Abstract
Objective
Interleukin (IL)-18 plays a crucial role in maintaining metabolic homeostasis and levels of this cytokine are influenced by gender, age, and sex hormones. The role of gender on IL-18 signaling, however, is unclear. We hypothesized that the presence of female sex hormone could preserve the metabolic phenotype of the IL-18R−/− animals.
Methods
We studied female mice with a global deletion of the α isoform of the IL-18 receptor (IL-18R−/−) and littermates control. Three studies were done: 1) animals fed a high fat diet (HFD) for 16 weeks; 2) animals fed chow diet for 72 weeks and 3) animals (3 weeks-old) randomized to either bilateral ovariectomy (OVX) or control surgery (SHAM) and followed for 16 weeks.
Results
Female IL-18R−/− mice gained less weight and maintained glucose homeostasis on a chow diet compared with HFD, but no differences between genotypes were observed. The maintenance of body weight and glucose homeostasis in IL-18R−/− mice was lost with aging. By 72 weeks of age, IL-18R−/− mice became heavier compared with WT mice due to an increase in both visceral and subcutaneous adiposity and displayed glucose intolerance. OVX did not affect body weight in IL-18R−/− mice but exacerbated glucose intolerance and impaired liver insulin signaling when compared with SHAM mice.
Conclusions
Female mice harboring a global deletion of the IL-18R, only present the same phenotype as reported in male IL-18R−/− mice if they are aged or have undergone OVX, in which circulating estrogen is likely to be blunted. The role of estrogen signaling in the protection against altered metabolic homeostasis in IL-18R−/− mice appears to be mediated by liver insulin signaling. We therefore suggest that the metabolic effects mediated by loss of IL-18 signaling are only present in a female sex hormone free environment.
Keywords: IL-18, Obesity, Insulin resistance, Gender
Highlights
-
•
Female mice harboring a global deletion of the IL-18R, only present the same phenotype as reported in male IL-18R−/− mice if they are aged or have undergone OVX, where circulating estrogen is likely to be blunted.
-
•
The role of estrogen signaling in the protection against altered metabolic homeostasis in IL-18R−/− mice appears to be mediated by liver insulin signaling.
-
•
These data have important implications for the role of IL-18 signaling in metabolism and the sexual dimorphism often observed in murine studies with respect to insulin resistance.
1. Introduction
Interleukin (IL)-18 is a cytokine belonging to the cytokine family of IL-1. It is known to signal through stimulation of IFN-γ [1] and is activated by pro-inflammatory stimuli such as LPS, Fas ligand, and TNF-α leading to caspase-1-mediated cleavage of pro-IL-18 into mature IL-18. IL-18 signals via a heterodimer of the transmembrane IL-18 receptors (α and β) and ultimately activates NFκB and subsequently regulates gene transcription. Besides this pathway, IL-18 is also involved in mitogen-activated protein kinase PIPs kinase and Stat 3 signaling.
Over the past decade, it has become apparent that IL-18 plays a crucial role in maintaining metabolic homeostasis. While IL-18 levels are increased in both type 2 diabetes [2] and obesity [3], work from us [4], [5] and others [6], [7] that employed both loss and gain of function in rodent models, demonstrated that IL-18 was both necessary and sufficient for the maintenance of metabolic homeostasis. In our recent study where we showed that mice that harbor a global deletion of the functional IL-18R are prone to gain weight, increased ectopic skeletal muscle lipid expression, inflammation and, ultimately insulin resistance, all experiments were performed in male mice [4]. It is important to note that IL-18 levels are influenced by gender [8], age [9], and sex hormones [10]. Moreover, lack of female sex hormones is associated with increased adiposity and insulin resistance in both rodents [11], [12] and humans, [13], [14]. The role of IL-18 signaling on metabolic homeostasis in female mice and, importantly, the role that the sex hormones may play in this experimental model is unclear. We, therefore, hypothesized that the presence of female sex hormone could preserve the metabolic phenotype of the IL-18R−/− animals. We tested the hypothesis in a diet-induced obesity model in both aged female mice and ovariectomized IL-18R−/− animals. We show that the lack of IL-18 signaling and female sex hormones combined, but not IL-18 or female sex hormone per se, leads to systemic insulin resistance and impaired insulin signaling in the liver.
2. Materials and methods
2.1. Animal experimental protocol
All experiments were approved by The Animal Experiments Inspectorate in Denmark. For the aging study, twelve-week-old IL18R−/− (mice deficient in IL-18 receptor 1, backcrossed 11 generations to C57BL/6J) and wild-type C57BL/6J mice were obtained from Charles River Laboratories (L'Arbresle, France). For the ovariohysterectomy (OVX) and diet-induced obesity (DIO) study, IL18R−/− and littermate control (WT) mice were obtained from heterozygous breeding. Mice were maintained on a 12-h light, 12-h dark cycle on a standard rodent chow diet (27%, 13%, and 60% kcal from protein, fat, and carbohydrate, respectively) for the aging and OVX study. For the DIO study, a high fat diet composed of 60% calories from fat (Research Diet 12492) was administered when the mice were 16 weeks old of age and continued for 16 weeks (DIO study). For the OVX study, 3 weeks old female, IL18R−/−, and WT mice were randomly divided into 2 groups, sham (SHAM) operated and OVX operated, and anesthetized with a Hypnorm (10 mg/ml) (vetPharma Ltd, Leeds, UK) and Midazolam (5 mg/ml) (Hameln pharmaceuticals, Hameln, Germany) (1:1:2) anesthetic mixture with sterile water as the last component, administered subcutaneously (s.c) (0.1 ml per 10 g body weight) and were OVX or SHAM operated. In the OVX animals, the ovary was isolated and allocated to the incised muscles; the oviduct was ligated with 6-0 absorbable Vicryl ligature (Ethicon, Norderstedt, Germany), and the ovary removed. This procedure was performed on both ovaries and the muscle incisions and the skin incision were closed with 5-0 absorbable Vicryl (Ethicon) with interrupted sutures. The SHAM operated females were incised as described above, through the skin and abdominal muscles, and the incisions were closed as described above.
