins A, DeSouza O. 2012. Termitarium volume as a determinant of invasion by obligatory termitophiles and inquilines in the nests of Constrictotermes cyphergaster (Termitidae, Nasutitermitinae). Insectes Soc. 59, 541–548. ( 10.1007/s00040-012-0249-3) [DOI] [Google Scholar]
- 25.Puker A, Ferreira FN, Rosa CS, Jameson ML, Vaz-De-Mello FZ. 2014. First record of the leaf chafer beetle Leucothyreus suturalis (Coleoptera: Scarabaeidae: Rutelinae) inhabiting termite nests, with notes on its life history. Ann. Entomol. Soc. Am. 108, 3–10. ( 10.1093/aesa/sau004) [DOI] [Google Scholar]
- 26.Korb J, Heinze J (eds) 2008. The ecology of social evolution. Berlin, Germany: Springer. [Google Scholar]
- 27.Cervo C. 2006. Polistes wasps and their social parasites: an overview. Ann. Zool. Fenn. 43, 531–549. [Google Scholar]
- 28.Oliveira RC, Oi CA, Vollet-Neto A, Wenseleers T. 2016. Intraspecific worker parasitism in the common wasp, Vespula vulgaris. Anim. Behav. 113, 79–85. ( 10.1016/j.anbehav.2015.12.025) [DOI] [Google Scholar]
- 29.Goudie F, Oldroyd BP. 2014. Thelytoky in the honey bee. Apidologie 45, 306–326. ( 10.1007/s13592-013-0261-2) [DOI] [Google Scholar]
- 30.Sumner S, Aanen DK, Delabie J, Boomsma JJ. 2004. The evolution of social parasitism in Acromyrmex leaf-cutting ants: a test of Emery's rule. Insectes Soc. 51, 37–42. ( 10.1007/s00040-003-0723-z) [DOI] [Google Scholar]
- 31.Van Oystaeyen A, Alves DA, Oliveira RC, Nascimento DL, Nascimento FS, Billen J, Wenseleers T. 2013. Sneaky queens in Melipona bees selectively detect and infiltrate queenless colonies. Anim. Behav. 86, 603–609. ( 10.1016/j.anbehav.2013.07.001) [DOI] [Google Scholar]
- 32.Lenoir A, D'Ettorre P, Errard C, Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. ( 10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 33.Ortolani I, Cervo R. 2010. Intra-specific body size variation in Polistes paper wasps as a response to social parasite pressure. Ecol. Entomol. 35, 352–359. ( 10.1111/j.1365-2311.2010.01187.x) [DOI] [Google Scholar]
- 34.Zimma B, Ayasse M, Tengö J, Ibarra F, Schulz C, Francke W. 2003. Do social parasitic bumblebees use chemical weapons? (Hymenoptera, Apidae). J. Comp. Physiol. A 189, 769–775. ( 10.1007/s00359-003-0451-x) [DOI] [PubMed] [Google Scholar]
- 35.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: The Belknap Press of Harvard University. [Google Scholar]
- 36.Beekman M, Oldroyd BP. 2008. When workers disunite: intraspecific parasitism by eusocial bees. Annu. Rev. Entomol. 53, 19–37. ( 10.1146/annurev.ento.53.103106.093515) [DOI] [PubMed] [Google Scholar]
- 37.Alves DA, Imperatriz-Fonseca VL, Francoy TM, Santos Filho PS, Nogueira-Neto P, Billen J, Wenseleers T. 2009. The queen is dead—long live the workers: intraspecific parasitism by workers in the stingless bee Melipona scutellaris. Mol. Ecol. 18, 4102–4111. ( 10.1111/j.1365-294X.2009.04323.x) [DOI] [PubMed] [Google Scholar]
- 38.Takahashi J-i, Martin SJ, Ono M, Shimizu I. 2010. Male production by non-natal workers in the bumblebee, Bombus deuteronymus (Hymenoptera: Apidae). J. Ethol. 28, 61–66. ( 10.1007/s10164-009-0155-y) [DOI] [Google Scholar]
- 39.Kleeberg I, Foitzik S. 2016. The placid slavemaker: avoiding detection and conflict as an alternative, peaceful raiding strategy. Behav. Ecol. Sociobiol. 70, 27–39. ( 10.1007/s00265-015-2018-6) [DOI] [Google Scholar]
