Abstract
The mucin-type O-glycome in cancer aberrantly expresses the truncated glycans Tn (GalNAcα1-Ser/Thr) and STn (Neu5Acα2,6GalNAcα1-Ser/Thr). However, the role of Tn and STn in cancer and other diseases is not well understood. Our recent discovery of the self-binding properties (carbohydrate–carbohydrate interactions, CCIs) of Tn (Tn–Tn) and STn (STn–STn) provides a model for their possible roles in cellular transformation. We also review evidence that Tn and STn are members of a larger family of glycan tumor antigens that possess CCIs, which may participate in oncogenesis.
Keywords: carbohydrate tumor antigens, carbohydrate–carbohydrate interactions, mechanism of action, Neu5Gc-GM3, optical tweezers, Tn and STn cancer antigens
Introduction
The human glycome is altered in many diseases including cancers (Ju et al. 2013). For example, the mucin-type O-glycome expresses the truncated glycans Tn (GalNAcα1-Ser/Thr) and STn (Neu5Acα2,6GalNAcα1-Ser/Thr) in over 80% of carcinomas (Springer 1984; Ju et al. 2008). Indeed, Tn and STn have been tumor markers for colorectal, lung, breast, cervical and gastric carcinomas for over three decades, and the expression level of Tn correlates with the metastatic potential and poor prognosis of patients (cf. (Ju et al. 2008)). Tn and STn are also expressed in other human diseases including the Tn syndrome and IgA nephropathy (Ju et al. 2013). However, the role of Tn and STn in cancer and other diseases is not well understood. In this paper, we review the recent discovery of the self-binding properties of Tn (Tn–Tn) and STn (STn–STn) (Haugstad et al. 2016), and how the binding and cross-linking activities of these two cancer antigens provide a model for their possible roles in cellular transformation and malignancy as well as other diseases. Finally, we discuss a family of carbohydrate tumor antigens that include Tn, STn and Neu5Gc-GM3, which possess carbohydrate–carbohydrate interactions (CCIs) and participate in cancer development.
Carbohydrate–carbohydrate interactions
CCIs have been observed for over 40 years, and have been largely associated with oligosaccharides and polysaccharides. For example, the oligosaccharide chains of specific glycosphingolipids such as GM3 interact with other glycolipid oligosaccharide chains (Gg3) (Kojima and Hakomori 1989), and with the N-glycans of membrane glycoproteins such as EGFR (heterotypic CCI) (Kawashima et al. 2009; Hayashi et al. 2013). This and many other examples led to the definition of the “glycosynapse”, which is a membrane microdomain of glycosphingolipids that was shown to be responsible for carbohydrate-dependent cell adhesion and signaling via transmembrane glycoprotein receptors (Hakomori 2002). This concept is analogous to the “immunological synapse”, which interconnects adhesion and signaling. CCIs are often characterized by lower affinities than carbohydrate–protein (lectin) interactions, but are often multivalent resulting in enhanced avidity (Handa and Hakomori 2012). Zhao et al. (2012) later made important contributions to this field by using fluorescent silica nanoparticles functionalized with carbohydrates in studies of carbohydrate–carbohydrate interactions. By studying the binding of nanoparticles coated with galactosyl (Gal), its 3-sulfo derivative (SGal) or Glc to galactolipids and glycolipids that had been immobilized in a multiwell plate, they revealed that the CCIs between the nanoparticles and the glycolipid is extremely specific for Gal-SGal. However, the number of published quantitative studies of carbohydrate self-interactions is limited. Tromas et al. (2001) studied the self-interaction (homotypic CCI) of the trisaccharide Lewisx determinant (Galβ1,4[Fucα1,3]GlcNAcα) using atomic force microscopy (AFM; Figure 1; Tromas et al. 2001). The AFM force-distance curves revealed multiple interactions, characterized by a bond strength between two Lewisx molecules equal to 20 ± 4 pN. CCIs as observed for the cell adhesion proteoglycans display an average adhesive force of 40 ± 15 pN (Dammer et al. 1995). Our AFM studies on the porcine submaxillary mucins (PSM) decorated with Tn (Tn–PSM) (i.e., trimmed to the Tn structure) shows self-interactions characterized by unbinding forces in the range of 30–50 pN when probed using loading rates below 10 nN/s (Haugstad et al. 2012). Furthermore, the observed bond strength was found to decrease with decreasing force loading rate (Haugstad et al. 2016), in accordance with the dynamic force spectroscopy theory (Evans 1998). These interaction strengths are lower than those reported for specific protein–carbohydrate (103–402 pN) for the SBA–mucin interaction (Sletmoen et al. 2009) and 73–144 pN for the alginate epimerase–alginate interaction (Sletmoen et al. 2004) or protein–protein interactions probed using the same range of loading rates (an overview is provided in Bizzarri and Cannistraro (2010)). In comparison, the strength of single ionic bonds between charged groups in aqueous solution is ~180 pN in physiological ionic strength (Spruijt et al. 2012), hydrogen bonds are reported to be 10 pN (Hoh et al. 1992) while forced dissociation of duplex DNA yields unbinding forces in the range 20–50 pN (Strunz et al. 1999). Using AFM, the average lifetime of the Tn–PSM self-interaction was determined to be 0.6 s (Haugstad et al. 2012). However, both the strength and the lifetime of these interactions are expected to increase significantly due to bond multiplicity.