2.2. Body composition measurements
Body weights were monitored weekly throughout the DIO and OVX study. At the cessation of the studies, we analyzed body composition by quantitative MRI using EchoMRI (Echo Medical Systems, Houston, TX, USA). Fat and lean mass percentages were calculated in relation to total body weight, which was measured prior to the MRI scan. To confirm the MRI findings, mice were killed and the gonadal, retroperitoneal, and inguinal fat pads were dissected and weighed.
2.3. Insulin tolerance tests
In the DIO-study, we performed an insulin tolerance test after 16 weeks of HFD, in the aging study in 16 months-old female mice, and in the OVX study 16 weeks after OVX/SHAM operations, 4 h after removal of food. Blood samples were obtained by tail cut and analyzed for glucose using a glucometer (Accu-chek Compact plus) immediately before and at 15, 30, 45, 60, 90, and 120 min after an intraperitoneal injection of insulin (0.75 U/kg lean body weight Actrapid, Novo Nordisk, DK).
2.4. Glucose tolerance tests
In the DIO study and the OVX study, we performed intraperitoneal glucose tolerance tests after 16 weeks of HFD feeding and 16 weeks after OVX, respectively, and after removal of food for 14 h. Blood samples were obtained by tail cut and analyzed for glucose using a glucometer (Accu-chek Compact plus) immediately before and at 15, 30, 45, 60, 90, and 120 min after an intraperitoneal injection of glucose (2 g/kg body weight). In the OVX and aging study, we performed an oral glucose tolerance test 16 weeks after OVX and in 9 months- (36 weeks) and in 18 (72 weeks) months-old female mice, respectively, after removal of food for 14 h. Blood samples were obtained by tail cut and analyzed for glucose content using a glucometer (Accu-chek Compact plus) immediately before and at 15, 30, 45, 60, 90, and 120 min after an oral gavage of glucose (2 g/kg body weight).
2.5. Measurement of blood samples
Blood samples were obtained from the tail vein, transferred into EDTA tubes, spun at 6000 g for 10 min at 4 C, and the plasma was removed. Plasma was stored at −80 C until analysis. Plasma insulin was determined by ELISA (Crystal Chem). Blood glucose was measured by glucometer (Accu-chek Compact Plus).
2.6. Insulin signaling tissue collection
In the OVX study, insulin signaling in liver, adipose tissue, and skeletal muscle was studied. Animals were given an intraperitoneal injection of insulin (1.5 units/kg lean body mass). After 5 min, the animals were sacrificed by cervical dislocation and the tissue removed.
2.7. Protein analyses
Tissues of interest were homogenized in an ice-cold lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 5 mM NaP, 2 mM NaV, 1 mM DTT, 0.2% Ipegal-CA-630, protease inhibitor (Roche Applied Science), 1% phosphatase inhibitor cocktail, pH 7.4) for 2 min using a tissue lysate with 30 oscillations/s (TissueLyser II; QIAGEN, Germany). Lysates were generated by centrifugation (16,000 g) for 15 min at 4 °C. Protein concentration in lysates was measured by the Bradford reagent (BioRad) [15].
Tissue lysates were subjected to SDS-PAGE using BioRad 4–15% precast gels and I-blot semi-dry transfer machine according to the manufacturer's instructions. Samples from all groups were loaded in even numbers on the same gel. PVDF membranes were probed with primary antibodies raised against the protein of interest; p-AMPK(Thr172) (#2535, Cell Signaling Technology, Danvers, MA), p-Akt(Ser473) (#9271, Cell Signaling Technology, Danvers, MA), p-GSK3 α/β (Ser21/9) (#9331 Cell Signaling Technology, Danvers, MA, USA), p-ACC(Ser79) (#3661, Cell Signaling Technology, Danvers, MA), p-JNK(Thr183/Tyr185) (#9251, Cell Signaling Technology, Danvers, MA), p-STAT3(Tyr705) (#9131, Cell Signaling Technology, Danvers, MA), total AKT (#9272, Cell Signaling Technology, Danvers, MA). Detection of primary antibodies was performed using appropriate peroxidase-conjugated IgG and protein signals visualized using FEMTO enhanced chemiluminescence and Biorad Chemidoc XRS imager. Total protein content was quantified using reactive brown 10 (Sigma–Aldrich, St-Louis, MO). Quantification of immunoblots was done using ImageJ (NIH, Bethesda, MD, http://rsb.info.nih.gov/ij).
2.8. Statistical analyses
All analyses were performed using SAS 9.2. Analyses with two variables were tested with a two way ANOVA followed by a Tukeys post hoc test. Time-series data were analyzed with PROC MIXED. Effects of insulin stimulation on genotype and OVX were analyzed using a three-way ANOVA. p < 0.05 was considered significant.
