Abstract
Partitioning defective (Par) proteins regulate cell polarity and differentiation. Par3, Par6β, and protein kinase Cζ (PKCζ), which are PAR complex members, have been shown to be associated with oncogenesis and progression. Herein, we report the expression pattern and clinical relevance of Par3, Par6β, and PKCζ in colorectal adenocarcinoma (CRAC). A total of 393 primary CRACs, 41 primary-metastatic CRAC pairs, 41 adenomas with low-grade dysplasia, and 41 nontumor colorectal tissue samples were examined by immunohistochemistry and Western blot assays for Par3, Par6β, and PKCζ protein expressions. The association Par3, Par6β, and PKCζ expressions and clinicopathologic factors, including patient survival, was evaluated. Primary CRACs and adenomas demonstrated higher levels of Par3, Par6β, and PKCζ than in nontumor colorectal epithelia. The expressions of Par3, Par6β, and PKCζ were higher in primary CRACs as compared to adenomas or in metastatic CRACs. Among primary CRACs, decreased Par3 expression was found to correlate with a high proliferation rate and poor histologic differentiation, decreased PKCζ expression was correlated with pathologic TNM stage (I-II vs III-IV) and lymph node metastasis, and decreased Par6β and PKCζ expressions were correlated with shortened overall survivals. In metastatic CRACs, decreased PKCζ expression was correlated with a shortened metastasis-free survival. While increased Par3, Par6β, and PKCζ expressions were implicated in tumorigenesis, decreased expressions of Par3, Par6β, and PKCζ were found to be associated with worse clinicopathologic factors in CRAC. In particular, the results of our study suggest that PKCζ down-expression is an independent poor prognostic and metastatic factor for CRAC.
Introduction
Polarity is a fundamental property of cells that is essential for the cell development and organization. Coordinated action of polarity regulatory protein complexes produces specific cell polarity. Polarity regulatory complexes were first discovered in Caenorhabditis elegans and were named as par-titioning-defective (Par) proteins [1]. Lethal mutations in PAR genes showed disruption in cell division and organization [2]. One of the polarity regulatory complexes, the PAR complex, is a tripartite composed of Par3/Par6/atypical protein kinase C (aPKC); the components are intimately connected and dynamically interacted to maintain epithelial structure and create spatial difference and functional asymmetry [3]. Association between the PAR complex and differentiation, tumorigenesis, progression, and metastasis has been observed in various cancers [4], [5], [6]. But little is known about the clinical relevance of the PAR complex in colorectal adenocarcinoma (CRAC).
CRAC is one of the most common cancer types and leading causes of cancer-related death [7]. The prognosis of CRAC has been improved through early detection and advanced surgical treatment. However, 30% of patients with CRAC develop distant metastasis, and the 5-year survival rate falls to 13% in patients with metastasis even after curative surgical resection [8], [9]. Considering that loss of polarity is a hallmark of cancer and metastasis, investigating the PAR complex in CRAC may help to identify potential targets for tumorigenic, prognostic, and therapeutic markers in CRAC.
To investigate expression patterns and the role of the PAR complex in CRAC, Par3, Par6β, and PKCζ protein expressions were evaluated. This study assessed Par3, Par6β, and PKCζ levels in nontumor colorectal mucosa, tubular adenoma, primary CRAC, and metastatic CRAC to evaluate differential expression during tumorigenesis and metastasis. The Par3, Par6β, and PKCζ expressions were analyzed in relation to clinicopathologic features, including patient overall survival and metastasis-free survival in CRAC.
Materials and Methods
Patients and Tissue Samples
A total of 393 paraffin-embedded primary CRAC samples were obtained from 393 patients who underwent surgical treatment and were histologically diagnosed with CRAC at the Samsung Medical Center (Seoul, South Korea) from June 1998 to December 2000 and at the Chungbuk National University Hospital (Cheongju, South Korea) from January 1994 to December 1998. Tissue samples were used for a uniform specimen processing and follow-up protocols. In a surgical specimen, one most representative and viable tumor area and one nontumor tissue area were selected and marked on the hematoxylin and eosin (H&E)–stained slides. To create a tissue microarray, tissue columns (3.0 mm in diameter) were punched from the original paraffin blocks and inserted into new recipient paraffin blocks (each containing 30 holes for tissue columns). Forty-one primary CRAC and matched metastatic CRAC samples, 41 adenomas with low-grade dysplasia, and 41 nontumor paraffin-embedded colorectal tissue samples were obtained from Chungnam National University Hospital (Daejeon, South Korea) from June 2004 to December 2010. Full H&E slides were reviewed, and full paraffin samples were used to compare protein expression pattern and distribution.
