Abstract
Measuring treatment efficacy in individuals with Autism Spectrum Disorder (ASD) relies primarily on behaviors, with limited evidence as to the neural mechanisms underlying these behavioral gains. This pilot study addresses this void by investigating neural and behavioral changes in a Phase I trial in young adults with high‐functioning ASD who received an evidence‐based behavioral intervention, Virtual Reality‐Social Cognition Training over 5 weeks for a total of 10 hr. The participants were tested pre‐ and post‐training with a validated biological/social versus scrambled/nonsocial motion neuroimaging task, previously shown to activate regions within the social brain networks. Three significant brain‐behavior changes were identified. First, the right posterior superior temporal sulcus, a hub for socio‐cognitive processing, showed increased brain activation to social versus nonsocial stimuli in individuals with greater gains on a theory‐of‐mind measure. Second, the left inferior frontal gyrus, a region for socio‐emotional processing, tracked individual gains in emotion recognition with decreased activation to social versus nonsocial stimuli. Finally, the left superior parietal lobule, a region for visual attention, showed significantly decreased activation to nonsocial versus social stimuli across all participants, where heightened attention to nonsocial contingencies has been considered a disabling aspect of ASD. This study provides, albeit preliminary, some of the first evidence of the harnessable neuroplasticity in adults with ASD through an age‐appropriate intervention in brain regions tightly linked to social abilities. This pilot trial motivates future efforts to develop and test social interventions to improve behaviors and supporting brain networks in adults with ASD. Autism Res 2018, 11: 713–725. © 2018 The Authors Autism Research published by International Society for Autism Research and Wiley Periodicals, Inc.
Lay Summary
This study addresses how the behavioral changes after treatment for ASD reflect underlying brain changes. Before and after receiving VR‐SCT, young adults with high‐functioning ASD passively viewed biological motion stimuli in a MRI scanner, tapping changes in the social brain network. The results reveal neuroplasticity in this age population, extending the window of opportunity for interventions to impact social competency in adults with ASD.
Keywords: neuroplasticity, adults with autism, emotion recognition, theory of mind, clinical trials, computerized treatment, virtual reality
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by challenges with social skills, speech and nonverbal communication, and repetitive behaviors [APA, 2013]. While the bulk of the work in ASD treatment has focused on early childhood, a critical stage that is believed to represent the most opportune time for treatment of social deficits to be achievable, treatment research in adults with ASD is much more limited [Howlin & Moss, 2012]. Moreover, evaluation of ASD treatment typically relies on behaviors, including externally observable behaviors (e.g., eye contact) and internal behaviors (e.g., self‐report of emotional and cognitive processes), leaving explanations elusive as to whether and how behavioral improvements reflect brain changes.
Neural markers associated with behavioral changes are particularly important because they serve to better inform our understanding of treatment mechanisms. Functional magnetic resonance imaging (fMRI) has been shown to be effective in revealing group differences between patients with ASD and healthy controls [Allison, Puce, & McCarthy, 2000; Anagnostou & Taylor, 2011; Carrington & Bailey, 2009; Harms, Martin, & Wallace, 2010; Kaiser et al., 2010, 2015 McKay et al., 2012; Yang, Rosenblau, Keifer, & Pelphrey, 2015]. Recently, fMRI has been further employed to understand the neural mechanisms of treatment in young children with ASD [Venkataraman et al., 2016; Ventola et al., 2015; Voos et al., 2012]. However, fMRI has not been applied to identify neural mechanisms of treatment effects in adults with ASD. There is an urgent need to do so, as intervention research and its effectiveness is limited beyond the age of adolescence in ASD [Bishop‐Fitzpatrick, Minshew, & Eack, 2013; Brugha, Doos, Tempier, Einfeld, & Howlin, 2015; Hedley et al., 2016; Walton & Ingersoll, 2013]. Moreover, conventional wisdom holds that the social deficits of ASD are difficult to near impossible to treat after childhood, although this stance remains relatively untested. Such a view has likely served to limit research initiatives focused on treatment for adults with autism [Blackwell, Trzesniewski, & Dweck, 2007]. The present study represents a preliminary effort to fill this void by examining whether young adults with high functioning ASD can benefit from age‐appropriate social training as manifested on brain and behavioral changes. We were particularly interested in measuring pre‐ to post‐training changes in brain regions previously linked to social skills and on related social measures. We compared before and after treatment the mean brain activation at a group level and whether there was a convergence between brain changes and improved social abilities to support changes at an individual level.
The fMRI measure used in this study is a well‐validated social perception, biological motion fMRI task [Kaiser et al., 2010], which robustly engages the neural circuits supporting both socio‐emotional and socio‐cognitive components of social information processing. The biological motion videos feature an adult engaging in children's games and social actions (e.g., waving, pat‐a‐cake). Prior research has shown that social orienting to biological motion is evolutionarily well‐conserved and fundamental to adaptive social engagement [Heberlein & Adolphs, 2004; Johnson, 2006; Simion, Regolin, & Bulf, 2008; Vallortigara, Regolin, & Marconato, 2005; Yang et al., 2015]. With the contrast of biological versus scrambled motion, the fMRI task has revealed neural circuits of key social brain regions implicated in core ASD deficits [Allison et al., 2000; Kaiser et al., 2010; McKay et al., 2012; Yang et al., 2015]. The regions include (a) key regions for socio‐emotional processing and emotion regulation [Etkin, Buchel, & Gross, 2015; Kanske, Heissler, Schonfelder, Bongers, & Wessa, 2011; Phelps & LeDoux, 2005]: the inferior frontal gyrus (IFG)/ventrolateral prefrontal cortex (vlPFC), ventromedial prefrontal cortex (vmPFC), and amygdala, and (b) key regions for socio‐cognitive processing and social information integration [Deen, Koldewyn, Kanwisher, & Saxe, 2015; Saggar, Shelly, Lepage, Hoeft, & Reiss, 2014; Yang et al., 2015]: the right posterior superior temporal sulcus (pSTS), and fusiform gyrus (FFG). These findings provide possible regions of interest for neuroplasticity in adults with ASD following social training.
In addition, the fMRI task affords a novel contrast of nonsocial versus social stimuli, which provides a window into the participants' neural responses to nonsocial stimuli. Previous research suggests that paying relatively more visual attention to nonsocial contingencies is a hallmark of autism [Klin, Lin, Gorrindo, Ramsay, & Jones, 2009], which may help account for why they fail to perceive social cues. We predicted that young adults with ASD would show reduced visual attention to nonsocial versus social stimuli, as one potential positive outcome of the social intervention.
