Abstract
Key points
Shift work is highly prevalent and is associated with significant adverse health impacts.
There is substantial inter‐individual variability in the way the circadian clock responds to changing shift cycles. The mechanisms underlying this variability are not well understood.
We tested the hypothesis that light–dark exposure is a significant contributor to this variability; when combined with diurnal preference, the relative timing of light exposure accounted for 71% of individual variability in circadian phase response to night shift work.
These results will drive development of personalised approaches to manage circadian disruption among shift workers and other vulnerable populations to potentially reduce the increased risk of disease in these populations.
Abstract
Night shift workers show highly variable rates of circadian adaptation. This study examined the relationship between light exposure patterns and the magnitude of circadian phase resetting in response to night shift work. In 21 participants (nursing and medical staff in an intensive care unit) circadian phase was measured using 6‐sulphatoxymelatonin at baseline (day/evening shifts or days off) and after 3–4 consecutive night shifts. Daily light exposure was examined relative to individual circadian phase to quantify light intensity in the phase delay and phase advance portions of the light phase response curve (PRC). There was substantial inter‐individual variability in the direction and magnitude of phase shift after three or four consecutive night shifts (mean phase delay −1:08 ± 1:31 h; range −3:43 h delay to +3:07 h phase advance). The relative difference in the distribution of light relative to the PRC combined with diurnal preference accounted for 71% of the variability in phase shift. Regression analysis incorporating these factors estimated phase shift to within ±60 min in 85% of participants. No participants met criteria for partial adaptation to night work after three or four consecutive night shifts. Our findings provide evidence that the phase resetting that does occur is based on individual light exposure patterns relative to an individual's baseline circadian phase. Thus, a ‘one size fits all’ approach to promoting adaptation to shift work using light therapy, implemented without knowledge of circadian phase, may not be efficacious for all individuals.
Keywords: shift work, circadian, light, melatonin, phase shift
Key points
Shift work is highly prevalent and is associated with significant adverse health impacts.
There is substantial inter‐individual variability in the way the circadian clock responds to changing shift cycles. The mechanisms underlying this variability are not well understood.
We tested the hypothesis that light–dark exposure is a significant contributor to this variability; when combined with diurnal preference, the relative timing of light exposure accounted for 71% of individual variability in circadian phase response to night shift work.
These results will drive development of personalised approaches to manage circadian disruption among shift workers and other vulnerable populations to potentially reduce the increased risk of disease in these populations.
Introduction
Night shift work causes misalignment between endogenous circadian rhythms and the imposed work–rest schedule, with negative consequences for daytime sleep and alertness on shift (Rajaratnam & Arendt, 2001; Jensen et al. 2016). Theoretically, the circadian system is able to adapt to night schedules by shifting the timing of the sleep propensity rhythm such that the peak occurs during the latter half of the daytime rest period (Eastman & Martin, 1999). Re‐aligning the circadian pacemaker with the imposed sleep–wake cycle leads to improved sleep and neurobehavioural outcomes (Baehr et al. 1999; Crowley et al. 2004; Boivin et al. 2012; Chapdelaine et al. 2012; Boudreau et al. 2013). Under some circumstances, such as the isolated environments of Antarctica (Ross et al. 1995) and North Sea oil platforms (Barnes et al. 1998a), complete circadian adaptation to shift work has been observed. Circadian adaptation, however, is reported to be rare in most typical field settings, even after numerous consecutive night shifts (Folkard, 2008; Ferguson et al. 2012; Jensen et al. 2016).
Preliminary findings in laboratory and field settings indicate there is substantial inter‐individual variability in the circadian phase shifting response to night shift work in terms of both the magnitude (Barnes et al. 1998a; Hansen et al. 2010) and the direction of the shift (Dumont et al. 2001; Gibbs et al. 2007). Variation in the magnitude of the phase shift is observed in offshore settings; even when all workers phase delay over multiple consecutive night shifts, some delay more than others (Hansen et al. 2010). At an individual level, phase advance and phase delay shifts can occur in response to the same abrupt change from night to day schedules (Deacon & Arendt, 1996; Gibbs et al. 2007). Factors that determine the direction and magnitude of phase resetting in shift workers have not been systematically examined.
The light–dark cycle is the primary determinant of resetting circadian phase (Duffy & Wright, 2005), capable of inducing phase shifts either by advance or delay depending on the timing of exposure relative to current circadian phase (Czeisler & Gooley, 2007). Light phase response curves (PRCs) describe the circadian response to light relative to the circadian phase at which light exposure occurs (Khalsa et al. 2003; St Hilaire et al. 2012). By abruptly shifting the timing of the sleep–wake cycle, night workers are exposed to more light during the biological night compared to day workers, as well as exposure to bright light during the morning commute home (Dumont et al. 2001, 2012). Seasonal differences in the direction and magnitude of phase shift are also observed in offshore shift work settings, suggesting differences in the timing of natural light exposure may influence the circadian response to night work (Barnes et al. 1998b). Dumont et al. (2001) examined 24‐h light exposure patterns in night workers, finding that workers with a later circadian phase were exposed to higher intensity light in the evening and darker sleeping environments during the day compared to those with advanced phase. This study did not assess circadian phase prior to the night shifts, however, so the possible influences of baseline phase differences were not considered.
Few studies have examined light exposure relative to individual circadian phase in night workers (e.g. Dumont et al. 2001). Large variability in phase is observed between individuals in both healthy controls (Sack et al. 1992; Duffy & Czeisler, 2002; Martin & Eastman, 2002; Crowley et al. 2003; Burgess et al. 2003b; Wright et al. 2005; Sletten et al. 2010) and night shift workers (Deacon & Arendt, 1996; Dumont et al. 2001; Hansen et al. 2010; Ftouni et al. 2015). As the circadian response to light is phase‐dependent, the substantial inter‐individual variability that exists in circadian phase in night workers means that circadian phase adjustment in response to night shift work is likely to differ between individuals irrespective of exposure to the same light–dark cycle.
The current study aimed to examine the temporal dynamics of circadian phase shifting from baseline, over multiple consecutive night shifts in nursing and medical staff, and to identify factors that predict individual phase shifting response, in particular the circadian timing of light exposure.
Methods
Ethical approval
The study protocol was approved by the Austin Health and Monash University Human Research Ethics Committees and conformed to the standards set by the latest revision of the Declaration of Helsinki. All participants provided written informed consent prior to enrolment and received AUS$100 payment upon completion of the study.
Participants
Nursing and medical staff were recruited from an intensive care unit (ICU) at Austin Health, Heidelberg, Australia. Nurses worked variable patterns of day (07:00–15:30), evening (15:00–21:30) and night (21:00–07:30) shifts. Medical staff worked a consistent rotating roster of seven consecutive day shifts (08:00–20:30), 7 days off, and seven consecutive night shifts (20:00–08:30). Participants were recruited via scheduled in‐service presentations, email advertising and targeted recruitment during shifts. Staff members were enrolled if their roster contained at least 4 days or evening shifts or days off followed by at least three or four consecutive night shifts during the study period. Work schedules were prospectively confirmed (detailed below): five participants had a documented night shift in the 3–4 weeks prior to participation. Of them, one worked a night shift 8 days prior to baseline, two had night shifts 14 days prior, one 16 days prior and another 28 days prior to baseline phase collection. Due to the rotating roster, doctors had 3 weeks in between night shift rotations.
