Abstract
A multi-center imaging trial by the American College of Radiology Imaging Network (ACRIN) “A Multicenter, phase II assessment of tumor hypoxia in glioblastoma using 18F Fluoromisonidazole (FMISO) with PET and MRI (ACRIN 6684)”, was conducted to assess hypoxia in patients with glioblastoma (GBM). The aims of this study were to support the role of proton magnetic resonance spectroscopic imaging (1H MRSI) as a prognostic marker for brain tumor patients in multi-center clinical trials. Seventeen participants from four sites had analyzable 3D MRSI datasets acquired on Philips, GE or Siemens scanners at either 1.5T or 3T. MRSI data were analyzed using LCModel to quantify metabolites N-acetylaspartate (NAA), creatine (Cr), choline (Cho), and lactate (Lac). Receiver operating characteristic curves for NAA/Cho, Cho/Cr, lactate/Cr, and lactate/NAA were constructed for overall survival at 1-year (OS-1) and 6-month progression free survival (PFS-6). The OS-1 for the 17 evaluable patients was 59% (10/17). Receiver operating characteristic analyses found the NAA/Cho in tumor (AUC = 0.83, 95% CI: 0.61 to 1.00) and in peritumoral regions (AUC = 0.95, 95% CI 0.85 to 1.00) were predictive for survival at 1 year. PFS-6 was 65% (11/17). Neither NAA/Cho nor Cho/Cr was effective in predicting 6-month progression free survival. Lac/Cr in tumor was a significant negative predictor of PFS-6, indicating that higher lactate/Cr levels are associated with poorer outcome. (AUC = 0.79, 95% CI: 0.54 to 1.00). In conclusion, despite the small sample size in the setting of a multi-center trial comprising different vendors, field strengths, and varying levels of expertise at data acquisition, MRS markers NAA/Cho, Lac/Cr and Lac/NAA predicted overall survival at 1 year and 6-month progression free survival. This study validates that MRSI may be useful in evaluating the prognosis in glioblastoma and should be considered for incorporating into multi-center clinical trials.
Introduction
Glioblastoma (GBM) is the most common and, unfortunately, the most aggressive type of primary malignant brain tumor. Despite treatments with surgery, radiation, and chemotherapy, the median overall survival is less than 15 months [1]. One of the pathologic hallmarks of GBM is tumor hypoxia resulting from an inefficient blood supply [2]. Tumor hypoxia limits the efficacy of radiation and chemotherapy and may select for a more aggressive tumor phenotype. It may also be a potent stimulator of abnormal angiogenesis [3]. Thus, a multi-center imaging trial, “American College of Radiology Imaging Network (ACRIN) 6684: A Multicenter, phase II assessment of tumor hypoxia in Glioblastoma using 18F Fluoromisonidazole (FMISO) with PET and MRI”, was conducted to assess hypoxia in patients with GBM.
The study found that increased tumor hypoxia as measured by 18F-FMISO standardized uptake values (SUV) peak and increased tumor vascular permeability (as measured by dynamic contrast enhanced MRI) were significantly associated with shorter overall survival at 1 year (OS-1). Furthermore, increased tumor perfusion (as measured by dynamic susceptibility contrast MRI) and increased vascular permeability (ktrans) were significantly associated with shorter progression free survival [4]. These findings highlight that hypoxia and abnormal tumor vasculature can have a significant impact on patient outcomes. Here, we report the results of the analysis of the proton magnetic resonance spectroscopic imaging (1H-MRSI) data for this trial in a subset of patients.
1H-MRSI is a magnetic resonance-based imaging modality that allows non-invasive sampling of metabolic changes in normal and abnormal brain parenchyma. It has also been proven useful in the diagnosis, prognosis, and management of brain tumor patients [5–12]. The most common metabolites evaluated in routine clinical practice include N-acetylaspartate (NAA), choline-containing compounds (Cho), lactate (Lac) and creatine (Cr).
N-Acetylaspartate is considered a neuronal metabolite and is decreased in processes with neuronal destruction or dysfunction [13]. NAA resonance is reduced or completely absent in malignant brain tumors as neurons are replaced by neoplastic tissue [14–16]. Choline is related to membrane turnover and their elevation is indicative of a process that results in increased glial proliferation and membrane synthesis (as seen with cellular proliferative disorders) [14, 17, 18]. Furthermore, an increase in Cho is directly related to tumor malignancy [19]. The dual effects of decreased NAA due to neuronal loss or dysfunction and increased Cho due to alterations in tumor membrane turnover make the NAA/Cho ratio a sensitive marker for brain tumors. Lactate is implicated in many cancer mechanisms: it is produced by enhanced glycolysis and glutaminolysis, it promotes cancer cell survival and proliferation, and it may also stimulate angiogenesis [20]. Consequently, the presence of Lac may indicate high level of malignancy [21, 22]. Creatine is a metabolite related to the cellular energy metabolism. It is considered relatively stable in different pathological processes affecting the central nervous system and useful as a reference metabolite. However, Cr is typically decreased in astrocytomas [16].
In this sub-study of the primary 6684 study, we sought to test whether the MRSI measures—e.g. NAA/Cho, Cho/Cr, Lac/Cr and Lac/NAA were predictive of outcome, and to evaluate how these MRSI measures relate to the previously analyzed MRI and PET markers.