- 40.Pohl S, Foitzik S. 2011. Slave-making ants prefer larger, better defended host colonies. Anim. Behav. 81, 61–68. ( 10.1016/j.anbehav.2010.09.006) [DOI] [Google Scholar]
- 41.Kleeberg I, Menzel F, Foitzik S. 2017. The influence of slavemaking lifestyle, caste and sex on chemical profiles in Temnothorax ants: insights into the evolution of cuticular hydrocarbons. Proc. R. Soc. B 284, 20162249 ( 10.1098/rspb.2016.2249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamminger T, Scharf T, Pennings PS, Foitzik S. 2011. Increased host aggression as an induced defense against slave-making ants. Behav. Ecol. 22, 255–260. ( 10.1093/beheco/arq191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleeberg I, Pamminger T, Jongepier E, Papenhagen M, Foitzik S. 2014. Forewarned is forearmed: aggression and information use determine fitness costs of slave raids. Behav. Ecol. 25, 1058–1063. ( 10.1093/beheco/aru084) [DOI] [Google Scholar]
- 44.Jongepier E, Kleeberg I, Job S, Foitzik S. 2014. Collective defence portfolios of ant hosts shift with social parasite pressure. Proc. R. Soc. B 281, 20140225 ( 10.1098/rspb.2014.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foitzik S, Fischer B, Heinze J. 2003. Arms races between social parasites and their hosts: geographic patterns of manipulation and resistance. Behav. Ecol. 14, 80–88. ( 10.1093/beheco/14.1.80) [DOI] [Google Scholar]
- 46.Jongepier E, Kleeberg I, Foitzik S. 2015. The ecological success of a social parasite increases with manipulation of collective host behaviour. J. Evol. Biol. 28, 2152–2162. ( 10.1111/jeb.12738) [DOI] [PubMed] [Google Scholar]
- 47.Achenbach A, Foitzik S. 2009. First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evolution 63, 1068–1075. ( 10.1111/j.1558-5646.2009.00591.x) [DOI] [PubMed] [Google Scholar]
- 48.Metzler D, Jordan F, Pamminger T, Foitzik S. 2016. The influence of space and time on the evolution of altruistic defence: the case of ant slave rebellion. J. Evol. Biol. 29, 874–886. ( 10.1111/jeb.12846) [DOI] [PubMed] [Google Scholar]
- 49.Pamminger T, Foitzik S, Metzler D, Pennings PS. 2014. Oh sister, where art thou? Spatial population structure and the evolution of an altruistic defence trait. J. Evol. Biol. 27, 2443–2456. ( 10.1111/jeb.12496) [DOI] [PubMed] [Google Scholar]
- 50.Buschinger A, Ehrhardt W, Winter U. 1980. The organization of slave raids in dulotic ants—a comparative study (Hymenoptera; Formicidae). Ethology 53, 245–264. ( 10.1111/j.1439-0310.1980.tb01053.x) [DOI] [Google Scholar]
- 51.Breed MD, Abel P, Bleuze TJ, Denton SE. 1990. Thievery, home ranges, and nestmate recognition in Ectatomma ruidum. Oecologia 84, 117–121. ( 10.1007/BF00665604) [DOI] [PubMed] [Google Scholar]
- 52.Downs SG, Ratnieks FLW. 2000. Adaptive shifts in honey bee (Apis mellifera L.) guarding behavior support predictions of the acceptance threshold model. Behav. Ecol. 11, 326–333. ( 10.1093/beheco/11.3.326) [DOI] [Google Scholar]
- 53.Adams RMM, Liberti J, Illum AA, Jones TH, Nash DR, Boomsma JJ. 2013. Chemically armed mercenary ants protect fungus-farming societies. Proc. Natl Acad. Sci. USA 110, 15 752–15 757. ( 10.1073/pnas.1311654110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allies AB, Bourke AFG, Franks NR. 1986. Propaganda substances in the cuckoo ant Leptothorax kutteri and the slave-maker Harpagoxenus sublaevis. J. Chem. Ecol. 12, 1285–1293. ( 10.1007/BF01012348) [DOI] [PubMed] [Google Scholar]