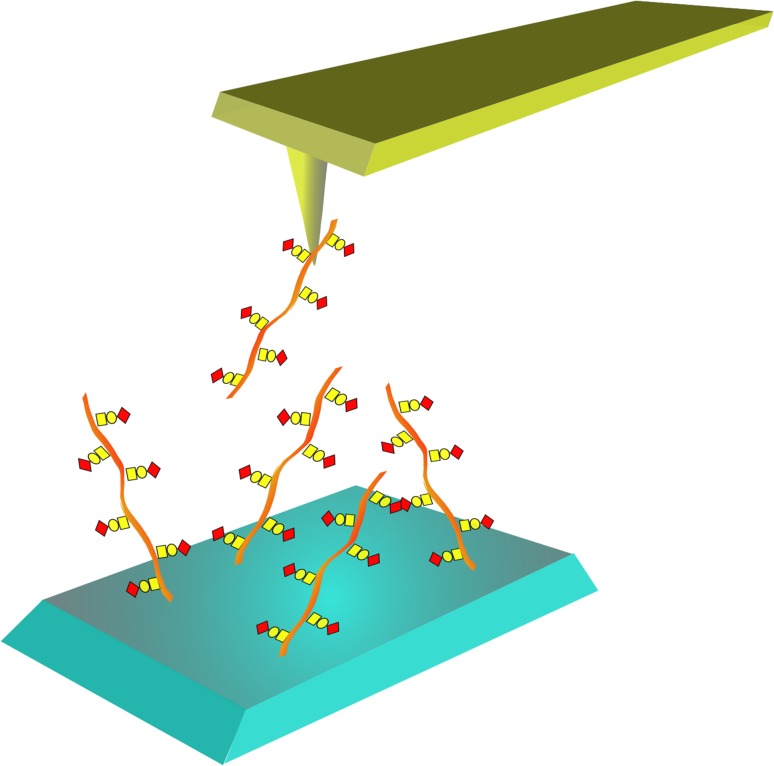
Fig. 1.
Schematic illustration of quantitative determination of carbohydrate–carbohydrate interactions employing AFM. One of the carbohydrates potentially involved in a carbohydrate–carbohydrate interaction is immobilized onto a small tip that is mounted onto the cantilever used in the AFM microscope. The other carbohydrate is immobilized onto a flat surface. The two molecules are brought into contact by, with the use of a piezo scanner, decreasing the distance between the tip and the surface. If the two carbohydrates have formed an interaction, this will be disrupted upon retraction of the tip from the surface. The degree of deflection of the cantilever holding the tip prior to bond rupture will reflect the strength of the interaction.
CCIs have been demonstrated to be involved in cis and trans interactions on cells and in dynamic processes such as adaptive immune responses, cell adhesion and recognition (Handa and Hakomori 2012). However, the scope and role of CCIs in both normal and disease states is not well understood.
Mucins with Tn and STn in cancer
Mucins represent a class of high molecular weight membrane and secreted glycoproteins that contain long heavily O-glycosylated domains, which are commonly composed of tandem repeats. Both membrane bound and secreted mucins are present on the cell surfaces that line body cavities including the respiratory, digestive and urogenital tracts. The mucosal surfaces have a close relationship with innate and adaptive immunity, and in interactions between internal and external environments including the microbiome. Several types of cancers are accompanied by overexpression of aberrantly glycosylated mucins such as the membrane tethered MUC1 (Corfield 2015). Overly expressed MUC1 in colon cancer, for example, is highly substituted with Tn and STn structures, which are believed to be due to changes in glycosyltransferase expression, their relative subcellular localization and even their activity. In addition, the loss of the chaperone COSMC is key to the aberrant overexpression of Tn and STn in some cancers (Ju et al. 2008; Radhakrishnan et al. 2014). This is because COSMC controls the folding and activity of T-synthase (C1GalT-1 or core 1 β1-3galactosyltransferase 1), which is required for the elongation of Tn to longer O-linked oligosaccharides (Aryal et al. 2010; Wang et al. 2010).
