Abstract
Ana M Valdes and colleagues discuss strategies for modulating the gut microbiota through diet and probiotics
Microbiome refers to the collective genomes of the micro-organisms in a particular environment, and microbiota is the community of micro-organisms themselves (box 1). Approximately 100 trillion micro-organisms (most of them bacteria, but also viruses, fungi, and protozoa) exist in the human gastrointestinal tract1 2—the microbiome is now best thought of as a virtual organ of the body. The human genome consists of about 23 000 genes, whereas the microbiome encodes over three million genes producing thousands of metabolites, which replace many of the functions of the host,1 3 consequently influencing the host’s fitness, phenotype, and health.2
Box 1. Glossary .
Microbiome—the collective genomes of the micro-organisms in a particular environment
Microbiota—the community of micro-organisms themselves
Microbiota diversity—a measure of how many different species and, dependent on the diversity indices, how evenly distributed they are in the community. Lower diversity is considered a marker of dysbiosis (microbial imbalance) in the gut and has been found in autoimmune diseases and obesity and cardiometabolic conditions, as well as in elderly people
Operational taxonomic unit—a definition used to classify groups of closely related organisms. DNA sequences can be clustered according to their similarity to one another, and operational taxonomic units are defined based on the similarity threshold (usually 97% similarity) set by the researcher
Colonocytes—epithelial cells of the colon
Germ-free animals—animals that have no micro-organisms living in or on them
Short chain fatty acids—fatty acids with two to six carbon atoms that are produced by bacterial fermentation of dietary fibres
Studying the gut microbiota
Twin studies have shown that, although there is a heritable component to gut microbiota, environmental factors related to diet, drugs, and anthropometric measures are larger determinants of microbiota composition.4 5
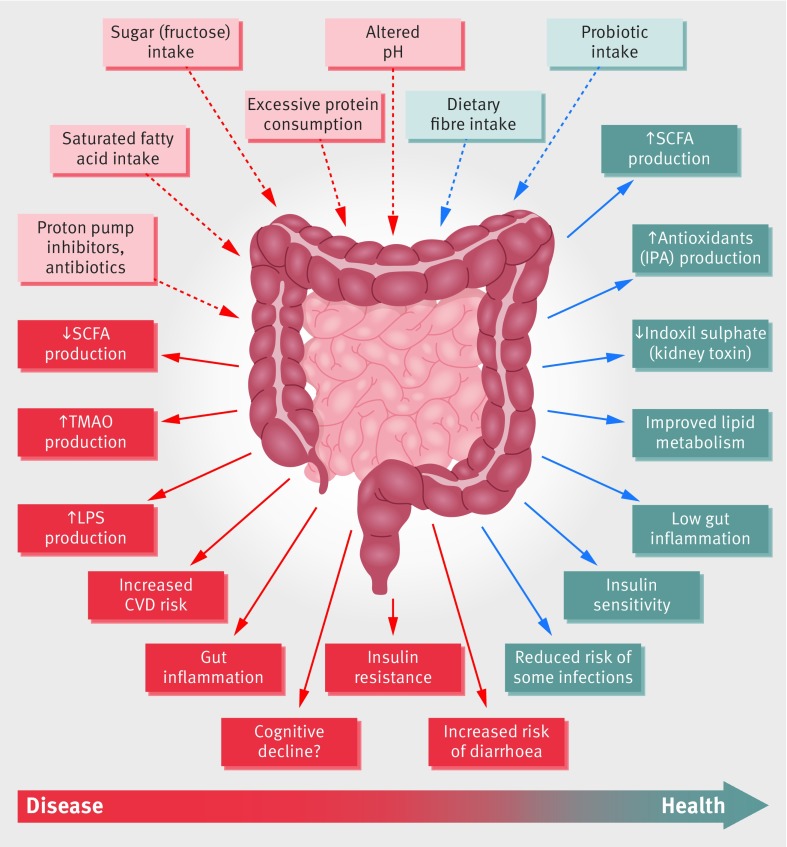
Gut microbes are key to many aspects of human health including immune,6 metabolic5 and neurobehavioural traits (fig 1).7 8 Different levels of evidence support the role of gut microbiota in human health, from animal models9 10 and human studies.4 11 12 13
Fig 1.
Schematic representation of the role of the gut microbiota in health and disease giving some examples of inputs and outputs. CVD=cardiovascular disease; IPA=indolepropionic acid; LPS=lipopolysaccharide; SCFA=short chain fatty acids; TMAO=trimethylamine N-oxide
Animal models can help identify gut microbes and mechanisms, though the degree to which findings translate to humans is unknown. In humans, observational studies can show cross-sectional associations between microbes and health traits but are limited by the inability to measure causal relations. The strongest level of evidence is obtained from interventional clinical studies—in particular, randomised controlled trials.
The composition of gut microbiota is commonly quantified using DNA based methods, such as next generation sequencing of 16S ribosomal RNA genes or whole genome shotgun sequencing, which also allow inference of microbiota functions.14 15 Metabolic products of the microbiota are now measurable in stool and serum using metabolomic methods.16
What does the gut microbiota do?
The gut microbiota provides essential capacities for the fermentation of non-digestible substrates like dietary fibres and endogenous intestinal mucus. This fermentation supports the growth of specialist microbes that produce short chain fatty acids (SCFAs) and gases.17 The major SCFAs produced are acetate, propionate, and butyrate.
Butyrate is the main energy source for human colonocytes, can induce apoptosis of colon cancer cells, and can activate intestinal gluconeogenesis, having beneficial effects on glucose and energy homeostasis.18 Butyrate is essential for epithelial cells to consume large amounts of oxygen through β oxidation, generating a state of hypoxia that maintains oxygen balance in the gut, preventing gut microbiota dysbiosis.19
Propionate is transferred to the liver, where it regulates gluconeogenesis and satiety signalling through interaction with the gut fatty acid receptors 18 Acetate—the most abundant SCFA and an essential metabolite for the growth of other bacteria—reaches the peripheral tissues where it is used in cholesterol metabolism and lipogenesis, and may play a role in central appetite regulation.20 Randomised controlled trials have shown that higher production of SCFAs correlates with lower diet-induced obesity21 and with reduced insulin resistance.22 Butyrate and propionate, but not acetate, seem to control gut hormones and reduce appetite and food intake in mice.21 Gut microbial enzymes contribute to bile acid metabolism, generating unconjugated and secondary bile acids that act as signalling molecules and metabolic regulators to influence important host pathways.23
Other specific products of the gut microbiota have been implicated directly in human health outcomes. Examples include trimethylamine and indolepropionic acid. The production of trimethylamine from dietary phosphatidylcholine and carnitine (from meat and dairy) depends on the gut microbiota and thus its amount in blood varies between people. Trimethylamine is oxidised in the liver to trimethylamine N-oxide, which is positively associated with an increased risk of atherosclerosis and major adverse cardiovascular events.24 Indolepropionic acid is highly correlated with dietary fibre intake25 and has potent radical scavenging activity in vitro,26 which seems to reduce the risk of incidence of type 2 diabetes.25
The gut microbiota and obesity
The gut microbiota seems to play a role in the development and progression of obesity. Most studies of overweight and obese people show a dysbiosis characterised by a lower diversity.31-39 Germ-free mice that receive faecal microbes from obese humans gain more weight than mice that receive microbes from healthy weight humans.4 A large study of UK twins found that the genus Christensenella was rare in overweight people and when given to germ free mice prevented weight gain.4 This microbe and others such as Akkermansia correlate with lower visceral fat deposits.12 Although much of the confirmatory evidence comes from mouse models, long term weight gain (over 10 years) in humans correlates with low microbiota diversity, and this association is exacerbated by low dietary fibre intake.28
Gut microbiota dysbiosis probably promotes diet induced obesity and metabolic complications by a variety of mechanisms including immune dysregulation, altered energy regulation, altered gut hormone regulation, and proinflammatory mechanisms (such as lipopolysaccharide endotoxins crossing the gut barrier and entering the portal circulation29 30; fig 1 ).