The treatment platform, Virtual Reality‐Social Cognition Training (VR‐SCT) [Kandalaft, Didehbani, Krawczyk, Allen, & Chapman, 2013] was utilized in this research, which is a short‐term, 10‐hr training, carried out over a 5‐week period. Virtual Reality (VR) in general has been shown to be an effective tool in training social skills for individuals with ASD [Bellani, Fornasari, Chittaro, & Brambilla, 2011; Kandalaft et al., 2013; Maskey, Lowry, Rodgers, McConachie, & Parr, 2014; Parsons & Mitchell, 2002; Wainer & Ingersoll, 2011]. Reviews on VR studies suggest there are several advantages of using VR environments to train social skills. Benefits include the abilities to (a) simulate real‐life experiences such as commonly encountered challenges, (b) offer a safe, repeatable, and controllable environment in which to practice, (c) increase motivation to practice by levering an engaging video‐game environment, and (d) be immersed into a situation to experience similarly evoked real‐life emotions related to the situation [Bellani et al., 2011; Parsons & Mitchell, 2002]. The current study followed the training platform in prior VR‐SCT studies [Didehbani, Allen, Kandalaft, Krawczyk, & Chapman, 2016; Kandalaft et al., 2013], comprehensively targeting socio‐emotional, socio‐cognitive and social functioning skills. Research findings from previous VR‐SCT studies showed that training had a positive impact on the ability to recognize emotions, understanding the intentions of others (theory of mind), and improved daily social functioning conversation abilities in both adults and children [Didehbani et al., 2016; Kandalaft et al., 2013]. Due to the live and immersive format of VR‐SCT, participants engage in a real‐time flexible conversation with a coach and confederate conversation partner while targeting multiple social‐cognitive strategies from one dynamic experience. In brief, VR‐SCT offers a comprehensive, engaging, interactive platform for training and improving a wide range of social abilities including socio‐emotional and socio‐cognitive abilities for individuals with ASD.
In the current study, we investigated how therapeutic response to VR‐SCT reflect brain changes in young adults with ASD. The biological motion fMRI task was utilized because it taps into key neural circuits of socio‐emotional and socio‐cognitive processing, which correspond to the treatment targets of VR‐SCT. Linking the biological motion fMRI task and VR‐SCT, we utilized two separate behavioral tasks to measure behavioral changes in both emotional and cognitive aspects of social information processing. For the emotional component, we measured behavioral changes in emotion‐recognition ability, whereas for the cognitive component, we measured behavioral changes in theory‐of‐mind ability. As VR‐SCT has been demonstrated to improve emotion recognition and theory of mind in prior research [Didehbani et al., 2016; Kandalaft et al., 2013], we expected that there would be similar behavioral improvement on average (one‐tailed). Moreover, central to the aim of the current study, we hypothesized that (a) brain regions linked to the social brain networks would show pre to post changes in response to the treatment at a group level across all participants, and (b) improvement on behavioral measures of emotion recognition and theory of mind would reflect changes in corresponding key social brain regions at the individual level, for socio‐emotional processing and emotion regulation [Etkin et al., 2015; Kanske et al., 2011; Phelps & LeDoux, 2005] and for socio‐cognitive processing and social information integration [Deen et al., 2015; Saggar et al., 2014; Yang et al., 2015], respectively.
We acknowledge that parts of a larger data set used in the current study, collected on the young adults with high‐functioning ASD, have been analyzed and previously reported in our neuroprediction study [Yang et al., 2017b]. However, it is important to point out that the goals and analyses represent clear distinctions between the previous study and the current study. First, with respect to goals, the previous study addressed the question: how informative fMRI is as a forecasting tool to predict treatment response and facilitate subject selection. In contrast, the current study addresses a different but equally important question: why VR‐SCT works on the brain level. Specifically, we wanted to elucidate the neural mechanisms underlying the behavioral changes. Second, in regard to analyses, the previous study examined the correlation between baseline brain activities and behavioral changes from baseline to endpoint, without examining any brain changes. In contrast, the current study examined exclusively the brain changes at the group level and the correlation between brain changes and behavioral changes from baseline to endpoint at the individual level. Thus, the main goals and analyses of the two studies are nonoverlapping.
Methods
Participants
Study participants included 17 young adults (age M = 22.50 years, SD = 3.89; 2 females, 15 males) with a primary diagnosis of high‐functioning (full‐scale IQ ≥ 80; M = 109.65, SD = 13.32, range = 88–131) ASD, recruited from two research sites: Yale Child Study Center (YCSC) for 7 participants, and Center for BrainHealth at The University of Texas at Dallas (CBH‐UTD) for the other 10 participants. IQ was measured using the Wechsler Abbreviated Scale of Intelligence (WASI) [Wechsler, 1999, 2011]. All participants met DSM‐V [APA, 2013] diagnostic criteria for ASD as determined by the results of a gold‐standard diagnostic instrument, the Autism Diagnostic Observation Schedule [Gotham et al., 2007; Hus & Lord, 2014; Lord et al., 2000]—administered by research‐reliable clinicians and licensed clinical psychologists. Unfortunately, the ADOS subdomain scores were not available for two participants because they were evaluated by psychologists involved in other projects, although their autism diagnosis was re‐confirmed and current before they were included in this project. Pretreatment clinical behavioral characterization was based on the self‐reported Social Responsiveness Scale, 2nd edition (SRS‐2) [Constantino, 2012] (M total raw = 82.41, SD = 33.43), which assesses ASD symptom severity in five domains: social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behavior. Comprehensive demographics and characterization information are provided in Table 1. This is a pretest‐posttest treatment‐only study and all of the 17 participants were assigned to receive VR‐SCT intervention. The study is registered at ClinicalTrials.gov (ID: NCT02139514; NCT02922400).
Table 1.
Participants Demographics and Pretreatment Autism Symptom Severity Profile (N = 17)
| Variable | Mean (S.D.) | Range |
|---|---|---|
| Pretreatment Age (years) | 22.50 (3.89) | 18.06–31.08 |
| Gender, male (0 = f, 1 = m) | 0.88 (0.33) | – |
| Full‐scale IQ | 109.65 (13.32) | 88–131 |
| Handedness (1 = right, 0 = ambi., −1 = left) | 1.00 (0.00) | – |
| ADOS Module 4 (n = 15)a | ||
| SA Domain | 10.73 (3.63) | 7–19 |
| RRB Domain | 0.93 (1.03) | 0–3 |
| Total | 11.67 (3.85) | 7–20 |
| Pretreatment SRS‐2 self‐reported raw scores | ||
| Social awareness | 9.59 (3.22) | 5–15 |
| Social cognition | 14.35 (5.79) | 6–28 |
| Social communication | 25.41 (11.77) | 6–47 |
| Social motivation | 15.18 (7.72) | 5–27 |
| Restricted interests and repetitive behavior | 17.88 (7.78) | 6–30 |
| Total | 82.41 (33.43) | 33–142 |
Note. This table was previously reported in our neuroprediction study [Yang et al., 2017b].
Unfortunately, the ADOS subdomain scores were not available for two participants because they were evaluated by psychologists involved in other projects, although their autism diagnosis was re‐confirmed and current before they were included in this project.
SA, social affect; RRB, restricted and repetitive behaviors.
Treatment Approach: Virtual Reality–Social Cognition Training (VR‐SCT)
VR‐SCT has been shown to be an effective intervention method for treating social deficits in both children and young adults with ASD [Didehbani et al., 2016; Kandalaft et al., 2013]. Results from this current study were expected to be similar to the outcomes from the prior studies, including improved emotion recognition and theory of mind after the 5‐week, 10‐hr training. During the training session, both the clinician and participant interacted entirely through virtual avatar characters (see Fig. 1 for sample). Participants engaged in real‐time, nonscripted, age‐appropriate scenarios such as job interviewing, dating, or negotiating with a college‐aged friend while receiving real‐time feedback from a coach clinician. Participants were given multiple opportunities within a session to practice a social skill within the conversations. More details regarding the VR‐SCT in this study had been described in a previous report [Yang et al., 2017b].