Self-binding studies of Tn and STn
Our laboratory recently examined the effects of the Tn antigen on the self-interactions of mucins using AFM (Figure 1) on a group of differently glycosylated PSM ranging from only Tn to the elongated core 1 blood group A antigen. Our results showed enhanced self-binding interactions for PSM possessing the Tn antigen (i.e., Tn–PSM) (Haugstad et al. 2012, 2015). Using optical tweezers (OT) experiments (Figure 2), we recently showed more that the enhanced binding of Tn–PSM is solely due to GalNAc–GalNAc interactions (Haugstad et al. 2016). Indeed, several mucins including the MUC1 human mucin possessing the Tn structure (Tn-MUC1) all showed similar self-binding interactions. Self-interactions were also present in STn decorated mucins, indicating that the α2,6-sialic acid group was not inhibitory to the GalNAc–GalNAc CCI, or that it indeed may also undergo CCIs (see A proposed mechanism of action of Tn and STn involving CCI in cancer). However, the addition of β1-3Gal to the Tn structure to form the T antigen disaccharide (Galβ1-3GalNAcα1-Ser/Thr) or the ST-antigen trisaccharide (Neu5Acα2-3Galβ1-3GalNAcα1-Ser/Thr) failed to show similar self-interactions (Haugstad et al. 2016). Accordingly, self-interactions were observed only after treatment of ST-MUC1 with both neuraminidase and β-galactosidase, which results in a MUC1 possessing only Tn (Haugstad et al. 2016). The GalNAc–GalNAc interactions observed for the Tn- and STn-mucins were also independent of the aglycone scaffold since polyethyleneglycol and polyacrylamide conjugates of Tn (in the α-linked form) showed similar binding strength and energy landscape characteristics. Control experiments with mucins or polymers lacking Tn or STn failed to show any such self-interactions. The GalNAc–GalNAc interactions for mucins and polymers possessing Tn also showed density dependent effects, while experiments performed with the addition of non-interacting ST-MUC1 molecules to dilute out the self-binding molecules allowed the observation of single binding events (Haugstad et al. 2016).
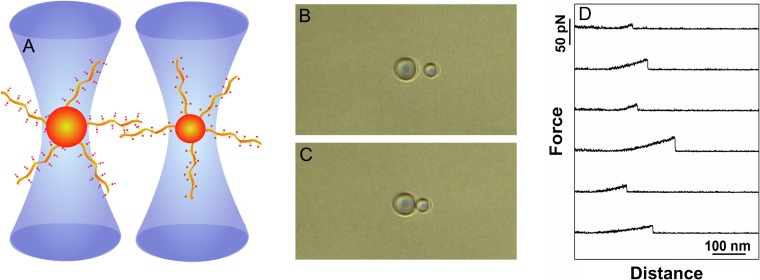
Fig. 2.
Schematic illustration of quantitative determination of carbohydrate–carbohydrate interactions employing dual beam optical tweezer. (A): A polystyrene bead functionalized with glycans or glycosylated molecules (the example depicts mucins of different but well-defined glycosylation pattern) is trapped in each of the two optical traps of the dual beam optical tweezers instrument. During the experiment, the two beads are brought into contact, allowing the molecules immobilized onto the bead surfaces to interact, before being separated. (B) and (C): Optical micrographs of the two optically trapped beads prior to (B) and in contact (C). The net displacements of the polystyrene beads from the center of the calibrated optical trap are continuously recorded and used for quantifying the force acting on the beads. (D): Examples of force-distance curves determined when separating two mucin-functionalized polystyrene beads. The selected force-distance curves reveal force jumps reflecting the rupture of CCIs formed between the immobilized mucin molecules.
Interestingly, polyacrylamide conjugated with Neu5Gc, the sialic acid present in many mammals in which the N-acetyl methyl group in humans (Neu5Ac) is replaced by the -CH2OH group, also showed self-binding interactions, and possessed higher rupture forces than the GalNAc self-binding interactions (Haugstad et al. 2016). Exogenous Neu5Gc incorporation into the tissues of humans via ingestion of red meat such as beef has been associated with inflammation and cancer (Samraj et al. 2015).
A proposed mechanism of action of Tn and STn involving CCI in cancer
The ability of Tn and STn to promote self-interactions may be important for understanding their possible molecular roles for initiating oncogenic pathways leading to many types of cancers. The self-interaction activities of Tn and STn are consistent with the hypothesis that they may be drivers or secondary promoters of carcinogenesis by their aggregation and subsequent activation of heavily O-glycosylated cell surface receptors that may be involved in a range of cellular signaling, including MUC1 and other O-glycosylated receptors. Furthermore, the observed density dependent avidity of Tn and STn on mucins and glycoconjugates (Haugstad et al. 2016) is consistent with their elevated expression levels observed in cancer associated MUC1 found in patients with poor prognosis. Indeed, engineered increased expression of STn in cancer cell lines increases their tumorigenicity (Julien et al. 2001; Ozaki et al. 2012).