Materials and methods
Study subjects
ACRIN, a cooperative group funded by the National Cancer Institute, conducted a prospective multi-institutional study in patients with newly diagnosed GBM (NCT00902577). The ACRIN 6684 trial enrolled 50 participants from 11 academic centers in the US between 2010 and 2013. Each participating site obtained approval by their local Institutional Review Board before subject accrual and obtained written informed consents for all subjects. Participants were referred by their treating neuro-oncologists or medical oncologists, who were made aware of the study by the site principal investigator, and were approached and consented by a member of the study team. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Forty-two patients had evaluable PET and MR imaging studies, and the results have been reported in the primary paper. [4].
Eligibility criteria included patients with a histological confirmed diagnosis of GBM with imaging verified residual disease after surgery who were scheduled to receive standard fractionated radiation therapy in addition to temozolomide chemotherapy. In addition to radiotherapy, patients could receive an investigational agent as part of a clinical trial. Other inclusion criteria included Karnofsky Performance Score > 60 and age > 18. Patients were followed every 3 months for tumor progression and survival for at least 1 year. The detailed inclusion and exclusion criteria can be viewed at https://www.acrin.org/Portals/0/Protocols/6684/ACRIN6684_Amend7_012412_master_ForOnline.pdf (section 6).
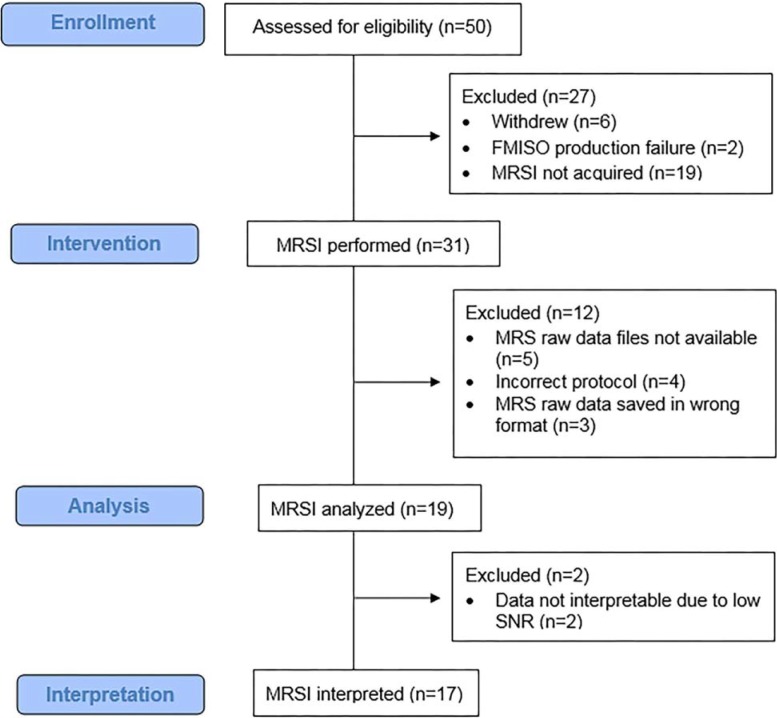
A subset of eight institutions initially qualified. MRSI was attempted in 31 cases. Of these 31 cases, 17 (55%) from four institutions had evaluable MRSI data. The 4 institutions, using 4 different MR scanners, collected MRSI data from the following numbers of patients: University of Washington (Philips 3T, 10 patients), Wake Forrest (GE 3T, 2 patients), Lee Moffitt (Siemens 1.5T, 4 patients), and Duke (Siemens 3T, 1 patient). The most common reasons for excluding data included: 1. The MRSI raw data files had not been saved at the time of data acquisition (N = 5), 2. The MRSI raw data had not been saved in the right format (N = 3) 3. Incorrect protocol has been chosen (N = 4), and 4. The data was not interpretable due to low SNR (N = 2) (Fig 1).
Fig 1. Consort flowchart.
The ACRIN 6684 trial enrolled 50 participants, 27 participants were excluded due to withdrawal (n = 6), technical difficulty in 18F-FMISO production (n = 2) and because MRSI was not performed (n = 19). MRSI was attempted in 31 cases. 17 (55%) had evaluable MRSI data. Reasons for excluding data included: 1. MRS raw data had not been saved at the time of data acquisition (N = 5), 2. Incorrect protocol had been chosen (N = 4), 3. MRS raw data had not been saved in the right format (N = 3), and 4. Data were not interpretable due to low SNR (N = 2).
MRI/MRSI acquisition
Research MRI and 18F-FMISO PET scans were performed after tumor diagnosis and within 2 weeks prior to initiation of chemo-radiation therapy. All sites were required to submit test scans to ensure image quality and the ability to perform required standardized image acquisition protocols prior to site participation The MRI requirements included anatomic pre-/post-contrast sequences along with dynamic contrast-enhanced (DCE) imaging, dynamic susceptibility contrast (DSC) perfusion, DTI, and MRSI. Acquisition and analysis of the DCE, DSC, and DTI data have been previously described [4]. The detailed MRI and PET imaging protocol can be viewed online at http://www.acrin.org/Portals/0/Protocols/6684/ACRIN6684_Amend7_012412_master_ForOnline.pdf (section 10).
Sites interested in participating in the exploratory study objectives related to MRSI were required to submit MRSI test data on phantoms and healthy subjects to the ACRIN core lab for quality control. All data were acquired using a 3D MRSI sequence with point-resolved spectroscopy excitation pulse sequence for signal localization. Acquisition parameters included TE = 140–144ms, TR = 1140–1180ms, phase encoding = 12x12x8, a FOV of >160 and nominal voxel sizes 2mL-3.5mL. Spectral bandwidth ranged from 1200 to 2000 Hz and number of points ranged from 512 to 1024 points. On the Philips scanner, 3 slices were acquired with 2D phase encoding of 12x11. The imaging time for MRSI was less than 10 minutes at all sites.
