Abstract
Malignant gliomas are the most common primary brain tumor and are characterized by rapid and highly invasive growth. Because of their poor prognosis, new therapeutic strategies are needed. Oncolytic virotherapy (OV) is a promising strategy for treating cancer that incorporates both direct viral replication mediated and immune mediated mechanisms to kill tumor cells. C134 is a next generation Δγ134.5 oHSV-1 with improved intratumoral viral replication. It remains safe in the CNS environment by inducing early IFN signaling which restricts its replication in non-malignant cells. We sought to identify how C134 performed in an immunocompetent tumor model that restricts its replication advantage over first generation viruses. To achieve this we identified tumors that have intact IFN signaling responses that restrict C134 and first generation virus replication similarly. Our results show that both viruses elicit a T cell mediated anti-tumor effect and improved animal survival but that subtle difference exist between the viruses effect on median survival despite equivalent in vivo viral replication. To further investigate this we examined the anti-tumor activity in immunodeficient mice and in syngeneic models with re-challenge. These studies show that the T cell response is integral to C134 replication independent anti-tumor response and that OV therapy elicits a durable and circulating anti-tumor memory. The studies also show that repeated intratumoral administration can extend both OV anti-tumor effects and induce durable anti-tumor memory that is superior to tumor antigen exposure alone.
Introduction
Cancer is the second leading cause of death in the United States, responsible for more than 1500 deaths a day [1]. High-grade gliomas are the most common primary malignant brain tumors in adults and represent about 10% of childhood brain tumors, with glioblastoma multiforme (GBM) being the most aggressive form [2], [3]. GBMs cells are invasive, diffuse, and infiltrating with no clear border between normal brain and tumor cells making the surgical resection always incomplete [4]. A multimodality therapy approach has been used for the GBM treatment including surgery, radiotherapy and chemotherapy with an alkylating agent (temozolomide). Despite this regimen, GBM patients have a poor prognosis with a median survival of 14.6 months and the outcome has not improved to a great extent over the last three decades [3]. Novel therapies are needed for this malignancy.
Oncolytic virotherapy is promising experimental therapeutic strategy especially for tumors that are resistant to conventional anticancer therapeutics. Previous studies showed that the HSV-1 γ134.5 encodes a multifunctional protein, infected cell protein 34.5 (ICP34.5) pivotal to neurovirulence. ICP34.5 suppresses autophagy, early IFN signaling, and late IFN stimulated protein kinase R mediated translational arrest in infected cells [5], [6], [7], [8], [9]. Disruption of the γ134.5 gene to produce a Δγ134.5 oHSV eliminates efficient replication in post-mitotic neuronal cells and eliminated its ability to cause encephalitis [5], [10], [11]. Recombinant Δγ134.5 HSVs have been investigated as a treatment for brain tumor and have been safely administered in the US and Europe at doses ranging from 5×105 to 3×109 PFU directly into the CNS tumors [12], [13], [14], [15]. While ~50% of treated patients developed clinical or radiographic anti-tumor responses following administration of the first-generation oHSV-1 in phase I trials, this conservatively designed OV did not produce a universal response. We postulated that diminished viral protein translation reduced viral replication and this contributed to its reduced efficacy. To counter these limitations, we created C134, a second-generation chimeric HCMV/HSV-1 oncolytic virus capable of late viral protein synthesis and improved viral replication in tumor by disrupting PKR and evading translational arrest; thus increasing viral antigen load [16] but with the same toxicity profile as first generation Δγ134.5 HSV [17], [18]. In addition to the direct anti-tumor activity caused by viral replication and lysis in infected cells, viral infection elicits an immune response that contributes to OV anti-tumor activity. The release of tumor antigens from viral lysed cells, danger signals, cytokine and chemokines produced during viral infection stimulate the immune response and reverse tumor associated immunosuppression [4], [19], [20].
To investigate this complex mechanistic system, an immunocompetent syngeneic model that recapitulates aspects of the human tumor was needed. We therefore examined the antigliomal activity of the both oncolytic viruses in highly resistant MG tumors where both viruses replicate similarly. We continue to hypothesize that the immune response contributes to oHSV activity and that oncolytic virotherapy can induce a long-term antitumor memory response. First, we assessed the susceptibility of the different glioma cell lines to infection by the oHSV. Our results show that oHSVs replicates differentially in the glioma tumor lines tested, allowing us to stratify tumor lines into those susceptible and resistant to direct oncolysis. Consistent with our finding in malignant peripheral nerve sheath tumors, we found that intact STAT signaling and ISGs accumulation predicts resistance to oncolytic virus infection and spread. Next we examined oHSV antitumor activity in two resistant tumor lines in vivo. We showed that both viruses prolonged mouse survival in 2 different mouse syngeneic brain tumor models where C134 has no replication advantage over the first generation oHSV and that the immune response contributes to this survival. Transfer of the tumor model to athymic nude mice led to loss of this survival advantage suggesting that the adaptive immune response contributes to the improved antitumor activity. We also show that OV treatment induced a circulating and durable anti-cancer immune memory response that surpassed tumor antigen exposure alone. Finally, our data also shows that repeated intratumoral administration of the OV improved survival of tumor-bearing mice.
Materials and Methods
Cell Lines and Viruses
Neuro2A (neuralglial tumor) cells were obtained from American Type Tissue Culture Collection and were propagated in Dulbecco's modified Eagle medium (DMEM)/F12 50/50 medium supplemented with 7.5% Fetal Bovine Serum (FBS) and 2.6 mM L-glutamine [18]. DBT Mouse glioma tumor lines were maintained in growth media containing DMEM with 10% fetal bovine serum and 10 mM L-glutamine (Sigma). Viruses have been previously described but in brief HSV-1(F) strain and R3616, the Δγ134.5 recombinant, was kindly provided by Dr. Bernard Roizman (University of Chicago, Chicago, IL) [5]. Recombinant viruses C101 and C134 have been described previously [17], [18]. Briefly, C101 is a Δγ134.5 virus derived from R3616 that expresses EGFP and C134 is a Δγ134.5 virus derived from C101 that contains the HCMV IRS1 gene under control of the HCMV IE promoter in the UL3/UL4 intergenic region [17] (Supplementary Figure 1).
Animal Methods: IC Tumor Implantation and Treatment
All studies were conducted in accordance with guidelines for animal use and care established by the University of Alabama at Birmingham and the Nationwide Children's Hospital Research Center Animal Resource Programs and the Institutional Animal Care and Use Committees (protocol number 050407478 and AR16–00057, AR16–00088). All mouse strains were obtained from Frederick Cancer Research and Development Center, NCI or Harlan/Envigo. For survival studies, 2×104 Neuro2A in 5% methylcellulose DMEM/F12 were intracerebrally injected into syngeneic A/J and treated 5 days later with optiprep virus (in 10 μl optiprep/PBS vehicle) as described previously [21]. For DBT tumors a similar approach was used, however 1×105 cells were stereotactically injected into syngeneic Balb/C mice and treated 7d later with optiprep virus using the same stereotactic coordinates. Direct oHSV anti-tumor activity was also assessed for each tumor line by repeating the same orthotopic method and treatment schema in athymic nude mice. Mice were assessed daily and moribund mice killed and date recorded. Survival curves determined using Kaplan–Meier analysis and median survivals and 95% confidence intervals calculated. Log-rank test was applied to compare survival between groups. Studies were repeated twice to ensure biologic validity.
Flank Tumor
To test for circulating anti-tumor memory in the long-term brain tumor survivors (survival >60 days), mice were re-challenged with flank tumors. Mice were injected subcutaneously with 5×105 cells/100 μl of 5% methylcellulose DBT in flank. Flank tumor growth was measured using calipers and tumor volume calculated based upon (L × W × H). Mice were monitored for more than 45 days or until all naïve mice developed tumors requiring euthanasia and ending the experiment.
Winn Assay
A modified Winn assay was performed by the intracerebral (IC) injection of 1×105 DBT cells/animal tumor cells that have been infected with R3616 or C134 (MOI 1) for 1 hour before injection into BALB/c mice. One week later, mice were treated with saline or 1×107 PFU R3616 or C134 IC. Mice were observed on a daily basis, and survival was recorded. Studies was repeated and long-term survivors (survival >60 days) from the C134-treated cohort and mice that have been implanted with mitomycin-treated DBT tumor cells in addition to naïve mice that have not been exposed to the tumor cells before as a control were challenged by subcutaneous injection with DBT at flank.
Viral Recovery
Assessment of viral replication In vitro and in vivo was performed as previously described [18]. In brief triplicate samples of tumor cells were infected in parallel with Δγ134.5 or C134, virus recovery measured by limiting plaque dilution from infected cultures at day 1, 2 and 3 post-infection. For in vivo viral recovery, mice bearing CNS tumors were treated with equivalent doses of a Δγ134.5 virus (R3616) or C134 (Δγ134.5, IRS), the animals were sacrificed on day 2 and 4 post-treatment for virus recovery. Brains were recovered, weighed, freeze–thawed, mechanically disrupted by homogenization and the samples were sonicated before quantifying the recovered virus by limiting dilution plaque assay or by Taqman quantitative PCR after DNA extraction. Average recovered virus and standard deviation were calculated for each virus and time point tested.
Flow Cytometry
Mice were saline perfused following sacrifice with 10–20 ml PBS. Brains were collected and homogenized by enzymatic digestion and mechanical disruption. Samples were passed through a cell strainer and the samples pelleted and then separated over a Percoll gradient (70%/30. The mononuclear cell infiltrate isolated at the interface after centrifugation at 2470 RPM at 4° for 30 min with no brake. FC block was performed followed by staining for the surface antigens (CD45-PE, CD3-AF647, CD4-PerCP Cy5.5, CD8-FITC) and measured by BD FACSCalibur and analysis by Flowjo (TreeStar).
IncuCyte ZOOM Viral Spread Assay
Cells were plated into 96-well flat clear bottom polystyrene tissue-culture treated microplates (Corning, NY, USA) and allowed to adhere for overnight. GFP-expressing 1st generation (C101), 2nd generation (C154) or the wild-type (M2001) oHSV-1 were added at indicated MOI and the plates were transferred into the IncuCyte ZOOM platform which was housed inside a cell incubator at 37 °C with 5% CO2, until the end of the assay. Four images per well from three technical replicates were taken every 3 hours for 3 days using a 10X objective lens and then analyzed using the IncuCyteTM Basic Software. Green channel acquisition time was 400 ms in addition to phase contrast.
Western Blotting
Cellular lysates were collected on ice in RIPA buffer (10 mM Tris-Cl pH 8.0, 1 mM EDTA, 1% Triton X100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl) with protease inhibitor cocktail (Roche) and diluted in 4× sample buffer (240 mM Tris-Cl pH 6.8, 40% glycerol, 4% SDS, 20% β-mercaptoethanol, 0.04% bromophenol blue). Samples were denatured at 98 °C for 5 minutes, chilled on ice, separated by PAGE, and transferred to a nitrocellulose membrane (Thermo Scientific) and blocked for 1 hour at room temperature with 5% dry milk (S.T. Jerrell Co.) or bovine serum albumin (Fisher). Membranes were incubated overnight at 4 °C with primary antibody diluted in Tris-buffered saline with 0.1% Tween-20 (TBST). Membranes were repeatedly washed with TBST, incubated for 1 hr. with secondary antibody (Thermo-Fisher) diluted in TBST (1:20,000) at room temperature, and subsequently washed with TBST. Membranes were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and exposed to x-ray film (Research Products International).