Wandall and coworkers (Radhakrishnan et al. 2014) investigated the effects of epigenetic silencing of COSMC, the chaperone for T-synthase, and overexpression of Tn and STn in a pancreatic cell line (T3M4). COSMC knockout cells exhibited enhanced invasive properties in culture, and enhanced growth and invasion as xenografts. These results show that truncation of O-glycans to give Tn and STn enhances malignant and metastatic tumor behavior in pancreatic cell line T3M4. Several signaling pathways regulating cellular homeostasis and oncogensis were identified, including adhesion and signaling molecules, which indicate that truncation and expression of O-glycans on cancer cells affect multiple pathways simultaneously to enhance tumor growth. However, a system-wide mechanism linking the presence of Tn and STn structures to the signaling pathways promoting tumor growth has not been suggested.
We suggest that such a system-wide mechanism may involve density dependent glycosylation of specific cell surface glycoproteins and receptors, such as MUC1, by Tn and STn, which via their CCIs induces hetero-/homotypic oligomerization and/or cross-linking. This could lead to the activation of multiple pathways associated with enhanced tumor growth and metastases. Importantly, the overexpression effects of Tn and STn in the pancreatic cancer cell line observed by Wandall and coworkers were fully reversible by re-expression of COSMC (Radhakrishnan et al. 2014), which suggest a possible therapeutic mechanism for reversing cancers driven by COSMC silencing and Tn and STn overexpression.
Binding of Tn and STn glycosylated proteins to the C-type macrophage galactose-binding lectin (MGL), a Gal/GalNAc specific lectin, may also play a role in cancer. The role of lectins on immune cells is thought to be in the recognition of pathogens, however, it is now clear that these glycan-binding proteins can recognize self-antigens. However, the engagement of MGL in the absence of a danger signal, for example, the triggering of a Toll receptor can lead to anergy (Beatson et al. 2015; van Vliet et al. 2006).
Tn and STn are members of a family of glycan tumor antigens possessing carbohydrate–carbohydrate interactions
The observations that Tn and STn show self-binding activities (homotypic CCI) that may be involved in oncogenesis appears to represent two members of a larger family of glycan tumor antigens that possess CCIs. Recently, Gildersleeve and coworkers reported that whole-cell cancer vaccines administered to human patients induce large antibody responses to carbohydrate and glycoprotein antigens (Xia et al. 2016). Using GVAX Pancreas (a granulocyte macrophage colony-stimulating factor-modified whole-cell tumor vaccine), they showed that the pancreatic cancer vaccine induces large immunoglobulin G and M responses in human patients including responses to tumor-associated carbohydrates and blood group antigens, many of which have been seen in other pancreatic cancer screens (Remmers et al. 2013). Table I list the glycan tumor and blood group antigens and their structures found in the Gildersleeve study. The largest and most frequent IgG responses to known tumor-associated antigens were directed toward the blood group determinant sialyl Lewisx, the Neu5Gc variant of the glycolipid GM3 and STn. The largest and most frequent IgM responses were directed toward the blood group determinant Lewisy. Large but infrequent IgG and IgM responses were also observed for glycopeptides from the cell surface tethered MUC4 carrying the T antigen as well as glycopeptides from MUC1 carrying the Tn antigen. In addition, the glycolipid GD3, the Neu5Gc variant of sialyl α2,6LacNAc and the Neu5Gc variant of sialyl α2,3LacNAc also induced antibody responses. Interestingly, many of these carbohydrate tumor and blood group epitopes have been shown to undergo CCIs (Table I).
Table I.