Forty primary CRAC and paired 40 nontumor frozen colorectal tissue samples stored in liquid nitrogen were obtained from the National Biobank of Korea, Chungnam National University Hospital, a member of the Korea Biobank Network, from January 2008 to December 2012. Under the review of H&E-stained frozen section, one vial (100 mg) of tumor sample and one nontumor frozen sample were obtained from the biobank.
All cases were clinicopathologically reviewed by two pathologists (M.K.Y. and K.H.K.), including overall survival (the length of time from the date of diagnosis to the date of identification of death) and metastasis-free survival (the length of time from the date of diagnosis to the date of identification of distant metastasis), from the archives of each hospital. None of the patients had received preoperative chemotherapy or radiotherapy. CRAC stages were determined according to the American Joint Committee on Cancer Staging System, eighth edition [10].
This study protocol was approved by the Institutional Review Board of Chungnam National University Hospital and complied with the tenets of the Declaration of Helsinki (CNUH 2015-05-025-002). The study was retrospective and was approved a waiver of consent from Institutional Review Board.
Immunohistochemical Staining Analysis
Tissue sections were cut from the tissue microarray paraffin blocks (393 primary CRACs) and from full paraffin blocks (41 primary CRACs, 42 matched metastatic CRACs, 41 adenomas, and 41 nontumor colorectal tissue samples). Tissue sections were mounted on the coated slides, deparaffinized with xylene, hydrated in serial solutions of alcohol, and heated in a pressure cooker containing 10 mmol/l sodium citrate (pH 6.0) for 3 minutes at full power for antigen retrieval. Peroxide blocking was performed using 3% H2O2 in methanol at room temperature for 10 minutes. Nonspecific protein-binding sites were blocked by incubation with serum-free protein for 20 minutes. The sections were incubated overnight at 4°C with the following primary antibodies: rabbit polyclonal anti-Par3 antibody (1:100, Clone 07-330, Millipore, Temecula, CA), rabbit polyclonal anti-Par6β antibody (1:400, catalog #B8062, Sigma, St. Louis, MO), rabbit polyclonal anti-PKCζ antibody (C-20) (1:300, catalog #sc-216, Santa Cruz Biotechnology, Santa Cruz, CA), and mouse monoclonal anti-Ki67 antibody (1:100, Dako, Glostrup, Denmark). After washing, the samples were incubated in Dako REAL EnVision/horseradish peroxidase rabbit/mouse detection reagent for an additional 20 minutes at room temperature followed by additional washing. After rinsing, the chromogen was developed for 2 minutes. The slides were then counterstained with Meyer's hematoxylin, dehydrated, and topped with coverslips. The primary antibody was omitted in the negative controls.
Immunohistochemical staining was scored using digitally scanned files by a scanscope program (Aperio ScanScope CS system, Vista, CA). The Allred et al. method was used to evaluate both the intensity of immunohistochemical staining and the proportion of stained neoplastic or nonneoplastic epithelial cells in each stained slide [11]. The proportion scores ranged from 0 to 5 (0, 0; 1, >0 to 1/100; 2, >1/100 to 1/10; 3, >1/10 to 1/3; 4, >1/3 to 2/3; 5, >2/3 to 1), while the intensity scores ranged from 0 to 3 (0, negative; 1, equivalent or weaker expression than in nontumor epithelial cells; 2, moderately higher than nontumor epithelial cells; 3, markedly higher than nontumor epithelial cells). The proportion and intensity scores were added to obtain the total score (range: 0-8). The total scores were categorized for analyses as follows: equivalent or weaker expression than that of nontumor epithelial cells was regarded as “low,” and higher expression than nontumor epithelial cells was regarded as “high”. The percentage of Ki67 antibody-positive nuclear-stained cells was determined, and the median score was 18%. For categorical analyses, Ki67 scores equivalent to or lower than the median score value were categorized as “low,” or scores higher the median score were categorized as “high.” The results were examined separately and scored by 2 pathologists (M.K.Y. and K.H.K.) who were blinded to the patients' details. Discrepancies in scores were discussed to obtain a consensus.