Figure 1.

The computer set‐up and example screenshots of a virtual reality training session. This figure was previously reported in our neuroprediction study [Yang et al., 2017b].
Primary Clinical Outcome: Changes in Emotion Recognition and Theory of Mind
Treatment effectiveness was measured by behavioral changes in two distinct domains of social abilities: emotion recognition (tapping change in socio‐emotional processing abilities) and theory of mind (tapping change in socio‐cognitive processing abilities), respectively.
Emotion recognition
The Advanced Clinical Solutions for WAIS‐IV and WMS‐IV Social Perception Subtest (ACS‐SP) [Kandalaft et al., 2012; Pearson, 2009], administered by trained research staff in our research centers, was utilized to measure emotion recognition abilities. Three subscales are generated from the subtest tasks: (a) SP‐Affect Naming, a measure of face emotion recognition; (b) SP‐Prosody, a measure of vocal affect recognition; (c) SP‐Pairs, a measure of nonliteral language interpretation. Across ACS‐SP scores, average internal consistency has been reported as r = 0.69–0.81, test–retest stability coefficient as corrected r = 0.60–0.70, and inter‐scorer agreement from 0.98 to 0.99. Normative scaled scores are available for all ACS‐SP subtests. Treatment effectiveness on emotion recognition is modeled as the Δ change scores of the ACS‐SP scaled scores, that is, post minus pre, such that positive (or negative) delta change scores indicate increase (or decrease) in emotion recognition abilities. In the prior pilot study involving VR‐SCT and young adults with autism [Kandalaft et al., 2013], emotion recognition changed significantly from pretreatment (M = 7.63, SD = 3.42) to posttreatment (M = 9.63, SD = 3.78), t (7)=2.83, P = 0.03, Hedges's gav [Lakens, 2013] = 0.53.
Theory of mind
The Social Attribution Task, also known as the triangles task [Abell, Happe, & Frith, 2000] was administered to measure a person's abilities of theory of mind or ability to understand others' intention. Videos were adapted from [Heider & Simmel, 1944], in which participants were asked to narrate the movements of triangles presented in six separate brief videos. Narratives were recorded, transcribed, and double‐scored by two blind raters. Based upon the scoring criteria established in previous research [Abell et al., 2000], participants' narratives were first scored on accuracy and attribution, respectively, and the two scores were then summed up to derive a total score. The accuracy and attribution aspects were scored separately using a 4‐point Likert scale (0–3 point scale) for each video, with 18 as the maximum possible score across all 6 videos for both accuracy and attribution, respectively. More points were awarded when the participant stated descriptions that were accurate to the nature of the video (for the accuracy aspect), or when more mentalizing or emotional words were utilized to describe the movement of the triangles (for the attribution aspect). The triangles task has been shown to have a high test–retest reliability of r = 0.76–0.88 and concurrent validity r = 0.78–0.93 [Hu et al., 2010]. The order of the videos was randomized and participants were presented with different sets of videos at pre‐ and post‐intervention testing. In the prior pilot study involving VR‐SCT and young adults with autism [Kandalaft et al., 2013], theory of mind changed significantly from pretreatment (M = 12.63, SD = 4.93) to posttreatment (M = 15.38, SD = 4.81), t (7) = 3.45, P = 0.01, Hedges's gav [Lakens, 2013] = 0.53.
fMRI Imaging Task
We measured the pretreatment and posttreatment BOLD (blood oxygen level dependent) responses using a well‐established biological motion fMRI task [Bjornsdotter, Wang, Pelphrey, & Kaiser, 2016; Kaiser et al., 2010; Ventola et al., 2015; Yang et al., 2016, 2017a]. We selected this paradigm because it measures the neural activities of two key components of social information processing [Kaiser et al., 2010], namely, socio‐emotional and socio‐cognitive processing, which correspond to the treatment targets of VR‐SCT and the two behavioral measures of emotion recognition and theory of mind, respectively. The participants were scanned while viewing coherent and scrambled point‐light displays of biological motion created from motion capture data. The coherent biological motion displays featured an adult male actor performing movements relevant to early childhood experiences, such as playing pat‐a‐cake [Klin et al., 2009], and contained 16 points corresponding to major joints. The scrambled motion animations were created by selecting all the 16 points from the biological motion displays and randomly plotting their trajectories on a black background (see Fig. S1 for sample fMRI stimuli). Thus, the coherent and scrambled displays contained the same local motion information, but only the coherent displays contained the configuration of a person [Johansso, 1973]. During the MRI scan, stimuli were presented using E‐Prime 2.0 software (Psychological Software Tools, Pittsburgh, PA, USA). Six coherent biological motion clips (BIO) and six scrambled (SCR) motion clips were presented once each in an alternating‐block design (time per block, ∼ 24 s). The experiment began with a 20‐s fixation period and ended with a 16‐s fixation period. The total duration was 328 s. The movies were presented without audio. The participants were asked to watch the videos and reminded to remain still and alert. The imaging task and stimuli are available from the authors upon reasonable request.
Imaging Acquisition and Processing
Scanning was performed on a Siemens MAGNETOM 3 Tesla Tim Trio scanner at the Yale Magnetic Resonance Research Center (for YCSC participants) or a Philips 3 Tesla MR system (for CBH‐UTD participants) within 1 week before and after the treatment. Details regarding the imaging parameters and individual‐level processing steps had been described in previous research [Yang et al., 2016, 2017a,b). The participant‐level contrast of interest is BIO > SCR, which served as inputs for the subsequent mass univariate fMRI analyses.
fMRI Analyses
We conducted mass univariate voxel‐wise GLM analyses across the whole brain to identify three kinds of clusters: first, pretreatment BOLD activation to the contrast of BIO > SCR significantly differs from posttreatment BOLD activation at the group level; second, brain changes in the contrast of BIO > SCR track with behavioral change in theory of mind at the individual level; third, brain changes in the contrast of BIO > SCR track with behavioral change in emotion recognition at the individual level. The analyses were conducted using mixed‐effects modeling with FSL's FLAME (FMRIB's Local Analysis of Mixed Effects) 1 + 2 inference algorithm, which provides highly accurate estimation of group‐level results that are generalizable to the population. We employed a stringent cluster‐defining threshold of Z > 2.33, P < 0.01, while correcting for multiple comparisons at a cluster‐level threshold of P < 0.05. Information about the surviving clusters was reported, including number of voxels in the cluster, the anatomical regions covered by the clusters based on the Desikan–Killiany atlas [Desikan et al., 2006], the coordinates of the peak voxels within each of the anatomical regions, and the Z‐statistics associated with the peak voxels. Site, age, IQ, sex, and pretreatment autism symptom severity using the SRS total raw scores were mean‐centered and controlled for as covariates of no interest in all group‐level univariate GLM analyses. This was to ensure that the results could be generalized to different sites, ages, IQ levels, sexes, and levels of pretreatment autism symptom severity. Finally, to understand the functional relevance of the surviving clusters, we performed quantitative reverse inferences using NeuroSynth (http://www.neurosynth.org/) [Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011]. Details regarding the procedure can be found in previous research [Yang et al., 2016, 2017b].