- 55.Corbara B, Dejean A. 2002. Paper stealing on an arboricolous ant nest by the wasp Agelaia fulvofasciata Degeer (Hymenoptera: Vespidae). Sociobiology 39, 281–284. [Google Scholar]
- 56.Cardoso DC, Cristiano MP, da Costa-Milanez CB, Heinze J. 2016. Agro-predation by Megalomyrmex ants on Mycetophylax fungus-growing ants. Insectes Soc. 63, 483–486. ( 10.1007/s00040-016-0487-x) [DOI] [Google Scholar]
- 57.Powell S, Del-Claro K, Feitosa RM, Brandão CR. 2014. Mimicry and eavesdropping enable a new form of social parasitism in ants. Am. Nat. 184, 500–509. ( 10.1086/677927) [DOI] [PubMed] [Google Scholar]
- 58.Cunningham JP, Hereward JP, Heard TA, De Barro PJ, West SA. 2014. Bees at war: interspecific battles and nest usurpation in stingless bees. Am. Nat. 184, 777–786. ( 10.1086/678399) [DOI] [PubMed] [Google Scholar]
- 59.Cerdá X, Retana J. 1998. Interference interactions and nest usurpation between two subordinate ant species. Oecologia 113, 577–583. ( 10.1007/s004420050411) [DOI] [PubMed] [Google Scholar]
- 60.Crespi B, Abbot P. 1999. The behavioral ecology and evolution of kleptoparasitism in Australian gall thrips. Florida Entomol. 82, 147–164. ( 10.2307/3496568) [DOI] [Google Scholar]
- 61.Cardinal S, Straka J, Danforth B. 2010. Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism. Proc. Natl Acad. Sci. USA 107, 16 207–16 211. ( 10.1073/pnas.1006299107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith JA, Tierney SM, Park YC, Fuller S, Schwarz MP. 2007. Origins of social parasitism: the importance of divergence ages in phylogenetic studies. Mol. Phylogen. Evol. 43, 1131–1137. ( 10.1016/j.ympev.2006.12.028) [DOI] [PubMed] [Google Scholar]
- 63.Choudhary M, Strassmann JE, Queller DC, Turillazzi S, Cervo R. 1994. Social parasites in polistine wasps are monophyletic: implications for sympatric speciation. Proc. R. Soc. Lond. B 257, 31–35. ( 10.1098/rspb.1994.0090) [DOI] [Google Scholar]
- 64.Emery C. 1909. Über den Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen. Biol. Centralbl. 29, 352–362. [Google Scholar]
- 65.Huang MH, Dornhaus A. 2008. A meta-analysis of ant social parasitism: host characteristics of different parasitism types and a test of Emery's rule. Ecol. Entomol. 33, 589–596. ( 10.1111/j.1365-2311.2008.01005.x) [DOI] [Google Scholar]
- 66.Härtel S, Neumann P, Raassen FS, Moritz RF, Hepburn HR. 2006. Social parasitism by Cape honeybee workers in colonies of their own subspecies (Apis mellifera capensis Esch.). Insectes Soc. 53, 183–193. ( 10.1007/s00040-005-0857-2) [DOI] [Google Scholar]
- 67.Foitzik S, Heinze J. 1998. Nest site limitation and colony takeover in the ant Leptothorax nylanderi. Behav. Ecol. 9, 367–375. ( 10.1093/beheco/9.4.367) [DOI] [Google Scholar]
- 68.d'Ettorre P, Heinze J. 2001. Sociobiology of slave-making ants. Acta Ethol. 3, 67–82. ( 10.1007/s102110100038) [DOI] [Google Scholar]
- 69.Brandt M, Foitzik S, Fischer-Blass B, Heinze J. 2005. The coevolutionary dynamics of obligate ant social parasite systems—between prudence and antagonism. Biol. Rev. 80, 251–267. ( 10.1017/S1464793104006669) [DOI] [PubMed] [Google Scholar]
- 70.Nunn C, Alitzer S. 2006. Infectious diseases in primates: behavior, ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 71.Hölldobler B, Wilson EO. 2009. The Superorganism: the beauty, elegance and strangeness of insect societies. New York: NY: W.W. Norton and Company. [Google Scholar]