Western Blot Assay
Proteins were extracted from 40 pairs of CRAC sample and nontumor tissue sample stored at −80°C in liquid nitrogen using PRO-PREP TM protein extraction solution (iNtRON Biotechnology, 17081, Kyungki-Do, South Korea). A total 50 μg of protein was separated using 10% SDS-polyacrylamide gel electrophoresis (Mini-PROTEAN TGXTM Gels, 456-1034, BIO-RAD, Hercules, CA) and then electrophoretically transferred to PVDF membrane (Immuno-Blot PVDF Membrane for Protein Blotting, 162-0177, BIO-RAD). After blotting, the membrane was incubated overnight at 4°C with rabbit polyclonal anti-PKCζ antibody (C-20) (1:300, catalog #sc-216, Santa Cruz Biotechnology, Santa Cruz, CA) followed by goat anti-rabbit IgG, H&L Chain Specific Peroxidase Conjugate secondary antibody (401353, Calbiochem, Darmstadt, Germany) at room temperature for 1 hour. Protein bands were enhanced with Immobilon TM Western chemiluminescent HRP substrate (WBKLS0500, Millipore, Billerica, MA), and the images were digitalized using an UVITEC Cambridge alliance mini 4M system (UVItec Limited, Cambridge, UK). The tissue sample was omitted in the negative control. Human colonic adenocarcinoma cell line COLO320HSR (KCLB 10020.1) was used as the positive control.
The PKCζ and β-actin bands were quantified by Image J program (https://imagej.nih.gov/ij/notes.html). The relative quantification value of PKCζ in each tissue sample was presented as the ratio of their PKCζ band value to that of β-actin band value. For categorical analysis, the relatively quantified PKCζ band value less than that of the paired nonneoplastic epithelia value was regarded as “low,” and value greater than that of the paired nonneoplastic epithelia value was regarded as “high.”
Statistical Analysis
Associations between Par3, Par6β, and PKCζ immunohistochemical and Western blot expressions with clinicopathologic variables for the colorectal neoplastic lesions were examined by Spearman rank correlation coefficients, Mann-Whitney U tests, and Kruskal-Wallis tests. The Wilcoxon signed rank test was used for group comparisons. For univariate analysis, overall survival curves with log-rank test were determined using the Kaplan-Meier method. Multivariate survival analysis was performed using Cox's proportional hazard regression model. Statistical significance was set at P < .05 (SPSS 22; SPSS Inc., Chicago, IL).
Results
Differential Immunohistochemical Expression of Par3, Par6β, and PKCζ in Nontumor Mucosa, Adenoma, and CRAC
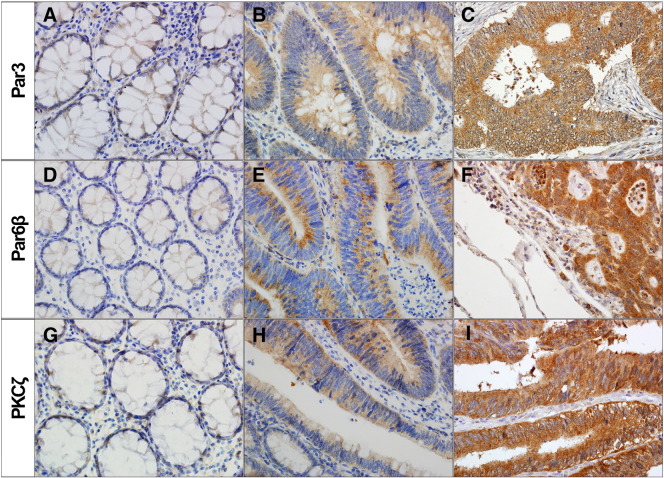
Par3, Par6β, and PKCζ immunostainings were detected in colonic epithelial cells but not in stromal cells. Par3, Par6β, and PKCζ stainings exhibited a cytoplasmic pattern without nuclear or membranous staining. Cytoplasmic Par3, Par6β, and PKCζ immunohistochemical expressions were compared in the 41 nontumor colorectal mucosae, 41 adenomas with low-grade dysplasia, 41 primary CRACs, and matched metastatic CRAC tissue samples (Figure 1).