Questionnaires
Participants completed an online sleep–health questionnaire including demographic information [age, sex, body mass index (BMI) and shift work history] and subjective measures of general health and sleep. The Pittsburgh Sleep Quality Index (PSQI; Cronbach's alpha = 0.80) is a 19‐item self‐rated questionnaire, and was used to assess sleep quality and disturbances over the past month (Buysse et al. 1989; Carpenter & Andrykowski, 1998). The Epworth Sleepiness Scale (ESS; Cronbach's alpha = 0.88) was used to assess daytime sleepiness, where participants were asked to rate on a four‐point scale the chances that they would fall asleep in eight common daily situations (Johns, 1991, 1992). The Morningness–Eveningness Composite Questionnaire (MEQ; Cronbach's alpha = 0.87) is a 13‐item questionnaire that was used to assess individual preferences associated with morning or evening activities (Smith et al. 1989). Participants maintained a work log for the duration of their involvement where they recorded the start and end times (including breaks) for all shifts. Shift types were prospectively confirmed by timesheet records maintained by hospital administration. Of the 346 shifts recorded by participants, 314 shifts matched timesheet records [level of agreement 90.8%; work diaries were used for 92.8% of shifts (321 entries), timesheet records were used for 6.4% of shifts (22 entries), scheduled roster for 0.29% of shifts (1 entry) and 0.58% could not be used (2 diary entries from separate participants)].
Individual light exposure
For the duration of the study, participants wore a wrist activity monitor (Actiwatch Spectrum or Spectrum Plus, Philips Respironics, Bend, OR, USA) on the non‐dominant wrist to monitor light exposure continuously in 1‐min epochs (medium sensitivity; 40 activity counts per epoch). Participants were asked to wear the device at all times, except when the device could be damaged (e.g. while showering) or would interfere with the hospital operational requirements, and to ensure that the light sensor remained uncovered at all times (e.g. by sleeves).
Sleep–wake activity
Participants maintained daily sleep diaries throughout the study, recording sleep and wake times for each sleep episode, which were used to determine the actigraphy analysis interval for each sleep episode (Actiware 6 software, Philips Respironics). If there was a substantial reduction in activity ≥30 min prior to or after self‐reported bedtime, bedtime was adjusted to occur at the start of the period of reduced activity. Similarly, if there was a substantial increase in activity ≥30 min prior to or after self‐reported wake time, wake time was adjusted to the start of the increase in activity (Ftouni et al. 2015). Actigraphy analysis was used to determine sleep and wake times when diary information was not available (10%, 12 sleep entries). Sleep and wake times were used to clean light data for artefacts (described below).
Circadian phase
The rhythm of the urinary melatonin metabolite, 6‐sulphatoxymelatonin (aMT6s), was used as a marker of circadian phase (Bojkowski et al. 1987; Lockley et al. 1997). Participants collected sequential urine samples at approximately 4‐h intervals (8 h during the sleep episode) for 48 h as previously described (Ftouni et al. 2015). Phase assessments were timed to occur at last rostered day shift (baseline), on their first night shift (night 1) and over the final consecutive night shift (night 3 or 4). In a subset of participants, circadian phase was also assessed on a 6th or 7th consecutive night shift. For each sample, participants recorded void times, sample collection time and the total volume. A 5 mL aliquot was stored frozen at −20°C and subsequently analysed for aMT6s concentration using radioimmunoassay (Aldhous & Arendt, 1988) at the Adelaide Research Assay Facility (University of Adelaide, Australia), using reagents purchased from Stockgrand Ltd (University of Surrey, Guildford, UK). Samples were assayed in two batches; the intra‐assay coefficients of variation (CV) were 7.4 and 6.7%, respectively, and the inter‐assay CVs were 8.7, 6.3 and 7.7% at 2.7, 11.5 and 19.7 ng mL−1, respectively (batch 1, 30% n = 376 samples), and 14.9, 3.5 and 5.4% at 5.7, 24.1 ng and 41.9 ng mL−1, respectively (batch 2, 70% n = 881 samples). The minimum detectable concentration was 0.5 ng mL−1.
Data analysis
The concentration of aMT6s in each sample (ng mL−1) was multiplied by the volume of the sample and divided by duration of the collection interval to obtain aMT6s concentration per hour (ng h−1), and then plotted relative to the midpoint of the time between the sample and the previous sample. Cosinor analysis of aMT6s values was conducted to determine the acrophase (peak) time of the aMT6s rhythm (Nelson et al. 1979). Circadian phase measured on the day shift was taken as baseline phase, and assumed to be the same immediately prior to night shifts: sleep and work patterns in between collection and night shifts were checked for major changes in behaviour that would be inconsistent with this assumption. The degree of phase shift was calculated as the difference in clock time between aMT6s acrophase at baseline and the final night shift.
Bright light exposure data were extracted from wrist actigraphy devices. To account for artefact data due to coverage of the sensor by clothing, wake time values <1 lux were excluded from analysis (Obayashi et al. 2016). Light data were also excluded when Actiware software indicated the device was off‐wrist. Light data were then log‐transformed as previously described (Dumont et al. 2001).
Given that the circadian response to light depends on an individual's phase at the time of exposure, light patterns were examined relative to individual circadian phase during the major advance and delay regions of the human PRC to light (Khalsa et al. 2003; Fig. 1). We assume aMT6s acrophase as the approximate ‘crossover point’ for the PRC, because previous studies have reported an average time difference of approximately 2 h between plasma melatonin and aMT6s acrophase (Nowak et al. 1987; Arendt, 1995; Ross et al. 1995; Benloucif et al. 2008), and the reported time difference of 1.8 h between plasma melatonin peak and core body temperature minimum (Shanahan & Czeisler, 1991). To examine light relative to circadian phase over each night shift, a daily aMT6s acrophase was estimated using linear interpolation between the clock time of acrophase on baseline and final night shifts. In participants with large differences in phase (>1 h) between baseline assessment (day shifts) and night 1 assessment, sleep and wake times were inspected to confirm whether baseline phase could be used as an accurate estimate of acrophase prior to commencing night shifts.
Figure 1. Phase response curve to light (adapted from Khalsa et al. 2003), where 0 on the abcissa represents timing of core body temperature minimum (CBTmin) in healthy subjects.
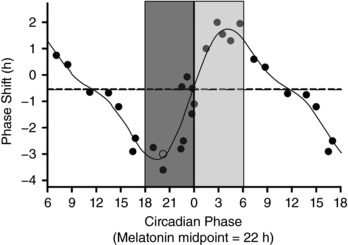
Light exposure prior to CBTmin induces phase delays, while exposure after CBTmin leads to phase advance shifts. We used aMT6s acrophase as a proxy for CBTmin. Light exposure was examined in the 90 deg before and after individual acrophase times, where light is predicted to induce the greatest phase delays or advances, depicted by the bars either side of 0. Dark grey indicates the delay zone, light grey the advance zone. Each 90 deg bin was approximately 6 h. Reproduced in part from Khalsa et al. (2003).
To determine the amount of light exposure during the delay and advance zones of the PRC, light data were averaged in 90 deg bins for the interval before (‘delay’ zone) and after (‘advance’ zone) acrophase for the day immediately prior to, and over each night shift. Each 90 deg bin was calculated individually for each subject to account for variability in phase shift over night shifts, such that 360 deg equalled 24 h plus the individual's phase shift per day. The difference in exposure to ‘delaying’ versus ‘advancing’ light (the difference in average lux 90 deg before versus 90 deg after acrophase) was examined at assumed baseline, over each night shift (night 1, night 2, night 3, night 4), and averaged across all night shifts. If more than 50% of data for a bin was missing, data for that bin were excluded from the analyses.
Statistical analyses were conducted using SPSS version 23 (SPSS Inc., Chicago, IL, USA). Independent t tests were used to compare acrophase time at baseline and final night shift, magnitude of phase shift, and number of days between baseline phase assessment and first night shift between participants who worked three versus four night shifts. Pearson correlations were used to examine the relationship between phase shift and potential explanatory factors (baseline phase, age, MEQ, BMI and difference in light between ‘delay’ and ‘advance’ zones on each night shift).
Based on the outcome of the correlation analysis, multiple linear regression was used to examine the proportion of variance in phase shift explained by the difference between delay and advance light exposure (at baseline and each night shift) in combination with morningness–eveningness score, BMI, age and acrophase at baseline.
