Abstract
Emetine is a small molecule protein synthesis inhibitor that is toxic to all cell types and therefore suitable for complete killing of all types of heterogeneous cancer cells within a tumor. It becomes significantly inactive (non-toxic) when derivatized at its N-2′ secondary amine. This provides a strategy for targeting emetine to cancerous tumor without killing normal cells. In this report, PSA activatable peptide prodrugs of emetine were synthesized. To overcome steric hindrances and enhance protease specific cleavage, a 2-stage prodrug activation process was needed to release emetine in cancer cells. In this 2-stage process, emetine prodrug intermediates are coupled to PSA peptide substrate (Ac-His-Ser-Ser-Lys-Leu-Gln) to obtain the full prodrug. Both prodrug intermediates 10 (Ala-Pro-PABC-Emetine) and 14 (Ser-Leu-PABC-Emetine) were evaluated for kinetics of hydrolysis to emetine and potency [Where PABC = p-aminobenzyloxycarbonyl]. While both intermediates quantitatively liberate emetine when incubated under appropriate conditions, upon coupling of PSA substrate to give the full prodrugs, only prodrug 16, the prodrug obtained from 14 was hydrolyzable by PSA. Cytotoxicity studies in PSA producing LNCaP and CWR22Rv1 confirm the activation of the prodrug by PSA with an IC50 of 75 nM and 59 nM respectively. The cytotoxicity of 16 is significantly reduced in cell lines that do not produce PSA. Further, in vivo toxicity studies are done on these prodrugs and other derivatives of emetine. The results show the significance of conformational modulation in obtaining safe emetine prodrugs.
Keywords: Emetine, Prostate specific antigen (PSA), Emetine-prodrugs, Prostate cancer, Emetine derivatives, Anticancer prodrugs
1. Introduction
The identification of tumor selective protein targets has enabled the discovery and development of many “targeted” cancer therapies. These therapies have produced improved survival rates in some patients with some types of cancer. However, within individual tumor sites the target proteins are heterogeneously expressed, thus, a relatively rapid development of “resistance” to these therapeutic inhibitors/treatments is observed due to the selection of cancer cells that lack or down-regulates the expression of the target protein. This heterogeneity of expression appreciably limits the usefulness of these therapies. One approach to overcome this heterogeneity problem is to identify a protein or mechanism that is critically needed for the survival of all cell types, and then develop a strategy to selectively inhibits the protein target only in cancer cells without affecting the normal cells.
General ribosomal and mitochondrial protein synthesis (translation) is critical to the survival of all cells. Any agent or process that inhibits protein translation will be cytotoxic to all types of cells. The natural product alkaloid emetine (Fig. 1A) is one such agent. Emetine is a small molecule found in the root of plant species Carapichea ipecacuanha (Brot.) L. Anderson but until recently this plant species was generally named as Psychotria ipecacuanha (Brot.) Stokes.1 It is the main active agent in ipecac syrup used as an emetic and expectorant.2 It is a ribosomal and mitochondrial protein synthesis inhibitor and also inhibits the synthesis of DNA and RNA.3 Emetine binds to the 40S ribosomal subunit to inhibit protein synthesis.4 Based on this mechanism, emetine is reported to be highly cytotoxic to all cells. It also causes the upregulation of pro-apoptotic and downregulation of anti-apoptotic gene products in various cancer cells.3 It is a potent and non-specific cellular toxin with a potent activity in the NCI 60 cancer cell screen (GI50 of 27 nM).
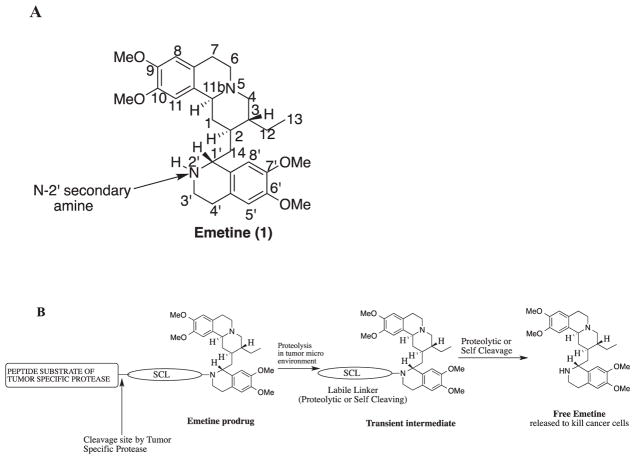
Fig. 1.
(A) Structure of emetine showing the numbering of the atoms; (B) Strategic design of emetine prodrug by derivatizing the N-2′ position for activation within tumor microenvironment.
Structure-activity relationship (SAR) studies have shown that the N-2′ position of emetine is crucial to its inhibition of protein synthesis and it must be a secondary amine.2,5 To identify emetine derivatives with reduced systemic toxicity, we have performed structure activity relationship (SAR) studies on N-2′ modified derivatives of emetine with diverse substituents.6–9 We have shown that modification at the N-2′ causes emetine to become significantly less cytotoxic (i.e. up to 300-fold reduction).6–9 Thus, the N-2′ position of emetine represents a potential cytotoxic “molecular switch” that would be turned “OFF” in an N-2′ modified emetine prodrug and then turned “ON” when the prodrug is activated in the cancerous tumor microenvironment to release emetine (Fig. 1B).
Previously we have described prodrugs of emetine that are activatable at the relatively low pH (5.5–6.8) within the microenvironment of cancerous tumor7 and prodrugs that are activated through sequential proteolysis by the proteases fibroblast activation protein (FAP) and (DPPIV).9 In the present study we describe the synthesis and evaluation of a peptidyl prodrug of emetine that is activated by Prostate Specific Antigen (PSA), a serine protease selectively expressed at high levels within sites of prostate cancer. PSA is enzymatically active within the tumor microenvironment but rapidly inactivated upon entering the circulation due to binding to abundant serum protease inhibitors α2-macroglobulin (A2M) and α1-antichymotrypsin (ACT).10–12
2. Results and discussion
2.1. Chemistry
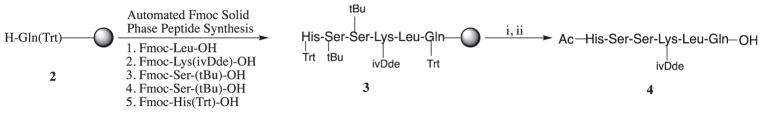
The peptide substrate of PSA, HSSKLQ contain only L-amino acids. This peptide was synthesized using automated Fmoc solid phase peptide synthesis using diisopropylcarbodiimide (DIC) and 1-hydroxybenzotriazole (HOBt) in N-methyl pyrolidinone with sequential removal of Fmoc with 25% piperidine in dimethylformamide (DMF) (Scheme 1). The N-terminal of histidine (His) was capped with an acetyl group and the acid sensitive side chains protecting groups (Trt, and t-Bu) were removed using 60% TFA in dichloromethane. The product obtained was purified with preparative HPLC to give peptide 4.
Scheme 1.
Synthetic scheme of the PSA substrate HSSK(ivDde)LQ 4. Reagents and conditions: (i) Ac2O, DIEA, DCM; (ii) 60% TFA in CH2Cl2.