3. Results
3.1. Female IL-18 receptor-deficient mice gain less weight and maintain glucose homeostasis
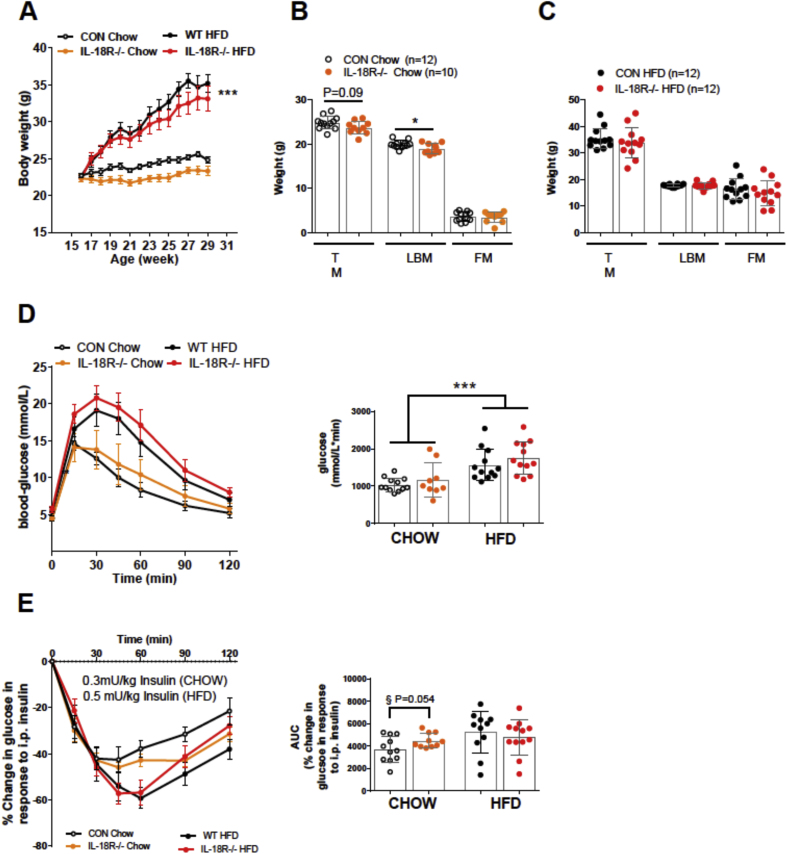
As discussed above, IL-18 levels are influenced by gender. Accordingly, we first measured the mRNA of the gene encoding IL-18R in skeletal muscle, white adipose tissue (WAT) and liver of male and female mice. While no differences were observed when comparing the skeletal muscle and WAT, the IL-18R mRNA expression was higher in the liver of females compared with males (Supplementary Figure 1). To evaluate the role of IL-18 signaling and body weight homeostasis in female mice, we performed loss-of-function studies in female mice with a global deletion of alpha-isoform of the IL-18 receptor and IL-18 (IL-18R−/−). In contrast with our previous observations in male IL-18R−/− [4], female IL-18R−/− mice did not become heavier compared with WT, irrespective of diet. In fact, there was a tendency for body weight to be lower in IL-18R−/− mice compared with WT mice (Figure 1A). In contrast with male IL-18R−/− mice [4], female IL-18R−/− mice had a reduced total lean body mass compared with CON on a chow (Figure 1B) but not HFD (Figure 1C). We next examined whether eight-month-old chow and HFD fed female IL-18R−/− mice exhibited whole body metabolic disturbances. No difference in glucose tolerance was observed between genotype irrespective of diet (Figure 1D), however, IL-18R−/− mice fed a chow diet tended to become more insulin sensitive (p = 0.056) compared with WT mice, but no differences were observed between genotypes when mice were fed a HFD (Figure 1E).
Figure 1.
Whole-body phenotype and glucose metabolism of chow-, and HFD-fed female IL-18R−/− mice. A: Growth curves during chow and HFD (CON-chow: n = 12; CON-HFD: n = 12; IL-18R−/−-chow: n = 10; IL-18R−/−-HFD: n = 12). B: Total mass (TM), lean body mass (LBM), and fat mass (FM) during chow diet (CON-chow: n = 12; IL-18R−/−-chow: n = 10). C: Total mass (TM), lean body mass (LBM), and fat mass (FM) during HFD (CON-HFD: n = 12; IL-18R−/−-HFD: n = 12). D: Intraperitoneal glucose tolerance during chow and HFD, 16 weeks after start of the study with area under the curve (AUC) inserted for chow and HFD (CON-chow: n = 12; IL-18R−/−-chow: n = 9; CON-HFD: n = 12; IL-18R−/−-HFD: n = 12). E: Intraperitoneal insulin tolerance test in females during chow and HFD, 16 weeks after start of the study, with area under the curve (AUC) inserted for chow and HFD (CON-chow: n = 11; IL-18R−/−-chow: n = 10; CON-HFD: n = 11; IL-18R−/−-HFD: n = 12). In the intraperitoneal insulin tolerance test 0.3 mU/kg and 0.5 mU/kg was used for chow and HFD, respectively. Results are presented as mean ± SEM. ***p < 0.0001 vs chow (A,D); *p < 0.05 (B); §p = 0.054 vs CON chow.