Results
Data retention
Of 41 participants who completed data collection, six (15%) did not have adequate aMT6s data (incomplete collection or poor quality aMT6s rhythm determined by cosinor analysis), eight (20%) participants did not collect sufficient urine samples for the final night shift, and two (5%) had documented use of melatonin during the urine collection period. Therefore, data from 25 participants [21 nurses (16 female); 4 doctors (3 female)], aged 33.4 ± 8.4 years (mean ± SD), were included in the analyses. Demographics, subjective sleep quality, shift work history and shift schedules immediately prior to baseline phase assessment are reported in Table 1. An additional three participants had aMT6s data at baseline and on a 6th or 7th night shift, plus one of the previous data set who had a repeated phase assessment on the 7th night shift; these participants were also included as a separate subset of circadian phase data, but were excluded from subsequent light analysis.
Table 1.
Characteristics of participants included in acrophase dataset (n = 25)
| 3 Night shifts | 4 Night shifts | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Min. | Max. | n | Mean (SD) | Min. | Max. | n | Mean (SD) | Min. | Max. | |
| Demographics | ||||||||||||
| Sex (male, female) | 3,9 | 3,10 | 6,19 | |||||||||
| Occupation (nurse, doctor) | 12,0 | 9,4 | 21,4 | |||||||||
| Age (years) | 33 (7) | 24 | 45 | 33 (10) | 25 | 58 | 33 (8) | 24 | 58 | |||
| BMI (kg/m2) | 24 (4) | 16 | 30 | 23 (3) | 17 | 30 | 24 (4) | 16 | 30 | |||
| PSQI | 7 (3) | 2 | 12 | 5 (2) | 3 | 8 | 6 (2) | 2 | 12 | |||
| ESS | 6 (4) | 1 | 11 | 6 (3) | 2 | 11 | 6 (3) | 1 | 11 | |||
| MEQ | 40 (4) | 35 | 49 | 37 (5) | 27 | 45 | 38 (5) | 27 | 49 | |||
| Circadian timing | ||||||||||||
| Baseline acrophase (h:min) | 4:11 (1:10) | 2:18 | 6:45 | 4:31 (1:02) | 2:45 | 6:19 | 4:21 (1:05) | 2:18 | 6:45 | |||
| 1st Night acrophase (h:min) | 4:34 (1:01) | 3:20 | 5:53 | 4:42 (1:04) | 2:49 | 6:19 | 4:39 (1:02) | 2:49 | 6:19 | |||
| 3rd or 4th Night acrophase (h:min) | 5:20 (1:11) | 3:37 | 6:46 | 5:16 (2:10) | 1:51 | 8:24 | 5:18 (1:37) | 1:51 | 8:24 | |||
| Phase shift (h:min) | −1:09 (1:31) | −2:41 | 3:07 | −00:45 (1:47) | −3:32 | 1:28 | −1:08 (1:39) | −3:43 | 3:07 | |||
| Shifts prior to baseline acrophase (days) | ||||||||||||
| No. day shifts | 5 (2) | 2 | 8 | 6* (4) | 0 | 14 | 6 (3) | 0 | 14 | |||
| No. evening shifts | 5 (2) | 1 | 9 | 3* (3) | 0 | 10 | 4 (3) | 0 | 10 | |||
| No. days off | 7 (2) | 3 | 10 | 6 (6) | 2 | 11 | 7 (2) | 2 | 11 | |||
| No. other | 1 (1) | 0 | 3 | 2* (3) | 0 | 9 | 1 (3) | 0 | 9 | |||
| No. night shifts | 0 (1) | 0 | 3 | 1* (3) | 0 | 7 | 1 (2) | 0 | 7 | |||
| Total days prior to baseline | 18 (4) | 6 | 21 | 17 (6) | 3 | 29 | 18 (5) | 3 | 29 | |||
| Shift work history (years) | ||||||||||||
| Day shifts | 10.50 (6.16) | 3 | 23 | 7.08 (8.03) | 0 | 30 | 8.72 (7.25) | 0 | 30 | |||
| Evening shifts | 10.50 (6.16) | 3 | 23 | 7.00 (8.12) | 0 | 30 | 8.68 (7.32) | 0 | 30 | |||
| Night shifts | 11.00 (5.86) | 3 | 23 | 7.54 (7.59) | 0 | 30 | 9.20 (6.90) | 0 | 30 | |||
Note: BMI = body mass index, PSQI = Pittsburgh Sleep Quality Index, ESS = Epworth Sleepiness Scale, MEQ = Morningness Eveningness Questionnaire. * P < 0.05, three night shifts versus four night shifts. Negative phase shift indicates phase delay; positive phase shift indicates phase advance.
For three participants (nurses), phase on day shifts was unavailable so the 48‐h urine collection including the first night shift was used as the baseline. For seven participants, one or both urine collection periods were shortened due to operational constraints, with urine collected over 24–30 h. In another seven participants one or both collections were shortened due to collection errors. Included collections (50 collections; 25 participants) had a significant cosinor fit (α set 0.10; 94% were P < 0.05, 98% were P < 0.10, one collection was P = 0.11).
Of the 25 participants with adequate aMT6s data, four had insufficient light data due to actiwatch malfunction or non‐compliance; one subject had no ‘delay’ light data at baseline, so could not be included in analyses of relative difference in amount of delaying and advancing light at baseline. Subsequently 20 participants were included in light analyses at baseline and the regression analysis.
Circadian phase
At baseline, the mean aMT6s acrophase time was 4:21 ± 1:05 h (range 2:18–6:45 h). After three or four consecutive night shifts, mean acrophase time was 5:18 ± 1:37 h (range 1:51 to 8:24 h; Fig. 2 A). We observed a greater range in acrophase time in workers after four night shifts compared to three (6:33 and 3:08 h represent the range in hours and minutes respectively). There were no significant differences in acrophase at baseline or final night shift between participants working three or four night shifts (t 23 = −0.74, P = 0.850 and t 23 = 0.09, P = 0.112, respectively; Table 1). There was no relationship between baseline acrophase and MEQ score (r = −0.05, P = 0.798), although there was a trend towards a relationship between acrophase on the final night shift and MEQ scores (r = −0.39, P = 0.052), indicating that individuals with greater eveningness scores had a later final night acrophase time.
Figure 2. aMT6s acrophase over the shift schedule.
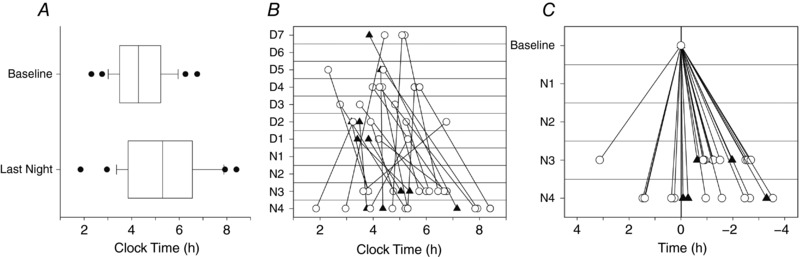
A, boxplots of aMT6s acrophase time at baseline and at the final successive night shift. Outliers are shown as closed circles. B, clock time of aMT6s acrophase at baseline (D1–D7) and on final night shift (N3 or N4) for each participant plotted by study day. Baseline acrophase is plotted on the day aMT6s was measured, and is assumed to be acrophase immediately prior to night shifts. C, change in aMT6s acrophase time from baseline to third (N3) or fourth (N4) night shift (acrophase at baseline minus acrophase at final night shift). Negative time indicates a phase delay (acrophase occurred at a later clock time after working night shifts compared to baseline), and positive indicates a phase advance (acrophase occurred at an earlier clock time after night shifts than at baseline). Men are represented by closed triangles and women by open circles.
Figure 2 B and C shows the variability in direction and magnitude of aMT6s phase shift between individuals. From baseline to the final night shift, 19 participants phase delayed (shift −1:40 ± 1:00 h, range −0:04 to −3:32 h), and six participants phase advanced (shift 1:20 ± 1:02 h, range 0:15–3:07 h). Sex differences in the direction of phase shift were observed, with no men phase advancing compared to a third (32%) of the women (male range: −0:04 h to −3:18 h delay; female range: +3:07 h advance to −3:32 h delay). The magnitude of the phase shift did not differ significantly between participants working three or four consecutive night shifts (t 23 = −0.58, P = 0.165).