MRSI data analysis
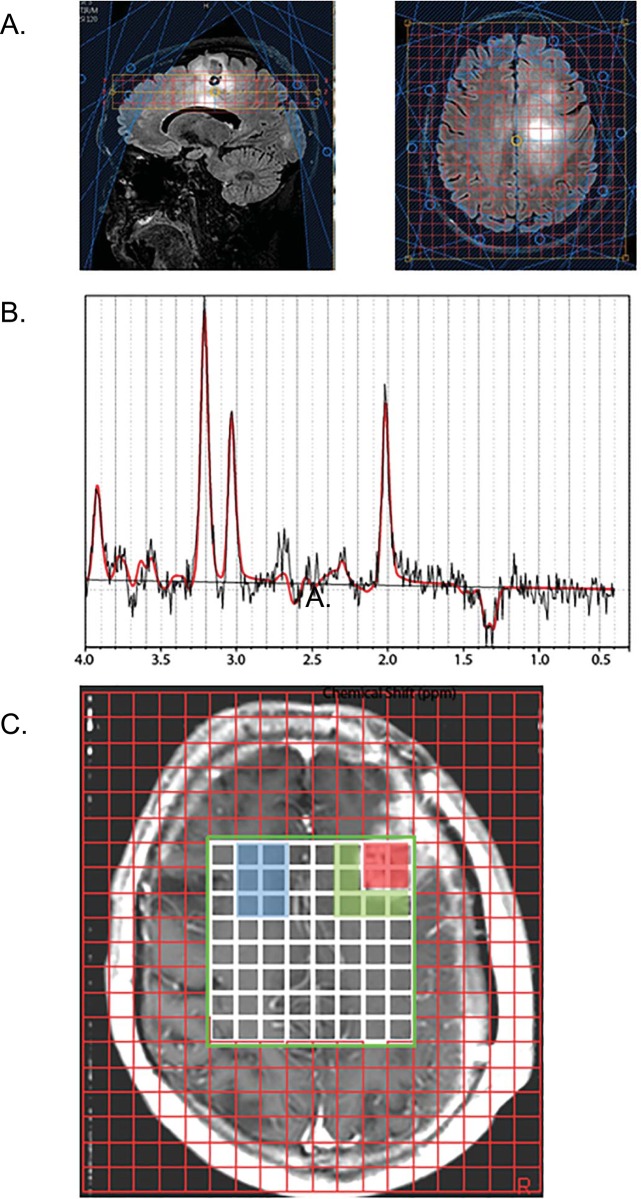
The spectroscopic raw data were centrally analyzed by ER who was blinded to the outcome of the participants. The data were analyzed using LCModel 6.1 Software [23] to determine the quantities of the metabolites NAA, Cr, Cho, and Lac. In addition, the presence of lipids (0.5–2 ppm) was recorded. Only fitted spectra with estimated standard deviations (Cramer–Rao lower bounds, automatically provided by the LCModel software) expressed in percent of the estimated concentrations lower than 25% were accepted [23]. In addition to the Cramer-Rao bounds criteria, spectra have been inspected by a spectroscopist for quality assurance. Data sets with low SNR (SNR <3) even on the contra lateral site were entirely excluded (N = 2). Also voxels containing subcutaneous fat from the scalp were excluded. Furthermore, MRSI datasets were vetted to ensure combability between the datasets as much as it was possible for a multi-center trial utilizing multiple vendors and magnetic field strengths. E.g. MRS datasets were excluded if they had been acquired using parameters that were outside the range of the stated protocol, e.g. 4 datasets were excluded because an incorrect echo time of TE = 45ms had been chosen. (Please see Results). For spectra classification, the MRSI data were overlaid on the post-contrast T1-weighted images. Voxels were classified into (i) contrast enhancing tumor; (ii) non-enhancing peritumoral parenchyma (periphery), and (iii) contralateral normal white matter, and mean metabolite ratios in those regions were calculated as previously described [5] (Fig 2).
Fig 2. MRSI methods.
A: Sagittal (left) and axial (right) MRSI voxel placement on a Philips scanner. Three slices were acquired with 2D phase encoding of 12x11. Data were acquired using a point-resolved spectroscopy excitation pulse sequence for signal localization. Acquisition parameters for all MRSI data included TE = 135–144 ms and TR = 1140–1180 ms. Also shown are the saturation bands for lipid suppression. B: The spectroscopic raw data were analyzed using LCModel 6.1 Software to determine the quantities of the metabolites NAA at 2 ppm, Cr at 3 ppm, Cho at 3.2 ppm, and Lac, a doublet, at 1.3 ppm. The gray line shows the spectrum; the red line indicates the fit of the MRS data. C: For spectra classification, MRSI data were overlaid on the post-contrast T1-weighted images. Voxels were classified into (i) enhancing tumor (red), (ii) non-enhancing peritumoral parenchyma approximately 1–1.5cm beyond the enhancing margin (green), and (iii) contralateral normal white matter (blue).
