Abstract
CYR61/CCN1 is a matricellular protein that resides in the extracellular matrix, but serves regulatory rather than structural roles. CYR61/CCN1 is found in mineralized tissues and has been shown to influence bone healing in vivo and osteogenic differentiation in vitro. In this study we generated Cyr61 bone-specific knockout mice to examine the physiological role of CYR61/CCN1 in bone development and maintenance in vivo. Extensive analysis of Cyr61 conditional knockout mice showed a significant decrease in both trabecular and cortical bone mass as compared to WT littermates. Our data suggest that CYR61/CCN1 exerts its effects on mature osteoblast/osteocyte function to modulate bone mass. Specifically, changes were observed in osteocyte/osteoblast expression of RankL, VegfA, and Sost. The increase in RankL expression was correlated with a significant increase in osteoclast number; decreased VegfA expression was correlated with a significant decrease in bone vasculature; increased Sost expression was associated with decreased Wnt signaling, as revealed by decreased Axin2 expression and increased adiposity in the bone marrow. Although the decreased number of vascular elements in bone likely contributes to the low bone mass phenotype in Cyr61 conditional knockout mice, this cannot explain the observed increase in osteoclasts and the decrease in Wnt signaling. We conducted in vitro assays using UMR-106 osteosarcoma cells to explore the role CYR61/CCN1 plays in modulating Sost mRNA and protein expression in osteocytes and osteoblasts. Overexpression of CYR61/CCN1 can suppress Sost expression in both control and Cyr61 knockout cells, and blocking Sost with siRNA can rescue Wnt responsiveness in Cyr61 knockout cells in vitro. Overall, our data suggests that CYR61/CCN1 modulates mature osteoblast and osteocyte function to regulate bone mass through both angiogenic effects as well as by modulating Wnt signaling, at least in part through the Wnt antagonist Sost.
Keywords: GENETIC ANIMAL MODELS, ANIMAL MODELS, MOLECULAR PATHWAYS, REMODELING, BONE MODELING AND REMODELING, WNT/BETA-CATENIN/LRPS, CELL/TISSUE SIGNALING, PARACRINE PATHWAYS, OSTEOCYTES, CELLS OF BONE
Introduction
Cyr61/CCN1 was first identified as an immediate early serum-inducible gene product in mouse fibroblasts.(1,2) Cyr61 is part of the CCN family of matricellular proteins, which is named for its founding members: CCN1/Cyr61, CCN2/Ctgf, CCN3/Nov, CCN4/Wisp1, CCN5/Wisp2, and CCN6/Wisp3. All six CCN members share similar modular protein structures, containing an insulin-like binding protein domain that has very low affinity for insulin, followed by von Willebrand type C, thrombospondin, and cysteine knot domains. As a matricellular protein, Cyr61 resides in the extracellular matrix (ECM), but serves regulatory rather than structural roles. Cyr61 is best known as a proangiogenic factor; Ccn1/Cyr61-null mice exhibit embryonic lethality as a result of placental vascular insufficiency and compromised vessel integrity.(3) However, Cyr61 regulates an array of cellular behaviors, including proliferation, differentiation, senescence, apoptosis, adhesion, and ECM synthesis in vitro.(1)
All six members of the CCN family are expressed in bone.(4,5) To date, functions in bone have been described for Ctgf, Nov, and Wisp1. Ablation of Ccn2/Ctgf in osteoblasts using Osteocalcin-Cre led to a mild low bone mass phenotype, seen in males but not in females; cortical bone was unaffected.(6) Nov is expressed in mature osteoblasts,(7) but no skeletal effects are seen in Ccn3/Nov−/− mice. However, they exhibit accelerated bone regeneration, consistent with studies showing that Nov inhibits osteoblast differentiation.(8) In contrast, in Ccn4/Wisp1−/− mice, cortical bone thickness, cross-sectional area, and endocortical mineral apposition rate are significantly reduced.(9) Hence, some CCN family members (Ctgf, Wisp) have anabolic functions in bone, whereas Nov has opposing functions.
Several studies have investigated the potential role of CCN1/Cyr61 in bone. Cyr61 was shown to play an important role in fracture healing in vivo, as local administration of Cyr61 improved healing(10,11) and inhibition of Cyr61 delayed healing.(11) The improved fracture healing in these studies could be attributed to the proangiogenic effects of Cyr61 produced by and acting on vascular cells. However, direct effects on osteoblast lineage cells are also possible. For example, Si and colleagues(12) showed that knockdown of Cyr61 blocked Wnt-induced osteoblast differentiation in vitro. Similarly, Si and colleagues(12) showed that Cyr61 binds to integrin αvβ3 in osteoblast-like cells in vitro to promote BMP2 expression and to enhance cell proliferation and differentiation. The Wnt pathway has essential anabolic functions in bone, and Cyr61 has been shown to be a direct target of Wnt signaling in osteoblast lineage cells.(12,13) Moreover, Cyr61 is among the most upregulated genes in bones of mice carrying the high bone mass (HBM) mutation in the Wnt co-receptor LRP5, and is among the most downregulated genes in Lrp5−/− mice.(14) A role for Cyr61 in vivo in bone is suggested by a report that a polymorphism within the promoter region of Cyr61 which is associated with reduced Cyr61 mRNA levels is also associated with increased risk of fracture nonunion in humans.(15) However, direct in vivo evidence for a role for Cyr61 in bone is lacking. The goal of this study was to investigate the role of Cyr61 in bone formation and maintenance in vivo through the use of an osteoblast-specific conditional knockout mouse model.
Materials and Methods
Reagents and materials
Recombinant full-length murine Cyr61 was from Abnova, Inc. (Taiwan) and recombinant murine Vegf-A was from R&D Systems, Inc (Minneapolis, MN, USA). Anti-Sclerostin and anti-VEGF antibodies were from R&D Systems, Inc.; anti-Active β-Catenin (nonphosphorylated Ser33/37/Thr41) and anti-Gapdh were from Cell Signaling, Inc. (Danvers, MA, USA); anti-CD31 was from BD Biosciences Inc. (San Jose, CA, USA); Fast Green, Leukocyte Acid Phosphatase 5, tartrate-resistant acid phosphatase (TRAP) staining kit, and siRNA against SOST were purchased from Qiagen (Germantown, MD, USA). Adeno-hCyr61 overexpression viral vector was from Addgene (Cambridge, MA, USA).
Vertebrate animals
Ccn1/Cyr61-floxed mice were a gift of Dr. Lester Lau.(16) To produce bone specific knockout mice, Cyr61-floxed mice were crossed with mice expressing Cre recombinase driven by an osteocalcin promoter (OCN-Cre; Tg(BGLAP-Cre)1Clem; The Jackson Laboratory, Bar Harbor, ME, USA)(17) to generate Cyr61fx/fx;OCN-Cre (hereafter referred to as Cyr61OCN) mice. Cyr61fx/fx mice were of the C57Bl/6J background, and OCN-Cre was on a B6.FVB background. Additionally, Cyr61fx/fx;Col1a1-Cre mice were generated using Tg(Col1a1-cre)1Kry(18) on a B6.FVB background; Cyr61fx/fx;Osx-Cre mice were generated using Tg(Sp7-tTA,tetO-EGFP/cre)1Amc(19) on a CD-1.B6 background; and Cyr61fx/fx;Prx1-Cre mice were generated using Tg(Prrx1-cre)1Cjt(20) on a B6.(C57BL/6J × SJL/J)F2 background.
Cyr61 reporter mice were obtained from the Mutant Mouse Regional Resource Center Repository (MMRRC; https://www.mmrrc.org/). They are a BAC transgenic line in which green florescent protein (GFP) is expressed under the control of the Cyr61eGFP locus.(21) All animals were treated in accordance with the National Institutes of Health guidelines for care and use of animals, and approved by the UCLA Institutional Animal Care and Use Committee.
Histology and immunostaining
Femurs or tibias were fixed with 4% paraformaldehyde (PFA) overnight at 4°C and decalcified in 19% EDTA for 2 weeks. The tissues were then dehydrated through a graded ethanol series and embedded in paraffin. Longitudinal 5-µm paraffin sections were prepared for histological analysis and immunohistochemistry (IHC) or immunofluorescence (IF). Specifically, TRAP staining was carried out using acid phosphatase, Leukocyte TRAP Kit (Sigma-Aldrich, St. Louis, MO, USA). IHC was performed using antibodies mentioned above.
All histology and IHC/IF images were analyzed on a microscope (Model BX60F; Olympus Optical Co., Japan) equipped with a digital camera (Model 01-RET-OEM-F-CLR-12; QImaging, Surrey, Canada). Photomicrographs were taken with a Nikon Ti-DH Microscope. Images were processed in PhotoShop (Adobe, San Jose, CA, USA).
µCT analysis
Bone parameters were quantified on femurs from mutant and control mice by µCT (Skyscan1172; Bruker MicroCT, Kontich, Belgium) using CTAn (v.1.14.4) and CTVol (v.2.2) software. The microradiography unit was set to an energy level of 55 kVp, intensity of 181 µA, and 900 projections; specimens were scanned at a 10-µm voxel resolution. A three-dimensional reconstruction was generated with NRecon software (Bruker MicroCT) from the set of scans. The regions of interest (ROIs) of trabecular bone were defined as the areas between 1 mm and 3 mm from the growth plate in the metaphyseal region of distal femurs. The ROI of cortical bone was defined as 0.75-mm segments of the femoral middle-diaphysis. All ROIs were drawn automatically and trabecular regions were assessed for bone mineral density (BMD), bone volume fraction (bone volume/total volume [BV/TV]), trabecular thickness (Tb.Th), and trabecular number (Tb.N). The midshaft cortical bone volume and thickness (Ct.Th) values were analyzed. All abbreviations and nomenclature are standardized according to published guidelines.(22)
Mechanical strength testing: three-point bending
An Instron 5564 materials testing machine (Instron, Inc., High Wycombe, UK) fitted with a 2-kN load cell was used to determine bone stiffness and breaking strength. The cross-head was lowered at 2 mm/min and data were recorded in load and in deflection. Each bone was tested for peak force and failure force, which were identified from the load-extension curve as the point of maximum load and where the load rapidly decreased to zero, respectively.
Bone histomorphometry
Static and dynamic histomorphometry was carried out on 3-month-old mice. Mice were injected intraperitoneally with calcein (10 µg/g of body weight; Sigma-Aldrich) two times at 7-day intervals, and euthanized 2 days after the last injection. Femurs were dissected and fixed in 4% PFA overnight, then embedded in methyl methacrylate. Longitudinal sections (5 µm) of the femur were prepared and stained with toluidine blue. All bone-specific parameters were measured and expressed in units following the guidelines established by the American Society for Bone and Mineral Research (ASBMR) Histomorphometry Nomenclature Committee using OsteoMeasure software (OsteoMetrics, Decatur, GA, USA). Cortical dynamic histomorphometry was performed where femurs were sectioned horizontally, and the distance between the two labels was measured on the cortical surface using OsteoMeasure Platform (OsteoMetrics). All abbreviations and nomenclature are standardized according to published guidelines.(23)
Imaging of blood vessels
Blood vessels in bone were imaged using microfil-perfusion angiogram in accordance with described methods.(24) Briefly, after animals were euthanized the vasculature was flushed with saline containing heparin sodium (100 U/mL) and then flushed with 10% neutral buffered formalin. After fixation the vasculature was injected with a radioopaque silicone rubber compound containing lead chromate (Microfil MV-122; Flow Tech, Inc., Carver, MA, USA). The samples were stored at 4°C overnight for contrast agent to polymerize. The hind limbs were then dissected, fixed and decalcified, and imaged using µCT as described.(24)
Fetal mouse metatarsal angiogenesis assay(25)
Cry61fx/fx female and Cyr61OCN male mice were time mated and embryonic day 17.5 (E17.5) embryos were collected and genotyped. Metatarsals of each embryo were dissected out and explant cultures were established using α-MEM supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin. Cultures were treated with exogenous recombinant mouse Vegf-A (50 ng/mL) or anti-VEGF blocking antibody. All cultures were maintained in a humidified incubator at 37°C and 5% CO2 and the media was changed every 3 days. After 14 days explants were fixed in 10% PBS-buffered formalin for 10 min at room temperature and subsequently stained for CD31 (BD Biosciences). There were six duplicates of metatarsals in each group, and all experiments were repeated at least twice.
RNA isolation and qRT-PCR
For in vivo samples, femurs and tibias were dissected and soft tissue was removed. Bones were then cut to remove both 3-mm termini, and the bone marrow was flushed out with cold PBS until the cortical bone became white. The resulting cortical bone was flash frozen with liquid nitrogen, homogenized with a grinder, and resuspended in 1 mL Trizol (Thermo Scientific, Waltham, MA, USA). Total RNA was isolated by the phenol-chloroform method and converted to cDNA. Total RNA was converted to cDNA using the Cells-to-Ct Kit following the manufacturer’s suggested protocol (Thermo Scientific). cDNA was amplified and quantified using Maxima SYBR Green qPCR master mix (Thermo Scientific). Analysis of relative gene expression was done using the 2−ΔΔCt method and results were normalized to the housekeeping gene Gapdh. All the primers used in the study are listed in Table 1.
Table 1.
Primer List
| Gene | Primer sequences (5′–3′) |
|---|---|
| mCyr61 | CTGCAGCAAAACTCAGCCCT |
| CACAGGGTCTGCCTTCTGAC | |
| rCyr61 | GCTGGAATGCAATTTCGGCG |
| CCCGTTCTGGTAGATCCTGG | |
| mAxin2 | TGCATCTCTCTCTGGAGCTG |
| TATGTCTTTGCACCAGCCAC | |
| mSclerostin | TGTCAGGAAGCGGGTGTAGT |
| GAGCCTCCTCCTGAGAACAA | |
| rSclerostin | CAACCAGACCATGAACCGGG |
| GTCACGAAGCGGGTGTAGTG | |
| mMEF2c | AGCTCAGTTCCCAAATCCCT |
| GTGCCAACAAAAGCATTGAA | |
| mDKK1 | GTCAGTGTGGTTCTTCTGGGA |
| CCGGGAACTACTGCAAAAAT | |
| mVEGF | AATGCTTTCTCCGCTCTGAA |
| CTCACCAAAGCCAGCACATA | |
| mRankl | CAGCCATTTGCACACCTCAC |
| CCCGATGTTTCATGATGCCG | |
| mOPG | TCACACAGGAGCTGATGACC |
| CCACAATGAACAAGTGGCTG | |
| mAdiponectin | AGGACATCCTGGCCACAATG |
| CTTAGGACCAAGAAGACCTGCAT | |
| mLPL | TCCTCAGCTGTGTCTTCAGG |
| CTGGTGGTCCTGGGAGTTT | |
| mGAPDH | CTTTGGCATTGTGGAAGGGC |
| CAGGGATGATGTTCTGGGCA | |
| rGAPDH | GGGTGTGAACCACGAGAAAT |
| CCTTCCACGATGCCAAAGTT |
Cell isolation and in vitro mineralization
Calvarial osteoblasts were isolated from P2–P5 Cyr61fx/fx mice following standard protocol.(26) Cells were treated with Cre adenovirus (AdCre) to ablate Cyr61 in these cells. Cells treated with GFP adenovirus (AdGFP) served as control. Bone marrow stromal cells (BMSCs) were isolated from adult Cyr61fx/fx and Cyr61fx/fx;Prx1Cre (Cyr61PRX) mice using standard protocols.(27) Osteogenic differentiation and adipogenic differentiation were performed on postnatal day 1 (P1) BMSCs using standard protocols. Briefly, for osteogenic differentiation cells were seeded in 24-well plates at a density of 5 × 104 cells/cm2 and then treated with osteogenic induction medium (α-MEM supplemented with 10% FBS, 10 mM β-glycerophosphate, and 50 µM L-ascorbic acid). For adipogenic differentiation, cells were seeded at a density of 2 × 105 cells/cm2 and then treated with adipogenic induction medium (α-MEM supplemented with 10% FBS, 1µM dexamethasone, 60µM indomethacin, 10 µg/mL insulin, and 0.5mM 3-isobutyl-1-methylxanthine) for 3 days and then switched to adipogenic maintenance medium (α-MEM with 10% FBS, 10 µg/mL insulin). Control cultures were grown in growth media (α-MEM with 10% FBS). All the media were changed every other day.
To examine osteogenic differentiation, cells were stained for alkaline phosphatase (ALP) activity (Sigma) and Alizarin Red S (Sigma) using manufacturers’ suggested protocols. Additionally, we quantified Alizarin Red S stain using 10% (wt/vol) cetylpyridinium chloride in 10mM sodium phosphate (pH 7.0). Alizarin Red S concentrations were determined by absorbance measurement at 562 nm and the values were normalized to total protein content. To examine adipogenic differentiation, cells were stained with Oil Red O stain (Sigma) using the manufacturer’s suggested protocol. We quantified Oil Red O stain using 100% methanol. Concentration of Oil Red O was determined by absorbance measurement at 500 nm and the values were normalized to total protein content. All in vitro experiments performed with primary cells were performed at least in triplicate and repeated at least twice.
Western blot analysis
Immunoblot analyses were performed to detect Sclerostin levels in cortical bone. In brief, femurs were dissected and flushed as described above. The resulting cortical bone was flash frozen and homogenized with pestle and mortar. Proteins were solubilized with radioimmunoprecipitation (RIPA) buffer containing protease inhibitor. Extracts (30 µg) were electrophoresed in a 10% SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride membrane. Immunoblot detection was performed with anti-Sclerostin, anti-active β-catenin (nonphosphorylated), and Gapdh antibody using an enhanced chemiluminescence (ECL) substrate (Thermo Scientific).
ELISA and plasma isolation
Adult mice (3 to 4 months old) were sedated under general inhalant anesthesia (3% isoflurane) for blood collection by cardiac puncture using a 1-mL syringe and 22G needle. Then 300 to 600 µL of blood was drawn into tubes coated with EDTA. The blood was gently mixed to prevent coagulation, kept on ice, and centrifuged at 2500g for 15 min at 4°C. The plasma (liquid portion) was immediately transferred to a 1.5-mL tube and stored at −80°C.
ELISA assay was conducted on the collected plasma using a commercially available kit for procollagen I N-terminal Peptide (P1NP; LifeSpan BioSciences, WA), and tartrate-resistant acid phosphatase 5b (TRAcP-5b; MyBioSource, CA).
siRNA, Clustered Regularly Interspaced Short Palindromic Repeats, DNA transfection, and assays
Lentiviral vector lentiCRISPRv2 (Clustered Regularly Interspaced Short Palindromic Repeats [CRISPR]) was obtained from Addgene (AddGene#52961).(28) We designed guide RNA (gRNA) 5′CUGCGCUAAACAACUCAACG for Cyr61 knockout in MC3T3-E1 cells and gRNA 5′CUGCGCGAAGCAACUCAACG for Cyr61 knockout in UMR-106 cells, then cloned these into lentiCRISPRv2 using restriction endonuclease BsmBI. To produce lentivirus, the transfer plasmids (lentiCRISPRv2-mcyr61, lentiCRISPRv2-rcyr61) were co-transfected into HEK293T cells with the packaging plasmids pVSVg (AddGene#8454) and psPAX2 (AddGene#12260). After infection, MC3T3-E1-Crispr-Cas9-Cyr61 cells were selected with puromycin (2 µg/mL; Sigma-Aldrich) for 5 days. Multiple clones were isolated. Data from a representative clone with the low levels of Ccn1 expression as measured by qRT-PCR were used.
Statistical analysis
For in vitro studies, the data represent results from at least three independent experiments, and are presented as the mean ± standard deviation (SD). Statistical significance between experimental and control groups was compared by Student’s t test. For experiments with more than two parameters, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis was used. For live animal studies, the data were presented as the mean ± SD. Statistical significance between mutant and WT control was compared by unpaired Student’s t test. A value of p < 0.05 was considered significant.
Results
Cyr61 expression in bone in vivo
Limited data are available on the expression of CCN1/Cyr61 in bone, although Cyr61 was detected in vivo in neonatal calvarial osteoblasts and osteocytes by immunostaining.(29) We therefore examined Cyr61 expression in vivo in adult bone using transgenic mice harboring a BAC construct in which the Cyr61 coding region has been replaced by enhanced GFP (eGFP) (GENSAT).(21) This analysis revealed Cyr61 expression in both trabecular and cortical bone. Expression was seen in bone lining cells, cuboidal osteoblasts, trabecular osteocytes, and cortical osteocytes (Fig. 1A, B). Expression was not observed in bone marrow stroma, sinusoids, or other vascular elements in bone (Fig. 1A, B).
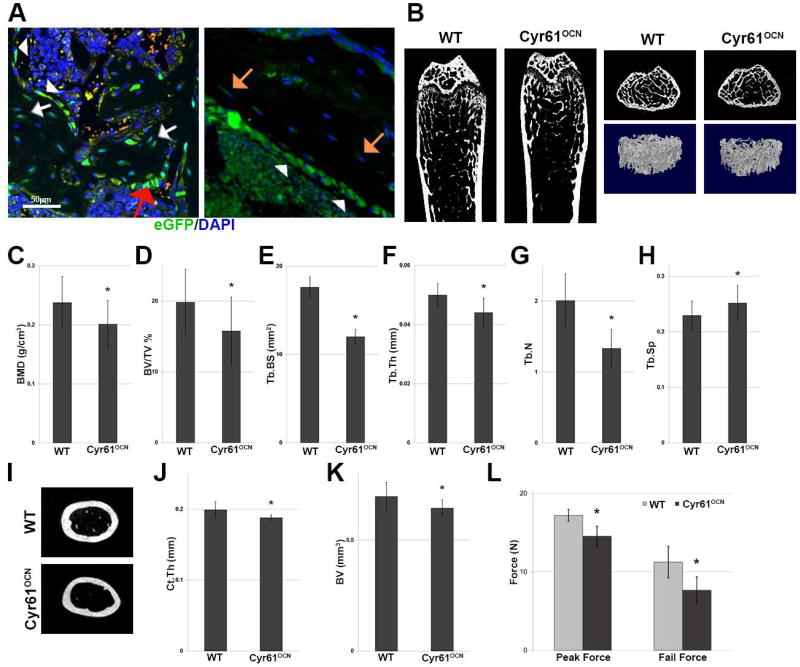
Fig. 1.
Cyr61 knockdown in mature osteoblasts and osteocytes results in decreased trabecular and cortical bone. (A) eGFP immunostaining in Cyr61eGFP mice; expression was seen in bone lining cells (white arrowheads), cuboidal osteoblasts (red arrow), trabecular osteocytes (white arrows), and cortical osteocytes (orange arrows). (B) µCT cross-sectional images and 3D reconstructions of the distal femurs of 3-month-old WT and Cyr61OCN mice (n = 6). (C–H) µCT analysis of trabecular bone showed significantly lower BMD, BV/TV, Tb.BS, Tb.Th, and Tb.N, as well as an increase in Tb.Sp (n = 6). (I) µCT cross-sectional images of mid-shafts of the femurs of WT and Cyr61OCN mice, (J, K) showed decreases in Ct.Th and BV in Cyr61OCN mice (n = 6). (L) Mechanical testing of the femurs of Cyr61OCN mice revealed significantly lower peak force as well as fail force during three-point bending tests as compared to control littermates (n = 6). BMD = bone mineral density; BV/TV = trabecular bone volume to total bone; Tb.BS = trabecular bone surface area; Tb.Th = trabecular bone thickness; Tb.N = trabecular bone number; Tb.Sp = trabecular bone spacing; Ct.Th = cortical bone thickness; BV = bone volume.
Decreased trabecular and cortical bone mass in Cyr61OCN mice
Given that the primary source of Cyr61 expression in bone is mature osteoblasts and osteocytes, we generated mice with a conditional deletion of Cyr61 in mature osteoblasts using OCN-Cre.(17) Analysis of Cyr61 mRNA expression in cortical bones of 3-month-old mice confirmed efficient knockdown in Cyr61OCN (Cyr61fx/fx;OCN-Cre) mice (Supplemental Fig. 1). No differences in the sizes or weights of control (Cyr61fx/fx) and Cyr61OCN littermates were observed. µCT analysis of trabecular bone revealed that 3-month-old male Cyr61OCN mice exhibited lower BMD and trabecular bone volume to total bone (BV/TV) compared to control littermates (Fig. 1B–D). Additionally, Cyr61OCN mice exhibited decreased trabecular bone surface area (Tb.BS), trabecular bone thickness (Tb.Th), and trabecular bone number (Tb.N), and an increase in trabecular spacing (Tb.Sp.) (Fig. 1E–H). µCT analysis also revealed a modest but statistically significant decrease in cortical bone thickness (Ct.Th) and volume (BV) in male Cyr61OCN mice (Fig. 1I–K). Mice lacking Cyr61 in osteoblast-lineage cells were generated using Osx-Cre (osteoprogenitors)(19) and Col1(2.3)-Cre (preosteoblasts).(18) µCT analysis of trabecular and cortical bone in male mice of these strains revealed decreased trabecular and cortical thickness in mutants that was indistinguishable from that observed in Cyr61OCN mice (Supplemental Fig. 2). The finding that ablation of Cry61 from osteoprogenitors or early osteoblasts does not lead to a more severe phenotype than does ablation in mature osteoblasts is consistent with the analysis in Fig. 1A showing that mature osteoblasts/osteocytes are the primary source of Cyr61 in bone. For this reason, Cyr61OCN mice were used for the remainder of the analysis. Due to the decreased cortical thickness, three-point bending tests were conducted to determine whether the observed cortical bone phenotype also resulted in significant loss of bone strength. Cyr61OCN mice had significantly lower peak force as well as fail force (Fig. 1L).
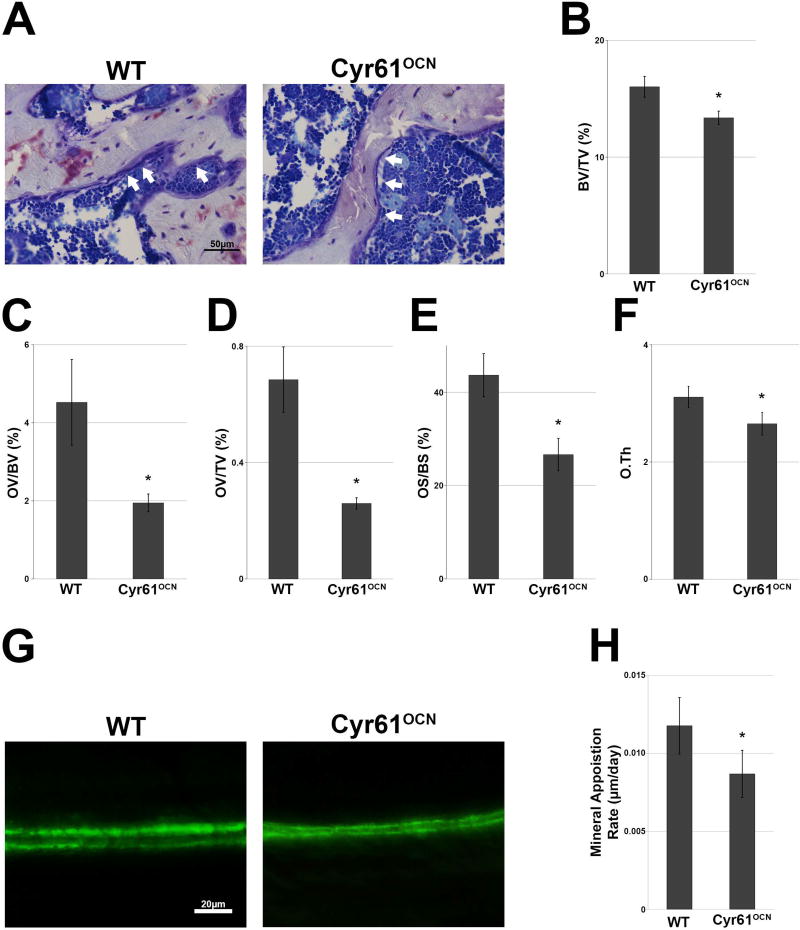
Histomorphometric analysis was performed in 3-month-old males in order to investigate the basis for the changes in bone morphology in Cyr61OCN mice. Consistent with the µCT analysis, histomorphometric analysis of trabecular bone revealed significant decreases in BV/TV, osteoid volume to bone volume (OV/BV), osteoid volume to total volume (OV/TV), osteoid surface to bone surface (OS/BS), and osteoid thickness (O.Th) in Cyr61OCN mice compared to control littermates (Fig. 2A–F). To analyze changes in cortical bone, calcein labeling was performed at 3 months of age, where the mice were injected twice with calcein 7 days apart and euthanized 2 days after the second injection. Calcein labeling revealed that Cyr61OCN mice exhibited a significantly lower mineral apposition rate on the endocortical surface compared to control littermates (Fig. 2G, H).
Fig. 2.
Histomorphometric analysis reveals decreases in trabecular bone and the rate of cortical bone apposition. (A) Toluidine blue staining of trabecular bones of 3-month-old adult mice showing changes in osteoid between Cyr61OCN and control (Cyr61fx/fx) mice. (B–F) Histomorphometric analysis of trabecular bone showed significant decrease in BV/TV (B), OV/BV, OV/TV, OS/BS, and O.Th in Cyr61OCN mice as compared to control littermates (n = 6). (G, H) Calcein labeling revealed a significant decrease in the cortical bone mineral deposition rate in Cyr61OCN mice as compared to control littermates (n = 4). OV/BV = osteoid volume to bone volume; OV/TV = osteoid volume to total volume; OS/BS = osteoid surface to bone surface; O.Th = osteoid thickness.
Increased osteoclast formation in Cyr61OCN mice
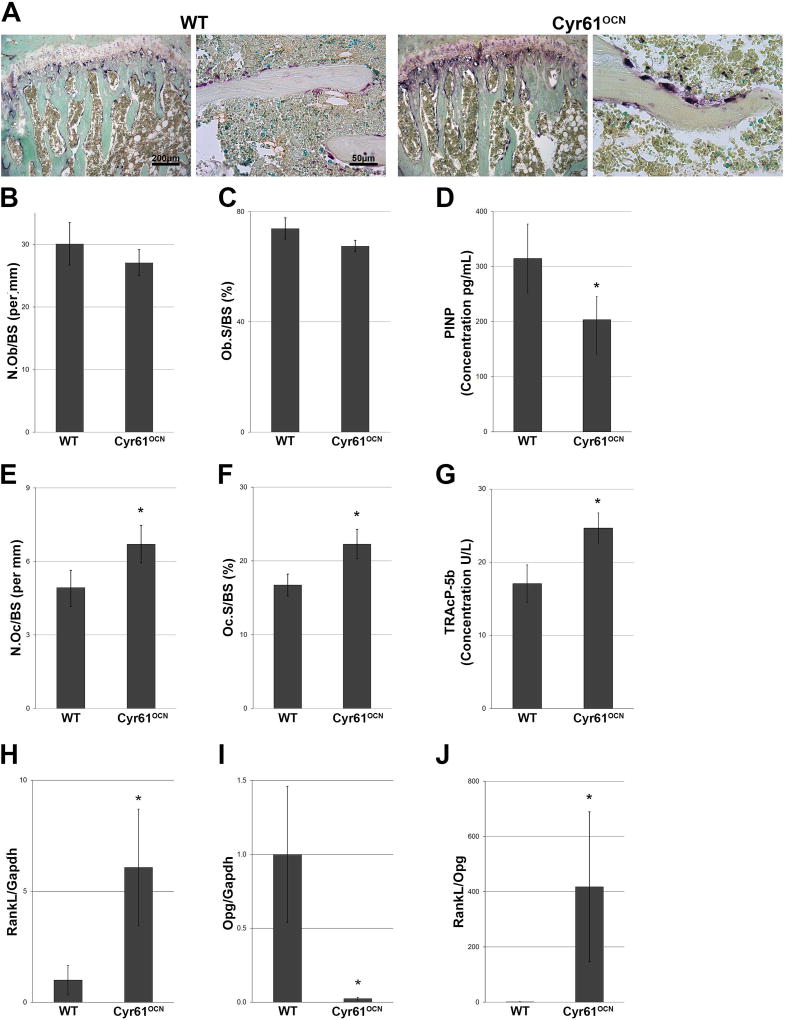
To examine the cellular basis for the lower bone mass phenotype in Cyr61OCN mice, a histomorphometric analysis of osteoblast and osteoclast numbers was performed using sections from 3-month-old male mice stained with H&E and TRAP (Fig. 3A). The analysis revealed no significant differences in the proportion of trabecular bone surface covered by activated osteoblasts (N.Ob/BS and Ob.S/BS) in Cyr61OCN and control littermates (Fig. 3B, C). However, we detected a significant decrease in plasma P1NP in Cyr61OCN mice (Fig. 3D), consistent with the decreased bone apposition rate (Fig. 2G, H). Given the decreased mineral apposition rate and impaired osteoid production (Fig. 2), these findings suggest that although osteoblast numbers are unchanged in mutants, osteoblast activity may be impaired. Bone mass is also determined by osteoclast activity, and Cyr61OCN mice showed significantly higher osteoclast numbers compared to control littermates (Fig. 3A, E, F), this increase in osteoclast number was confirmed by plasma TRAcP-5b ELISA (Fig. 3G). Osteoclast numbers are regulated by osteoblasts and osteocytes through the production of the stimulatory factor RANKL, which binds to the RANK receptor on osteoclasts, and to its antagonist osteoprotegerin (OPG).(30) The RANKL/OPG ratio produced by osteoblasts/osteocytes thus controls osteoclast formation in vivo. The increased osteoclast number in 3-month-old Cyr61OCN mice was accompanied by significantly higher RankL mRNA expression and significantly lower Opg mRNA expression compared to control littermates (Fig. 3H, I); as a consequence, the RankL/Opg ratio was increased over 400-fold in Cyr61OCN mice. To examine if this effect is cell autonomous, we confirmed changes in RankL and Opg expression in UMR106 cells in vitro (Supplemental Fig. 3). Taken together, these findings suggest that both decreased osteoblast activity and increased osteoclast formation contribute to the low bone mass phenotype of Cyr61OCN mice.
Fig. 3.
Increased osteoclast formation in Cyr61OCN mice. (A) TRAP staining of the proximal tibia in 3-month-old mice. Right photomicrographs for each genotype show the chondro-osseous junction. Photomicrographs on the left for each genotype are magnified images of trabeculae. (B, C) Histomorphometric image analysis of osteoblasts revealed no significant differences in the N.Ob/BS or in Ob.S/BS in Cyr61OCN mice compared to control (Cyr61fx/fx) littermates (n = 6). (D) P1NP ELISA showed significant decrease in plasma P1NP in Cyr61OCN mice as compared to control littermates (n = 6). (E, F) Image analysis of osteoclasts revealed significant increases in both the N.Oc/BS and Oc.S/BS in Cyr61OCN mice compared to control littermates (n = 6). (G) TRAcP-5b ELISA showed significant increase in plasma TRAcP-5b in Cyr61OCN mice as compared to control littermates (n = 7). (H–J) mRNA expression of cortical bone in 3-month-old mice showed a significant increase in RankL expression and a significant decrease in Opg expression in Cyr61OCN mice compared to control littermates, which resulted in over 400-fold increase in the RankL/Opg ratio in Cyr61OCN mice (n = 6). N.Ob/BS = number of osteoblasts relative to total bone surface; Ob.S/BS = bone surface area that was covered by osteoblasts relative to total surface; P1NP = procollagen I N-terminal peptide; N.Oc/BS = number of osteoclasts relative to total bone surface; Oc.S/BS = bone surface area that was covered by osteoblasts relative to total surface.
Cyr61OCN mice have decreased vasculature and VegfA expression
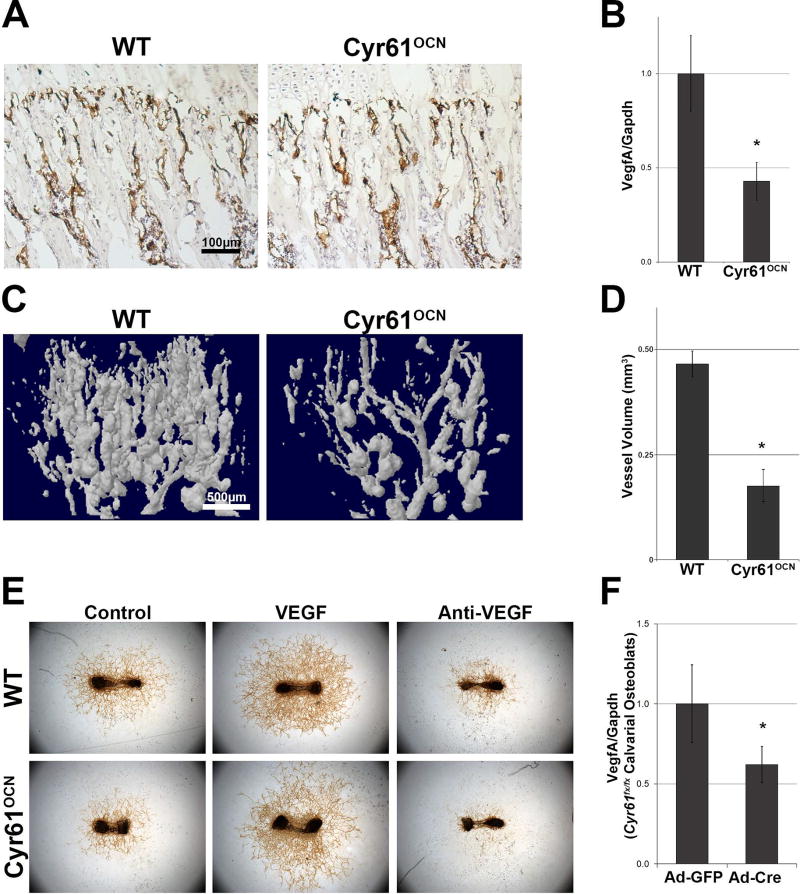
Angiogenesis and osteogenesis are highly coupled processes.(31,32) As discussed, previous reports have attributed improved fracture healing upon administration of exogenous Cyr61 to its pro-angiogenic effects.(10,11) Therefore, we examined the vasculature in Cyr61OCN mice. CD31/platelet endothelial cell adhesion molecule (PECAM) staining revealed a decrease in vasculature at the chondro-osseous junction in Cyr61OCN mice (Fig. 4A). One mechanism by which Cyr61 modulates angiogenesis is by inducing VegfA expression.(33) VegfA mRNA expression in adult cortical bone was found to be more than twofold lower in Cyr61OCN mice compared to control littermates (Fig. 4B). In contrast, expression levels of mRNA for the angiogenic regulator Fgf1 were elevated in Cyr61OCN mice, while no changes were seen in mRNA levels of Pdgf1, Hif1α, or Angpt1 in cortical bone (Supplemental Fig. 4). To quantify changes in vasculature we performed microfil-perfusion angiograms on 1-month-old mice, and found a significant decrease in the vasculature of Cyr61OCN mice compared to control littermates (Fig. 4C, D). Furthermore, ex vivo blood vessel outgrowth assays revealed a considerable decrease in vascular outgrowth from Cyr61OCN femurs, which was rescued by exogenous VEGF in the culture media (Fig. 4E). These findings indicate that osteoblast-derived Cyr61 plays an essential role in coupling angiogenesis and osteogenesis through regulation of VegfA levels. To examine if the change in VegfA expression is cell autonomous to osteoblasts, we investigated whether VegfA levels are altered in isolated calvarial osteoblasts. As seen in Fig. 4F, when primary Cyr61fx/fx calvarial osteoblasts were treated with AdCre virus, we observed a significant decrease in VegfA expression as compared to cells treated with AdGFP control virus. The observation that exogenous VegfA can rescue the angiogenic defect suggests that decreased VegfA production by osteoblasts most likely leads to the angiogenesis defects seen in vivo. However, increased Fgf1 expression may also impact the angiogenic phenotype in Cyr61 mutants. Additional studies will be needed to determine if altered Fgf1 expression modulates the phenotype, is a direct effect of loss of Cry61 expression, or is an indirect compensatory response to lower VegfA levels.
Fig. 4.
Cyr61OCN mice have decreased vasculature and VegfA expression. (A) PECAM immunostaining of 1-month-old mice demonstrating a slight decrease in vasculature at the chondro-osseous junctions in Cyr61OCN mice compared to control (Cyr61fx/fx) littermates. (B) VegfA mRNA expression of 3-month-old cortical bone was decreased more than twofold in Cyr61OCN mice as compared to control littermates (n = 6). (C) Microfil-perfusion angiograms of proximal tibia in 1 month old mice showed a significant decrease in vessel volume (D) in Cyr61OCN mice (n = 4). (E) Ex vivo fetal (E17.5) metatarsal blood vessel outgrowth assay demonstrated a considerable decrease in vascular outgrowth from Cyr61OCN mice compared to WT (Cyr61fx/fx) littermates. This decrease in vessel outgrowth was rescued by addition of exogenous VEGF (n = 4). (F) Deletion of Cyr61 using AdCre resulted in a significant decrease in VegfA expression in vitro (n = 3).
Decreased Wnt signaling and increased bone marrow adiposity in Cyr61OCN mice in vivo
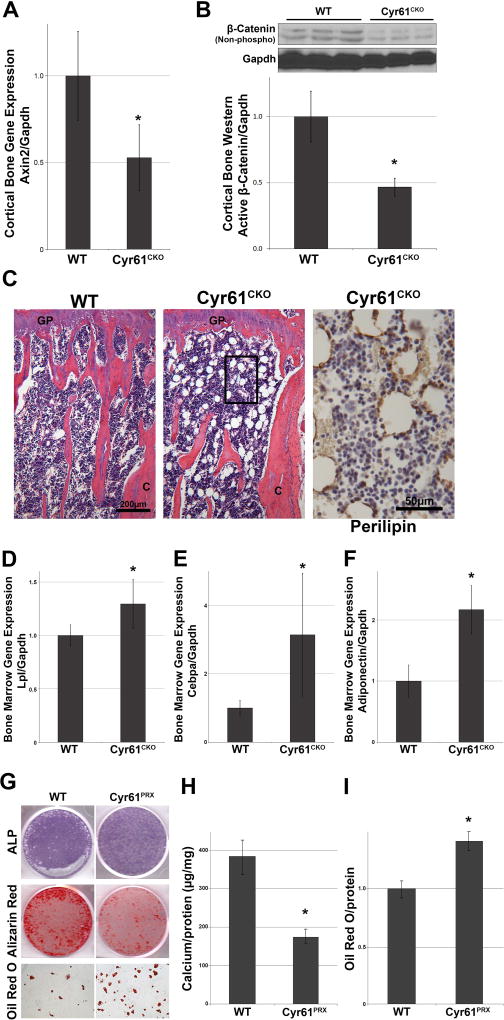
Although the decreased vascular outgrowth and lower VegfA mRNA expression likely contributes to the low bone mass phenotype in Cyr61OCN mice, this cannot explain the observed increase in osteoclast numbers in vivo.(31,34) We therefore considered other mechanisms of CCN1/Cyr61 action. As discussed previously, Cyr61 is a direct target of canonical Wnt signaling, and has been shown to modulate Wnt-mediated osteoblast differentiation in vitro.(12) Although most studies have focused on Cyr61 as a downstream target of Wnt signaling, others have indicated that Cyr61 can act upstream to modulate Wnt signaling positively or negatively.(35) Given that Wnt signaling plays an essential role in regulating the RANKL/OPG ratio in osteoblasts and osteocytes,(36,37) we investigated the impact of loss of Cyr61 on Wnt pathway output in bone. The expression level of the Wnt target Axin2 was decreased twofold in protein extracts from cortical bones of adult Cyr61OCN mice compared to control (Cyr61fx/fx) littermates (Fig. 5A). In agreement, we also observed a significant decrease in activated β-catenin (nonphosphorylated) from cortical bone protein extracts from Cyr61OCN mice compared to control littermates (Fig. 5B).
Fig. 5.
Decreased Wnt signaling in Cyr61OCN mice. (A) qRT-PCR analysis of expression of the Wnt responsive gene Axin2 in cortical bone of 3-month-old Cyr61OCN mice as compared to control (Cyr61fx/fx) littermates (n = 6). (B) Western analysis of active β-catenin (nonphosphorylated) levels in cortical bone of 3-month-old Cyr61OCN and control littermates (n = 3). (C) H&E staining of the distal tibias of 3-month-old mice revealed abundant fat droplets in Cyr61OCN mice, which were not present in WT (Cyr61fx/fx) mice. (D–F) qRT-PCR analysis of bone marrow mRNAs revealed a significant increase in expression of adipogenic genes, Lpl, Cebpa, and Adiponectin in Cyr61OCN mice compared to control littermates (n = 6). (G–I) In vitro osteogenic and adipogenic differentiation of BMSCs showed a significant decrease in mineralization and significant increase in adipogenesis in Cyr61PRX cells as compared to WT control (n = 5).