Results
Primary Clinical Outcome
As previously reported in our neuroprediction study [Yang et al., 2017b], VR‐SCT significantly improved emotion recognition in terms of the ACS‐SP scaled scores from pretreatment (M = 11.41, SD = 4.42) to posttreatment (M = 12.94, SD = 3.51), Δ = 1.53, S.D. of Δ = 2.72, t (16) = 2.32, P = 0.03 (two‐tailed), Hedges's gav [Lakens, 2013] = 0.38, and the CL (Common Language) effect size [Mcgraw & Wong, 1992] indicates that after controlling for individual differences, the likelihood that a person's emotional recognition score is higher at post‐treatment than pre‐treatment is 71%. In addition, VR‐SCT marginally improved theory of mind in terms of the total scores from the triangles task from pre‐treatment (M = 19.41, SD = 3.89) to post‐treatment (M = 20.35, SD = 3.84), Δ = 0.94, S.D. of Δ = 1.95, t (16) = 1.99, P = 0.06 (two‐tailed), Hedges's gav = 0.24, and the CL effect size indicates that after controlling for individual differences, the likelihood that a person's theory of mind total score is higher at post‐treatment than pretreatment is 69%. This effect was significant one‐tailed, P = 0.03, and therefore also as hypothesized. The change scores of emotion recognition were not significantly correlated with those of theory of mind, r(15) = −0.22, P = 0.40, suggesting that they were tapping improvement in distinctively different domains.
Neural Mechanisms of Change
Based on the evidence that VR‐SCT induced behavioral changes, we continued to answer the question of neural mechanisms underlying the behavioral changes. This question can be addressed in two ways. First, at the group level, we compared the mean brain activations before and after VR‐SCT. Second, at the individual level, we analyzed what brain changes tracked with behavioral changes induced by VR‐SCT.
Group mean brain change after VR‐SCT
At the group level, as seen in Fig. S2, BIO > SCR activated similar brain regions before and after treatment. However, when specifically comparing the mean BOLD responses to BIO > SCR before and after VR‐SCT, as shown in Fig. 2, there was a cluster (253 voxels) in the left superior parietal lobule showing significant increase in the contrast of BIO > SCR. One‐sample t‐test further indicates that the region tended to be deactivated to BIO > SCR (or activated to SCR > BIO) before VR‐SCT, t (16) = −2.42, P = 0.03, Cohen's d = −0.57, but relatively neutral to BIO > SCR after VR‐SCT, t = 0.14, P = 0.89, Cohen's d = 0.03. Table 2 lists the peak significance and peak coordinates of this cluster. To further interpret the possible functions associated with this cluster, we conducted a NeuroSynth‐based reverse inference analysis. The top 10 NeuroSynth‐decoded feature terms were eye movements (0.18), reaching (0.15), saccades (0.14), representation (0.13), observation (0.13), visually (0.13), eye fields (0.12), execution (0.11), attention (0.11), and foci (0.11); numbers within the parentheses are correlation coefficients between the surviving cluster and the meta‐analysis maps of the feature terms in NeuroSynth. This suggests that the cluster is primarily associated with visual observation and attention.
Figure 2.
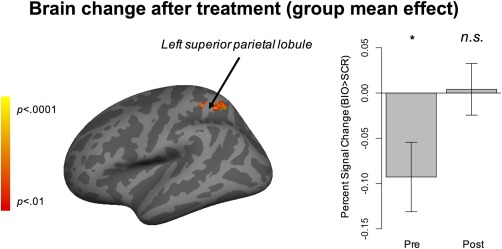
The group‐level brain activation change before and after virtual reality‐social cognition training. The brain map on the left illustrates the region of significant change, which is in the left superior parietal lobule. The bar graph on the right illustrates the activation in terms of percent signal change in response to BIO > SCR at pretreatment and posttreatment time points, respectively. Error bars represent ±1 standard error. The analysis was corrected, with voxel‐level threshold Z > 2.33, P < 0.01, and cluster‐level threshold P < 0.05. Site, IQ, age, sex, and pretreatment autism symptoms severity were included as covariates of no interest. *P < 0.05.
Table 2.
Neural Mechanisms Underlying Behavioral Changes Induced by VR‐SCT
| Local maxima | |||||||
|---|---|---|---|---|---|---|---|
| Cluster | N voxels | Anatomical Region | x | y | z | Z peak | |
| Mean brain changes after VR‐SCT | 253 | L | Superior parietal gyrus | −30 | −50 | 54 | 3.93 |
| Brain changes tracking with theory‐of‐mind changes | 591 | R | Supramarginal gyrus | 60 | −40 | 18 | 3.78 |
| R | Superior temporal gyrus | 64 | −32 | 16 | 3.74 | ||
| R | Inferior parietal gyrus | 50 | −56 | 14 | 3.66 | ||
| R | Banks of the STS | 58 | −36 | 14 | 3.61 | ||
| R | Middle temporal gyrus | 48 | −58 | 10 | 3.04 | ||
| Brain changes tracking with emotion‐recognition changes | 232 | L | Pars triangularis | −42 | 32 | 0 | 3.50 |
| L | Pars opercularis | −36 | 26 | 10 | 3.44 | ||
| L | Insula | −28 | 26 | 10 | 3.19 | ||
| L | Lateral orbitofrontal cortex | −28 | 28 | 8 | 2.86 | ||
Note. The coordinates are in MNI152 space, mm.
The analysis was corrected, with voxel‐level threshold Z > 2.33, P < 0.01, and cluster‐level threshold P < 0.05. Site, IQ, age, sex, and pretreatment autism symptoms severity were included as covariates of no interest.
L, left; R, right; STS, superior temporal sulcus; VR‐SCT, virtual reality‐social cognition training.
Tracking theory‐of‐mind change with brain change
At the individual level, we correlated changes in behavioral measure and changes in brain activation to BIO > SCR, for theory of mind and emotion recognition, respectively. First, as seen in Fig. 3A, there was a significant positive correlation in a cluster (591 voxels) in the right posterior superior temporal sulcus. The scatterplot illustrates the nature of the relationship, where improvement in theory of mind was associated with increase activation to BIO > SCR in this region, and the estimated effect size was Pearson's |r| = 0.67. There was no surviving region that showed negative correlation between change in theory of mind and change in brain activation. To further interpret the possible functions associated with this cluster, we conducted a NeuroSynth‐based reverse inference analysis. The top 10 NeuroSynth‐decoded feature terms were biological (0.10), multisensory (0.10), sense (0.10), action observation (0.10), tone (0.09), observing (0.08), auditory visual (0.08), attribute (0.07), observation (0.07), and intentions (0.07). This suggests that the cluster is primarily associated with social information processing, and multisensory integration. Table 2 lists the peak significance, peak coordinates, and anatomical locations encompassed by the cluster.
Figure 3.