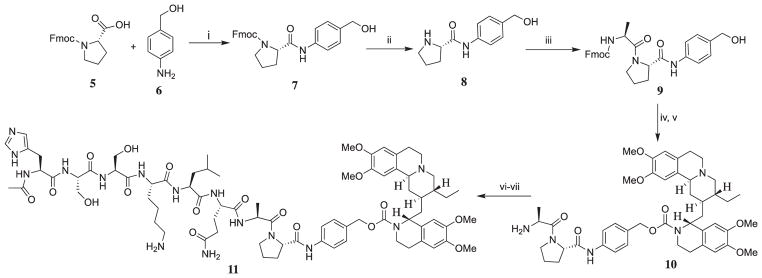
Previously we reported on the design of a general precursor for emetine prodrug which can be activated by the ubiquitous DPPIV to liberate emetine, and we established the need for a self immolative linker p-aminobenzyloxycarbonyl (PABC) between emetine and the dipeptide Ala-Pro.9 This precursor (represented as compound 10 in this present study) demonstrated an efficient quantitative release of emetine within 24 h, and this is further confirmed by its equipotency with emetine when tested in cancer cell lines.9 We have previously reported the synthesis of compound 10.9 Fmoc-protected amino acid (Fmoc-Pro-OH) was dissolved in 50% dichloromethane (CH2Cl2) in DMF followed by the addition of 4-amino benzyl alcohol and N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) was used as the coupling reagents. The crude products were purified by flash column chromatography to obtain the para-amidobenzyl alcohol intermediates in 84–92% yield.
In our preliminary investigation of the kinetics of the use of EEDQ as coupling reagent for amide bond formation, we found that extending the reaction time to 24 h did not cause any significant change in yield of product and/or the type of impurities (side products). EEDQ is also more attractive for our amide bond formation from free amine and carboxylic acid group because the reaction does not require any organic base. The use of EEDQ also eliminates the competing ester formation reaction that may occur between the primary alcohol of the para-aminobenzyl alcohol (PABA) and the amino group of the amino acid if other amide coupling reagents are used.13
Emetine was coupled with the alcohol group of the para-amidobenzyl alcohol intermediate in the presence of N,N′-disuccinimidyl carbonate (DSC) to afford the carbamate moiety in a two-step but one-pot reaction under inert atmosphere (Scheme 2). First, the intermediate benzyl alcohol was treated with a mixture of N,N′-disuccinimidyl carbonate (DSC) and DMAP in CH2Cl2. The reaction was monitored by TLC, and the para-amidobenzyl alcohol 9 was completely consumed after about 15–20 min. The succinimidyl carbonate produced is unstable and decomposed upon exposure to air while being purified by flash chromatography. It decomposed while still in the chromatographic eluent, CH2Cl2 solution, within 1 h of elution from the column. To overcome this problem, we re-designed the synthesis of carbamate to a one-pot synthetic strategy. First, we monitored the consumption of the para-amidobenzyl alcohol 9 and the formation of the intermediate succinimidyl carbonate by TLC. When the TLC showed that 9 had been completely consumed, a solution of emetine in DCM/DIPEA was then added to the reaction mixture containing the succinimidyl carbonate under argon atmosphere. The reaction mixture was then monitored by TLC at 15 min interval, and complete disappearance of the intermediate was observed in about 1.5 h. Removal of Fmoc from the product was done in 25% piperidine in DMF while it is in the crude product to give product 10 which was purified using preparative HPLC to furnish compound 10 in 50% yield.
Scheme 2.
Synthetic path for prodrug 11. Reagents and conditions: (i) EEDQ, DCM-DMF, 87%; (ii) 25% Piperidine/DMF, 65% (iii) Fmoc-Ala-OH, EEDQ, DCM-DMF, RT, 80%; (iv) DMAP, DSC, DCM, Emetine·2HCl, DIEA, Argon; (v) 25% Piperidine/DMF, 50%; (vi) HATU, 4, DIEA, DMF. (vii) 2% Hydrazine in DMF, 40%.
Compound 10 was coupled to peptide 4 in DMF in the presence of HATU and DIEA followed by the removal of the 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)isovaleryl (ivDde) with 2% hydrazine in DMF followed by purification by preparative HPLC to afford prodrug 11.
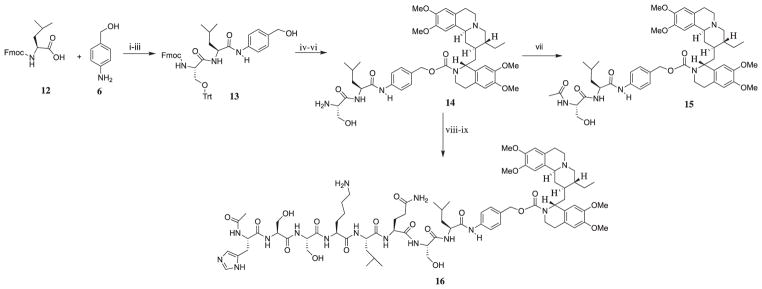
As shown in Scheme 3, the synthesis of prodrug intermediate 14 and 16 followed a similar route as that of 10 and 11. The amino acids alanine and proline were replaced with serine and leucine. Attempts to use 1% TFA in CH2Cl2 to remove the Trtyl group (Trt) in 14 was futile, because it decomposed the carbamate bond to emetine also. Thus, we carried out a stepwise reduction of the amount of TFA to obtain a TFA-dichloromethane mixture with less than 1% TFA that will remove the Trt group without decomposing the carbamate. After several experimenting attempts, 0.5% TFA was found suitable. After removal of the Fmoc group, compound 14 was treated with acetic anhydride to obtain 15 with the N-terminal capped with acetyl group. Compound 14 was coupled to 4 in the presence of HATU and DIEA. The protecting group iv-Dde group was removed from the ε amino group of lysine by treating the final product 16 with 2% hydrazine.
Scheme 3.
Synthetic path for prodrug intermediate 14 and prodrug 15. Reagents and conditions: (i) EEDQ, DCM-DMF, 92%; (ii) 25% Piperidine/DMF, 74% (iii) Fmoc-Ser(Trt)-OH, EEDQ, DCM-DMF, RT, 88%; (iv) DMAP, DSC, DCM, Emetine·2HCl, DIEA, Argon; (v) 25% Piperidine/DMF, 65%; (vi) 0.5% TFA in DCM. (vii) Ac2O, DIEA; (viii) HATU, 4, DIEA, DMF; (ix) 2% Hydrazine in DMF, 55%.
2.2. Biological studies
2.2.1. Investigation of PSA cleavage of prodrug 11
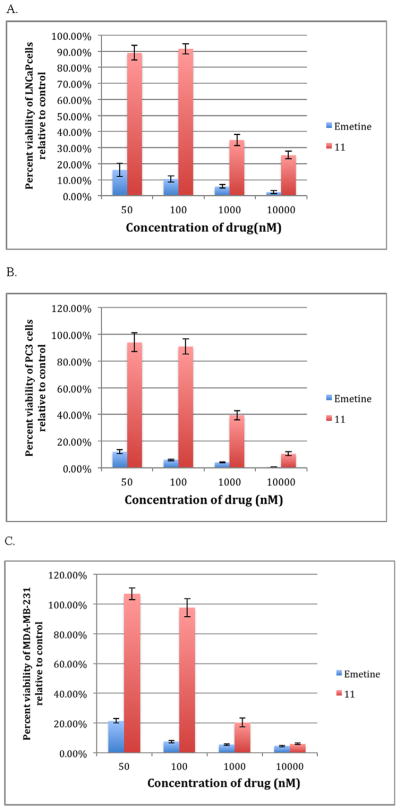
We have previously reported the utilization of an efficient and specific peptide substrate (His-Ser-Ser-Lys-Leu-Gln) of PSA in targeting small molecule to prostate cancer.10,11 Further it was shown that PSA could not easily cleave its substrates if the P1 position of the peptide substrate is directly coupled to bulky leaving groups due to steric hindrance.11 The inclusion of a P1′ amino acid greatly improved substrate hydrolysis. Thus, we coupled the PSA substrate Ac-His-Ser-Ser-Lys-Leu-Gln, 4 (HSSKLQ) to compound 10 via amide bond formation to give prodrug 11. As shown in the synthetic Scheme 1, the N-terminal of peptide 4 was capped with acetyl group to protect it from being degraded by amino-peptidases in the serum. Thus, we reasoned that prodrug 11 would be activated to emetine via a two-step enzyme activation process involving cleavage of HSSKLQ by PSA followed by cleavage of Ala-Pro by DPPIV. However, incubation of 11 with PSA in PSA buffer at 37 °C over a period of 24 h and monitored with LCMS did not release the expected precursor 10. This indicates that the presence of Alanine at the P1′ position makes it difficult for PSA to cleave its substrate. For in vitro cytotoxicity assay, LNCaP was used as the PSA producing human prostate cancer cell line while PC3 was used as non-PSA-producing cell.14 Consistent with the lack of activation to free emetine, when tested in PSA producing LNCaP in a 3-day exposure, prodrug 11 is relatively inactive compared to emetine, (Fig. 2A). The IC50 of 11 against LNCaP is 757 nM (about 24-fold higher than the IC50 of emetine, 31.6 nM). This remains the same even upon exogenous addition of increased PSA to the assay. 11 was also tested against two non-PSA producing cancer cell lines PC3 (human prostate cancer) and MDA-MB-231 (human breast cancer) and was also found similarly less active compared to emetine (Fig. 2B, C).