Microbiota diversity and health
Lower bacterial diversity has been reproducibly observed in people with inflammatory bowel disease,31 psoriatic arthritis,32 type 1 diabetes,33 atopic eczema,34 coeliac disease,35 obesity,36 type 2 diabetes,37 and arterial stiffness,38 than in healthy controls. In Crohn’s disease smokers have even lower gut microbiome diversity.39 The association between reduced diversity and disease indicates that a species-rich gut ecosystem is more robust against environmental influences, as functionally related microbes in an intact ecosystem can compensate for the function of other missing species. Consequently, diversity seems to be a generally good indicator of a “healthy gut.”40 41 But recent interventional studies indicate that major increases in dietary fibre can temporarily reduce diversity, as the microbes that digest fibre become specifically enriched, leading to a change in composition and, through competitive interactions, reduced diversity.22
The functional role of the gut microbiome in humans has been shown using faecal microbiota transplantation.42 This procedure is effective in cases of severe drug refractory Clostridium difficile infection and is now routinely used for this purpose around the world.43 For other pathologies, faecal transplants are not yet clinical practice but have been explored.44 For example, transplanting faeces from a lean healthy donor (allogeneic) to recipients with metabolic syndrome resulted in better insulin sensitivity, accompanied by altered microbiota composition, than using autologous faeces.45
Effects of food and drugs on the gut microbiota
Specific foods and dietary patterns can all influence the abundance of different types of bacteria in the gut, which in turn can affect health (table 1).
Table 1.
Examples of foods, nutrients, and dietary patterns that influence human health linked to their effect on the gut microbiota
| Dietary element | Effect on gut microbiome | Effect on health outcomes mediated by gut microbiome | Human observational studies | Human interventional studies |
|---|---|---|---|---|
| Low FODMAP diet | Low FODMAP diet increased Actinobacteria; high FODMAP diet decreased abundance of bacteria involved in gas consumption58 | Reduced symptoms of irritable bowel syndrome56 | Yes | Yes |
| Cheese | Increased Bifidobacteria,97 98 which are known for their positive health benefits to their host through their metabolic activities.99 Decrease in Bacteroides and Clostridia, some strains of which are associated with intestinal infections98 | Potential protection against pathogens.100 Increased production of SCFA and reduced production of TMAO99 | Yes | Yes |
| Fibre and prebiotics | Increased microbiota diversity and SCFA production 22 101 102 | Reduced type 2 diabetes22 and cardiovascular disease103 | Yes | Yes |
| Artificial sweeteners | Overgrowth of Proteobacteria and Escherichia coli.104 Bacteroides, Clostridia, and total aerobic bacteria were significantly lower, and faecal pH was significantly higher47 | Induced glucose intolerance105 | No | No |
| Polyphenols (eg, from tea, coffee, berries, and vegetables such as artichokes, olives, and asparagus) | Increased intestinal barrier protectors (Bifidobacteria and Lactobacillus), butyrate producing bacteria (Faecalibacterium prausnitzii and Roseburia) and Bacteroides vulgatus and Akkermansia muciniphila.107 Decreased lipopolysaccharide producers (E coli and Enterobacter cloacae).106 | Gut micro-organisms alter polyphenol bioavailability resulting in reduction of metabolic syndrome markers and cardiovascular risk markers108 | Yes | Yes |
| Vegan | Very modest differences in composition and diversity in humans and strong differences in metabolomic profile compared with omnivore diet in humans50 | Some studies show benefit of vegetarian over omnivore diet,109 others fail to find a difference110 | Yes | Yes |
FODMAP=fermentable oligosaccharides, disaccharides, monosaccharides and polyols; SCFA=small chain fatty acids; TMAO= trimethylamine N-oxide
High-intensity sweeteners are commonly used as sugar alternatives, being many times sweeter than sugar with minimal calories. Despite being “generally recognised as safe” by regulatory agencies, some animal studies have shown that these sugar substitutes may have negative effects on the gut microbiota.46 Sucralose, aspartame, and saccharin have been shown to disrupt the balance and diversity of gut microbiota.46 Rats given sucralose for 12 weeks had significantly higher proportions of Bacteroides, Clostridia, and total aerobic bacteria in their guts and a significantly higher faecal pH than those without sucralose.47 Mice given sucralose for six months had an increase in the expression in the gut of bacterial pro-inflammatory genes and disrupted faecal metabolites.48
Food additives, such as emulsifiers, which are ubiquitous in processed foods, have also been shown to affect the gut microbiota in animals.49 Mice fed relatively low concentrations of two commonly used emulsifiers—carboxymethylcellulose and polysorbate-80—showed reduced microbial diversity compared with mice not fed with emulsifiers. Bacteroidales and Verrucomicrobia were decreased and inflammation promoting Proteobacteria associated with mucus was enriched .49
Other areas of concern include the side effects of popular restrictive diets on gut health. These include some strict vegan diets, raw food or “clean eating” diets, gluten-free diets, and low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diets used to treat irritable bowel syndrome.