One of the major functions of Wnt signaling in bone is to regulate the differentiation of progenitor cells. Activation of the canonical Wnt pathway drives progenitor cell self-renewal and osteoblast differentiation, whereas inhibition of Wnt signaling promotes adipogenic differentiation of progenitors in bone.(38) Histological examination of 3-month-old males revealed a considerable increase in adiposity in the marrow space of Cyr61OCN mice compared to control littermates, as monitored by the presence of perilipin-positive vesicles throughout the bone marrow (Fig. 5C). This increase in adipogenesis coincided with a significant increase in expression of the adipogenic genes lipoprotein lipase (LPL), CCAAT/Enhancer Binding Protein Alpha (Cebpα), and adiponectin in the bone marrow of Cyr61OCN mice (Fig. 5D–F). In accordance, in vitro mineralization assays of BMSCs demonstrated a significant decrease in the osteogenic potential of Cyr61PRX BMSCs compared to control Cyr61fx/fx BMSCs. This decrease in osteogenic potential was accompanied by an increase in adipogenic differentiation (Fig. 5G–I).
CYR61 deletion results in increased Sclerostin in vivo and in vitro
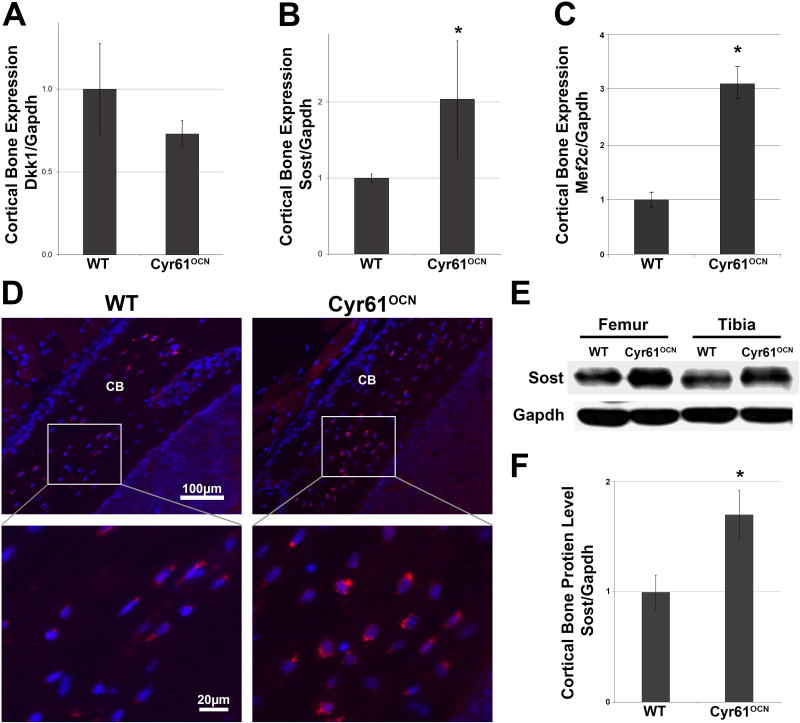
The data reveal a decrease in Wnt signaling in Cyr61OCN mice. The observed alterations in osteoclast formation, maintenance of progenitors, and osteoblastic versus adipogenic differentiation support the conclusion that this decrease contributes to the Cyr61OCN low bone mass phenotype. Cyr61 is deleted in mature osteoblasts and osteocytes in Cyr61OCN mice; this suggests that Cyr61 produced by these cells modulates levels of Wnt signaling in progenitor cells within the bone. Osteocytes can modulate bone homeostasis by secreting the Wnt inhibitors DKK1 and SOST, which regulate Wnt signaling in multiple cell types in bone.(39–41) Levels of expression of DKK1 and SOST were therefore examined. No differences in Dkk1 mRNA levels were found in cortical bones from 3-month-old Cyr61OCN mice compared to WT littermates (Fig. 6A). In contrast, increased Sost mRNA expression was observed in Cyr61OCN mice (Fig. 6B).
Fig. 6.
Increased Sost expression in Cyr61OCN mice. (A–C) qRT-PCR analysis of (A) Dkk1, (B) Sost, and (C) Mef2c mRNA expression in cortical bone revealed no change in Dkk1 expression but a significant increase in Sost and Mef2c mRNA levels (n = 6). (D) Immunohistochemistry for Sost protein revealed increased staining in osteocytes in Cyr61OCN mice. (E, F) Western blot analysis of Sost protein levels in cortical bone of 3-month-old Cyr61OCN and control (WT; Cyr61fx/fx) littermates (n = 3).
The transcription factor MEF2C is a direct activator of Sost expression.(42,43) Levels of Mef2C mRNA expression were significantly elevated in cortical bone of Cyr61OCN mice (Fig. 6C). To confirm whether Mef2C activity is increased, we examined the expression of the Mef2C transcriptional target Sfrp3, and found that it is also significantly increased in Cyr61OCN mice (Supplemental Fig. 5). IHC and Western blot analysis were performed to examine the potential impact of elevated Sost mRNA on protein levels in vivo. IHC showed that Cyr61OCN mice exhibited an increase in Sclerostin staining, especially around osteocytes within the cortical bone, as compared to control littermates (Fig. 6D). Additionally, Western blot analysis of protein lysates of cortical bone revealed a significant increase in Sost protein in Cyr61OCN mice (Fig. 6E, F). These findings suggest that Cyr61, which is expressed in osteocytes (Fig. 1), is required to suppress Sost mRNA and protein levels in bone in vivo, and this is at least in part to due to Cyr61-mediated suppression of Mef2C expression.
CYR61 can inhibit SOST expression in vitro
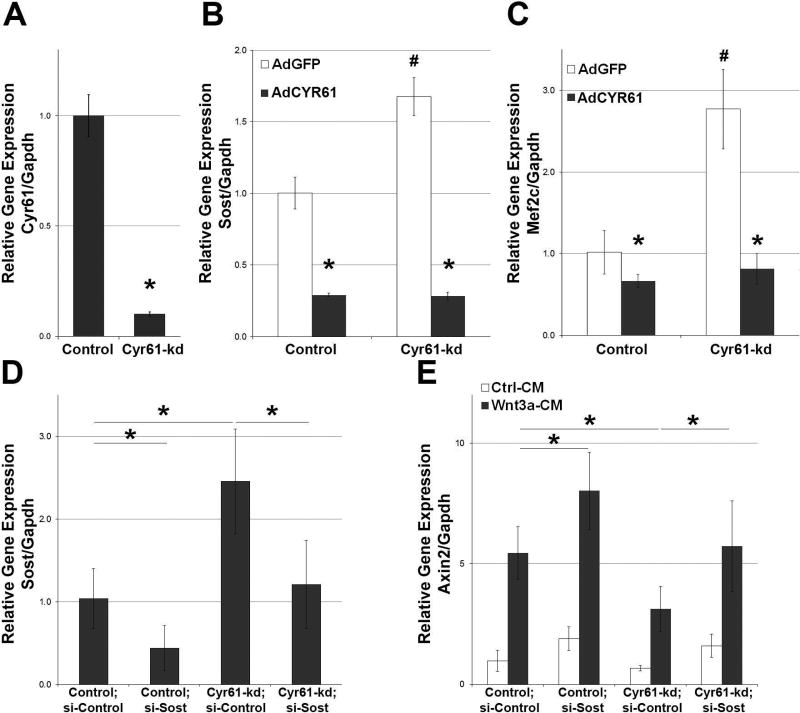
As discussed above, loss of Cyr61 in osteoblasts/osteocytes is associated with increased expression of Mef2c and its direct transcriptional target Sost. The UMR106 osteoblastic cell line, known to express Sost,(43) was used to examine the effect of Cyr61 on Sost expression. Deletion of Cyr61 in UMR-106 cells (Cyr61-kd) using CRISPR/Cas gene editing led to a significant increase in Sost mRNA expression (Fig. 7A, B). This was observed in multiple Cyr61-kd UMR-106 cell lines. In accordance, overexpression of Cyr61 reduced levels of Sost mRNA expression in Cyr61-kd and WT cells by more than fourfold (Fig. 7C). Similar results were observed for Mef2c mRNA expression (Fig. 7D). To investigate whether Cyr61 can modulate Wnt signaling at least in part by regulating Sost expression, a rescue experiment was conducted by knocking down Sost in Cyr61-kd cells using siRNA (Fig. 7E). As seen in Fig. 7E, stimulation with Wnt3a for 24 hours leads to a strong induction of the Wnt responsive gene Axin2. Sost knockdown led to a further induction of Axin2. In contrast, the level of Axin2 induced by Wnt3A is reduced in Cyr61-kd cells. However, levels of Axin2 induction in Cyr61-kd cells were restored to WT levels by Sost knockdown.
Fig. 7.
Cyr61 inhibits Sost expression in vitro. (A) Cyr61 was significantly suppressed in UMR106 knockdown cells as compared to control (n = 3). (B, C) Cyr61 knockdown (Cyr61-kd) in UMR106 osteoblasts led to a significant decrease in Cyr61, increase in (B) Sost and (C) Mef2c expression. Rescue with AdCYR61 resulted in a significant decrease in Sost and Mef2c expression in both Cyr61-kd and control cells. *p < 0.05 AdGDF versus AdCYR61; #p < 0.05 UMR106::AdGFP versus Cyr61-kd cells (n = 3). (D) Successful knockdown of Sost using siRNA in Cyr61-kd cells and control cells (n = 3). (E) qRT-PCR analysis of Axin2 mRNA expression in UMR106 control and Cyr61-kd cells demonstrates that knockdown of Sost rescues Wnt responsiveness in Cyr61-kd cells (n = 3). AdCYR61 = Cyr61 adenovirus.
These data are consistent with the conclusion that elevated Sost expression plays a major role in the impaired Wnt signaling observed in Cyr61OCN mice in vivo. However, we cannot rule out Sost-independent mechanisms. For example, Wnt10b mRNA levels are also decreased in Cyr61OCN mice (Supplemental Fig. 5B). Although we cannot at present determine the precise contributions of elevated Sost, reduced Wnt10b, and/or other changes, the reduced levels of active β-catenin in cortical bone of Cyr61OCN mice indicate that reduced Wnt signaling is a key component of the phenotype.
Discussion
Previous studies have shown that Cyr61 can influence bone healing in vivo and osteogenic differentiation in vitro.(10–12) However, the physiological sources of Cyr61 in bone, whether Cyr61 exerts direct effects on osteoblasts/osteocytes in vivo, and its mechanism of action, were unknown. Osteoblast-specific Cyr61 knockout mice were generated and used to address these questions. Deletion of Cyr61 in osteoblasts and osteocytes resulted in altered trabecular microarchitecture, decreased cortical bone thickness, and decreased bone strength.
The analysis of CYR61-eGFP expression suggests that the major sources of CYR61/CCN1 are mature osteoblasts and osteocytes in bone. Functional evidence that these cells represent the major source of CYR61 in osteolineage cells comes from the finding that µCT analyses of conditional knockout mice generated using Osx-Cre (preosteoblasts), Col1(2.3)-Cre (osteoblasts), and OCN-Cre (mature osteoblasts) all led to similar low bone mass phenotypes.
Osteocytes play a major role in maintaining bone homeostasis by signaling to both osteoblasts and osteoclasts.(44,45) Cyr61OCN mice exhibited a low bone mass phenotype accompanied by decreased osteoblast activity and increased adipogenic differentiation of osteoprogenitors. The reduced osteoblast activity and low bone mass in Cyr61OCN mice may result in part from reduced VegfA expression. The finding that loss of VegfA from osteoprogenitors leads to decreased bone mass and increased adipogenic differentiation at the expense of osteogenic differentiation,(46) as was observed in Cyr61OCN mice, is consistent with this hypothesis. Cyr61 has been shown to modulate angiogenesis directly(46) as well as indirectly through the regulation of VEGF expression and activity.(47) Our data are consistent with the notion that the changes in vasculature in Cyr61OCN mice are caused by decreased VEGF expression in osteoblasts/osteocytes, as vascular outgrowth in Cyr61OCN mice was rescued by the addition of exogenous VEGF. However, it is unknown whether exogenous VEGF alone can restore bone mass and decrease adipogenic differentiation in Cyr61OCN mice, and other aspects of the Cyr61OCN phenotype cannot be attributed to reduced VEGF expression and activity. For example, VEGF has no impact on SOST expression in osteoblasts,(48) whereas Cyr61OCN mice exhibit elevated SOST expression. Moreover, VEGF induces osteoclastogenesis directly and is required for osteoclast development in vivo.(49,50) Thus, reduced VEGF levels in bone usually lead to reduced osteoclast numbers.(46) In contrast, bones from Cyr61OCN mice exhibit increased osteoclast numbers.
Wnt signaling pathways play a central role in bone development, homeostasis, and healing.(37,51,52) Reduction of Wnt signaling causes bone loss(53–55) while enhancement of Wnt signaling results in increased bone volume and abnormal bone density.(40,56–58) Wnt signals achieve these effects through promoting osteoblast activity,(59,60) inhibiting osteoclast function,(36,61) and modulating the differentiation and self-renewal of many types of multipotent stem cells.(62–64) Cyr61 has been well established to be a direct target of canonical Wnt signaling and to play an important role in Wnt-induced osteogenic differentiation in vitro.(12) Our data suggest that Cyr61 is also an essential mediator of Wnt signaling in vivo. Although Cyr61 was known to be a Wnt target, whether Cyr61 regulated Wnt pathway output in bone in vivo was unclear. Here we show that loss of Cyr61 in osteoblasts and osteocytes led to decreased Wnt signaling. The effect of Cyr61 on Wnt signaling is reminiscent of the matricellular protein periostin (Postn), where periostin knockout mice also showed a defect in Wnt signaling.(65) Similar to Postn, Cyr61 can bind to integrin αvβ3. A previous study showed that deletion of integrin αv from osteogenic lineage cells led to inability to downregulate Sost expression in mechanically loaded bones.(66) This raises the possibility that both Cyr61 and Postn are essential ligands for integrin αvβ3 and regulate Wnt signaling via an effect on Sost expression through this integrin in bone. A major difference between Cyr61OCN and Postn−/− knockout mice is that Cyr61OCN mice showed changes in Wnt signaling during normal bone growth and maintenance, whereas in Postn−/− mice the change in Wnt signaling was only evident with PTH treatment. Additionally, Postn is mainly expressed in the periosteum, whereas Cyr61 is expressed throughout the bone and is most important in mature osteoblasts and osteocytes. These data suggest that Cyr61 and Postn might share similar pathways in regulating the bone microenvironment, but differ in location and physiological responsiveness to stimuli. Further studies are warranted to test the shared and unique features of Cyr61 and Postn function in bone.
Several lines of evidence suggest that Cyr61 modulates Wnt pathway output at least in part by repressing expression of the Wnt inhibitor Sost in mature osteoblasts/osteocytes. The extent to which elevated Sost expression contributes to the low bone mass phenotype of Cyr61OCN mice remains unknown, because we also detected reduced Wnt10b expression, and there may be unknown effects on other anabolic pathways. However, knockdown of Sost expression in vitro did rescue Wnt responsiveness in Cyr61-deficient osteoblasts. The suppression of Sost expression by osteocytes(67) is required for the stimulation of Wnt signaling within lining osteoblasts to initiate new bone growth.(68) Cyr61 has been shown to bind to Sost directly, and Sost appears to block some activities of Cyr61.(69) Although it is unknown whether Cyr61 reciprocally blocks Sost activity, Cyr61 may exert anabolic effects in bone by blocking not only the expression of Sost, but also by blocking its activity. Sost blocking antibodies are currently in phase 3 clinical trials and will likely to become the gold standard for osteoporosis therapy.(70) Cyr61 administration might be a useful adjuvant therapy.
Osteocytes control osteoclast formation through the secretion of RANKL and OPG, a decoy receptor for RANKL.(45) We observed a drastic increase in RANKL/OPG ratio in cortical bone mRNA in Cyr61OCN mice that resulted in a concomitant increase in osteoclast numbers. Cyr61 was shown to directly inhibit OC formation in vitro through an unknown mechanism that does not affect RANK signaling.(71) The reason that there is a relatively mild increase in osteoclast numbers observed in vivo in spite of a major change in RANKL/OPG ratio is unknown, but could be due to several factors. First is the fact that RANKL and OPG are expressed in many different compartments; OPG is known to be primarily expressed in bone lining and bone marrow stromal cells,(72) which are not affected by our knockout and were not included in our bone marrow–depleted cortical bone mRNA extract. Thus the mRNA ratio shown in Fig. 3 may not directly correlate with the overall protein levels of RANKL and OPG that are available to osteoclasts. In addition, we cannot rule out the involvement of other pathways that might mitigate the effects of RANKL on osteoclast numbers in Cyr61 mutants. Finally, as mentioned above, Cyr61OCN mice also showed decreased bone vasculature, which would be predicted to blunt the increase in osteoclast numbers. Regardless of the precise mechanism, the current findings warrant further investigation of direct and indirect effects of Cyr61 on osteoclast formation and function in vivo in the future.
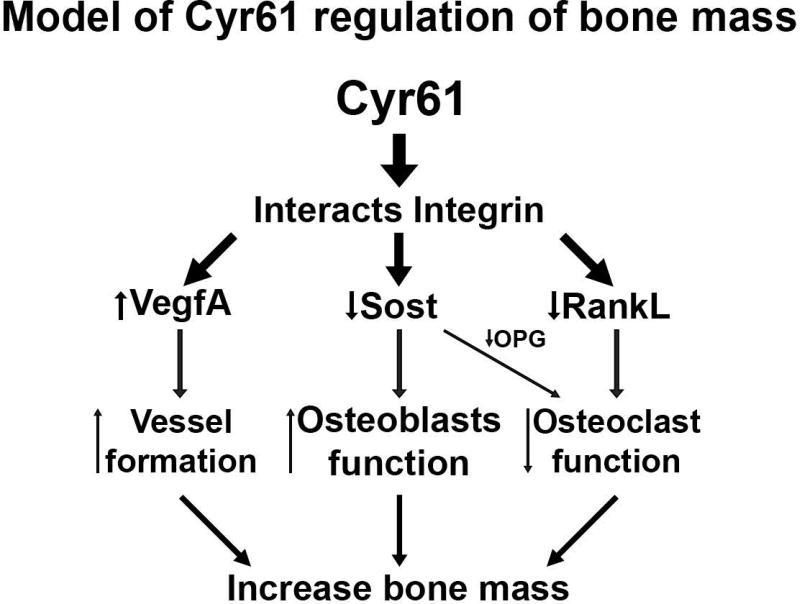
Taken together, our data suggest that Cyr61 plays a critical role in the bone microenvironment by regulating mature osteoblast/osteocyte function. The observed changes are most likely attributed to changes in osteocyte/osteoblast expression of RankL, VegfA, and Sost. The precise mechanism by which Cyr61 regulates expression of these genes in osteoblast lineage cells remains unknown. However, current literature suggests Cyr61 exerts the majority of its effects as a ligand for integrins αvβ3, αvβ5, and α6β1.(73) This is also likely to be the case in bone. Thus we postulate that Cyr61 interacts with integrins to down regulate Sost expression and in turn increase Wnt signaling, downregulating RankL expression and osteoclast numbers (Fig. 8). The roles of these integrins in mature osteoblasts/osteocytes in vivo remain unknown, but consistent with the proposed model of Cyr61 action as a ligand for αvβ3, deletion of integrin αv from osteogenic lineage cells led to the inability to downregulate Sost expression in mechanically loaded bones.(66) Future studies are therefore of interest to determine whether Cyr61 is an essential ligand that mediates the effects of αv integrin in mechanotransduction in vivo.