Figure 1.
Representative immunohistochemical expressions of Par3, Par6β, and PKCζ in CRAC. (A, D, G) Faint or weak expression on nontumor colorectal mucosa, (B, E, H) moderate expression on tubular adenoma, and (C, F, I) marked high expression on primary CRAC.
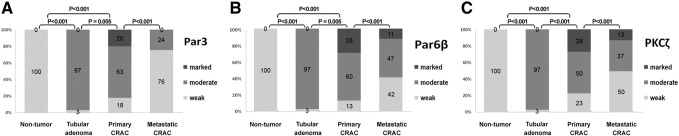
All nontumor mucosae were negative or weak stained for Par3, Par6β, and PKCζ. Primary CRACs and adenomas showed upregulation of Par3, Par6β, and PKCζ expression compared with the nontumor colorectal mucosa (P < .001, P < .001, and P < .001, respectively) (Figure 2). Primary CRAC showed significantly higher levels of Par3, Par6β, and PKCζ than did the adenomas (P = .005, P = .005, and P < .001, respectively). Metastatic CRAC showed significantly lower levels of Par3, Par6β, and PKCζ compared with primary CRAC (P < .001, P < .001, and P < .001, respectively). Par3, Par6β, and PKCζ levels were positively correlated with each other (P < .001, P < .001, and P < .001, respectively) (Supplementary Table 1).
Figure 2.
Comparison of the expressions of Par3, Par6β, and PKCζ by immunohistochemistry among nontumor colorectal mucosa, tubular adenoma, primary CRAC, and matched metastatic CRAC (n = 41).
Clinicopathologic Features and Par3, Par6β, and PKCζ Immunohistochemical Expression Patterns in Primary CRACs
A total of 393 CRAC cases were evaluated; the patients' age ranged from 25 to 91 years, with the mean of 58.7 years. CRACs were located in the colon and rectum at a ratio of 1.1:1. Primary CRACs were mostly well or moderately differentiated (93%). Patients with CRAC had lymph nodal metastasis in 41% of cases and distant metastasis in 17%. Immunohistochemical expression of Par3, Par6β, and PKCζ with clinicopathologic features of a total of 393 CRACs was assessed (Table 1). Par3 expression was negatively correlated with poor histologic differentiation and high proliferation (Ki67 index) (P = .043 and P < .001, respectively). PKCζ expression was negatively correlated with pathologic stage (I-II vs III-IV) and lymph node metastasis (P = .028 and P = .039, respectively).
Table 1.
Correlation between Par3, Par6β, and PKCζ Immunohistochemical Expressions and Clinicopathologic Factors in CRAC Patients (n = 393)
| Characteristics | Patients No. (%) | Par3 |
Patients No. (%) | Par6β |
Patients No. (%) | PKCζ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | P | Low | High | P | Low | High | P | ||||
| Sex | .643 | .629 | .664 | |||||||||
| Male | 215 (57) | 166 (58) | 49 (55) | 222 (57) | 147 (58) | 75 (56) | 217 (57) | 130 (57) | 87 (59) | |||
| Female | 161 (43) | 121 (42) | 40 (45) | 166 (43) | 106 (42) | 60 (44) | 161 (43) | 100 (44) | 61 (41) | |||
| Age, years (mean) | 393 | 58 | 60 | .594 | 393 | 58 | 60 | .691 | 393 | 59 | 59 | .441 |
| Pathologic stage | .499 | .381 | .028 | |||||||||
| I-II | 189 (51) | 141 (50) | 48 (54) | 196 (51) | 123 (49) | 73 (54) | 191 (51) | 105 (47) | 86 (58) | |||
| III-IV | 183 (49) | 142 (52) | 41 (46) | 188 (49) | 126 (51) | 62 (46) | 183 (49) | 121 (54) | 62 (42) | |||
| Differentiation | .043 | .608 | .408 | |||||||||
| WD | 85 (23) | 58 (20) | 27 (31) | 89 (23) | 56 (22) | 33 (25) | 86 (23) | 49 (22) | 37 (25) | |||
| MD + PD | 288 (77) | 227 (80) | 61 (69) | 296 (77) | 195 (78) | 101 (75) | 289 (77) | 179 (79) | 110 (75) | |||
| LN metastasis | .217 | .552 | .039 | |||||||||
| Absent | 207 (56) | 153 (54) | 54 (61) | 214 (56) | 136 (55) | 78 (58) | 208 (51) | 116 (51) | 92 (62) | |||
| Present | 165 (44) | 131 (46) | 34 (39) | 170 (44) | 113 (45) | 57 (42) | 166 (49) | 110 (49) | 56 (38) | |||
| Ki67 index | .000 | .853 | .863 | |||||||||
| Low | 223 (62) | 157 (57) | 69 (79) | 232 (63) | 149 (63) | 83 (64) | 224 (62) | 134 (62) | 90 (63) | |||
| High | 135 (88) | 117 (43) | 18 (21) | 1358 (37) | 88 (37) | 47 (36) | 135 (38) | 82 (38) | 53 (37) |
WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; LN, lymph node.