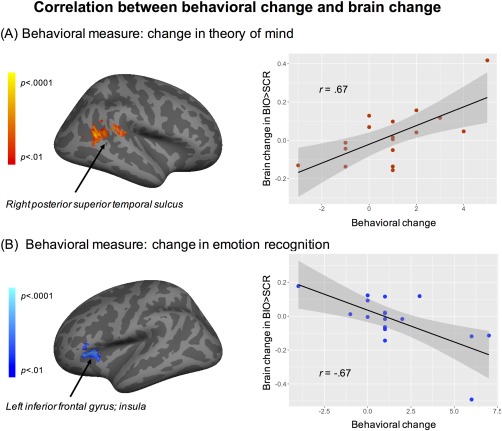
The individual‐level correlation between brain activation change and behavioral change in (A) theory of mind and (B) emotion recognition, after virtual reality‐social cognition training. The brain maps on the left illustrate the region of significant correlation, while the scatterplots on the right illustrate the relationship between behavioral change (x‐axis) and brain change in BIO > SCR (y‐axis). The regression lines and the 95% confidence intervals were also plotted. The analysis was corrected, with voxel‐level threshold Z > 2.33, P < 0.01, and cluster‐level threshold P < 0.05. Site, IQ, age, sex, and pretreatment autism symptoms severity were included as covariates of no interest.
Tracking emotion‐recognition change with brain change
Second, as seen in Fig. 3B, there was a significant negative correlation in a cluster (232 voxels) in the left inferior frontal gyrus/ventrolateral prefrontal cortex. The scatterplot illustrates the nature of the relationship, where improvement in emotion recognition was associated with decrease activation to BIO > SCR in this region, and the estimated effect size was Pearson's |r| = 0.67. There was no surviving region that showed positive correlation between change in emotion recognition and change in brain activation. To further interpret the possible functions associated with this cluster, we conducted a NeuroSynth‐based reverse inference analysis. The top 10 NeuroSynth‐decoded feature terms were competing (0.20), conflicting (0.10), shifting (0.09), verb (0.08), phonological (0.07), semantically (0.07), nouns (0.06), lexical (0.06), words (0.06), and orthographic (0.05). This suggests that the cluster is primarily associated with conflict resolution and language processing. Table 2 lists the peak significance, peak coordinates, and anatomical locations encompassed by the cluster.
Comparison with neural predictive biomarkers of treatment effectiveness
With this same dataset, we had previously identified a set of brain regions in which pretreatment activation predicted treatment effectiveness, particularly on predicting improvement in emotion recognition [Yang et al., 2017b]. Specifically, for emotion‐recognition change, the neural predictive biomarkers (cluster‐corrected) were located to (a) the left posterior superior temporal sulcus, superior temporal gyrus, and middle temporal gyrus (regions implicated in processing verbal emotions), and (b) the right insula, orbitofrontal cortex, and inferior frontal gyrus (regions implicated in processing nonverbal emotions). Importantly, these predictive regions did not overlap with the mechanistic regions for emotion‐recogniton change (cluster‐corrected) as identified in the current study, which were localized to the left inferior frontal gyrus and ventrolateral prefrontal cortices. Nonetheless, across all participants, the pretreatment activation to BIO > SCR in the predictive regions was highly and positively correlated with decreased activation to BIO > SCR in the mechanistic regions, r(15) = 0.82, P < 0.0001, that is, the stronger the activation in predictive regions was at baseline, the greater the activation reduction in mechanistic regions was from baseline to endpoint.
Discussion
Neural Mechanisms of Change
This Phase I pilot trial evaluated behavioral and brain changes in adults with high functioning ASD in response to a virtual reality social cognition training protocol (VR‐SCT) delivered over 5 weeks for a total of 10 hr. Specifically, we measured pre‐ to post‐ changes in the behavioral domains of socio‐emotional (i.e., emotional recognition) and socio‐cognitive (theory of mind as reflected in describing intentionality) and the neural aspects of brain activation comparing treatment response changes to social versus nonsocial motion. In contrast to our previous neuroprediction study [Yang et al., 2017b] that focused on how pretreatment brain activity may be used to forecast behavioral changes, we were particularly interested in the convergence between behavioral and brain changes in the current study. Our preliminary results can be summarized in four key findings:
Group mean brain change after VR‐SCT
First of all, we found the trained adults with ASD as a group showed a significantly decreased brain activation to nonsocial versus social stimuli in the left superior parietal lobule (SPL). This parietal region has been linked to the heightened visual attention to nonsocial features in preference above social aspects [Klin et al., 2009], which has been a pathognomonic and purportedly disabling feature of autism. It has been suggested that individuals with ASD tend to have difficulty in predicting events in the social world and may compensate for this by relying on the nonsocial, predictable contingencies aspects of the world to understand the world [Sinha et al., 2014; Van de Cruys et al., 2014]. Our results suggest that before the treatment, participants might have engaged in significantly more visual attention for the scrambled/nonsocial versus biological/social stimuli; with changes after the treatment showing the adults with ASD attended roughly similarly to both types of visual stimuli. Importantly, VR‐SCT features repeatable social practices in a motivating computer‐based environment that offers a safe place to attempt interaction without the real‐world consequences of failure. Although speculative, it is possible that VR‐SCT had made social events more predictable after treatment, which in turn might have reduced the ASD participants' visual attention to the nonsocial contingencies of the stimuli.
Tracking theory‐of‐mind change with brain change
In addition, our results provide some of the first evidence that brain activation to social versus nonsocial in the right posterior superior temporal sulcus (pSTS) tracks training‐induced behavioral change in theory of mind as measured by the Triangle task in adults with ASD. This region, purportedly, is a hub for socio‐cognitive processing and involved in the temporal integration of visual, auditory and somatosensory cues of other's behaviors [Pelphrey & Morris, 2006; Redcay, 2008; Yang et al., 2015]. Specifically, temporal integration is about the ability to construct and integrate multi‐dimensional information over time into a coherent whole so that one can understand and predict the happening of events over time (e.g., the ability to understand and predict what people will do next when they walk toward you and wave at you) [Kilner et al., 2007; Koster‐Hale & Saxe, 2013; Nakano et al., 2010; Stevenson et al., 2014; Yang et al., 2015]. In our study, it would require the ability of temporal integration to understand the interactions of the objects in the depicted scenarios in the Triangle task. The brain‐behavior relationship identified in this particular brain region also raises the possibility that researchers may test whether concurrent intervention procedures in this brain region may serve to facilitate even higher levels of performance on the theory of mind task. For example, oxytocin [Domes et al., 2014] has been found to be able to increase the function of the right pSTS region in adults with autism. This possibility to combine two or more treatment to enhance treatment effectiveness warrants testing in future studies.
Tracking emotion‐recognition change with brain change
Furthermore, our results showed that decreased brain activation to social versus nonsocial in the left inferior frontal gyrus (IFG) region tracked gains in socio‐emotional processing of identifying emotions. It has been reported that there is a right‐ versus left‐hemisphere advantageous difference in face recognition and processing emotional stimuli [Adolphs, 2002; Adolphs et al., 2000; Ahern et al., 1991; Blonder et al., 1991; Buchanan et al., 2000; Ley & Bryden, 1979; Morris et al., 1999; Rama et al., 2001]. Although speculative, it is possible that to successfully process emotional stimuli, one needs to shift from left‐hemisphere processing to right‐hemisphere processing, and decreasing the use of implicated regions on the left hemisphere may contribute to better emotion recognition. Our result is consistent with this possibility. Anecdotally, some VR‐SCT participants stated that prior to training, they had a constant “inner self‐dialogue” preventing them from focusing on the intent of what was being said in the conversation. As part of the training, participants were instructed to utilize strategies to focus on the social cues and implications of other's words and use that information to generate a follow‐up response. It is plausible that VR‐SCT helps facilitate a shift away from a language‐centered approach toward a compensatory cognitive strategy to increase attention to social information. Future research may examine whether a left‐to‐right processing shift takes place as a result of social training.