Statistical Analysis
Statistical analysis was performed using Prism 6 (GraphPad Software). Survival was assessed using log-rank test: the data are shown using Kaplan Meier survival curves with median survival for each cohort included. For multi-cohort comparisons (immune cell infiltrates measurements, tumor growth upon re-challenge), non-parametric one-way ANOVA was used (Kruskal-Wallace with Dunn’ Correction for multiple comparisons) with standard error of the mean shown in graphs. For all analyses, the cutoff for statistical significance was set at P ≤ .05. The following notation was used: (NS) P > .05, *P ≤ .05, **P ≤ .01, ***P ≤ .001, ****P < .0001. Statistical comparisons and methods used are provided in the figures.
Results
Screening of Malignant Glioma Cell Lines Reveals Differential Sensitivities to Δγ134.5 oHSV Infection and Lysis
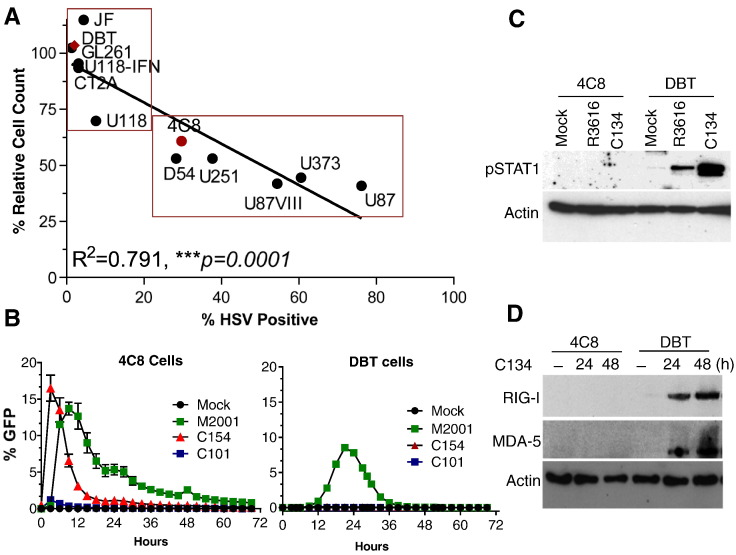
Historically, oHSV therapeutic efficacy was solely attributed to direct replication-based lysis of tumor cells [22]. Increasingly however the antiviral immune is an important contributor to OV anti-tumor efficacy [19], [20], [23]. We sought to evaluate the immune system contribution to the antitumor effect of oHSV. We chose malignant glioma tumor lines resistant to OHSV replication so we can exclude direct oncolytic effect. First, to identify the relative susceptibility of different MG cell lines to OV and to determine if viral replication directly correlates with tumor lysis in cell culture, we performed a multistep spread assay using Δγ134.5 oHSV (as described previously) [16] and screened 12 established GBM cell line for their susceptibility to Δγ134.5 HSV infection and cytolysis. The results showed a significant correlation (R2 = 0.7911: ***P = .0001) between Δγ134.5 oHSV infection and cell death (Figure 1A). While six established GBM cell lines were resistant to the infection (DBT, GL261, U118-IFN, CT2A and JF), the remaining cell lines had variable sensitivity. The U87VIII, U373 and U87 cell lines were highly permissive to oHSV infection while U118, 4C8, D54 and U251 had intermediate sensitivity. Real time evaluation of viral spread using Incuyte zoom measurement of cell to cell spread using EGFP encoding viruses M2001, C101 and C154 (EGFP-expressing wild type HSV, R3616 and C134, respectively) showed that C134 had improved replication and spread in the permissive 4C8 cells over the 1st generation virus (Figure 1B and Supplementary Figure 1B). However, in the restrictive tumor line, DBT, replication of the next generation virus was similar to a 1st generation virus (Figure 1B) and neither of them produced significant amount glycoprotein D upon infection (Supplementary Figure 2). We recently showed that peripheral tumor cells with intact STAT signaling responses can restrict C134 replication to that of a 1st generation Δγ134.5 oHSVs [16], [24]. To further interrogate whether this mechanism restricts C134 and Δγ134.5 OV replication in CNS tumor lines, we examined viral induction of JAK/STAT signaling in representative cell lines. We examined 4C8 (sensitive) and DBT (resistant) cells and as anticipated identified that both R3616 and C134 induce Stat-1 signaling (Figure 1C) and the surrogate ISGs (RIG-I and MDA-5) production in the more oHSV resistant DBT cells (Figure 1D). In contrast, neither oHSV elicits STAT1 signaling nor ISG production in the more susceptible 4C8 cells (Figure 1, C & D).
Figure 1.
Glioma cell line susceptibility to oHSV infection.
(A) Viral infection and tumor lysis directly correlate (***P = .0001, R2 = 0.7911: Pearson's Correlation). Twelve glioma cell lines were infected at a multiplicity of infection (MOI) of 0.1 and cellular lysates collected 48 h post infection for viral recovery analysis. Chart showing the relative percentage of cells infected with oHSV and the absolute numbers of cells remaining (% relative cell count). (B) 4C8 and DBT cells were infected by the GFP-expression R3616 (C101), C134 (C154) and the WT-virus (M2001) at an MOI of 1 then viral spread measured by the Incucyte Zoom® over time. (C) 4C8 and DBT cells were infected by the R3616 and C134 at MOI 1. At 6 hpi, cells were harvested and analyzed by immunoblotting for phosphorylated STAT-1 (top panel) and actin (lower panel). (D) 4C8 and DBT cells infected with R3616 or C134 (MOI 1) were collected at 24 and 48 hpi and analyzed by immunoblotting for RIG-I, MDA-5 and actin.