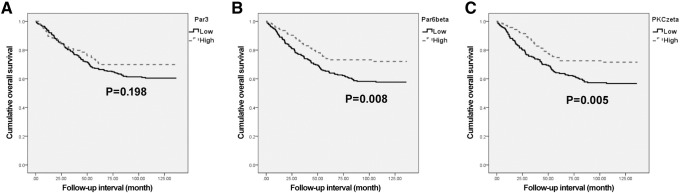
Overall survival analyses were performed with data from 373 patients with CRAC. The Kaplan-Meier survival curves and log-rank tests showed significant association of low Par6β and PKCζ expressions with shortened overall survival (P = .008 and P = .005, respectively) (Figure 3). The Kaplan-Meier overall survival curves for cases with low expression of Par3 showed a tendency towards shortened survival times, but the trend did not reach statistical significance (P = .198). The multivariate analyses using the Cox's proportional hazard model were performed on age; sex; stage (I-II vs III-IV); and Par3, Par6β, and PKCζ expressions (Table 2, Table 3 and 4). The multivariate analysis showed low Par6β and PKCζ expressions to be significant poor prognostic factors indicative of poor overall survival (P = .021 and P = .027, respectively). Par3 expression was not a significant prognostic factor for overall survival (P = .301).
Figure 3.
Kaplan-Meier curves according to Par3, Par6β, and PKCζ expressions in CRAC (n = 393); overall survival according to (A) Par3 (P = .198), (B) Par6β (P = .008), and (C) PKCζ (P = .005).
Table 2.
Multivariate Analysis Results for Overall Survival in CRAC Patients (n = 393)
| Overall Survival |
|||
|---|---|---|---|
| P | HR | 95% CI | |
| Par3 (low vs high) | .301 | 0.793 | (0.512-1.230) |
| Sex (female vs male) | .573 | 1.106 | (0.778-1.573) |
| Age (under 60 years vs over 60 years) | .013 | 1.566 | (1.099-2.231) |
| Stage (I + II vs III + IV) | <.001 | 4.692 | (3.127-7.040) |
HR, hazard ratio; CI, confidence index.
Table 3.
Multivariate Analysis Results for Overall Survival in CRAC Patients (n = 393)
| Overall Survival |
|||
|---|---|---|---|
| P | HR | 95% CI | |
| Par6β (low vs high) | .021 | 0.632 | (0.427-0.933) |
| Sex (female vs male) | .499 | 1.127 | (0.797-1.594) |
| Age (under 60 years vs over 60 years) | .015 | 1.537 | (1.086-2.176) |
| Stage (I + II vs III + IV) | <.001 | 4.943 | (3.300-7.404) |
Table 4.