Comparison with neural predictive biomarkers of treatment effectiveness
Finally, adding to our prior findings, the current results identified distinctly different sets of brain regions as the neural mechanistic biomarkers associated with the emotion‐recognition change (left IFG/vlPFC), than those previously reported as the neural predictive biomarker brain region (left temporal regions; right IFG, OFC, and insula) [Yang et al., 2017b]. This pattern of results suggest that the two functions—that is, predicting who may be primed to respond more advantageously from the treatment versus mediating how the treatment works—may be localized to separate regions. Interestingly, we found that the stronger the baseline activation was in the predictive biomarker regions before treatment, the greater the reduction in activation was from treatment baseline to endpoint in the mechanistic biomarker regions. Although speculative, this relation between the predictive and mechanistic biomarker brain regions supports the possibility that these regions may be functionally connected in the context of behavioral changes. We offer one interpretation proposing that stronger pretreatment brain responses in the predictive regions may facilitate the participants to engage in a left‐to‐right hemisphere shift in the IFG region when they were trained to better process emotions. This and other possible explanations need further investigation.
Clinical Implications
Overall, the present findings support a potential for virtual reality social scenario training to harness the socio‐emotional and socio‐cognitive capacity and neuroplasticity in adults with longstanding diagnosis of ASD. The neurobiological mechanistic markers identified in this research may have a number of clinically relevant research implications. First, they help advance our scientific understanding of how the treatment works to improve supporting brain systems, perhaps suggesting that interventionists could focus on the related psychological functions to refine the treatment, for example, the temporal integration function implicated in the right pSTS region for training theory of mind. Second, the neural mechanisms identified in this study potentially may serve as a possible target to monitor and evaluate the purported mechanisms of the treatment, for example, increased reliance on the right pSTS for change in theory of mind. However, such an approach might depend on clinicians' ability to gather the data using imaging methods relatively inexpensive, such as functional near‐infrared spectroscopy. Third, the social brain regions provide a possible target for concurrent interventions (e.g., oxytocin) to boost the effectiveness of the primary treatment. Overall, the pattern of findings from this Phase I pilot trial offers promising directions for follow‐up randomized control trials research and open new avenues for treatment development in adults with high functioning ASD, a heretofore relatively neglected area of focus, despite immense potential for improvement.
Limitations
There are several limitations to consider regarding this research. First, the findings were limited to one single treatment‐only group in a pretest‐posttest design. Future work should conduct randomized controlled trials to further establish these findings. Second, the size of our preliminary sample (N = 17) was relatively small. A larger sample would allow possible stratification of the sample, which is important given the heterogeneity of autism. Third, the primary clinical outcomes are limited to changes in emotion recognition and theory of mind, respectively. Although these two abilities are among the most basic abilities in socio‐emotional and socio‐cognitive processing [Baron‐Cohen et al., 1985; Gallese et al., 2004] that underlie a wide range of social skills and are also central to our understanding of ASD deficits [Uljarevic & Hamilton, 2013], there is a need for future research to include other ASD‐related measures, such as interaction behaviors [Rice & Redcay, 2016] and conversation skills [Scattone, 2008].
Fourth, for the purpose of this study, we implemented 5‐week intervention [Kandalaft et al., 2013] and measured behavioral and brain changes only during this period. However, there is a need for future studies to vary the duration of intervention and test how well the behavioral and brain changes via the intervention may be maintained and generalized after intervention. It is possible that longer intervention time and/or a maintenance plan (e.g., a brief course every 3 months after the end of intervention) could lead to more lasting and broadly generalized changes after the posttreatment endpoint. Fifth, prior research showed that males and females with ASD tend to differ in their severity and ASD‐related phenotypic presentation [Werling & Geschwind, 2013]. Although we controlled for symptom severity and gender, our sample is predominantly male (15 out of 17). Future research should recruit equal and larger number of participants in both gender groups to address possible gender‐specific neural mechanisms. Sixth, all participants were high‐functioning (IQ range = 88–131) and it remains unclear whether the findings may apply to lower‐functioning individuals. Importantly, the VR‐SCT has an inclusion criterion of IQ ≥ 80 and future research needs to investigate other treatment approaches for those who may be cognitively impaired. Finally, the results are limited to young adults with ASD and it is unclear how they are compared to treatment work in children with ASD. In a related project, we have collected neuroimaging data from young children with ASD who received Pivotal Response Treatment. We expect to compare the results to the current work in a separate paper.
Conclusions
Despite the limitations, our study is the first to advance the knowledge of neural mechanisms of response to treatment in adults with autism receiving VR‐SCT. These findings extend the window of critical time periods where individuals with ASD may be able to benefit from even short term (10 hr) of intervention focused on commonly encountered social exchanges during young adulthood such as interviewing for a job, asking someone for a date or negotiating with a friend when disagreements occur. Such interventions may not only improve social cognition skills at a critical stage when adults with ASD are needing to develop social skills that support independence, but also strengthen the underlying brain networks to support higher social functioning capacity. There is currently limited intervention research in adults with ASD. This study moves the field one step closer to the goal of providing scientifically‐based precise intervention for individuals with ASD into adulthood.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting FigureS1
Supporting FigureS2
Acknowledgments
We thank the participants and their families included in this study for their time and participation and the research assistants in our research centers, making this research possible. This work was supported by the Harris Professorship at Yale Child Study Center to KAP, Autism Speaks Meixner Postdoctoral Fellowship in Translational Research (#9284) to DY, a gift from the Autism Society–Northwestern Pennsylvania to DY, and the Yale Center for Research Computing for guidance and use of the research computing infrastructure (NIH grants RR19895 and RR029676‐01). Additionally, we thank the Rees‐Jones Foundation, Vin and Caren Prothro Foundation, and the Crystal Charity Ball for their generous support of Center for BrainHealth's research. We also thank Jeffrey Spence for feedback with statistical analyses.