Fig. 8.
Proposed mechanism of Cyr61 modulation of bone mass via regulation of Sost expression.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIAMS grants R01 AR052686 and R21 AR071734 to KML.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68(19):3149–63. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4(12):3145–51. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22(24):8709–20. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen PC, Cheng HC, Yang SF, Lin CW, Tang CH. The CCN family proteins: modulators of bone development and novel targets in bone-associated tumors. Biomed Res Int. 2014;2014:437096. doi: 10.1155/2014/437096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parisi MS, Gazzerro E, Rydziel S, Canalis E. Expression and regulation of CCN genes in murine osteoblasts. Bone. 2006;38(5):671–7. doi: 10.1016/j.bone.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Canalis E, Zanotti S, Beamer WG, Economides AN, Smerdel-Ramoya A. Connective tissue growth factor is required for skeletal development and postnatal skeletal homeostasis in male mice. Endocrinology. 2010;151(8):3490–501. doi: 10.1210/en.2010-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita Y, Sakamoto K, Tamamura Y, et al. CCN3 protein participates in bone regeneration as an inhibitory factor. J Biol Chem. 2013;288(27):19973–85. doi: 10.1074/jbc.M113.454652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rydziel S, Stadmeyer L, Zanotti S, Durant D, Smerdel-Ramoya A, Canalis E. Nephroblastoma overexpressed (Nov) inhibits osteoblastogenesis and causes osteopenia. J Biol Chem. 2007;282(27):19762–72. doi: 10.1074/jbc.M700212200. [DOI] [PubMed] [Google Scholar]
- 9.Maeda A, Ono M, Holmbeck K, et al. WNT1-induced Secreted Protein-1 (WISP1), a novel regulator of bone turnover and Wnt signaling. J Biol Chem. 2015;290(22):14004–18. doi: 10.1074/jbc.M114.628818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey SP, Doht S, Eden L, et al. Cysteine-rich matricellular protein improves callus regenerate in a rabbit trauma model. Int Orthop. 2012;36(11):2387–93. doi: 10.1007/s00264-012-1659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Athanasopoulos AN, Schneider D, Keiper T, et al. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282(37):26746–53. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si W, Kang Q, Luu HH, et al. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26(8):2955–64. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Sheu TJ, Hoak D, et al. CCN1 regulates chondrocyte maturation and cartilage development. J Bone Miner Res. 2016;31(3):549–59. doi: 10.1002/jbmr.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayturk UM, Jacobsen CM, Christodoulou DC, et al. An RNA-seq protocol to identify mRNA expression changes in mouse diaphyseal bone: applications in mice with bone property altering Lrp5 mutations. J Bone Miner Res. 2013;28(10):2081–93. doi: 10.1002/jbmr.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schütze N, Rücker N, Müller J, Adamski J, Jakob F. 5′ Flanking sequence of the human immediate early responsive gene ccn1 (cyr61) and mapping of polymorphic CA repeat sequence motifs in the human ccn1 (cyr61) locus. Mol Pathol. 2001;54(3):170–5. doi: 10.1136/mp.54.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33(10):2078–90. doi: 10.1128/MCB.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–12. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 18.Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224(2):245–51. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 19.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 20.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 21.Heintz N. Gene expression nervous system atlas (GENSAT) Nat Neurosci. 2004;7(5):483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- 22.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 23.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Wan C, Deng L, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117(6):1616–26. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuiper EJ, Roestenberg P, Ehlken C, et al. Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem. 2007;55(11):1139–47. doi: 10.1369/jhc.7A7258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol. 2012;816:19–29. doi: 10.1007/978-1-61779-415-5_2. [DOI] [PubMed] [Google Scholar]
- 27.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4(1):102–6. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 28.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11(8):783–4. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaki H, Kubota S, Suzuki A, et al. Differential roles of CCN family proteins during osteoblast differentiation: involvement of Smad and MAPK signaling pathways. Bone. 2011;49(5):975–89. doi: 10.1016/j.bone.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Xiong J, O’Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res. 2012;27(3):499–505. doi: 10.1002/jbmr.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–8. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saran U, Gemini Piperni S, Chatterjee S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109–17. doi: 10.1016/j.abb.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Zhou D, Herrick DJ, Rosenbloom J, Chaqour B. Cyr61 mediates the expression of VEGF, alphav-integrin, and alpha-actin genes through cytoskeletally based mechanotransduction mechanisms in bladder smooth muscle cells. J Appl Physiol (1985) 2005;98(6):2344–54. doi: 10.1152/japplphysiol.01093.2004. [DOI] [PubMed] [Google Scholar]
- 34.Nakai T, Yoshimura Y, Deyama Y, Suzuki K, Iida J. Mechanical stress up-regulates RANKL expression via the VEGF autocrine pathway in osteoblastic MC3T3-E1 cells. Mol Med Rep. 2009;2(2):229–34. doi: 10.3892/mmr_00000088. [DOI] [PubMed] [Google Scholar]
- 35.Latinkic BV, Mercurio S, Bennett B, et al. Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling. Development. 2003;130(11):2429–41. doi: 10.1242/dev.00449. [DOI] [PubMed] [Google Scholar]
- 36.Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8(5):751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi Y, Uehara S, Udagawa N, Takahashi N. Regulation of bone metabolism by Wnt signals. J Biochem. 2016;159(4):387–92. doi: 10.1093/jb/mvv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan Z, Li Q, Luo S, et al. PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2016;11(3):216–25. doi: 10.2174/1574888x10666150519093429. [DOI] [PubMed] [Google Scholar]
- 39.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 40.Morvan F, Boulukos K, Clément-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–45. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–9. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 42.Collette NM, Genetos DC, Economides AN, et al. Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci U S A. 2012;109(35):14092–7. doi: 10.1073/pnas.1207188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leupin O, Kramer I, Collette NM, et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22(12):1957–67. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437–43. doi: 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell2026; and more. Endocr Rev. 2013;34(5):658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Berendsen AD, Jia S, et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122(9):3101–13. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61) Angiogenesis. 2002;5(3):153–65. doi: 10.1023/a:1023823803510. [DOI] [PubMed] [Google Scholar]
- 48.Genetos DC, Toupadakis CA, Raheja LF, et al. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem. 2010;110(2):457–67. doi: 10.1002/jcb.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa M, Kaneda T, Arakawa T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000;473(2):161–4. doi: 10.1016/s0014-5793(00)01520-9. [DOI] [PubMed] [Google Scholar]
- 50.Niida S, Kondo T, Hiratsuka S, et al. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proc Natl Acad Sci U S A. 2005;102(39):14016–21. doi: 10.1073/pnas.0503544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Whetstone HC, Lin AC, et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4(7):e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minear S, Leucht P, Jiang J, et al. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2(29):29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 53.Kim JB, Leucht P, Lam K, et al. Bone regeneration is regulated by wnt signaling. J Bone Miner Res. 2007;22(12):1913–23. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- 54.Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 56.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–9. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 58.Babij P, Zhao W, Small C, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18(6):960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 59.Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 60.Bodine PV, Zhao W, Kharode YP, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18(5):1222–37. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 61.Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280(22):21162–8. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 62.Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006;133(7):1219–29. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- 63.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 65.Bonnet N, Conway SJ, Ferrari SL. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc Natl Acad Sci U S A. 2012;109(37):15048–53. doi: 10.1073/pnas.1203085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaneko K, Ito M, Naoe Y, Lacy-Hulbert A, Ikeda K. Integrin αv in the mechanical response of osteoblast lineage cells. Biochem Biophys Res Commun. 2014;447(2):352–7. doi: 10.1016/j.bbrc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 68.Sawakami K, Robling AG, Ai M, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 69.Craig TA, Bhattacharya R, Mukhopadhyay D, Kumar R. Sclerostin binds and regulates the activity of cysteine-rich protein 61. Biochem Biophys Res Commun. 2010;392(1):36–40. doi: 10.1016/j.bbrc.2009.12.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 71.Crockett JC, Schütze N, Tosh D, et al. The matricellular protein CYR61 inhibits osteoclastogenesis by a mechanism independent of alphavbeta3 and alphavbeta5. Endocrinology. 2007;148(12):5761–8. doi: 10.1210/en.2007-0473. [DOI] [PubMed] [Google Scholar]
- 72.Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal. 2016;10(2):121–7. doi: 10.1007/s12079-016-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.