Due to the variability in shift schedules, there were a different number of days between the baseline phase assessment and first night shift [2.6 ± 2.4 days (mean ± SD), range 0–7 days]. There was no relationship in number of days between baseline and night shift measurements and the magnitude of phase shift (r = −0.11, P = 0.613).
In a small subset of participants who worked six (n = 1) or seven (n = 3) consecutive night shifts the mean acrophase at baseline was 4:24 ± 2:22 h (range 2:46 to 7:54 h). After six or seven night shifts there was mean acrophase time of 9:16 ± 4:15 h (range 5:04 to 14:54 h). All four participants phase delayed, with a large range in the magnitude of observed phase shift (range −1:57 to −7:14 h).
Variables associated with phase shift
Figure 3 illustrates light exposure in key phase shifting zones for each individual. A significant positive association was found between phase shift and light exposure relative to circadian phase, with those receiving a greater proportion of light in the advance zone more likely to phase advance, and those with more delay‐zone light were more likely to phase delay (Figure 4). This relationship was observed when measuring light exposure at baseline (r 2 = 0.27, P = 0.019), during the second night shift (r 2 = 0.41, P = 0.003), third night shift (r 2 = 0.24, P = 0.032), and light averaged over all night shifts (r 2 = 0.33, P = 0.007). There was no statistical difference in the amount of light (difference in average delaying and advancing light, relative to individual phase) over baseline (t 18 = −0.44, P = 0.663) or the first three night shifts (night 1, t 18 = −0.49, P = 0.628; night 2, t 18 = −0.68, P = 0.507; night 3, t 17 = −0.10, P = 0.926) between those who worked three or four night shifts. The magnitude of phase shift was not significantly associated with the acrophase time at baseline (r 2 = 0.12, P = 0.084), age (r 2 = 0.02, P = 0.533), BMI (r 2 = 0.13, P = 0.073) or diurnal preference (MEQ; r 2 = 0.12, P = 0.084). Acrophase on the final night shift was strongly associated with phase shift (r 2 = 0.61, P < 0.0001), indicating the individuals with later acrophase had responded to night shifts with larger phase delays.
Figure 3. Light exposure relative to circadian phase for each participant.
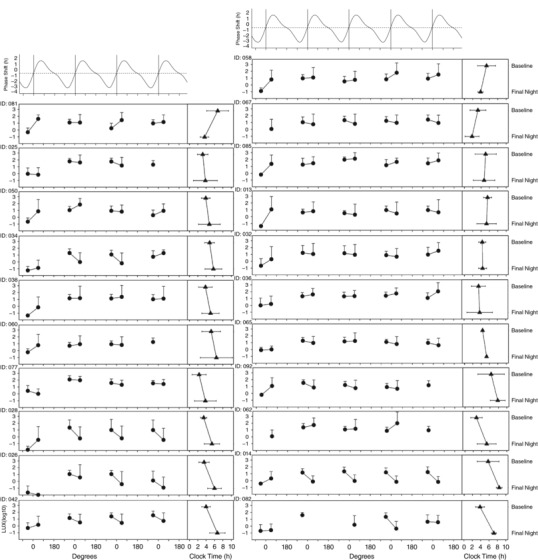
The circles represent log10 lux averaged in each delay (90 degs before acrophase) and advance (90 degs after acrophase) zone at baseline, first night shift (N1), second night shift (N2), third night shift (N3) and fourth night shift (N4). Light is averaged relative to individual circadian phase: the x‐axis represents circadian degrees (360 deg = 24+daily phase shift), such that 0 indicates acrophase each day. Triangles represent aMT6s acrophase in clock time at baseline and final night shift (error bars show 95% confidence intervals). The difference between light in delay compared to advance zones was examined at baseline and each night shift to examine the relationship with the direction of phase shift for each participant.
Figure 4. Relationship between phase shift and the difference between delay and advance light in the 90 deg either side of acrophase time at baseline, first night shift (N1), second night shift (N2), third night shift (N3), fourth night shift (N4) and averaged over all night shifts (Mean Night).
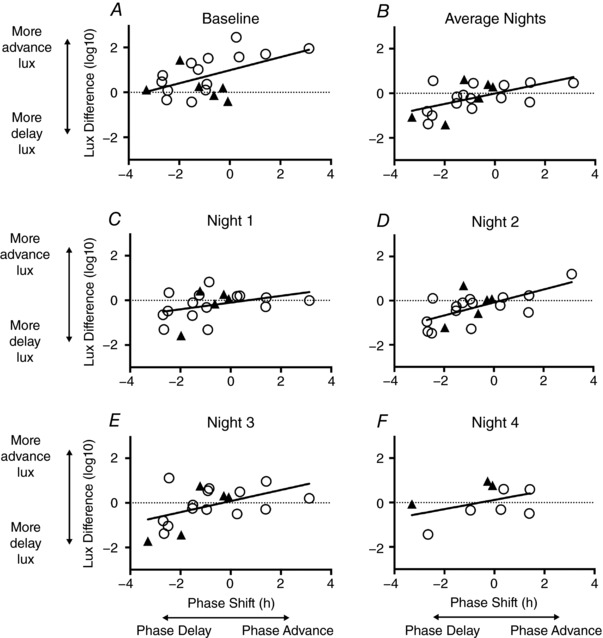
Negative phase shift indicates phase delay; positive phase shift indicates phase advance. Negative lux difference indicates a greater amount of delaying light (90 deg before acrophase), while positive lux difference indicates a greater amount of advancing light exposure (90 deg after acrophase) compared to delaying. Men are represented as closed triangles, women as open circles.
Regression analysis
Multiple linear regression demonstrated that the difference between advance‐ and delay‐zone light exposure at baseline, and averaged across all night shifts, accounted for 47% of the variance in phase shift between individuals (adjusted r 2 = 0.54, P = 0.001). Adding MEQ score improved the model, accounting for 71% of the variability in phase shift (adjusted r 2 = 0.71, P = < 0.001; Fig. 5). The regression model predicted a phase shift within ±30 min in 45% of individuals, and within ±60 min in 85%. For additional regression models fitted, see the Supporting Information (Table S1).
Figure 5. Predicted vs. actual phase shift for each participant from two models.
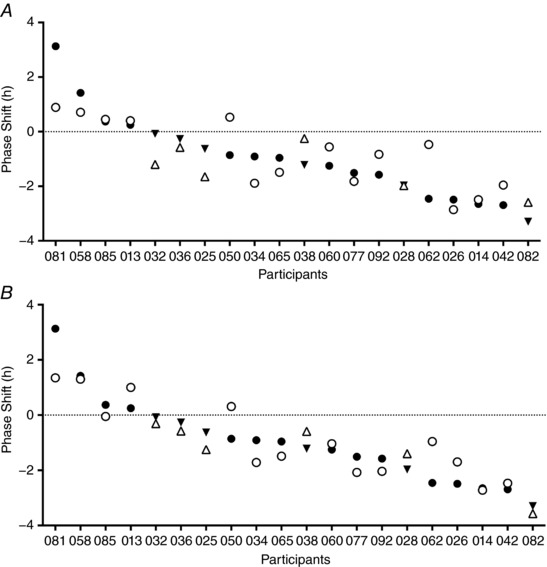
A, phase shift predictions calculated using regression model incorporating the difference between light in the delay and advance zones at baseline, and averaged across the night shifts; B, phase shift predictions calculated using a regression model using the difference between the delay and advance zones at baseline, averaged across the night shifts, and MEQ score. Predictions using this model (B) show less prediction error compared to predictions obtained without including MEQ score (A). Filled symbols indicate actual phase shift; open symbols indicate predicted phase shift. Men are represented by triangles, women by circles. Negative phase shift = phase delay, positive = phase advance. Participants are ranked according to their phase shift.