3.2. The maintenance of body weight and glucose homeostasis in female IL-18 receptor deficient mice is lost with aging
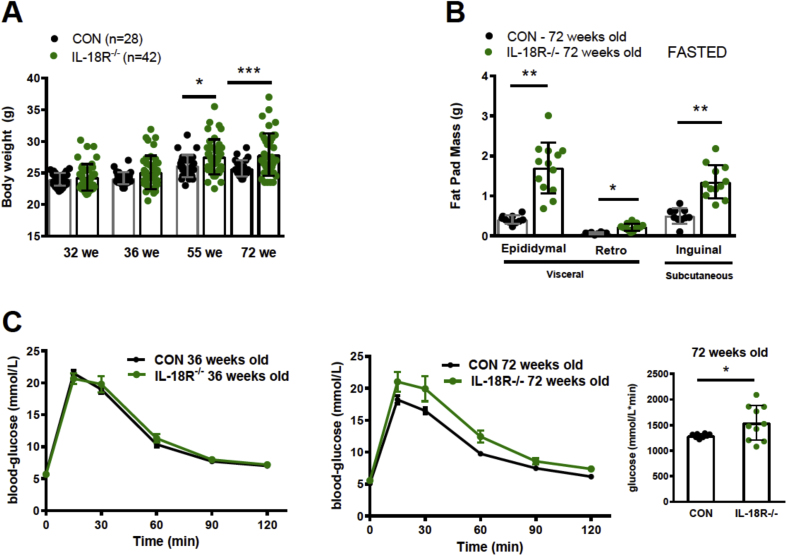
As no differences in obesity or glucose tolerance were observed between female CON and IL-18R−/− animals, we hypothesized that this effect was mediated by female sex hormones. Although mice do not undergo a true menopause, female mice exhibit ovarian failure, which is significant around 14 month of age [16] and develops many of the same age-associated health complications, including bone and muscle loss and excess fat deposition, as is observed in postmenopausal women [17]. Therefore, we initially studied the effect of aging on the phenotype and followed chow fed, female CON and IL-18R−/− mice from 32 to 72 weeks of age. While normal body weight was maintained up to 36 weeks of age, by 55 weeks of age, IL-18R−/− mice became heavier compared with CON (Figure 2A). The increase in body weight was due to an increase in both visceral and subcutaneous adiposity when fat pads were obtained and weighed at 72 weeks of age (Figure 2B). We next evaluated glucose homeostasis over the same time-course. Consistent with body mass data, no difference was observed between genotypes at 36 weeks of age (Figure 2C; left panel), but by the age of 72 weeks, when IL-18R−/− had increased fat mass, we also observed glucose intolerance in these mice (Figure 2C; right panels). Somewhat paradoxically, at this age, the IL-8R−/− animals had a lower liver weight (Supplementary Figure 2A).
Figure 2.
Whole-body phenotype and glucose metabolism of aging female IL-18R−/− mice. A: Body weight for 32, 36, 55, and 72 weeks old animals (CON-: n = 28 IL-18R−/− n = 42). B: Gonadal, retroperitoneal, and inguinal fat pads in 72 weeks old fasted animals (CON-: n = 10; IL-18R−/− n = 13); C: Oral glucose tolerance with area under the curve (AUC) inserted in 72 weeks old animals (CON: n = 8; IL-18R−/− n = 10). Results are presented as mean ± SEM. *p < 0.05 (A, B, C) and **p < 0.01 vs CON (B).
3.3. Ovariectomy exacerbates glucose intolerance and impaired liver insulin signaling but not body weight, in IL-18R−/− mice
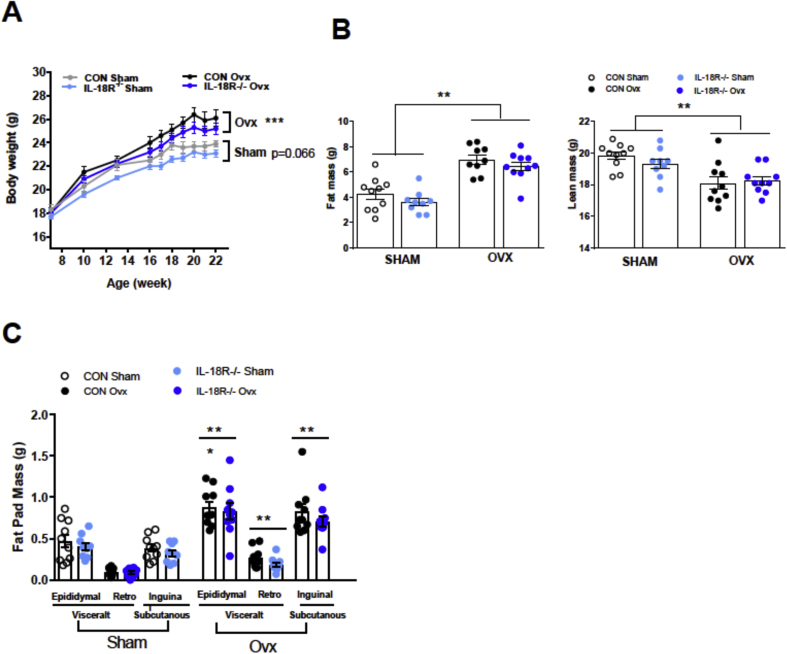
As the phenotype in aging mice may have been explained by the progressive loss of circulating female sex hormones, we next performed an OVX study in which chow fed IL-18R−/− female and WT animals underwent surgery at 7 weeks and were followed subsequently for 16 weeks. Irrespective of genotype, OVX mice gained more weight compared with SHAM, but no differences were observed between genotypes (Figure 3A). The increase in body mass was due to a marked increase in adiposity, since OVX resulted in a loss of lean mass (Figure 3B). The increased adiposity was attributable to increases in both visceral and subcutaneous fat mass, with no difference between genotype (Figure 3C).
Figure 3.
Whole-body phenotype of ovariectomized female IL-18R−/− mice. A: Growth curve for sham and Ovariectomized (OVX) mice (CON-Sham: n = 10; CON-Ovx n = 10; IL-18R−/−-Sham: n = 10; IL-18R−/−-Ovx: n = 10). B: Fat and lean mass for Sham and Ovariectomized (OVX) mice; C: Gonadal, retroperitoneal, and inguinal fat pads in Sham and Ovariectomized (OVX) mice. Results are presented as mean ± SEM. ***p < 0.001 vs Sham (A, B, C).