Fig. 2.
A comparison of the cytotoxicity of compounds 11 and emetine at different concentrations in (A) LNCaP; (B) PC3 and (C) MDA-MB-231.
Thus, for emetine to be released as free secondary amine at the N-2′, we need to develop an intermediary molecule similar to 10, that provides a more favorable P1′ for cleavage by PSA. It was earlier reported that in the analysis of the cleavage products of purified semenogelin I (SgI) and II (SgII) by PSA, the amino acid Ser was found at position P1′ at a high frequency among the products. 15 Also, the dipeptide Ser-Leu has been inserted at position P1′ and P2′ in some PSA-activated prodrugs of doxorubicin16,17 and TGX-D1.18 The Ser-Leu linker appears to be removed in these studies by aminopeptidases present ubiquitously in the extracellular fluid. Thus, we synthesized the emetine dipeptide Ser-Leu-PABC-Emetine, 14 as the general precursor.
In all the studies previously reported on the cleavage of Ser-Leu from small molecule drugs, the prodrugs have been reported to be equipotent with the small molecule drugs. However, the enzyme responsible for the cleavage of Ser-Leu dipeptide is not specified. Elsadek et al. carried out the cleavage in LNCaP tumor homogenate,17 but the homogenate has a mixture of enzymes that can cause diverse biochemical processes. Hence, it is difficult to know which enzyme in the mixture specifically does the cleavage. Tai et al. provided a cyclization mechanism for the cleavage when the Leu is coupled to an alcohol functional group to make an ester.18 Thus, to investigate the cleavage and stability of Ser-Leu-PABC-Emetine, 14, we incubated it for 24–72 h at 37 °C in PBS, purified PSA, 10% FBS containing media and serum containing LNCaP conditioned media. Incubation of 14 in these media conditions did not result in its hydrolysis to emetine or any product of hydrolysis upon analysis with LCMS (Table 1).
Table 1.
Hydrolysis of Ser-Leu-PABC-Emetine, 14.
| Incubation conditions at 37 °C | % After 72 h incubation
|
|
|---|---|---|
| Emetine | Ser-Leu-PABC-Emetine, 14 | |
| PBS | 0.0% | 100% |
| 10% FBS | 0.0% | 100% |
| Conditioned media | 0.0% | 100% |
| Purified PSA | 0.0% | 100% |
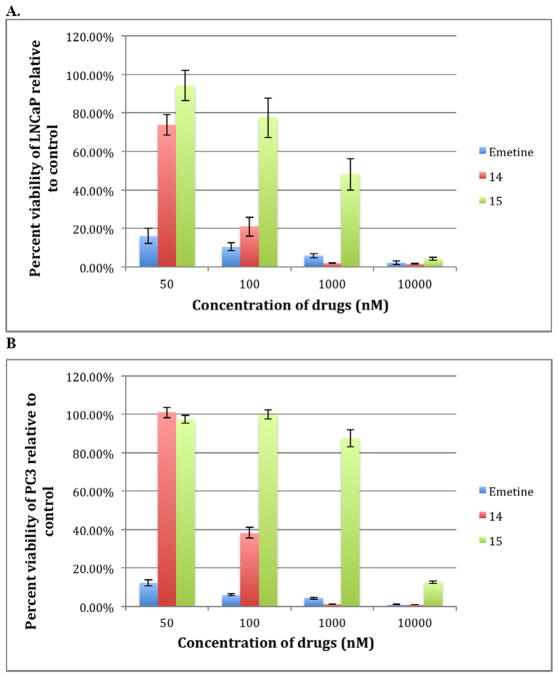
Since 14 did not hydrolyze to emetine in LNCaP conditioned media we hypothesized that the cleavage of Ser-Leu may require intracellular enzymatic action. If 14 is intracellularly converted to emetine, it would be equipotent with emetine in cytotoxicity. Thus, we tested the cytotoxicity potency of 14 in the presence of LNCaP and PC3 cells line (Fig. 3). Over a 3-day exposure, 14 showed significant potency in both cell lines that is comparable to emetine consistent with intracellular release of emetine as the potential mechanism of activation. Compound 14 showed a relatively similar cytotoxicity effect in both LNCaP and PC3 with IC50 below 100 nM in both cell lines.
Fig. 3.
Concentration dependent cytotoxicity of compounds 14 and 15 relative to emetine in (A) LNCaP–PSA producing cell line and (B) PC3 which does not produce PSA.
To further evaluate Ser-Leu linker release, we capped the N-terminal of 14 with an acetyl group to obtain 15 and a significant loss of potency was observed (Fig. 3). As observed with other N-2′ modified emetine derivatives, significant cytotoxicity for 15 was demonstrated only at 10,000 nM. This suggests that the enzyme cleaving the Ser-Leu dipeptide is an exopeptidase, requiring a free terminal amino group.
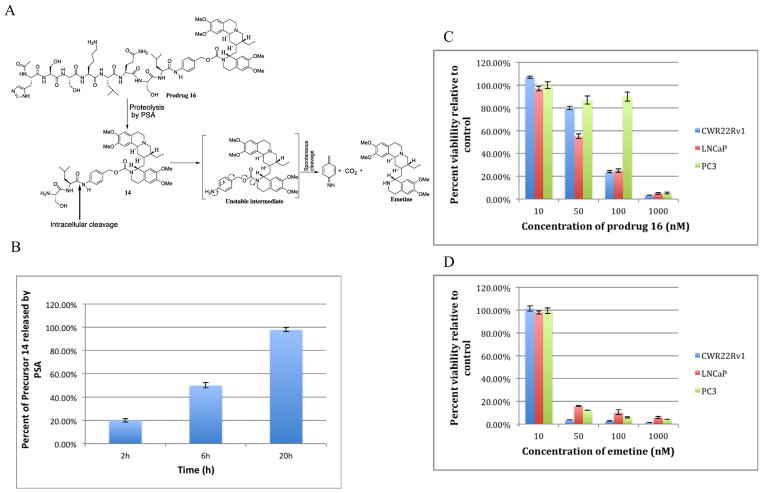
The next step in generating a PSA-activated prodrug of emetine is to couple 14 to the peptide 4 (Ac-His-Ser-Ser-Lys-Leu-Gln) to produce prodrug 16 [Ac-His-Ser-Ser-Lys-Leu-Gln-Ser-Leu-PABC-Emetine] (Fig. 4A). Prodrug 16 will only be activated to emetine when PSA cleaves the peptide substrate to produce 14 followed by intracellular removal of Ser-Leu leading to conversion to the unstable intermediate PABC-emetine which then undergoes spontaneous 1,6-benzyl elimination to release active emetine (Fig. 4A). Upon incubation with purified PSA, prodrug 16 was cleaved to the intermediate precursor 14 in a time dependent manner (Fig. 4B). As analyzed by LCMS, 20% of 14 was observed after 2 h incubation with PSA, 50% in 6 h and 98% at 20 h incubation. Further conversion of 14 to emetine was not observed with incubation with purified PSA. Stability studies on 16 were done in PBS and 10% FBS over a five-day incubation at 37 °C, and no sign of cleavage or any form of hydrolysis/decomposition was detectable. Since the final prodrug will be administered through the blood, we incubated 16 in human plasma for 48 h. Neither precursor 14 nor emetine was detected in the plasma.