Endogenous and exogenous glycan tumor and blood group antigens with known or potential carbohydrate–carbohydrate interactions. The list is derived from the large IgM and IgG carbohydrate antibody responses observed upon vaccination of human patients using GVAX pancreas (a granulocyte macrophage colony-stimulating factor-modified whole-cell tumor vaccine) (Xia et al. 2016)
| Glycan tumor antigens | Structure | Graphical structure representation | CCI |
|---|---|---|---|
| Endogenous glycans | |||
| Tn | GalNAcα1-Ser/Thr |  |
Yes |
| STn | Neu5Acα2,6GalNAcα1-Ser/Thr | 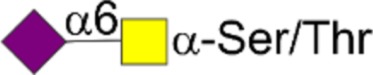 |
Yes |
| Lewisx | Galβ1-4[Fucα1-3]GlcNAcβ1-3Galβ1-R | 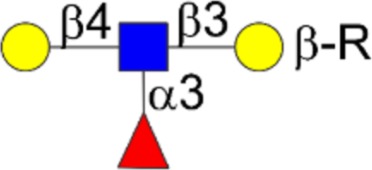 |
Yes |
| Sialyl Lewisx | Neu5Acα2-3Galβ1-4[Fucα1-3]GlcNAcβ1-3Galβ1-R | 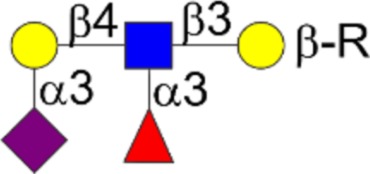 |
Not known |
| Lewisy | Fucα1-2Galβ1-4[Fucα1-3]GlcNAcβ1-3βGalβ1-R | 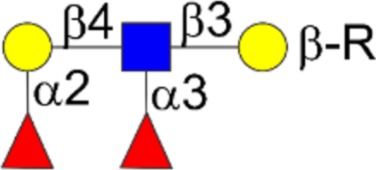 |
Yes |
| GD3 | Neu5Acα2-8Neu5Acα2-3Galβ1-4Glcβ1-Cer | 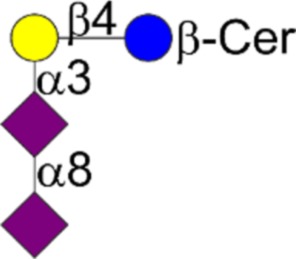 |
Not known |
| T | Galβ1-3GalNAcα1-Ser/Thr |  |
No |
| Exogenous glycans | |||
| Neu5Gc variant of GM3 | Neu5Gcα2-3Galβ1-4Glcβ1-Cer | 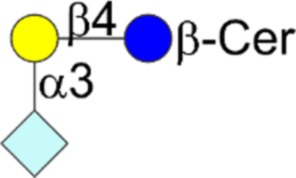 |
Highly likely |
| Neu5Gc variant sialyl α2,6LacNAc | Neu5Gcα2-6Galβ1-4GlcNAcβ1-R | 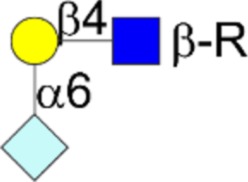 |
Likely |
| Neu5Gc variant sialyl α2,3LacNAc | Neu5Gcα2-3Galβ1-4GlcNAcβ1-R | 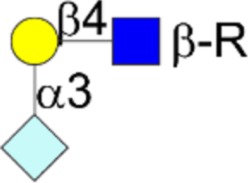 |
Likely |
Endogenous glycan tumor antigens in Table I
The list of endogenous glycan tumor antigens shown in Table I includes Tn and STn, which have been recently demonstrated by us to undergo homotypic CCIs (Haugstad et al. 2016). Both Tn and STn are known to be early pan-carcinoma markers including in pancreatic cancer (Remmers et al. 2013). Tn and STn glycosylated proteins have been shown to bind to the C-type lectin MGL, a Gal/GalNAc specific lectin, which also may also play a role in oncogenesis (Beatson et al. 2015; van Vliet et al. 2006).
Lewisx in Table I has been demonstrated by Hakomori and coworkers (cf. (Handa and Hakomori 2012)) to undergo homotypic CCI and has been shown to be involved in embryonic compaction in the mouse, and autoaggregation of mouse embryonal carcinoma F9 cells. It is also an embryonic stage antigen in the mouse (Fenderson et al. 1990).
We are unaware of any CCI data for sialyl Lewisx shown in Table I. On the other hand, sialyl Lewisx is a known ligand for E-selectin, and their interactions are associated with promotion of tumor cell invasion and metastasis (Ono and Hakomori 2003). In addition, sialyl Lewisx with neighboring sulfated tyrosine residues on the mucin PSGL-1 is a ligand for the P-, L- and E-selectins that are involved in leukocyte, endothelial cells and platelet adhesion interactions (Cummings and McEver 2017).
Lewisy in Table I, which is similar in structure to Lewisx, has been shown to undergo heterotypic CCI with a blood group H glycosphingolipid (Hakomori, 2004). Lewisy, like Lewisx, is also an embryonic stage antigen (Fenderson et al. 1990), and is found in a variety of tumors including pancreatic cancer cells (Remmers et al. 2013).
The disialoganglioside GD3 is similar in structure to GM3 in Table I, but with an extra Neu5Ac moiety attached to the glycan. We are unaware of any CCI data for GD3, but it is a ligand for siglec7 (Nicoll et al. 2003). Hakomori and coworkers have suggested that the interaction of GD3 with siglec7 may play a role in the metastatic potential of renal cell carcinoma, particularly in the lung (Ito et al. 2001).
The T antigen is also found as an endogenous tumor glycan (Table I). However, we recently showed that the T antigen does not undergo homotypic CCI (Haugstad et al. 2016). However, it is a ligand for certain lectins such as galectin-3 (Zhao et al. 2009). Indeed, the role of some of the CCIs above may have in common certain lectin (galectin) lattices, which have been shown to regulate the diffusion and turnover of cell surface receptors, the dynamics and turnover of the formation of the immune synapse and the formation of focal adhesions (Dennis 2015). Thus, four of the seven endogenous tumor glycans in Table I are known to undergo CCI.