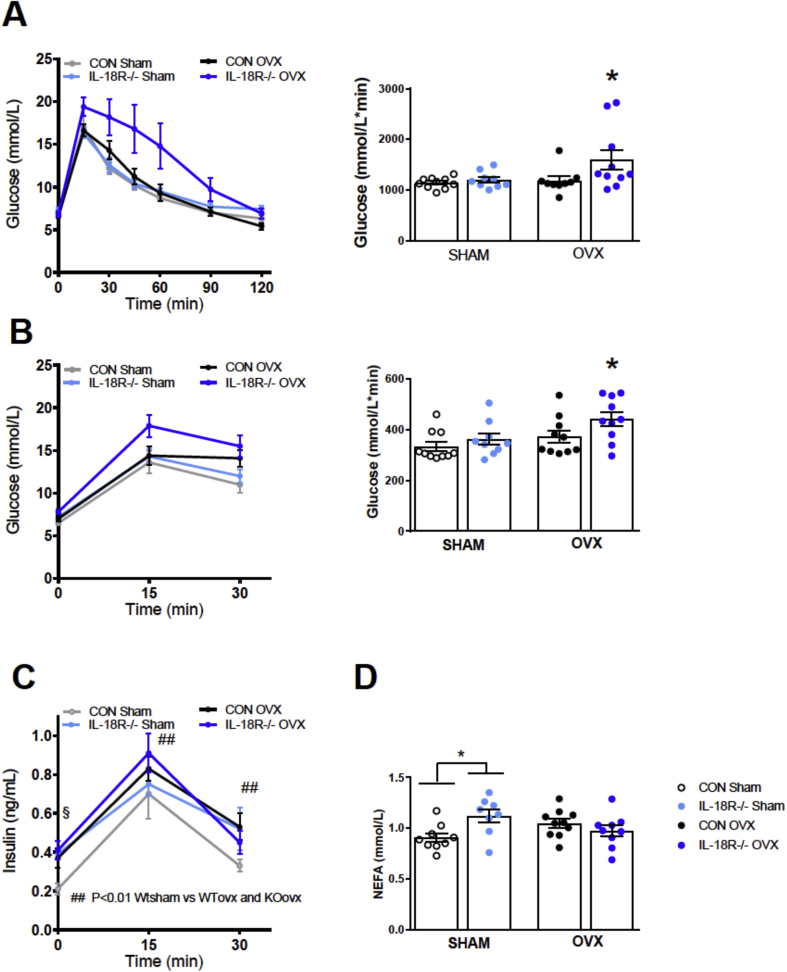
Despite the fact that OVX did not affect body weight when comparing IL-18R−/− with WT mice, we also examined metabolic homeostasis as we have previously observed uncoupling between body weight and glucose homeostasis with gene deletion in mice [18]. Consistent with such uncoupling of metabolism to body mass, OVX caused glucose intolerance in IL-18R−/− compared with WT mice, as measured by IPGTT (Figure 4A) and during an oral glucose tolerance test (OGTT) (Figure 4B). The impairment in glucose tolerance observed in IL-18R−/− was likely to be due to insulin resistance rather than a defect in insulin secretion since no differences were observed in insulin concentration when comparing genotypes during the OGTT (Figure 4C).
Figure 4.
Ovariectomized IL-18R−/− mice exhibit impaired glucose tolerance and insulin resistance. A: Intraperitoneal glucose tolerance with area under the curve (AUC) inserted in Sham and ovariectomized mice 16 weeks after operation (CON-Sham: n = 10; CON-OVX n = 9; IL-18R−/−-Sham: n = 9; IL-18R−/−-Ovx: n = 10). B: Oral glucose tolerance with area under the curve (AUC) inserted in Sham and Ovx animals 17 weeks after operation (CON-Sham: n = 10; CON-OVX n = 10; IL-18R−/−-Sham: n = 9; IL-18R−/−-OVX: n = 10); C: Plasma insulin during an oral glucose tolerance test in Sham and Ovx animals 17 weeks after operation (CON-Sham: n = 10; CON-OVXx n = 10; IL-18R−/−-Sham: n = 9; IL-18R−/−-OVX: n = 10); D: Fasted NEFA concentration in Sham and Ovx animals 17 weeks after operation (CON-Sham: n = 10; CON-OVX n = 10; IL-18R−/−-Sham: n = 9; IL-18R−/−-OVX: n = 10). Results are presented as mean ± SEM. *p < 0.05 vs CON Sham (A, B, D: one-way ANOVA, difference between four groups); *p < 0.05 vs CON Sham/KO Sham and CON OVX (A, B: No difference between CON Sham and KO sham and the groups were pooled to one group. One-way ANOVA between three groups); ##p < 0.01 (C: CON OVX and KO OVX vs CON Sham); §p = 0.056 versus CON OVX, CON Sham and KO sham, two-way ANOVA (4 groups (CON-sham, CON-OVX, KO-sham, KO-OVX) and Time (15 and 30 min)).