Fig. 4.
(A) Schematic representation of the tandem activation of prodrug 16 beginning with cleavage of PSA substrate by PSA followed by intracellular dipeptide cleavage and spontaneous release of emetine; (B) Cleavage of the PSA substrate in prodrug 16 to liberate precursor 14 measured over 20 h; (C) Concentration dependent cytotoxicity studies of prodrug 16 in different prostate cancer cell lines; (D) Concentration dependent cytotoxicity studies of emetine in different prostate cancer cell lines to provide a simple comparison with prodrug 16 (Fig. 4C).
Having established the cleavage of 16 by PSA to produce 14, we then evaluated the cytotoxicity of prodrug 16 relative to emetine over a 3-day exposure in PSA producing LNCaP and CWR22Rv1 (human low PSA-producing prostate cancer cell line) and PC3 was used as non-PSA-producing human prostate cancer cell line (Fig. 4C, D, Table 2). As expected, the concentration dependent cytotoxicity of prodrug 16 shows a pattern that is consistent with the potency of 14. This indicates that the PSA expressed by the PSA producing cells caused the hydrolysis of 16 to 14. The activity of 16 depends not only on the cleavage of the PSA substrate, but also on the hydrolysis of 14 to liberate emetine. Overall the IC50 of prodrug 16 in PSA producing CWR22Rv1 and LNCaP are 75 nM and 59 nM respectively. These are about 2-fold higher than the IC50 of emetine in these cell line (Table 2). In the non-PSA producing PC3 cell employed in this study, the IC50 indicates that prodrug 16 is about 7–9 times less cytotoxic in the non-PSA producing cells than in the PSA producing cells.
Table 2.
In vitro cytotoxicity to compare emetine and prodrug 16 in different cell lines.
| IC50 (nM)
| |||
|---|---|---|---|
| CWR22Rv1 | LNCaP | PC3 | |
| Emetine | 30.80 ± 0.9 | 31.60 ± 2.4 | 29.43 ± 3.2 |
| Prodrug 16 | 75.81 ± 1.1 | 59.01 ± 2.9 | 537.7 ± 11.1 |
2.2.2. In vivo toxicity study of emetine and its N-2′ derived prodrugs and derivatives
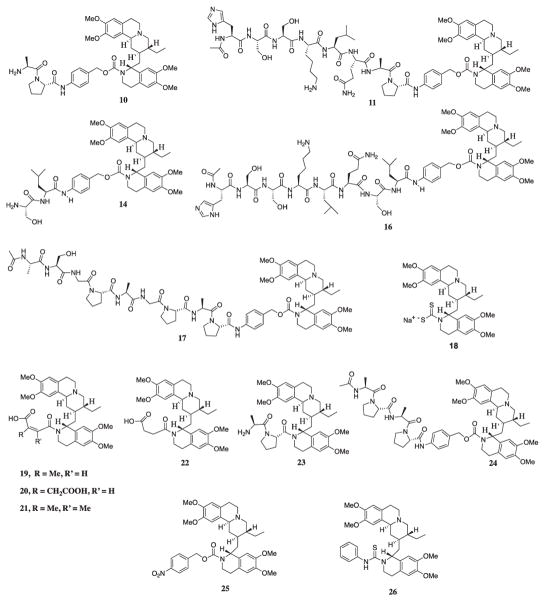
The toxicities associated with the use of emetine in clinical studies are dose dependent. Among them, the most relevant is its well-documented cardiotoxicity. We determined the maximum tolerable dose (MTD) of emetine by IV injection in mice of average body weight of 25 g to be 8 mg/kg (416 nmol/injection). Doses higher than this (i.e. 16 mg/kg) produced convulsions and death within 1 min of injection. In earlier studies, a dose of 8 mg/kg produced no therapeutic efficacy against PC-3 xenografts. Previously, we determined that the N-2′ modified emetine derivatives 18, 19, 20, 22 that were much less toxic in vitro compared to emetine were also significantly less toxic in vivo with MTD ≥ 100 mg/kg. These results coupled with the selective stability of 16 in human plasma and toxicity of the emetine prodrug 16 against PSA producing vs. non-PSA producing cell lines suggested further in vivo efficacy studies with 16 were warranted. However, prior to efficacy studies, we needed to establish the MTD for 16 to determine whether this prodrug strategy would sufficiently mitigate the in vivo toxicity of emetine to generate a therapeutic index for this compound.
Somewhat unexpectedly, a single injection of 16 at a dose of 50 mg/kg (804 nmol) which is only twice the MTD for emetine on an equimolar basis resulted in the same acute toxicity seen in emetine with convulsions observed within 1–2 min of IV injection followed by death. The MTD for 16 was established at 25 mg/kg (402 nmol) with mice showing acute distress followed by recovery within an hour of injection (Table 3). Similar results were observed in mice given 50 mg/kg prodrug 11 (822 nmol) and the FAP-activated prodrug 17 (908 nmol). While the in vitro studies demonstrated that these N-2′ modified emetine prodrugs were not cytotoxic until sufficient emetine was proteolytically released, the in vivo kinetics of convulsion and death within a few minutes was too rapid to be due to proteolytic conversion of these prodrugs to free emetine. The results suggested that in vivo toxicities such as cardiotoxicity described for emetine are not inhibited by modification of the N-2′ position.
Table 3.
In vivo toxicity evaluation of emetine and its derivatives/prodrugs in nude mice.a
| Compounds (drugs) | MTD mg/kg (nmol injected) | Effects/observations at higher doses |
|---|---|---|
| Emetine, 1 | 8 (416 nmol) | Convulsion and death within 1 min of injection at 16 mg/kg |
| 10 | 32 (1002 nmol) | Convulsion and death at higher doses |
| 11 | 25 (411 nmol) | Convulsion and death at higher doses |
| 14 | 50 (1541 nmol) | Toxic effect was observed at higher doses |
| 16 | 25 (402 nmol) | Convulsion started about 1 min post injection at higher doses |
| 17 | 25 (454 nmol) | Convulsion and death within 1 min at higher doses |
| 18 | >100 (4320 nmol) | No remarkable behavior even at higher doses. Mice remained alive |
| 19 | >100 (4218 nmol) | No remarkable behavior even at higher doses. Mice remained alive |
| 20 | >100 (3926 nmol) | No remarkable behavior even at higher doses. Mice remained alive |
| 21 | ND | 32 mg/kg (1318 nmol) is toxic like emetine |
| 22 | >100 (4305 nmol) | No remarkable behavior. Mice remained alive |
| 23 | ND | Convulsion and death within 1 min of injection at 16 mg/kg (617 nmol) |
| 24 | ND | Intermittent convulsion started 1 min post injection of 33 mg/kg dose (818 nmol). Death occurred after about 8 min |
| 25 | >64 (2425 nmol) | No remarkable behavior, no sign of toxicity. Mice remained alive |
| 26 | >100 (4063 nmol) | No remarkable behavior, no sign of toxicity. Mice remained alive |
ND = Not Determined.
To further evaluate and understand how this toxicity may be affected by the structure of emetine derivatives, we also tested the prodrug precursors or intermediates 10 and 14. The MTD for 10 was 32 mg/kg (1003 nmol) and 50 mg/kg for 14 (1541 nmol). The main difference between 10 and 14 is that 10 has Ala-Pro dipeptide group and 14 has Ser-Leu group. The difference in these two dipeptide groups may have caused conformational change that made 14 relatively less toxic (Fig. 5, Table 3). In earlier studies we demonstrated that while compounds 19 and 20 were well tolerated in vivo with MTD > 100 mg/kg, compound 21, which differed by only a methyl group, was lethal at a dose of 32 mg/kg. These results suggested that specific conformations might impart toxicity. We proceeded to generate 23, which differs from 10 in that it lacks the PABC linker and therefore adopts a conformation that causes steric hindrance, which prevents it from being activated by DPPIV. This causes 23 to be significantly less active in in vitro cytotoxicity assays compared to 10.9 However, compound 23 is lethal within 1–2 min of injection at a dose of 16 mg/kg (617 nmol). Giving that the removal of PABC from 10 to obtain 23 resulted in a molecule that is very toxic/lethal to animal like emetine, we next produced 24 to evaluate whether by adding an additional Ala-Pro dipeptide group to 10 we would change the conformation to a less toxic prodrug. However, 24 produced rapid death at a dose of 33 mg/kg (818 nmol).
Fig. 5.
Structure of different N-2′ derived emetine prodrugs and derivatives studied for in vivo toxicity.
At this point we proceeded to generate other emetine analogs that are not protease-activated prodrug to evaluate them for in vivo toxicity. So, we synthesized the carbamate derivative 25 which has almost the same linker (PABC) used in the peptidyl prodrugs (10, 11, 14, 16, 17, and 24) but with a nitro group at the para position. This molecule is about 200-fold less cytotoxic than emetine in vitro against different cancer cell lines. Upon IV injection, mice that received 64 mg/kg (2425 nmol) did not show any observable sign of toxicity. We also tested a thiourea derivative 26, that is also less cytotoxic in vitro than emetine because the N-2′ amine was derivatized, but upon in vivo test, no sign of toxicity was seen in mice that received up to 100 mg/kg (4063 nmol) of this molecule.
Based on these observations, we conclude that, while the functional activity of the N-2′ secondary amine of emetine does determine its in vitro cytotoxicity, it does not determine its acute in vivo cardiotoxicity. However, our data suggests that this in vivo toxicity is caused by the solution phase conformation adopted by emetine or its derivatives. We propose that such conformation may determine its potency to block the calcium or sodium channel. It appears that compounds 18, 19, 20, 22, 25 and 26 have adopted a solution phase conformation in which they no longer caused the toxicity that results in convulsion and death of the animals. The potency to block calcium and/or sodium channel has been significantly removed.
2.2.3. Moving forward: how will the emetine prodrugs succeed?
Emetine has been reported to block both the calcium and sodium channels in the heart, and this contributes to its cardiotoxic effect.19,20 From the structure activity relationship studies of in vivo toxicity of emetine and its derivatives (prodrugs), it is conclusive that the solution phase conformation of emetine is a critical factor in eliminating the in vivo cardiotoxicity. Thus, we hypothesize that to obtain a prodrug of emetine that will not present acute in vivo toxicity, we need to modify emetine on the aromatic ring of the tetrahydroisoquinoline ring (position C-5′, C-6′, C-7′, or C-8′, Fig. 1A) to modulate the conformation.
3. Conclusions
Overall, we have established a strategy for activating emetine prodrugs by the PSA for the purpose of obtaining PSA activatable emetine prodrugs within metastatic prostate tumor microenvironment. Although the precursor 10 (Ala-Pro-PABC-Emetine) is efficiently activated by DPPIV to give emetine, and it is a good intermediate for FAP activated emetine prodrug,9 it is not adaptable for PSA activated prodrug. We established in this study that precursor 14 (Ser-Leu-PABC-Emetine) is a good intermediate for PSA cleavable prodrug 16 which is activated in two steps. While PSA activation of 16 to 14 occurs extracellularly in the presence of PSA, we believe that the activation of 14 to emetine occurs intra-cellularly.
This study also includes an extensive study on the in vivo toxicity of emetine and its derivatives. It is evident that all the N-2′ derived emetine derivatives have a significantly reduced cytotoxicity. 6–9 However, our in vivo studies show that while some of these derivatives do not produce acute lethal toxicity like emetine, some of them are still toxic. We propose here that the in vivo toxicity (cardiotoxicity, lethality) of emetine, and its derivatives may not be controlled by the N-2′ secondary amine alone but by the solution phase conformation adopted by the molecule. Thus, modifying emetine derivatives on the tetrahydroisoquinoline ring will produce new derivatives with a solution phase conformation that does not induce the acute lethal toxicity. N-2′ derived prodrugs will then be synthesized from these derivatives.
4. Experimental section
4.1. Chemistry
4.1.1. General information
All solvents and reagents used were bought from commercial sources and used without further purification. Peptide synthesis was done as solid-phase synthesis using AAPPTec Apex 396 40-well peptide synthesizer. The 1H and 13C NMR spectra were obtained on a Bruker Avance III 500 MHz NMR spectrometer at 500 MHz and 125 MHz, respectively in deuterated chloroform (CDCl3) or deuterated methanol (CD3OD). Chemical shifts are in δ units (ppm) with TMS (0.00 ppm), CHCl3 (7.27 ppm), or CH3OH (3.34 ppm) as the internal standard for 1H NMR, and CDCl3 (77.00 ppm) or CD3OD (49.90 ppm) for 13C NMR. Mass spectra were obtained on Bruker Esquire 3000 Mass Spectrometer equipped with ESI. Analytical thin-layer chromatography was performed using 0.25 mm precoated silica gel 60 F254 plates (Analtech Uniplates). Flash column chromatography was performed using silica gel 60 (200 × 400 mesh, Sorbent Technologies) with the indicated solvent. Purity of the compounds was determined with reverse phase-HPLC. The purity of all the compounds was determined to be >95%.
4.1.1.1. HPLC methods
In this study, the reverse phase HPLC studies were carried out using the following methods.
4.1.1.2. HPLC method 1
This method was used for analytical reversed-phase HPLC for the analysis of the synthetic emetine analogs and prodrugs (to determine purity) and for analysis of cleavage studies of emetine prodrugs.
4.1.1.3. HPLC unit
Waters Delta 600 Controller equipped with a variable wavelength UV–vis detector (Waters 2487 Dual λ Absorbance Detector) set to detect at 215 nm and 285 nm and a Vydac 218TP54 (C18, 5 μm, 4.6 mm. i.d. × 250 mm) analytical column.
4.1.1.4. Software
Empower 2.
4.1.1.5. HPLC conditions
Flow rate: 1.3 mL/min; mobile phase A: 5% MeCN, 95% water and 0.1% TFA. Mobile phase B: 100% MeCN, 0.1% TFA. Gradient: 0–2 min 100 % mobile phase A; 2–20 min gradual change to 100% mobile phase B; 20–25, 100% mobile phase B; 25–27 min gradual change to 100% mobile phase A; 27–30 min, 100% mobile phase A. Injection volume: 100 μL
4.1.1.6. HPLC method 2
This method was used for preparative purification of compounds.
4.1.1.7. HPLC unit
Waters Delta 600 Controller equipped with a variable wavelength UV–vis detector (Waters 2487 Dual λ Absorbance Detector) set to detect at 215 nm and 285 nm and a Luna (C18, 10 μm, 21.20 mm. i.d. × 250 mm) preparative column.
4.1.1.8. Software
Empower 2.
4.1.1.9. HPLC conditions
Flow rate: 25 mL/min; mobile phase A: 5% MeCN, 95% water and 0.1% TFA. Mobile phase B: 100% MeCN, 0.1% TFA radient: 0–4.0 min, 100% mobile phase A; 4.0–20.0 min gradual change to 100% mobile phase B; 20.0–20.5 min 100% mobile phase B; 20.5–21.0 min, sharp change to mobile phase A; 21.0–24.0 min mobile phase A. Injection volume: 1.0 mL.
4.1.2. General procedure for peptide synthesis (Scheme 1)
Peptides were prepared by solid-phase synthesis using AAPPTec Apex 396 40-well peptide synthesizer. The product was obtained using standard Fmoc protocol, starting with preloaded H- Gln (Trt)-OH onto a 2-ClTrt resin. The amino acids (0.1 mmol) were coupled for 1 h using double coupling conditions. HOBt (5 equiv) was used as activating reagent; 5 equiv of Fmoc-protected amino acids were used with 0.5 M DIC in NMP. Upon reaction completion, the peptide on the resin was washed consecutively with NMP, methanol, and CH2Cl2. The N-terminal of the peptide was then capped with Ac2O (6 M equivalent) in the presence of DIEA (6 M equivalent) in 10 mL CH2Cl2. The product obtained was washed with CH2Cl2. Cleavage of peptide from the resin together with the removal of tBu and Trt groups was accomplished with 60% TFA in CH2Cl2. All solvents and volatiles were evaporated from the reaction, and the peptide was precipitated in cold diethyl ether. The residue obtained was purified by the reverse phase preparative HPLC using the HPLC method 2. Eluent fraction was lyophilized to give desired compounds as off-white powder.
4.1.3. General procedure for the synthesis of the N-2′ derived analogs of emetine with self-cleaving PABC linker (Schemes 2 and 3)
Respective Fmoc-protected amino acid (1.2 M equivalent) was dissolved in N,N dimethylformamide (DMF). To this was added 1.2 M equivalent of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) followed by a quantity of dichloromethane (CH2Cl2) equal to the volume of DMF. The solution was stirred for 5 min at room temperature and 4-aminobenzyl alcohol (1.0 M equivalent) was added which turned the colorless solution to yellow. The mixture was stirred at room temperature for 8 h. Solvent was evaporated under reduced pressure and the residue was purified with flash column chromatography on silica gel using gradient elution with 5% ethyl acetate in CH2Cl2 to elute less polar impurities followed by elution with 50% EtOAc in CH2Cl2 to elute the desired product (compound 7 or 13).
To remove Fmoc, each desired product was dissolved in a solution of 25% piperidine in dimethylformamide (DMF), and the mixture was stirred at room temperature for 15 min. Solvent was evaporated from the mixture under reduced pressure. 10.0 mL DMF was added to the oily residue and 200 mL hexanes was added to it. The mixture was stirred for 1.0 min in hexanes and allowed to settle. The hexane layer was removed using separatory funnel. This treatment with hexanes was repeated 3 times and the DMF was evaporated from the DMF layer to give an oily residue. Pure product is then precipitated from cold diethyl ether to give the desired amino acid amide with from alpha amino group.
A viscous solid was obtained and this was triturated in hexanes to obtain a free amine group to which the next amino acid was coupled using the same EEDQ coupling process described above to obtain 9 and 13.
4.1.3.1 (9H-Fluoren-9-yl)methyl (S)-2-((4-(hydroxymethyl)phenyl)-carbamoyl)pyrrolidine-1-carboxylate (7)
This was first synthesized as described above: Yield 87%, white powder, ESI-MS m/z 443.1 ([C27H26N2O4+1]+ calcd. 443.5; HPLC (method 1), tR is 10.2 min, purity 97.8%.
4.1.3.2. (S)-N-(4-(Hydroxymethyl)phenyl)pyrrolidine-2-carboxamide (8)
Yield 65%, pale yellow solid, 1H NMR (500 MHz, CDCl3) δ 1.78–1.86 (1H, m), 1.87–1.94 (1H, m), 2.18–2.27 (2H, m), 2.85 (1H, s), 2.95–3.04 (1H, m), 3.05–3.12 (1H, m), 3.39–3.35 (1H, m), 3.79 (1H, dd, J = 2.5, 6.3 Hz), 4.52 (2H, s), 4.58 (1H, s), 7.33 (2H, d, J = 7.5 Hz), 7.58 (2H, d, J = 7.5 Hz); 13C NMR (125 MHz, CDCl3) δ 29.6, 34.8, 50.7, 64.5, 67.5, 123.6 (2C), 131.2 (2C), 141.1, 141.6, 177.9. HPLC (method 1) purity 98.6%.
4.1.3.3. Compound 8 was coupled to Fmoc-Ala-OH using the same amide bond formation method with EEDQ to produce 9. (9H-Fluoren-9-yl)methyl ((S)-1-((S)-2-((4-(hydroxymethyl)phenyl)carbamoyl) pyrrolidin-1-yl)-1-oxopropan-2-yl)carbamate (9)
Yield 80%, white powder; 1H NMR (500 MHz, CD3OD) δ 1.37 (3H, d, J = 7.5 Hz), 1.93–2.09 (2H, m), 2.10–2.21 (1H, m), 2.23–2.35 (1H, m), 3.31–3.35 (2H, m), 3.60–3.69 (1H, m), 3.78–3.87 (1H, m), 4.20–4.27 (2H, m), 4.31–4.50 (2H, m), 4.58 (3H, br s), 7.25–7.32 (4H, m), 7.34–7.49 (4H, m), 7.56 (2H, d, J = 7.5 Hz), 7.81 (2H, d, J = 7.5 Hz), 7.99 (1H, s). 13C NMR (125 MHz, CD3OD) δ 17.4, 26.3, 30.8, 48.2, 49.8, 50.2, 62.5, 64.8, 68.1, 120.9 (2C), 121.2 (2C), 126.3 (2C), 128.1, 128.3, 129.0 (2C), 129.3 (2C), 138.6, 138.9, 142.7 (2C), 145.1, 145.4, 158.4, 172.8, 174.1. ESI-MS m/z 513.1 ([C30H31N3O5+1]+ calcd. 513.6 and m/z 536.3 ([C30H31N3O5+Na]+ calcd. 536.6); Purity was determined by HPLC to be 99%.
4.1.4. Conversion of emetine secondary amine to carbamate to afford compounds 10 and 14 (Schemes 2 and 3)
Reaction of primary alcohol and amine to form carbamate moiety was utilized in this synthesis. Each of the dipeptide coupled para amido benzyl alcohol 9 and 13 was coupled to emetine as described below: 4-(dimethylamino) pyridine (DMAP) (1 M equivalent) was added to a solution of N,N′-disuccinimidyl carbonate (DSC) (1.2 M equivalent) solution in 20% DMF/DCM mixture under argon atmosphere. The respective alcohol (1.0 M equivalent) solution in dichloromethane was then added to this DMAP and DSC mixture and the reaction was stirred at room temperature for 15–20 min. TLC indicated the complete consumption of the alcohol between 15 and 20 min of the reaction. Then, emetine.2HCl (1.2 M equivalent) solution in 15 mL CH2Cl2 and 4 M equivalent of N,N-diisopropylethyl amine (DIEA) was added. The reaction mixture was stirred at room temperature under argon and the progress was monitored with TLC and ESI-MS. TLC indicated the completion of the reaction in about 1.5 h. Solvent was then evaporated completely from the reaction mixture under reduced pressure and the residue obtained was purified with flash column chromatography using gradient elution. 100% ethyl acetate eluted the impurities less polar than the desired compound followed by elution with 10% MeOH in EtOAc to obtain Fmoc protected product. This product was dissolved in a solution of 25% piperidine in dimethylformamide (DMF) and the mixture was stirred at room temperature for 15 min. Solvent was evaporated from the mixture under reduced pressure. A viscous solid was obtained and this was triturated in hexanes. The final residue obtained was purified with preparative HPLC using method 2 to give the desired compound.
4.1.4.1. 4-((S)-1-(L-Alanyl)pyrrolidine-2-carboxamido)benzyl (R)-1-(((2R,3R,11bS)-3-ethyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[ 2,1-a]isoquinolin-2-yl)methyl)-6,7-dimethoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate (10)
Yield: 50%, pale yellow powder; 1H NMR (500 MHz, CD3OD) δ 0.90 (3H, t, J = 7.4 Hz), 1.08–1.20 (3H, m), 1.23–1.42 (6H, m), 1.52–1.68 (1H, m), 1.92–2.09 (2H, m), 2.11–2.35 (3H, m), 2.51–2.82 (3H, m), 2.82–2.96 (1H, m), 3.02–3.18 (4H, m), 3.2–3.38 (3H, m), 3.62–3.73 (4H, m), 3.81 (3H, s), 3.83 (3H, s), 3.87 (3H, s), 3.99 (3H, s), 4.21–4.38 (1H, m), 4.45–4.65 (1H, m), 4.90–5.12 (1H, m), 5.30 (1H, d, J = 12.5 Hz), 5.38 (1H, d, J = 10.8 Hz), 6.71 (2H, s), 6.77 (1H, s), 6.80 (1H, s), 6.91 (1H, s), 7.34 (2H, d, J = 8.0 Hz), 7.50 (2H, d, J = 8.0 Hz); 13C NMR (125 MHz, CD3OD) δ 10.1, 19.2, 24.5, 26.1, 30.1, 31.0, 31.5, 35.2, 35.8, 37.1, 38.2, 39.9, 42.2, 47.9, 50.1, 51.2, 56.2 (2C), 56.9 (2C), 62.1, 63.2, 64.6, 70.5, 111.1, 112.3, 112.9, 113.8, 121.8 (2C), 127.0, 127.8, 128.0 (2C), 130.1, 131.9, 135.0, 139.8, 147.1 (2C), 149.2 (2C), 157.8, 172.7, 175.2. ESI-MS m/z 798.8, ([C45H59N5O8+-H]+ calcd. 799.0), HPLC tR is 13.9 min and purity is 98.6%.
4.1.4.2. 4-((S)-2-((S)-2-Amino-3-hydroxypropanamido)-4-methyl-pentanamido) benzyl (R)-1-(((2R,3R,11bS)-3-ethyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)methyl)-6,7-dimethoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate (14)
After the synthesis was completed in a manner similar to 10 above, removal of Trtyl group was required. In a typical experiment, 300 mg of sample was dissolved in 10 mL DCM and to this was added a solution of 1% TFA in DCM (10 mL) gradually. The reaction mixture was then stirred at room temperature for 30 min. Solvent was evaporated and the mixture was purified by preparative HPLC using method 2. This was lyophilized to give desired compound 14.
Yield: 65%, off white powder; 1H NMR (500 MHz, CD3OD) δ 0.91 (3H, t, J = 7.4 Hz), 0.95–1.17 (6H, m), 1.30–1.65 (6H, m), 1.67–1.85 (6H, m), 2.15–2.35 (2H, m), 2.71–2.89 (5H m), 2.91–3.1 (2H, m), 3.20–3.47 (4H, m), 3.6–3.75 (1H, m), 3.81 (6H, s), 3.87 (3H, s), 3.91 (3H, s), 4.1–4.2 (2H, m), 4.32–4.40 (1H, m), 4.53–4.62 (1H, m), 4.91 (2H, m), 5.2 (1H, m), 6.75 (2H, s), 6.82 (1H, s), 6.91 (1H, s), 7.35 (2H, d, J = 8.0 Hz), 7.48 (2H, d, J = 8.0 Hz).
13C NMR (125 MHz, CD3OD) δ 13.8, 22.9 (2C), 24.1, 27.3, 28.5, 34.5 (2C), 38.5, 39.5, 41.4 (2C), 48.7, 52.3, 54.2, 56.5 (6C), 60.6 (2C), 67.8, 70.2, 109.3 (2C), 111.5 (2C), 121.2 (2C), 125.7 (2C), 129.3 (2C), 131.4 (3C), 137.8, 143.5 (2C), 147.8 (2C), 161.2, 173.8 (2C).
MALDI-MS m/z 831.17, ([C46H63N5O9+H]+ calcd. 831.04), HPLC tR is 15.30 min and purity is 98.9%.
4.1.4.3. 4-((S)-2-((S)-2-Acetamido-3-hydroxypropanamido)-4-methyl-pentanamido) benzyl (R)-1-(((2R,3R,11bS)-3-ethyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)methyl)-6,7-dimethoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate (15)
150 mg of 14 was dissolved in DCM (10 mL) and 5 mL of acetic anhydride was added followed by 0.5 mL of DIPEA. The reaction mixture was stirred at room temperature for 1.5 h. Solvent was then evaporated. The crude residue was purified using preparative HPLC (method 2) and solvent evaporated. Yield 83.9%, yellow powder; MALDI-MS mz 872.12, ([C48H65N5O10]+ calcd. 872.07). HPLC tR is 16.00 min and purity is 97.7%.
4.1.5. General procedure for the synthesis of peptidyl-emetine prodrugs with the self immolative linker (Schemes 2 and 3)
The peptide, compound 4 (3 M equivalent) was dissolved in dimethyl formamide and DIEA (8 M equivalents) was added. This was followed by HATU (3 M equivalents). The mixture was stirred for 5 min at room temperature and 1 M equivalent of either compound 10 or 14 was added. The reaction mixture was stirred at room temperature overnight. Solvent was evaporated from the mixture under reduced pressure and the crude product was treated with 2% hydrazine in DMF (v/v) for 5 min. Solvent was evaporated and residue obtained was purified by the reverse phase preparative HPLC using the HPLC method 2 to afford the intermediate from which the ivDde protecting group was removed by treating the compound with 2% hydrazine in DMF. This was further purified with preparative HPLC to give the desired compounds (11 or 16) which was lyophilized to give powdered products.
4.1.5.1
Ac-His-Ser-Ser-Lys-Leu-Gln-Ala-Pro-PABC-Emetine (11): Yield 45%, yellow powder; ESI-MS m/z 1521.15, ([C76H109N15O18+H]+ calcd. 1521.80) HPLC tR is 14.70 min and purity is 97.6%.
4.1.5.2
Ac-His-Ser-Ser-Lys-Leu-Gln-Ser-Leu-PABC-Emetine (16): Yield 35%, light yellow powder; MALDI-MS m/z 1553.98, ([C77H113N15O17+H]+ calcd. 1553.85) HPLC tR is 14.5 min and purity is 98.5%.
4.1.5.3
The full description of the synthesis of compounds 17–26 have been previously report.6–9
4.2. Biology
Purified native human prostate specific antigen was purchased from Bio Rad (Hercules, CA).
The human androgen receptor positive (LNCaP), androgen independent CRW22Rv1, androgen receptor negative PC3 prostate cancer cell lines, the human breast cancer cell lines MDA-MB-231 were all purchased from the American Type Culture Collection (Manassas, VA). Dulbecco’s modified Eagle medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 medium, L-glutamine, penicillin- streptomycin were all obtained from Invitrogen (Carlsbad, CA); and the fetal bovine serum (FBS) from Thermo Scientific (Waltham, MA). LNCaP, CRW22Rv1 and PC3 cells were grown in cell culture flasks in RPMI culture medium and MDA-MB-231 was grown in DMEM with phenol red (GIBCO) supplemented with only 10% fetal bovine serum, 1% L-glutamine and 1% Penicillin-Streptomycin. Cells were cultured in a humidified atmosphere of 95% air and 5% carbon dioxide at 37 °C. To sub-culture cells for experiments, cells growing as monolayer cultures were released from the tissue culture flasks by treatment with 0.05% trypson/EDTA. Cell population density was determined with the aid of hemocytometer. For all the in vitro experiments, cell-growth was maintained in the log phase and the cells were used while still in this logarithmic growth phase.
4.2.1. Stability of test compounds in buffer
The stability of all tested compounds was evaluated in aqueous solutions to ascertain that hydrolysis observed are due to enzyme mediated hydrolysis and not poor aqueous stability. Solutions of tested compounds (10 mM in DMSO) were added to PBS buffer without any enzyme to make a final concentration of 200 μM and 2% DMSO (v/v). The mixtures were incubated at 37 °C for 48 h in a shaking incubator. To measure the stability of the compounds 50 μL of cold acetonitrile was added and the mixture was then centrifuged at 13,000 rpm for 6 min. The supernatant was analyzed by the HPLC (using method 1, Section 4.1.1) and ESI mass spectroscopy.
4.2.2. PSA-mediated hydrolysis of prodrug ex-vivo
Solutions of each compound tested (10 mM in DMSO) were diluted in PSA buffer (50 mM Tris.HCl, 100 mM NaCl, pH 7.4) to give a final concentration of 200 μM. Purified PSA was then added to give a final PSA concentration of 20 μg/mL. These prodrug-PSA mixtures were incubated at 37 °C for 24 h in a shaking incubator. To evaluate the extent of hydrolysis at different time points, 50 μL was withdrawn from the mixture and 100 μL of cold acetonitrile was added to stop the reaction. The mixture was placed on ice for 10 min and then centrifuged at 13,000 rpm for 6 min. This was followed by analytical HPLC and mass spectrometry analysis. Similar experimental design was done in the absence of PSA using PBS buffer, 10% FBS in PBS, and conditioned RPMI growth media. This conditioned RPMI media was obtained by centrifuging the growth medium in which LNCaP cell lines have grown to about 95% confluency. The supernatant obtained from centrifuging the medium forms the conditioned medium. In all these assays the final DMSO composition is 2.0% (v/v).
4.2.3. In vitro efficacy (cytotoxicity) of emetine analogs and prodrugs in cancer cell lines
This was done as reported in our previous papers.6–9 To determine the efficacy of emetine and its prodrug analogs in cancer cell lines LNCaP was plated at a density of 5 × 103 in 100 μL of growth medium per well. PC3, CWR22Rv1 and MDA-MB-231 were plated at a density of 2.5 × 103 cells in 100 μL of appropriate growth medium per well in 96-well plates. These cells were incubated for 24 h to allow attachment to the tissue culture plates. Serial dilutions (10 μM, 50 μM, 100 μM, 1000 μM, 10,0000 μM) of each drug (emetine and its derivatives) were made in sterile DMSO and these were further diluted 500 times (20 nM, 100 nM, 200 nM, 2000 nM, 20,000 nM) using respective medium. 100 μL of the growth medium was taken from each of these drug concentrations and added (in eight replicates) to the cells in the 96 well-plate to give final drug concentrations of 10 nM, 50 nM, 100 nM, 1000 nM, and 10,000 nM of the drug. This gives a uniform 0.1% v/v DMSO concentration in all drug treated cells. A vehicle control of 0.2% v/v and blank of 0.0% v/v DMSO in the growth medium were made. 100 μL of these was also added in eight replicates to the cells plated in 100 μL of medium in the 96 well-plate to make vehicle of 0.1% DMSO and blank control of 0% DMSO. Each experiment was repeated at least twice. Population of viable cells was determined on day zero and each of the 3rd and 5th day of incubating the cells with the drugs using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay.
The Cell Titer 96® Non-Radioactive Cell Proliferation Assay manufactured by Promega Corporation was employed for this study. This is based on cellular conversion of a tetrazolium salt into a formazan product that can be measured easily using a 96-well plate reader. The proliferation assays were performed according to the manufacturers instructions. Absorbance was recorded at 570 nm with a correction wavelength of 650 nm on a SpectraMax Plus384 absorbance micro-plate reader (Molecular Devices, Sunnyvale, CA). In both the vehicle and blank control, the cell growth was in the logarithmic phase from day zero to the 5th day.
4.2.4. In vivo toxicity studies of emetine derivatives and prodrugs in mice
Mice were purchased from Taconic. Mouse care and treatment was done in line with the guidelines of the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. Eight weeks old male Balb/c mice were injected intravenously with different doses of emetine and its derivatives. The average weight for the mice used is 25 g. Each drug molecule was given to the mice at different doses ranging from 8 MPK to 100 MPK by intravenous (IV) injection. Each experiment or dosage was done in triplicate. Drug solutions were made either in 1% DMSO in saline or in 10% cremophor in saline. Mice received 200 μL bolus of each dose. Mice in the control experiment received 200 μL ml of 1% DMSO in saline or 10% cremophor in saline at every dosage.
Supplementary Material
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bmc.2017.11.015.
References
- 1.Júnior WSF, Cruz MP, dos Santos LL, Medeiros MFT. J Herb Med. 2012;2:103–112. [Google Scholar]
- 2.Grollman AP. Proc Natl Acad Sci USA. 1966:1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinboye ES, Bakare O. Open Nat Prod J. 2011;4:8–15. [Google Scholar]
- 4.Jimenez A, Carrasco L, Vazquez D. Biochemistry. 1977;16:4727–4730. doi: 10.1021/bi00640a030. [DOI] [PubMed] [Google Scholar]
- 5.Troconis M, Ma W, Nichols DE, McLaughlin J. J Comput Aided Mol Des. 1998;12:411–418. doi: 10.1023/a:1008019720578. [DOI] [PubMed] [Google Scholar]
- 6.Akinboye ES, Rosen DM, Denmeade SR, Kwabi-Addo B, Bakare O. J Med Chem. 2012;55:7450–7459. doi: 10.1021/jm300426q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akinboye ES, Bamji ZD, Kwabi-Addo B, et al. Bioorg Med Chem. 2015;23:5839–5845. doi: 10.1016/j.bmc.2015.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamji ZD, Washington KN, Akinboye E, Bakare O, Kanaan YM, Copeland RL. Anticancer Res. 2015;35:4723–4732. [PubMed] [Google Scholar]
- 9.Akinboye ES, Brennen WN, Rosen DM, Bakare O, Denmeade SR. Prostate. 2016;76:703–714. doi: 10.1002/pros.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denmeade SR, Jakobsen C, Janssen S, et al. J Natl Cancer Inst. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- 11.Denmeade SR, Nagy A, Gao J, Lilja H, Schally AV, Isaacs JT. Cancer Res. 1998;58:2537–2540. [PubMed] [Google Scholar]
- 12.Denmeade SR, Lou W, Malm J, Lovgren J, Lilja H, Isaacs JT. Cancer Res. 1997;57:4924–4930. [PMC free article] [PubMed] [Google Scholar]
- 13.Zacharie B, Connolly TP, Penney CL. J Org Chem. 1995;60:7072–7074. [Google Scholar]
- 14.Tai S, Sun Y, Squires JM, et al. Prostate. 2011;71:1668–1679. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malm J, Hellman J, Hogg P, Lilja H. Prostate. 2000;45:132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Garsky VM, Lumma PK, Feng D-M, et al. J Med Chem. 2001;44:4216–4224. doi: 10.1021/jm0101996. [DOI] [PubMed] [Google Scholar]
- 17.Elsadek B, Graeser R, Warnecke A, et al. ACS Med Chem Lett. 2010;1:234–238. doi: 10.1021/ml100060m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai W, Shukla RS, Qin B, Li B, Cheng K. Mol Pharm. 2011;8:901–912. doi: 10.1021/mp200007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemmens-Gruber R, Karkhaneh A, Studenik C, Heistracher P. Br J Pharmacol. 1996;117:377–383. doi: 10.1111/j.1476-5381.1996.tb15202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmens-Gruber R, Studenik C, Karkhaneh A, Heistracher P. J Cardiovasc Pharmacol. 1997;30:554–561. doi: 10.1097/00005344-199711000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.