- 72.d'Ettorre P, Mondy N, Lenoir A, Errard C. 2002. Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc. R. Soc. Lond. B 269, 1911–1918. ( 10.1098/rspb.2002.2110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brandt M, Foitzik S. 2004. Community context and specialization influence coevolution between a slavemaking ant and its hosts. Ecology 85, 2997–3009. ( 10.1890/03-0778) [DOI] [Google Scholar]
- 74.Wilson EO. 1963. Social modifications related to rareness in ant species. Evolution 17, 249–253. ( 10.1111/j.1558-5646.1963.tb03274.x) [DOI] [Google Scholar]
- 75.Herbers JM, Foitzik S. 2002. The ecology of slavemaking ants and their hosts in North temperate forests. Ecology 83, 148–163. ( 10.1890/0012-9658(2002)083%5B0148:TEOSAA%5B2.0.CO;2) [DOI] [Google Scholar]
- 76.Bonelli S, Witek M, Canterino S, Sielezniew M, Stankiewicz-Fiedurek A, Tartally A, Baletto E, Schönrogge K. 2011. Distribution, host specificity, and the potential for cryptic speciation in hoverfly Microdon myrmicae (Diptera: Syrphidae), a social parasite of Myrmica ants. Ecol. Entomol. 36, 135–143. ( 10.1111/j.1365-2311.2010.01253.x) [DOI] [Google Scholar]
- 77.Buschinger A. 1989. Evolution, speciation, and inbreeding in the parasitic ant genus Epimyrma (Hymenoptera, Formicidae). J. Evol. Biol. 2, 265–283. ( 10.1046/j.1420-9101.1989.2040265.x) [DOI] [Google Scholar]
- 78.Wittmann D. 1985. Aerial defense of the nest by workers of the stingless bee Trigona (Tetragonisca) angustula. Behav. Ecol. Sociobiol. 16, 111–114. ( 10.1007/BF00295143) [DOI] [Google Scholar]
- 79.Nogueira-Neto P. 1970. Behavior problems related to the pillages made by some parasitic stingless bees (Meliponinae, Apidae). In Development and evolution of behavior: essays in memory of TC Schneirla (ed. Aronson LR.), pp. 416–434. San Francisco, CA: W. H. Freeman. [Google Scholar]
- 80.Sumner S, Nash DR, Boomsma JJ. 2003. The adaptive significance of inquiline parasite workers. Proc. R. Soc. Lond. B 270, 1315–1322. ( 10.1098/rspb.2003.2362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foitzik S, Herbers JM. 2001. Colony structure of a slavemaking ant. II. Frequency of slave raids and impact on the host population. Evolution 55, 316–323. ( 10.1111/j.0014-3820.2001.tb01296.x) [DOI] [PubMed] [Google Scholar]
- 82.Foitzik S, Achenbach A, Brandt M. 2009. Locally adapted social parasite affects density, social structure, and life history of its ant hosts. Ecology 90, 1195–1206. ( 10.1890/08-0520.1) [DOI] [PubMed] [Google Scholar]
- 83.Keller L, Passera L. 1989. Influence of the number of queens on nestmate recognition and attractiveness of queens to workers in the Argentine ant, Iridomyrmex humilis (Mayr). Anim. Behav. 37, 733–740. ( 10.1016/0003-3472(89)90059-6) [DOI] [Google Scholar]
- 84.Buschinger A. 1986. Evolution of social parasitism in ants. Trends Ecol. Evol. 1, 155–160. ( 10.1016/0169-5347(86)90044-3) [DOI] [PubMed] [Google Scholar]
- 85.Buschinger A. 1990. Sympatric speciation and radiative evolution of socially parasitic ants—heretic hypotheses and their factual background. J. Zool. Syst. Evol. Res. 28, 241–260. ( 10.1111/j.1439-0469.1990.tb00379.x) [DOI] [Google Scholar]
- 86.Roubik DW. 1989. Ecology and natural history of tropical bees. New York, NY: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- 87.Grüter C, Kärcher M, Ratnieks FLW. 2011. The natural history of nest defence in a stingless bee, Tetragonisca angustula (Latreille) (Hymenoptera: Apidae), with two distinct types of entrance guards. Neotrop. Entomol. 40, 55–61. ( 10.1590/S1519-566X2011000100008) [DOI] [PubMed] [Google Scholar]