An Immune-Mediated Mechanism Contributes to R3616 and C134 Antitumor Activity
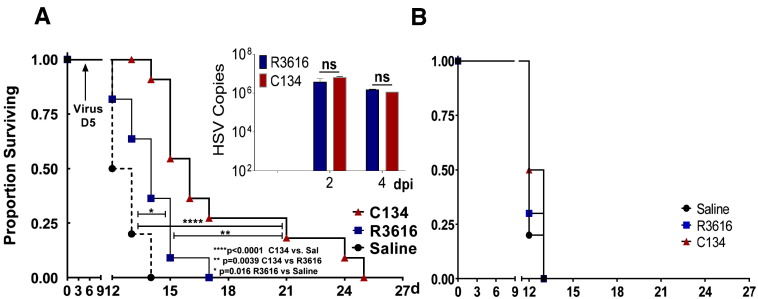
Next, we sought to evaluate the contribution of the immune system to the antitumor effect of the R3616 and C134. We chose DBT and N2A because C134 has no replication or infection advantage over a 1st generation virus in either of these tumor lines. One tumor line permits Δγ134.5 late gene expression (N2A) and viral replication similar to the next generation; C134 (Supplementary Figure 2 and Figure 2 insert) [24]. In contrast, the DBT tumor line induces a rapid IFN response (data not shown) that restricts both viruses' infection and gene expression (Figure 1B and Supplementary Figure 2). Consistent with our previous findings in A/J mice bearing orthotopic Neuro2A (N2A) tumors, we found that both viruses enhanced animal survival significantly compared to saline-treated cohort however despite equivalent replication in this tumor (Figure 2A insert), the second generation virus (C134) significantly extended animal survival over that of the Δγ134.5 1st generation virus (**P = .0039, Figure 2A). These data suggest that a replication-independent mechanism contributes to the difference in the antitumor activity of both oHSVs. To determine if this anti-tumor effect requires a T cell response, we repeated the N2A orthotopic studies in athymic nude mice and we found that in the absence of T cells, oHSV's survival advantage was also lost in this model (Figure 2B).
Figure 2.
oHSV antitumor efficacy and T cell involvement
(A) A/J mice were implanted with (2X104) N2A brain tumors orthotopically using sterotactic injection (10 mice/cohort). Cohorts were Saline or oHSV treated (5×105 PFU) 1 week later and survival monitored. Both viruses improved animal survival when compared to saline treatment (R3616 vs. Saline: *P = .016, C134 vs. Saline: ****P < .0001). C134 also improved survival over R3616 treatment (**P = .0039). (A: Insert) Two and four days post-oHSV treatment, mice were sacrificed and their brains harvested, homogenized and analyzed by Taqman qPCR for viral DNA: there was no difference in C134 or R3616 viral replication. (B) Studies were then performed in athymic nude mice. Animals were implanted with N2A tumor cells intracranially and treated with oHSV (5×105 PFU) or saline and survival was monitored. The results show a reduction in R3616 and C134.
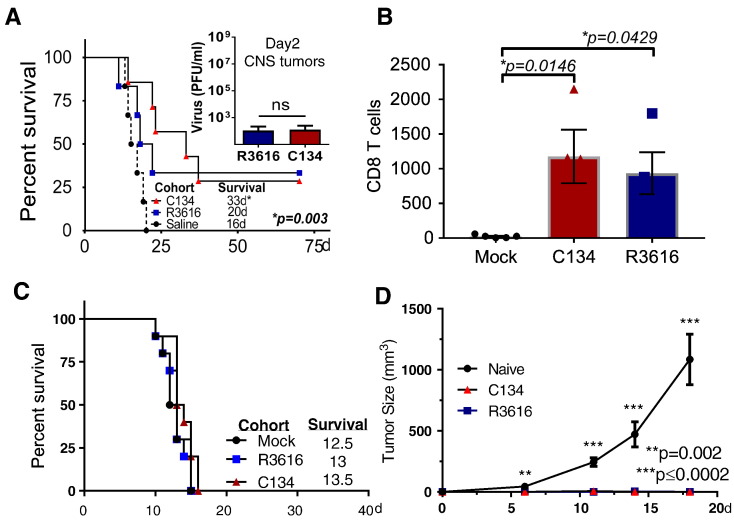
Next we sought to evaluate the antitumor activity of oHSVs in the more restrictive DBT tumor model. The DBT model is more resistant to oHSV gene expression and replication as confirmed by limited viral spread (Figure 1B) and gD production (Supplemental Figure 2). Mice bearing orthotopic DBT tumors were treated with oHSV (1 × 107 PFU) or saline and mouse survival was monitored. Similar to our in vitro studies, C134 and R3616 replicate equally poorly in this tumor model in vivo (Figure 3A insert). Notably, both viruses improved overall animal survival but only C134 produced a statistically significant improvement in survival over saline cohort (**P = .003, C134 vs. Saline, Figure 3A). Interestingly, flow cytometric analysis of the brain tumor from both R3616 and C134 treated mice showed a significant increase in the number of the CD8 T lymphocytes over the saline-treated cohort (**P = .0025 ANOVA, [C134 vs. Mock, *P = .0145], [R3616 vs. Mock, *P = .0415] Figure 3B) but not CD4 T cells (Supplementary Figure 2B) suggesting a role of T cells in the oHSV-mediated antitumor effect. We sought to confirm the contribution of T cells to survival in this model and repeated the in vivo studies in athymic nude mice. The results showed that again when T cells are eliminated, neither of the oHSVs improved median or long-term survival (Figure 3C). These data combined with the athymic nude N2A results suggest that a replication-independent mechanism contributes to survival in this resistant orthotopic brain tumor model and that these require T lymphocytes.