Statistical data analysis
Receiver operating characteristic (ROC) curves for the spectroscopic markers NAA/Cho, Cho/Cr, Lac/Cr, and Lac/NAA were constructed for two binary outcomes: OS-1 and 6-month progression free survival (PFS-6) as outlined under the study objectives/specific aims of the ACRIN 6684 trial (https://www.acrin.org/Portals/0/Protocols/6684/ACRIN6684_Amend7_012412_master_ForOnline.pdf). The areas under the ROC curves (AUC) and the associated 95% CIs were estimated empirically. A marker was considered effective in classification of an outcome status when its lower 95% CI of the AUC was at least 0.50. Correlations between parameters were assessed using Pearson correlation coefficients. All analyses were done with SAS 9.4 (Cary, NC, USA). Of note, the MRSI exam was a secondary and an exploratory aim in the study. Therefore, the study was not originally powered for this secondary objective. The data generated from this study may help us to accurately power future studies of MRS in glioblastoma.
Results
Study cohort
Patient demographic information, tumor size, O6-methylguanin-DNA-methyltransferase (MGMT) status, and initial treatment as well as salvage therapy received for the 17 evaluable patients are presented in Table 1. Of note, the patient characteristics in the 17 evaluable patients were similar to the entire cohort of 42 patients included in the primary paper [4]; e.g. the median age in this cohort was 59 y/o, the same as in the previous publication; the male gender percentage was 65% vs. 64%, and the median residual tumor size was 11.97 vs. 13.27. Furthermore, the OS-1 for the 17 evaluable patients was 59% vs. 60% in the larger cohort. These results suggest that the rejection or non-availability of some of the MRSI data has not introduced any significant bias into patient selection that might impact the analysis and results.
Table 1. Patient cohort.
| Total Evaluable N = 17 | |
|---|---|
| Patient Characteristics | |
| Median age (range) | 59 (45,77) |
| Gender, male (%) | 11 (65%) |
| Median residual CE tumor volume (range) | 11.97 (0.84, 59.5) |
| MGMT methylated* | 1 (6%) |
| MGMT unmethylated | 3 (18%) |
| MGMT unknown/not tested | 13 (76%) |
| Initial Treatment | |
| Temozolomide + RT | 12 (71%) |
| Temozolomide + RT+ clinical trial drug | 4 (23%) |
| Temozolomide +BSI (PARP inhibitor), but no RT | 1 (6%) |
| Salvage Therapy# | |
| None | 4 (24%) |
| RT | 1 (6%) |
| Surgery | 1 (6%) |
| Bevacizumab | 4 (24%) |
| Chemotherapy | 9 (53%) |
| NovoTTF | 9 (53%) |
| Survival | |
| Median survival time, days (95%CI) | 403 (209, 642) |
| OS-1 (%) | 10 (59%) |
| Median PFS, days (range) | 201 (128,335) |
| PFS-6 (%) | 11 (65%) |
CE contrast enhancement; RT radiation therapy
*Central review of tissue was not required and not all sites tested MGMT or patient underwent biopsy so there was insufficient tissue for MGMT testing.
# Patients could be counted more than once if received multiple salvage therapies.
MRSI metabolic ratios as predictor of 1-year overall survival (OS-1)
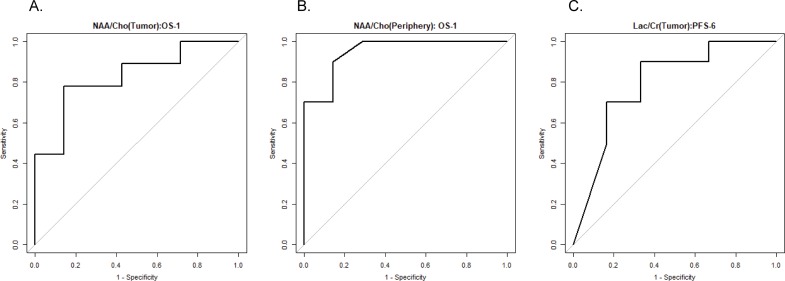
The OS-1 for the 17 evaluable patients was 59% (10/17). ROC analyses found the NAA/Cho in tumor (AUC = 0.83, 95% CI: 0.61 to 1.00) and in peritumoral parenchyma (AUC = 0.95, 95% CI 0.85 to 1.00) were predictive of survival at 1 year with higher values predicting increased OS-1 (Table 2). In addition, Lac/Cr in tumor showed a trend towards association with OS-1, with higher Lac values predicting poorer outcome (AUC = 0.78, 95% CI: 0.49 to 1.00). Cho/Cr was not effective in predicting OS-1. ROC curves with the corresponding AUC for the significant predictors using OS-1 are shown in Fig 3.
Table 2. Receiver operating characteristic (ROC) analysis.
| Metabolic ratio | ROI | OS-1 AUC (95% CI) |
PFS-6 AUC (95% CI) |
|---|---|---|---|
| NAA/Cho(n = 16) | Tumor | 0.83 (0.61–1.00) | 0.67 (0.39–0.95) |
| Cho/Cr(n = 16) | Tumor | 0.65 (0.35–0.96) | 0.60 (0.27–0.93) |
| Lac/Cr (n = 16) | Tumor | 0.73 (0.45–1.00) | 0.79 (0.54–1.00) |
| Lac/NAA(n = 16) | Tumor | 0.78 (0.49–1.00) | 0.76 (0.49–1.00) |
| NAA/Cho(n = 17) | Periphery | 0.95 (0.85–1.00) | 0.67 (0.40–0.95) |
| Cho/Cr(n = 17) | Periphery | 0.63 (0.34–0.92) | 0.53 (0.23–0.83) |
| Lac/Cr(n = 17) | Periphery | 0.56 (0.25–0.86) | 0.53 (0.26–0.80) |
| Lac/NAA(n = 17) | Periphery | 0.57 (0.26–0.88) | 0.53 (0.24–0.82) |
ROI Region of Interest; OS-1 one-year overall survival; PFS-6 Six-month progression free survival; AUC area under the ROC curve; NAA N-acetylaspartate; Cr creatine; Cho choline-containing compounds; Lac lactate
Bold AUCs indicate significant predictions of outcome and italics AUCs indicate trends towards predicting outcome.