To investigate insulin resistance in adipose tissue, we measured circulating non-esterified fatty acid (NEFA) concentrations during the OGTT as a proxy marker. IL-18R−/− SHAM animals display increased circulating NEFA concentrations after fasting compared with WT SHAM-operated animals (Figure 4D). However, consistent with a normal glucose tolerance during an OGTT in SHAM-operated IL-18R−/− animals, SHAM-operated IL-18R−/− animals exhibited similar NEFA concentrations and percentage drop in NEFA concentrations during an OGTT compared with SHAM-operated WT animals, indicative of normal suppression of lipolysis during an OGTT and, therefore, normal insulin sensitivity of the adipose tissue. In contrast, and consistent with impaired glucose tolerance in IL-18R−/− OVX mice, we observed an impaired suppression of NEFA during the OGTT in these animals compared with other groups, indicating insulin resistance in the adipose tissue (data not shown). Somewhat surprisingly, OVX animals had lower liver triacylglycerol levels irrespective of genotype (Supplementary Figure 2B). Taken together and coupled with the data in aged animals, these data indicate that the IL-18 signaling mediated defect in glucose metabolism is dependent upon the loss of female sex hormones.
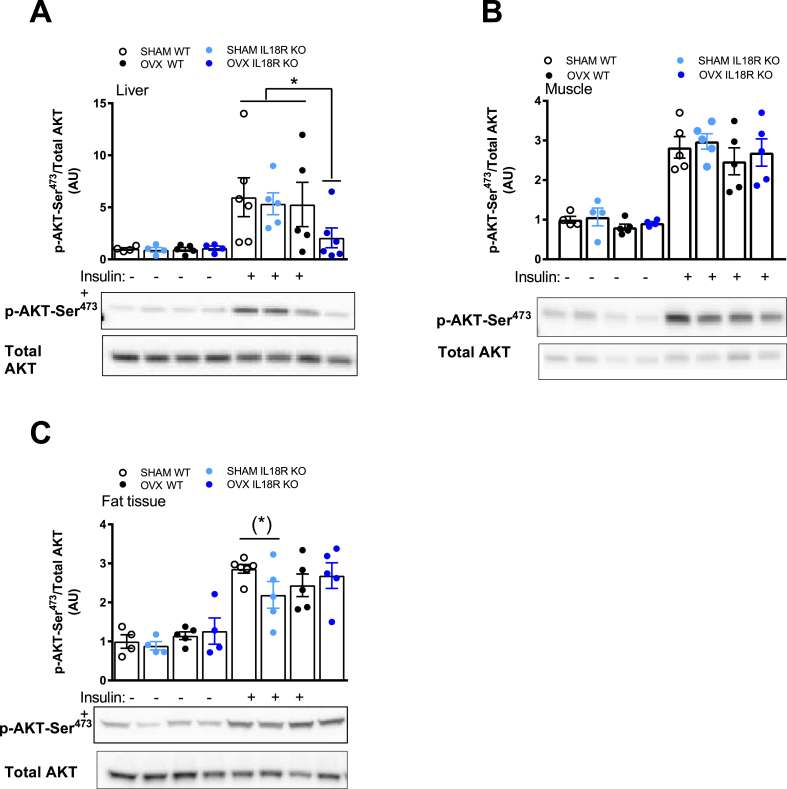
In order to gain insight into a possible mechanism for the aforementioned observations, we next interrogated insulin signaling in liver, skeletal muscle, and adipose tissue by measuring phosphorylation of Akt at the Serine 473 site, 2 min after a bolus of insulin in SHAM and OVX IL-18R−/− and WT animals. In liver, OVX IL-18R−/− animals had impaired Akt phosphorylation relative to other groups (Figure 5A). In contrast, no such effect was observed in adipose tissue. (Figure 5B). We also measured the phosphorylation of the downstream target of Akt, namely GSK3. Consistent with the data for Akt phosphorylation, we saw little effect of either IL18R−/− or OVX on this measure (Supplementary Figure 3). We previously demonstrated that IL-18 activates AMPK [4], while others [19] have demonstrated that estradiol activates AMPK. Accordingly, we measured the phosphorylation of AMPK in liver, skeletal muscle and white adipose tissue in this study. In contrast with our previous work [4], we observed no effect of OVX or genotype on AMPK phosphorylation (Supplementary Figure 4).
Figure 5.
Ovariectomized IL-18R−/− mice exhibit impaired insulin signaling in liver. Total and phosphorylated Ser473 Akt during Sham and OVX in liver (A), muscle (B), and adipose tissue (C) before and after insulin stimulation. No Insulin (−); Insulin stimulation (+). Samples from Sham and OVX were run on the same gel and related to total Akt. (n = 4–6 per group). Results are presented as mean ± SEM. *p < 0.05 vs CON Sham, KO Sham, CON OVX (two-way ANOVA: Class: 4 groups (CONsham CON-OVX KOsham KO-OVX) and Insulin × 4 groups). (*) = p = 0.056 vs CONsham.
4. Discussion
Rates of obesity, type 2 diabetes, and cardiovascular disease have steadily risen over the past five decades. Obesity is a risk factor for type 2 diabetes and cardiovascular disease; however, it has become apparent that the relationship is not linear. While obesity rates are higher in females [20], men have higher rates of related diseases [21]. It is also well known that female mice are protected from HFD-induced metabolic phenotypes and, for this reason, most murine studies in diet-induced obesity have been conducted in male mice (for review see Ref. [22]). In the current study we demonstrate that, in direct contrast with our previous studies in male mice [4], IL-18R−/− female mice do not display obesity, insulin resistance or impaired liver insulin sensitivity. However, in advanced aging, when mice lose circulating estrogen or after OVX, the protection observed in female IL-18 receptor deficiency is lost, suggesting that the protection against metabolic disease in these mice is driven by circulating female sex hormones.