Vegans are viewed by some as healthier than omnivores. A study of 15 vegans and 16 ominvores found striking differences in serum metabolites generated by the gut microbes but very modest differences in gut bacterial communities.50 A controlled feeding experiment of 10 human omnivores randomised to receive either a high fat and low fibre diet or a low fat and high fibre for 10 days found very modest effects on gut microbiome composition and no difference in short chain fatty acid production. Together these data support a greater role for diet influencing the bacterial derived metabolome than just the short term bacterial community.50
Animal and in vitro studies indicate that gluten-free bread reduces the microbiota dysbiosis seen in people with gluten sensitivity or coeliac disease.51 52 But most people who avoid gluten do not have coeliac disease or proved intolerance, and a recent large observational study showed an increased risk of heart disease in gluten avoiders, potentially because of the reduced consumption of whole grains.53 One study showed that 21 healthy people had substantially different gut microbiota profiles after four weeks on a gluten-free diet. Most people showed a lower abundance of several key beneficial microbe species.54
The low FODMAP diet has been shown in six randomised controlled trials to reduce symptoms of irritable bowel syndrome.55 56 It is associated with a reduced proportion of Bifidobacterium in patients with irritable bowel syndrome, and responsiveness to this diet can be predicted by faecal bacterial profiles.57 Low FODMAP diets lead to profound changes in the microbiota and metabolome, the duration and clinical relevance of which are as yet unknown.58 59
In addition to diet, medication is a key modulator of the gut microbiota composition. A large Dutch-Belgian population study showed that drugs (including osmotic laxatives, progesterone, TNF-α inhibitors and rupatadine) had the largest explanatory power on microbiota composition (10% of community variation).13 Other studies have shown major effects of commonly prescribed proton pump inhibitors on the microbial community, which could explain higher rates of gastrointestinal infection in people taking these drugs.60 Antibiotics clearly have an effect on gut microbes, and low doses are routinely given to livestock to increase their growth and weight. A large proportion of antibiotic use in many countries is for agriculture—particularly intensive farming of poultry and beef.61 Several observational human studies as well as many rodent studies have pointed to an obesogenic effect of antibiotics in humans even in tiny doses found in food.61 But humans have very variable responses to antibiotics, and intervention studies have not shown consistent metabolic consequences.62 Pesticides and other chemicals are commonly sprayed on foods, but, although levels can be high, solid evidence for their harm on gut health and the effects of organic food is currently lacking.63
Insufficient clinical evidence exists to draw clear conclusions or recommendations for these or other dietary preferences based on gut microbiota. But future studies of food additives, drugs, and the safety and efficacy of dietary modifications must take into account these advances and their effect on the gut microbiota. This is becoming clear in patients with cancer treated with immunochemotherapy, bone marrow recipients, and patients with autoimmune disorders on biologics, where small changes in their microbiota can cause major changes in their response.64 Moreover, animal experiments have shown the protective effects of phytoestrogens on breast cancer depend on the presence of gut microbes (such as Clostridium saccharogumia, Eggerthella lenta, Blautia producta, and Lactonifactor longoviformis) that can transform isoflavones into the bioactive compounds.65
Box 2 summarises our current knowledge on the interactions between gut microbiota, nutrition, and human health.
Box 2. Consensus and uncertainties .
What we know
Probiotic supplementation has several beneficial effects on human health
The microbes in our gut influence and human energy metabolism22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45
Diet and medication have a strong influence on gut microbiota composition
Microbiota composition influences response to chemotherapy and immunotherapy96
Microbiome composition defines glucose response to foods and can be used to personalise diet94
Dietary fibre intake influences gut microbiota composition and is related to better health86 87 104
What we don’t know
Are natural probiotics in food better than probiotic supplements? Should we take them preventively?
Can microbes influence food choices and appetite?
Do low dose antibiotics in food affect human health?
What is the effect of pesticides in food on the gut microbiome? Is organic food better for the gut microbiota?
Should all new drugs and food chemicals be tested on the gut microbiota?
Manipulating the gut microbiota through diet
Changes to the gut microbiota can occur within days of changing diet; remarkable differences were found after African Americans and rural Africans switched diets for only two weeks.66 Increased abundance of known butyrate producing bacteria in the African Americans consuming a rural African diet caused butyrate production to increase 2.5 times and reduced synthesis of secondary bile acid.66 Another study comparing extreme shifts between plant and animal protein based diets showed these changes after only five days.67 But healthy microbiota are resilient to temporal changes by dietary interventions, meaning that homeostatic reactions restore the original community composition, as recently shown in the case of bread 68
Prebiotic foods and dietary fibre
Most national authorities define dietary fibre as edible carbohydrate polymers with three or more monomeric units that are resistant to the endogenous digestive enzymes and thus are neither hydrolysed nor absorbed in the small intestine.69 A subset of dietary fibre sources is fermentable, which means that they serve as growth substrates for microbes in the distal bowel.70 Some non-digestible carbohydrates have been referred to as “prebiotics,” which are defined as food components or ingredients that are not digestible by the human body but specifically or selectively nourish beneficial colonic micro-organisms (box 3).71 The prebiotic concept has been criticised for being poorly defined and unnecessarily narrow,72 and some scientists prefer the term “microbiota accessible carbohydrates,”11 which are essentially equivalent to fermentable dietary fibre in that they become available as growth substrates for gut microbes that possess the necessary enzymatic capacity to use them.70
Box 3. What are prebiotics and probiotics?
Dietary amounts of protein, saturated and unsaturated fats, carbohydrates, and dietary fibre influence the abundance of different types of bacteria in the gut. The microbiota can also be modified by adding live micro-organisms to food or by periods of fasting.
Probiotics are live bacteria and yeasts that, when administrated in a viable form and in adequate amounts, are beneficial to human health. They are usually added to yoghurts or taken as food supplements.
Prebiotics are defined as a substrate that is selectively used by host micro-organisms conferring a health benefit. Although all compounds considered prebiotics are microbiota accessible carbohydrates or fermentable dietary fibre, the reverse is not true. The prebiotic concept is an area of current debate70
Synbiotics contain a mixture of prebiotics and probiotics
Consuming resistant starches has been shown to enrich specific bacterial groups (Bifidobacterium adolescentis, Ruminococcus bromii, and Eubacterium rectale) in some people.74 75 The taxa enriched differ depending on the type of resistant starches and other dietary fibres,75 indicating that shifts are dependent on the carbohydrate’s chemical structure and the microbes’ enzymatic capacity to access them. Microbes need also to “adhere” to a substrate and tolerate the conditions generated from fermentation (such as low pH).76
The effect of microbiota accessible carbohydrates on the gastrointestinal microbiome composition can be substantial, with specific species becoming enriched to constitute more than 30% of the faecal microbiota.75 77 Thus, microbiota accessible carbohydrates provide a potential strategy to enhance useful minority members of the microbiome. These changes only last as long as the carbohydrate is consumed, and they are highly individual, which provides a basis for personalised approaches. Many short term feeding trials with purified dietary fibres or even whole plant based diets either have no effect on microbiota diversity or reduce it,22 but can still have clinical benefits, potentially through metabolites such as small chain fatty acids.22 67
Low fibre intake reduces production of small chain fatty acids and shifts the gastrointestinal microbiota metabolism to use less favourable nutrients,78 leading to the production of potentially detrimental metabolites.79 80 Convincing evidence shows that the low fibre Western diet degrades the colonic mucus barrier, causing microbiota encroachment, which results in pathogen susceptibility81and inflammation,82 providing a potential mechanism for the links of Western diet with chronic diseases. Two recent studies showed that the detrimental effects of high fat diets on penetrability of the mucus layer and metabolic functions could be prevented through dietary administration of inulin.83 84 Overall, these findings, together with the role of butyrate in preventing oxygen induced gut microbiota dysbiosis,19 provide a strong rational to enrich dietary fibre consumption to maintain intact mucosal barrier function in the gut.85
Considerable observational evidence shows that fibre intake is beneficial for human health. Two recent meta-analyses found clear links between dietary fibre and health benefits in a wide range of pathologies,86 87 and a recent intervention study found dietary fibres significantly reduced insulin resistance in patients with type 2 diabetes, with clear links to the shifts in the microbiota and beneficial metabolites (such as butyrate).45
Probiotic foods
Probiotics are live micro-organisms that, when administered in adequate amounts, confer a health benefit on the host).88 Probiotics (mostly Bifidobacterium and Lactobacillus species) can be included in a variety of products, including foods, dietary supplements, or drugs.
There are concerns that most microbe supplements are unable to establish themselves in the gut and fail to exert an effect on the resident community.89 90 But probiotics can affect health independently of the gut microbiota through direct effects on the host; for example, through immune modulation or the production of bioactive compounds. The therapeutic effect of probiotic supplementation has been studied in a broad range of diseases.
We searched the Cochrane library of systematic reviews for “probiotic*”, yielding 39 studies, and searched Medline for “systematic review” or “meta-analysis” and “probiotic*”, yielding 31 studies. We included information on systematic reviews of randomised controlled trials published in the past five years where the main treatment was probiotics (not dietary supplements in general). Only studies that focused on comparisons of probiotics with a control group, that contained at least some moderate or high quality randomised controlled trials in the estimation of the authors of the systematic review, which resulted in a total of 22 systematic reviews (table 2 ). The analysis of 313 trials and 46 826 participants showed substantial evidence for beneficial effects of probiotic supplementation in preventing diarrhoea, necrotising enterocolitis, acute upper respiratory tract infections, pulmonary exacerbations in children with cystic fibrosis, and eczema in children. Probiotics also seem to improve cardiometabolic parameters and reduced serum concentrationof C reactive protein in patients with type 2 diabetes. Importantly, the studies were not homogeneous and were not necessarily matched for type or dose of probiotic supplementation nor length of intervention, which limits precise recommendations. Emerging areas of probiotic treatment include using newer microbes and combinations, combining probiotics and prebiotics (synbiotics),91 and personalised approaches based on profiles of the candidate microbes in inflammation, cancer, lipid metabolism, or obesity.92 Stable engraftment of a probiotic Bifidobacterium longum, for example, has been shown to depend on individualised features of the gut microbiota, providing a rationale for the personalisation of probiotic applications.93
Table 2.
Summary of systematic reviews analysing the role of probiotics on clinical outcomes
| Outcome | Reference | No of studies/participants | Evidence of benefit? | Results/conclusions |
|---|---|---|---|---|
| Clostridium difficile associated diarrhoea in adults and children | Goldenberg et al (2017)111 | 39/9955 | Yes | Moderate quality evidence that probiotics are safe and effective for preventing C difficile associated diarrhoea. (RR 0.30, 95% CI 0.21 to 0.42) |
| Necrotising enterocolitis | Al Faleh et al (2014)112Rees et al (2017)113 | 17/5338 | Yes | Enteral supplementation of probiotics prevents severe necrotising enterocolitis (RR 0.43, 95%CI 0.33 to 0.56) and all cause mortality in preterm infants (RR 0.65, 95% CI 0.25 to 0.81) |
| Antibiotic associated diarrhoea in children | Goldenberg et al (2015)114 | 26/3898 | Yes | Moderate evidence of a fall in the incidence of antibiotic associated diarrhoea in the probiotic v control group (RR 0.46, 95% CI 0.35 to 0.61; I2=55%, 3898 participants) |
| Probiotics for preventing acute upper respiratory tract infections | Hao et al (2015)115 | 12/3720 | Yes | Probiotics were better than placebo in reducing the number of participants experiencing episodes of acute upper respiratory tract infections, the mean duration of an episode , antibiotic use, and related school absence (12 trials, 3720 participants including children, adults, and older people) |
| Urinary tract infections | Schwenger et al (2015)116 | 9/735 | No | No significant benefit for probiotics compared with placebo or no treatment |
| Prevention of asthma and wheeze in infants | Azad et al (2013)117 | 6/1364 | No | No evidence to support a protective association between perinatal use of probiotics and doctor diagnosed asthma or childhood wheeze |
| Prevention of eczema in infants and children | Mansfield et al (2014) | 16/2797 | Yes | Probiotic supplementation in the first several years of life did have a significant impact on development of eczema (RR 0.74, 95% CI 0.67 to 0.82) |
| Prevention of invasive fungal infections in preterm neonates | Agrawal et al (2015) 119 | 19/4912 | Unclear | Probiotic supplementation reduced the risk of invasive fungal infections (RR 0.50, 95% CI 0.34 to 0.73, I2=39%) but there was high heterogeneity between studies. Analysis after excluding the study with a high baseline incidence (75%) showed that probiotic supplementation had no significant benefits (RR 0.89, 95% CI 0.44 to 1.78) |