Figure 3.
oHSV-induced antitumor memory response in resistant glioma
(A) Balb/c mice were implanted with DBT tumor cells (1×105) intracranially then treated 7 days later with Saline or with oHSV (1×107 PFU of R3616 or C134) and survival was monitored (median survival is shown in the graph). While both viruses produced long-term survivors, only C134 significantly improved survival (C134 vs. Saline, **P = .0031: R3616 vs. Saline NS: P = .1115) (A insert) Mice were sacrificed and viral recovery was measured from harvested brains by limiting plaque dilution. Virus was recovered only on D2 post-treatment (with no statistical difference between the R3616 or C134 treated groups). (B) Tumor bearing mice were treated as described above and on D5 post treatment sacrificed, saline perfused, and their CNS infiltrating leukocyte populations analyzed by flow cytometry for CD8 T cells. (C) Athymic nude mice were implanted with DBT tumor cells intracranially then treated with oHSV (1×107 PFU of R3616 or C134) or saline and survival monitored. (D) Long-term survivors (>50d) from both the C134 or R3616-treated cohorts and naïve mice (Control) were re-challenged with subcutaneous injection of 1X106 DBT tumors in flank and tumor growth was monitored. Tumor size at each time point was compared by one way ANOVA (Kruskal-Wallis with Dunn's multiple comparisons correction).
R3616 and C134 Antitumor Effect Elicits Durable Immunity
In contrast to the N2A model, the DBT tumor model produced long-term survivors. This provided us with an opportunity to test whether oncolytic viral therapy elicited an immune memory response in these long-term survivors. After the mice had survived more than 70 days from their initial tumor challenge, we re-challenged them with 1 × 106 DBT tumor cells in their flank subcutaneously. An age matched naive mouse cohort injected with equivalent DBT tumor cells served as a positive control. Tumor volumes were measured over 20 days. Both the R3616 and C134 long-term survivor mice inhibited tumor growth on re-challenge while the naive mice had an average tumor size of 1050 mm3 (Figure 3D). These results demonstrate that the long-term survivors developed a circulating memory immune response against DBT tumors.
Repeated Treatment with oHSV Further Improves Mouse Survival
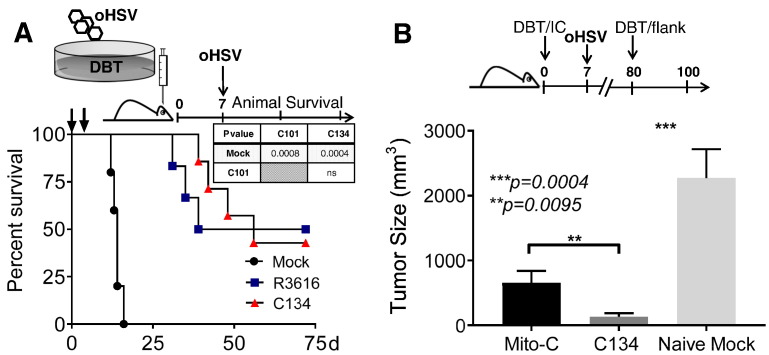
Prime and boost contributes to successful vaccination has been used in OV therapy to enhance the immune-mediated antitumor effect [25], [26], [27], [28]. To test whether repeated oHSV administration improves OV activity using the DBT model, mice were administered oHSV twice using a modified Winn-type assay. As described previously [25], DBT tumor cells were infected at an MOI of 1 for 1.5 hours and then immediately implanted (D0). Mice were then retreated on day 7 with oHSVs. As shown previously, DBT tumor cells do not support oHSV replication and oHSV did not produce cytolysis in this tumor line with 100% cell survival after infection (Figure 1B). Repeated administration of either R3616 or C134 further improved oHSV-mediated anti-tumor activity and animal survival when compared to saline (Figure 4A).
Figure 4.
Repeated intratumoral oHSV administration improved mice survival
(A) Using a modified Winn assay, cohorts of Balb/C mice were randomized into 3 cohorts and implanted with 1×105 DBT tumor cells that had been mock or oHSV infected (R3616 or C134) at an MOI of 1 for 1 h prior to implantation. One week later, the respective mice cohorts were saline or oHSV re-treated (1×107 PFU of R3616 or C134) and survival of mice was monitored. (B) DBT cells were either pretreated with Mitomycin C (Mito-C) or oHSV (R3616 or C134: MOI 1) before intracerebral implantation in Balb/C mice. Seven days later, the Mito-C tumor bearing cohort was treated with saline and the oHSV treated cohorts was re-treated with their respective oHSV (R3616 or C134 1×107 PFU). After 50d, an age matched DBT-naïve cohort and the long-term survivors (both oHSV-treated and Mito-C tumor implanted mice) were then challenged with 1×106 DBT tumors in flank and tumor growth monitored. Results show that C134 long-term survivors resist tumor re-challenge at a distant site better than naïve (***P = .0004, C134 vs. Naïve Mock) or tumor antigen experienced mice (**P = .0095: C134 vs. Mito-C) based upon one way ANOVA (Kruskal-Wallis with Dunn's correction for multiple comparisons).
To discern whether the oHSV infection was integral to the improved anti-tumor immune response or whether natural immune response to tumor antigen exposure was responsible for tumor control, we included a matched cohort stereotactically injected with equivalent numbers of Mitomycin C pre-treated DBT tumor cells. Mitomycin C eliminates tumor cell replication while maintaining other tumor cell functions (e.g. surface antigen expression). We then re-challenged oHSV- and mitomycin C (Mito-C)-treated long-term survivors with DBT tumors 80 days post antigen exposure with flank tumors similar to the prior study. As anticipated, the C134 long-term survivors significantly reduced tumor growth on re-challenge when compared to the tumor antigen naïve group (Figure 4B, ***P = .0004 [C134 vs. Naïve]). What is new and significant however was that the C134 treated long-term survivors had improved anti-tumor activity when compared to mice with prior tumor antigen exposure (**P = .0095 [C134 vs. Mito-C]). This shows that that C134 treatment produces durable immune mediated anti-tumor protection in survivors and that this protection is superior to prior antigen exposure.
Discussion
Malignant gliomas are aggressive poor prognosis primary brain tumors characterized by rapid and highly invasive growth. Due to its infiltrative, insidious nature within the central nervous system, the survival rate of patient is very low despite decades of research in developing sophisticated surgical and radiation techniques and new chemotherapeutics. Oncolytic viruses are promising experimental therapeutics for tumors that are resistant to conventional anticancer therapeutics. Both naturally occurring and engineered recombinant oncolytic viruses have been investigated as potential therapeutics for brain tumors. This diverse group of viral therapeutics includes DNA viruses (HSV, Adenovirus), RNA viruses (poliovirus, respiratory and enteric orphan virus, measles virus, vesiculostomatitis virus) and certain retroviruses.
HSV-1 is a neurotropic virus that has been extensively studied. It is neurotropic and anti-viral medications exist to control the infection if required. First generation oncolytic HSVs were derived by deleting one or more copies of a virulence gene (γ134.5 gene) important for efficient viral replication in the CNS environment. The γ134.5 gene is not required for viral replication in cell culture but is an important pathogenesis gene and indispensable for virus replication in the CNS. The Δγ134.5 mutation restricts replication in some tumor environments which potentially impacts antitumor efficacy; therefore next generation viruses sought ways to improve oHSV replication through conditional γ134.5 gene expression in tumor cells or through selective complementation of γ134.5 functions. C134 is a chimeric oncolytic virus developed in our lab and derived from Δγ134.5 background that has IRS1 (PKR-evasion gene from Human Cytomegalovirus) transferred from HCMV to enhance its replication in tumor cells [17], [18]. C134 has improved late gene expression, viral replication, and anti-tumor activity without restoring the wild-type neurovirulence [18].
Our previous studies showed that C134 replicates better than 1st generation Δγ134.5 OVs in vitro and in vivo and that this replication advantage improved animal survival in vivo. The purpose of these studies was to further characterize the anti-tumor activity in an immunocompetent and restrictive tumor environment where C134 has no replication advantage over Δγ134.5 virus (R3616). By doing this we can evaluate additional mechanisms that contribute to oHSV antitumor effect. Because the immune response is often integral to the indirect OV anti-tumor activity, we avoided patient derived xenografts and orthotopic human xenograft models because they require immunocompromised athymic nude or SCID mice for in vivo evaluation. Instead we used aggressive syngeneic tumor models that 1) restrict C134 replication to that of a 1st gen virus and 2) permit evaluation of the immune mediated anti-tumor response. The results show that both C134 and the 1st generation oHSV elicit a T cell dependent immune mediated anti-tumor effect in these models. The syngeneic models however demonstrate some subtle differences between the viruses. C134 significantly improves median survival in both of the tumor models. The first generation virus, R3616, only produces a statistically significant improvement in survival in the N2A model but not in the DBT restrictive model. While it extended median survival, it was less effective than C134 in the N2A model. In the more OV resistant DBT model, only C134 significant improved median survival over saline whereas R3616 did not (despite equivalent replication). Yet both viruses were capable of eliciting long-term immune mediated antitumor effect and survival in a proportion of the treated cohort.
We hypothesized that the anti-viral immune response contributed to this improved survival and therefore tested the anti-tumor activity in an athymic nude mouse model. When the T cells are eliminated, R3616 and C134 produce similar survival curves and are no better than saline therapy. T cells therefore are essential for C134 and R3616 anti-tumor activity in these restrictive syngeneic models. The adaptive immune response's importance is further supported by the fact that long-term survivors generate a circulating immune memory response that restricts tumor growth on re-challenge in the DBT studies. These results also show that treatment with C134 primes and improves this anti-tumor immune response over simple tumor antigen exposure alone. Interestingly, the results do not demonstrate any difference in durable anti-tumor response between long-term survivors treated with a 1st generation virus or with a next generation virus (data not shown). The principal difference identified between these viruses is in terms of median survival and this occurs both with single injection and with repeated exposure. This suggests that in addition to the T cell mediated response additional immune mediated mechanisms may also delay tumor growth in the C134 treated cohort but their effect is not durable like the T cell mediated activity. There are several possibilities for this difference in tumor growth between the viruses that we are currently investigating. Based upon our studies, the CD8 T cell adaptive immune response plays a crucial role in fighting and limiting tumor growth however, cytotoxic T cell numbers infiltrating into the tumor are similar between the viruses which suggests functional differences in the immune response after treatment with the first generation and next generation virus.