Multivariate Analysis Results for Overall Survival in CRAC Patients (n = 393)
| Overall Survival |
|||
|---|---|---|---|
| P | HR | 95% CI | |
| PKCζ (low vs high) | .027 | 0.653 | (0.448-0.952) |
| Sex (female vs male) | .536 | 1.117 | (0.787-1.586) |
| Age (under 60 years vs over 60 years) | .035 | 1.460 | (1.027-2.075) |
| Stage (I + II vs III + IV) | <.001 | 4.718 | (3.144-7.079) |
Comparison of Metastasis-Free Survival with Par3, Par6β, and PKCζ Immunohistochemical Expressions in Primary and Metastatic CRACs
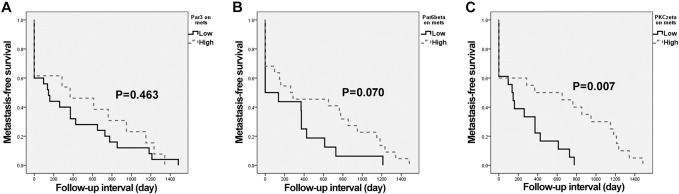
Par3, Par6β, and PKCζ expressions of primary and metastatic CRACs with metastasis-free survival were assessed. Analyses of metastasis-free survival were performed with data from 41 patients with primary CRAC and matched metastatic CRAC. The Kaplan-Meier survival curves and log-rank tests showed a significant association between low PKCζ expression on metastatic CRAC and shortened metastasis-free survival (P = .007) (Figure 4). The multivariate analysis also showed low PKCζ expression on metastatic CRAC to be a significant predictor of shortened metastasis-free survival (P = .028) (Table 5). Low expressions of Par3 and Par6β on metastatic CRAC tended to correspond to earlier metastasis; however, this trend did not reach statistical significance in the univariate and multivariate survival analyses (Figure 4) (Supplementary Tables 2 and 3). Par3, Par6β, and PKCζ expressions on primary CRAC were not correlated with metastasis-free survival (P = .342, P = .244, and P = .211, respectively).
Figure 4.
Kaplan-Meier curves according to Par3, Par6β, and PKCζ expressions in metastatic CRAC (n = 41); metastasis-free survival according to (A) Par3 (P = .463), (B) Par6β (P = .070), and (C) PKCζ (P = .007).
Table 5.
Multivariate Analysis Results for Metastasis-Free Survival in CRAC (n = 41)
| Overall Survival |
|||
|---|---|---|---|
| P | HR | 95% CI | |
| PKCζ on metastatic CRAC (low vs high) | .028 | 0.399 | (0.176-0.904) |
| Sex (female vs male) | .341 | 1.427 | (0.686-2.968) |
| Age (under 60 years vs over 60 years) | .600 | 0.799 | (0.347-1.844) |
| Stage (I vs II-IV) | .002 | 26.276 | (3.323-207.766) |
Western Blot Assay of PKCζ Expression and Correlation with Clinicopathologic Features and Prognostic Significance in Primary CRACs
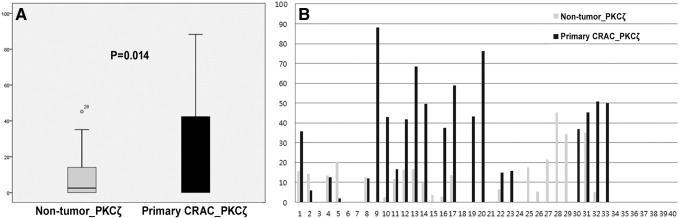
PKCζ Western blot assays were performed using 40 pairs of CRAC sample and nontumor tissue sample stored at −80°C in liquid nitrogen. PKCζ Western blots showed that CRAC expressed significantly higher levels of PKCζ than the nontumor colorectal mucosa (P = .014) (Figure 5). Decreased PKCζ Western blot expression was correlated with poor histologic differentiated CRAC (P = .001) (Supplementary Table 4). Kaplan-Meier overall survival curves and multivariate analysis using the Cox's proportional hazard model were performed on data from 40 patients with CRAC. Cases with low PKCζ expression tended to be associated with shortened overall survival times, but this trend did not reach statistical significance (Supplementary Figure 1, Supplementary Table 5).
Figure 5.
Comparison of the expression of PKCζ by Western blot analysis between nontumor colorectal mucosa and primary CRAC.
Discussion
In the present study, expression of the PAR complex proteins (Par3, Par6β, PKCζ) was assessed by immunohistochemistry and Western blot assays in CRAC. Primary CRACs and adenomas showed upregulated Par3, Par6β, and PKCζ compared with nontumor colorectal mucosa. Primary CRACs exhibited significantly upregulated Par3, Par6β, and PKCζ versus adenomas. Metastatic CRACs showed decreased levels of Par3, Par6β, and PKCζ expression compared with primary CRAC samples. Aberrant expression of Par3, Par6β, and PKCζ might be involved in tumorigenesis at an early stage of CRAC, while alterations in the PAR complex expression might be related to tumor progression and metastasis. Expression of the PAR complex has been reported in several cancers including CRAC. Increased Par3 protein expression has been shown in hepatocellular and renal cell carcinoma [12], [13]. Decreased Par3 protein expression in metastatic breast cancers compared with matched primary tumors has also been reported [4]. Overexpression of Par6β protein and its transcript has been reported in breast cancer [5], [14]. PKCζ protein expression is highly expressed in breast, ovary, and head and neck cancers [15], [16], [17]. PKCζ expression has been shown to be up- or down-expressed in CRAC [18], [19].