Fig 3.
ROC curves with the corresponding AUC for predictors (A. tumor NAA/Cho, B. peripheral NAA/Cho, and C. tumor Lac/Cr) using 1-year overall survival (OS-1) and of 6-month progression free survival (PFS-6).
MRSI metabolic ratios as predictor of 6-month progression free survival (PFS-6)
Six-month progression free survival was 65% (11/17). Neither NAA/Cho nor Cho/Cr was effective in predicting PFS-6 (Table 2). Lac/Cr in tumor was a significant negative predictor of PFS-6, indicating that higher Lac/Cr levels are associated with poorer outcome (AUC = 0.79, 95% CI: 0.54 to 1.00). In addition, Lac/NAA in tumor showed a trend as a negative predictor of PFS-6, indicating that higher Lac/NAA levels are associated with poorer outcome (AUC = 0.76, 95% CI: 0.49 to 1.00). ROC curves with the corresponding AUC for the significant predictors using PFS-6 are shown in Fig 3.
Correlations between MRSI and other imaging markers
In addition, we explored the correlations between MRS markers and MR imaging markers of vascularity (CBV, CBF, and ktrans) as well as between MRS markers and PET markers of tumor hypoxia (SUVmax). None of these markers were significantly associated with MRS markers (P >0.1) and Pearson correlation coefficient absolute values were below 0.42 (Table 3).
Table 3. Person Correlations between MRS markers and MRI markers of vascularity, CBV, CBF, and ktrans) as well as between MRS markers and PET markers of tumor hypoxia (SUVmax).
| Metabolic ratio | ROI | nrCBV (R) | nCBF (R) | Median ktrans (R) |
SUV max (R) |
|---|---|---|---|---|---|
| NAA/Cho | Tumor | -0.38 | -0.41 | -0.08 | -0.33 |
| Cho/Cr | Tumor | 0.24 | 0.28 | -0.02 | 0.03 |
| Lac/Cr | Tumor | -0.27 | -0.25 | -0.06 | -0.29 |
| Lac/NAA | Tumor | -0.02 | -0.01 | 0.23 | -0.18 |
| NAA/Cho | Periphery | -0.33 | -0.34 | 0.14 | -0.41 |
| Cho/Cr | Periphery | 0.17 | 0.20 | -0.27 | 0.11 |
| Lac/Cr | Periphery | -0.09 | -0.11 | 0.10 | -0.15 |
| Lac/NAA | Periphery | -0.01 | -0.01 | 0.33 | -0.04 |
(nRCBV: relative cerebral blood volume, corrected for leakage effects and normalized to normal-appearing white matter; nCBF: cerebral blood flow, normalized to normal-appearing white matter; ktrans: vascular permeability; SUV standardized uptake value
Discussion
This study reports the results of the 1H-MRSI data of the ACRIN 6684 multi-center imaging trial to assess tumor hypoxia in GBM using 18F-FMISO PET and MRI. We previously showed that increased tumor hypoxia as measured by 18F-FMISO SUVpeak was significantly associated with shorter OS-1. Furthermore, increased tumor perfusion (rCBV and rCBF,) and increased vascular permeability (median ktrans) were significantly associated with shorter PFS [4]. Since MRSI is able to measure metabolites that reflect tumor burden, it may be able to provide additional information to the previously reported MRI and PET parameters.
Our data suggest that the MRS marker NAA/Cho in the tumor and in the peritumoral periphery were significant predictors of survival. NAA/Cho or its inverse Cho/NAA have previously been described as reliable biomarkers of tumor prognosis and biomarkers for treatment response [7–10]. In our study, peritumoral NAA/Cho showed the highest prediction of OS-1. This may indicate that increased tumor infiltration into the peritumoral parenchyma reflects active tumor growth and subsequently poorer outcomes [24]. On the other hand, tumor spectra within the contrast enhancement reflect less active tumor where growth is stalled because of necrosis, lack of blood supply and/or hypoxia. These results also emphasize that MRSI, compared to a single voxel approach, is essential in the evaluation of brain tumors as it simultaneously covers multiple areas of the tumor environment.
In our study, increase in Lac/Cr in the tumor was a marker of poor prognosis for PFS-6 and increases in Lac/NAA in the tumor showed a trend associated with poor prognosis for PFS-6 and OS-1. The production of lactate, tumor acidosis, and hypoxia are commonly thought to be linked. However, some studies have shown that high lactate concentrations are not necessarily associated with hypoxia [20, 25, 26]. Cancer cells will produce Lac in the absence of hypoxia. Low blood perfusion rates, aerobic glycolysis, and increased glutaminolysis may also contribute to lactate accumulation in non-hypoxic areas [20, 27–29]. With the entire cohort of 42 patients included in the primary paper [4], our group showed that increased tumor hypoxia as measured by 18F-FMISO SUVpeak was significantly associated with poor outcome. This study, on a subset of the same subjects, shows increased Lac/Cr levels were significantly associated with shorter PFS-6. Interestingly, no correlation between Lac/Cr and 18F-FMISO SUVpeak was observed in this study, which could be due to a limited sample size, measured values assessed in different locations within the tumor and peritumoral parenchyma, or the possibility that both markers provide complementary information.