In conclusion, MG tumors differ in their susceptibility to oHSV. We have identified that similar to our results in peripheral tumors, CNS tumors with intact STAT signaling and an ISG response to virus are predictors of resistance to oHSV infection. Both R3616 and C134 improved the overall survival of animal bearing restrictive brain tumors by immune-mediated mechanisms but only C134 consistently extended median survival and was superior to the 1st gen virus in the other tumor model. Moreover, C134 has the advantage of improved replication in other MG models [18], [29]. Further studies are required to better understand the immune mechanisms contributing to the enhanced immune mediated survival and this difference in median survival observed between the first and second generation oncolytic HSV-1.
Acknowledgments
Acknowledgements
Funding for these studies was provided by: Alex's Lemonade Stand Foundation, Hyundai Hope on Wheels, NIH P20 CA151129.
Footnotes
Funding: SEBT, Alex's Lemonade, NIH SPORE P20.
Conflict of interest: None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2017.10.005
Appendix A. Supplementary data
Supplemental Figure 1: Table describing the genetics of the different viruses used in the study.
Supplemental Figure 2: Immunoblotting studies of HSV glycoprotein D in Neuro2A and DBT cell lines. DBT and N2A cells were infected by the R3616 and C134 at MOI 1 overnight then cells were harvested and analyzed by immunoblotting for HSV-1 glycoprotein-D production (Top panel) and actin (lower panel).
Supplemental Figure 3: (A) Examples of representative flow cytometry plots from the treatment groups and our gating strategy. (B) CNS infiltrating leukocyte populations analyzed by flow cytometry showed no significant difference in CD4 infiltrates.
References
- 1.Davis JJ, Fang B. Oncolytic virotherapy for cancer treatment: challenges and solutions. J Gene Med. 2005;7(11):1380–1389. doi: 10.1002/jgm.800. [Epub 2005/07/19. PubMed PMID: 16025557] [DOI] [PubMed] [Google Scholar]
- 2.Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E, Pollack IF. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol. 2011;13(4):410–416. doi: 10.1093/neuonc/noq205. [Epub 2011/02/25. PubMed PMID: 21345842; PubMed Central PMCID: PMC3064697] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [Epub 2005/03/11. PubMed PMID: 15758009] [DOI] [PubMed] [Google Scholar]
- 4.Foreman PM, Friedman GK, Cassady KA, Markert JM. Oncolytic Virotherapy for the Treatment of Malignant Glioma. Neurotherapeutics. 2017 doi: 10.1007/s13311-017-0516-0. [Epub 2017/03/08. PubMed PMID: 28265902] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250(4985):1262–1266. doi: 10.1126/science.2173860. [Epub 1990/11/30. PubMed PMID: 2173860] [DOI] [PubMed] [Google Scholar]
- 6.Chou J, Roizman B. Herpes simplex virus 1 gamma(1)34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc Natl Acad Sci U S A. 1994;91(12):5247–5251. doi: 10.1073/pnas.91.12.5247. [Epub 1994/06/07. PubMed PMID: 8202476; PubMed Central PMCID: PMC43971] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin H, Ma Y, Prabhakar BS, Feng Z, Valyi-Nagy T, Yan Z, Verpooten D, Zhang C, Cao Y, He B. The gamma 1 34.5 protein of herpes simplex virus 1 is required to interfere with dendritic cell maturation during productive infection. J Virol. 2009;83(10):4984–4994. doi: 10.1128/JVI.02535-08. [Epub 2009/03/13. PubMed PMID: 19279105; PubMed Central PMCID: PMC2682079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verpooten D, Ma Y, Hou S, Yan Z, He B. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J Biol Chem. 2009;284(2):1097–1105. doi: 10.1074/jbc.M805905200. [Epub 2008/11/18. PubMed PMID: 19010780; PubMed Central PMCID: PMC2613634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1(1):23–35. doi: 10.1016/j.chom.2006.12.001. [Epub 2007/11/17. PubMed PMID: 18005679] [DOI] [PubMed] [Google Scholar]
- 10.Bolovan CA, Sawtell NM, Thompson RL. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J Virol. 1994;68(1):48–55. doi: 10.1128/jvi.68.1.48-55.1994. [Epub 1994/01/01. PubMed PMID: 8254758; PubMed Central PMCID: PMC236262] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest. 1993;91(6):2837–2843. doi: 10.1172/JCI116527. [Epub 1993/06/01. PubMed PMID: 8390490; PubMed Central PMCID: PMC443352] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markert JM, Gillespie GY, Weichselbaum RR, Roizman B, Whitley RJ. Genetically engineered HSV in the treatment of glioma: a review. Rev Med Virol. 2000;10(1):17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [Epub 2000/02/02. PubMed PMID: 10654002] [DOI] [PubMed] [Google Scholar]
- 13.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, Nabors LB, Markiewicz M, Lakeman AD, Palmer CA. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17(1):199–207. doi: 10.1038/mt.2008.228. [Epub 2008/10/30. PubMed PMID: 18957964; PubMed Central PMCID: PMC2834981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, Maclean A, Harland J, Mckie E. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [Epub 2000/06/14. PubMed PMID: 10845724] [DOI] [PubMed] [Google Scholar]
- 15.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, Hadley D, Patterson J, Brown SM, Rampling R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11(22):1648–1658. doi: 10.1038/sj.gt.3302289. [Epub 2004/08/31. PubMed PMID: 15334111] [DOI] [PubMed] [Google Scholar]