References
- Abell, F. , Happe, F. , & Frith, U. (2000). Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cognitive Development, 15, 1–16. [Google Scholar]
- Adolphs, R. (2002). Neural systems for recognizing emotion. Current Opinion Neurobiology, 12, 169–177. [DOI] [PubMed] [Google Scholar]
- Adolphs, R. , Damasio, H. , Tranel, D. , Cooper, G. , & Damasio, A.R. (2000). A role for somatosensory cortices in the visual recognition of emotion as revealed by three‐dimensional lesion mapping. Journal of Neuroscience, 20, 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern, G.L. , Schomer, D.L. , Kleefield, J. , Blume, H. , Cosgrove, G.R. , Weintraub, S. , & Mesulam, M.M. (1991). Right hemisphere advantage for evaluating emotional facial expressions. Cortex, 27, 193–202. [DOI] [PubMed] [Google Scholar]
- Allison, T. , Puce, A. , & McCarthy, G. (2000). Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences, 4, 267–278. [DOI] [PubMed] [Google Scholar]
- Anagnostou, E. , & Taylor, M.J. (2011). Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Molecular Autism, 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA , 2013. Diagnostic and statistical manual of mental disorders: DSM‐5, 5th ed American Psychiatric Publishing, Washington, D.C. [Google Scholar]
- Baron‐Cohen, S. , Leslie, A.M. , & Frith, U. (1985). Does the autistic child have a “theory of mind”?. Cognition, 21, 37–46. [DOI] [PubMed] [Google Scholar]
- Bellani, M. , Fornasari, L. , Chittaro, L. , & Brambilla, P. (2011). Virtual reality in autism: state of the art. Epidemiology Psychiatry Sciences, 20, 235–238. [DOI] [PubMed] [Google Scholar]
- Bishop‐Fitzpatrick, L. , Minshew, N.J. , & Eack, S.M. (2013). A systematic review of psychosocial interventions for adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43, 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter, M. , Wang, N. , Pelphrey, K. , & Kaiser, M.D. (2016). Evaluation of quantified social perception circuit activity as a neurobiological marker of autism spectrum disorder. JAMA Psychiatry, 73, 614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell, L.S. , Trzesniewski, K.H. , & Dweck, C.S. (2007). Implicit theories of intelligence predict achievement across an adolescent transition: A longitudinal study and an intervention. Child Development, 78, 246–263. [DOI] [PubMed] [Google Scholar]
- Blonder, L.X. , Bowers, D. , & Heilman, K.M. (1991). The role of the right hemisphere in emotional communication. Brain, 114, 1115–1127. [DOI] [PubMed] [Google Scholar]
- Brugha, T.S. , Doos, L. , Tempier, A. , Einfeld, S. , & Howlin, P. (2015). Outcome measures in intervention trials for adults with autism spectrum disorders; A systematic review of assessments of core autism features and associated emotional and behavioural problems. International Journal of Methods in Psychiatric Research, 24, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan, T.W. , Lutz, K. , Mirzazade, S. , Specht, K. , Shah, N.J. , Zilles, K. , & Jancke, L. (2000). Recognition of emotional prosody and verbal components of spoken language: An fMRI study. Brain Research Cognitive Brain Research, 9, 227–238. [DOI] [PubMed] [Google Scholar]
- Carrington, S.J. , & Bailey, A.J. (2009). Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum Brain Mapping, 30, 2313–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J.N. 2012. Social responsiveness scale (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Deen, B. , Koldewyn, K. , Kanwisher, N. , & Saxe, R. (2015). Functional organization of social perception and cognition in the superior temporal sulcus. Cerebral Cortex, 25, 4596–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R.S. , Segonne, F. , Fischl, B. , Quinn, B.T. , Dickerson, B.C. , Blacker, D. , … Killiany, R.J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Didehbani, N. , Allen, T. , Kandalaft, M. , Krawczyk, D. , & Chapman, S. (2016). Virtual reality social cognition training for children with high functioning autism. Computers in Human Behavior, 62, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes, G. , Kumbier, E. , Heinrichs, M. , & Herpertz, S.C. (2014). Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with asperger syndrome. Neuropsychopharmacology, 39, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Buchel, C. , & Gross, J.J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Gallese, V. , Keysers, C. , & Rizzolatti, G. (2004). A unifying view of the basis of social cognition. Trends in Cognitive Sciences, 8, 396–403. [DOI] [PubMed] [Google Scholar]
- Gotham, K. , Risi, S. , Pickles, A. , & Lord, C. (2007). The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37, 613–627. [DOI] [PubMed] [Google Scholar]
- Harms, M.B. , Martin, A. , & Wallace, G.L. (2010). Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychology Reviews, 20, 290–322. [DOI] [PubMed] [Google Scholar]
- Heberlein, A.S. , & Adolphs, R. (2004). Impaired spontaneous anthropomorphizing despite intact perception and social knowledge. Proceedings of the National Academy of Sciences of the United States of America, 101, 7487–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley, D. , Uljarevic, M. , Cameron, L. , Halder, S. , Richdale, A. , & Dissanayake, C. (2016). Employment programmes and interventions targeting adults with autism spectrum disorder: A systematic review of the literature. Autism, 21, 929–941. [DOI] [PubMed] [Google Scholar]
- Heider, F. , & Simmel, M. (1944). An experimental study of apparent behavior. American Journal of Psychology, 57, 243–259. [Google Scholar]
- Howlin, P. , & Moss, P. (2012). Adults with autism spectrum disorders. Canadian Journal of Psychiatry, 57, 275–283. [DOI] [PubMed] [Google Scholar]
- Hu, Z. , Chan, R.C. , & McAlonan, G.M. (2010). Maturation of social attribution skills in typically developing children: An investigation using the social attribution task. Behavioral and Brain Functions, 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus, V. , & Lord, C. (2014). The autism diagnostic observation schedule, module 4: Revised algorithm and standardized severity scores. Journal of Autism and Developmental Disorders, 44, 1996–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansso, G. (1973). Visual‐perception of biological motion and a model for its analysis. Perception & Psychophysics, 14, 201–211. [Google Scholar]
- Johnson, M.H. (2006). Biological motion: A perceptual life detector?. Current Biology, 16, R376–R377. [DOI] [PubMed] [Google Scholar]
- Kaiser, M.D. , Hudac, C.M. , Shultz, S. , Lee, S.M. , Cheung, C. , Berken, A.M. , … Pelphrey, K.A. (2010). Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America, 107, 21223–21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, M.D. , Yang, D.Y. , Voos, A.C. , Bennett, R.H. , Gordon, I. , Pretzsch, C. , … Pelphrey, K.A. (2015). Brain mechanisms for processing affective (and nonaffective) touch are atypical in autism. Cereb Cortex, 26, 2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalaft, M.R. , Didehbani, N. , Cullum, C.M. , Krawczyk, D.C. , Allen, T.T. , Tamminga, C.A. , & Chapman, S.B. (2012). The Wechsler ACS social perception subtest: A preliminary comparison with other measures of social cognition. Journal of Psychoeducational Assessment, 30, 455–465. [Google Scholar]
- Kandalaft, M.R. , Didehbani, N. , Krawczyk, D.C. , Allen, T.T. , & Chapman, S.B. (2013). Virtual reality social cognition training for young adults with high‐functioning autism. Journal of Autism and Developmental Disorders, 43, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske, P. , Heissler, J. , Schonfelder, S. , Bongers, A. , & Wessa, M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21, 1379–1388. [DOI] [PubMed] [Google Scholar]