It is well known that OVX in mice leads to obesity and insulin resistance, and this appears to be due to loss of estrogen receptor signaling since restoration of estrogen at physiological concentrations maintains insulin action and glucose tolerance in OVX mice [23]. Moreover, estrogen receptor alpha-deficient mice (ERα ko) are glucose intolerant and insulin resistant [24], [24], [25]. However, as recently discussed, the organs/tissues responsible for the defect are unclear. Work from the Hevener laboratory has recently found that skeletal muscle [26] and myeloid cell [27] specific ERα deficiency both lead to impaired metabolic homeostasis. As IL-18 is an inflammatory cytokine and we recently demonstrated that IL-18 production from the Nod-Like receptor 1 (NLRP1) inflammasome prevents HFD-induced metabolic dysregulation [5], we examined whether inflammation may have mediated the impaired metabolic homeostasis observed in OVX IL-18R−/− mice (Figure 4). However, we saw no differences in several markers of inflammation we measured when comparing OVX IL-18R−/− with SHAM IL-18R−/− mice (data not shown) indicating that the phenotype was unlikely to be due to modification to inflammatory signaling. Likewise, we measured muscle insulin signaling and saw no effect when comparing OVX IL-18R−/− with SHAM IL-18R−/− mice (data not shown), suggesting that skeletal muscle was unlikely to be mediating the observed phenotype. Given that we observed changes in glucose tolerance independent of insulin secretion (Figure 4) and near complete blunting of insulin-stimulated AKT phosphorylation in liver (Figure 5), it is likely that a lack of estrogen receptor signaling in OVX IL-18R−/− was mediated by insulin resistance in this tissue. However, as recently discussed [28], the role of estrogen receptor signaling on liver insulin resistance is still unclear since some [29] but not others [25] find a defect in hepatic insulin action in ERα ko mice.
It must be noted that we studied IL-18R−/− mice and not IL-18 deficient mice in the current study. A previous study has demonstrated some differences in the phenotypes of these two strains of mice [30], raising the possibility that some of the actions of IL-18 may be receptor independent. Accordingly, we performed GTT and ITT experiments in female IL-18 KO mice and compared those data with the data reported herein on IL-18R−/− female mice. Consistent with a previous report in male mice [6], we observed no differences when comparing strains (data not shown), suggesting that the possibility of IL-18 acting via mechanisms not involving it's receptor is unlikely.
In conclusion, we have demonstrated that, unlike our previous observations in male mice harboring a whole body deletion of the IL-18R [4], female mice harboring the same deletion do not present with this phenotype unless they are aged or have undergone OVX where circulating estrogen is likely to be blunted. Moreover, the role of estrogen signaling in the protection against altered metabolic homeostasis in IL-18R−/− mice appears to be mediated by liver insulin signaling. These data have important implications for the role of IL-18 signaling in metabolism and the sexual dimorphism often observed in murine studies with respect to insulin resistance.
Acknowledgments
We would like to acknowledge the assistance of Betina Mentz, Ruth Rousing, Hanne Villumsen, Lone Nielsen, Noemi G. James, and the Rodent Metabolic Phenotyping Center. The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden. During the study period, the Centre of Inflammation and Metabolism (CIM) was supported by a grant from the Danish National Research Foundation (DNRF55). The study was further supported by grants from the Novo Nordisk Foundation, Hørslevfonden, the Danish National Research Foundation (#10-083807), Højmosegårdlegatet, Fonden for Lægevidenskabens Fremme, Direktør Jacob Madsen og Hustru Olga Madsens Fond. CIM/CFAS is a member of DD2 – the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant nos. 09-067009 and 09-075724). The Rodent Metabolic Phenotyping Center is part of the UNIK Project: Food, Fitness & Pharma for Health and Disease (see www.foodfitnesspharma.ku.dk), supported by the Danish Ministry of Science, Technology, and Innovation. BL postdoctoral fellowship was supported by a grant from the Danish National Research Foundation. This study was supported, in part, by a grant from the National Health and Medical Research Council of Australia (NHMRC project grant 526606). MAF is a Senior Principal Research Fellow of the NHMRC (APP1116936).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.04.005.
Contributor Information
Birgitte Lindegaard, Email: birgitte.lindegaard.madsen@regionh.dk.
Mark A. Febbraio, Email: m.febbraio@garvan.org.au.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Figure 1. Female mice exhibit a higher level of IL-18R mRNA in liver compared to male mice. IL-18R mRNA expression in the gastrocnemius muscle (A), white adipose tissue (B), and in liver (C). Samples were related to 18S. No differences in the expression of 18S between male and female were observed. **P < 0.01.
Supplementary Figure 2. IL-18R−/− mice exhibit a lower liver weight and ovariectomized mice lower liver triacylglycerol levels irrespective of genotype. Liver weight (A) and triacylglycerol levels on liver (B). **P < 0.01.
Supplementary Figure 3. Insulin signaling in liver, muscle, and adipose tissue in ovariectomized and IL-18R−/− mice. Phosphorylated Ser21/9 GSK3 α/β during Sham and OVX in liver (A), muscle (B), and adipose tissue (C) before and after insulin stimulation. No Insulin (−); Insulin stimulation (+). Samples from Sham and OVX were run on the same gel and related to total protein (n = 4–6 per group). Results are presented as mean ± SEM. *P < 0.05, **P < 0.01 effect of insulin.
Ovariectomized IL-18R−/− mice exhibit no change in AMPK phosphorylation. Phosphorylated Thr172 AMPK in liver (A), muscle (B), and adipose tissue (C). Samples from Sham and OVX were run on the same gel and related to total AMPK (n = 4–6 per group).