- 88.Camargo JM, Pedro SR. 2003. Neotropical Meliponini: the genus Partamona Schwarz, 1939 (Hymenoptera, Apidae, Apinae)—bionomy and biogeography. Rev. Bras. Entomol. 47, 311–372. ( 10.1590/S0085-56262003000300001) [DOI] [Google Scholar]
- 89.Kerr WE. 1984. Virgilio de Portugal Brito Araújo (1919–1983). Acta Amazonica 14, 327–328. ( 10.1590/1809-43921984142327) [DOI] [Google Scholar]
- 90.Nunes TM, von Zuben LG, Costa L, Venturieri GC. 2015. Defensive repertoire of the stingless bee Melipona flavolineata Friese (Hymenoptera: Apidae). Sociobiology 61, 541–546. ( 10.13102/sociobiology.v61i4.541-546) [DOI] [Google Scholar]
- 91.Powell S. 2016. A comparative perspective on the ecology of morphological diversification in complex societies: nesting ecology and soldier evolution in the turtle ants. Behav. Ecol. Sociobiol. 70, 1075–1085. ( 10.1007/s00265-016-2080-8) [DOI] [Google Scholar]
- 92.d'Ettorre P, Lenoir A.. 2010. Nestmate recognition. In Ant ecology (eds Lach L, Parr C, Abbott K), pp. 194–209, Oxford, UK: Oxford University Press. [Google Scholar]
- 93.Gamboa GJ. 2004. Kin recognition in eusocial wasps. Ann. Zool. Fenn. 41, 789–808. [Google Scholar]
- 94.Howard RW, Blomquist GJ. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393. ( 10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- 95.van Zweden JS, D'Ettorre P.. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 96.Cini A, Bruschini L, Poggi L, Cervo R. 2011. Fight or fool? Physical strength, instead of sensory deception, matters in host nest invasion by a wasp social parasite. Anim. Behav. 81, 1139–1145. ( 10.1016/j.anbehav.2011.02.017) [DOI] [Google Scholar]
- 97.Jones SM, van Zweden JS, Grüter C, Menezes C, Alves D, Nunes-Silva P, Czaczkes TJ, Imperatriz-Fonseca VL, Ratnieks FLW. 2012. The role of wax and resin in the nestmate recognition system of a stingless bee, Tetragonisca angustula. Behav. Ecol. Sociobiol. 66, 1–12. ( 10.1007/s00265-011-1246-7) [DOI] [Google Scholar]
- 98.Stuart RJ. 1987. Individually-produced nestmate recognition cues and a colony odour ‘gestalt’ in leptothoracine ants. In Chemistry and biology of social insects (eds Eder J, Rembold H), p. 480 Munich, Germany: J. Peperny. [Google Scholar]
- 99.Vienne C, Soroker V, Hefetz A. 1995. Congruency of hydrocarbon patterns in heterospecific groups of ants: transfer and/or biosynthesis? Insectes Soc. 42, 267–277. ( 10.1007/BF01240421) [DOI] [Google Scholar]
- 100.Boulay R, Hefetz A, Soroker V, Lenoir A. 2000. Camponotus fellah colony integration: worker individuality necessitates frequent hydrocarbon exchanges. Anim. Behav. 59, 1127–1133. ( 10.1006/anbe.2000.1408) [DOI] [PubMed] [Google Scholar]
- 101.Couvillon MJ, Caple JP, Endsor SL, Kärcher M, Russell TE, Storey DE, Ratnieks FL. 2007. Nest-mate recognition template of guard honeybees (Apis mellifera) is modified by wax comb transfer. Biol. Lett. 3, 228–230. ( 10.1098/rsbl.2006.0612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reeve HK. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. ( 10.1086/284926) [DOI] [Google Scholar]
- 103.van Zweden JS, Grüter C, Jones SM, Ratnieks FLW. 2011. Hovering guards of the stingless bee Tetragonisca angustula increase colony defensive perimeter as shown by intra- and inter-specific comparisons. Behav. Ecol. Sociobiol. 65, 1277–1282. ( 10.1007/s00265-011-1141-2) [DOI] [Google Scholar]