Discussion
This study demonstrates significant inter‐individual variability in the direction and magnitude of circadian phase shift in response to multiple consecutive night shifts in nursing and medical staff working rotating shift schedules in the ICU. Furthermore, we showed that this variability can largely be explained by individual differences in the amount of light exposure in key phase shifting times, predicted by the human PRC to light. Finally, we found that 71% of the observed variability in the phase shift response to night shift work can be explained by a combination of the distribution of light exposure relative to individual circadian phase and diurnal preference. Using these variables, we are able to predict the magnitude of phase shift to within ±60 min in 85% of individuals (which is substantial given the 6.5 h range in phase shifts observed). These findings support the concept that differences in light exposure relative to circadian phase strongly contribute to inter‐individual variability in circadian response to night shift work.
We observed a range of ∼4.5 h in the timing of aMT6s acrophase at baseline, which is consistent with previous findings in individuals on regular diurnal schedules assessed in the laboratory (Wright et al. 2005; Sletten et al. 2010). Although it is difficult to determine a true baseline phase in shift workers, all workers had at least 7 days of confirmed day‐active schedules prior to their baseline phase assessment. This variability at baseline may reflect differences in underlying circadian physiology such as intrinsic period (Duffy et al. 2001; Wright et al. 2001), differing light–dark exposure on day shifts or longer term differences in work schedules not captured in the work rosters studied.
The range in acrophase time after night shifts we observed is similar to that reported in previous simulated night shift and field assessments of phase in shift workers (Barnes et al. 1998a; Crowley et al. 2003; Hansen et al. 2010; Ftouni et al. 2015). While we saw a larger range in acrophase time after four night shifts compared to three nights, this was not explained by group differences in average phase shift, or light exposure patterns. After six or seven night shifts, the range in acrophase was even larger (9.8 h), despite the small subset sample size. Previous work in operational settings has observed larger phase shifts with increased number of consecutive night shifts (Barnes et al. 1998a; Jensen et al. 2016). It may be that the additional time on the changed light–dark cycle allowed for some of our participants to continue phase shifting over the fourth night shift. Together, these findings provide evidence that the timing of aMT6s acrophase varies considerably in night shift workers, both on day and on night shift schedules.
A large range in both the direction and the magnitude of phase shift was observed over 3–4 night shifts, indicating considerable inter‐individual variability in the circadian response to night shifts. While previous findings indicate there is inter‐individual variability in circadian response to changes in light–dark schedules in highly controlled laboratory (Deacon & Arendt, 1996) or geographically isolated field settings (Barnes et al. 1998a,b; Gibbs et al. 2007), this study extends previous work to demonstrate considerable variation between individuals in the direction and magnitude of phase shift response to night work in a naturalistic setting. The variability in phase shifts observed in this study highlights the importance of investigating individual‐level data when assessing circadian response to shift schedules and the subsequent implications for safety on shift. Significant performance impairments have been observed in night workers tested within ±3 h either side of aMT6s acrophase (Ftouni et al. 2015). Based on these findings, most workers in our sample would therefore have been at work or commuting home at a biologically high‐risk time, particularly if the aMT6s acrophase had shifted to occur in the first hours of the daytime sleep. Conversely, individuals who phase advanced would be shifting the nadir in performance earlier in the evening, and thus be at work or possibly commuting to work at a similarly high‐risk biological time.
Eastman & Martin (1999) propose that partial adaptation (i.e. shifting melatonin peak into the first half of the daytime sleep episode) is sufficient to see improvements in performance and safety on shift. After three or four consecutive night shifts we observed one subject with acrophase occurring after 08.00 h, and two later than 07.00 h; none of the participants started their daytime sleep prior to aMT6s acrophase time. Therefore, by this criterion, none of our subjects could be considered even partially adapted to night work after three or four nights. In the subset of participants who worked six or seven night shifts, two met criteria for partial adaptation to night shift, with acrophase time occurring within the first half of the daytime sleep episode. One of these participants showed a clear gradual delay in sleep timing in the 7 days prior to the night shift, indicating a delay in the timing of light exposure conducive to phase delays. Previous findings of partial adaptation to night shift were observed after a larger number of night shifts (more than seven nights) and/or followed interventions specifically designed to promote phase shifts by manipulating light exposure (Eastman & Martin, 1999; Boivin & James, 2002; Crowley et al. 2003), or were in remote offshore settings with limited exposure to natural sunlight (Barnes et al. 1998a,b; Gibbs et al. 2007; Hansen et al. 2010). Our findings indicate that it is unlikely that workers in a hospital setting will adapt to night schedules within three or four nights without a substantial targeted intervention that modifies light–dark exposure. This may have implications for the wellbeing of such night shift workers. Gumenyuk et al. (2012) found that circadian phase in workers who met criteria for Shift Work Disorder was within the range of that observed for day‐active workers after being exposed to at least three consecutive night shifts. In contrast, those without Shift Work Disorder symptoms showed markedly delayed circadian phase in response to the same exposure to night shifts. These findings suggest that lack of circadian adaptation to night shift may contribute to shift work intolerance and clinical manifestations such as sleepiness and insomnia, which are the hallmark symptoms of Shift Work Disorder.
We did not observe any phase advances in the six males included in the analysis, compared to a range in phase shift direction in females (6 phase advanced, 13 phase delayed). This apparent sex difference was not explained by differences in the number of night shifts, occupation or diurnal preference, nor by differing light exposure patterns. Phase advances have been previously observed in males in response to light pulses in the laboratory (Warman et al. 2003) and in offshore shift work settings (Gibbs et al. 2007). It is not clear whether the difference in direction of phase shift between sexes is due to differences in underlying physiology [e.g. sex differences in length of the intrinsic period (Duffy et al. 2011; Eastman et al. 2017) or sensitivity to phase shifting effects of light (Goel & Lee, 1995; Karatsoreos et al. 2011)], or whether we did not see phase advances in males due to the small sample size.
The difference between amount of phase delaying and phase advancing light exposure showed a strong relationship with the direction and magnitude of phase shift across the schedule, supporting the hypothesis that the individual pattern of light exposure relative to circadian phase is a major determinant of circadian response to shift work. Previous work has documented the 24‐h profile of light exposure in night workers, and compared the light profiles with circadian phase position at the end of a series of night shifts (Dumont et al. 2001). This study found that workers with a delayed circadian phase had greater light exposure during the evening compared to those with advanced phase. We have extended this work to examine light exposure patterns relative to individual circadian phase, over a measured phase shift across consecutive night shifts. In doing so we quantified the pattern of light exposure, based on a PRC established in the laboratory (Khalsa et al. 2003), to make predictions about when the circadian pacemaker is likely to be most sensitive to the phase shifting effects of light. By examining the difference between the amount of phase delaying and phase advancing light, we were able to estimate the net phase shifting signal of light exposure for each individual. This suggests that, as expected, light exposure plays a critical role in determining circadian response to shift work in a field setting, in a manner that is predictable from a PRC.
By extension, these results indicate that manipulation of light exposure is an important component to any intervention attempting to promote circadian phase shifts to reduce the negative consequences of night shift work. Given the large variability in circadian phase at baseline, generalised recommendations of light exposure based on clock time is not necessarily a suitable approach for all workers, and may actually be counterproductive. For example, an individual with aMT6s acrophase occurring early in the night shift (e.g. 03.00 h) being advised to seek light during the night and avoid bright morning light exposure on the commute home (which can be difficult to achieve in practice; Lockley, 2005) will not be reducing the phase advancing effects of light as intended. Instead they will probably be exposed to phase advancing light in the latter part of the night shift, after the aMT6s acrophase, and avoiding light during the commute home would have minimal impact in counteracting these effects. Accordingly, interventions aimed at promoting circadian adaptation to night work would probably have greater efficacy if light exposure recommendations are based on the timing of an individual's circadian phase rather than generalised management based on clock time. Conversely, an intervention approach that has been proposed to account for individual variability in circadian phase is to systematically shift the timing of light exposure so as to facilitate maximal phase shifting in individuals across a range of circadian phases (Mitchell et al. 1997; Crowley et al. 2003). Notwithstanding the potential utility of this approach, we suggest that knowledge of circadian phase can inform improved personalised intervention strategies for shift work, to promote either adaptation (followed by re‐adaptation) or non‐adaptation, depending on the specific shift rotations.