- Kilner, J.M. , Friston, K.J. , & Frith, C.D. (2007). Predictive coding: an account of the mirror neuron system. Cognitive Processes, 8, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin, A. , Lin, D.J. , Gorrindo, P. , Ramsay, G. , & Jones, W. (2009). Two‐year‐olds with autism orient to non‐social contingencies rather than biological motion. Nature, 459, 257–U142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster‐Hale, J. , & Saxe, R. (2013). Theory of mind: A neural prediction problem. Neuron, 79, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t‐tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley, R.G. , & Bryden, M.P. (1979). Hemispheric differences in processing emotions and faces. Brain and Language, 7, 127–138. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Risi, S. , Lambrecht, L. , Cook, E.H., Jr. , Leventhal, B.L. , DiLavore, P.C. , Pickles, A. , & Rutter, M. (2000). The autism diagnostic observation schedule‐generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Maskey, M. , Lowry, J. , Rodgers, J. , McConachie, H. , & Parr, J.R. (2014). Reducing specific phobia/fear in young people with autism spectrum disorders (ASDs) through a virtual reality environment intervention. PLoS One, 9, e100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgraw, K.O. , & Wong, S.P. (1992). A common language effect size statistic. Psychological Bulletin, 111, 361–365. [Google Scholar]
- McKay, L.S. , Simmons, D.R. , McAleer, P. , Marjoram, D. , Piggot, J. , & Pollick, F.E. (2012). Do distinct atypical cortical networks process biological motion information in adults with autism spectrum disorders?. Neuroimage, 59, 1524–1533. [DOI] [PubMed] [Google Scholar]
- Morris, J.S. , Scott, S.K. , & Dolan, R.J. (1999). Saying it with feeling: Neural responses to emotional vocalizations. Neuropsychologia, 37, 1155–1163. [DOI] [PubMed] [Google Scholar]
- Nakano, T. , Ota, H. , Kato, N. , & Kitazawa, S. (2010). Deficit in visual temporal integration in autism spectrum disorders. Proceedings Biological Sciences, 277, 1027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, S. , & Mitchell, P. (2002). The potential of virtual reality in social skills training for people with autistic spectrum disorders. Journal of Intellectual Disability Research, 46, 430–443. [DOI] [PubMed] [Google Scholar]
- Pearson , 2009. Advanced clinical soultions (ACS) for the WAIS‐IV and WMS‐IV. San Antonio, TX: Pearson. [Google Scholar]
- Pelphrey, K.A. , & Morris, J.P. (2006). Brain mechanisms for interpreting the actions of others from biological‐motion cues. Current Directions in Psychological Science, 15, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, E.A. , & LeDoux, J.E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175–187. [DOI] [PubMed] [Google Scholar]
- Rama, P. , Martinkauppi, S. , Linnankoski, I. , Koivisto, J. , Aronen, H.J. , & Carlson, S. (2001). Working memory of identification of emotional vocal expressions: An fMRI study. Neuroimage, 13, 1090–1101. [DOI] [PubMed] [Google Scholar]
- Redcay, E. (2008). The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neuroscience and Biobehavioral Reviews, 32, 123–142. [DOI] [PubMed] [Google Scholar]
- Rice, K. , & Redcay, E. (2016). Interaction matters: A perceived social partner alters the neural processing of human speech. Neuroimage, 129, 480–488. [DOI] [PubMed] [Google Scholar]
- Saggar, M. , Shelly, E.W. , Lepage, J.F. , Hoeft, F. , & Reiss, A.L. (2014). Revealing the neural networks associated with processing of natural social interaction and the related effects of actor‐orientation and face‐visibility. Neuroimage, 84, 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattone, D. (2008). Enhancing the conversation skills of a boy with Asperger's disorder through social stories and video modeling. Journal of Autism and Developmental Disorders, 38, 395–400. [DOI] [PubMed] [Google Scholar]
- Simion, F. , Regolin, L. , & Bulf, H. (2008). A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences of the United States of America, 105, 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, P. , Kjelgaard, M.M. , Gandhi, T.K. , Tsourides, K. , Cardinaux, A.L. , Pantazis, D. , Diamond, S.P. , & Held, R.M. (2014). Autism as a disorder of prediction. Proceedings of the National Academy of Sciences of the United States of America, 111, 15220–15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, R.A. , Siemann, J.K. , Schneider, B.C. , Eberly, H.E. , Woynaroski, T.G. , Camarata, S.M. , & Wallace, M.T. (2014). Multisensory temporal integration in autism spectrum disorders. Journal of Neuroscience, 34, 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarevic, M. , & Hamilton, A. (2013). Recognition of emotions in autism: A formal meta‐analysis. Journal of Autism and Developmental Disorders, 43, 1517–1526. [DOI] [PubMed] [Google Scholar]
- Vallortigara, G. , Regolin, L. , & Marconato, F. (2005). Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biology, 3, e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys, S. , Evers, K. , Van der Hallen, R. , Van Eylen, L. , Boets, B. , de‐Wit, L. , & Wagemans, J. (2014). Precise minds in uncertain worlds: Predictive coding in autism. Psychological Review, 121, 649–675. [DOI] [PubMed] [Google Scholar]
- Venkataraman, A. , Yang, D.Y. , Dvornek, N. , Staib, L.H. , Duncan, J.S. , Pelphrey, K.A. , & Ventola, P. (2016). Pivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorder. Neuroreport, 27, 1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola, P. , Yang, D.Y. , Friedman, H.E. , Oosting, D. , Wolf, J. , Sukhodolsky, D.G. , & Pelphrey, K.A. (2015). Heterogeneity of neural mechanisms of response to pivotal response treatment. Brain Imaging Behavior, 9, 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos, A.C. , Pelphrey, K.A. , Tirrell, J. , Bolling, D.Z. , Wyk, B.V. , Kaiser, M.D. , … Ventola, P. (2012). Neural mechanisms of improvements in social motivation after pivotal response treatment: Two case studies. Journal of Autism and Developmental Disorders, 43, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer, A.L. , & Ingersoll, B.R. (2011). The use of innovative computer technology for teaching social communication to individuals with autism spectrum disorders. Research in Autism Spectrum Disorders, 5, 96–107. [Google Scholar]
- Walton, K.M. , & Ingersoll, B.R. (2013). Improving social skills in adolescents and adults with autism and severe to profound intellectual disability: A review of the literature. Journal of Autism and Developmental Disorders, 43, 594–615. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. 1999. Wechsler abbreviated scale of intelligence (WASI). San Antonio, TX: Pearson. [Google Scholar]
- Wechsler, D. 2011. Wechsler abbreviated scale of intelligence—second edition (WASI‐II). San Antonio, TX: Pearson. [Google Scholar]
- Werling, D.M. , & Geschwind, D.H. (2013). Sex differences in autism spectrum disorders. Current Opinion Neurology, 26, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D. , Pelphrey, K.A. , Sukhodolsky, D.G. , Crowley, M.J. , Dayan, E. , Dvornek, N.C. , … Ventola, P. (2016). Brain responses to biological motion predict treatment outcome in young children with autism. Translational Psychiatry, 6, e948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D.Y. , Rosenblau, G. , Keifer, C. , & Pelphrey, K.A. (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience and Biobehavioral Reviews, 51, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y.J. , Sukhodolsky, D.G. , Lei, J. , Dayan, E. , Pelphrey, K.A. , & Ventola, P. (2017a). Distinct neural bases of disruptive behavior and autism symptom severity in boys with autism spectrum disorder. Journal of Neurodevelopmental Disorders, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y.J.D. , Allen, T. , Abdullahi, S.M. , Pelphrey, K.A. , Volkmar, F.R. , & Chapman, S.B. (2017b). Brain responses to biological motion predict treatment outcome in young adults with autism receiving virtual reality social cognition training: Preliminary findings. Behaviour Research and Therapy, 93, 55–66. [DOI] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R.A. , Nichols, T.E. , Van Essen, D.C. , & Wager, T.D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting FigureS1
Supporting FigureS2