Thus, the lack of correlations between metabolic markers measured by MRSI, hypoxia measured by 18F-FMISO PET, and MRI markers of tumor perfusion (e.g. nrCBV, nrCBF and median K trans) remains an interesting area for further study. The interactions between perfusion, hypoxia, and metabolism reflect the complexity of tumor biology and likelihood of response to chemotherapy or radiation. The lack of correlation may also be attributable to the limited sample size of 17 patients. Currently, we have too few data to study whether the combination of hypoxia information from 18F-FMISO PET and lactate information from MRSI might be complementary and helpful. Thus, complementary multimodal PET and MR imaging that captures diverse biological information simultaneously may be needed to tease out the complexities of tumor biology and may warrant further investigations.
One of the major limitations of this study is the limited sample size, with only 17 out of 31 cases having evaluable MRSI data. However, the rejection of some of the MRSI data does not appear to have introduced significant bias into the analysis since this smaller subset was similar to the larger cohort based on demographic and survival characteristics. Another limitation of this study pertains to possible partial volume effects because each voxel may contain partial volume fractions of more than one tissue type. Finally, the MRSI scans in our study came from 4 different institutions with different magnetic field strengths and with 3 different vendor magnets and MRSI acquisition software packages, which may limit comparisons.
Clinical multi center trials that use MRS as a tumor biomarker have proven to be challenging [30]. Typical challenges include the spectral quality of MRS data including low SNR, incorrect voxel placements at the time of scan acquisition, volume averaging due to large voxel sizes (averaging of surrounding benign tissue), and insufficient separation of metabolite peaks, as well as inadequate water suppression due to suboptimal shimming [5, 18]. Specifically, from our study sites, the most common reasons for excluding data were the logistical difficulties where MRSI raw data files had not been saved or sites had not adhered to the acquisition protocol. Only 2 datasets were not interpretable due to low SNR. Consequently, better education and closer monitoring during clinical trials are needed to increase the number of analyzable MRSI data sets. For example, site visits by a MR spectroscopist or a point person at a site may help educating MR technologists to improve MRSI data acquisition and saving of the datasets. Finally, the implementation of state-of-the art MRSI methods that run with minimal user interaction may also help to alleviate these challenges which calls for more effort from the MRS user community and vendors to implement these advanced tools in clinical protocols.
However, despite these challenges, there have been several successful clinical trials using MRS as biomarkers [31]. One of the utilizations of MRSI in multi-center clinical trials pertains to the automatic classifications of brain tumors from MR spectra in adults [21, 32–37] and pediatric populations [38, 39]. Furthermore, MRSI has been used for prognoses in patients with glial tumors [40] or for therapeutic monitoring to determine the efficacy of medications [5]. In a prior trial by ACRIN (Protocol RTOG 0625/ACRIN 6677 “A Randomized Trial of Bevacizumab with Temozlomide in Recurrent Glioblastoma”), our group reported that increased levels of NAA/Cho and decreased levels of Cho/Cr at 8 weeks post bevacizumab-based therapy in patients with recurrent GBM correlated with PFS-6 and OS-1 [5].
In summary, despite the small sample size in the setting of a multi-center trial comprising different vendors, field strengths, and varying levels of expertise at data acquisition, MRS markers NAA/Cho, Lac/Cr and Lac/NAA predicted OS-1 and PFS-6 well. Thus, given the positive results from both ACRIN6677 and ACRIN 6684, optimizing MRSI acquisition for implementation of these promising biomarkers appears warranted in evaluating patients’ prognosis. More sophisticated analysis approaches using machine learning techniques to look at the entire spectra rather than individual metabolites from MRS and MRSI also hold great promise to build on our promising results.
Conclusion
Despite all aforementioned challenges, 1H MRSI measures were predictive of outcome in a group of patients with GBM treated using radiotherapy and temozolomide with information that appears distinct from and complementary to DCE/DSC-MRI and hypoxia PET. Therefore, standardized MRSI should be considered for incorporation into future multi-center clinical trials and may be useful in diagnosis and in risk stratifying patients.
Supporting information
(PDF)
“Multicenter, phase II assessment of tumor hypoxia in glioblastoma using 18F Fluoromisonidazole (FMISO) with PET and MRI”.
(PDF)
Acknowledgments
We like to thank the ACRIN team including Donna Hartfeil for critical project oversight and the ACRIN Data and Imaging Core, Lindsey Dymond, Bola Shodunke, James Gimpel and Lisa Cimino. Oversite of the trial was provided by Study Co-Chair Elizabeth R. Gerstner, MD (Massachusetts General Hospital, Email: egerstner@partners.org), Study Co-Chair James R. Fink, MD, (University of Washington Medical Center, Email: jrfink@u.washington.edu), Co-Chair David Mankoff, MD, PhD (University of Pennsylvania, Email: David.Mankoff@uphs.upenn.edu) and Study Statistician Zheng Zhang, PhD (Brown University, Email: zzhang@stat.brown.edu).