- 104.Turillazzi S, Sledge MF, Dani FR, Cervo R, Massolo A, Fondelli L. 2000. Social hackers: integration in the host chemical recognition system by a paper wasp social parasite. Naturwissenschaften 87, 172–176. ( 10.1007/s001140050697) [DOI] [PubMed] [Google Scholar]
- 105.Uboni A, Bagneres AG, Christidès JP, Lorenzi MC. 2012. Cleptoparasites, social parasites and a common host: chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 58, 1259–1264. ( 10.1016/j.jinsphys.2012.06.013) [DOI] [PubMed] [Google Scholar]
- 106.Quezada-Euán JJG, Ramírez J, Eltz T, Pokorny T, Medina R, Monsreal R. 2013. Does sensory deception matter in eusocial obligate food robber systems? A study of Lestrimelitta and stingless bee hosts. Anim. Behav. 85, 817–823. ( 10.1016/j.anbehav.2013.01.028) [DOI] [Google Scholar]
- 107.Brandt M, Heinze J, Schmitt T, Foitzik S. 2005. A chemical level in the coevolutionary arms race between an ant social parasite and its hosts. J. Evol. Biol. 18, 576–586. ( 10.1111/j.1420-9101.2004.00867.x) [DOI] [PubMed] [Google Scholar]
- 108.Couvillon MJ, Robinson EJ, Atkinson B, Child L, Dent KR, Ratnieks FL. 2008. En garde: rapid shifts in honeybee, Apis mellifera, guarding behaviour are triggered by onslaught of conspecific intruders. Anim. Behav. 76, 1653–1658. ( 10.1016/j.anbehav.2008.08.002) [DOI] [Google Scholar]
- 109.Martin SJ, Helanterä H, Drijfhout FP. 2011. Is parasite pressure a driver of chemical cue diversity in ants? Proc. R. Soc. B 278, 496–503. ( 10.1098/rspb.2010.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jongepier E, Foitzik S. 2016. Ant recognition cue diversity is higher in the presence of slavemaker ants. Behav. Ecol. 27, 304–311. ( 10.1093/beheco/arv153) [DOI] [Google Scholar]
- 111.Davies NB, Brooke MDL. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 58, 225–236. ( 10.2307/4996) [DOI] [Google Scholar]
- 112.Spottiswoode CN, Stevens M. 2011. How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. R. Soc. B 278, 3566–3573. ( 10.1098/rspb.2011.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schorkopf DLP, Hrncir M, Mateus S, Zucchi R, Schmidt VM, Barth FG. 2009. Mandibular gland secretions of meliponine worker bees: further evidence for their role in interspecific and intraspecific defence and aggression and against their role in food source signalling. J. Exp. Biol. 212, 1153–1162. ( 10.1242/jeb.021113) [DOI] [PubMed] [Google Scholar]
- 114.Ono M, Igarashi T, Ohno E, Sasaki M. 1995. Unusual thermal defence by a honeybee against mass attack by hornets. Nature 377, 334–336. ( 10.1038/377334a0) [DOI] [Google Scholar]
- 115.Grüter C, Menezes C, Imperatriz-Fonseca VL, Ratnieks FLW. 2012. A morphologically specialized soldier caste improves colony defense in a neotropical eusocial bee. Proc. Natl Acad. Sci. USA 109, 1182–1186. ( 10.1073/pnas.1113398109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grüter C, Segers FHID, Menezes C, Vollet-Neto A, Falcon T, von Zuben L, Bitondi MMG, Nascimento FS, Almeida EAB. 2017. Repeated evolution of soldier sub-castes suggests parasitism drives social complexity in stingless bees. Nat. Commun. 8, 4 ( 10.1038/s41467-016-0012-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grüter C, Segers FHID, Santos LLG, Hammel B, Zimmermann U, Nascimento FS. 2017. Enemy recognition is linked to soldier size in a polymorphic stingless bee. Biol. Lett. 13, 20170511 ( 10.1098/rsbl.2017.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gill KP, Van Wilgenburg E, Macmillan DL, Elgar MA. 2013. Density of antennal sensilla influences efficacy of communication in a social insect. Am. Nat. 182, 834–840. ( 10.1086/673712) [DOI] [PubMed] [Google Scholar]