- 16.Jackson JD, Markert JM, Li L, Carroll SL, Cassady KA. STAT1 and NF-kappaB Inhibitors Diminish Basal Interferon-Stimulated Gene Expression and Improve the Productive Infection of Oncolytic HSV in MPNST Cells. Mol Cancer Res. 2016;14(5):482–492. doi: 10.1158/1541-7786.MCR-15-0427. [Epub 2016/02/18. PubMed PMID: 26883073; PubMed Central PMCID: PMC4867290] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassady KA. Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection. J Virol. 2005;79(14):8707–8715. doi: 10.1128/JVI.79.14.8707-8715.2005. [PubMed PMID: 15994764] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AC, Parker JN, Gillespie GY, Lakeman FD, Meleth S, Markert JM, Cassady KA. Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses. Gene Ther. 2007;14(13):1045–1054. doi: 10.1038/sj.gt.3302942. [PubMed PMID: 17429445] [DOI] [PubMed] [Google Scholar]
- 19.Cassady KA, Haworth KB, Jackson J, Markert JM, Cripe TP. To Infection and Beyond: The Multi-Pronged Anti-Cancer Mechanisms of Oncolytic Viruses. Virus. 2016;8(2) doi: 10.3390/v8020043. [Epub 2016/02/11. PubMed PMID: 26861381; PubMed Central PMCID: PMC4776198] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leddon JL, Chen CY, Currier MA, Wang PY, Jung FA, Denton NL, Cripe KM, Haworth KB, Arnold MA, Gross AC. Oncolytic HSV virotherapy in murine sarcomas differentially triggers an antitumor T-cell response in the absence of virus permissivity. Mol Ther Oncolytics. 2015;1:14010. doi: 10.1038/mto.2014.10. [Epub 2015/01/01PubMed PMID: 27119100; PubMed Central PMCID: PMC4782947] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A. 2000;97(5):2208–2213. doi: 10.1073/pnas.040557897. [Epub 2000/02/19. PubMed PMID: 10681459; PubMed Central PMCID: PMC15779] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [Epub 2012/07/12. PubMed PMID: 22781695; PubMed Central PMCID: PMC3888062] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellums EK, Markert JM, Parker JN, He B, Perbal B, Roizman B, Whitley RJ, Langford CP, Bharara S, Gillespie GY. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol. 2005;7(3):213–224. doi: 10.1215/S1152851705000074. [Epub 2005/08/02. PubMed PMID: 16053696; PubMed Central PMCID: PMC1871915] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassady KA, Saunders U, Shimamura M. Deltagamma(1)134.5 herpes simplex viruses encoding human cytomegalovirus IRS1 or TRS1 induce interferon regulatory factor 3 phosphorylation and an interferon-stimulated gene response. J Virol. 2012;86(1):610–614. doi: 10.1128/JVI.05099-11. [PubMed PMID: 22072777; PubMed Central PMCID: PMC3255867] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer DF, Pereboeva L, Gillespie GY, Cloud GA, Elzafarany O, Langford C, Markert JM, Lamb LS., Jr. Effect of HSV-IL12 Loaded Tumor Cell-Based Vaccination in a Mouse Model of High-Grade Neuroblastoma. J Immunol Res. 2016;2016:2568125. doi: 10.1155/2016/2568125. [Epub 2016/09/10. PubMed PMID: 27610392; PubMed Central PMCID: PMC5005549] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang HR, Gilham DE, Mulryan K, Kirillova N, Hawkins RE, Stern PL. Combination of vaccination and chimeric receptor expressing T cells provides improved active therapy of tumors. J Immunol. 2006;177(7):4288–4298. doi: 10.4049/jimmunol.177.7.4288. [Epub 2006/09/20. PubMed PMID: 16982863] [DOI] [PubMed] [Google Scholar]
- 27.Schell JB, Rose NF, Bahl K, Diller K, Buonocore L, Hunter M, Marx PA, Gambhira R, Tang H, Montefiori DC. Significant protection against high-dose simian immunodeficiency virus challenge conferred by a new prime-boost vaccine regimen. J Virol. 2011;85(12):5764–5772. doi: 10.1128/JVI.00342-11. [Epub 2011/04/15. PubMed PMID: 21490100; PubMed Central PMCID: PMC3126289] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nistal-Villan E, Bunuales M, Poutou J, Gonzalez-Aparicio M, Bravo-Perez C, Quetglas JI, Carte B, Gonzalez-Aseguinolaza G, Prieto J, Larrea E. Enhanced therapeutic effect using sequential administration of antigenically distinct oncolytic viruses expressing oncostatin M in a Syrian hamster orthotopic pancreatic cancer model. Mol Cancer. 2015;14:210. doi: 10.1186/s12943-015-0479-x. [Epub 2015/12/17. PubMed PMID: 26671477; PubMed Central PMCID: PMC4681018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman GK, Nan L, Haas MC, Kelly VM, Moore BP, Langford CP, Xu H, Han X, Beierle EA, Markert JM. gamma(1)34.5-deleted HSV-1-expressing human cytomegalovirus IRS1 gene kills human glioblastoma cells as efficiently as wild-type HSV-1 in normoxia or hypoxia. Gene Ther. 2015;22(4):348–355. doi: 10.1038/gt.2014.107. [Epub 2014/11/28. PubMed PMID: 25427614; PubMed Central PMCID: PMC4383690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Table describing the genetics of the different viruses used in the study.
Supplemental Figure 2: Immunoblotting studies of HSV glycoprotein D in Neuro2A and DBT cell lines. DBT and N2A cells were infected by the R3616 and C134 at MOI 1 overnight then cells were harvested and analyzed by immunoblotting for HSV-1 glycoprotein-D production (Top panel) and actin (lower panel).
Supplemental Figure 3: (A) Examples of representative flow cytometry plots from the treatment groups and our gating strategy. (B) CNS infiltrating leukocyte populations analyzed by flow cytometry showed no significant difference in CD4 infiltrates.