The clinical and prognostic implications of PAR complex protein expression were assessed in the present study. Par3 expression was inversely correlated with poor histologic differentiation and high proliferation. PKCζ expression was inversely correlated with pathologic stage, poor histologic differentiation, and lymph node metastasis. Par6β and PKCζ protein levels were predictive of overall survival in CRAC. Interestingly, lower PKCζ expression was significantly related to shortened metastasis-free survival. Alterations in PAR complex expression and the clinical significance in cancers have been described. Par3 overexpression was associated with reduced survival in hepatocellular and renal cell carcinomas [12], [20]. Decreased expression of Par6β showed a tendency to be associated with poor histologic differentiation in breast cancer [5]. Low PKCζ expression was a predictor of shortened disease-free survival in CRAC [21], [22]. Reduced expression of PKCζ was related to frequent recurrence in bladder cancer [6]. Changes in the expression of individual Par3, Par6β, and PKCζ proteins have been implicated as prognostic factor for cancers.
The PAR complex (Par3, Par6β, and PKCζ) are known to colocalize apicolateral junction of the cell membrane and to dynamically interact with other regulatory proteins, including other polarity complexes [23]. Par3 is regulated by atypical PKC (aPKC)–dependent phosphorylation. Separated from Par3, an activated Par6/aPKC is involved in cell migration and signaling [24]. In the present study, protein levels of Par3, Par6β, and PKCζ were found to statistically positively correlate with each other. Even though Par3, Par6β, and PKCζ proteins had different clinical impact, alteration patterns of the protein levels during tumorigenesis and metastasis showed a similar tendency. PKCζ, one of the aPKCs, showed a significant clinical and prognostic significance, suggesting that PKCζ could be a key contributing factor of the PAR complex for CRAC.
Conclusion
Overexpressions of cytoplasmic Par3, Par6β, and PKCζ occurred in malignant transformation but appeared to be reduced in metastatic CRAC. Decreased expressions of Par3, Par6β, and PKCζ in CRAC were associated with worse clinicopathologic features. Especially, decreased Par6β and PKCζ expressions were associated with shortened overall survival. Decreased PKCζ expression was significantly associated with shortened metastasis-free survival in CRAC. Although the clinical significance of the PAR complex is not completely understood, aberrant PKCζ expression can be a challenging prognostic marker in predicting metastasis and survival and may be a potential therapeutic target. The roles of the PAR complex are variable depending on the cell types, and their clinical implications are still unclear. Further investigation into underlying mechanism of the PAR complex and related signaling pathways in CRAC is required.
The following are the supplementary data related to this article.
Supplementary tables
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2016R1D1A1B01014311) and the Basic Science Research Program through the NRF funded by the Ministry of Education, Science, and Technology (NRF-2017R1D1A1B04031187).