Previous work has found relationships between diurnal preference and phase response to abrupt changes in sleep–wake (and consequently light–dark) schedules, with greater eveningness tendency associated with larger phase delays following a delay in the light–dark cycle (Mitchell et al. 1997; Dumont et al. 2001). While we did not find a significant association, participants who had the greatest eveningness scores showed the largest phase delays. No participants in our sample were morning‐types, possibly because these individuals have self‐selected out of shift work due to lower tolerance (Saksvik et al. 2011). We suggest that the relationship between phase shift and diurnal preference may have been stronger with a larger range in MEQ scores. Furthermore, diurnal preference is associated with individual differences in circadian period in healthy volunteers (Duffy et al. 2001). Differences in circadian period are associated with the magnitude of phase shift following a 9‐h delay in the light–dark cycle (Eastman et al. 2016). In our study, including diurnal preference in a regression model with light exposure accounted for an additional 17% of variability in phase shift in our sample, a larger individual contribution than other factors such as BMI (8%) or aMT6s acrophase at baseline (5%) when each factor was tested in combination with light data. This observation suggests that diurnal preference provides important additional information in predicting circadian response to night work. Further investigation is, however, required to determine whether there are differences in the phase response curve to light as a function of diurnal preference.
It is possible that shift workers’ diurnal preference is related to activity‐rest behaviours which influence light–dark exposure, thereby influencing circadian phase shift response to night shifts. For example, individuals with strong evening‐type tendency may inadvertently seek more light throughout the night, increasing the likelihood of greater exposure during phase delaying times. We did not, however, see a relationship between MEQ score and acrophase at baseline. This may be due to a number of reasons, including unstable phase relationships whilst on rotating shift schedules, inadequate sample size and/or the influence of homeostatic processes on morningness/eveningness scores due to differences in dissipation of sleep pressure (Mongrain et al. 2006).
The large proportion of variability in phase shift explained by our regression model has potential applications for individualised shift work management, with customised phase shift goals based on initial phase position to distribute sleepiness between individuals across the night shift. For example, for evening‐type individuals with late phase the goal would be a phase delay in preparation for the night shift and sleep after the shift, whereas morning‐type individuals could adapt by phase advance and sleep before the shift. Complementary shift scheduling could utilise this approach, such that individuals with a mixture of advanced and delayed phase position are on shift together, to reduce accident risk on shift by spreading aMT6s acrophase time (and thus time of reduced alertness and poor performance) across the shift schedule. Shift work interventions may also incorporate phase shifting strategies in preparation for night shifts to reduce the risk of alertness impairment in the first few shifts, similar to schedules for phase delay or advance prior to transmeridian travel to reduce jetlag (Burgess et al. 2003a).
While this study has high ecological validity, there are a number of limitations. Due to the naturalistic setting we had limited control over confounding factors such as drug use, which may influence the measurement of urinary aMT6s (Arendt, 2005). Participants with self‐reported use of sleep medications were excluded from analysis, however. We classified delay and advance zones based on aMT6s acrophase, relying on Khalsa's human PRC (Khalsa et al. 2003) which was developed in a controlled laboratory setting, referenced to core body temperature minimum relative to the midpoint of light exposure. Given that recent work suggests that the majority of the light effect occurs toward the start of a light exposure (Chang et al. 2012) we tested whether realignment of the PRC to light onset would improve our estimate of light exposure, but we did not find any significant improvement in our phase predictions. We also assumed a linear phase shift across night shifts in order to make predictions about individual delay and advance zones of the PRC. Future work collecting urine continuously across all night shifts would more accurately map the temporal dynamics of phase shifting and light exposure. Given that we include light relative to phase over each night shift, calculated using knowledge of circadian phase after successive night shifts, our regression model must be considered explanatory rather than predictive of phase shift. Additionally, we measured light using a wrist‐worn device, which is a potential source of error as this is not necessarily equivalent to the light hitting the cornea, and ultimately the retina.
This study is potentially limited by the impact of light suppression of melatonin synthesis on aMT6s profiles. Prior work has, however, reported a strong relationship between circadian phase measured from urinary cortisol (less impacted by light exposure) and aMT6s (n = 7; mean ± SD Pearson's r = 0.98 ± 0.01, P < 0.01) (Barger et al. 2012). Measuring circadian timing via urinary aMT6s is well established in the literature as a reliable assessment of phase in field settings, and has been used successfully in numerous prior studies of shift workers including nurses (Dumont et al. 2001, 2012; Hansen et al. 2006; Ftouni et al. 2015) on North Sea oil rigs (Barnes et al. 1998a,b; Gibbs et al. 2002, 2007; Thorne et al. 2008) and in Antarctica (Midwinter & Arendt, 1991; Ross et al. 1995). This method has also been used in several clinical populations and laboratory settings (e.g. Bearn et al. 1989; Skene et al. 1990; Deacon & Arendt, 1994, 1996; Lockley et al. 1997).
Circadian response to light depends on multiple factors, including intensity, timing, wavelength and light history (Czeisler & Gooley, 2007). In this study we have considered intensity, timing and to some extent light history by examining light across the night shift schedule; however, we have not factored in light wavelength. The circadian pacemaker is particularly sensitive to phase resetting by short‐wavelength light (Lockley et al. 2003; Gooley et al. 2010). We used estimated photopic lux from the Actiwatch spectrum rather than measurements from the blue light channel for the following reasons: (i) familiarity with photopic lux measure in the field generally; (ii) the strong correlation observed between photopic lux and data from the blue irradiance channel measured in a subset of our participants (n = 8, mean ± SD Pearson's r = 0.97 ± 0.01, P < 0.0001); and (iii) because the blue channel data from the Actiwatch spectrum are not sufficient at this stage for assessing the impact of changes in the spectra of light exposure on biological responses. We recognise that in the future it may be possible to measure melanopic lux or melanopic/photopic lux ratios (Lucas et al. 2014) using wearable devices. Such devices were not available at the time that we undertook this work.
Our findings indicate that ICU workers do not show partial circadian adaptation to night shifts over 3–4 consecutive night shifts, although there are large individual differences in both the direction and the magnitude of circadian phase shift. These findings provide strong evidence that baseline circadian phase and the individual light exposure patterns over the night shifts largely determine circadian adaptation to shift schedules, and that current ‘one size fits all’ approaches to shift work interventions may be counterproductive for some individuals.
Additional information
Competing interests
JES, SG, MDH and AC have no conflicts to declare. TLS and MM serve as a Project Leaders in the Cooperative Research Centre (CRC) for Alertness, Safety and Productivity. MH serves as a Theme Leader in the CRC for Alertness, Safety and Productivity, which funded this work; and has received grants from Prevention Express and TEVA, which are not related to the work reported in this paper. SWL has had a number of commercial interests in the last 12 months (2016–17). He is a Program Leader for the CRC for Alertness, Safety and Productivity, Australia, which funded this work. No other interests are directly related to the research or topic reported in this paper but, in the interests of full disclosure, are outlined below. SWL has received consulting fees from the Atlanta Falcons, Atlanta Hawks, BHP Billiton and Slingshot Insights; has current consulting contracts with Akili Interactive; Consumer Sleep Solutions; Delos Living LLC; Environmental Light Sciences LLC; Headwaters Inc.; Hintsa Performance AG; Light Cognitive; Mental Workout; OpTerra Energy Services Inc.; Pegasus Capital Advisors LP; PlanLED; and Wyle Integrated Science and Engineering; has received unrestricted equipment gifts from Biological Illuminations LLC, Bionetics Corporation and F. Lux Software LLC; royalties from Oxford University Press; and has served as a paid expert in legal proceedings related to light, sleep and health. He holds a patent through Harvard University and Brigham and Women's Hospital for ‘Systems and methods for determining and/or controlling sleep quality’. SMWR is also a Program Leader for the CRC for Alertness, Safety and Productivity, Australia, which funded this work. SMWR reports grants from Vanda Pharmaceuticals, Philips Respironics, Cephalon, Rio Tinto and Shell, and has received equipment support and consultancy fees through his institution from Optalert, Tyco Healthcare, Compumedics, Mental Health Professionals Network, and Teva Pharmaceuticals, which are not related to this paper.