Exogenous glycan tumor antigens in Table I
Neu5Gc, the sialic acid analog found in red meat from a variety of mammals but not humans, is present in several of the exogenous tumor glycans as shown in Table I. Importantly, Neu5Gc has been recently shown by us to undergo homotypic CCI (Haugstad et al. 2016). Interestingly, the Neu5Gc variant of GM3 is present in Table I. Neu5Ac-GM3, which is present in humans, has been reported to be involved in heterotypic CCIs with other gangliosides such as Gg3, and in regulating the activity of EGFR via its N-linked glycans in cancer (Kawashima et al. 2009; Hayashi et al. 2013). The Neu5Gc variant of GM3 is detected in a variety of human cancers including colon carcinomas, breast cancers (Hakomori and Handa 2015) and pancreatic cancer (Xia et al. 2016). The Neu5Gc variant of GM3 is also reported to be less effective than Neu5Ac-GM3 in inhibiting the in vitro EFG-induced EGFR phosphorylation in A431 human epidermoid carcinoma cells (Hayashi et al. 2013). Interestingly, de-N-acetyl-GM3 (NeuNH2), enhances EGFR kinase activity (Hanai et al. 1988).
Importantly, the Neu5Gc variant of GM3 is likely to undergo CCI as reported for Neu5Ac-GM3 (Kojima and Hakomori 1989; Hakomori and Handa 2015). However, the interaction of Neu5Gc-GM3 with EGFR appears different from that of Neu5Ac-GM3 (Casadesus et al. 2013; Hayashi et al. 2013), which may contribute to the formers greater oncogenic properties and its presence in other tumors. Thus, the role of the Neu5Gc moiety in Neu5Gc-GM3 appears important in its role in oncogenesis.
Neu5Gc is also incorporated into the structures of sialyl α2,6LacNAc and sialyl α2,3LacNAc shown in Table I. Due to the presence of Neu5Gc in these glycans, they are also likely to undergo CCIs. This suggests that the CCIs of the three exogenous Neu5Gc modified tumor glycans in Table I may contribute to the increased cancer and elevated inflammatory response associated with the Neu5Gc epitope in engineered mice as observed by Varki and coworkers (Samraj et al. 2015).
Importantly, the list of glycan tumor antigens or markers in Table I is not exhaustive of the literature (Stowell et al. 2015). For example, sialyl Lewisa, another Lewis blood group determinant, is a well-known gastrointestinal and pancreatic tumor marker (Stowell et al. 2015).
Conclusions
Our analysis indicates that 7 of the 10 glycan tumor antigens in Table I either exhibit or are likely to exhibit CCIs. Of the seven endogenous glycan tumor antigens, two are O-linked glycans (Tn and STn), and two are Lewis blood group determinants that may be N- or O-linked or glycolipid (Lewisx, Lewisy). Of the three exogenous glycan tumor antigens, one is a glycosphingolipid (Neu5Gc variant of GM3), and the other two (Neu5Gc variant of sialyl α2,6LacNAc and Neu5Gc variant of sialyl α2,3LacNAc) may be associated with N- or O-linked carbohydrate structures. All three exogenous tumor glyans are likely to undergo CCI. Thus, it is possible that the variety of N- and O-glycan, glycolipid and blood group epitopes present in Table I that undergo CCI and lectin mediated interactions may participate in a variety of cellular signaling pathways leading to oncogenic transformation. Furthermore, the presence of both endogenous and exogenous glycan tumor antigens (Table I) raises the question of the possible role(s) of these two types of glycans in cellular transformation.
Importantly, IgG responses by patients to the Neu5Gc variant of GM3 and sialyl Lewisx in Table I showed a trend toward a correlation with survival, and continued evaluation of these responses has been suggested (Xia et al. 2016). Hence, further work is required to investigate the physical and biological roles of these and the other glycan tumor antigens as shown in Table I.
Lastly, the current model for Tn and STn self-binding of glycoconjugates in cancer may also help to explain the roles of these epitopes in other diseases such as the Tn syndrome and IgA nephropathy (Ju et al. 2008).
Abbreviation
- AFM
atomic force microscopy
- OT
optical tweezers
- CCI
carbohydrate–carbohydrate interaction
- Tn
GalNAcα1-Ser/Thr
- STn
Neu5Acα2,6GalNAcα1-Ser/Thr
- PSM
porcine submaxillary mucin.
Funding
This work was supported in part by the National Institutes of Health grants R01CA078834 and U01GM113534 to TAG and MRC grant reference MR/R000026/1 to JB.
Conflict of interest statement
None declared.