- 119.Spaethe J, Brockmann A, Halbig C, Tautz J. 2007. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 94, 733–739. ( 10.1007/s00114-007-0251-1) [DOI] [PubMed] [Google Scholar]
- 120.Foitzik S, Deheer CJ, Hunjan DN, Herbers JM. 2001. Coevolution in host-parasite systems: behavioural strategies of slave-making ants and their hosts. Proc. R. Soc. Lond. B 268, 1139–1146. ( 10.1098/rspb.2001.1627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jongepier E, Foitzik S. 2016. Fitness costs of worker specialization for ant societies. Proc. R. Soc. B 283, 20152572 ( 10.1098/rspb.2015.2572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruschini C, Cervo R. 2011. Venom volatiles of the paper wasp social parasite Polistes sulcifer elicit intra-colonial aggression on the nest of the host species Polistes dominulus. Insectes Soc. 58, 383–390. ( 10.1007/s00040-011-0155-0) [DOI] [Google Scholar]
- 123.von Zuben L, Schorkopf D, Elias L, Vaz A, Favaris A, Clososki G, Bento J, Nunes T. 2016. Interspecific chemical communication in raids of the robber bee Lestrimelitta limao. Insectes Soc. 63, 339–347. ( 10.1007/s00040-016-0474-2) [DOI] [Google Scholar]
- 124.Topoff H, Zimmerli E. 1993. Colony takeover by a socially parasitic ant, Polyergus breviceps: the role of chemicals obtained during host-queen killing. Anim. Behav. 46, 479–486. ( 10.1006/anbe.1993.1216) [DOI] [Google Scholar]
- 125.d'Ettorre P, Errard C, Ibarra F, Francke W, Hefetz A. 2000. Sneak in or repel your enemy: Dufour's gland repellent as a strategy for successful usurpation in the slave-maker Polyergus rufescens. Chemoecology 10, 135–142. ( 10.1007/PL00001815) [DOI] [Google Scholar]
- 126.Mori A, Grasso DA, Visicchio R, Le Moli F. 2000. Colony founding in Polyergus rufescens: the role of the Dufour's gland. Insectes Soc. 47, 7–10. ( 10.1007/s000400050002) [DOI] [Google Scholar]
- 127.Visicchio R, Sledge MF, Mori A, Grasso DA, Le Moli F, Turillazzi S, Moneti G, Spencer SH, Jones GR. 2000. Dufour's gland contents of queens of the slave-making ant Polyergus rufescens and its host species Formica cunicularia. Ethol. Ecol. Evol. 12, 67–73. ( 10.1080/03949370.2000.9728323) [DOI] [Google Scholar]
- 128.Ruano F, Hefetz A, Lenoir A, Francke W, Tinaut A. 2005. Dufour's gland secretion as a repellent used during usurpation by the slave-maker ant Rossomyrmex minuchae. J. Insect Physiol. 51, 1158–1164. ( 10.1016/j.jinsphys.2005.06.005) [DOI] [PubMed] [Google Scholar]
- 129.Brandt M, Heinze J, Schmitt T, Foitzik S. 2006. Convergent evolution of the Dufour's gland secretion as a propaganda substance in the slave-making ant genera Protomognathus and Harpagoxenus. Insectes Soc. 53, 291–299. ( 10.1007/s00040-006-0871-z) [DOI] [Google Scholar]
- 130.Bauer S, Witte V, Böhm M, Foitzik S. 2009. Fight or flight? A geographic mosaic in host reaction and potency of a chemical weapon in the social parasite Harpagoxenus sublaevis. Behav. Ecol. Sociobiol. 64, 45–56. ( 10.1007/s00265-009-0817-3) [DOI] [Google Scholar]