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA180794. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
In addition we would like to thank the participating site Radiology PIs and Neuro-oncologists that enrolled patients: Jason Rockhill (University of Washington), Glenn Lesser (Wake Forest University), Alexis Demopoulous (Mount Sinai), Ryan Murtagh (Moffitt Cancer Center), Richard Wahl (John Hopkins), Lilja Solnes (Weill Cornell), Jaishri Blakeley (John Hopkins), Jacob Dubroff (University of Pennsylvania), Arati Desai (University of Pennsylvania), Tammie Benzinger (Washington University), Joseph Simpson (Washington University), Janis O’Malley (University of Alabama Birmingham), John Fiveash (University of Alabama Birmingham), Jenny Hoang (Duke University), John Go (University of Southern California), and James Hu (University of Southern California), and the MRI/PET teams as well as all patients and their families for participating in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
American College of Radiology Imaging Network receives funding from the National Cancer Institute through the grants U01CA079778 and U01CA080098. DM receives funding from the National Cancer Institute through the grants U01CA190254 and U01CA148131. ER receives funding from the National Cancer Institute through the grant R01CA190901. OA receives funding from the National Cancer Institute through the grants K22CA178269 and R01CA211080. The URL of the National Cancer Institute is https://www.cancer.gov/. National Cancer Institute grants also include U01-CA080098, U01-CA079778, and NCTN-CA180794.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330 . [DOI] [PubMed] [Google Scholar]
- 2.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–37. doi: 10.1038/nrc2397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819 . [DOI] [PubMed] [Google Scholar]
- 4.Gerstner ER, Zhang Z, Fink JR, Muzi M, Hanna L, Greco E, et al. ACRIN 6684: Assessment of Tumor Hypoxia in Newly Diagnosed Glioblastoma Using 18F-FMISO PET and MRI. Clin Cancer Res. 2016;22(20):5079–86. doi: 10.1158/1078-0432.CCR-15-2529 ; PubMed Central PMCID: PMCPMC5065740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratai EM, Zhang Z, Snyder BS, Boxerman JL, Safriel Y, McKinstry RC, et al. Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677. Neuro Oncol. 2013;15(7):936–44. doi: 10.1093/neuonc/not044 ; PubMed Central PMCID: PMCPMC3688017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapalino O, Ratai EM. Multiparametric Imaging Analysis: Magnetic Resonance Spectroscopy. Magn Reson Imaging Clin N Am. 2016;24(4):671–86. doi: 10.1016/j.mric.2016.06.001 . [DOI] [PubMed] [Google Scholar]
- 7.Dyke JP, Sanelli PC, Voss HU, Serventi JV, Stieg PE, Schwartz TH, et al. Monitoring the effects of BCNU chemotherapy Wafers (Gliadel) in glioblastoma multiforme with proton magnetic resonance spectroscopic imaging at 3.0 Tesla. J Neurooncol. 2007;82(1):103–10. doi: 10.1007/s11060-006-9254-6 . [DOI] [PubMed] [Google Scholar]
- 8.Zhang K, Li C, Liu Y, Li L, Ma X, Meng X, et al. Evaluation of invasiveness of astrocytoma using 1H-magnetic resonance spectroscopy: correlation with expression of matrix metalloproteinase-2. Neuroradiology. 2007;49(11):913–9. doi: 10.1007/s00234-007-0271-8 . [DOI] [PubMed] [Google Scholar]
- 9.McKnight TR, Lamborn KR, Love TD, Berger MS, Chang S, Dillon WP, et al. Correlation of magnetic resonance spectroscopic and growth characteristics within Grades II and III gliomas. J Neurosurg. 2007;106(4):660–6. doi: 10.3171/jns.2007.106.4.660 . [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Catana C, Ratai EM, Andronesi OC, Jennings DL, Batchelor TT, et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71(11):3745–52. doi: 10.1158/0008-5472.CAN-10-2991 ; PubMed Central PMCID: PMCPMC3107375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankar T, Caramanos Z, Assina R, Villemure JG, Leblanc R, Langleben A, et al. Prospective serial proton MR spectroscopic assessment of response to tamoxifen for recurrent malignant glioma. J Neurooncol. 2008;90(1):63–76. doi: 10.1007/s11060-008-9632-3 . [DOI] [PubMed] [Google Scholar]
- 12.Deviers A, Ken S, Filleron T, Rowland B, Laruelo A, Catalaa I, et al. Evaluation of the lactate-to-N-acetyl-aspartate ratio defined with magnetic resonance spectroscopic imaging before radiation therapy as a new predictive marker of the site of relapse in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2014;90(2):385–93. doi: 10.1016/j.ijrobp.2014.06.009 . [DOI] [PubMed] [Google Scholar]
- 13.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson SJ. Multivoxel magnetic resonance spectroscopy of brain tumors. Mol Cancer Ther. 2003;2(5):497–507. . [PubMed] [Google Scholar]
- 15.Oh J, Henry RG, Pirzkall A, Lu Y, Li X, Catalaa I, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging. 2004;19(5):546–54. doi: 10.1002/jmri.20039 . [DOI] [PubMed] [Google Scholar]
- 16.Howe FA, Barton SJ, Cudlip SA, Stubbs M, Saunders DE, Murphy M, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003;49(2):223–32. doi: 10.1002/mrm.10367 . [DOI] [PubMed] [Google Scholar]
- 17.Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. 2005;2(2):197–214. doi: 10.1602/neurorx.2.2.197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–79. doi: 10.1148/radiol.13130531 ; PubMed Central PMCID: PMCPMC4263653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill SS, Thomas DG, Van Bruggen N, Gadian DG, Peden CJ, Bell JD, et al. Proton MR spectroscopy of intracranial tumours: in vivo and in vitro studies. J Comput Assist Tomogr. 1990;14(4):497–504. . [DOI] [PubMed] [Google Scholar]
