Abstract
Objective
To determine the potential impact of erenumab, a human anti‐calcitonin gene‐related peptide (CGRP) receptor monoclonal antibody, on total exercise time (TET), time to exercise‐induced angina, and ST depression in a double‐blind, placebo‐controlled study in patients with stable angina due to documented coronary artery disease.
Background
The relative importance of the CGRP receptor pathway during myocardial ischemia has not been established.
Methods
An exercise treadmill test was conducted following a single IV infusion of erenumab 140 mg or placebo. The primary endpoint was the change from baseline in exercise duration as measured by TET with a noninferiority margin of −90 seconds. Safety follow‐up visits occurred through week 12. Eighty‐eight participants were included in the analysis.
Results
LS mean (SE) change in TET was −2.9 [14.8] seconds in the erenumab group and 8.1 [14.4] seconds in placebo; adjusted mean (90% CI) treatment difference was −11.0 (–44.9, 22.9) seconds. The CI lower bound (–44.9 sec) did not reach pre‐defined non‐inferiority margin of −90 seconds, demonstrating that TET change from baseline in the erenumab group was non‐inferior to placebo. There was no difference in time to exercise‐induced angina in erenumab and placebo groups (median [90% CI] time of 500 [420, 540] vs 508 [405, 572] seconds; hazard ratio [90% CI]: 1.11 [0.73, 1.69], P = .69) or time to onset of ≥1 mm ST‐segment depression (median [90% CI] time of 407 [380, 443] vs 420 [409,480] seconds; hazard ratio [95% CI]: 1.14 [0.76, 1.69], P = .59). Adverse events were reported by 27% and 32% of patients in erenumab and placebo groups.
Conclusions
Erenumab did not adversely affect exercise time in a high cardiovascular risk population of patients, supporting that inhibition of the canonical CGRP receptor does not worsen myocardial ischemia.
Keywords: calcitonin gene‐related peptide, clinical trial, safety, cardiovascular
Abbreviations
- AE
adverse event
- CGRP
calcitonin gene‐related peptide
- CI
confidence interval
- CV
cardiovascular
- ETT
exercise treadmill test
- SE
standard error
- TET
total exercise time
INTRODUCTION
Erenumab, a human monoclonal antibody targeting the canonical calcitonin gene‐related peptide (CGRP) receptor, is being developed for preventive treatment of migraine. Migraine is a chronic disease, and while prevalence decreases with age after a peak around 40 years, it may overlap with the development of cardiovascular co‐morbidities.1, 2, 3 Therefore, it is important to determine the potential impact of CGRP inhibition on coronary vasodilatory capacity, especially during myocardial ischemia.
During myocardial ischemia, cardiac sensory nerves release a number of vasodilatory and cytoprotective mediators, including CGRP.4 Further, when administered exogenously, supra‐physiologic concentrations of CGRP can increase total exercise time (TET) during an exercise treadmill test (ETT).5 However, the concentrations of exogenous CGRP required to increase TET or protect against myocardial ischemia far exceed the endogenous physiological levels of CGRP that are released during a response to ischemia.5, 6 The precise role played by the canonical CGRP receptor in mediating these vasodilatory mechanisms remains unknown as CGRP binds to other distinct receptors in the calcitonin receptor family, such as the amylin 1 receptor to which it binds with similar potency as amylin.7, 8 Further, the relative importance of the CGRP receptor pathway compared with the other vasodilatory pathways that may be activated during myocardial ischemia has not been established. Although certain experimental protocols have suggested a potential detrimental effect,9, 10 the majority of in vitro and in vivo studies have reported no effect of inhibition of the CGRP receptor during myocardial ischemic injury.10, 11, 12, 13 Possibly, compensatory vasodilatory responses during myocardial ischemia can be preserved with inhibition of the CGRP receptor via redundancies in vasodilatory mechanisms or due to activation of other receptors responsive to CGRP.
To date there has been no clinical evidence suggesting that selective blockade of the CGRP receptor during ischemia worsens myocardial ischemia. When tested in an ETT as a model to determine the physiologic effect of vasoactive agents, the small molecule CGRP receptor antagonist, telcagepant, did not significantly reduce TET or time to ST‐segment depression when administered at supra‐therapeutic doses.14 Using an in vitro model of isolated human coronary artery, erenumab alone did not induce vasoconstriction up to the highest concentration tested (1 µM), nor did it affect sumatriptan‐induced contraction of coronary arteries.15
Clinical trials of patients with migraine typically use exclusion criteria that would result in few, if any, enrolled patients with diagnosed cardiovascular (CV) disease. Here, we elected to directly assess any potential deleterious anti‐vasodilatory effects of erenumab in a dedicated study of high‐risk patients with known CV disease undergoing an ETT to detect myocardial ischemia. The primary objective of this study was to evaluate the effect of erenumab compared with placebo on exercise capacity as measured by TET, a surrogate marker of myocardial ischemia, during an ETT as in patients with stable angina and limited exercise tolerance.
METHODS
Trial Population
Adults (18 to 85 years old) with a history of chronic stable angina for at least 3 months prior to screening, with at least 1 angina episode/month, on average over that period were eligible. The presence of ischemic heart disease was documented by history of biomarker release and/or electrocardiographically proven myocardial infarction, coronary angiography demonstrating at least 1 major epicardial coronary artery with a stenosis of at least 50% diameter or greater, or coronary revascularization procedure at least 3 months prior to screening. Use of cardiac medications for the treatment of angina or high blood pressure was allowed to continue during the trial if the dose had remained stable for at least 30 days prior to randomization and these medications were not expected to change during the study. Patients with medical conditions that might prevent study completion, interfere with interpretation of results, or constitute a safety risk per the investigator's opinion were excluded. Women of childbearing potential were required to use an effective contraceptive method.
Patients needed to complete 2 qualifying ETTs during the screening period demonstrating reproducible exercise limited TET to be enrolled in the study. A qualifying ETT was defined as exercise duration of 3‐12 minutes limited by symptoms related to myocardial ischemia (eg, angina) and ≥ 1.0 mm ST‐segment depression, or ≥ 3 mm ST‐segment depression alone (without requirement of angina‐related symptoms); and ≤ 1 minute difference or within 20% duration (using the longest duration qualifying ETT) in TET between the 2 qualifying ETTs. The second screening ETT was to be performed > 48 hours and ≤ 14 days after the first ETT.
Trial Oversight
Institutional review boards at each center approved the study protocol. All patients provided written informed consent. Sites maintained compliance with Health Insurance Portability and Accountability Act or relevant regional regulations. The study was conducted in accordance with the International Conference on Harmonisation Tripartite Guideline on Good Clinical Practice. An independent data monitoring committee reviewed and made recommendations regarding the safety of study participants throughout the double‐blind treatment phase and until treatment assignment information was available to the study team for the primary analysis. Amgen funded the study. This elective study was conducted in accordance to the original protocol with amendments to: include a 12‐lead electrocardiogram 4 hours after the completion of the ETT on day 1; add an assessment of anti‐erenumab antibodies at day 1, week 4, and end of study; increase the number of study centers; allow for the use of 2 out of 3 screening ETTs to qualify patients for enrollment; remove the restriction for antianginal medication on the morning of the ETT; and to change the noninferiority margin from 60 seconds to 90 seconds based on pre‐planned blinded analysis of within‐subject change during the baseline period to accommodate the within‐subject TET variation of 60 seconds or 20% difference allowed in qualifying TETs for each participant. Site investigators collected the data and Amgen conducted the data analyses according to a pre‐specified statistical analysis plan. All authors interpreted the data and collaborated in manuscript preparation with support from a professional medical writer, funded by Amgen. All authors made the decision to submit the manuscript and attested to the veracity and completeness of data and analyses and the fidelity of this report to the study protocol. A copy of the protocol is available at https://www.clinicaltrialsregister.eu EudraCT number 2015‐002322‐40.
Trial Design
This was a multicenter, randomized, double‐blind, placebo‐controlled phase 2a trial conducted at 40 sites across North America, Europe, South Africa, and New Zealand from November 2015 (first subject randomized) to April 2017 (end of study). The study was conducted in outpatient clinic facilities. Eligible participants were recruited into the study by participating sites, where they were regularly followed, and via advertisement. Following screening (up to 6 weeks), patients were randomized 1:1 to a single intravenous infusion of erenumab 140 mg or placebo (Fig. 1). Randomization was based on a computer‐generated schedule created by the sponsor before study initiation and was centrally executed using an interactive response system. Eligibility was evaluated and confirmed by the principal investigator. Randomization was stratified based on the TET average (<7 minutes or ≥ 7 minutes) of the 2 qualifying ETTs performed during screening. Patients, site personnel, and study sponsor personnel were blinded to treatment group assignment through the 12‐week double‐blind on‐study period. Safety follow‐up was completed at week 12.
Figure 1.
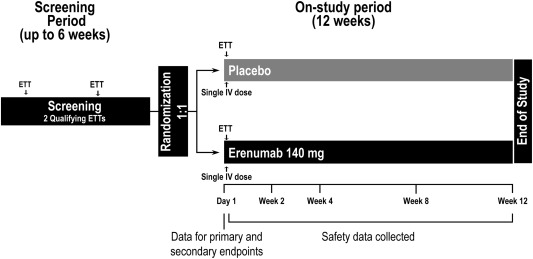
Study schema. Randomization was stratified by baseline TET (<7 minutes or ≥ 7 minutes) defined as the average TET of 2 qualifying ETTs performed during screening. ETT, exercise treadmill test; IV, intravenous; TET, total exercise time.
Assessments and Safety Evaluations
We conducted primary and secondary endpoint assessments as part of the ETT on Day 1. Safety was evaluated from the date of randomization through the end of the study (week 12). ETT was conducted using the standard Bruce protocol.16, 17 All ETTs for a given patient were to be performed under approximately the same conditions (time, temperature, food intake, caffeine, and nicotine consumption before the exercise test). Enrolled patients had one post‐randomization ETT that was conducted on Day 1 approximately 30 min following administration of investigational product. The depth of the ST‐segment depression was measured at 60 msec after the J‐point based on the average of at least 3 consecutive ST‐segments. A ≥ 1 mm ST‐segment depression was defined as horizontal or down‐sloping ST depression using baseline ST level as the reference if baseline ST level was below the isoelectric line and the isoelectric line as the reference if baseline ST level was above the isoelectric line. We evaluated safety by monitoring adverse events (MedDRA v19.0),18 serious adverse events,19 laboratory assessments, vital signs, and electrocardiograms.
Objectives and Endpoints
The primary objective was to evaluate the effect of erenumab compared to placebo for the primary endpoint of change from baseline in TET with a non‐inferiority (NI) margin of −90 seconds. Prespecified secondary endpoints were time to onset of exercise‐induced angina and time to onset of ≥ 1 mm ST‐segment depression during ETT.
Statistical Analysis
The primary endpoint was analyzed using an analysis of variance model with treatment and randomization strata (<7 or ≥ 7 minutes). The lower bound of a 2‐sided 90% confidence interval for mean difference in change from baseline in exercise duration was calculated and compared with an NI margin of −90 seconds to support the hypothesis that erenumab does not decrease exercise duration. NI margin of −90 seconds was selected based on 2 published cross‐over studies with small molecule inhibitors of CGRP telcagepant (NI = −60 seconds) and tadalafil (NI = −90 seconds).14, 20 This predetermined NI margin is supported by the mean within patient difference in TET of 67 seconds calculated as a pre‐planned blinded analysis of screening ETTs after 45 subjects completed 2 screening ETTs. Secondary endpoints were analyzed using a stratified (< 7 or ≥ 7 minutes randomization strata) log‐rank test statistic comparing the 2 treatment groups at a significance level of 0.10. Hazard ratio estimates were obtained from the Cox proportional hazard model including treatment group and baseline TET as a continuous measure. Primary analyses were conducted after all patients completed the post‐randomization ETT and safety analyses were conducted after all patients completed end of study at the week 12 visit.
Sample size was based on the primary endpoint of change from baseline in TET. Assuming a common standard deviation of 130 seconds and a difference in change from baseline in TET of 0 seconds between erenumab and placebo groups, the planned sample size of at least 27 participants per group would provide 80% power that the lower bound of the 2‐sided 90% confidence interval would exceed −90 seconds with a 10% significance level. A 5% dropout rate was assumed.
The full analysis set included all patients randomized in the study. The efficacy analysis set included patients who received randomized treatment and completed the post‐randomization ETT, analyzed according to randomized treatment. The safety analysis set included all randomized patients who received investigational product, analyzed according to actual treatment received.
RESULTS
Patients
Demographics and baseline disease characteristics were balanced between treatment groups and were representative of a high‐risk patient population with 100% of patients having cardiovascular disease and approximately 40% of patients with a history of myocardial infarction (Table 1). Of 255 patients screened, 89 patients were randomized, 88 (99%) received investigational product (1 patient randomized to receive erenumab withdrew consent prior to administration of investigational product), and 87 (98%) completed the week 12 safety follow‐up visit (1 patient in the placebo group was lost to follow‐up). Median (Q1, Q3) age of the patients was 65.0 (57.0, 70.0) years, and 78% of patients were male. All patients had reproducible exercise‐induced angina and ST‐segment depression at screening. Concomitant cardiovascular medication use was reported by all patients at baseline (Table 1).
Table 1.
Baseline Characteristics
| Placebo (N = 44) | Erenumab 140 mg IV (N = 45) | |
|---|---|---|
| Age (years, median (Q1, Q3) | 65.0 (58.5, 69.5) | 64.0 (56.0, 70.0) |
| Female, n (%) | 11 (25) | 9 (20) |
| White, n (%) | 37 (84) | 43 (96) |
| Cardiovascular history, n (%) | ||
| Coronary artery disease (CAD) | 44 (100) | 45 (100) |
| Myocardial infarction | 20 (46) | 16 (36) |
| Coronary artery bypass | 15 (34) | 14 (31) |
| Percutaneous coronary artery intervention | 28 (64) | 27 (60) |
| Cerebrovascular or peripheral arterial disease | 10 (23) | 11 (24) |
| Transient ischemic attack | 2 (5) | 2 (4) |
| Cerebrovascular accident | 2 (5) | 1 (2) |
| Carotid or vertebro‐basilar artery disease | 2 (5) | 6 (13) |
| Other cerebrovascular conditions | 3 (7) | 2 (4) |
| Peripheral artery disease | 5 (11) | 4 (9) |
| Cardiac risk factors | ||
| Hypertension | 41 (93) | 40 (89) |
| Type 2 diabetes mellitus | 13 (30) | 12 (27) |
| Dyslipidemia | 9 (21) | 14 (31) |
| Obesity | 5 (11) | 4 (9) |
| Baseline concomitant medication use | 44 (100) | 45 (100) |
| Beta blockers | 36 (92) | 39 (87) |
| Nitrates | 25 (67) | 31 (69) |
| ACE inhibitors | 27 (61) | 29 (64) |
| Calcium channel blockers | 14 (32) | 17 (38) |
| Angiotensin receptor blockers | 10 (23) | 7 (16) |
| Ranolazine | 1 (2) | 0 (0) |
| Other | 44 (100) | 45 (100) |
Full analysis set; all randomized patients.
Primary Endpoint
Erenumab was non‐inferior to placebo with respect to the primary endpoint (Fig. 2). The least squares mean (90% CI) change from baseline in TET was −2.9 (–27.5, 21.7) seconds for erenumab and 8.1 (–15.8, 32.0) seconds for placebo. The adjusted mean (90% CI) treatment difference was −11.0 (–44.9, 22.9) seconds. The lower limit of the 90% confidence interval of the difference was above the prespecified NI margin of −90 seconds, supporting the hypothesis that erenumab does not decrease exercise capacity as measured by change in TET compared to placebo.
Figure 2.
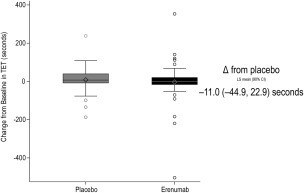
Change from baseline in total exercise time (TET). Effect of erenumab on total exercise time (TET) during the post‐treatment exercise treadmill test (ETT). The box plot and whisker plot illustrate the median (line in middle of the box), mean (diamond), interquartile range (box), 1.5 interquartile range (whiskers), and outliers (open circles). CI, confidence interval.
Secondary and Other Endpoints
No difference was observed between erenumab compared to placebo on time to ≥ 1 mm ST‐segment depression with a median (90% CI) time of 407 (380, 443) seconds for erenumab and 420 (409, 480) seconds for placebo (Fig. 3). The adjusted hazard ratio was 1.14 (0.76, 1.69) (P = .59). No difference was observed between erenumab compared to placebo on time to exercise‐induced angina with a median (90% CI) time of 500 (420, 540) seconds for erenumab and 508 (405, 572) seconds for placebo (Fig. 4). The adjusted hazard ratio (90% CI) was 1.11 (0.73, 1.69) (P = .69). Moreover, there was no difference between the erenumab and placebo groups in peak systolic and diastolic blood pressure or heart rate during the ETT or in functional recovery following completion of the ETT (Table 2).
Figure 3.
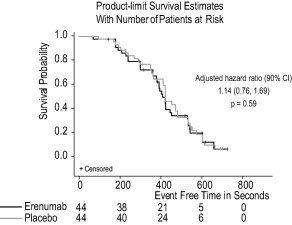
Time to onset of ≥ 1 mm ST‐segment depression. Kaplan–Meier product limit survival estimates for onset of ≥ 1 mm ST‐segment depression during the exercise treadmill test (ETT) in patients receiving erenumab or placebo. CI, confidence interval.
Figure 4.
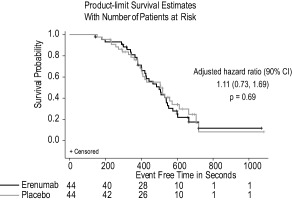
Time to onset of exercise‐induced angina. Kaplan–Meier product limit survival estimates for onset of exercise‐induced angina during the exercise treadmill test (ETT) in patients receiving erenumab or placebo. CI, confidence interval.
Table 2.
Blood Pressure and Heart Rate During Post‐Randomization Exercise Treadmill Test
| Placebo (N = 44) | Erenumab 140 mg IV (N = 44) | |
|---|---|---|
| Systolic blood pressure, mmHg | ||
| Baseline (Post‐study drug, prior to ETT) | 132.1 (18.3) | 129.3 (12.0) |
| Peak during ETT | 172.2 (22.4) | 172.1 (22.9) |
| Last measurement (Post‐ETT) | 135.0 (18.5) | 134.5 (15.2) |
| Diastolic blood pressure, mmHg | ||
| Baseline (Post‐study drug, prior to ETT) | 80.0 (11.8) | 77.8 (13.6) |
| Peak during ETT | 90.3 (15.4) | 90.1 (15.1) |
| Last measurement (Post‐ETT) | 79.2 (11.6) | 76.2 (9.3) |
| Heart rate, beats per minute | ||
| Baseline (Post‐study drug, prior to ETT) | 70.5 (13.4) | 69.6 (10.0) |
| Peak during ETT | 122.6 (22.0) | 122.2 (19.7) |
| Last measurement (Post‐ETT) | 78.8 (13.7) | 73.3 (8.7) |
Data are mean (SD). Safety analyses set; all patients randomized who received at least 1 dose of study drug.
Safety
The proportion of patients reporting at least one adverse event (AE) during the double‐blind treatment phase was similar for the erenumab and placebo groups (Table 3). The majority of AEs reported were singularly reported. Only one serious AE (atrial fibrillation in placebo) was reported (Table 3). Disease‐related events expected in a patient population with stable angina were reported in 2 patients in the erenumab group (Table 3). One patient experienced an event of angina pectoris starting on day 1 and ending on day 3. A second patient experienced unstable angina starting on day 15 and ending on day 18, with a second event that began on day 52 and ended on day 54. No deaths occurred during the study. Changes from baseline in systolic and diastolic blood pressure and heart rate during the safety follow‐up period were similar between the 2 groups. No patient developed anti‐erenumab binding or neutralizing antibodies during this study. No clinically significant changes in serum chemistry or hematology laboratory values were observed.
Table 3.
Summary of Adverse Events – 12‐week Follow‐Up
| Placebo (N = 44) | Erenumab 140 mg IV (N = 44) | |
|---|---|---|
| Adverse events, n (%) | ||
| Any | 14 (32) | 12 (27) |
| Serious | 1 (2) | 0 (0) |
| Fatal | 0 (0) | 0 (0) |
Safety analyses set; all patients randomized who received at least 1 dose of study drug.
DISCUSSION
In this study, patients with stable angina treated with a single dose of erenumab 140 mg IV did not experience significant changes in exercise time, a surrogate of tolerance to myocardial ischemia, compared to placebo. The change in TET from baseline was non‐inferior for erenumab compared to placebo and no difference was observed in the time to onset of ≥ 1 mm ST‐segment depression or exercise‐induced angina. In addition to the lack of effect observed during the ETT, there were no significant differences between treatment groups in reported adverse events through the 12‐week safety follow‐up.
We used an erenumab dose of 140 mg IV for this safety testing, which, due to increased bioavailability, results in circulating concentrations higher than the maximal concentration achieved with a 140 mg subcutaneous dose that has been proven efficacious in clinical studies of migraine prevention.21, 22 Mean serum concentrations of erenumab following 140 mg IV administration were highest following the infusion. Following completion of the ETT mean erenumab concentration was at least 2‐fold higher than the C max reached following 140 mg SC administration. Thus, the use of 140 mg IV dose of erenumab ensured rapid and robust blockade of the CGRP receptor, providing a substantial margin over concentrations achieved by subcutaneous administration of 140 mg.23
Using exercise testing to assess the potential effects of vasoactive drugs to affect tolerance to myocardial ischemia has benefits over alternative approaches. Coronary angiography with coronary blood flow reserve measurement, for example, requires an invasive procedure and could be confounded by iatrogenic vessel spasms. Invasive measurements may also have limited ability to detect changes related to small vessel coronary disease. Other methodologies to evaluate ischemia such as PET or MRI perfusion imaging are less widely available and more difficult to standardize across sites compared to ECG treadmill tests. Additional findings with erenumab support that CGRP receptor inhibition with erenumab does not pose a cardiovascular risk; no effects on blood pressure or increased risk for CV events have been observed in a large clinical program for erenumab.21, 22, 24
In addition to demonstrating safety of erenumab in cardiovascular patients, these results also support the existence of redundant vasodilatory mechanisms in patients with chronic stable angina. Erenumab blocks a single receptor, the canonical CGRP receptor, without effect on other receptors, including the amylin 1 receptor, to which the CGRP ligand also binds with high affinity.7, 8 Because erenumab blocks a single receptor the results of this study may not be extrapolated to therapeutic approaches that inhibit the CGRP ligand.
Limitations
While this study enrolled patients with documented ischemic heart disease, study results may not apply to patients not meeting the inclusion/exclusion criteria for our study and may not account for all variations in the extent of coronary artery disease or other vascular diseases such as peripheral artery disease. This study population differed from the clinical trial populations studied in migraine prevention (ie, mostly middle‐aged female) and it remains unknown whether various hormonal factors might impact CV safety of erenumab. Further trials, including the ongoing open‐label extensions of prior erenumab clinical trials, are needed to determine the long‐term safety of erenumab and the durability of its effects. Further, because migraine itself has been reported as a risk factor for ischemic stroke or cardiovascular events, better understanding of the long‐term cardiovascular safety of erenumab in migraine patients appears relevant to clinical practice.
CONCLUSIONS
These results demonstrate that inhibition of the CGRP receptor with erenumab does not impact exercise tolerance or interfere with homeostatic vasodilatory mechanisms with stable chronic myocardial ischemia. These results in addition to the functional improvements and favorable safety and tolerability profiles of erenumab demonstrated in multiple clinical studies suggest that inhibition of the CRGP receptor by erenumab is a promising preventive treatment approach in patients with episodic and chronic migraine.
STATEMENT OF AUTHORSHIP
Category 1
(a) Conception and Design
Christophe Depre
(b) Acquisition of Data
Lubomir Antalik, Michael Koren
(c) Analysis and Interpretation of Data
Christophe Depre, Lubomir Antalik, Amaal Starling, Michael Koren, Osaro Eisele, Robert A. Lenz, Daniel D. Mikol
Category 2
(a) Drafting the Manuscript
Christophe Depre, Lubomir Antalik, Amaal Starling, Michael Koren, Osaro Eisele, Robert A. Lenz, Daniel D. Mikol
(b) Revising It for Intellectual Content
Christophe Depre, Lubomir Antalik, Amaal Starling, Michael Koren, Osaro Eisele, Robert A. Lenz, Daniel D. Mikol
Category 3
(a) Final Approval of the Completed Manuscript
Christophe Depre, Lubomir Antalik, Amaal Starling, Michael Koren, Osaro Eisele, Robert A. Lenz, Daniel D. Mikol
Acknowledgments
This study was fully funded by Amgen. Erenumab is co‐developed in partnership with Amgen and Novartis. Medical writing support was provided by Jon Nilsen, PhD, of Amgen.
Conflict of Interest: Amaal Starling reports consulting fees from eNeura, Eli Lilly & Company, Amgen, and Alder. Michael Koren is an employee of Jacksonville Center for Clinical Research, which has received research funds and consulting fees from Amgen, Inc. Christophe Depre, Osaro Eisele, Robert A. Lenz, and Daniel D. Mikol are employees and stockholders of Amgen Inc. Lubomir Antalik has no conflict of interest to declare.
Trial registration: EudraCT No. 2015‐002322‐40
REFERENCES
- 1. Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: A life‐span study. Cephalalgia. 2010;30:1065‐1072. [DOI] [PubMed] [Google Scholar]
- 2. Stewart WF. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64‐69. [PubMed] [Google Scholar]
- 3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache. 2015;55:21‐34. [DOI] [PubMed] [Google Scholar]
- 4. Franco‐Cereceda A, Liska J. Potential of calcitonin gene‐related peptide in coronary heart disease. Pharmacology. 2000;60:1‐8. [DOI] [PubMed] [Google Scholar]
- 5. Uren NG, Seydoux C, Davies GJ. Effect of intravenous calcitonin gene related peptide on ischaemia threshold and coronary stenosis severity in humans. Cardiovasc Res. 1993;27:1477‐1481. [DOI] [PubMed] [Google Scholar]
- 6. Mair J, Lechleitner P, Längle T, et al. Plasma CGRP in acute myocardial infarction. Lancet. 1990;335:168. [DOI] [PubMed] [Google Scholar]
- 7. Bailey RJ, Walker CS, Ferner AH, et al. Pharmacological characterization of rat amylin receptors: Implications for the identification of amylin receptor subtypes. Brit J Pharmacol. 2012;166:151‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juaneda C, Dumont Y, Quirion R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol Sci. 2000;21:432‐438. [DOI] [PubMed] [Google Scholar]
- 9. Lu R, Li YJ, Deng HW. Evidence for calcitonin gene‐related peptide‐mediated ischemic preconditioning in the rat heart. Regul Pept. 1999;82:53‐57. [DOI] [PubMed] [Google Scholar]
- 10. Chai W, Mehrotra S, Jan Danser AH, et al. The role of calcitonin gene‐related peptide (CGRP) in ischemic preconditioning in isolated rat hearts. Eur J Pharmacol. 2006;531:246‐253. [DOI] [PubMed] [Google Scholar]
- 11. Regan CP, Stump GL, Kane SA, et al. Calcitonin gene‐related peptide receptor antagonism does not affect the severity of myocardial ischemia during atrial pacing in dogs with coronary artery stenosis. J Pharmacol Exp Ther. 2009;328:571‐578. [DOI] [PubMed] [Google Scholar]
- 12. Wu DM, van Zwieten PA, Doods HN. Effects of calcitonin gene‐related peptide and BIBN4096BS on myocardial ischemia in anesthetized rats. Acta Pharmacol Sin. 2001;22:588‐594. [PubMed] [Google Scholar]
- 13. Sekiguchi N, Kanatsuka H, Sato K, et al. Effect of calcitonin gene‐related peptide on coronary microvessels and its role in acute myocardial ischemia. Circulation. 1994;89:366‐374. [DOI] [PubMed] [Google Scholar]
- 14. Chaitman BR, Ho AP, Behm MO, et al. A randomized, placebo‐controlled study of the effects of telcagepant on exercise time in patients with stable angina. Clin Pharmacol Ther. 2012;91:459‐466. [DOI] [PubMed] [Google Scholar]
- 15. Rubio‐Beltrán E, et al. Effects of AMG 334 on human isolated coronary artery [abstract]. Cephalalgia. 2016;36:1‐185. [Google Scholar]
- 16. Bruce RA, Lovejoy FW, Pearson R Jr, and, et al. Normal respiratory and circulatory pathways of adaptation in exercise. J Clin Invest. 1949;28:1423‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruce RA, Pearson R, Lovejoy FW, and, et al. Variability of respiratory and circulatory performance during standardized exercise. J Clin Invest. 1949; 28:1431‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goux S, et al. MedDRA Maintenance and Support Services Organization Annual Report 2014; 2014.
- 19.U.S Department of Health and Human Services NIoH, N.C.I., Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; 2010.
- 20. Patterson D, Kloner R, Effron M, et al. The effect of tadalafil on the time to exercise‐induced myocardial ischaemia in subjects with coronary artery disease. Br J Clin Pharmacol. 2005;60:459‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Neurol. 2017;16:425‐434. [DOI] [PubMed] [Google Scholar]
- 22. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol. 2016;15:382‐390. [DOI] [PubMed] [Google Scholar]
- 23. Vu T, Ma P, Chen JS, et al. Pharmacokinetic‐pharmacodynamic relationship of erenumab (AMG 334) and capsaicin‐induced dermal blood flow in healthy and migraine subjects. Pharm Res. 2017;34:1784‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goadsby PJ, Reuter U, Bonner J, et al. Phase 3, randomised, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of erenumab (amg 334) in migraine prevention: Primary results of the STRIVE trial [Abstract PF52]. J Neurol Neurosurg Psychiatry. 2017;88:e1.62‐e1‐e1. [Google Scholar]