- 131.Råberg L. 2014. How to live with the enemy: understanding tolerance to parasites. PLoS Biol. 12, e1001989 ( 10.1371/journal.pbio.1001989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lorenzi MC, Filippone F. 2000. Opportunistic discrimination of alien eggs by social wasps (Polistes biglumis, Hymenoptera Vespidae): a defense against social parasitism? Behav. Ecol. Sociobiol. 48, 402–406. ( 10.1007/s002650000251) [DOI] [Google Scholar]
- 133.Hare JF. 1996. Discrimination of nestmate larvae by the ant Leptothorax longispinosus. Can. J. Zool. 74, 2055–2061. ( 10.1139/z96-233) [DOI] [Google Scholar]
- 134.Cini A, Nieri R, Dapporto L, Monnin T, Cervo R. 2014. Almost royal: incomplete suppression of host worker ovarian development by a social parasite wasp. Behav. Ecol. Sociobiol. 68, 467–475. ( 10.1007/s00265-013-1661-z) [DOI] [Google Scholar]
- 135.Feeney WE, Welbergen JL, Langmore NE. 2012. The frontline of avian brood parasite–host coevolution. Anim. Behav. 84, 3–12. ( 10.1016/j.anbehav.2012.04.011) [DOI] [Google Scholar]
- 136.Gilman RT, Nuismer SL, Jhwueng D. 2012. Coevolution in multidimensional trait space favours escape from parasites and pathogens. Nature 483, 328–330. ( 10.1038/nature10853) [DOI] [PubMed] [Google Scholar]
- 137.Thompson JN. 1994. The coevolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 138.Kleeberg I, Jongepier E, Job S, Foitzik S. 2015. Geographic variation in social parasite pressure predicts intraspecific but not interspecific aggressive responses in hosts of a slavemaking ant. Ethology 121, 694–702. ( 10.1111/eth.12384) [DOI] [Google Scholar]
- 139.Ortolani I, Cervo R. 2009. Coevolution of daily activity timing in a host-parasite system. Biol. J. Linn. Soc. 96, 399–405. ( 10.1111/j.1095-8312.2008.01139.x) [DOI] [Google Scholar]
- 140.Segers FHID, Von Zuben LG, Grüter C. 2016. Local differences in parasitism and competition shape defensive investment in a polymorphic eusocial bee. Ecology 97, 417–426. ( 10.1890/15-0793.1) [DOI] [PubMed] [Google Scholar]
- 141.Johnson CA, Herbers JM. 2006. Impact of parasite sympatry on the geographic mosaic of coevolution. Ecology 87, 382–394. ( 10.1890/05-1093) [DOI] [PubMed] [Google Scholar]
- 142.Fischer B, Foitzik S. 2004. Local co-adaptation leading to a geographical mosaic of coevolution in a social parasite system. J. Evol. Biol. 17, 1026–1034. ( 10.1111/j.1420-9101.2004.00749.x) [DOI] [PubMed] [Google Scholar]
- 143.Nuismer SL, Thompson JN, Gomulkiewicz R. 2003. Coevolution between hosts and parasites with partially overlapping geographic ranges. J. Evol. Biol. 16, 1337–1345. ( 10.1046/j.1420-9101.2003.00609.x) [DOI] [PubMed] [Google Scholar]
- 144.Fischer-Blass B, Heinze J, Foitzik S. 2006. Microsatellite analysis reveals strong but differential impact of a social parasite on its two host species. Mol. Ecol. 15, 863–872. ( 10.1111/j.1365-294X.2005.02798.x) [DOI] [PubMed] [Google Scholar]
- 145.Brandt M, Fischer-Blass B, Heinze J, Foitzik S. 2007. Population structure and the coevolution between social parasites and their hosts. Mol. Ecol. 16, 2063–2078. ( 10.1111/j.1365-294X.2007.03300.x) [DOI] [PubMed] [Google Scholar]
- 146.Pennings P, Achenbach A, Foitzik S. 2011. Similar evolutionary potentials in an obligate ant parasite and its two host species. J. Evol. Biol. 24, 871–886. ( 10.1111/j.1420-9101.2010.02223.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baer B, Schmid-Hempel P. 1999. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397, 151–154. ( 10.1038/16451) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.