| Prevention of nosocomial infections | Manzanares et al (2015)120 | 30/2972 | Yes | Probiotics were associated with a significant reduction in infections (RR 0.80, 95%CI 0.68 to 0.95, P=0.009; I2=36%, P=0.09). A significant reduction in the incidence of ventilator associated pneumonia was found (RR 0.74, 95% CI 0.61 to 0. 90, P=0.002; I2=19%) |
| Treatment of rotavirus diarrhoea in infants and children | Ahmadi et al (2015)121 | 14/1149 | Yes | Probiotic supplementation resulted in a mean difference of −0.41 (CI 95% −0.56 to −0.25; P<0.001) in the duration of diarrhoea. Probiotics exert positive effect on reducing the duration of acute rotavirus diarrhoea compared with control |
| Prevention and treatment of Crohn’s disease and ulcerative colitis | Saez Lara et al (2015)122 | 14/821 ulcerative colitis 8/374 Crohn’s disease |
Yes | The use of probiotics and/or synbiotics has positive effects in the treatment and maintenance of ulcerative colitis, whereas in Crohn’s disease clear effectiveness has only been shown for synbiotics (no meta- analysis was performed) |
| Pulmonary exacerbations in children with cystic fibrosis | Ananathan et al (2016)123 | 9/275 | Yes | Significant reduction in the rate of pulmonary exacerbation (two parallel group randomised controlled trials and one crossover trial: RR 0.25, 95% CI 0.15 to 0.41; P< 0.00001) |
| Type 2 diabetes (fasting glucose, glycated haemoglobin test) | Akbari et al (2016)124 | 13/805 | Yes | Probiotics significantly reduced fasting blood glucose compared with placebo (8 studies; standardised mean difference −1.583; 95% CI −4.18 to 4.18; P = 0.000). Significant reduction in HbA1c was also seen (6 studies; SMD −1.779; 95% CI, −2.657 to −0.901; P = 0.000) |
| Type 2 diabetes (insulin resistance, insulin levels) | Zhang et al (2016)125 | 7/425 | Yes | Probiotic therapy significantly decreased homeostasis model assessment of insulin resistance (HOMA-IR) and insulin concentration (WMD: −1.08, 95% CI −1.88 to −0.28; and weighted mean difference −1.35mIU/L, 95% CI -−2.38 to −0.31, respectively |
| Necrotising enterocolitis in pre-term neonates with focus on Lactobacillus reuteri | Athalye-Jape et al (2016)126 | 6/1778 | Yes | Probiotic reduced duration of hospitalisation (mean difference = −10.77 days, 95% CI −13.67 to −7.86; in 3 randomised controlled trials), and late onset sepsis (RR 0.66; 95% CI, 0.52 to 0.83; 4 RCTs) were reduced in the |
| Reduction of serum concentration of C reactive protein | Mazidi et al (2017)127 | 19/935 | Yes | Significant reduction in serum C reactive protein after probiotic administration with a WMD −1.35 mg/L, (95% CI −2.15 to −0.55, I2 65.1%) |
| Cardiovascular risk factors in patients with type 2 diabetes | Hendijani et al (2017)128 | 11/641 | Yes | Probiotic consumption significantly decreased systolic blood pressure (−3.28 mm Hg; 95% CI −5.38 to −1.18), diastolic (WMD −2.13 mm Hg; 95% CI −4.5 to 0.24), low density lipoprotein cholesterol (WMD 8.32 mg/dL; 95% CI −15.24 to −1.4), total cholesterol (WMD −12.19 mg/dL; 95% CI −17.62 to −6.75) and triglycerides(WMD −24.48 mg/dL; 95% CI −33.77 to −11.18) compared with placebo |
| Reduction of total cholesterol and low density lipoprotein cholesterol | Wu et al (2017)129 | 15/976 | Yes | Lactobacillus consumption significantly reduced total cholesterol by 0.26 mmol/L (95% CI −0.40 to −0.12) and LDL-C by 0.23 mmol/L (95% CI, −0.36 to −0.10) |
| Depressive symptoms | Wallace, and Milev (2017)79,130 | 6/1080 | Yes | No quantitative analysis was performed. Most studies found positive results, and the authors conclude that compelling evidence shows that probiotics alleviate depressive symptoms |
| Vulvovaginal candidiasis in non-pregnant women | Xie et al (2018)131 | 10/1656 | Yes | Probiotics increased the rate of short term clinical cure (RR 1.14, 95% CI 1.05 to 1.24, low quality evidence) and mycological cure (RR 1.06, 95% CI 1.02 to 1.10, low quality evidence) and decreased relapse rate at one month (RR 0.34, 95% CI 0.17 to 0.68, low quality evidence) |
| Chronic periodontitis | Ikram et al (2018) 132 | 7/220 | Yes | The overall mean difference for gaining clinical attachment level gain between probiotics and placebo was significant (weighted mean difference 1.41, 95% CI 0.15 to 2.67, P=0.028) |
RR=risk ratio, SBP systolic blood pressure, DBP= diastolic blood pressure, TC= total cholesterol, TG=serum triglycerides, SMD=standardised mean difference, WMD=weighted mean difference’ CI=confidence interval
Personalised nutrition and future directions
Given the variation in the gut microbiota between people, the optimal diet of a person may need to be tailored to their gut microbiota. Zeevi et al.94 obtained a multidimensional microbiota profile in 900 people and monitored food intake, continuous blood glucose levels, and physical activity for one week. The researchers devised a machine learning algorithm to predict personalised glucose responses after meals based on clinical and gut microbiome data and showed that it achieved significantly higher predictions than approaches such as carbohydrate counting or glycaemic index scores. In a follow-up double blinded randomised crossover trial of 26 participants, personalised dietary interventions based on the algorithm successfully normalised blood glucose levels.94
A study on response to bread68 using a randomised crossover trial of one week long dietary interventions showed significant interpersonal variability in the glycaemic response to different bread types. The type of bread that induced the lower glycaemic response in each person could be predicted based solely on microbiome data collected before the intervention.68 Much more research is needed to establish whether these kinds of personalised approaches are feasible, sustainable, and have a positive effect on clinical outcomes.
Conclusions
We are entering an era where we can increasingly modify health through food and measure the effects through our microbes or metabolites. Fibre is a key nutrient for a healthy microbiome and has been overlooked while debates have raged about sugar and fat. The adverse effects on the microbiome of drugs and processed food ingredients can no longer be ignored. Given the current gaps in knowledge, we need clinical evidence that can be translated into clinical practice, ideally through randomised controlled studies that use consistent matrices of prebiotics or probiotics or faecal microbiota transplantation to assess changes in gut microbiota composition and in health outcomes.
Key messages.
Gut microbiota influences many areas of human health from innate immunity to appetite and energy metabolism
Targeting the gut microbiome, with probiotics or dietary fibre, benefits human health and could potentially reduce obesity
Drugs, food ingredients, antibiotics, and pesticides could all have adverse effects on the gut microbiota
Microbiota should be considered a key aspect in nutrition; the medical community should adapt their education and public health messages
Fibre consumption is associated with beneficial effects in several contexts
Contributors and sources: AMV studies the molecular basis of ageing and complex disease and has recently investigated the role of gut microbiome composition on cardiometabolic disorders. JW has studied and reported widely on the microbial ecology of the gut microbiota, its role in host health, and how it can be modulated by diet. ES heads a multidisciplinary lab of computational biologists and experimental scientists focusing on nutrition, genetics, microbiome, and their effect on health and disease. His aim is to develop personalised nutrition and medicine. TDS leads the TwinsUK registry and British gut project as the head of a multidisciplinary team studying the genetic, dietary, and lifestyle determinants of human gut microbiome composition and its relationship to common diseases. All authors contributed, read, and approved the final version.