References
- 1.Kemphues KJ, Priess JR, Morton DG, Cheng N. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52(3):311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 2.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35(1):747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 3.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778(3):614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Xue B, Krishnamurthy K, Allred DC, Muthuswamy SK. Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol. 2013;15(2):189–200. doi: 10.1038/ncb2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunliffe HE, Jiang Y, Fornace KM, Yang F, Meltzer PS. PAR6B is required for tight junction formation and activated PKCzeta localization in breast cancer. Am J Cancer Res. 2012;2(5):478–491. [PMC free article] [PubMed] [Google Scholar]
- 6.Namdarian B, Wong E, Galea R, Pedersen J, Chin X, Speirs R. Loss of APKC expression independently predicts tumor recurrence in superficial bladder cancers. Urol Oncol. 2013;31(5):649–655. doi: 10.1016/j.urolonc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Bell GP, Thompson BJ. Colorectal cancer progression: lessons from Drosophila? Semin Cell Dev Biol. 2014;28:70–77. doi: 10.1016/j.semcdb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 9.Malafosse R, Penna C, Cunha AS, Nordlinger B. Surgical management of hepatic metastases from colorectal malignancies. Ann Oncol. 2001;12(7):887–894. doi: 10.1023/a:1011126028604. [DOI] [PubMed] [Google Scholar]
- 10.Jessup J, Goldberg R, Asare E, Benson A, III, Brierley J, Chang G. AJCC cancer staging manual. Eighth edition. Chapter 20. 2017;20:251–274. [Google Scholar]
- 11.Kojima Y, Akimoto K, Nagashima Y, Ishiguro H, Shirai S, Chishima T. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 2008;39(6):824–831. [Google Scholar]
- 12.Jan YJ, Ko BS, Liu TA, Wu YM, Liang SM, Chen SC. Expression of partitioning defective 3 (par-3) for predicting extrahepatic metastasis and survival with hepatocellular carcinoma. Int J Mol Sci. 2013;14(1):1684–1697. doi: 10.3390/ijms14011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugay F, Le Goff X, Rioux-Leclerq N, Chesnel F, Jouan F, Henry C. Overexpression of the polarity protein PAR-3 in clear cell renal cell carcinoma is associated with poor prognosis. Int J Cancer. 2014;134(9):2051–2060. doi: 10.1002/ijc.28548. [DOI] [PubMed] [Google Scholar]
- 14.Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008;68(20):8201–8209. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin J, Liu Z, Li H, Sun J, Chang X, Liu J. Association of PKCzeta expression with clinicopathological characteristics of breast cancer. PLoS One. 2014;9(6):e90811. doi: 10.1371/journal.pone.0090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nazarenko I, Jenny M, Keil J, Gieseler C, Weisshaupt K, Sehouli J. Atypical protein kinase C zeta exhibits a proapoptotic function in ovarian cancer. Mol Cancer Res. 2010;8(6):919–934. doi: 10.1158/1541-7786.MCR-09-0358. [DOI] [PubMed] [Google Scholar]
- 17.Cohen EE, Lingen MW, Zhu B, Zhu H, Straza MW, Pierce C. Protein kinase C zeta mediates epidermal growth factor-induced growth of head and neck tumor cells by regulating mitogen-activated protein kinase. Cancer Res. 2006;66(12):6296–6303. doi: 10.1158/0008-5472.CAN-05-3139. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Park M, Shin N, Kim G, Kim YG, Shin JS. High mobility group box-1 is phosphorylated by protein kinase C zeta and secreted in colon cancer cells. Biochem Biophys Res Commun. 2012;424(2):321–326. doi: 10.1016/j.bbrc.2012.06.116. [DOI] [PubMed] [Google Scholar]
- 19.Mustafi R, Cerda S, Chumsangsri A, Fichera A, Bissonnette M. Protein kinase-ζ inhibits collagen I–dependent and anchorage-independent growth and enhances apoptosis of human Caco-2 cells. Mol Cancer Res. 2006;4(9):683–694. doi: 10.1158/1541-7786.MCR-06-0057. [DOI] [PubMed] [Google Scholar]
- 20.Dagher J, Dugay F, Rioux-Leclercq N, Verhoest G, Oger E, Bensalah K. Cytoplasmic PAR-3 protein expression is associated with adverse prognostic factors in clear cell renal cell carcinoma and independently impacts survival. Hum Pathol. 2014;45(8):1639–1646. doi: 10.1016/j.humpath.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF. Repression of intestinal stem cell function and tumorigenesis through direct phosphorylation of β-Catenin and Yap by PKCζ. Cell Rep. 2015;10(5):740–754. doi: 10.1016/j.celrep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF. Control of nutrient stress-induced metabolic reprogramming by PKCzeta in tumorigenesis. Cell. 2013;152(3):599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khursheed M, Bashyam MD. Apico-basal polarity complex and cancer. J Biosci. 2014;39(1):145–155. doi: 10.1007/s12038-013-9410-z. [DOI] [PubMed] [Google Scholar]
- 24.Forteza R, Wald FA, Mashukova A, Kozhekbaeva Z, Salas PJ. Par-complex aPKC and Par3 cross-talk with innate immunity NF-kappaB pathway in epithelial cells. Biol Open. 2013;2(12) doi: 10.1242/bio.20135918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables