INTRODUCTION
Globally, 37 million people are living with the HIV virus.1 Since the year 2000, the number of individuals with access to antiretroviral therapy (ART) has significantly increased from 700,000 to over 16 million.1,2 Wide-spread ART use has halved the HIV-related mortality rate, from an estimated 2 million deaths in 2005 to 1 million in 2016.1,2 During the same time period though, cardiovascular disease mortality rates more than doubled in people living with HIV (PLWH).3
Hypertension, the leading risk factor for mortality worldwide, is a growing problem in HIV-infected adults.4–11 HIV-infected adults on ART have a higher prevalence of hypertension when compared with HIV-uninfected individuals.4–6,12–15 A recent meta-analysis of data from around the globe demonstrated that 35% of all HIV-infected adults on ART have hypertension, compared to an estimated 30% of HIV-uninfected adults.6 Among ART-experienced individuals older than 50 years, more than 50% have hypertension.6
In addition, HIV-infected adults with hypertension have a higher risk of cardiovascular events and all-cause mortality than HIV-uninfected adults with hypertension or HIV-infected adults with normal blood pressure.8,13,16–18 A prospective cohort study of over 80,000 HIV-infected and uninfected American veterans followed over a median six-year period, for example, found that HIV-infected adults with hypertension had a 2-fold higher risk of incident acute myocardial infarction as compared with HIV-uninfected adults with hypertension.17
Although the epidemiologic problem of hypertension in HIV-infected adults is well defined,6,7,9 fewer studies have evaluated the pathophysiologic mechanisms leading to hypertension in PLWH. Traditional cardiovascular risk factors explain some, but not all, of the increased hypertension risk among HIV-infected adults.14,19,20 A number of virologic- and treatment-related factors have been implicated, among them chronic inflammation, immune reconstitution, and lipodystrophy, all of which uniquely influence common downstream pathways such as the sympathetic and renin-angiotensin-aldosterone systems.21–25
Therefore, in this review we explore the mechanisms of hypertension in HIV infection. Understanding the mechanisms of hypertension in HIV-infected adults is important for two reasons. First, increased knowledge of the mechanisms of hypertension in HIV-infected adults will be critical to public health efforts to prevent hypertension, cardiovascular disease and premature mortality in HIV-infected adults. Second, the study of HIV-specific pathophysiology of hypertension may reveal important immunologic and inflammatory mechanisms of hypertension in the general population. Our review is not intended to be a comprehensive analysis of the numerous mechanisms of hypertension. Instead, we have focused on those mechanisms which might be particularly important in HIV-infected adults. An enhanced understanding of these processes may thereby aid in the development of new preventative and therapeutic interventions for hypertension in HIV-infected adults and for the general population.
MECHANISMS
Table 1 lists the published, in-vivo human studies describing possible mechanisms for hypertension in HIV-infected adults. Table 2 describes the findings for each of these studies. Figure 1 provides a central, schematic representation of the mechanisms of hypertension in HIV infection. The sections below describe each mechanism individually including data from the studies in these tables and data from other human and animal studies. We searched PubMed (up to December 2017) and EMBASE (up to December 2017) for relevant articles using the terms “HIV” and “hypertension” in combination with the following medical subjecting heading terms and keywords: “pathophysiology”, “blood pressure”, “kidney/renal injury”, “kidney/renal dysfunction”, “endothelial cell”, “cytokine”, “lipodystrophy”, “sympathetic activity”, “CD4 count”, “ART”, “ARV”, and “weight gain”. We included any papers which included previously unreported data regarding mechanisms for the relationship between HIV and hypertension, the metabolic syndrome, or cardiovascular disease. Human, animal, and ex-vivo studies were included. We also reviewed bibliographies of included articles to identify additional studies which were not included in the original search.
Table 1.
Published human studies of novel mechanisms for hypertension in HIV-infected adults
| Year | First Author | Journal | Study Design | Country | Sample Size | % of HIV-infected on ART | |
|---|---|---|---|---|---|---|---|
| HIV-infected | HIV-uninfected | ||||||
| 2005 | Thiebaut | Antivir Ther | Prospective Cohort | 21 countries (Europe, US and Australia) | 17,179 | 0 | 84% |
| 2006 | Crane | AIDS | Prospective Cohort | U.S.A. | 444 | 0 | 100% |
| 2006 | Palacios | HIV Med | Prospective Cohort | Spain | 95 | 0 | 100% |
| 2008 | Baekken | Nephrol Dial | Cross-sectional | Norway | 495 | 2091 | 72% |
| 2008 | Baekken | J Hypertens | Prospective Cohort | Norway | 542 | 24,968 | 71% |
| 2009 | Crane | HIV Med | Cross-Sectional | U.S.A. | 347 | 0 | 69% |
| 2012 | Freitas | J Clin Hypertens | Cross-Sectional | Portugal | 368 | 0 | 100% |
| 2013 | Glyn | J Hum Hypertens | Cross-Sectional | South Africa | 53 | 129 | 0% |
| 2013 | Hadigan | Am J Nephrol | Prospective Cohort | U.S.A. | 182 | 0 | 100% |
| 2013 | Manner | HIV Med | Prospective Cohort | Norway | 42 | 0 | 0% |
| 2013 | Manner | J Clin Hypertens | Prospective Cohort | Norway | 434 | 0 | 57% |
| 2014 | Morimoto | Nutrition | Cross-Sectional | Brazil | 285 | 0 | 79% |
| 2014 | Peck | BMC Medicine | Cross-Sectional | Tanzania | 301 | 153 | 50% |
| 2014 | Tenorio | J Infect Dis | Case-Control | U.S.A. | 458 | 0 | 100% |
| 2015 | Rokx | AIDS Res Hum | Clinical Trial | Netherlands | 50 | 0 | 100% |
| 2015 | Wensink | PLoS One | Cross-Sectional | South Africa | 903 | 0 | 87% |
| 2016 | Castley | PLoS One | Cross-Sectional | Australia | 475 | 0 | 77% |
| 2016 | Maffongelli | AIDS | Prospective Cohort | Italy | 116 | 0 | 100% |
| 2016 | Nduka | Int J Cardiol | Cross-sectional | Nigeria | 406 | 0 | 75% |
| 2016 | Pirro | Sci Rep | Cross-Sectional | Italy | 170 | 0 | 100% |
| 2016 | van Zoest | Clin Infect Dis | Prospective Cohort | Netherlands | 527 | 517 | 95% |
| 2017 | Ascher | Hypertension | Prospective Cohort | U.S.A. | 823 | 267 | 59% |
| 2017 | Ding | AIDS Res Hum | Cross-Sectional | China | 345 | 345 | 87% |
| 2017 | Rodriguez-Arboli | PLoS One | Prospective Cohort | Tanzania | 834 | 0 | 76% |
Table 2.
Summary of results for published human studies of novel mechanisms for hypertension in HIV-infected adults
| Study | Results | Novel mechanism supported |
|---|---|---|
| Thiebaut 2005 (Antivir Ther) |
Factors associated with new onset hypertension included: male sex, higher BMI, older age, higher BP at baseline and clinical lipodystrophy | Lipodystrophy |
| Crane 2006 (AIDS) |
Lopinavir/ritonavir was significantly associated with an increased incidence of new-onset hypertension (OR=2.5, p=0.03) | Antiretroviral therapy (protease inhibitors) |
| Palacios 2006 (HIV Med) |
Higher SBP at follow-up was significantly associated with: older age, higher baseline SBP, high total cholesterol, and lower baseline CD4 T-cell count | Dyslipidemia, immune suppression/reconstitution |
| Baekken 2008 (Nephrol Dial) |
Microalbuminuria was more common in HIV-infected adults (compared to HIV-negative adults) and was associated with higher blood pressure | Renal disease (microalbuminuria) |
| Baekken 2008 (J Hypertens) |
Statistically significant predictors of new-onset hypertension: older age, higher BMI, higher total cholesterol, longer duration of ART, and microalbuminuria | Dyslipidemia, antiretroviral therapy, renal disease |
| Crane 2009 (HIV Med) |
Lipohypertrophy (OR 4.3, p=0.006) and lipoatrophy (OR=5.5, p=0.01) were both associated with hypertension | Lipodystrophy |
| Freitas 2012 (J Clin Hypertens) |
Compared to normotensive HIV-infected adults, HIV-infected adults with hypertension had higher total fat, central, and central/peripheral fat mass ratios | Lipodystrophy |
| Glyn 2013 (J Hum Hypertens) |
Low eGFR was associated with higher blood pressure and higher L-arginine levels in HIV-infected African men but not uninfected men | Renal disease (L-arginine) |
| Hadigan 2013 (Am J Nephrol) |
Microalbuminuria was associated with new-onset hypertension, low CD4 T-cell counts (< 200 cells/μl) and ritonavir use | Renal disease, immune suppression, ART |
| Manner 2013 (HIV Med) |
LPS and sCD14, both markers of microbial translocation, independently predicted new-onset hypertension in ART-naïve, HIV-infected adults | Microbial translocation, chronic inflammation |
| Manner 2013 (J Clin Hypertens) |
Nadir CD4 cell count < 50 cells/μl (aOR 2.48; 95% CI 1.27-4.83) and ART duration (aOR 1.13; 95% CI 1.03-1.24) independently predicted new-onset hypertension | Immune suppression, antiretroviral therapy |
| Morimoto 2014 (Nutrition) |
HIV-infected adults with metabolic syndrome had higher SBP and DBP measurements and lower plasma adiponectin levels than those without | Dyslipidemia, adipokines |
| Peck 2014 (BMC Medicine) |
Age, alcohol use, BMI, microalbuminuria, low eGFR and higher current CD4 T-cell count were independently associated with hypertension. | Renal disease, immune reconstitution, ART |
| Tenorio 2014 (J Infect Dis) |
Among HIV-infected adults on ART, elevated IL-6 were strongly associated with hypertension at baseline (OR 1.6, p <0.001) and at one year (OR 1.8, p <0.001) | Chronic inflammation |
| Rokx 2015 (AIDS Res Hum) |
Switching from a nevirapine to a rilpivirine-based regimen led to a 6 mm Hg reduction in SBP at 24 and 48 weeks (95% CI −1.7 to −10.3, p=0.007) | Antiretroviral therapy (NNRTI) |
| Wensink 2015 (PLoS One) |
In HIV-infected adults on ART, albuminuria was significantly associated with hypertension, diminished eGFR, and increased HIV viral load | Renal disease (microalbuminuria) |
| Castley 2016 (PLoS One) |
CXCL10, sCD163 and sCD14 remained elevated despite ART use and were associated with total cholesterol and LDL-c levels, but not hypertension | Chronic inflammation, dyslipidemia |
| Maffongelli 2016 (AIDS) |
X4-tropic HIV (but not R5-tropic virus) independently predicted new-onset hypertension (HR 2.29, 95% CI 1.39-3.76, p=0.001) | HIV tropism |
| Nduka 2016 (Int J Cardiol) |
A propensity score matching model estimated the average treatment effect of ART on SBP and DBP to be 7.85 mm Hg and 7.45 mm Hg, respectively (p <0.001) | Antiretroviral therapy |
| Pirro 2016 (Sci Rep) |
Endothelial dysfunction was independently associated with hypertension, HIV RNA levels, and microalbuminuria in HIV-infected adults | Renal disease, chronic vascular inflammation |
| van Zoest 2016 (Clin Infect Dis) |
Prior stavudine use independently predicted new-onset hypertension among HIV-infected adults. The effect was attenuated after adjustment for abdominal obesity | Antiretroviral therapy (NRTI), lipodystrophy |
| Ascher 2017 (Hypertension) |
Higher urine albumin-to-creatinine levels and lower eGFR independently predicted new-onset hypertension in HIV-infected, but not HIV-uninfected, women | Renal disease (microalbuminuria) |
| Ding 2017 (AIDS Res Hum) |
Lower nadir CD4 T-cell count (< 50 cells/μl) was independently associated with hypertension, but only in HIV-infected adults who were underweight or obese | Immune suppression/reconstitution |
| Rodriguez-Arboli 2017 (PLoS One) |
Age, BMI, and eGFR, but not ART exposure or CD4 count, were found to be independent predictors of new-onset hypertension among HIV-infected adults | Renal disease |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LDL-c, low density lipoprotein cholesterol; LPS, lipopolysaccharide; sCD14, soluble CD14; SBP, systolic blood pressure
Figure 1. Schematic representation of HIV-related mechanisms of hypertension.
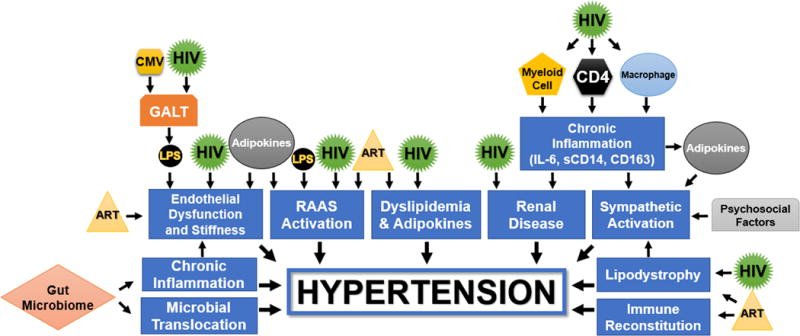
ART = Antiretroviral Therapy; CD163 = Cluster of Differentiation 163 Protein; CD4 = Cluster of Differentiation 4 Helper T-Cell; CMV = Cytomegalovirus; GALT = Gut-Associated Lymphoid Tissue; HIV = Human Immunodeficiency Virus; IL-6 = Interleukin-6; LPS = Lipopolysaccharide; RAAS = Renin Angiotensin Aldosterone System; sCD14 = Soluble Cluster of Differentiation 14 Protein
Microbial Translocation
Microbial gut translocation has been implicated in the pathophysiology of hypertension in HIV-infected adults.23,26 HIV preferentially infects CD4 T-cells in gut-associated lymphoid tissue (GALT),27 leading to disruption of the body’s natural mucosal defenses and passage of microbes into the systemic circulation.23,26,27,28 Lipopolysaccharide (LPS) and soluble CD14 (sCD14), both markers of microbial gut translocation, have been shown to be associated with hypertension in the context of HIV infection.23,29 In a nested, case-control study of HIV-infected adults, new-onset hypertension was associated with higher baseline levels of LPS (P <0.001) and sCD14 (P=0.024).23 Plasma concentrations of LPS and sCD14 were strongly correlated (Spearman’s rho=0.62, P=0.01) among hypertensive HIV-infected but not normotensive HIV-infected or uninfected controls, implying a that a common process such as microbial translocation is uniquely associated with these biomarkers in the context of hypertension.23 Similarly, a case-control study of 458 virologically suppressed HIV-infected adults found elevations in sCD14 preceded and predicted incident hypertension (OR=1.9; 95% CI 1.3-2.6; P<0.001).29
LPS is increased in the plasma of HIV-infected participants both before and after initiation of ART.23,26 LPS elevation may cause hypertension in HIV-infected adults through several different pathways. In the general population, LPS has been associated with both arterial stiffness and endothelial cell apoptosis.23,30,31 Furthermore, centrally administered LPS has been shown to promote an inflammatory cascade that ultimately produces prostaglandin E2 and activates the sympathetic nervous system in wildtype mice.32 LPS has also been associated with activation of the renin-angiotensin-aldosterone system (RAAS). In wildtype mice, LPS has been shown to promote angiotensin II activity by acting on adhesion leukocytes of the endothelium.33 LPS also activates RAAS through the peripheral increase of endothelial angiotensin receptors.33 In mouse models of hypertension, these receptors were found to trigger an NADPH-mediated cascade that produces reactive oxygen species (ROS) and induces endothelial dysfunction and hypertension.33 These findings were corroborated by Zhang et al., who studied the effects of LPS in wildtype mice. The investigators found that cerebroventricular administration of LPS led to increased expression of central angiotensin II receptors, precipitating NADPH-mediated ROS production and activation of the sympathetic nervous system.32
The mechanism by which LPS induces endothelial dysfunction may be mediated in part by long-term ART use. One cross-sectional study found that, among the 46 participants on long-term ART (mean duration = 2 years), LPS concentrations were significantly associated with brachial artery flow-mediated dilation (FMD), a marker of endothelial function (r= − 0.33; P=0.02). Elevated LPS was not associated with endothelial function in ART-naive adults or those on ART for only 6 months.26
Chronic Inflammation
Inflammatory Biomarkers
Several studies have demonstrated that inflammatory markers of HIV-related chronic immune activation are associated with hypertension. Elevated interleukin-6 (IL-6) levels have been shown to precede and predict hypertension in HIV-infected adults (OR=1.8; 95% CI 1.4-2.5; P<0.001).29 Elevated IL-6 levels have similarly been associated with hypertension,34,35 as well as cardiovascular mortality among HIV-uninfected adults.36 In a genome-wide association analysis of over 3,000 older HIV-uninfected adults, sCD14, also a marker of monocyte activation, was shown to be associated with cardiovascular risk factors that included hypertension.37 sCD14 was characterized by significant genetic polymorphisms that influenced its expression among different ethnic populations.37 In contrast, a cross-sectional study of 109 ART-naïve and 365 ART-exposed HIV-infected individuals found that higher plasma concentrations of sCD14 persisted despite ART use, but were not associated with hypertension.38
Increased CD163 has also been associated with hypertension in HIV-infected adults, although this may be more of a marker of a mechanism underlying hypertension rather than a causative factor in hypertension. One cross-sectional study investigated 27 virologically controlled HIV-infected adults without known cardiovascular disease.39 Using FDG-PET and coronary CT imaging, the investigators found that compared with HIV-uninfected control groups without atherosclerotic disease, HIV-infected participants demonstrated increased arterial inflammation of the aorta (prevalence odds ratio of 2.23 versus 1.89 (P<0.001)) which was associated with elevated CD163 levels.39 The investigators concluded that elevated CD163 may indicate the activation and infiltration of macrophages into the wall of the aorta in these HIV-infected adults.38,39
For other markers associated with cardiovascular disease in HIV-infected adults, such as retinol-binding protein 4 (RBP-4), a direct association with hypertension has not yet been tested.40 Additionally, some investigators have described the influence of other co-infections such as cytomegalovirus (CMV) on the disruption of mucosal barriers and activation of downstream inflammatory pathways that later influence cardiovascular events.29 While CMV has been associated with a greater risk of non-AIDS defining events including cardiovascular disease among HIV-infected adults (HR=1.53; 95% CI 1.05-2.16; P=0.016),41 its association with hypertension has not been specifically examined. Numerous data has shown that premature cardiovascular disease among HIV-infected adults is at least in part due to immune activation and chronic inflammation despite viral suppression,42,43 but the specific role of immune activation and chronic inflammation in hypertension was not examined in most of these studies.
ART-Mediated Inflammation
Protease inhibitors (PIs) have been shown to trigger inflammatory pathways. An in-vitro study done on PI-treated human adipocytes demonstrated PI-mediated generation of ROS both through mitochondrial effects as well as macrophage accumulation, leading to alterations of adipocyte-native cytokines.44 The relationship between vascular ROS and hypertension was established in murine models of genetically modified mice, in which ROS over-production was shown to also be associated with vascular collagen deposition, arterial stiffening, and kidney injury.45
The Role of Macrophages
The role of macrophages in hypertension among the general population has been previously demonstrated in murine models of hypertension. For instance, when compared with wildtype mice, genetically modified mice incapable of producing macrophage-derived 12/15 lipoxygenase did not develop elevated blood pressure when exposed to various hypertensive precipitants, including deoxycorticosterone acetate (DOCA)/high-salt environment and NG-nitro-L-arginine-methyl ester (L-NAME), an inhibitor of nitric oxide (NO) synthase.46 Furthermore, knockout mice demonstrated elevated blood pressures in response to these stimuli following adoptive transfer of wildtype macrophages, while wildtype mice became resistant to the hypertensive effects of L-NAME following clodronate-induced macrophage depletion.46 Such findings suggest an association between innate immunity and hypertension,47 and warrant further exploration in the context of HIV infection.
The Role of Myeloid Cells
Myelo-monocytic and other cells of myeloid origin have been shown to be directly involved in the pathogenesis of arterial hypertension and deserves greater attention as a possible mechanism for hypertension in the context of HIV infection. For instance, in three mechanistically distinct murine models of hypertension, male mice with hypertension were observed to have higher levels of myeloid-derived suppressor cells (MDSCs), which in turn attenuated the pathologic splenic hyperactivity of T-cells and pro-inflammatory cytokines interferon-gamma, tumor necrosis factor-alpha, and interleukin-17.48 When wild-type MDSCs were transferred into these hypertensive mice, the blood pressure decreased.48 HIV infection has been shown to effect MDSCs in multiple ways. Advanced HIV disease is associated with both increased MDSC activity49 and MDSC deficiency.50 In addition, persistently elevated levels of MDSC activity have been observed even in aviremic HIV-infected individuals on ART.51 To the best of our knowledge, no published research has yet explored the relationship between MDSCs and hypertension in the context of HIV infection.
The Gut Microbiome
A growing body of literature has implicated abnormalities in the gut microbiome as an important mechanism for hypertension, and this deserves greater attention as a possible mechanism in the context of HIV infection. Animal models of hypertension have shown that when compared with conventionally raised mice, germ-free mice exposed to angiotensin II infusion had a less robust systemic inflammatory response, lower systolic blood pressure, and less end-organ damage.52 Two human studies have confirmed that gut dysbiosis is more common in adults with hypertension when compared with normotensive controls.53,54 There is likely a complex relationship between hypertension, the gut microbiome and immune activation. In one study, for example, the administration of a high-salt diet to healthy volunteers was associated with increased blood pressure, reduction of intestinal Lactobacillus spp., and increase in CD4+ TH17 cell activity.55 Furthermore, Lactobacillus murinus supplementation in salt-sensitive, hypertensive mice exposed to a high-salt diet was associated with attenuated TH17 activity and decreased blood pressures.55 HIV itself has multiple effects on the gut microbiome and there are several ongoing trials examining the effects of probiotics on the gut microbiome and systemic inflammatory state of HIV-infected adults.56 We hope that these studies have included hypertension as a rigorously measured outcome.
Immune Reconstitution
CD4 T-cells have been shown to be critical in the pathophysiology of hypertension in the general population.57,58 Knockout mice incapable of producing T-cells do not develop hypertension in response to angiotensin II infusion.57,58
In HIV-infected adults, CD4 T-cell counts drop dramatically and then rise rapidly after the initiation of ART. Lower nadir CD4 T-cell counts have been associated with higher incidence of hypertension after ART initiation in several studies.5,22,59–61 Replicated by multiple investigators, these findings suggest that hypertension in HIV-infected adults on ART may be a phenomenon of immune suppression and reconstitution. This theory was supported by a sub-analysis of 332 HIV-infected adults enrolled in a large randomized clinical trial of immediate versus deferred ART initiation among immune reconsititute HIV-infected individuals.62 Investigators found that in individuals with baseline CD4 counts greater than 500 cells/mm3, ART exposure did not increase blood pressures or increase arterial stiffness.62 Another cross-sectional study done on 300 ART-experienced and 45 ART-naïve HIV-infected adults in China found that a nadir CD4 T-cell count less than 50 cells/mm3 was associated with increased prevalence of hypertension in underweight participants (body mass index (BMI) < 18.5 kg/m2) with an adjusted OR of 18.91 (P=0.043), but not in normal or overweight participants.63
The influence of nadir CD4 count on hypertension can be explained in part by the concept of early aging or immunosenescence. HIV-infected young adults have immunologic profiles that are similar to older HIV-uninfected adults,64 and this “senescent” immunologic profile persists in HIV-infected adults even after the initiation of ART and viral suppression.65 In HIV-uninfected populations, shortened telomere length is a genetically-mediated marker of immune senescence.66 The HIV virus seems to induce premature telomeric shortening through mitochondrial dysfunction.25 While immune senescence, through telomere shortening, has been proposed to explain the increased cardiovascular risk in HIV-infected adults,25 its specific relationship to hypertension has not yet been evaluated.
The relationship between immunosuppression and hypertension may also be related to HIV tropism.25 Investigators demonstrated that ART-experienced patients infected with an R5-tropic virus were less likely to experience hypertension than those infected with an X4-tropic virus (ARR = 47.6%).25 This difference may be due to the more aggressive behavior of the X4-tropic viral strain which has been associated with lower nadir CD4 counts.25,67
Lipodystrophy, Dyslipidemia, and Adipocytokines
Lipodystrophy
Both ART and HIV itself can cause lipodystrophy, an umbrella term encompassing lipoatrophy and lipohypertrophy. Lipodystrophy may cause hypertension through simultaneous accumulation of central adiposity and atrophy of peripheral adiposity.68,69 In a cross-sectional study of HIV-infected adults on ART, this association remained statistically significant even when accounting for BMI and other confounders.68 Furthermore, a cross-sectional study of HIV-infected adults with variable ART-exposure found that both lipoatrophy and lipohypertrophy independently predicted hypertension.21 After adjusting for BMI, patients with moderate lipoatrophy had a five-fold greater risk of hypertension as compared to those without lipodystrophy (aOR=5.5, P=0.01).21
HIV- and ART-related lipoatrophy and lipohypertrophy have also been associated with the RAAS dysregulation that causes hypertension.21,70 For instance, a study of angiotensin II and adrenocorticotropic hormone (ACTH) infusion into HIV-infected adults found that HIV-related visceral lipohypertrophy was independently associated with RAAS activation even in low-sodium conditions, with elevated median plasma renin activity among those with increased visceral adipose tissue (3.50 ng/mL·h) compared to those without increased adipose tissue (1.45 ng/mL·h) (P=0.002).71
The use of PIs specifically has been associated with lipodystrophy and perturbations in lipid homeostasis that may be linked to hypertension and cardiovascular disease.61,72,73 An in-vitro study of mouse macrophages found that PIs disturb protein folding, causing imbalances in intracellular cholesterol and calcium stores, promoting gene expression of certain proteins involved in lipid metabolism of macrophages, and triggering pathways that ultimately result in cell apoptosis.72 These mechanisms may be implicated in the pathophysiology of cardiovascular disease.72 The effects on lipid metabolism were also suggested by a sub-analysis of a prospective cohort study on HIV-infected individuals on PI-based regimens, which found an association between PI-related lipodystrophy and diminished levels of peroxisome proliferator activator receptor-gamma (PPAR-gamma), which regulate genes involved in lipid homeostasis and inflammation.74 These findings were supported by an in-vitro study on human and murine adipocytes, which found that PPAR-gamma agonist exposure attenuated PI-induced RAAS activation but that PPAR antagonists increased RAAS activation.24 The relationship between PPAR-gamma and RAAS has been extensively explored in the general population using murine models of dominant negative mutations of PPAR-gamma, which are associated with hypertension in humans.75 For instance, when exposed to angiotensin II infusions, transgenic, but not wild-type, mice demonstrated attenuated vasodilatory responses to acetylcholine exposure, although this effect was reversed in the presence of a superoxide scavenger, suggesting that the protective mechanisms of PPAR-gamma may be related to RAAS-mediated oxidative stress.76 Similar in-vitro studies examining the downstream vascular effects of PIs on PPAR-gamma and angiotensin II are warranted to explore their potential role as possible mediators of hypertension in the setting of HIV infection.
The Role of Adiponectin
The pathophysiologic mechanisms by which HIV-related dyslipidemias induce hypertension are likely related to the adipocytokines adiponectin and leptin.77,78,79,80,81 Adiponectin, an adipose tissue-derived cytokine or “adipokine”, acts as a vasodilator by stimulating endothelial nitric oxide,77,78,80 and thus its deficiency has been shown to be critical in the pathogenesis of obesity-related hypertension in the general population.77 In the context of HIV infection, decreased plasma levels of adiponectin have similarly been associated with metabolic syndrome.78,79 For instance, in a cross-sectional study of 54 HIV-infected children and adolescents, adiponectin levels were significantly lower among those with metabolic syndrome (P=0.004).78 Similarly, in a case-control study of 285 HIV-infected individuals (226 on ART; 59 ART-naïve) stratified by metabolic syndrome, lower adiponectin levels (P<0.0001) and higher concentrations of ROS (P<0.0001) were observed in the plasma of patients with metabolic syndrome, irrespective of ART status.79 Notably, no studies have directly analyzed the relationship between adiponectin and hypertension in HIV infection.
The Role of Leptin
Leptin, an adipokine generated by inflammatory cascades that are also activated in HIV infection, acts primarily on the aorta and hypothalamus.77,81 HIV-infected individuals on ART have been shown to have elevated leptin concentrations irrespective of BMI.82 In the aorta and hypothalamus, leptin receptors trigger the activation of both the RAAS and the sympathetic nervous system.77,81 In a family-based association analysis of the leptin gene among 695 individuals from 82 families, several single nucleotide polymorphisms (SNPs) that were significantly associated with higher plasma leptin concentrations were also associated with hypertension in women (β=0.48; 95% CI: 0.25-0.72; P<0.001), but not in men.83 Several mechanisms may explain the association between elevated leptin and hypertension. Firstly, murine models of hypertension have shown that leptin centrally activates the RAAS system.77,84 In addition, elevated leptin activates the sympathetic nervous system.81,83 Leptin also activates the RAAS and the central nervous system through direct effects on the medulla oblongata and by inducing production of acute phase reactants from the liver.81,85
The Neuroendocrine Response
HIV-mediated activation of the sympathetic nervous system may also be important in the pathophysiology of hypertension in HIV-infected adults. Already mentioned are the effects of adipokines on increased sympathetic tone.77,81,84,85 Additionally, both LPS and centrally acting macrophage-derived inflammatory cytokines appear to generate a similar neuroendocrine response.23,81,86 Also, HIV-infected adults with lipodystrophy have localized elevations of noradrenaline concentrations within adipose tissue and skeletal muscle.87 ART may also directly affect monoamine oxidase activity through mitochondrial derangements, or possibly influencing post-synaptic norepinephrine reuptake.87
This neuro-endocrine hyperactivity may be partially explained by psychosocial influences that have been shown to a play a role in hypertension, with investigators implicating stressors such as HIV-related stigma and mood disorders in autonomic dysfunction and disturbances of circadian rhythm.88–90 The influence of circadian rhythm derangement is further supported by the observation that, even among HIV-infected individuals who did not meet diagnostic criteria for hypertension, there was a greater likelihood of elevated nocturnal pressures and a lesser incidence of dipping systolic blood pressure, both of which may be harbingers of impending hypertension.88,91,92
HIV-related Renal Disease
Microalbuminuria
Microalbuminuria, a marker of renal injury, has been shown in multiple studies to be independently associated with hypertension in HIV-infected participants.5,93–96 HIV-infected adults have been shown to have higher rates of albuminuria than uninfected adults,5,95,97 with a prevalence estimated to be up to four times greater than that of the general population.95 These higher rates of albuminuria in HIV-infected adults are partly attributable to direct HIV viral effect and partly due to chronic inflammation.5,96,98 A recent multicenter, prospective cohort study of 823 HIV-infected and 267 HIV-uninfected women followed over a median of 10 years found microalbuminuria to be an independent predictor of incident hypertension (RR 1.13 per urine albumin-to-creatinine ratio doubling; 95% CI: 1.07-1.29).96 These studies suggest that hypertension ultimately occurs through renally-mediated mechanisms of sodium imbalance and RAAS activation.94–96
The Role of L-Arginine
L-arginine, a nitric oxide precursor, may also be an important mediator in the relationship between renal disease and hypertension in HIV-infected adults.99 In the general population, L-arginine deficiency has previously been shown to cause vasoconstriction and hypertension.100 One cross-sectional analysis of HIV-infected and HIV-uninfected South African men demonstrated that low estimated glomerular filtration rate (eGFR) was common in HIV-infected men and was associated with higher L-arginine levels (P=0.002).99
Other
HIV-related RAAS Activation
Abnormal RAAS activation may play a critical role in the pathophysiology of hypertension in HIV infection. HIV-infected persons demonstrated increased RAAS activity as a result of several upstream pathways.25,71 HIV-infected adults have high plasma renin activity, even in low-sodium environments.71 This may be explained by the structure of HIV-1 protease, which is similar to renin.101,102 The virus has been shown to promote the production of renin by CD4 T-cells, which are a source of endogenous RAAS activation.102 Reciprocally, renin interacts directly with the HIV virus to promote viral replication.101,102 In one in-vitro study, renin was shown to be critical to HIV replication within T cells, presumably by adopting the role of HIV-protease and interacting with its receptor (P)RR.102 Investigators found that HIV-infected T-cells incubated in a renin-rich medium, compared with those in a renin-free buffer, exhibited a 3-fold increase in the concentration of p24 (an essential viral protein).102 Furthermore, the addition of a direct renin inhibitor was shown to decrease the production of both HIV viral proteins.102
Arterial Stiffness
PIs may cause arterial stiffness, an established independent risk factor for hypertension. Arterial stiffness has been shown to induce elevated central pulse pressure, sympathetic activation, and left ventricular hypertrophy.77 A large, prospective cohort study of ART-treated HIV-infected patients without hypertension found that patients on the PIs lopinavir/ritonavir were twice as likely as those on other ART drugs to develop hypertension.61 Schillaci et al. showed that compared to HIV-uninfected controls, HIV-infected patients on PIs had a greater degree of aortic stiffness, as measured by higher aortic pulse wave velocity (7.6±1.1 versus 6.8±1.2 m×s−1, P=0.015) and aortic augmentation (6.8±5 versus 4.6±4 mm Hg, P=0.037).73 The end-organ effects of arterial stiffness and endothelial dysfunction are likely the direct consequence of vascular inflammation.98
Protease Inhibitors
PIs have been implicated in the pathophysiology of hypertension in HIV-infected adults through numerous mechanisms including: RAAS activation, endothelial dysfunction, arterial stiffness, lipodystrophy, and dyslipidemia.5,24,44,61,72,73,98 The effects on endothelial dysfunction, arterial stiffness, lipodystrophy, and dyslipidemia have already been each discussed in dedicated sub-sections above.
The use of PIs is associated with RAAS activation.24 Acting on adipocytes directly, the PI combinations ritonavir/lopinavir and ritonavir/atazanavir were shown to activate adipokine-mediated inflammatory pathways that led to activation of adipose RAAS.24 An in-vitro study of human and murine adipocytes demonstrated up to a 4-fold increase of angiotensin receptor protein expression following only 5 days of exposure to lopinavir/ritonavir or atazanavir/ritonavir.24 This effect was prevented by the use of RAAS antagonists.24
Nucleoside and Non-Nucleoside Reverse Transcriptase Inhibitors
Certain nucleoside reverse transcriptase inhibitors (NRTIs) may also play some role in the pathophysiology of hypertension in HIV-infected adults, although data are conflicting. One prospective cohort study of 444 HIV-infected adults without hypertension at baseline found that combination therapy with lamivudine and tenofovir as compared with lamivudine and zidovudine was associated with an increased risk of hypertension (OR 2.3; 95% CI 1.0-5.2; P=0.046).61 Similarly, a sub-analysis of a prospective cohort study of 527 HIV-infected and 517 HIV-uninfected adults found that prior stavudine exposure was independently associated with hypertension (OR=1.54; 95% CI 1.04-2.30). Other studies, including our own, have shown no relationship between NRTI use and hypertension.5
There have been fewer studies exploring the association between non-nucleoside reverse transcriptase inhibitors (NNRTIs) and hypertension. However, a prospective open-label clinical trial that evaluated the cardiometabolic outcomes after HIV-infected participants were switched from an older generation (nevirapine) to a newer generation NNRTI (rilpivirine) demonstrated a mean systolic blood pressure decrease of 6.0 mm Hg (95% CI −1.7 to −10.3; P=0.007) after 24 weeks of therapy.103
How much of HTN in HIV-infected adults is simply attributable to ART?
Direct ART effect may explain some but not all of the increased risk of hypertension in HIV-infected adults. HIV-infected participants on ART have higher rates of hypertension than ART-naïve HIV-infected persons.6,9,10,15,24,59–61,69,98,104,105 While no published studies have quantified the hypertensive risk directly attributed to ART use, many studies done on ART-experienced HIV-infected participants have identified other independent risk factors for hypertension, including nadir CD4 count < 50 cells/μL (aOR 2.48; 95% CI 1.27-4.83),22 lipodystrophy (OR 4.80; 95% CI 2.43-9.85; P<0.0001),4 lipoatrophy (OR 4.3; 95% CI 1.5-12.4; P=0.006),21 renal dysfunction,5,59,96,98 and HIV tropism.25 In addition, the higher prevalence of hypertension in ART-experienced adults may be due to the immune reconstitution caused by these drugs rather than direct drug effect on blood pressure.5,26
Findings from observational studies describing HIV-infected adults on different ART regimens should be interpreted with caution for several reasons. First, there is the possibility of selection bias since there may be underlying clinical factors justifying the use of a particular anti-retroviral regimen that may consequently influence the development of hypertension. Furthermore, the studies evaluating ART and hypertension also suffer from potential confounding by heterogeneity between and within ART classes, and relatively sparse information on mechanisms by which ART may cause hypertension. Finally, the comparison of people on ART versus not on ART may be problematic because several studies have postulated that poor HIV control is actually associated with lower blood pressure due to greater vascular permeability and a peri-septic state.5,18,106 Randomized trials are warranted to account for potential confounders and better understand the independent cardiovascular effects of ART.
FUTURE DIRECTIONS AND TREATMENT IMPLICATIONS
In this review, we have summarized what is known about the mechanisms for hypertension in HIV-infected adults. It is important to note that most of the published research that we reviewed described more generally the pathophysiology of cardiovascular disease in the context of HIV and did not look more specifically at the pathophysiology of hypertension. More basic science and clinical research is needed to investigate whether these pathways that have been proven to lead to cardiovascular disease in HIV-infected populations are the same pathways that lead to hypertension, or whether other pathways are important for hypertension.
Larger, multinational prospective studies are needed to determine the mechanistic factors that precede and predict hypertension in HIV-infected adults. Most human data regarding hypertension in HIV-infected adults comes from cross-sectional studies, and debates in the literature regarding potential mechanisms for hypertension in HIV-infected adults have resulted from conflict between cross-sectional studies.63,107 Surprisingly few of the large cohorts of HIV-infected adults have included rigorous measurement of blood pressure.
The best method for screening for hypertension and the optimal threshold for starting antihypertensive medications should also be determined. None of the current guidelines provide specific recommendations related to HIV-infected adults.108,109 There may be a role for universal ambulatory blood pressure monitoring (ABPM), which has been validated in the general population as an independent predictor of cardiovascular disease outcomes,92 as studies have demonstrated a high prevalence of abnormal diurnal blood pressure patterns and masked hypertension among HIV-infected adults undergoing ABPM screening.88,91
Interventional studies of novel approaches to prevent and/or treat HIV-infected adults targeting the pathways described above are needed. Small studies of RAAS antagonists have yielded promising results.110,111 Telmisartan, which acts as both an angiotensin receptor blocker as well as a PPAR-gamma agonist, is particularly effective. One longitudinal prospective study of 13 hypertensive, HIV-infected males on ART found that telmisartan use reduced systolic (−9.2 ± 3.4 mm Hg; P=0.006) and diastolic blood pressure (−11.9 ± 2.9 mm Hg; P=0.001) over a 3-year period.111 In another prospective study of 18 virologically-suppressed HIV-infected males, telmisartan use greatly reduced systolic (20.00 mmHg, P<0.001) and diastolic (13.34 mmHg, P<0.001) blood pressures after 6 months.110
To date, though, there have not been any large scale trials evaluating the role of RAAS antagonists in the prevention and treatment of hypertension in HIV. Given the pivotal role that renin plays both in the initiation of the RAAS cascade as well as mediation of viral replication, RAAS antagonists could result in significant clinical benefit in HIV-infected adults. Additionally, interventional studies exploring therapies that target chronic inflammation, LPS and leptin are likely warranted.
CONCLUSIONS
In summary, hypertension is common in HIV-infected adults and is likely due to a combination of traditional risk factors, HIV-specific factors, and ART. We have described the current evidence for possible mechanisms of hypertension in HIV-infected adults related to HIV-specific factors and ART. Novel pathophysiologic mechanisms for hypertension in HIV-infected adults may include: microbial translocation, chronic inflammation, immune suppression and reconstitution, viral tropism, lipodystrophy, adipokines, and HIV-related renal disease. Large, multinational cohort studies are needed to solidify our knowledge. In addition, interventional studies are needed to discover new interventions for preventing and treating hypertension and hypertension-related cardiovascular disease in HIV-infected adults.
Acknowledgments
We are grateful to Kevin Pain, Research Specialist, Weill Cornell Medical Library, for his assistance in developing and performing the literature search upon which our review is based.
Sources of Funding: SF is supported by the post-doctoral global health research fellowship at Weill Cornell Medical College. RP is supported by a career development grant from the Fogarty International Institute of the NIH (K01 TW01281).*
Footnotes
Disclosure of Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.WHO. HIV/AIDS Fact Sheet. WHO Media Centre; 2017. [Google Scholar]
- 2.WHO. Global Health Sector Response to HIV. 2000-20152015. [Google Scholar]
- 3.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–220. doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. Journal of hypertension. 2003;21(7):1377–1382. doi: 10.1097/01.hjh.0000059071.43904.dc. [DOI] [PubMed] [Google Scholar]
- 5.Peck RN, Shedafa R, Kalluvya S, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC medicine. 2014;12(1):125. doi: 10.1186/s12916-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. Journal of the American Society of Hypertension. 2017;11(8):530–540. doi: 10.1016/j.jash.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Ryscavage P, Still W, Nyemba V, Stafford K. Prevalence of Systemic Hypertension Among HIV-Infected and HIV-Uninfected Young Adults. presented at: Open Forum Infectious Diseases. 2017 doi: 10.14423/SMJ.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 8.Nuesch R, Wang Q, Elzi L, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV-infected patients: Swiss HIV Cohort Study (SHCS) J Acquir Immune Defic Syndr. 2013;62(4):396–404. doi: 10.1097/QAI.0b013e3182847cd0. [DOI] [PubMed] [Google Scholar]
- 9.Nduka CU, Stranges S, Sarki AM, Kimani PK, Uthman OA. Evidence of increased blood pressure and hypertension risk among people living with HIV on antiretroviral therapy: a systematic review with meta-analysis. J Hum Hypertens. 2016;30(6):355–362. doi: 10.1038/jhh.2015.97. [DOI] [PubMed] [Google Scholar]
- 10.Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. Aids. 2005;19(9):953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 11.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Önen NF, Overton ET, Seyfried W, et al. Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV clinical trials. 2010;11(2):100–109. doi: 10.1310/hct1102-100. [DOI] [PubMed] [Google Scholar]
- 13.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen KA, Peer N, Mills EJ, Kengne AP. Burden, Determinants, and Pharmacological Management of Hypertension in HIV-Positive Patients and Populations: A Systematic Narrative Review. AIDS reviews. 2015;17(2):83–95. [PubMed] [Google Scholar]
- 15.van Zoest RA, Wit FW, Kooij KW, et al. Higher Prevalence of Hypertension in HIV-1-Infected Patients on Combination Antiretroviral Therapy Is Associated With Changes in Body Composition and Prior Stavudine Exposure. Clin Infect Dis. 2016;63(2):205–213. doi: 10.1093/cid/ciw285. [DOI] [PubMed] [Google Scholar]
- 16.Phillips AN, Carr A, Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13(2):177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 17.Armah KA, Chang CC, Baker JV, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis. 2014;58(1):121–129. doi: 10.1093/cid/cit652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloomfield GS, Hogan JW, Keter A, et al. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14:284. doi: 10.1186/1471-2334-14-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clinical Infectious Diseases. 2014;59(12):1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 20.van Zoest RA, van den Born B-JH, Reiss P. Hypertension in people living with HIV. Current Opinion in HIV and AIDS. 2017;12(6):513–522. doi: 10.1097/COH.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 21.Crane HM, Grunfeld C, Harrington RD, Kitahata MM. Lipoatrophy and lipohypertrophy are independently associated with hypertension. HIV Med. 2009;10(8):496–503. doi: 10.1111/j.1468-1293.2009.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manner IW, Troseid M, Oektedalen O, Baekken M, Os I. Low nadir CD4 cell count predicts sustained hypertension in HIV-infected individuals. J Clin Hypertens (Greenwich) 2013;15(2):101–106. doi: 10.1111/jch.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manner IW, Baekken M, Kvale D, et al. Markers of microbial translocation predict hypertension in HIV-infected individuals. HIV Med. 2013;14(6):354–361. doi: 10.1111/hiv.12015. [DOI] [PubMed] [Google Scholar]
- 24.Boccara F, Auclair M, Cohen A, et al. HIV protease inhibitors activate the adipocyte renin angiotensin system. Antivir Ther. 2010;15(3):363–375. doi: 10.3851/IMP1533. [DOI] [PubMed] [Google Scholar]
- 25.Maffongelli G, Alteri C, Gentilotti E, et al. Impact of HIV-1 tropism on the emergence of non-AIDS events in HIV-infected patients receiving fully suppressive antiretroviral therapy. Aids. 2016;30(5):731–741. doi: 10.1097/QAD.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blodget E, Shen C, Aldrovandi G, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7(8):e42624. doi: 10.1371/journal.pone.0042624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chun T-W, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. The Journal of infectious diseases. 2008;197(5):714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 28.Desai S, Landay A. Early immune senescence in HIV disease. Current HIV/AIDS Reports. 2010;7(1):4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2003;284(6):L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- 31.Amar J, Ruidavets JB, Sollier CBD, et al. Soluble CD14 and aortic stiffness in a population-based study. Journal of hypertension. 2003;21(10):1869–1877. doi: 10.1097/00004872-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens. 2010;28(4):806–816. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund DD, Brooks RM, Faraci FM, Heistad DD. Role of angiotensin II in endothelial dysfunction induced by lipopolysaccharide in mice. Am J Physiol Heart Circ Physiol. 2007;293(6):H3726–3731. doi: 10.1152/ajpheart.01116.2007. [DOI] [PubMed] [Google Scholar]
- 34.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19(2):149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 35.Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007;49(2):304–310. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- 36.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. Jama. 2001;286(17):2107–2113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 37.Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33(1):158–164. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castley A, Williams L, James I, Guelfi G, Berry C, Nolan D. Plasma CXCL10, sCD163 and sCD14 Levels Have Distinct Associations with Antiretroviral Treatment and Cardiovascular Disease Risk Factors. PLoS One. 2016;11(6):e0158169. doi: 10.1371/journal.pone.0158169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. Jama. 2012;308(4):379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong SJ, Chin BS, Chae YT, et al. Serum retinol-binding protein-4 levels are increased in HIV-infected subjects with metabolic syndrome receiving highly active antiretroviral therapy. Yonsei Med J. 2012;53(6):1211–1215. doi: 10.3349/ymj.2012.53.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lichtner M, Cicconi P, Vita S, et al. Cytomegalovirus coinfection is associated with an increased risk of severe non–AIDS-defining events in a large cohort of HIV-infected patients. The Journal of infectious diseases. 2014;211(2):178–186. doi: 10.1093/infdis/jiu417. [DOI] [PubMed] [Google Scholar]
- 42.Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Curr Opin HIV AIDS. 2016;11(2):216–225. doi: 10.1097/COH.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3(3):e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagathu C, Eustace B, Prot M, et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antiviral therapy. 2007;12(4):489. [PubMed] [Google Scholar]
- 45.Wu J, Saleh MA, Kirabo A, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126(1):50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kriska T, Cepura C, Magier D, Siangjong L, Gauthier KM, Campbell WB. Mice lacking macrophage 12/15-lipoxygenase are resistant to experimental hypertension. Am J Physiol Heart Circ Physiol. 2012;302(11):H2428–2438. doi: 10.1152/ajpheart.01120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215(1):21–33. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah KH, Shi P, Giani JF, et al. Myeloid Suppressor Cells Accumulate and Regulate Blood Pressure in Hypertension. Circ Res. 2015;117(10):858–869. doi: 10.1161/CIRCRESAHA.115.306539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang ZN, Yi N, Zhang TW, et al. Myeloid-Derived Suppressor Cells Associated With Disease Progression in Primary HIV Infection: PD-L1 Blockade Attenuates Inhibition. J Acquir Immune Defic Syndr. 2017;76(2):200–208. doi: 10.1097/QAI.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 50.Sui Y, Frey B, Wang Y, et al. Paradoxical myeloid-derived suppressor cell reduction in the bone marrow of SIV chronically infected macaques. PLoS Pathog. 2017;13(5):e1006395. doi: 10.1371/journal.ppat.1006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Zhao J, Ren JP, et al. Expansion of myeloid-derived suppressor cells promotes differentiation of regulatory T cells in HIV-1+ individuals. Aids. 2016;30(10):1521–1531. doi: 10.1097/QAD.0000000000001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karbach SH, Schonfelder T, Brandao I, et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J Am Heart Assoc. 2016;5(9) doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilck N, Matus MG, Kearney SM, et al. Salt-responsive gut commensal modulates T H 17 axis and disease. Nature. 2017;551(7682):585. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim CJ, Walmsley SL, Raboud JM, et al. Can Probiotics Reduce Inflammation and Enhance Gut Immune Health in People Living with HIV: Study Designs for the Probiotic Visbiome for Inflammation and Translocation (PROOV IT) Pilot Trials. HIV Clin Trials. 2016;17(4):147–157. doi: 10.1080/15284336.2016.1184827. [DOI] [PubMed] [Google Scholar]
- 57.Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Arboli E, Mwamelo K, Kalinjuma AV, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania– A prospective cohort study. PLoS One. 2017;12(3):e0172089. doi: 10.1371/journal.pone.0172089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palacios R, Santos J, Garcia A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7(1):10–15. doi: 10.1111/j.1468-1293.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 61.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. Aids. 2006;20(7):1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 62.Baker JV, Hullsiek KH, Engen NW, et al. Early Antiretroviral Therapy at High CD4 Counts Does Not Improve Arterial Elasticity: A Substudy of the Strategic Timing of AntiRetroviral Treatment (START) Trial. Open Forum Infect Dis. 2016;3(4):ofw213. doi: 10.1093/ofid/ofw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding Y, Lin H, Liu X, et al. Hypertension in HIV-Infected Adults Compared with Similar but Uninfected Adults in China: Body Mass Index-Dependent Effects of Nadir CD4 Count. AIDS Res Hum Retroviruses. 2017;33(11):1117–1125. doi: 10.1089/AID.2017.0008. [DOI] [PubMed] [Google Scholar]
- 64.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. The Journal of infectious diseases. 2003;187(12):1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 65.Desai S, Usuga X, Martinson J. Immune senescence, activation and abnormal T cell homeostasis despite effective HAART, a hallmark of early aging in HIV. presented at: 16th Conference on Retroviruses and Opportunistic Infections [Google Scholar]
- 66.von Kanel R, Malan NT, Hamer M, Malan L. Comparison of telomere length in black and white teachers from South Africa: the sympathetic activity and ambulatory blood pressure in Africans study. Psychosom Med. 2015;77(1):26–32. doi: 10.1097/PSY.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 67.Seclen E, Soriano V, Gonzalez MM, et al. Impact of baseline HIV-1 tropism on viral response and CD4 cell count gains in HIV-infected patients receiving first-line antiretroviral therapy. J Infect Dis. 2011;204(1):139–144. doi: 10.1093/infdis/jir218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freitas P, Carvalho D, Santos AC, et al. Central/Peripheral fat mass ratio is associated with increased risk of hypertension in HIV-infected patients. J Clin Hypertens (Greenwich) 2012;14(9):593–600. doi: 10.1111/j.1751-7176.2012.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thiebaut R, El-Sadr WM, Friis-Moller N, et al. Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antivir Ther. 2005;10(7):811–823. doi: 10.1177/135965350501000706. [DOI] [PubMed] [Google Scholar]
- 70.Yiannikouris F, Gupte M, Putnam K, et al. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60(6):1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srinivasa S, Fitch KV, Wong K, et al. RAAS Activation Is Associated With Visceral Adiposity and Insulin Resistance Among HIV-infected Patients. J Clin Endocrinol Metab. 2015;100(8):2873–2882. doi: 10.1210/jc.2015-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou H, Pandak WM, Jr, Lyall V, Natarajan R, Hylemon PB. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol. 2005;68(3):690–700. doi: 10.1124/mol.105.012898. [DOI] [PubMed] [Google Scholar]
- 73.Schillaci G, De Socio GV, Pirro M, et al. Impact of treatment with protease inhibitors on aortic stiffness in adult patients with human immunodeficiency virus infection. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(11):2381–2385. doi: 10.1161/01.ATV.0000183744.38509.de. [DOI] [PubMed] [Google Scholar]
- 74.Nazih H, Raffi F, Taieb A, et al. Peroxisome proliferator activating receptor alpha and gamma polymorphisms and metabolic abnormalities in HIV-infected patients receiving highly active antiretroviral therapy: the ANRS CO8 APROCO-COPILOTE study. AIDS Res Hum Retroviruses. 2012;28(4):393–399. doi: 10.1089/aid.2010.0311. [DOI] [PubMed] [Google Scholar]
- 75.Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 76.Hu C, Lu KT, Mukohda M, Davis DR, Faraci FM, Sigmund CD. Interference with PPARgamma in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol Genomics. 2016;48(2):124–134. doi: 10.1152/physiolgenomics.00087.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sabbatini AR, Fontana V, Laurent S, Moreno H. An update on the role of adipokines in arterial stiffness and hypertension. J Hypertens. 2015;33(3):435–444. doi: 10.1097/HJH.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 78.Espiau M, Yeste D, Noguera-Julian A, et al. Adiponectin, Leptin and Inflammatory Markers in HIV-associated Metabolic Syndrome in Children and Adolescents. Pediatr Infect Dis J. 2017;36(2):e31–e37. doi: 10.1097/INF.0000000000001394. [DOI] [PubMed] [Google Scholar]
- 79.Morimoto HK, Simao AN, de Almeida ER, et al. Role of metabolic syndrome and antiretroviral therapy in adiponectin levels and oxidative stress in HIV-1 infected patients. Nutrition. 2014;30(11-12):1324–1330. doi: 10.1016/j.nut.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 80.Kim DH, Kim C, Ding EL, Townsend MK, Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension. 2013;62(1):27–32. doi: 10.1161/HYPERTENSIONAHA.113.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kathyayani T, Reddy AH, Lakshmi BS, Venkatappa B. Neuro-endocrine immune networks leading to HIV-associated cardiovascular abnormalities: Role of leptin. HIV & AIDS Review. 2015;14(3):53–60. [Google Scholar]
- 82.Pinzone, Fox ML, Sastry MK, Parenti DM, Simon GL. Plasma leptin concentration increases early during highly active antiretroviral therapy for acquired immunodeficiency syndrome, independent of body weight. J Endocrinol Invest. 2005;28(3):Rc1–3. doi: 10.1007/BF03345372. [DOI] [PubMed] [Google Scholar]
- 83.Ma D, Feitosa MF, Wilk JB, et al. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension. 2009;53(3):473–479. doi: 10.1161/HYPERTENSIONAHA.108.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Wang H, Luo W, et al. Leptin-induced endothelial dysfunction is mediated by sympathetic nervous system activity. Journal of the American Heart Association. 2013;2(5):e000299. doi: 10.1161/JAHA.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41(3):763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 86.Norbiato G, Bevilacqua M, Vago T, Taddei A, Clerici M. Glucocorticoids and the immune function in the human immunodeficiency virus infection: a study in hypercortisolemic and cortisol-resistant patients. The Journal of Clinical Endocrinology & Metabolism. 1997;82(10):3260–3263. doi: 10.1210/jcem.82.10.4304. [DOI] [PubMed] [Google Scholar]
- 87.van Gurp PJ, Tack CJ, van der Valk M, et al. Sympathetic nervous system function in HIV-associated adipose redistribution syndrome. Aids. 2006;20(5):773–775. doi: 10.1097/01.aids.0000216379.91936.84. [DOI] [PubMed] [Google Scholar]
- 88.Kent ST, Schwartz JE, Shimbo D, et al. Race and sex differences in ambulatory blood pressure measures among HIV+ adults. J Am Soc Hypertens. 2017;11(7):420–427.e423. doi: 10.1016/j.jash.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schutte AE, Botha S, Fourie CMT, et al. Recent advances in understanding hypertension development in sub-Saharan Africa. J Hum Hypertens. 2017;31(8):491–500. doi: 10.1038/jhh.2017.18. [DOI] [PubMed] [Google Scholar]
- 90.Wang T, Jiang Z, Hou W, et al. HIV Tat protein affects circadian rhythmicity by interfering with the circadian system. HIV medicine. 2014;15(9):565–570. doi: 10.1111/hiv.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kent ST, Bromfield SG, Burkholder GA, et al. Ambulatory blood pressure monitoring in individuals with HIV: a systematic review and meta-analysis. PloS one. 2016;11(2):e0148920. doi: 10.1371/journal.pone.0148920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kent ST, Burkholder GA, Tajeu GS, Overton ET, Muntner P. Mechanisms influencing circadian blood pressure patterns among individuals with HIV. Current hypertension reports. 2015;17(11):88. doi: 10.1007/s11906-015-0598-1. [DOI] [PubMed] [Google Scholar]
- 93.Wensink GE, Schoffelen AF, Tempelman HA, Rookmaaker MB, Hoepelman AI, Barth RE. Albuminuria Is Associated with Traditional Cardiovascular Risk Factors and Viral Load in HIV-Infected Patients in Rural South Africa. PLoS One. 2015;10(8):e0136529. doi: 10.1371/journal.pone.0136529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pirro M, Mannarino MR, Francisci D, et al. Urinary albumin-to-creatinine ratio is associated with endothelial dysfunction in HIV-infected patients receiving antiretroviral therapy. Sci Rep. 2016;6:28741. doi: 10.1038/srep28741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baekken M, Os I, Sandvik L, Oektedalen O. Microalbuminuria associated with indicators of inflammatory activity in an HIV-positive population. Nephrol Dial Transplant. 2008;23(10):3130–3137. doi: 10.1093/ndt/gfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ascher SB, Scherzer R, Peralta CA, et al. Association of Kidney Function and Early Kidney Injury With Incident Hypertension in HIV-Infected Women. Hypertension. 2017;69(2):304–313. doi: 10.1161/HYPERTENSIONAHA.116.08258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Msango L, Downs JA, Kalluvya SE, et al. Renal dysfunction among HIV-infected patients starting antiretroviral therapy in Mwanza, Tanzania. AIDS (London, England) 2011;25(11):1421. doi: 10.1097/QAD.0b013e328348a4b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV-positive population compared with the general population: influence of combination antiretroviral therapy. J Hypertens. 2008;26(11):2126–2133. doi: 10.1097/HJH.0b013e32830ef5fb. [DOI] [PubMed] [Google Scholar]
- 99.Glyn MC, Van Rooyen JM, Schutte R, et al. A comparison of the association between glomerular filtration and L-arginine status in HIV-infected and uninfected African men: the SAfrEIC study. J Hum Hypertens. 2013;27(9):557–563. doi: 10.1038/jhh.2013.14. [DOI] [PubMed] [Google Scholar]
- 100.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine–nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94(6):1298–1303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 101.Tzoupis H, Leonis G, Megariotis G, Supuran CT, Mavromoustakos T, Papadopoulos MG. Dual inhibitors for aspartic proteases HIV-1 PR and renin: advancements in AIDS-hypertension-diabetes linkage via molecular dynamics, inhibition assays, and binding free energy calculations. J Med Chem. 2012;55(12):5784–5796. doi: 10.1021/jm300180r. [DOI] [PubMed] [Google Scholar]
- 102.Chandel N, Ayasolla K, Lan X, et al. Renin modulates HIV replication in T cells. J Leukoc Biol. 2014;96(4):601–609. doi: 10.1189/JLB.2A0414-192R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rokx C, Verbon A, Rijnders BJ. Short communication: Lipids and cardiovascular risk after switching HIV-1 patients on nevirapine and emtricitabine/tenofovir-DF to rilpivirine/emtricitabine/tenofovir-DF. AIDS Res Hum Retroviruses. 2015;31(4):363–367. doi: 10.1089/AID.2014.0278. [DOI] [PubMed] [Google Scholar]
- 104.Nduka CU, Stranges S, Bloomfield GS, et al. A plausible causal link between antiretroviral therapy and increased blood pressure in a sub-Saharan African setting: A propensity score-matched analysis. Int J Cardiol. 2016;220:400–407. doi: 10.1016/j.ijcard.2016.06.210. [DOI] [PubMed] [Google Scholar]
- 105.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. International journal of epidemiology. 2013;42(6):1754–1771. doi: 10.1093/ije/dyt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okeahialam BN, Sani MU. Heart disease in HIV/AIDS. How much is due to cachexia? Afr J Med Med Sci. 2006;35(Suppl):99–102. [PubMed] [Google Scholar]
- 107.Gupta SK, Shen C, Mather KJ, Agarwal R, Dube MP. Neither proteinuria nor albuminuria is associated with endothelial dysfunction in HIV-infected patients without diabetes or hypertension. J Infect Dis. 2011;204(12):1946–1950. doi: 10.1093/infdis/jir668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association. Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2017 doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 109.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood pressure. 2013;22(4):193–278. doi: 10.3109/08037051.2013.812549. [DOI] [PubMed] [Google Scholar]
- 110.Vecchiet J, Ucciferri C, Falasca K, Mancino P, Di Iorio A, De Caterina R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antiviral therapy. 2011;16(5):639. doi: 10.3851/IMP1809. [DOI] [PubMed] [Google Scholar]
- 111.Ucciferri C, Falasca K, Vignale F, Di Nicola M, Vecchiet J. Long term effect of telmisartan in HIV-positive male patients with high blood pressure. Braz J Infect Dis. 2015;19(6):668–669. doi: 10.1016/j.bjid.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