- 20.Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155–71. doi: 10.1007/s00109-015-1307-x ; PubMed Central PMCID: PMCPMC4762928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerand ME, Pipas JM, Mamourian A, Tosteson TD, Dunn JF. Classification of biopsy-confirmed brain tumors using single-voxel MR spectroscopy. AJNR Am J Neuroradiol. 1999;20(1):117–23. . [PubMed] [Google Scholar]
- 22.Opstad KS, Ladroue C, Bell BA, Griffiths JR, Howe FA. Linear discriminant analysis of brain tumour (1)H MR spectra: a comparison of classification using whole spectra versus metabolite quantification. NMR Biomed. 2007;20(8):763–70. doi: 10.1002/nbm.1147 . [DOI] [PubMed] [Google Scholar]
- 23.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–4. . [DOI] [PubMed] [Google Scholar]
- 24.Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, et al. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia. 2009;57(12):1306–15. doi: 10.1002/glia.20850 . [DOI] [PubMed] [Google Scholar]
- 25.Hunt TK, Aslam R, Hussain Z, Beckert S. Lactate, with oxygen, incites angiogenesis. Adv Exp Med Biol. 2008;614:73–80. doi: 10.1007/978-0-387-74911-2_9 . [DOI] [PubMed] [Google Scholar]
- 26.Yaromina A, Quennet V, Zips D, Meyer S, Shakirin G, Walenta S, et al. Co-localisation of hypoxia and perfusion markers with parameters of glucose metabolism in human squamous cell carcinoma (hSCC) xenografts. Int J Radiat Biol. 2009;85(11):972–80. doi: 10.3109/09553000903232868 . [DOI] [PubMed] [Google Scholar]
- 27.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–65. . [PubMed] [Google Scholar]
- 28.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809 ; PubMed Central PMCID: PMCPMC2849637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–4. doi: 10.1126/science.1193494 . [DOI] [PubMed] [Google Scholar]
- 30.Weinreb JC, Blume JD, Coakley FV, Wheeler TM, Cormack JB, Sotto CK, et al. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy—results of ACRIN prospective multi-institutional clinicopathologic study. Radiology. 2009;251(1):122–33. doi: 10.1148/radiol.2511080409 ; PubMed Central PMCID: PMCPMC2663583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin AP, Rowland B, Griffiths JR. Clinical Trials that Utilize MRS as a Biomarker. eMagRes. 2016;5:1–8. [Google Scholar]
- 32.Julia-Sape M, Griffiths JR, Tate AR, Howe FA, Acosta D, Postma G, et al. Classification of brain tumours from MR spectra: the INTERPRET collaboration and its outcomes. NMR Biomed. 2015;28(12):1772–87. doi: 10.1002/nbm.3439 and erratum in Julia-Sape M, Griffiths JR, Tate RA, Howe FA, Acosta D, Postma G, et al. Classification of brain tumours from MR spectra: the INTERPRET collaboration and its outcomes. NMR Biomed. 2016;29(3):371. doi: 10.1002/nbm.3483. PubMed PMID: 26915795. [DOI] [PubMed] [Google Scholar]
- 33.De Stefano N, Caramanos Z, Preul MC, Francis G, Antel JP, Arnold DL. In vivo differentiation of astrocytic brain tumors and isolated demyelinating lesions of the type seen in multiple sclerosis using 1H magnetic resonance spectroscopic imaging. Ann Neurol. 1998;44(2):273–8. doi: 10.1002/ana.410440222 . [DOI] [PubMed] [Google Scholar]
- 34.Lukas L, Devos A, Suykens JA, Vanhamme L, Howe FA, Majos C, et al. Brain tumor classification based on long echo proton MRS signals. Artif Intell Med. 2004;31(1):73–89. doi: 10.1016/j.artmed.2004.01.001 . [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Gomez JM, Luts J, Julia-Sape M, Krooshof P, Tortajada S, Robledo JV, et al. Multiproject-multicenter evaluation of automatic brain tumor classification by magnetic resonance spectroscopy. MAGMA. 2009;22(1):5–18. doi: 10.1007/s10334-008-0146-y ; PubMed Central PMCID: PMCPMC2797843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortega-Martorell S, Ruiz H, Vellido A, Olier I, Romero E, Julia-Sape M, et al. A novel semi-supervised methodology for extracting tumor type-specific MRS sources in human brain data. PLoS One. 2013;8(12):e83773 doi: 10.1371/journal.pone.0083773 ; PubMed Central PMCID: PMCPMC3871596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tate AR, Majos C, Moreno A, Howe FA, Griffiths JR, Arus C. Automated classification of short echo time in in vivo 1H brain tumor spectra: a multicenter study. Magn Reson Med. 2003;49(1):29–36. doi: 10.1002/mrm.10315 . [DOI] [PubMed] [Google Scholar]
- 38.Vicente J, Fuster-Garcia E, Tortajada S, Garcia-Gomez JM, Davies N, Natarajan K, et al. Accurate classification of childhood brain tumours by in vivo (1)H MRS—a multi-centre study. Eur J Cancer. 2013;49(3):658–67. doi: 10.1016/j.ejca.2012.09.003 . [DOI] [PubMed] [Google Scholar]
- 39.Orphanidou-Vlachou E, Auer D, Brundler MA, Davies NP, Jaspan T, MacPherson L, et al. (1)H magnetic resonance spectroscopy in the diagnosis of paediatric low grade brain tumours. Eur J Radiol. 2013;82(6):e295–301. doi: 10.1016/j.ejrad.2013.01.030 . [DOI] [PubMed] [Google Scholar]
- 40.Negendank WG, Sauter R, Brown TR, Evelhoch JL, Falini A, Gotsis ED, et al. Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg. 1996;84(3):449–58. doi: 10.3171/jns.1996.84.3.0449 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
“Multicenter, phase II assessment of tumor hypoxia in glioblastoma using 18F Fluoromisonidazole (FMISO) with PET and MRI”.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.