Author contributions
All authors contributed to the work presented and have given final approval for its publication. JES contributed to the conception of design, data collection, analysed data and compiled the manuscript. TLS contributed to the conception of study design, coordinated study implementation, provided critical insight and comments on data analysis interpretation and manuscript draft. MM coordinated study implementation, provided critical insight and comments on data analysis, interpretation and manuscript draft. SG supported data collection and provided comments on interpretation and manuscript draft. MDM supported data collection and provided comments on interpretation and manuscript draft. AC facilitated data collection and study implementation and provided comments on interpretation and manuscript draft. MH contributed to the conception of study design, facilitated data collection and study implementation and provided critical insight and comments on interpretation and manuscript draft. SWL contributed to the conception of study design, provided critical insight and comments on data analysis, interpretation and manuscript draft. SWR contributed to the conception of study design, provided critical insight and comments on data analysis, interpretation and manuscript draft.
Funding
This research was funded by the Cooperative Research Centre for Alertness, Safety and Productivity, Melbourne, Victoria, Australia. Grant number P2.1.01‐15.
Supporting information
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Table S1. Linear regression model results.
Acknowledgements
We thank Jessica Papaleo, Matthew McLaren, Aaron Johnson, Kaitlyn Crocker, Niamh McDonald, Emma Giliberto and Trisha D'Lima from the Sleep and Circadian Medicine Laboratory, School of Psychological Sciences and Monash Institute of Cognitive and Clinical Neurosciences, Monash University, Melbourne, Australia, for their assistance with data collection. We thank Dr Glenn Eastwood, Helen Young, Dr Graeme Hart and the staff of the Intensive Care Unit at Austin Health, Melbourne, Australia, for their support. With thanks to Mark Salkeld at the Adelaide Research Assay Facility, Adelaide, Australia, for conducting the aMT6s radioimmunoassays.
Biography
Julia Stone is a PhD candidate in the Alertness CRC; School of Psychological Sciences at Monash University, Australia; and Monash Institute of Cognitive and Clinical Neurosciences. Her research interests lie in circadian rhythms and shift work, particularly measuring and predicting circadian response to changes in light/dark cycles. Julia has a Bachelor of Arts with Honours (Psychology) from the University of Melbourne.

Edited by: Kim Barrett & William Taylor
Linked articles This article is highlighted by a Perspective by Grønli & Mrdalj. To read this Perspective, visit https://doi.org/10.1113/JP276043.
References
- Aldhous ME & Arendt J (1988). Radioimmunoassay for 6‐sulphatoxymelatonin in urine using an iodinated tracer. Clin Biochem 25, 298–303. [DOI] [PubMed] [Google Scholar]
- Arendt J (1995). Melatonin and the mammalian pineal gland. Springer Science & Business Media, Berlin. [Google Scholar]
- Arendt J (2005). Melatonin: characteristics, concerns, and prospects. J Biol Rhythms 20, 291–303. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Fogg LF & Eastman CI (1999). Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol Regul Integr Comp Physiol 277, R1598–R1604. [DOI] [PubMed] [Google Scholar]
- Barger LK, Sullivan JP, Vincent AS, Fiedler ER, McKenna LM, Flynn‐Evans EE, Gilliland K, Sipes WE, Smith PH & Brainard GC (2012). Learning to live on a Mars day: fatigue countermeasures during the Phoenix Mars Lander mission. Sleep 35, 1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes RG, Deacon S, Forbes MJ & Arendt J (1998a). Adaptation of the 6‐sulphatoxymelatonin rhythm in shiftworkers on offshore oil installations during a 2‐week 12‐h night shift. Neurosci Lett 241, 9–12. [DOI] [PubMed] [Google Scholar]
- Barnes RG, Forbes MJ & Arendt J (1998b). Shift type and season affect adaptation of the 6‐sulphatoxymelatonin rhythm in offshore oil rig workers. Neurosci Lett 252, 179–182. [DOI] [PubMed] [Google Scholar]
- Bearn J, Franey C, Arendt J & Checkley S (1989). A study of the effects of desipramine treatment alone and in combination with l‐triiodothyronine on 6‐sulphatoxymelatonin excretion in depressed patients. Br J Psychiatry 155, 341–347. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL & Revell VL (2008). Measuring melatonin in humans. J Clin Sleep Med 4, 66–69. [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, Boudreau P, James FO & Kin NY (2012). Photic resetting in night‐shift work: impact on nurses’ sleep. Chronobiol Int 29, 619–628. [DOI] [PubMed] [Google Scholar]
- Boivin DB & James FO (2002). Circadian adaptation to night‐shift work by judicious light and darkness exposure. J Biol Rhythms 17, 556–567. [DOI] [PubMed] [Google Scholar]
- Bojkowski CJ, Arendt J, Shih MC & Markey SP (1987). Melatonin secretion in humans assessed by measuring its metabolite, 6‐sulfatoxymelatonin. Clin Chem 33, 1343–1348. [PubMed] [Google Scholar]
- Boudreau P, Dumon G & Boivin DB (2013). Circadian adaptation to night shift work influences sleep, performance, mood and the autonomic modulation of the heart. PLoS One 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda C, Fogg LF & Eastman CI (2003a). Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms 18, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Savic N, Sletten TL, Roach GD, Gilbert SS & Dawson D (2003b). The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med 1, 102–114. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Carpenter JS & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosom Res 45, 5–13. [DOI] [PubMed] [Google Scholar]
- Chang A, Santhi N, St Hilaire MA, Gronfier C, Bradstreet DS, Duffy JF, Lockley SW, Kronauer RE & Czeisler CA (2012). Human responses to bright light of different durations. J Physiol 590, 3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapdelaine S, Paquet J & Dumont M (2012). Effects of partial circadian adjustments on sleep and vigilance quality during simulated night work. J Sleep Res 21, 380–389. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee CW, Tseng CY, Fogg LF & Eastman CI (2003). Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms 18, 513–523. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee CW, Tseng CY, Fogg LF & Eastman CI (2004). Complete or partial circadian re‐entrainment improves performance, alertness, and mood during night‐shift work. Sleep 27, 1077–1088. [DOI] [PubMed] [Google Scholar]
- Czeisler CA & Gooley JJ (2007). Sleep and circadian rhythms in humans In Cold spring harbor symposia on quantitative biology, pp. 579–597. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. [DOI] [PubMed] [Google Scholar]
- Deacon SJ & Arendt J (1996). Adapting to phase shifts, I. An experimental model for jet lag and shift work. Physiol Behav 59, 665–673. [DOI] [PubMed] [Google Scholar]
- Deacon SJ & Arendt J (1994). Phase‐shifts in melatonin, 6‐sulphatoxymelatonin and alertness rhythms after treatment with moderately bright light at night. Clin Endocrinol (Oxf) 40, 413–420. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang A, Phillips A, Münch M, Gronfier C, Wyatt JK, Dijk D, Wright KP & Czeisler CA (2011). Sex difference in the near‐24‐hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA 108, 15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF & Czeisler CA (2002). Age‐related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett 318, 117–120. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW & Czeisler CA (2001). Association of intrinsic circadian period with morningness‐eveningness, usual wake time, and circadian phase. Behav Neurosci 115, 895–899. [DOI] [PubMed] [Google Scholar]
- Duffy JF & Wright KP (2005). Entrainment of the human circadian system by light. J Biol Rhythms 20, 326–338. [DOI] [PubMed] [Google Scholar]
- Dumont M, Benhaberou‐Brun D & Paquet J (2001). Profile of 24‐h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms 16, 502–511. [DOI] [PubMed] [Google Scholar]