Competing interests: We have read and understood BMJ policy on competing interests and declare the following: AMV and TS are consultants to Zoe Global. JW has received research funding from industry sources involved in the manufacture and marketing of prebiotics and dietary fibres and is a co-owner of Synbiotics Solutions, a developer of synbiotic products. ES is a consultant of DayTwo Inc. AMV is funded by the NIHR Nottingham Biomedical Research Centre. JW is supported through the Campus Alberta Innovates programme and grants of the Canadian Institute of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), the JPI HDHL, and the Canadian Foundation for Innovation. ES is supported by the Crown Human Genome Center; the Else Kroener Fresenius Foundation; Donald L. Schwarz, Sherman Oaks, CA; Jack N Halpern, New York, NY; Leesa Steinberg, Canada; and grants funded by the European Research Council and the Israel Science Foundation. TwinsUK was funded by the Wellcome Trust; European Community’s Seventh Framework Programme (FP7/2007-2013). The study also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. TDS is an NIHR senior investigator.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is one of a series commissioned by The BMJ. Open access fees for the series were funded by Swiss Re, which had no input on the commissioning or peer review of the articles. The BMJ thanks the series advisers, Nita Forouhi and Dariush Mozaffarian, for valuable advice and guiding selection of topics in the series.
References
- 1. Bull MJ, Plummer NT. Part 1: The human gut microbiome in health and disease. Integr Med (Encinitas) 2014;13:17-22. [PMC free article] [PubMed] [Google Scholar]
- 2. Rath CM, Dorrestein PC. The bacterial chemical repertoire mediates metabolic exchange within gut microbiomes. Curr Opin Microbiol 2012;15:147-54. 10.1016/j.mib.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vyas U, Ranganathan N. Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol Res Pract 2012;2012:872716. 10.1155/2012/872716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell 2014;159:789-99. 10.1016/j.cell.2014.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018;555:210-5. 10.1038/nature25973 [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Sparks JB, Karyala SV, Settlage R, Luo XM. Host adaptive immunity alters gut microbiota. ISME J 2015;9:770-81. 10.1038/ismej.2014.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients 2011;3:637-82. 10.3390/nu3060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol 2017;17:219-32. 10.1038/nri.2017.7 [DOI] [PubMed] [Google Scholar]
- 9. De Palma G, Lynch MD, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med 2017;9:9. 10.1126/scitranslmed.aaf6397 [DOI] [PubMed] [Google Scholar]
- 10. Wiley NC, Dinan TG, Ross RP, Stanton C, Clarke G, Cryan JF. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health. J Anim Sci 2017;95:3225-46. [DOI] [PubMed] [Google Scholar]
- 11. Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014;20:779-86. 10.1016/j.cmet.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beaumont M, Goodrich JK, Jackson MA, et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol 2016;17:189. 10.1186/s13059-016-1052-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science 2016;352:560-4. 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- 14. Vogtmann E, Hua X, Zeller G, et al. Colorectal cancer and the human gut microbiome: Reproducibility with whole-genome shotgun sequencing. PLoS One 2016;11:e0155362. 10.1371/journal.pone.0155362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranjan R, Rani A, Metwally A, McGee HS, Perkins DL. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun 2016;469:967-77. 10.1016/j.bbrc.2015.12.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao L, Ni Y, Su M, et al. High throughput and quantitative measurement of microbial metabolome by gas chromatography/mass spectrometry using automated alkyl chloroformate derivatization. Anal Chem 2017;89:5565-77. 10.1021/acs.analchem.7b00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 2006;40:235-43. 10.1097/00004836-200603000-00015 [DOI] [PubMed] [Google Scholar]
- 18. De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014;156:84-96. 10.1016/j.cell.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 19. Byndloss MX, Olsan EE, Rivera-Chávez F, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017;357:570-5. 10.1126/science.aam9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 2014;5:3611. 10.1038/ncomms4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin HV, Frassetto A, Kowalik EJ, Jr, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 2012;7:e35240. 10.1371/journal.pone.0035240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018;359:1151-6. 10.1126/science.aao5774 [DOI] [PubMed] [Google Scholar]
- 23. Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med 2017;56:54-65. 10.1016/j.mam.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 24. Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575-84. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Mello VD, Paananen J, Lindström J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep 2017;7:46337. 10.1038/srep46337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chyan YJ, Poeggeler B, Omar RA, et al. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem 1999;274:21937-42. 10.1074/jbc.274.31.21937 [DOI] [PubMed] [Google Scholar]
- 27. Thaiss CA, Itav S, Rothschild D, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016. 10.1038/nature20796 [DOI] [PubMed] [Google Scholar]
- 28. Menni C, Jackson MA, Pallister T, Steves CJ, Spector TD, Valdes AM. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int J Obes (Lond) 2017;41:1099-105. 10.1038/ijo.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gäbele E, Dostert K, Hofmann C, et al. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol 2011;55:1391-9. 10.1016/j.jhep.2011.02.035 [DOI] [PubMed] [Google Scholar]
- 30. Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis 2016;15:108. 10.1186/s12944-016-0278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006;55:205-11. 10.1136/gut.2005.073817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 2015;67:128-39. 10.1002/art.38892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Goffau MC, Luopajärvi K, Knip M, et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013;62:1238-44. 10.2337/db12-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang M, Karlsson C, Olsson C, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol 2008;121:129-34. 10.1016/j.jaci.2007.09.011 [DOI] [PubMed] [Google Scholar]
- 35. Schippa S, Iebba V, Barbato M, et al. A distinctive ‘microbial signature’ in celiac pediatric patients. BMC Microbiol 2010;10:175. 10.1186/1471-2180-10-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480-4. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lambeth SM, Carson T, Lowe J, et al. Composition, diversity and abundance of gut microbiome in prediabetes and type 2 diabetes. J Diabetes Obes 2015;2:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menni C, Lin C, Cecelja M, et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur Heart J 2018;•••:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Opstelten JL, Plassais J, van Mil SW, et al. Gut microbial diversity is reduced in smokers with Crohn’s disease. Inflamm Bowel Dis 2016;22:2070-7. 10.1097/MIB.0000000000000875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sommer F, Rühlemann MC, Bang C, et al. Microbiomarkers in inflammatory bowel diseases: caveats come with caviar. Gut 2017;66:1734-8. 10.1136/gutjnl-2016-313678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 2017;15:630-8. 10.1038/nrmicro.2017.58 [DOI] [PubMed] [Google Scholar]