References
- 1.Kim R.J., Wilson C.G., Wabitsch M., Lazar M.A., Steppan C.M. HIV protease inhibitor-specific alterations in human adipocyte differentiation and metabolism. Obesity (Silver Spring) 2006;14:994–1002. doi: 10.1038/oby.2006.114. [DOI] [PubMed] [Google Scholar]
- 2.Aso Y., Okumura K., Takebayashi K., Wakabayashi S., Inukai T. Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care. 2003;26:2622–2627. doi: 10.2337/diacare.26.9.2622. [DOI] [PubMed] [Google Scholar]
- 3.Esposito K., Pontillo A., Ciotola M., Di Palo C., Grella E., Nicoletti G. Weight loss reduces interleukin-18 levels in obese women. The Journal of Clinical Endocrinology & Metabolism. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 4.Lindegaard B., Matthews V.B., Brandt C., Hojman P., Allen T.L., Estevez E. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes. 2013;62:3064–3074. doi: 10.2337/db12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy A.J., Kraakman M.J., Kammoun H.L., Dragoljevic D., Lee M.K., Lawlor K.E. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metabolism. 2016;23:155–164. doi: 10.1016/j.cmet.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Netea M.G., Joosten L.A., Lewis E., Jensen D.R., Voshol P.J., Kullberg B.J. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nature Medicine. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 7.Zorrilla E.P., Sanchez-Alavez M., Sugama S., Brennan M., Fernandez R., Bartfai T. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyama M., Kotani J., Usami M. Gender difference in granulocyte dynamics and apoptosis and the role of IL-18 during endotoxin-induced systemic inflammation. Shock. 2009;32:401–409. doi: 10.1097/SHK.0b013e31819c358a. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L., Corsi A., Lauretani F., Bandinelli S., Bartali B., Taub D.D. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cioffi M., Esposito K., Vietri M.T., Gazzerro P., D'Auria A., Ardovino I. Cytokine pattern in postmenopause. Maturitas. 2002;41:187–192. doi: 10.1016/s0378-5122(01)00286-9. [DOI] [PubMed] [Google Scholar]
- 11.Rincon J., Holmang A., Wahlstrom E.O., Lonnroth P., Bjorntorp P., Zierath J.R. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes. 1996;45:615–621. doi: 10.2337/diab.45.5.615. [DOI] [PubMed] [Google Scholar]
- 12.D'Eon T.M., Souza S.C., Aronovitz M., Obin M.S., Fried S.K., Greenberg A.S. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. Journal of Biological Chemistry. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 13.Abildgaard J., Pedersen A.T., Green C.J., Harder-Lauridsen N.M., Solomon T.P., Thomsen C. Menopause is associated with decreased whole body fat oxidation during exercise. American Journal of Physiology. Endocrinology and Metabolism. 2013;304:E1227–E1236. doi: 10.1152/ajpendo.00492.2012. [DOI] [PubMed] [Google Scholar]
- 14.Lovejoy J.C., Champagne C.M., de J.L., Xie H., Smith S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. International Journal of Obesity. 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 16.Gosden R.G., Laing S.C., Felicio L.S., Nelson J.F., Finch C.E. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biology of Reproduction. 1983;28:255–260. doi: 10.1095/biolreprod28.2.255. [DOI] [PubMed] [Google Scholar]
- 17.Perez G.I., Jurisicova A., Wise L., Lipina T., Kanisek M., Bechard A. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5229–5234. doi: 10.1073/pnas.0608557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews V.B., Allen T.L., Risis S., Chan M.H., Henstridge D.C., Watson N. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- 19.Martinez de Morentin P.B., Gonzalez-Garcia I., Martins L., Lage R., Fernandez-Mallo D., Martinez-Sanchez N. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabolism. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onat A., Karadeniz Y., Tusun E., Yuksel H., Kaya A. Advances in understanding gender difference in cardiometabolic disease risk. Expert Review of Cardiovascular Therapy. 2016;14:513–523. doi: 10.1586/14779072.2016.1150782. [DOI] [PubMed] [Google Scholar]
- 22.Griffin C., Lanzetta N., Eter L., Singer K. Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2016;311:R211–R216. doi: 10.1152/ajpregu.00136.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stubbins R.E., Holcomb V.B., Hong J., Nunez N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. European Journal of Nutrition. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 24.Riant E., Waget A., Cogo H., Arnal J.F., Burcelin R., Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 25.Ribas V., Nguyen M.T., Henstridge D.C., Nguyen A.K., Beaven S.W., Watt M.J. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. American Journal of Physiology. Endocrinology and Metabolism. 2010;298:E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribas V., Drew B.G., Zhou Z., Phun J., Kalajian N.Y., Soleymani T. Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Science Translational Medicine. 2016;8 doi: 10.1126/scitranslmed.aad3815. 334ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribas V., Drew B.G., Le J.A., Soleymani T., Daraei P., Sitz D. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Reviews. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryzgalova G., Gao H., Ahren B., Zierath J.R., Galuska D., Steiler T.L. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 30.Pazos P., Lima L., Tovar S., Gonzalez-Touceda D., Dieguez C., Garcia M.C. Divergent responses to thermogenic stimuli in BAT and subcutaneous adipose tissue from interleukin 18 and interleukin 18 receptor 1-deficient mice. Scientific Reports. 2015;5:17977. doi: 10.1038/srep17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ovariectomized IL-18R−/− mice exhibit no change in AMPK phosphorylation. Phosphorylated Thr172 AMPK in liver (A), muscle (B), and adipose tissue (C). Samples from Sham and OVX were run on the same gel and related to total AMPK (n = 4–6 per group).