- Dumont M, Lanctôt V, Cadieux‐Viau R & Paquet J (2012). Melatonin production and light exposure of rotating night workers. Chronobiol Int 29, 203–210. [DOI] [PubMed] [Google Scholar]
- Eastman CI & Martin S (1999). How to use light and dark to produce circadian adaptation to night shift work. Ann Med 31, 87–98. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA & Crowley SJ (2016). Circadian rhythms of European and African‐Americans after a large delay of sleep as in jet lag and night work. Sci Rep 6, 36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA & Crowley SJ (2017). Sex and ancestry determine the free‐running circadian period. J Sleep Res, 26, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Kennaway DJ, Baker A, Lamond N & Dawson D (2012). Sleep and circadian rhythms in mining operators: limited evidence of adaptation to night shifts. Appl Ergon 43, 695–701. [DOI] [PubMed] [Google Scholar]
- Folkard S (2008). Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int 25, 215–224. [DOI] [PubMed] [Google Scholar]
- Ftouni S, Sletten TL, Nicholas CL, Kennaway DJ, Lockley SW & Rajaratnam SW (2015). Ocular measures of sleepiness are increased in night shift workers undergoing a simulated night shift near the peak time of the 6‐sulfatoxymelatonin rhythm. J Clin Sleep Med 11, 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M, Hamptom S, Morgan L & Arendt J (2002). Adaptation of the circadian rhythm of 6‐sulphatoxymelatonin to a shift schedule of seven nights followed by seven days in offshore oil installation workers. Neurosci Lett 325, 91–94. [DOI] [PubMed] [Google Scholar]
- Gibbs M, Hampton S, Morgan L & Arendt J (2007). Predicting circadian response to abrupt phase shift: 6‐sulphatoxymelatonin rhythms in rotating shift workers offshore. J Biol Rhythms 22, 368. [DOI] [PubMed] [Google Scholar]
- Goel N & Lee TM (1995). Sex differences and effects of social cues on daily rhythms following phase advances in Octodon degus . Physiol Behav 58, 205–213. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SW, Brainard GC, Kronauer RE, Czeisler CA & Lockley SW (2010). Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med 2, 31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumenyuk V, Roth T & Drake C (2012). Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder. Chronobiol Int 29, 928–936. [DOI] [PubMed] [Google Scholar]
- Hansen ÅM, Garde AH & Hansen JH (2006). Diurnal urinary 6‐sulfatoxymelatonin levels among healthy danish nurses during work and leisure time. Chronobiol Int 23, 1203–1215. [DOI] [PubMed] [Google Scholar]
- Hansen JH, Geving IH & Reinertsen RE (2010). Adaptation rate of 6‐sulfatoxymelatonin and cognitive performance in offshore fleet shift workers: a field study. Int Arch Occup Environ Health 83, 607–615. [DOI] [PubMed] [Google Scholar]
- Jensen MA, Garde AH, Kristiansen J, Nabe‐Nielsen K & Hansen ÅM (2016). The effect of the number of consecutive night shifts on diurnal rhythms in cortisol, melatonin and heart rate variability (HRV): a systematic review of field studies. Int Arch Occup Environ Health, 89, 531–545. [DOI] [PubMed] [Google Scholar]
- Johns MW (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545. [DOI] [PubMed] [Google Scholar]
- Johns MW (1992). Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 15, 376–381. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Butler MP, LeSauter J & Silver R (2011). Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology 152, 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S, Jewett ME, Cajochen C & Czeisler CA (2003). A phase response curve to single bright light pulses in human subjects. J Physiol 549, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley SW (2005). Timed melatonin treatment for delayed sleep phase syndrome: the importance of knowing circadian phase. Sleep 28, 1214–1216. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC & Czeisler CA (2003). High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 88, 4502–4502. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC & Defrance R (1997). Relationship between melatonin rhythms and visual loss in the blind 1. J Clin Endocrinol Metab 82, 3763–3770. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW & O'Hagan JB (2014). Measuring and using light in the melanopsin age. Trends Neurosci 37, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S & Eastman CI (2002). Sleep logs of young adults with self‐selected sleep times predict the dim light melatonin onset. Chronobiol Int 19. [DOI] [PubMed] [Google Scholar]
- Midwinter MJ & Arendt J (1991). Adaptation of the melatonin rhythm in human subjects following nightshift work in Antarctica. Neurosci Lett 122, 195–198. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Hoese EK, Liu L, Fogg LF & Eastman CI (1997). Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms 12, 5–15. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J & Dumont M (2006). Circadian and homeostatic sleep regulation in morningness–eveningness. J Sleep Res 15, 162–166. [DOI] [PubMed] [Google Scholar]
- Nelson W, Tong L, Lee J & Halberg F (1979). Methods for cosinor‐rhythmometry. Chronobiologia 6, 305. [PubMed] [Google Scholar]
- Nowak R, McMillen IC, Redman J & Short RV (1987). The correlation between serum and salivary melatonin concentrations and urinary 6‐hydroxymelatonin sulphate excretion rates: two non‐invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol (Oxf) 27, 445–452. [DOI] [PubMed] [Google Scholar]
- Obayashi K, Saeki K & Kurumatani N (2016). Ambient light exposure and changes in obesity parameters: a longitudinal study of the HEIJO‐KYO cohort. J Clin Endocrinol Metab 101, 3539–3547. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SW & Arendt J (2001). Health in a 24‐h society. Lancet 358, 999–10005. [DOI] [PubMed] [Google Scholar]
- Ross JK, Arendt J, Horne J & Haston W (1995). Night‐shift work in Antarctica: sleep characteristics and bright light treatment. Physiol Behav 57, 1169–1174. [DOI] [PubMed] [Google Scholar]
- Sack RL, Blood ML & Lewy AJ (1992). Melatonin rhythms in night shift workers. Sleep 15, 434–441. [DOI] [PubMed] [Google Scholar]
- Saksvik IB, Bjorvatn B, Hetland H, Sandal GM & Pallesen S (2011). Individual differences in tolerance to shift work – a systematic review. Sleep Med Rev 15, 221–235. [DOI] [PubMed] [Google Scholar]
- Shanahan TL & Czeisler CA (1991). Light exposure induces equivalent phase shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. J Clin Endocrinol Metab 73, 227–235. [DOI] [PubMed] [Google Scholar]
- Skene DJ, Bojkowski CJ, Currie JE, Wright J, Boulter PS & Arendt J (1990). 6‐Sulphatoxymelatonin production in breast cancer patients. J Pineal Res 8, 269–276. [DOI] [PubMed] [Google Scholar]
- Sletten TL, Vincenzi S, Redman J, Lockley SW & Rajaratnam SW (2010). Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol 1, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Reilly C & Midkiff K (1989). Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol 74, 728. [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa S, Kronauer RE, Czeisler CA & Lockley SW (2012). Human phase response curve to a 1 h pulse of bright white light. J Physiol 590, 3035–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne H, Hampton S, Morgan L, Skene DJ & Arendt J (2008). Differences in sleep, light, and circadian phase in offshore 18: 00–06: 00 h and 19: 00–07: 00 h shift workers. Chronobiol Int 25, 225–235. [DOI] [PubMed] [Google Scholar]
- Warman V, Dijk D, Warman G, Arendt J & Skene DJ (2003). Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett 342, 37–40. [DOI] [PubMed] [Google Scholar]
- Wright KP, Gronfier C, Duffy JF & Czeisler CA (2005). Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms 20, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Hughes RJ, Kronauer RE, Dijk D & Czeisler CA (2001). Intrinsic near‐24‐h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Natl Acad Sci 98, 14027–14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Table S1. Linear regression model results.