- 42. DeFilipp Z, Peled JU, Li S, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv 2018;2:745-53. 10.1182/bloodadvances.2018017731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schneider KM, Wirtz TH, Kroy D, et al. Successful fecal microbiota transplantation in a patient with severe complicated Clostridium difficile infection after liver transplantation. Case Rep Gastroenterol 2018;12:76-84. 10.1159/000481937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cammarota G, Ianiro G, Tilg H, et al. European FMT Working Group European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017;66:569-80. 10.1136/gutjnl-2016-313017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kootte RS, Levin E, Salojarvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell metabolism. 2017; 26: 611-9 e6. [DOI] [PubMed]
- 46. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol Behav 2016;164(Pt B):488-93. 10.1016/j.physbeh.2016.04.029 [DOI] [PubMed] [Google Scholar]
- 47. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, McLendon RE, Schiffman SS. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J Toxicol Environ Health A 2008;71:1415-29. 10.1080/15287390802328630 [DOI] [PubMed] [Google Scholar]
- 48. Bian X, Chi L, Gao B, Tu P, Ru H, Lu K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front Physiol 2017;8:487. 10.3389/fphys.2017.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92-6. 10.1038/nature14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu GD, Compher C, Chen EZ, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016;65:63-72. 10.1136/gutjnl-2014-308209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mohan M, Chow CT, Ryan CN, et al. Dietary gluten-induced gut dysbiosis is accompanied by selective upregulation of microRNAs with intestinal tight junction and bacteria-binding motifs in rhesus macaque model of celiac disease. Nutrients 2016;8:8. 10.3390/nu8110684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bevilacqua A, Costabile A, Bergillos-Meca T, et al. Impact of gluten-friendly bread on the metabolism and function of in vitro gut microbiota in healthy human and coeliac subjects. PLoS One 2016;11:e0162770. 10.1371/journal.pone.0162770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lebwohl B, Cao Y, Zong G, et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ 2017;357:j1892. 10.1136/bmj.j1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonder MJ, Tigchelaar EF, Cai X, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med 2016;8:45. 10.1186/s13073-016-0295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Halmos EP. When the low FODMAP diet does not work. J Gastroenterol Hepatol 2017;32(Suppl 1):69-72. 10.1111/jgh.13701 [DOI] [PubMed] [Google Scholar]
- 56. Gibson PR. The evidence base for efficacy of the low FODMAP diet in irritable bowel syndrome: is it ready for prime time as a first-line therapy? J Gastroenterol Hepatol 2017;32(Suppl 1):32-5. 10.1111/jgh.13693 [DOI] [PubMed] [Google Scholar]
- 57. Bennet SMP, Bohn L, Storsrud S, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 2017. [DOI] [PubMed] [Google Scholar]
- 58. McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut 2017;66:1241-51. 10.1136/gutjnl-2015-311339 [DOI] [PubMed] [Google Scholar]
- 59. Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017;66:1517-27. 10.1136/gutjnl-2017-313750 [DOI] [PubMed] [Google Scholar]
- 60. Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749-56. 10.1136/gutjnl-2015-310861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science 2016;352:544-5. 10.1126/science.aad9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reijnders D, Goossens GH, Hermes GD, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: A randomized double-blind placebo-controlled trial. Cell Metab 2016;24:63-74. 10.1016/j.cmet.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 63. Lee YM, Kim KS, Jacobs DR, Jr, Lee DH. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev 2017;18:129-39. 10.1111/obr.12481 [DOI] [PubMed] [Google Scholar]
- 64. Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 2017;14:356-65. 10.1038/nrgastro.2017.20 [DOI] [PubMed] [Google Scholar]
- 65. Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 2016;14:273-87. 10.1038/nrmicro.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Keefe SJ, Li JV and Lahti L. Fat, fibre and cancer risk in African Americans and rural Africans. 2015; 6: 6342. [DOI] [PMC free article] [PubMed]
- 67. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559-63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korem T, Zeevi D, Zmora N, et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metabolism. 2017; 25: 1243-53 e5. [DOI] [PubMed]
- 69. Jones JM. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr J 2014;13:34. 10.1186/1475-2891-13-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deehan EC, Duar RM, Armet AM, Perez-Muñoz ME, Jin M, Walter J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to Improve human health. Microbiol Spectr 2017;5:5. 10.1128/microbiolspec.BAD-0019-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 2015;12:303-10. 10.1038/nrgastro.2015.47 [DOI] [PubMed] [Google Scholar]
- 72. Olle B. Medicines from microbiota. Nat Biotechnol 2013;31:309-15. 10.1038/nbt.2548 [DOI] [PubMed] [Google Scholar]
- 73. Walter J. Murine gut microbiota-diet trumps genes. Cell Host Microbe 2015;17:3-5. 10.1016/j.chom.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 74. Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016;4:33. 10.1186/s40168-016-0178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One 2010;5:e15046. 10.1371/journal.pone.0015046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 2012;10:323-35. 10.1038/nrmicro2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2011;5:220-30. 10.1038/ismej.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 1991;70:443-59. 10.1111/j.1365-2672.1991.tb02739.x [DOI] [PubMed] [Google Scholar]
- 79. Russell WR, Gratz SW, Duncan SH, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr 2011;93:1062-72. 10.3945/ajcn.110.002188 [DOI] [PubMed] [Google Scholar]
- 80. Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 2007;73:1073-8. 10.1128/AEM.02340-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016;167:1339-1353.e21. 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Earle KA, Billings G, Sigal M, et al. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 2015;18:478-88. 10.1016/j.chom.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zou J, Chassaing B, Singh V, et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 2018;23:41-53.e4. 10.1016/j.chom.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schroeder BO, Birchenough GMH, Ståhlman M, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018;23:27-40.e7. 10.1016/j.chom.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ray K. Gut microbiota: Filling up on fibre for a healthy gut. Nat Rev Gastroenterol Hepatol 2018;15:67. 10.1038/nrgastro.2018.2 [DOI] [PubMed] [Google Scholar]
- 86. Veronese N, Solmi M, Caruso MG, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr 2018;107:436-44. 10.1093/ajcn/nqx082 [DOI] [PubMed] [Google Scholar]
- 87. Thompson SV, Hannon BA, An R, Holscher HD. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2017;106:1514-28. 10.3945/ajcn.117.163246 [DOI] [PubMed] [Google Scholar]
- 88. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506-14. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 89. Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 2016;8:52. 10.1186/s13073-016-0300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Walter J, Maldonado-Gómez MX, Martínez I. To engraft or not to engraft: an ecological framework for gut microbiome modulation with live microbes. Curr Opin Biotechnol 2018;49:129-39. 10.1016/j.copbio.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 2017;23:107-13. 10.1038/nm.4236 [DOI] [PubMed] [Google Scholar]