References
- Aryal RP, Ju T, Cummings RD. 2010. The endoplasmic reticulum chaperone cosmc directly promotes in vitro folding of T-synthase. J Biol Chem. 285:2456–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatson R, Maurstad G, Picco G, Arulappu A, Coleman J, Wandell HH, Clausen H, Mandel U, Taylor-Papadimitriou J, Sletmoen M et al. 2015. The breast cancer-associated glycoforms of MUC1, MUC1-Tn and sialyl-Tn, are expressed in COSMC wild-type cells and bind the C-Type Lectin MGL. PLoS One. 10:e0125994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarri AR, Cannistraro S. 2010. The application of atomic force spectroscopy to the study of biological complexes undergoing a biorecognition process. Chem Soc Rev. 39:734–749. [DOI] [PubMed] [Google Scholar]
- Casadesus AV, Fernandez-Marrero Y, Clavell M, Gomez JA, Hernandez T, Moreno E, Lopez-Requena A. 2013. A shift from N-glycolyl- to N-acetyl-sialic acid in the GM3 ganglioside impairs tumor development in mouse lymphocytic leukemia cells. Glycoconjugate J. 30:687–699. [DOI] [PubMed] [Google Scholar]
- Corfield AP. 2015. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim Biophys Acta. 1850:236–252. [DOI] [PubMed] [Google Scholar]
- Cummings R, McEver P. 2017. C-type Lectins In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH et al. editors. Essentials of Glycobiology, 3rd ed New York: Cold Springs Harbor Laboratory Press. [PubMed] [Google Scholar]
- Dammer U, Popescu O, Wagner P, Anselmetti D, Güntherodt HJ, Misevic GN. 1995. Binding strength between cell adhesion proteoglycans measured by atomic force microscopy. Science. 26:1173–1175. [DOI] [PubMed] [Google Scholar]
- Dennis JW. 2015. Many light touches convey the message. Trends Biochem Sci. 40:673–686. [DOI] [PubMed] [Google Scholar]
- Evans E. 1998. Energy landscapes of biomolecular adhesion and receptor anchoring at interfaces explored with dynamic force spectroscopy. Faraday Discuss. 111:1–16. [DOI] [PubMed] [Google Scholar]
- Fenderson BA, Eddy EM, Hakomori S. 1990. Glycoconjugate expression during embryogenesis and its biological significance. BioEssays. 12:173–179. [DOI] [PubMed] [Google Scholar]
- Hakomori SI. 2002. The glycosynapse. Proc Natl Acad Sci USA. 99:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. 2004. Carbohydrate-to-carbohydrate interaction in basic cell biology: a brief overview. Arch Biochem Biophys. 426:173–181. [DOI] [PubMed] [Google Scholar]
- Hakomori S-I, Handa K. 2015. GM3 and cancer. Glycoconjugate J. 32:1–8. [DOI] [PubMed] [Google Scholar]
- Hanai N, Dohi T, Nores GA, Hakomori S. 1988. A novel ganglioside, de-N-acetyl-GM3 (II3-NeuNH2LacCer), acting as a strong promotor for epidermal growth-factor receptor kinase and as stimulator for cell-growth. J Biol Chem. 263:6296–6301. [PubMed] [Google Scholar]
- Handa K, Hakomori S-i. 2012. Carbohydrate to carbohydrate interaction in development process and cancer progression. Glycoconjugate J. 29:627–637. [DOI] [PubMed] [Google Scholar]
- Haugstad KE, Gerken TA, Stokke BT, Dam TK, Brewer CF, Sletmoen M. 2012. Enhanced self-association of mucins possessing the T and Tn carbohydrate cancer antigens at the single-molecule level. Biomacromolecules. 13:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugstad KE, Hadjialirezaei S, Stokke BT, Brewer CF, Gerken TA, Burchell J, Picco G, Sletmoen M. 2016. Interactions of mucins with the Tn or Sialyl Tn cancer antigens including MUC1 are due to GalNAc-GalNAc interactions. Glycobiology. 26:1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugstad KE, Stokke BT, Brewer CF, Gerken TA, Sletmoen M. 2015. Single molecule study of heterotypic interactions between mucins possessing the Tn cancer antigen. Glycobiology. 25:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Chiba H, Kuronuma K, Go S, Hasegawa Y, Takahashi M, Gasa S, Watanabe A, Hasegawa T, Kuroki Y et al. 2013. Detection of N-glycolyated gangliosides in non-small-cell lung cancer using GMR8 monoclonal antibody. Cancer Sci. 104:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh JH, Cleveland JP, Prater CB, Revel JP, Hansma PK. 1992. Quantized adhesion detected with the atomic force microscope. J Am Chem Soc. 114:4917–4918. [Google Scholar]
- Ito A, Handa K, Withers DA, Satoh M, Hakomori S. 2001. Binding specificity of siglec7 to disialogangliosides of renal cell carcinoma: possible role of disialogangliosides in tumor progression. FEBS Lett. 498:116–120. [DOI] [PubMed] [Google Scholar]
- Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE et al. 2008. Human tumor antigens to and sialyl Tn arise from mutations in Cosmc. Cancer Res. 68:1636–1646. [DOI] [PubMed] [Google Scholar]
- Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X, Kudelka MR, Cutler C, Zeng J, Wang J, Sun X et al. 2013. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteom Clin Appl. 7:618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Krzewinski-Recchi MA, Harduin-Lepers A, Gouyer V, Huet G, Le Bourhis X, Delannoy P. 2001. Expression of Sialyl-Tn antigen in breast cancer cells transfected with the human CMP-Neu5Ac: GalNAc alpha 2,6-sialyltransferase (ST6GalNAc 1) cDNA. Glycoconjugate J. 18:883–893. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Yoon SJ, Itoh K, Nakayama K. 2009. Tyrosine kinase activity of epidermal growth factor receptor is regulated by GM3 binding through carbohydrate to carbohydrate interactions. J Biol Chem. 284:6147–6155. [DOI] [PubMed] [Google Scholar]
- Kojima N, Hakomori S. 1989. Specific interaction between gangliotriaosylceramide (Gg3) and sialosyllactosylceramide (GM3) as a basis for specific cellular recognition between lymphoma and melanoma cells. J Biol Chem. 264:20159–20162. [PubMed] [Google Scholar]
- Nicoll G, Avril T, Lock K, Furukawa K, Bovin N, Crocker PR. 2003. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur J Immunol. 33:1642–1648. [DOI] [PubMed] [Google Scholar]
- Ono M, Hakomori S. 2003. Glycosylation defining cancer cell motility and invasiveness. Glycoconjugate J. 20:71–78. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Matsuzaki H, Ando H, Kaji H, Nakanishi H, Ikehara Y, Narimatsu H. 2012. Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin Exp Metastasis. 29:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, Vakhrushev SY, Olsen JV, Hansen L, Bennett EP et al. 2014. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci USA. 111:E4066–E4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers N, Anderson JM, Linde EM, DiMaio DJ, Lazenby AJ, Wandall HH, Mandel U, Clausen H, Yu F, Hollingsworth MA. 2013. Aberrant expression of mucin core proteins and O-Linked glycans associated with progression of pancreatic cancer. Clin Cancer Res. 19:1981–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samraj AN, Pearce OMT, Laeubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL et al. 2015. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. 112:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletmoen M, Dam TK, Gerken TA, Stokke BT, Brewer CF. 2009. Single-molecule pair studies of the interactions of the alpha-GalNAc (Tn-antigen) form of porcine submaxillary mucin with soybean agglutinin. Biopolymers. 91:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletmoen M, Skjak-Braek G, Stokke BT. 2004. Single-molecular pair unbinding studies of mannuronan C-5 epimerase AlgE4 and its polymer substrate. Biomacromolecules. 5:1288–1295. [DOI] [PubMed] [Google Scholar]
- Springer GF. 1984. T and Tn, general carcinoma auto-antigens. Science. 224:1198–1206. [DOI] [PubMed] [Google Scholar]
- Spruijt E, Van Den Berg SA, Cohen Stuart MA, Van Der Gucht J. 2012. Direct measurement of the strength of single ionic bonds between hydrated charges. ACS Nano. 6:5297–5303. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Ju TZ, Cummings RD. 2015. Protein glycosylation in cancer Annu Rev Pathol. 10:473–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunz T, Oroszlan K, Schafer R, Guntherodt HJ. 1999. Dynamic force spectroscopy of single DNA molecules. Proc Natl Acad Sci USA. 96:11277–11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromas C, Rojo J, de la Fuente JM, Barrientos AG, Garcia R, Penades S. 2001. Adhesion forces between Lewis(x) determinant antigens as measured by atomic force microscopy. Angew Chem, Int Ed. 40:3052–3055. [DOI] [PubMed] [Google Scholar]
- van Vliet SJ, Gringhuis SI, Geijtenbeek TBH, van Kooyk Y. 2006. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 7:1200–1208. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M, Cummings RD. 2010. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci USA. 107:9228–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Schrump DS, Gildersleeve JC. 2016. Whole-cell cancer vaccines induce large antibody responses to carbohydrates and glycoproteins. Cell Chem Biol. 23:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Guo X, Nash GB, Stone PC, Hilkens J, Rhodes JM, Yu L-G. 2009. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 69:6799–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Liu Y, Park HJ, Boggs JM, Basu A. 2012. Carbohydrate-coated fluorescent silica nanoparticles as probes for the galactose/3-sulfogalactose carbohydrate–carbohydrate interaction using model systems and cellular binding studies. Bioconjugate Chem. 23:1166–1173. [DOI] [PubMed] [Google Scholar]