- 92. Chua KJ, Kwok WC, Aggarwal N, Sun T, Chang MW. Designer probiotics for the prevention and treatment of human diseases. Curr Opin Chem Biol 2017;40:8-16. 10.1016/j.cbpa.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 93. Maldonado-Gómez MX, Martínez I, Bottacini F, et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 2016;20:515-26. 10.1016/j.chom.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 94. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079-94. 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 95. Deehan EC, Walter J. The fiber gap and the disappearing gut microbiome: Implications for human nutrition. Trends Endocrinol Metab 2016;27:239-42. 10.1016/j.tem.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 96. Hakim H, Dallas R, Wolf J, et al. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin Infect Dis 2018. 10.1093/cid/ciy153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Uchida M, Mogami O, Matsueda K. Characteristic of milk whey culture with Propionibacterium freudenreichii ET-3 and its application to the inflammatory bowel disease therapy. Inflammopharmacology 2007;15:105-8. 10.1007/s10787-007-1557-5 [DOI] [PubMed] [Google Scholar]
- 98. Foligné B, Breton J, Mater D, Jan G. Tracking the microbiome functionality: focus on Propionibacterium species. Gut 2013;62:1227-8. 10.1136/gutjnl-2012-304393 [DOI] [PubMed] [Google Scholar]
- 99. Zheng H, Yde CC, Clausen MR, et al. Metabolomics investigation to shed light on cheese as a possible piece in the French paradox puzzle. J Agric Food Chem 2015;63:2830-9. 10.1021/jf505878a [DOI] [PubMed] [Google Scholar]
- 100. Montel MC, Buchin S, Mallet A, et al. Traditional cheeses: rich and diverse microbiota with associated benefits. Int J Food Microbiol 2014;177:136-54. 10.1016/j.ijfoodmicro.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 101. Sasaki D, Sasaki K, Ikuta N, et al. Low amounts of dietary fibre increase in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Sci Rep 2018;8:435. 10.1038/s41598-017-18877-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cheng W, Lu J, Li B, et al. Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Front Microbiol 2017;8:1750. 10.3389/fmicb.2017.01750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Administration FaD Code of Federal Regulations Title 21 Subpart E: Specific Requirements for Health Claims [23 pp.] EFSA J 2016;14:4369. [Google Scholar]
- 104. Rodriguez-Palacios A, Harding A, Menghini P, et al. The artificial sweetener Splenda promotes gut Proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease-like ileitis. Inflamm Bowel Dis 2018;24:1005-20. 10.1093/ibd/izy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181-6. 10.1038/nature13793 [DOI] [PubMed] [Google Scholar]
- 106. Moreno-Indias I, Sánchez-Alcoholado L, Pérez-Martínez P, et al. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct 2016;7:1775-87. 10.1039/C5FO00886G [DOI] [PubMed] [Google Scholar]
- 107. Etxeberria U, Arias N, Boqué N, et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem 2015;26:651-60. 10.1016/j.jnutbio.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 108. Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and rffects on bioaccessibility. Nutrients 2016;8:78. 10.3390/nu8020078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med 2013;173:1230-8. 10.1001/jamainternmed.2013.6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mihrshahi S, Ding D, Gale J, Allman-Farinelli M, Banks E, Bauman AE. Vegetarian diet and all-cause mortality: Evidence from a large population-based Australian cohort - the 45 and Up Study. Prev Med 2017;97:1-7. 10.1016/j.ypmed.2016.12.044 [DOI] [PubMed] [Google Scholar]
- 111. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2017;12:CD006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014;(4):CD005496. [DOI] [PubMed] [Google Scholar]
- 113. Rees CM, Hall NJ, Fleming P, Eaton S. Probiotics for the prevention of surgical necrotising enterocolitis: systematic review and meta-analysis. BMJ Paediatr Open 2017;1:e000066. 10.1136/bmjpo-2017-000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Goldenberg JZ, Lytvyn L, Steurich J, Parkin P, Mahant S, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 2015;(12):CD004827. [DOI] [PubMed] [Google Scholar]
- 115. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev 2015;(2):CD006895. [DOI] [PubMed] [Google Scholar]
- 116. Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev 2015;(12):CD008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ 2013;347:f6471. 10.1136/bmj.f6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mansfield JA, Bergin SW, Cooper JR, Olsen CH. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med 2014;179:580-92. 10.7205/MILMED-D-13-00546 [DOI] [PubMed] [Google Scholar]
- 119. Agrawal S, Rao S, Patole S. Probiotic supplementation for preventing invasive fungal infections in preterm neonates--a systematic review and meta-analysis. Mycoses 2015;58:642-51. 10.1111/myc.12368 [DOI] [PubMed] [Google Scholar]
- 120. Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care 2016;19:262. 10.1186/s13054-016-1434-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ahmadi E, Alizadeh-Navaei R, Rezai MS. Efficacy of probiotic use in acute rotavirus diarrhea in children: A systematic review and meta-analysis. Caspian J Intern Med 2015;6:187-95. [PMC free article] [PubMed] [Google Scholar]
- 122. Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int 2015;2015:505878. 10.1155/2015/505878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ananthan A, Balasubramanian H, Rao S, Patole S. Probiotic supplementation in children with cystic fibrosis-a systematic review. Eur J Pediatr 2016;175:1255-66. 10.1007/s00431-016-2769-8 [DOI] [PubMed] [Google Scholar]
- 124. Akbari V, Hendijani F. Effects of probiotic supplementation in patients with type 2 diabetes: systematic review and meta-analysis. Nutr Rev 2016;74:774-84. 10.1093/nutrit/nuw039 [DOI] [PubMed] [Google Scholar]
- 125. Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas) 2016;52:28-34. 10.1016/j.medici.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 126. Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: A strain-specific systematic review. JPEN J Parenter Enteral Nutr 2016;40:783-94. 10.1177/0148607115588113 [DOI] [PubMed] [Google Scholar]
- 127. Mazidi M, Rezaie P, Ferns GA, Vatanparast H. Impact of probiotic administration on serum C-reactive protein concentrations: Systematic review and meta-Aanalysis of randomized control trials. Nutrients 2017;9:9. 10.3390/nu9010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis. Clin Nutr 2017. [DOI] [PubMed] [Google Scholar]
- 129. Wu Y, Zhang Q, Ren Y, Ruan Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS One 2017;12:e0178868. 10.1371/journal.pone.0178868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 2017;16:14. 10.1186/s12991-017-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xie HY, Feng D, Wei DM, et al. Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst Rev 2017;11:CD010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ikram S, Hassan N, Raffat MA, Mirza S, Akram Z. Systematic review and meta-analysis of double-blind, placebo-controlled, randomized clinical trials using probiotics in chronic periodontitis. J Investig Clin Dent 2018;•••:e12338. 10.1111/jicd.12338 [DOI] [PubMed] [Google Scholar]
- 104. So D, Whelan K, Rossi M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr 2018. 10.1093/ajcn/nqy041 [DOI] [PubMed] [Google Scholar]